Abstract
The endoplasmic reticulum (ER) is responsible for regulating proteome integrity throughout the secretory pathway. The ER protects downstream secretory environments such as the extracellular space by partitioning proteins between ER protein folding, trafficking and degradation pathways in a process called ER quality control. In this process, ER quality control factors identify misfolded, aggregation-prone protein conformations and direct them towards ER protein folding or degradation, reducing their secretion to the extracellular space where they could further misfold or aggregate into proteotoxic conformations. Despite the general efficiency of ER quality control, many human diseases, such as the systemic amyloidoses, involve aggregation of destabilized, aggregation-prone proteins in the extracellular space. A common feature for all systemic amyloid diseases is the ability for amyloidogenic proteins to evade ER quality control and be efficiently secreted. The efficient secretion of these amyloidogenic proteins increases their serum concentrations available for the distal proteotoxic aggregation characteristic of these diseases. This indicates that ER quality control, and the regulation thereof, is a critical determinant in defining the onset and pathology of systemic amyloid diseases. Here, we discuss the pathologic and potential therapeutic relationship between ER quality control, protein secretion and distal deposition of amyloidogenic proteins involved in systemic amyloid diseases. Furthermore, we present evidence that the Unfolded Protein Response, the stress-responsive signaling pathway that regulates ER quality control, is involved in the pathogenesis of systemic amyloid diseases and represents a promising emerging therapeutic target to intervene in this class of human disease.
Systemic Amyloid Diseases are Dependent on Secretion of Amyloidogenic Proteins
The ability for proteins to attain their native three-dimensional conformation is critical for human health. The inability for proteins to maintain this folded conformation can lead to misfolding and subsequent proteotoxic aggregation associated with the onset and pathology of many diseases including the systemic amyloidoses. Systemic amyloid diseases are a class of human disorders characterized by the extracellular misfolding and proteotoxic aggregation of proteins that deposit as amyloid on tissues such as the heart, gut, and peripheral nerves (Blancas-Mejia and Ramirez-Alvarado 2013, Gillmore and Hawkins 2013). The deposition of amyloid fibrils is causatively associated with organ malfunction and eventual death in the pathogenesis of these diseases. Fourteen structurally-diverse proteins (or fragments thereof) deposit as amyloid in association with multiple systemic amyloid diseases (Table 1) (Sipe, Benson et al. 2014). The majority of these diseases are caused by inherited or acquired mutations in an amyloidogenic protein that destabilize the native protein structure and promote their extracellular misfolding and/or aggregation into proteotoxic soluble oligomers and amyloid fibrils. Despite the similar involvement of proteotoxic aggregation and distal deposition of amyloidogenic proteins, the pathologies of systemic amyloid diseases are highly variable, presenting with distinct ages of onset, organ involvement, and severity. This heterogeneity challenges the development of therapeutic approaches to intervene in these diseases.
Table 1.
List of amyloidogenic precursor proteins and their associating systemic amyloid disease. Adapted from (Sipe, Benson et al. 2014).
| Precursor Protein | Disease | Organ Involvement |
|---|---|---|
| α-fibrinogen Variants | Fibrinogen Amyloidosis | Kidney |
| Apolipoprotein A-I Variants | ApoAI Amyloidosis | Kidney, Heart, Liver |
| Apolipoprotein A-II Variants | ApoAII Amyloidosis | Kidney |
| Apolipoprotein A-IV wild-type | ApoAIV Amyloidosis | Kidney |
| β2-microglobulin Variants | Dialysis Related Amyloidosis | Heart, GI tract, lung |
| BriPP Variants | Familial British Dementia | Brain |
| Cystatin C Variants | Cystatin C Amyloid Angiopathy | Cerebral Blood vessels |
| Gelsolin Variants | Finnish Hereditary Amyloidosis | Eyes, peripheral nerves, skin |
| Immuboglobulin Light Chain | Light Chain Amyloidosis | Heart, kidney, liver, gastrointestinal tract |
| Immunoglobulin Heavy Chain | Heavy Chain Amyloidosis | Kidney, Liver |
| Leukocyte Chemotactic Factor 2 | ALECT2 Amyloidosis | Kidney |
| Lysozyme Variants | Lysozyme Amyloidosis | Kidney, Liver, Spleen |
| Serum Amyloid A | Secondary Amyloidosis | Kidney, Liver, spleen |
| Transthyretin Variants | Familial Amyloid Polyneuropathy, Familial Amyloid Cardiomyopathy | Heart, Peripheral Nerves, lem ptomeninges |
| Transthyretin wild-type | Senile Systemic Amyloidosis | Heart |
Currently, few strategies exist to treat systemic amyloid diseases. Highly-invasive liver transplantation is employed to treat systemic amyloidoses caused by destabilized, amyloidogenic variants of proteins including TTR, α-fibrinogen, and apolipoprotein A-II (Holmgren, Ericzon et al. 1993, Suhr, Herlenius et al. 2000, Gillmore, Stangou et al. 2006, Gillmore, Lachmann et al. 2009). In this strategy, a liver expressing a destabilized, amyloidogenic protein is replaced with a liver secreting the wild-type protein, avoiding hepatic synthesis of the amyloidogenic aggregation-prone protein responsible for the distal proteotoxicity. Similarly, chemotherapeutic ablation of dyscratic plasma cells expressing an amyloidogenic immunoglobulin light chain (LC) reduces circulating concentrations of proteotoxic LC sequences and improves patient prognosis in Light Chain Amyloidosis (AL) (Merlini, Seldin et al. 2011, Merlini, Comenzo et al. 2014). Alternatively, the small molecule Tafamidis, a kinetic stabilizer of the native TTR tetramer, is approved in Europe and Japan as a noninvasive strategy to treat Familial Amyloid Polyneuropathy caused by the secretion and subsequent proteotoxic aggregation of destabilized, amyloidogenic TTR variants (Bulawa, Connelly et al. 2012, Coelho, Maia et al. 2012, Coelho, Maia et al. 2013). In this strategy, Tafamadis binding to the TTR tetramer prevents TTR tetramer dissociation and subsequent misfolding required for proteotoxic TTR aggregation. The establishment of similar strategies to prevent misfolding and proteotoxic protein aggregation in other systemic amyloidoses is challenged by the lack of small molecule binding sites on many amyloidogenic proteins. Furthermore, small molecule strategies to ameliorate proteotoxicity at distal tissues are limited by our poor understanding of the proteotoxic mechanism(s) by which amyloidogenic proteins induce toxicity, although it is clear that misfolded proteins and/or small soluble oligomers are the predominant proteotoxic species (Kayed, Head et al. 2003, Reixach, Deechongkit et al. 2004, Haass and Selkoe 2007, Lashuel, Overk et al. 2013). The lack of non-invasive strategies to ameliorate distal proteotoxicity involved in many systemic amyloidoses has led to a significant amount of experimental effort to identify new biologic pathways and processes that can be therapeutically targeted to intervene in these disorders.
A critical factor in dictating systemic amyloid disease pathogenesis is the secretion of amyloidogenic proteins from effector tissues (i.e., tissues that synthesize the amyloidogenic protein). The secretion of amyloidogenic proteins defines their serum concentrations available for proteotoxic, concentration-dependent aggregation and distal deposition. The importance of amyloidogenic protein serum concentrations in disease pathogenesis is evident in patients receiving liver transplantation. Replacing a liver secreting destabilized, amyloidogenic variants of proteins such as TTR, apolipoprotein A-II or α-fibrinogen with a liver secreting the corresponding wild-type protein decreases serum concentrations of the amyloidogenic protein variant and corresponds with marked improvement in patients (Holmgren, Ericzon et al. 1993, Suhr, Herlenius et al. 2000, Gillmore, Stangou et al. 2006, Gillmore, Lachmann et al. 2009). Similarly, reducing hepatic TTR synthesis, and subsequently serum levels, of amyloidogenic TTRs using siRNA or anti-sense RNA technologies also shows significant potential to attenuate distal toxicity and improve patient prognosis (Adams, Lozeron et al. 2012, Obici and Merlini 2014, Castano, Drachman et al. 2015). Since secretion of amyloidogenic proteins from effector tissues is a primary determinant in dictating circulating serum concentrations of amyloidogenic proteins, the activity and regulation of biologic pathways that mediate amyloidogenic protein secretion is both relevant to the pathogenesis of systemic amyloid diseases and represents a potential therapeutic target to reduce serum concentrations of amyloidogenic proteins causatively associated with distal proteotoxicity in these disorders.
Protein Secretion is Dictated by the Activity of ER Quality Control Pathways Involved in Protein Folding, Trafficking and Degradation
Nearly one-third of the human proteome, including all proteins involved in systemic amyloid diseases, is targeted to the endoplasmic reticulum (ER) for folding and trafficking to downstream secretory environments such as the extracellular space. These proteins are directed to the ER by N-terminal targeting sequences that mediate their co-translational import into the ER (Fig. 1A). In the ER, these newly-synthesized unfolded proteins engage ER-localized folding enzymes and ATP-dependent chaperones that facilitate both posttranslational modification of the polypeptide chain (e.g., N-glycosylation, disulfide bond formation) and proper folding into the native three-dimensional conformation (Braakman and Bulleid 2011). Folded proteins are packaged into the COPII vesicles for trafficking to the Golgi where they are sorted and targeted to downstream secretory environments. Proteins unable to attain a folded conformation through interactions with ER chaperones and folding enzymes are retained in the ER and directed towards degradation through mechanisms such as ER-associated degradation (ERAD) (Smith, Ploegh et al. 2011, Guerriero and Brodsky 2012). In ERAD, non-folded or misfolded protein conformations are recognized by ERAD receptors in the ER lumen and targeted for retrotranslocation to the cytosol where they are ubiquitinated and degraded by the proteasome. Misfolded proteins can also be degraded through a mechanism involving trafficking to the lysosome via trafficking to the Golgi or autophagic removal of misfolded ER proteins (Kruse, Brodsky et al. 2006).
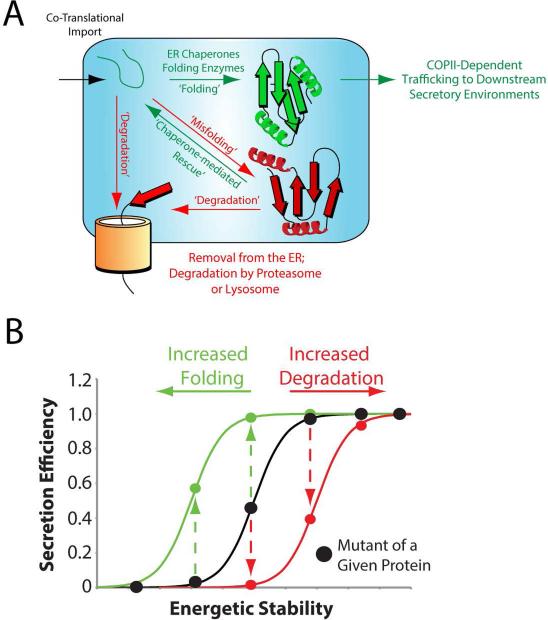
Figure 1. Secretion of proteins through the secretory pathway is dependent on the relative activity of ER protein folding, trafficking and degradation pathways.
A. Illustration showing the partitioning of ER-targeted proteins between ER protein folding, trafficking and degradation pathways.
B. Plot relating the secretion efficiency from mammalian cells to the inherent energetic stability for a series of destabilized variants of a single protein. The impact of increasing ER folding capacity (green) or degradation capacity (red) on the secretion of these variants is also shown.
The partitioning of proteins between ER protein folding, trafficking and degradation pathways, also referred to as ER quality control, serves a critical role in regulating downstream secretory environments including the extracellular space. ER quality control functions to prevent the trafficking of misfolded or non-folded protein conformations to the extracellular environment where they could further misfold or aggregate into proteotoxic conformations. In this ER quality control mechanism, the secretion of proteins to the extracellular space is dictated by two primary determinants (Wiseman, Powers et al. 2007). The first is the inherent energetic stability of the protein fold, which includes both the thermodynamic stability (i.e., the propensity to attain a folded conformation) and the kinetic stability (i.e., the rate of folding) of the polypeptide chain – two parameters predominantly dictated by the genetically-encoded amino acid sequence. The energetic stability of a protein defines its ability to attain folded conformations in the steady-state ER environment, and thus is an important determinant in defining protein partitioning between ER protein folding/trafficking and degradation pathways. The importance of protein stability in secretion can be visualized by relating the energetic stability for a series of destabilized variants of a single protein to the secretion efficiency of these same protein variants from a mammalian cell (Fig. 1B). Above a certain energetic stability, protein variants are efficiently secreted to levels similar to that observed for the wild-type protein, reflecting their ability to attain a folded conformation in the ER environment. Alternatively, highly destabilized proteins are not efficiently secreted, but instead retained in the ER and/or targeted for degradation. This relationship between secretion and protein stability has been demonstrated for destabilized variants of amyloidogenic proteins including TTR and lysozyme (Sekijima, Wiseman et al. 2005, Kumita, Johnson et al. 2006, Wiseman, Powers et al. 2007).
The second factor that dictates protein secretion through the secretory pathway is the relative activity of ER protein folding and degradation pathways (Wiseman, Powers et al. 2007). These pathways compete for misfolded or non-folded protein conformations in the ER to facilitate their partitioning towards either protein folding or degradation (Fig. 1A). Thus, altering the activity of ER protein folding or degradation pathways significantly influences the partitioning of non-folded proteins between these two pathways. The impact of increasing ER folding or degradation pathway activity on protein secretion can be visualized using the relationship between the energetic stability and secretion for the destabilized protein variants shown in Fig. 1B. Increasing the activity of ER protein folding pathways can increase the ability for protein variants to attain a folded conformation through interactions with ER chaperones and folding enzymes, resulting in the more efficient secretion of moderately stable protein variants (Fig. 1B, green). Alternatively, increasing the activity of ER degradation pathways will increase partitioning of misfolded or non-folded protein conformations towards degradation, preventing their interactions with pro-folding ER chaperones and folding enzymes, and decreasing the secretion of protein variants with moderate levels of stability (Fig. 1B, red). This ability for cells to influence protein secretion through altering the activity of ER protein folding, trafficking and degradation pathways provides a mechanism to adapt ER quality control and secretory function to tissue-specific, environmental, or metabolic demands. This is achieved through the activation of stress-responsive signaling pathways such as the Unfolded Protein Response (UPR).
ER Quality Control is Regulated by the Unfolded Protein Response (UPR)
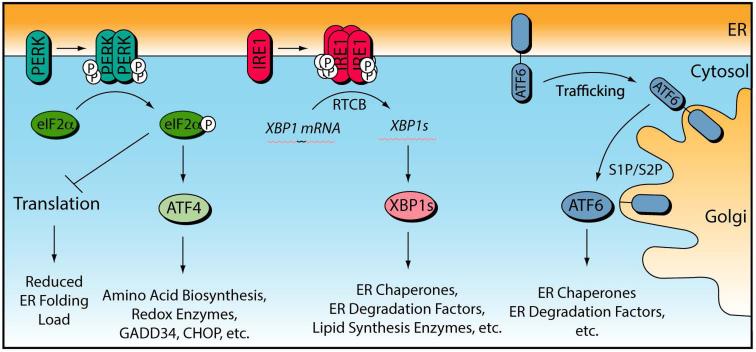
The UPR is a stress-responsive signaling pathway that regulates ER quality control in response to developmental cues or genetic, environmental or aging related insults that increase accumulation of misfolded proteins in the ER (i.e., ER stress) (Ron and Walter 2007, Walter and Ron 2011, Wang and Kaufman 2012). The UPR is a collective term for three stress signaling pathways activated downstream of the ER stress-sensing proteins Protein Kinase R-like ER kinase (PERK), inositol requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) (Fig. 2). These three signaling pathways function to relieve ER stress and reestablish efficient ER quality control in response to pathologic insult by adjusting two regulatory parameters that dictate ER protein homeostasis: protein load and ER quality control capacity (Wiseman, Koulov et al. 2007, Powers, Morimoto et al. 2009, Calamini and Morimoto 2012).
Figure 2.
Illustration showing the signaling pathways activated downstream of the three UPR sensor proteins PERK, IRE1 and ATF6.
PERK is activated through a mechanism involving PERK autophosphorylation and dimerization (Ron and Walter 2007, Walter and Ron 2011, Wang and Kaufman 2012). Activated PERK contains an active cytosolic kinase that phosphorylates the α subunit of eukaryotic initiation factor 2 (eIF2α). Phosphorylated eIF2α inhibits the eIF2B GTP exchange factor required for translation initiation, resulting in a reduction in new protein synthesis. Reducing protein synthesis through PERK activation decreases the load of newly-synthesized, unfolded proteins entering into the ER during the initial phase of ER stress. This functions to promote ER protein homeostasis by freeing ER quality control factors including chaperones, folding enzymes, and degradation factors to alleviate the misfolded protein load in the ER that initiated UPR activation (i.e., the ER stress).
PERK-dependent eIF2α phosphorylation also leads to the downstream activation of stress-responsive transcription factors such activating transcription factor 4 (ATF4). These transcription factors induce genes involved in global cellular protein homeostasis maintenance including amino acid biosynthesis enzymes and cellular redox factors (Harding, Zhang et al. 2003, Lu, Jousse et al. 2004). PERK-regulated transcription factors also induce expression of the eIF2α phosphatase regulatory subunit GADD34 that associates with protein phosphatase 1 to de-phosphorylate eIF2α and restore translational integrity in a negative feedback loop of PERK signaling (Novoa, Zeng et al. 2001, Ma and Hendershot 2003). Furthermore, ATF4 induces the transcription factor CHOP, which is involved in the ER-stress dependent expression of pro-apoptotic factors including death receptor 5 (DR5), PUMA, Bax, and Bak during prolonged or severe ER stress (Tabas and Ron 2011, Urra, Dufey et al. 2013). Importantly, while PERK-dependent eIF2α phosphorylation is activated by ER stress as part of the UPR, other stress-regulated eIF2α kinases phosphorylate eIF2α in response to other stresses such as amino acid deprivation, viral infection, and oxidative stress (Wek, Jiang et al. 2006, Sonenberg and Hinnebusch 2009). This ability for eIF2α phosphorylation to be activated by a variety of cellular insults is consistent with the global impact of eIF2α phosphorylation on cellular proteome maintenance.
The IRE1 pathway is the most conserved arm of the UPR found in all eukaryotes from yeast to humans (Ron and Walter 2007, Walter and Ron 2011, Wang and Kaufman 2012). IRE1 activation proceeds through a mechanism involving autophosphorylation and oligomerization. Active IRE1 contains a cytosolic endoribonuclease domain that cleaves XBP1 mRNA at two sites 26 nt apart. Cleaved XBP1 is religated by the RtcB tRNA ligase, resulting in a new mRNA encoding the active transcription factor spliced XBP1 (XBP1s) (Jurkin, Henkel et al. 2014, Kosmaczewski, Edwards et al. 2014, Lu, Liang et al. 2014). XBP1s localizes to the nucleus and induces expression of genes involved in a variety of ER functions including lipid homeostasis, protein folding and protein degradation (Lee, Iwakoshi et al. 2003, Yamamoto, Yoshida et al. 2004, Shoulders, Ryno et al. 2013). Apart from XBP1s activation, active IRE1 also functions to degrade mRNA localized to the plasma membrane in a process called Regulated IRE1-mediated mRNA decay (RIDD) (Hollien and Weissman 2006, Han, Lerner et al. 2009, Hollien, Lin et al. 2009, Gaddam, Stevens et al. 2013). While the functional implications of RIDD remain to be defined, RIDD has been proposed to influence ER function through multiple mechanisms including reducing the load of newly-synthesized proteins entering into the ER, regulating the activity of UPR signaling pathways and promoting apoptosis (Maurel, Chevet et al. 2014). Active IRE1 can also promote apoptosis through recruitment of TRAF2, activating the JNK signaling pathway (Urano, Wang et al. 2000, Tabas and Ron 2011).
ATF6 activation proceeds through a mechanism distinct from IRE1 and PERK. In response to ER stress, ATF6 is trafficked to the Golgi (Ron and Walter 2007, Walter and Ron 2011, Wang and Kaufman 2012). The signals that induce ATF6 trafficking are largely undefined, but have been proposed to involve alterations in ATF6 disulfide bonding and oligomerization (Nadanaka, Okada et al. 2007). In the Golgi, ATF6 is proteolytically processed by Site 1 and Site 2 proteases, releasing the active N-terminal ATF6 transcription factor domain. The active cleaved N-terminal ATF6 transcription factor (henceforth referred to as ATF6) induces expression of genes involved in ER functions including ER protein quality control (Yamamoto, Yoshida et al. 2004, Shoulders, Ryno et al. 2013).
XBP1s and ATF6 transcriptionally induce overlapping, but distinct, sets of genes involved in ER quality control (Lee, Iwakoshi et al. 2003, Yamamoto, Yoshida et al. 2004, Shoulders, Ryno et al. 2013). These UPR-associated transcription factors can also heterodimerize to synergistically induce certain ER quality control factors including those involved in protein degradation (Yamamoto, Yoshida et al. 2004, Shoulders, Ryno et al. 2013). As such, differential activation of XBP1s and/or ATF6 results in a continuum of ER quality control environments with distinct capacities that can be used to sensitively adapt ER quality control and function to specific cellular demands.
ER Quality Control is a Critical Determinant in Systemic Amyloid Disease Pathology
ER quality control regulates the folding, trafficking and degradation of all proteins involved in systemic amyloid diseases. This indicates that ER quality control could significantly impact the secretion and subsequent distal proteotoxicity of destabilized, amyloidogenic proteins associated with these disorders. The importance of ER quality control in systemic amyloid diseases has been best demonstrated for the familial TTR amyloidoses. These diseases are causatively associated with the expression and secretion of >100 destabilized TTR variants (Johnson, Connelly et al. 2012). Amyloidogenic TTR variants are predominantly secreted from the liver and deposit as proteotoxic oligomers and amyloid fibrils on distal tissues including the heart and peripheral nerves in association with Familial Amyloid Cardiomyopathy (FAC) and Familial Amyloid Polyneuropathy (FAP), respectively (Buxbaum 2004, Adams, Lozeron et al. 2012, Johnson, Connelly et al. 2012).
Clinical presentation of TTR amyloid diseases is influenced by the ability for ER quality control pathways to identify destabilized TTR variants and prevent their hepatic secretion. Patients expressing highly-destabilized, highly-aggregation prone TTR variants such as TTRD18G present with a relatively mild systemic amyloid disease pathology that is inconsistent with the extremely high aggregation propensity of these variants (Garzuly, Vidal et al. 1996, Hammarstrom, Sekijima et al. 2003, Sekijima, Hammarstrom et al. 2003, Sekijima, Wiseman et al. 2005). Interestingly, these highly-destabilized, highly-aggregation-prone TTR variants are recognized by ER quality control pathways in the liver and targeted for degradation (Sekijima, Wiseman et al. 2005, Sato, Susuki et al. 2007, Chen, Genereux et al. 2014). The recognition of these variants decreases their secretion and subsequently serum concentrations, slowing proteotoxic aggregation of these highly-destabilized TTRs on distal tissues (Garzuly, Vidal et al. 1996, Hammarstrom, Sekijima et al. 2003, Sekijima, Hammarstrom et al. 2003).
Alternatively, moderately-destabilized, but still aggregation-prone, TTR variants such as TTRL55P escape ER quality control and are secreted from the liver at levels identical to those observed for the stable wild-type TTR (Sekijima, Wiseman et al. 2005, Chen, Genereux et al. 2014). This leads to high serum concentrations for these amyloidogenic variants, which facilitates proteotoxic aggregation in the serum. Patients expressing these TTR variants present with a severe, early onset disease pathology (Jacobson, McFarlin et al. 1992). These results indicate that the ability for destabilized, aggregation-prone TTRs to escape ER quality control influences the onset and severity of TTR amyloid disease pathology. Similar relationships between ER quality control efficiency and destabilized protein secretion have also been proposed to influence disease pathology in other systemic amyloid diseases such as Lysozyme Amyloidosis (Kumita, Johnson et al. 2006, Ryno, Wiseman et al. 2013), although further studies are required to better correlate secretion efficiency, amyloidogenic protein serum concentration, and disease severity for these other disorders.
Since ER quality control dictates amyloidogenic protein secretion, imbalanced regulation of ER quality control pathways in effector tissues, through mechanisms such as impaired UPR activity, could also contribute to systemic amyloid disease pathology. Correlative evidence suggests that alterations in hepatic ER quality control regulation can influence distal deposition of amyloidogenic proteins. A longitudinal study measuring hepatic gene expression in mice overexpressing wild-type TTR – a model of senile systemic amyloidosis (Table 1) – showed that aging-dependent cardiac deposition of TTR correlates with reduced hepatic expression of stress-responsive genes including the UPR markers XBP1s and TRIB3 (Buxbaum, Tagoe et al. 2012). Similarly, ER quality control genes showed differential expression in livers of FAP patients as compared to controls, suggesting that altered ER quality control regulation may be involved in the proteotoxic TTR deposition observed in patients (Norgren, Olsson et al. 2014). These results suggest that stress-signaling in the liver directly impacts distal TTR deposition.
Similar relationships are also suggested by the clinical presentations of acquired systemic amyloid disease pathology in recipients of domino liver transplantations. In these types of transplantations, patients suffering from severe liver damage receive a liver from a patient suffering from a TTR amyloid disease caused by the hepatic secretion of a destabilized, amyloidogenic TTR variant. Interestingly, the recipients of the mutant TTR expressing liver present with TTR deposition on the heart or peripheral nerves on a timescale considerably faster than that observed in the donor (Stangou, Heaton et al. 2005, Goto, Yamashita et al. 2006, Takei, Gono et al. 2007, Llado, Baliellas et al. 2010). This suggests that alterations in the ability for the liver to regulate secretion of amyloidogenic TTR may contribute to the more rapid distal deposition of proteotoxic TTR conformations observed in patients suffering from this acquired form of disease.
While we are still learning of the relationship between ER quality control and distal proteotoxicity in systemic amyloid disease pathology, the relationship provides a potential mechanism to explain the involvement of aging in the onset and pathology of systemic amyloid diseases. The ability to regulate ER quality control through UPR signaling declines during aging (Naidoo 2009, Lindquist and Kelly 2011). Furthermore, overexpression of destabilized, disease-associated variants of amyloidogenic proteins including TTR and lysozyme can induce ER stress and UPR activation in model systems (Sato, Susuki et al. 2007, Kumita, Helmfors et al. 2012, Kamada, Kusakabe et al. 2015), suggesting that the expression of these amyloidogenic proteins can challenge ER quality control in effector tissues. Thus, age-dependent reductions in the ability for cells to regulate ER quality control could impact the secretion of destabilized, amyloidogenic proteins and facilitate their distal deposition. As new models and approaches are being developed to probe the role of ER quality control in the distal proteotoxic aggregation of amyloidogenic proteins, we anticipate that ER quality control and the maintenance thereof will continue to be identified as a critical factor in defining the onset and pathogenesis for pathologically diverse systemic amyloid diseases.
UPR-Dependent Regulation of ER Quality Control as a Potential Therapeutic Target in Systemic Amyloid Disease
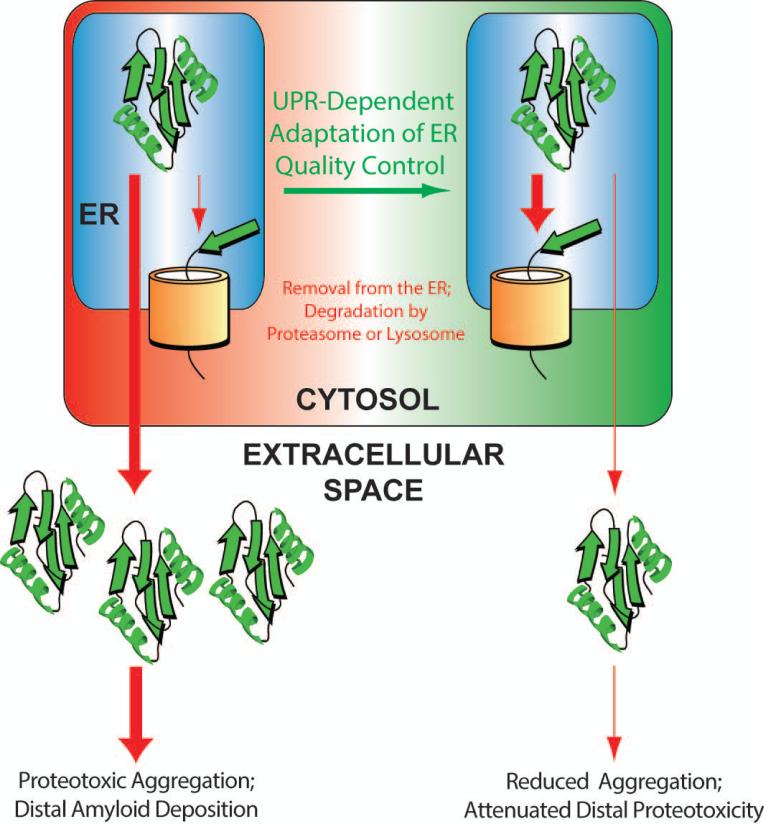
Our view of systemic amyloid diseases is that imbalances in ER quality control influence the secretion and subsequent distal proteotoxicity of amyloidogenic proteins. Thus, we view ER quality control as an upstream determinant in defining distal proteotoxicity in amyloid disease pathogenesis. This suggests the possibility that promoting ER quality control to reduce secretion of amyloidogenic proteins offers an opportunity to ameliorate the distal proteotoxicity involved in these diseases (Fig. 3).
Figure 3. Illustration showing the therapeutic potential for UPR-dependent adaptation of ER quality control to attenuate secretion of destabilized, aggregation-prone proteins.
In disease pathophysiology (left), destabilized proteins are folded and efficiently secreted. The efficient secretion of these proteins increases their extracellular concentrations available for proteotoxic aggregation and distal deposition involved in disease pathogenesis. UPR-dependent remodeling of ER quality control pathways (right) can reduce secretion of these destabilized proteins through increased ER retention or increased partitioning to degradation pathways. This reduced secretion decreases extracellular populations of destabilized, aggregation-prone protein available for proteotoxic aggregation and attenuates the distal proteotoxicity causatively associated with systemic amyloid disease pathogenesis.
ER quality control is predominantly regulated through the activity of the UPR-associated transcription factors XBP1s and ATF6 (Fig. 2) (Lee, Iwakoshi et al. 2003, Yamamoto, Yoshida et al. 2004, Shoulders, Ryno et al. 2013). These transcription factors have evolved to induce a transcriptional program that promotes ER quality control in response to challenges to the ER environment. As such, activation of these transcription factors globally adapts ER quality control pathways to prevent secretion of misfolding-prone protein conformations that accumulate during ER stress. This suggests that activating XBP1s and/or ATF6 offers a potential strategy to sensitively reduce secretion of destabilized, amyloidogenic proteins without globally impacting secretion of endogenous wild-type proteins.
Recently, the advantage of activating XBP1s and/or ATF6 to reduce secretion and extracellular aggregation of amyloidogenic proteins has been demonstrated. Stress-independent activation of the UPR-associated transcription factor ATF6 was shown to preferentially reduce secretion and extracellular aggregation of destabilized, amyloidogenic TTR variants from cell culture models (Shoulders, Ryno et al. 2013, Chen, Genereux et al. 2014). This decrease in TTR variant secretion corresponds to increased degradation of these destabilized proteins, indicating that ATF6 activation increases partitioning of destabilized TTRs towards degradation pathways. Alternatively, neither the secretion nor degradation of wild-type TTR was significantly affected by ATF6 activation. Interestingly, the decreased TTR variant secretion and increased TTR variant degradation observed following ATF6 activation correlates with the energetic stability, and thus aggregation-propensity, for each variant, demonstrating that ATF6 activation increases ER quality control stringency for TTR secretion (Chen, Genereux et al. 2014). This indicates that ATF6 activation could be broadly applied to reduce secretion of the >100 destabilized TTR variants involved in TTR amyloid diseases. Furthermore, ATF6-dependent reductions in destabilized TTR secretion synergizes with strategies to stabilize the native TTR tetramer using molecules such as Tafamidis, indicating that these two approaches could potentially used in combination to treat TTR amyloid disease in vivo.
Stress-independent activation of XBP1s and/or ATF6 also preferentially reduced secretion and extracellular aggregation of a destabilized, amyloidogenic immunoglobulin light chain (LC), as compared to a stable, non-amyloidogenic LC (Cooley, Ryno et al. 2014). Interestingly, XBP1s- or ATF6-dependent remodeling of the ER environment reduced amyloidogenic LC secretion through distinct mechanisms. XBP1s activation increased amyloidogenic LC degradation. Alternatively, ATF6 activation increased ER retention of the amyloidogenic LC through a mechanism involving associations with ATP-dependent chaperones such as BiP or GRP94. The ability to influence the secretion and extracellular aggregation of amyloidogenic TTRs and LCs by activating ATF6 and/or XBP1s suggests that similar strategies can be employed to attenuate secretion and extracellular aggregation of other destabilized amyloidogenic proteins involved in systemic amyloid diseases.
Similar strategies could also be employed to intervene in other diseases caused by the aggregation of destabilized, aggregation-prone proteins targeted to the ER. ATF6 activation attenuates pathologic intracellular aggregation of the destabilized, aggregation-prone Z-variant of α-1-antitrypsin (A1AT) that induces hepatic dysfunction in association with A1AT deficiency (Smith, Granell et al. 2011). Similarly, activating IRE1/XBP1s or ATF6 reduces intracellular aggregation of the P23H rhodopsin variant involved in retinal degeneration (Chiang, Hiramatsu et al. 2012, Chiang, Messah et al. 2012). In neither case was trafficking of the wild-type protein significantly affected. Thus, adapting ER quality control through ATF6 and/or IRE1/XBP1s activation has the potential to decrease the intra- and/or extracellular proteotoxic aggregation of multiple destabilized proteins involved in highly diverse human protein aggregation diseases.
Establishing IRE1/XBP1s or ATF6 activation as strategy to intervene in systemic amyloid diseases is challenged by the lack of small molecules available to preferentially activate these UPR-associated signaling pathways. Global activators of the UPR such as thapsigargin or tunicamycin are not therapeutically valuable since they induce global UPR activation and subsequent apoptotic signaling primarily through the PERK signaling pathway (Tabas and Ron 2011, Urra, Dufey et al. 2013). Small molecules that bind to the IRE1 nucleotide binding pocket and activate IRE1 endoribonuclease activity and subsequent XBP1 splicing have been reported, although the selectivity of these molecules for the IRE1/XBP1s pathway remain to be established (Han, Lerner et al. 2009, Korennykh, Egea et al. 2009). Similarly, the small molecule BiX was reported to activate the ATF6 arm of the UPR, although the selectivity of this molecule also remains to be defined (Kudo, Kanemoto et al. 2008). While these molecules offer a potential strategy to pharmacologically target IRE1/XBP1s or ATF6 signaling, new small molecule strategies to activate the IRE1/XBP1s or ATF6 transcriptional programs are required to define the therapeutic potential for UPR-dependent adaptation of ER quality control to intervene in systemic amyloid disease pathology.
A potential limitation in targeting UPR signaling pathways in the context of disease is the impact of adapting ER quality control on physiologic ER function. Initial experiments show that activating XBP1s or ATF6 does not globally impact secretion of the endogenous secreted proteome from HEK293 cells (Shoulders, Ryno et al. 2013). Furthermore, IRE1/XBP1s or ATF6 activation do not influence secretion/trafficking of wild-type proteins including TTR, A1AT or rhodopsin (Smith, Granell et al. 2011, Chiang, Hiramatsu et al. 2012, Chiang, Messah et al. 2012, Shoulders, Ryno et al. 2013, Chen, Genereux et al. 2014). While this suggests that adapting ER quality control through UPR-associated transcription factor activation allows for preferential reduction in secretion of destabilized, amyloidogenic proteins, as compared to stable, wild-type proteins, further studies will be required to define the potential impact for activating these pathways on the secretion and activity of the endogenous secreted proteome in vivo.
Activating UPR-associated transcriptional signaling pathways could also influence other aspects of ER function such as lipid metabolism or the regulation of apoptotic signaling. High levels of ATF6 activation are sufficient to induce fatty liver disease in zebrafish (Howarth, Lindtner et al. 2014). Alternatively, increasing IRE1 RIDD activity increases apoptosis in INS-1 pancreatic beta cells (Han, Lerner et al. 2009). While therapeutic adaptations of ER quality control in vitro can be achieved with lower levels of ATF6 or IRE1/XBP1s activation that do not induce these detrimental consequences (Shoulders, Ryno et al. 2013, Chen, Genereux et al. 2014)(Cooley, Ryno et al. 2014), evaluating the impact of activating these UPR-associated transcription factors on other aspects of ER function and organismal health will be critical for the establishment of this potential strategy to ameliorate proteotoxic protein aggregation involved in protein aggregation diseases.
Concluding Remarks
ER quality control is involved in defining the secretion efficiency of destabilized, amyloidogenic proteins associated with systemic amyloid diseases. Thus, ER quality control is an upstream determinant that can influence the extracellular, concentration-dependent aggregation of amyloidogenic proteins into the proteotoxic oligomers and amyloid fibrils that induce toxicity at distal tissues in systemic amyloid disease pathogenesis. Taking this view, it is clear that alterations in ER quality control can contribute to the onset and pathogenesis of systemic amyloid diseases. Furthermore, promoting ER quality control in effector tissues through mechanisms such as arm-selective UPR activation could reveal a broadly-applicable therapeutic strategy to intervene in this class of protein aggregation diseases. This approach can synergize with other established and developing therapeutic approaches to intervene in these diseases such as small molecules that stabilize the native protein structure (e.g., Tafamidis) (Bulawa, Connelly et al. 2012, Coelho, Maia et al. 2012, Coelho, Maia et al. 2013) or molecules that disrupt fibril formation (e.g., doxycycline) (Adams, Lozeron et al. 2012, Castano, Drachman et al. 2015). As we continue to explore the pathologic and therapeutic involvement of ER quality control in systemic amyloid diseases, we will begin to learn more of the intricate relationship between ER function and extracellular protein homeostasis that can be applied to define pathologic and potentially therapeutic roles for ER quality control in other protein aggregation diseases that involve secretory proteins such as Alzheimer's disease, retinal degeneration and A1AT deficiency.
Acknowledgements
We thank Justine Lebeau for critical reading of this review. RLW thanks NIH (NS079882 and DK102635), the Ellison Medical Foundation, and the Amyloidosis Foundation for funding. JCG thanks NIH (HL099245) and the American Heart Association for funding.
References
- Adams D, Lozeron P, Lacroix C. Amyloid neuropathies. Curr Opin Neurol. 2012;25(5):564–572. doi: 10.1097/WCO.0b013e328357bdf6. [DOI] [PubMed] [Google Scholar]
- Blancas-Mejia LM, Ramirez-Alvarado M. Systemic amyloidoses. Annu Rev Biochem. 2013;82:745–774. doi: 10.1146/annurev-biochem-072611-130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, Packman J, Powers ET, Wiseman RL, Foss TR, Wilson IA, Kelly JW, Labaudiniere R. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109(24):9629–9634. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JN. The systemic amyloidoses. Curr Opin Rheumatol. 2004;16(1):67–75. doi: 10.1097/00002281-200401000-00013. [DOI] [PubMed] [Google Scholar]
- Calamini B, Morimoto RI. Protein homeostasis as a therapeutic target for diseases of protein conformation. Curr Top Med Chem. 2012;12(22):2623–2640. doi: 10.2174/1568026611212220014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163–178. doi: 10.1007/s10741-014-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Genereux JC, Qu S, Hulleman JD, Shoulders MD, Wiseman RL. ATF6 activation reduces the secretion and extracellular aggregation of destabilized variants of an amyloidogenic protein. Chem Biol. 2014;21(11):1564–1574. doi: 10.1016/j.chembiol.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, Hiramatsu N, Messah C, Kroeger H, Lin JH. Selective activation of ATF6 and PERK endoplasmic reticulum stress signaling pathways prevent mutant rhodopsin accumulation. Invest Ophthalmol Vis Sci. 2012;53(11):7159–7166. doi: 10.1167/iovs.12-10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, Messah C, Lin JH. IRE1 directs proteasomal and lysosomal degradation of misfolded rhodopsin. Mol Biol Cell. 2012;23(5):758–770. doi: 10.1091/mbc.E11-08-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho T, Maia LF, da Silva AM, Cruz MW, Plante-Bordeneuve V, Suhr OB, Conceicao I, Schmidt HH, Trigo P, Kelly JW, Labaudiniere R, Chan J, Packman J, Grogan DR. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260(11):2802–2814. doi: 10.1007/s00415-013-7051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Plante-Bordeneuve V, Lozeron P, Suhr OB, Campistol JM, Conceicao IM, Schmidt HH, Trigo P, Kelly JW, Labaudiniere R, Chan J, Packman J, Wilson A, Grogan DR. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–792. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley CB, Ryno LM, Plate L, Morgan GJ, Hulleman JD, Kelly JW, Wiseman RL. Unfolded protein response activation reduces secretion and extracellular aggregation of amyloidogenic immunoglobulin light chain. Proc Natl Acad Sci U S A. 2014;111(36):13046–13051. doi: 10.1073/pnas.1406050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddam D, Stevens N, Hollien J. Comparison of mRNA localization and regulation during endoplasmic reticulum stress in Drosophila cells. Mol Biol Cell. 2013;24(1):14–20. doi: 10.1091/mbc.E12-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzuly F, Vidal R, Wisniewski T, Brittig F, Budka H. Familial meningocerebrovascular amyloidosis, Hungarian type, with mutant transthyretin (TTR Asp18Gly). Neurology. 1996;47(6):1562–1567. doi: 10.1212/wnl.47.6.1562. [DOI] [PubMed] [Google Scholar]
- Gillmore JD, Hawkins PN. Pathophysiology and treatment of systemic amyloidosis. Nat Rev Nephrol. 2013;9(10):574–586. doi: 10.1038/nrneph.2013.171. [DOI] [PubMed] [Google Scholar]
- Gillmore JD, Lachmann HJ, Rowczenio D, Gilbertson JA, Zeng CH, Liu ZH, Li LS, Wechalekar A, Hawkins PN. Diagnosis, pathogenesis, treatment, and prognosis of hereditary fibrinogen A alpha-chain amyloidosis. J Am Soc Nephrol. 2009;20(2):444–451. doi: 10.1681/ASN.2008060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillmore JD, Stangou AJ, Lachmann HJ, Goodman HJ, Wechalekar AD, Acheson J, Tennent GA, Bybee A, Gilbertson J, Rowczenio D, O'Grady J, Heaton ND, Pepys MB, Hawkins PN. Organ transplantation in hereditary apolipoprotein AI amyloidosis. Am J Transplant. 2006;6(10):2342–2347. doi: 10.1111/j.1600-6143.2006.01507.x. [DOI] [PubMed] [Google Scholar]
- Goto T, Yamashita T, Ueda M, Ohshima S, Yoneyama K, Nakamura M, Nanjo H, Asonuma K, Inomata Y, Watanabe S, Uchino M, Tanaka K, Ando Y. Iatrogenic amyloid neuropathy in a Japanese patient after sequential liver transplantation. Am J Transplant. 2006;6(10):2512–2515. doi: 10.1111/j.1600-6143.2006.01484.x. [DOI] [PubMed] [Google Scholar]
- Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92(2):537–576. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hammarstrom P, Sekijima Y, White JT, Wiseman RL, Lim A, Costello CE, Altland K, Garzuly F, Budka H, Kelly JW. D18G transthyretin is monomeric, aggregation prone, and not detectable in plasma and cerebrospinal fluid: a prescription for central nervous system amyloidosis? Biochemistry. 2003;42(22):6656–6663. doi: 10.1021/bi027319b. [DOI] [PubMed] [Google Scholar]
- Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Holmgren G, Ericzon BG, Groth CG, Steen L, Suhr O, Andersen O, Wallin BG, Seymour A, Richardson S, Hawkins PN, et al. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet. 1993;341(8853):1113–1116. doi: 10.1016/0140-6736(93)93127-m. [DOI] [PubMed] [Google Scholar]
- Howarth DL, Lindtner C, Vacaru AM, Sachidanandam R, Tsedensodnom O, Vasilkova T, Buettner C, Sadler KC. Activating transcription factor 6 is necessary and sufficient for alcoholic fatty liver disease in zebrafish. PLoS Genet. 2014;10(5):e1004335. doi: 10.1371/journal.pgen.1004335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson DR, McFarlin DE, Kane I, Buxbaum JN. Transthyretin Pro55, a variant associated with early-onset, aggressive, diffuse amyloidosis with cardiac and neurologic involvement. Hum Genet. 1992;89(3):353–356. doi: 10.1007/BF00220559. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol. 2012;421(2-3):185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkin J, Henkel T, Nielsen AF, Minnich M, Popow J, Kaufmann T, Heindl K, Hoffmann T, Busslinger M, Martinez J. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. EMBO J. 2014;33(24):2922–2936. doi: 10.15252/embj.201490332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Kusakabe T, Sugimoto Y. Amyloidogenic lysozymes accumulate in the endoplasmic reticulum accompanied by the augmentation of ER stress signals. Biochim Biophys Acta. 2015;1850(6):1107–1119. doi: 10.1016/j.bbagen.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457(7230):687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmaczewski SG, Edwards TJ, Han SM, Eckwahl MJ, Meyer BI, Peach S, Hesselberth JR, Wolin SL, Hammarlund M. The RtcB RNA ligase is an essential component of the metazoan unfolded protein response. EMBO Rep. 2014;15(12):1278–1285. doi: 10.15252/embr.201439531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse KB, Brodsky JL, McCracken AA. Autophagy: an ER protein quality control process. Autophagy. 2006;2(2):135–137. doi: 10.4161/auto.2.2.2388. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, Tabira T, Imaizumi K, Takeda M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15(2):364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- Kumita JR, Helmfors L, Williams J, Luheshi LM, Menzer L, Dumoulin M, Lomas DA, Crowther DC, Dobson CM, Brorsson AC. Disease-related amyloidogenic variants of human lysozyme trigger the unfolded protein response and disturb eye development in Drosophila melanogaster. FASEB J. 2012;26(1):192–202. doi: 10.1096/fj.11-185983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumita JR, Johnson RJ, Alcocer MJ, Dumoulin M, Holmqvist F, McCammon MG, Robinson CV, Archer DB, Dobson CM. Impact of the native-state stability of human lysozyme variants on protein secretion by Pichia pastoris. FEBS J. 2006;273(4):711–720. doi: 10.1111/j.1742-4658.2005.05099.x. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist SL, Kelly JW. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol. 2011;3(12) doi: 10.1101/cshperspect.a004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llado L, Baliellas C, Casasnovas C, Ferrer I, Fabregat J, Ramos E, Castellote J, Torras J, Xiol X, Rafecas A. Risk of transmission of systemic transthyretin amyloidosis after domino liver transplantation. Liver Transpl. 2010;16(12):1386–1392. doi: 10.1002/lt.22174. [DOI] [PubMed] [Google Scholar]
- Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23(1):169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Liang FX, Wang X. A synthetic biology approach identifies the mammalian UPR RNA ligase RtcB. Mol Cell. 2014;55(5):758–770. doi: 10.1016/j.molcel.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278(37):34864–34873. doi: 10.1074/jbc.M301107200. [DOI] [PubMed] [Google Scholar]
- Maurel M, Chevet E, Tavernier J, Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci. 2014;39(5):245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Merlini G, Comenzo RL, Seldin DC, Wechalekar A, Gertz MA. Immunoglobulin light chain amyloidosis. Expert Rev Hematol. 2014;7(1):143–156. doi: 10.1586/17474086.2014.858594. [DOI] [PubMed] [Google Scholar]
- Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29(14):1924–1933. doi: 10.1200/JCO.2010.32.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka S, Okada T, Yoshida H, Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol. 2007;27(3):1027–1043. doi: 10.1128/MCB.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev. 2009;8(3):150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Norgren N, Olsson M, Nystrom H, Ericzon BG, de Tayrac M, Genin E, Plante-Bordeneuve V, Suhr OB. Gene expression profile in hereditary transthyretin amyloidosis: differences in targeted and source organs. Amyloid. 2014;21(2):113–119. doi: 10.3109/13506129.2014.894908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici L, Merlini G. An overview of drugs currently under investigation for the treatment of transthyretin-related hereditary amyloidosis. Expert Opin Investig Drugs. 2014;23(9):1239–1251. doi: 10.1517/13543784.2014.922541. [DOI] [PubMed] [Google Scholar]
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- Reixach N, Deechongkit S, Jiang X, Kelly JW, Buxbaum JN. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc Natl Acad Sci U S A. 2004;101(9):2817–2822. doi: 10.1073/pnas.0400062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Ryno LM, Wiseman RL, Kelly JW. Targeting unfolded protein response signaling pathways to ameliorate protein misfolding diseases. Curr Opin Chem Biol. 2013;17(3):346–352. doi: 10.1016/j.cbpa.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Susuki S, Suico MA, Miyata M, Ando Y, Mizuguchi M, Takeuchi M, Dobashi M, Shuto T, Kai H. Endoplasmic reticulum quality control regulates the fate of transthyretin variants in the cell. EMBO J. 2007;26(10):2501–2512. doi: 10.1038/sj.emboj.7601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekijima Y, Hammarstrom P, Matsumura M, Shimizu Y, Iwata M, Tokuda T, Ikeda S, Kelly JW. Energetic characteristics of the new transthyretin variant A25T may explain its atypical central nervous system pathology. Lab Invest. 2003;83(3):409–417. doi: 10.1097/01.lab.0000059937.11023.1f. [DOI] [PubMed] [Google Scholar]
- Sekijima Y, Wiseman RL, Matteson J, Hammarstrom P, Miller SR, Sawkar AR, Balch WE, Kelly JW. The biological and chemical basis for tissue-selective amyloid disease. Cell. 2005;121(1):73–85. doi: 10.1016/j.cell.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, Yates JR, 3rd, Su AI, Kelly JW, Wiseman RL. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013;3(4):1279–1292. doi: 10.1016/j.celrep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P. Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid. 2014;21(4):221–224. doi: 10.3109/13506129.2014.964858. [DOI] [PubMed] [Google Scholar]
- Smith MH, Ploegh HL, Weissman JS. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science. 2011;334(6059):1086–1090. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Granell S, Salcedo-Sicilia L, Baldini G, Egea G, Teckman JH, Baldini G. Activating transcription factor 6 limits intracellular accumulation of mutant alpha(1)-antitrypsin Z and mitochondrial damage in hepatoma cells. J Biol Chem. 2011;286(48):41563–41577. doi: 10.1074/jbc.M111.280073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangou AJ, Heaton ND, Hawkins PN. Transmission of systemic transthyretin amyloidosis by means of domino liver transplantation. N Engl J Med. 2005;352(22):2356. doi: 10.1056/NEJM200506023522219. [DOI] [PubMed] [Google Scholar]
- Suhr OB, Herlenius G, Friman S, Ericzon BG. Liver transplantation for hereditary transthyretin amyloidosis. Liver Transpl. 2000;6(3):263–276. doi: 10.1053/lv.2000.6145. [DOI] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Gono T, Yazaki M, Ikeda S, Ikegami T, Hashikura Y, Miyagawa S, Hoshii Y. Transthyretin-derived amyloid deposition on the gastric mucosa in domino recipients of familial amyloid polyneuropathy liver. Liver Transpl. 2007;13(2):215–218. doi: 10.1002/lt.20954. [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. Biochim Biophys Acta. 2013;1833(12):3507–3517. doi: 10.1016/j.bbamcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197(7):857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34(Pt 1):7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Wiseman RL, Koulov A, Powers E, Kelly JW, Balch WE. Protein energetics in maturation of the early secretory pathway. Curr Opin Cell Biol. 2007;19(4):359–367. doi: 10.1016/j.ceb.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman RL, Powers ET, Buxbaum JN, Kelly JW, Balch WE. An adaptable standard for protein export from the endoplasmic reticulum. Cell. 2007;131(4):809–821. doi: 10.1016/j.cell.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136(3):343–350. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]