Abstract
Objectives
High prevalence of lower urinary tract symptoms (LUTS) consistent with benign prostate hyperplasia (BPH) is associated with obesity and prostatic inflammation. Here, we investigated whether chemokines associated with obesity and prostatic inflammation can be measured in normally voided urine of BPH/LUTS patients to demonstrate the mechanistic association between obesity and BPH/LUTS.
Methods
Frozen urine specimens of BPH/LUTS patients enrolled in the Nashville Men's Health Study were sent for blinded analysis to University of Pittsburgh. Thirty patients were blocked by their AUA-SI (>7 or ≤7) and prostatic enlargement (<40, 40–60, >60 cc). Clinical parameters including age, prostate size, and medications were derived from chart review. CXC chemokines (CXCL-1, CXCL-8, and CXCL-10), CC chemokines (CCL2 and CCL3), and sIL-1ra were measured in thawed urine using Luminex™ xMAP® technology and ELISA for NGF.
Results
Urinary CCL2 levels were several fold higher compared with the other six proteins, of which CCL3 was detectable in less than one-fourth of patients. Urine levels of sIL-1ra and CXCL-8 were significantly associated with increasing BMI and waist circumference in BPH patients. CXCL-8 showed a marginal association with overall AUA-SI scores, as well as obstructive (p = 0.08) symptom sub-scores. Prostate volume was inversely and marginally associated with urinary CXCL-10 (p = 0.09).
Conclusions
Urine levels of CXCL-8, CXCL-10, and sIL-1ra were associated with varying degrees with LUTS severity, prostate size, and obesity, respectively. These findings in urine are consistent with past studies of chemokine levels from expressed prostatic secretions and demonstrate the potential of noninvasively measured chemokine in urine to objectively classify BPH/LUTS patients.
Keywords: AUA-SI, BPH, Chemokines, Obesity, Urine
Introduction
BPH/LUTS was earlier thought to be related to prostatic enlargement, but the relationship did not find support in recent studies [1]. Evidence from a large clinical studies including Medical Therapy of Prostatic Symptoms MTOPS and REDUCE trial suggested that risk for BPH/LUTS is associated with intra-prostatic infiltration of inflammatory cells [2]. Inverse association of LUTS with daily use of nonsteroidal anti-inflammatory drugs by BPH patients in a large Olmsted county study [3] further supported the role of inflammation.
In addition, there is epidemiological evidence for a modest overall increased prevalence of BPH/LUTS in obese subjects [4]. Although the precise mechanisms by which obesity influences the risk of BPH/LUTS remain unclear, a theory of causation can be supported by animal studies which provide biological underpinnings for the association between the two disorders [5]. Obesity is characterized by enlarged depots of adipose tissue, which are hormonally active and secrete pro-inflammatory cytokines and chemokines that can influence local adipocyte biology as well as prostate health [6].
Since obesity-associated inflammation is not localized to one organ, it is phenotypically different from acute inflammation and is more akin to the chronic, low-grade inflammation [6]. Inflammation originating in adipose tissue involves activation of inflammatory pathways following nutrient sensing by inflammasome [6]. Chemotactic recruitment of macrophages mediated by chemokine CCL2 [7] is considered a key step in adipose tissue-mediated inflammation. Considering the limitations of clinical research, chemokines levels in urine represent a potential means to clarify the pathogenic mechanisms underlying the link between obesity and BPH/LUTS.
Furthermore, paracrine signaling mediated by chemokines is also considered essential for normal organogenesis as well as diseases afflicting prostate [8]. These characteristics of chemokines make them suitable for assessing the transition from healthy to disease states involving inflammation [9]. Moreover, the continuous scale of chemokine expression can improve the classification of BPH/LUTS patients and overcome the constraints of binary outcomes dependent on detecting infiltration of inflammatory cells in prostate biopsy [10]. Previous studies have already reported association of elevated chemokines in prostate tissue and prostatic fluid with clinical attributes of BPH/LUTS patients [11, 12]. Here, we investigated whether chemokines associated with obesity and prostatic inflammation can be measured in normally voided urine of BPH/LUTS patients and whether obesity alters the pheno-type of BPH/LUTS.
Methods
Study population
We randomly selected 30 participants from Nashville Men's Health Study, who previously provided written informed consent with guarantees of confidentiality prior to data collection in accordance with the Vanderbilt University IRB. Men scheduled for a diagnostic prostate biopsy between 2010 and 2011 were selected for this urine analysis study. Demographic and clinical data including medications were obtained by chart review of eligible patients. Measures of body size and weight were collected by a trained research staff member at the time of recruitment using standardized protocols. LUTS severity was assessed by the AUA symptom index (AUA-SI), and prostate volume (cm3) was measured by transrectal ultrasound and all subjects recorded their current medications on a medication record form. Limited tissue material obtained from prostate needle biopsy was only enough for diagnosis and not available for this research. We also collected from the chart review all the data on prescription and nonprescription medications to treat cardiovascular disease and BPH.
Study design
Eligible participants were 40 years or older with BPH/ LUTS, and patients with any evidence of cancer, high-grade prostatic intraepithelial neoplasia, or a suspicious, atypical, or other lesion in biopsy were excluded. Patients who were diabetic, currently smoking, or taking medications for BPH including alpha blocker and finasteride were excluded to avoid their confounding effect on chemokine expression.
For inclusion, patients had to have prostate volume >20 and <100 cc with documented AUA-SI. From the eligible biopsy-negative men, we randomly selected 30 patients for urinary biomarker assay blocked on three prostate volume categories (<40, 40 to <60, and 60 cc or more) and two categories of LUTS severity (0–7 and 8–35), such that there were five participants within each volume × LUTS cell. Following informed consent, a spot urine sample was collected from patients and aliquoted into cryovials on the day of collection and preserved at −80 °C.
Urine assay
Frozen urine aliquots were shipped overnight on dry ice to University of Pittsburgh for blinded analysis. Aliquots were thawed prior to analysis, and 50 μL of urine specimen was analyzed in duplicate for seven proteins. CXC chemokines (CXCL-1, CXCL-8, and CXCL-10), CC chemokines (CCL2 and CCL3), and sIL-1ra were analyzed using Luminex™ xMAP® technology, and enzyme-linked immunosorbent assay (ELISA) was used for NGF analysis as previously described [9].
Statistical analysis
The association of chemokines with prostate volume or LUTS severity was evaluated using linear regression, adjusting for the covariates of age and body mass index (BMI). Urine chemokine values were natural log-transformed prior to analysis to meet model assumptions, and back-transformed with geometric means presented between different categories of BPH patients. The independent relationship of chemokines with AUA-SI and clinical attributes of patients including prostate volume and BMI was ascertained by beta-coefficients (β) using regression analysis. Correlation among chemokines was analyzed by Spearman's correlation coefficient (rs). p values less than 0.05 were considered significant (two-tailed test).
Results
Clinical characteristics
Only Caucasians met the inclusion criteria for the study, and there were not enough patients from other races to meet the inclusion criteria. Quartile distribution of clinical characteristics for patients in each block is summarized in Table 1, where age, BMI, and PSA were not statistically different among different blocks. However, the patients blocked by low and high AUA-SI and patients blocked by prostate volume differed significantly in AUA-SI and prostate volume, respectively (Table 1). Several patients were taking an NSAID (n = 11) or a statin (7), and patients taking these medications were evenly spread in each study block. Analysis by Mantel–Haenszel Chi-square test did not show any significant difference in medication use between blocks (data not shown).
Table 1.
Patient demographics and clinical characteristics of 30 eligible biopsy-negative men, randomly selected for urinary biomarker assay blocked on two categories of LUTS severity (0-7 and 8-35) and three prostate volume categories (<40, 40 to <60, and 60 cc or more), such that there were five participants within each volume × LUTS cell
| Factors | AUA-SI: 0-7 (n = 15) |
AUA-SI: 8-35 (n = 15) |
p * | ||||
|---|---|---|---|---|---|---|---|
| Med | 25th | 75th | Med | 25th | 75th | ||
| Age (years) | 60 | 57 | 63 | 60 | 57 | 63 | 0.97 |
| BMI | 27.8 | 25.9 | 32.6 | 29.0 | 27.3 | 31.6 | 0.36 |
| Height (cm) | 176 | 169 | 184 | 182 | 177 | 182 | 0.20 |
| Waist (cm) | 104 | 99 | 112 | 104 | 98 | 116 | 0.90 |
| Waist-hip ratio | 1.03 | 0.98 | 1.06 | 0.99 | 0.97 | 1.03 | 0.32 |
| PSA (ng/ml) | 4.6 | 4.1 | 6.1 | 4.1 | 3.4 | 5.9 | 0.66 |
| AUA-SI | 5 | 3 | 6 | 16 | 11 | 18 | <0.01** |
| PV (mls) | 55 | 36 | 69 | 46 | 36 | 63 | 0.76 |
| Prostate volume |
p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PV: <40 cc (n = 10) |
PV: 40-60 cc (n = 10) |
PV: 60-100 cc (n = 10) |
||||||||
| Med | 25th | 75th | Med | 25th | 75th | Med | 25th | 75th | ||
| Age (years) | 59 | 57 | 62 | 60 | 54 | 62 | 63 | 60 | 64 | 0.11 |
| BMI | 28.8 | 25.9 | 30.8 | 29.0 | 28.1 | 32.6 | 27.5 | 25.8 | 28.7 | 0.32 |
| Height (cm) | 175 | 170 | 182 | 182 | 171 | 187 | 180 | 179 | 182 | 0.23 |
| Waist (cm) | 103 | 98 | 112 | 105 | 104 | 116 | 102 | 99 | 109 | 0.39 |
| Waist-hip ratio | 1.01 | 0.94 | 1.03 | 1.02 | 0.99 | 1.11 | 1.01 | 0.98 | 1.03 | 0.62 |
| PSA (ng/ml) | 4.0 | 2.6 | 5.3 | 4.1 | 3.5 | 4.9 | 5.1 | 4.2 | 8.1 | 0.14 |
| AUA-SI | 7.5 | 4 | 16 | 9 | 6 | 17 | 7.5 | 5 | 16 | 0.77 |
| PV (mls) | 33 | 28 | 36 | 48 | 44 | 55 | 72 | 68 | 89 | <0.01** |
p for AUA-SI by Wilcoxon's rank sums test and for PV by Kruskal-Wallis test
The p value for the patients blocked by AUA-SI was calculated based on Wilcoxon's rank sums test and using Kruskal-Wallis test for patients blocked by prostate volume
p < 0.01
Urinary chemokines
CXC chemokines (CXCL-1, CXCL-8, and CXCL-10), CCL2, sIL-1ra, and NGF were present in measurable amounts in all 30 specimens with skewed distribution, where median ranged from 11.5 to 361.7 pg/mL (Table 2). The median of CCL3 was much lower, and it was only present in seven out of 30 specimens. Expression of chemokines correlated with each other, where the expression of CXCL-1 and CXCL-8 belonging to the CXC chemokine family positively correlated with sIL-1ra expression with rs of 0.48 and 0.49, respectively (**p < 0.01, Table 2). CXCL-1 also positively correlated with CXCL-8 and CXCL-10 with rs of 0.84 and 0.47, respectively (**p < 0.01). CCL2 levels were several folds higher than other chemokines, and its expression positively correlated with all three members of CXC chemokine family (*p < 0.05; **p < 0.01). In contrast, expression of another chemokine CCL3, was lowest and without any correlation with any other tested chemokine.
Table 2.
Quartile distribution of urine chemokines irrespective of blocks and correlation matrix, except for CCL3, median expression of chemokines ranged from 11.5 to 361.7 pg/mL
| n | Median | Min | 25th | 75th | Max | |
|---|---|---|---|---|---|---|
| CXCL-1 | 30 | 26.7 | 9.5 | 18.9 | 45.5 | 395.4 |
| sIL-1ra | 30 | 43.9 | 13.1 | 32.2 | 62.4 | 894.1 |
| CXCL-8 | 30 | 11.5 | 0.6 | 4.0 | 48.9 | 543.2 |
| CXCL-10 | 30 | 75.2 | 14.4 | 51.7 | 107.2 | 921.5 |
| CCL2 | 30 | 361.7 | 45.2 | 189.9 | 539.3 | 4716 |
| CCL3 | 7 | 1.4 | 0.8 | 1.0 | 12.1 | 19.5 |
| NGF | 30 | 79.5 | 12.3 | 47.2 | 125.2 | 576.9 |
| CXCL-1 | sIL-1ra | CXCL-8 | CXCL-10 | CCL2 | CCL3 | |
|---|---|---|---|---|---|---|
| sIL-1ra | 0.48** | |||||
| CXCL-8 | 0.84** | 0.49** | ||||
| CXCL-10 | 0.47** | 0.22 | 0.34 | |||
| CCL2 | 0.63** | 0.28 | 0.58** | 0.41* | ||
| CCl3 | 0.57 | 0.64 | 0.61 | 0.29 | 0.21 | |
| NGF | –0.09 | –0.20 | –0.06 | 0.26 | –0.16 | 0.07 |
Expression of chemokines correlated between members of same family and others, where correlation is shown by Spearman's correlation coefficient rs
p < 0.05
p < 0.01
Blocked by AUA-SI
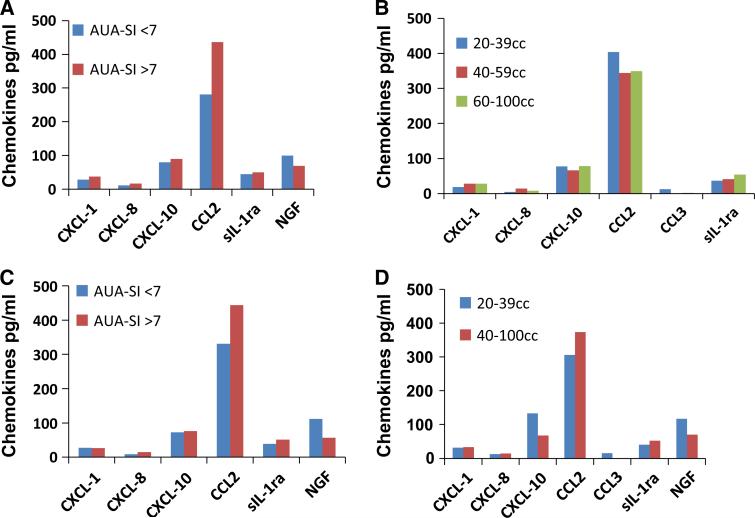
Median unadjusted chemokine values in patients blocked on AUA-SI are shown in Fig. 1a. The CCL2 levels were increased in BPH/LUTS patients with AUA-SI score >7 relative to patients having AUA-SI score <7, but the increase was not significant (p > 0.05). After adjusting for covariates of age and BMI, urine CCL2 levels remained high in patients with higher AUA-SI score, but the difference remained insignificant (Fig. 1c). Similarly, the slightly higher NGF in BPH/LUTS patients with AUA-SI score >7 (Fig. 1a) also remained unchanged after adjustment (Fig. 1c). Levels of other chemokines were similar in the two blocks with or without adjustment.
Fig. 1.
a Median urine chemokine levels of BPH/LUTS patients blocked on LUTS severity based on AUA-SI. CCL2 was slightly elevated and NGF was slightly lowered in block with higher AUA-SI, but difference was not significant. b Median urine chemokine levels of BPH/LUTS patients when blocked by prostate volume. CCL2 and NGF levels were slightly higher and levels of CXCL-1, CXCL-8, and sIL-1ra were slightly lower in patients with smaller prostate (<39 cc) relative to patients with enlarged prostate, without any significance. c Geometric means for adjusted chemokine levels in BPH/LUTS patients blocked on LUTS severity based on AUA-SI. Adjusted CCL2 levels were also slightly elevated and NGF was slightly lowered in block with higher AUA-SI, but difference was not significant. d Geometric means for adjusted chemokine levels in BPH/LUTS patients blocked of prostatic enlargement. Adjusted CCL2 levels were also slightly elevated and NGF levels were slightly lower in patients with higher prostate volume, but difference was not significant. CXCL-10 (#p = 0.09, n = 30) and CCL3 (**p < 0.01; n = 7) were significantly higher in patients with smaller prostate volume
Blocked by prostate volume
Patients with a small prostate (<39 cc) had slightly higher unadjusted CCL2 and NGF levels, whereas urine from patients with a larger prostate had slightly higher CXCL-1, CXCL-8, and sIL-1ra levels without any significance (Fig. 1b). After adjusting for covariates of age and BMI, patients with a smaller prostate showed slightly higher levels of CXCL-10, CCL3, and NGF (p > 0.05). Consolidation of prostate volume groups into two cohorts, showed that CXCL-10 remained high with a smaller prostate with trend toward significance (p = 0.09) (Fig. 1d) and analysis for CCL3 reached significance (**p = 0.01).
Association of chemokines with LUTS and obesity measures
Regression analysis revealed association of selected chemokines with AUA-SI, BMI and waist circumference (Table 3). Urine levels of CXCL-8 showed marginal association with obstructive symptoms (p = 0.07) measured within AUA-SI. Each log-transformed unit increase in age-adjusted levels of CXCL-8 increased the AUA-SI of BPH/LUTS patients by 1.29 unit (p = 0.08) and obstructive symptoms (or emptying predominant LUTS) by 0.87 unit (p = 0.08). CXCL-8 also showed marginal association with BMI (p = 0.06) and waist circumference with trend toward significance (p = 0.07). The relationship of age-adjusted CCL2 with AUA-SI showed high values of β coefficient without any significance.
Table 3.
Association of urine chemokines with clinical attributes
| AUA-SI |
Irritative symptoms |
Obstructive symptoms |
Prostate volume |
|||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| CXCL-1 | 1.87 | 0.17 | 0.56 | 0.36 | 1.31 | 0.15 | 2.93 | 0.47 |
| CXCL-8 | 1.29 | 0.08 | 0.42 | 0.21 | 0.87 | 0.08 | 0.08 | 0.97 |
| CXCL-10 | 0.10 | 0.93 | –0.02 | 0.97 | 0.12 | 0.88 | –1.62 | 0.66 |
| sIL-1ra | 1.09 | 0.48 | 0.14 | 0.83 | 0.94 | 0.37 | 5.38 | 0.24 |
| CCL2 | 2.42 | 0.34 | 0.44 | 0.47 | 0.85 | 0.36 | 2.08 | 0.61 |
| BMI |
Waist circumference |
Waist/hip ratio |
Height |
|||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| CXCL-1 | 0.97 | 0.37 | 2.98 | 0.30 | 0.01 | 0.34 | –0.84 | 0.62 |
| CXCL-8 | 1.08 | 0.06 | 2.83 | 0.07 | 0.01 | 0.14 | –0.49 | 0.60 |
| CXCL-10 | –0.23 | 0.81 | –0.20 | 0.94 | 0.00 | 0.75 | 1.01 | 0.50 |
| sIL-1ra | 4.41 | <0.01** | 12.6 | <0.01** | 0.01 | 0.52 | 2.63 | 0.16 |
| CCL2 | 1.23 | 0.23 | 1.34 | 0.64 | –0.01 | 0.44 | 0.64 | 0.70 |
The strength of independent relationship between chemokines and clinical attributes of patients is indicated by value of corresponding beta-coefficients (β) and respective p values calculated by regression model. sIL-1ra was significantly associated with BMI and waist circumference with
p < 0.01, whereas CXCL-8 showed trend toward significance (underlined p values) with LUTS measured by AUA-SI and obesity measures of BMI and waist circumference
Analysis for sIL-1ra revealed that each unit rise of sIL-1ra in urine significantly increased the BMI and waist circumference of BPH/LUTS patient by 4.41 and 12.6 unit, respectively (**p < 0.01). Most of the chemokines including sIL-1ra showed high values of β coefficient for prostate volume, but none of them reached significance.
Discussion
This is a first study to investigate the relationship of paracrine signaling molecules, chemokines excreted into urine with various clinical attributes of BPH/LUTS patients enrolled in a large clinical study. We found that adipose tissue-derived chemokine sIL-1ra showed significant association with inflammatory chemokines (CXCL-1 and CXCL-8) and obesity measures of BPH/LUTS patients. The association of sIL-1ra with obesity in BPH/LUTS patients was independent of age or prostate volume, which suggests that obesity is an independent risk factor and it is considered to promote BPH-associated inflammation [13].
Obesity is associated with altered adipokine production, and enhanced production of leptin, which can act both locally through autocrine and paracrine mode and systemically through endocrine pathways [6]. Earlier findings from our group found positive association of serum leptin levels with BMI of overactive bladder patients of either gender from Boston Area Community Health (BACH) study [14]. Together, serum and urine analyses of LUTS patients corroborate findings from epidemiologic studies which predict that increased prevalence of metabolic diseases and general greying of population is likely to further increase the prevalence of BPH/LUTS in US population [15]. Understanding the chemokines involved in obesity and BPH/LUTS may pave the way for design of preventative and therapeutic strategies that may stem this progress.
The directionality of the relationship is uncertain as it remains unknown whether the rise in urinary sIL-1ra is the result or the cause of increased BMI or waist circumference. A positive linear association of increased serum levels of sIL-1ra with obesity and inflammatory diseases [16] was previously reported in the literature. Elevated fatty acids in the serum due to obesity and the cytokines can converge to activate the same downstream inflamma-tory pathways [17]. The activation of these pathways leads to production of transcription factors that enter the nucleus and activate inflammatory cytokine gene expression leading to propagation of inflammation [6]. We can therefore surmise that increased centralized obesity as reflected by increased waist circumference is likely responsible for the increased sIL-1ra expression in BPH patients. A signifi-cant association between the genotype distribution of the IL-1Ra gene and the risk of BPH has also been reported [18].
Other major finding of this pilot study is that CXCL-8 previously measured in seminal plasma can also be noninvasively measured in urine of BPH/LUTS patients. CXCL-8, like other CXC chemokines, is induced by IFN-γ, and therefore, the elevated CXCL-8 in seminal plasma [19] and that in the urine of BPH/LUTS patients reported here were anticipated following reports of upregulated IFN-γ expression in inflamed regions of BPH prostate [20]. The relationship of elevated CXCL-8 in seminal plasma with higher BMI has been reported earlier [21], and here, we found that elevated urinary CXCL-8 is also associated with BMI and waist circumference of BPH/LUTS. The association of pro-inflammatory chemokines with BMI is consistent with reported activation of normally resident T-lymphocytes and macrophages in adipose tissue leading to a secretion of proinflammatory cytokines and chemokines, such as CXCL-8 attracting neutrophils, CXCL-10 attracting T-lymphocytes, and CCL2 attracting monocytes in a feed-forward loop [22].
In agreement with higher CCL2 levels reported in prostatic fluids with enlarged prostate [11], we report here that higher levels of CCL2 in urine of BPH/LUTS patients positively correlated with enlarged prostate and expression of inflammatory CXC chemokines. CCL2 was anticipated to be higher in BPH patients with enlarged prostate, owing to its stromal origin [11]. Increase in age-adjusted urine levels of CCL2 supports the stromal hyperplasia as the source of increased expression of CCL2. It is also worth noting that without age adjustment, urine CCL2 levels were slightly lower in patients with enlarged prostate. In contrast, urine levels of CXCL-10 showed an inverse association with prostate volume, which hints at different tissue origin for CXCL-10 and CCL2 within BPH prostate. Interestingly, just as noted for urinary CXCL-10 here, a recent study reported inverse association between serum levels of another CXC chemokine (CXCL-5) with prostate volume of BPH patients [23]. Epithelial origin for CXC chemokines (CXCL-10) [24] is therefore consistent with reports of higher epithelial proliferation coincident in regions of inflammatory infiltration noted in prostate biopsy of BPH patients [25].
The finding of higher CCL3 and CXCL-10 levels in patients with smaller prostate indicates that chronic inflammation of prostate can be independent of prostate volume. CXC chemokines are direct mediators of leukocyte accumulation and promotion of fibrotic changes in prostate tissue [26]. Chemokines can be secreted by multiple cell types within the prostatic microenvironment, including endothelial cells, inflammatory cells, epithelial cells, and fibroblastic cells [23]. These pro-inflammatory chemokines upon binding to their cognate receptors expressed in prostate can mediate prostatic enlargement and can possibly drive phenotypic changes in sensory neurons innervating diseased prostate.
Elevated levels of chemokines in urine can trace their origin to pelvic organs (prostate and bladder) directly secreting chemokines into lower urinary tract. Since molecular weight of chemokines is much lower than that of cystatin C [27], a protein filtered by glomerulus, the elevated levels of chemokines in urine can also be traced to secretion of chemokines from prostate into systemic circulation, which are then filtered into urine by kidney to maintain homeostatic equilibrium for serum chemokine levels. Relatively higher levels of CCL2 in urine compared with other six proteins measured here do suggest potential contribution of CCL2 from bladder tissue [28] into urine. Secretion of CCL2 by lower urinary tract is also supported by lower serum levels of CCL2 in OAB patients of either gender found in analysis of serum specimens collected as part of BACH study [14].
It is innate nature of systemic circulation to maintain homeostasis of blood constituents including chemokines, so any excess of chemokines contributed by prostate or bladder of BPH/LUTS or OAB patients are likely to be filtered into urine via kidney [27]. Furthermore, a recent study reported that serum levels of CXCL-1, CXCL-8, CXCL-10, and CCL2 chemokines were indifferent between BPH patients and controls [23]. In fact, serum levels of these chemokines in BPH patients were comparable to the urine levels of respective chemokines reported here in BPH/LUTS patients. Likewise, serum levels of CCL2 and CCL3 in prostatitis patients were indifferent from healthy controls, but the corresponding levels in urine collected post-prostatic massage showed elevation only in prostatitis [29]. Prostate massage is supposed to increase the contribution of prostate and accessory sex organs into the urine [30], but our studies demonstrate that prostate contribution can be detected in BPH/LUTS patients even in the absence of prostate massage.
Strict exclusion criteria used here led us to derive an association that is independent of confounding variables. Patients who were diabetic, currently smoking, or taking medications for BPH including alpha blocker and finasteride were excluded to avoid their confounding effect on chemokine expression. But this was a cross-sectional study, which precludes drawing firm conclusions on causality or directionality of the relationship. Notwithstanding the limitations, we were able to detect adipose tissue-derived chemokines in urine and report their association with obesity measures of patients. These findings demonstrate the stability of urine chemokines in their utility as biomarkers because urine was preserved for at least 2–3 years prior to analysis.
Biopsy-based studies of MTOPS and REDUCE trials have demonstrated the role of inflammation in BPH/LUTS [2], but biopsy is unlikely to be a preferred option in daily practice to inform the clinical management of BPH/LUTS patients. The procedure of biopsy itself may induce inflammation, and wrong site selection can lead to erroneous clinical judgment.
Conclusions
These findings support normally voided urine as a suitable matrix for detecting adipose tissue-derived chemokines and identifying markers for phenotyping and risk classification of BPH/LUTS patients. There is an unmet need for a noninvasive approach to facilitate risk classification and medical management of BPH/LUTS and for more accurate prognosis. Further research in a larger sample size is warranted to synthesize these disparate observations into a cohesive understanding of the relationship between obesity and BPH/LUTS.
Acknowledgments
The work was partly supported by grants; NIH P20 DK090919, NIH R01CA12060, NIH R01DK087962 (PI Fowke).
Footnotes
Conflict of interest None.
References
- 1.Michel M, de la Rosette J. Medical treatment of lower uri-nary tract symptoms suggestive of benign prostatic hyperplasia. Euro Urol Suppl. 2009;8(6):496–503. [Google Scholar]
- 2.Roehrborn CG. BPH progression: concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int. 2008;101(Suppl 3):17–21. doi: 10.1111/j.1464-410X.2008.07497.x. doi:10.1111/j.1464-410X.2008.07497.x. [DOI] [PubMed] [Google Scholar]
- 3.St Sauver JL, Jacobson DJ, McGree ME, Lieber MM, Jacobsen SJ. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. Am J Epidemiol. 2006;164(8):760–768. doi: 10.1093/aje/kwj258. doi:10.1093/aje/kwj258. [DOI] [PubMed] [Google Scholar]
- 4.Raheem OA, Parsons JK. Associations of obesity, physical activity and diet with benign prostatic hyperplasia and lower uri-nary tract symptoms. Curr Opin Urol. 2014;24(1):10–14. doi: 10.1097/MOU.0000000000000004. doi:10.1097/MOU.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 5.Jiang M, Strand DW, Franco OE, Clark PE, Hayward SW. PPARgamma: a molecular link between systemic metabolic disease and benign prostate hyperplasia. Differentiation. 2011;82(4–5):220–236. doi: 10.1016/j.diff.2011.05.008. doi:10.1016/j.diff.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li HB, Jin C, Chen Y, Flavell RA. Inflammasome activation and metabolic disease progression. Cytokine Growth Factor Rev. 2014;25(6):699–706. doi: 10.1016/j.cytogfr.2014.07.020. doi:10.1016/j.cytogfr.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Perez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlondorff D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12(7):1369–1382. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 8.Macoska JA. Chemokines and BPH/LUTS. Differentiation. 2011;82(4–5):253–260. doi: 10.1016/j.diff.2011.04.003. doi:10.1016/j.diff.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyagi P, Killinger K, Tyagi V, Nirmal J, Chancellor M, Peters KM. Urinary chemokines as noninvasive predictors of ulcerative interstitial cystitis. J Urol. 2012;187(6):2243–2248. doi: 10.1016/j.juro.2012.01.034. doi:10.1016/j.juro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung SF, Chung SD, Kuo HC. Increased serum C-reactive protein level is associated with increased storage lower urinary tract symptoms in men with benign prostatic hyperplasia. PLoS ONE. 2014;9(1):e85588. doi: 10.1371/journal.pone.0085588. doi:10.1371/journal.pone.0085588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita K, Ewing CM, Getzenberg RH, Parsons JK, Isaacs WB, Pavlovich CP. Monocyte chemotactic protein-1 (MCP-1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate. 2010;70(5):473–481. doi: 10.1002/pros.21081. doi:10.1002/pros.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penna G, Mondaini N, Amuchastegui S, Innocenti SD, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/ chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51(2):524–533. doi: 10.1016/j.eururo.2006.07.016. discussion 533. [DOI] [PubMed] [Google Scholar]
- 13.Vignozzi L, Gacci M, Cellai I, Santi R, Corona G, Morelli A, Rastrelli G, Comeglio P, Sebastanelli A, Maneschi E, Nesi G, De Nunzio C, Tubaro A, Mannucci E, Carini M, Maggi M. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate. 2013;73(8):789–800. doi: 10.1002/pros.22623. doi:10.1002/pros.22623. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi P, Chancellor M, Kupelian V, Araujo A, Rosen R, McKinlay J. Obesity, inflammation and overactive bladder: preliminary results from a pilot study of serum leptin and MCP-1 levels in men and women with and without OAB symptoms. J Urol. 2011;185(4, Supplement):e462–e463. doi:10.1016/j. juro.2011.02.763. [Google Scholar]
- 15.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 179(5 Suppl):S75–S80. 2008 doi: 10.1016/j.juro.2008.03.141. doi:10.1016/j.juro.2008.03.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cartier A, Bergeron J, Poirier P, Almeras N, Tremblay A, Lemieux I, Despres JP. Increased plasma interleukin-1 receptor antagonist levels in men with visceral obesity. Ann Med. 2009;41(6):471–478. doi: 10.1080/07853890903022801. doi:10.1080/07853890903022801. [DOI] [PubMed] [Google Scholar]
- 17.Weber K, Schilling JD. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J Biol Chem. 2014;289(13):9158–9171. doi: 10.1074/jbc.M113.531202. doi:10.1074/jbc. M113.531202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konwar R, Gara R, Singh M, Singh V, Chattopadhyay N, Bid HK. Association of interleukin-4 and interleukin-1 receptor antagonist gene polymorphisms and risk of benign prostatic hyperplasia. Urology. 2008;71(5):868–872. doi: 10.1016/j.urology.2007.12.072. doi:10.1016/j. urology.2007.12.072. [DOI] [PubMed] [Google Scholar]
- 19.Kramer G, Steiner GE, Handisurya A, Stix U, Haitel A, Knerer B, Gessl A, Lee C, Marberger M. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002;52(1):43–58. doi: 10.1002/pros.10084. doi:10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- 20.Steiner GE, Stix U, Handisurya A, Willheim M, Haitel A, Reithmayr F, Paikl D, Ecker RC, Hrachowitz K, Kramer G, Lee C, Marberger M. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83(8):1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 21.Lotti F, Corona G, Colpi GM, Filimberti E, Degli Innocenti S, Mancini M, Baldi E, Noci I, Forti G, Adorini L, Maggi M. Elevated body mass index correlates with higher seminal plasma interleukin 8 levels and ultrasonographic abnormalities of the prostate in men attending an andrology clinic for infertility. J Endocrinol Invest. 2011;34(10):e336–e342. doi: 10.3275/7855. doi:10.3275/7855. [DOI] [PubMed] [Google Scholar]
- 22.Antoniou KM, Tzanakis N, Tzortzaki EG, Malagari K, Koutsopoulos AV, Alexandrakis M, Wells AU, Siafakas NM. Different angiogenic CXC chemokine levels in bronchoalveolar lavage fluid after interferon gamma-1b therapy in idiopathic pulmonary fibrosis patients. Pulm Pharmacol Ther. 2008;21(6):840–844. doi: 10.1016/j.pupt.2008.06.005. doi:10.1016/j.pupt.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Macoska JA, Begley LA, Dunn RL, Siddiqui J, Wei JT, Sarma AV. Pilot and feasibility study of serum chemokines as markers to distinguish prostatic disease in men with low total serum PSA. Prostate. 2008;68(4):442–452. doi: 10.1002/pros.20717. doi:10.1002/pros.20717. [DOI] [PubMed] [Google Scholar]
- 24.Schauer IG, Ressler SJ, Tuxhorn JA, Dang TD, Rowley DR. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology. 2008;72(1):205–213. doi: 10.1016/j.urology.2007.11.083. doi:10.1016/j.urology.2007.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feneley MR, Span PN, Schalken JA, Harper M, Griffiths K, Holmes K, Kirby RS. A prospective randomized trial evaluating tissue effects of finasteride therapy in benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1999;2(5/6):277–281. doi: 10.1038/sj.pcan.4500377. doi:10.1038/sj.pcan.4500377. [DOI] [PubMed] [Google Scholar]
- 26.Gharaee-Kermani M, Kasina S, Moore BB, Thomas D, Mehra R, Macoska JA. CXC-type chemokines promote myofibroblast phenoconversion and prostatic fibrosis. PLoS ONE. 2012;7(11):e49278. doi: 10.1371/journal.pone.0049278. doi:10.1371/journal.pone.0049278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjork J, Grubb A, Larsson A, Hansson LO, Flodin M, Sterner G, Lindstrom V, Nyman U. Accuracy of GFR estimating equations combining standardized cystatin C and creatinine assays: a cross-sectional study in Sweden. Clin Chem Lab Med. 2014 doi: 10.1515/cclm-2014-0578. doi:10.1515/cclm-2014-0578. [DOI] [PubMed] [Google Scholar]
- 28.Bouchelouche K, Alvarez S, Andersen L, Nordling J, Horn T, Bouchelouche P. Monocyte chemoattractant protein-1 production by human detrusor smooth muscle cells. J Urol. 2004;171(1):462–466. doi: 10.1097/01.ju.0000090192.36436.d5. [DOI] [PubMed] [Google Scholar]
- 29.Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S, Thumbikat P, Pope RM, Landis JR, Koch AE, Schaeffer AJ. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179(5):1857–1861. doi: 10.1016/j.juro.2008.01.028. doi:10.1016/j.juro.2008.01.028 discussion 1861–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vicentini C, Gravina GL, Angelucci A, Pascale E, D'Ambrosio E, Muzi P, Di Leonardo G, Fileni A, Tubaro A, Festuccia C, Bologna M. Detection of telomerase activity in prostate massage samples improves differentiating prostate cancer from benign prostatic hyperplasia. J Cancer Res Clin Oncol. 2004;130(4):217–221. doi: 10.1007/s00432-003-0525-8. doi:10.1007/s00432-003-0525-8. [DOI] [PubMed] [Google Scholar]