Abstract
Purpose
To both evaluate the frequency of eleven commonly cited barriers to optimal glaucoma medication adherence among glaucoma patients and identify barriers contributing to poor adherence.
Design
Prospective, cross-sectional survey.
Participants
190 adults with glaucoma taking ≥1 glaucoma medication who received care in glaucoma clinics in Ann Arbor, MI and Baltimore, MD.
Methods
Participants completed a survey on demographic and disease characteristics, barriers to optimal glaucoma medication adherence, interest in an eye drop aid, and self-reported adherence (measured by the Morisky Adherence Scale). Descriptive statistics and logistic regression analyses were performed.
Main Outcome Measures
Frequency and number of barriers to adherence among both adherent and non-adherent patients. Odds ratios with 95% confidence intervals identifying barriers associated with poor adherence.
Results
27% of the sample reported poor adherence. 61% of all participants cited multiple barriers and 10% cited a single barrier as impediments to optimal adherence. 29% of subjects cited no barriers, though only 13% of patients who cited no barriers were non-adherent. Among non-adherent patients, ≥31% cited each of the eleven barriers as important. Logistic regression analysis, adjusted for age, revealed that the following barriers were associated with higher odds of non-adherence: decreased self-efficacy, OR = 4.7 [95% CI 2.2–9.7, p= < 0.0001]; difficulty instilling drops, OR = 2.3 [95% CI 1.1–4.9, p= 0.03]; forgetfulness, OR = 5.6 [95% CI 2.6–12.1, p= < 0.0001]; and difficulties with the medication schedule, OR = 2.9 [1.4–6.0, p= 0.006]. For each additional barrier cited as important, there was a 10% increased odds of being non-adherent, OR = 1.1 [95% CI 1.0–1.2, p= 0.01].
Conclusion
Each of the eleven barriers was important to at least 30% of surveyed patients with poor adherence, with the majority identifying multiple barriers to adherence. Low self-efficacy, forgetfulness, and difficulty with drop administration and the medication schedule were all barriers associated with poor adherence. Interventions to improve medication adherence must address each patient’s unique set of barriers.
Keywords: glaucoma, medication adherence, barriers, technology, forgetfulness
Open angle glaucoma is the leading cause of blindness in black and Latino adults and the third leading cause of blindness in white adults.1–3 Intraocular pressure (IOP) lowering is the only proven method of minimizing both the development and progression of glaucoma4–5. IOP lowering is almost always achieved through eye drop administration. However, adherence to medical therapies is notoriously poor, with reported rates of non-adherence ranging from 30–80%.6, 7 Poor adherence has been shown to be associated with disease progression and blindness.8–11 In order to improve the clinical management of glaucoma, it is critically important to understand the reasons that glaucoma patients do not adhere to their medications.
Numerous qualitative studies have examined adherence behaviors among glaucoma patients. We identified eleven principal reasons cited in the literature for poor glaucoma medication adherence. These included skepticism that glaucoma will cause vision loss;12–14 skepticism that glaucoma medications are effective;12–15 poor knowledge about glaucoma;13, 14, 16, 17 poor self-efficacy;14, 18 forgetfulness;13, 14, 17, 19 cost;13, 17, 20 difficulties with the medication schedule;17, 19 side effects;15, 17, 19 difficulty with eye drop administration,13–15, 17, 19 mistrust in the physician,21 and perceived life stress (Table 1).22
Table 1.
Barriers to Glaucoma Medication Adherence
| Barriers to Glaucoma Medication Adherence | Literature Sources |
|---|---|
| Beliefs about glaucoma, skepticism that glaucoma will cause vision loss | Friedman 200812; Lacey 200913; Tsai 200317; Sleath 201026 |
| Beliefs about glaucoma medications, skepticism that glaucoma medications will mitigate vision loss | Friedman 200812; Lacey 200913; Stryker 201015; Tsai 200317; Sleath 201026 |
| Poor self-efficacy | Sleath 201218; Sleath 201026 |
| Poor knowledge about glaucoma | Friedman 200812; Lacey 200913; Stryker 201015; Lunnela 201016; Tsai 200317 |
| Mistrust of physician | Stryker 201015; Lunnela 201016; Tsai 200317; Taylor 200219 |
| Difficulty with eye drop administration | Lacey 200913; Tsai 200317; Taylor 200219; Sleath 201026 |
| Medication cost | Friedman 200812; Tsai 200317; Taylor 200219 |
| Medication-induced side effects | Friedman 200812; Tsai 200317; Taylor 200219 |
| Forgetfulness | Lacey 200913; Stryker 201015; Tsai 200317; Taylor 200219 |
| Difficulties with the medication schedule | Lacey 200913; Tsai 200317; Taylor 200219 |
| Life Stress | Hall 201427; Kretchy 201428; Cohen 198329 |
Though many studies have identified barriers to adherence, there is limited information on the relative frequency of these different barriers and on whether patients tend to have a single barrier or multiple barriers. It is important to understand whether a single barrier is most often related to poor adherence or whether each patient with poor adherence has their own unique set of barriers. If all patients with poor adherence have the same few barriers, designing group-based health behavior interventions to improve adherence would be optimal. If patients with poor adherence all have multiple different barriers, it will be important to individualize our strategies for supporting these patients. The goal of this study was to evaluate whether adherence was overwhelmingly affected by a single barrier or whether each person had a unique set of barriers to address. Additionally, we aimed to identify barriers that place patients at higher risk of non-adherence. Finally, we examined interest in eye drop aids to explore views on potential adherence support systems.
Methods
Participants and Sample Selection
This was a prospective survey study. We recruited a convenience sample of glaucoma patients from two glaucoma clinics by approaching all patients in the clinic waiting rooms once weekly. One glaucoma clinic was at the University of Michigan in Ann Arbor, MI, and the other was a private practice in Baltimore, MD. Recruitment took place from January 2013 – April 2013. We included any patient who reported taking ≥1 intraocular pressure (IOP) lowering medications who was interested in completing the survey. Formal informed consent was deemed unnecessary by the IRB as no personal health identifiers were collected in the survey. We excluded patients who were non-English speaking. A trained staff member was available to assist the subject in answering the questionnaire if needed. The questionnaire response rate was 69% with 273 questionnaires handed out and 190 returned. Five questionnaires were returned blank for a total of 185 questionnaires included in the analysis.
IRB
This study was approved prospectively as an exempt study by the University of Michigan Institutional Review Board (IRB # HUM00064465) and adhered to the tenets of the Declaration of Helsinki.
Questionnaire
The printed questionnaire consisted of 33 questions and took approximately 20 minutes to complete. The survey was written at ≤8th grade reading level as determined by Fleish-Kincaid software in Microsoft Office. The questionnaire had four sections: 1) demographic information; 2) barriers to medication adherence; 3) the Morisky Adherence Scale; and 4) interest in a medication assist device. The demographic information included age, sex, length of time with glaucoma, number of glaucoma medications, medical co-morbidities, overall health status, overall vision status, educational level, and social support.
In the second section on barriers to medication adherence, subjects were asked to use a visual analogue scale to rate the importance of eleven commonly cited reasons12, 13, 15–17, 19 that make it “hard for patients to take glaucoma eye drops.” The visual analogue scale had five major hatch-marks anchored between “strongly disagree” and “strongly agree.” The barriers included were informed by the Health Belief Model. The Health Belief Model postulates that a health behavior will occur if a person believes a disease will affect them, it will have important consequences, the treatment will help mitigate their risk, they do not have too many barriers to overcome to implement the treatment, and they have sufficient self-efficacy to carry out the treatment strategy23–25
Ten commonly cited reasons were chosen for inclusion after an extensive literature review (Table 1).12, 13, 15–19, 26 In addition, life stress was added as a barrier as it has been identified as a barrier to medication adherence among asymptomatic adults with hypertension,27, 28 a very similar disease to glaucoma as both diseases are often asymptomatic until something catastrophic occurs, like a stroke or severe vision loss. The content of the barrier scenarios was based on validated scales including the Glaucoma Medication Self-Efficacy and Outcome Expectations Scale,26 the Perceived Stress Scale29 and the Trust in Physician Scale.30 The survey items were then simplified into single barrier scenarios (Appendix 1, available at http://aaojournal.org). The barrier scenarios were simplified in order to reduce respondent burden as the intent of the questionnaire was to provide a description of the frequency of various barriers to medication adherence. The face validity of the barrier statements were tested through expert review by three glaucoma specialists (ALR, PPL, PANC). After subjects rated the importance of each barrier, they were asked to rank their top reasons for difficulties with adherence.
In the third section of the survey, subjects completed the Morisky Adherence Scale, a validated instrument for measuring self-reported adherence.31 The Morisky scale has been used to measure adherence in a wide variety of chronic conditions (including generally asymptomatic conditions such as diabetes and osteoporosis)32–34 by adapting it to the relevant disease and this was done for glaucoma in this study (Appendix 2, available at http://aaojournal.org). In the fourth section of the survey, subjects were asked about the problems they experience when applying eye drops and about their use of and interest in a medication assist device.
Analyses
We analyzed the data using SAS software, version 9.3 (SAS Institute, Cary, NC). We summarized participant characteristics using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. The questionnaire results were summarized with frequency distributions. We defined non-adherence as a Morisky adherence score >2, according to the standardized scoring of the validated instrument.31 Because the majority of subjects rated the barriers as either “strongly agree” or “strongly disagree” on the visual analogue scale, we dichotomized the data defining a barrier as important if the subject rated the barrier as ≥ the midpoint of the visual analogue scale. We also reported on the subjects’ ranking of the barriers.
We ran univariate logistic regression analyses to evaluate the association between clinically important predictor variables (e.g. age, sex, education level, length of glaucoma diagnosis, number of glaucoma medications, number of chronic medical conditions, subjective overall health status,35 subjective eyesight status, and social support) and poor adherence to glaucoma medications. As age was the only covariate significantly associated with adherence (p=0.006), we ran analyses evaluating the association between the different barriers and poor adherence adjusted for age. We used Spearman correlation to assess the relationships between the barriers associated with poor adherence and all eleven barriers.
Results
Subject Characteristics
Of 185 subjects, 49 (26.5%) were non-adherent by self-report on the Morisky Adherence Scale (a higher odds of a Morisky score ≥2). The level of non-adherence in our subject population is fairly representative of the larger glaucoma patient population, which in multiple studies has been found to be at least 30% non-adherent.6, 7 However, it is on the lower end of the range of estimates for glaucoma medication non-adherence (5%–80%) found in a recent meta-analysis.6 It is likely on the lower end because rates of self-reported non-adherence have been shown to be lower than rates of non-adherence measured with electronic dosing monitors.36 There was no significant difference between the percent of non-adherent patients between the two sites (p=0.4). Subjects who were non-adherent were significantly younger (61.5±17.3) than subjects who were adherent (66.3±14.4) (p=0.006) (Table 2). Sex, length of glaucoma diagnosis, number of glaucoma medications, overall heath status, overall eyesight status, educational attainment, and living alone were not significantly different between subjects who were and were not adherent (p>0.1 for all comparisons, Table 2). Overall, the subject population had glaucoma for a mean of 11.5±10.8 years and took an average of 2.4±1.3 glaucoma medications. The subjects reported good health with 97.7% reporting “excellent,” “very good,” or “good” health even though the total sample population had a mean of 2.3±1.6 chronic diseases. The majority (66.5%) of the population reported good vision. Twenty-five percent of the subjects lived alone. The subjects had high levels of educational attainment with 60.4% of the population having graduated from college or graduate school.
Table 2.
Description of Study Population by Adherence Status
| Variable Mean±Standard Deviation |
Value | Non- Adherent N (%) |
Adherent N (%) |
Overall N (%) |
p- value |
|---|---|---|---|---|---|
| Study population | 49 (26.5) | 136 (73.5) | 185 | ||
| Site | Ann Arbor | 25 (51.0) | 79 (58.1) | 104 (56.2) | 0.4a |
| Baltimore | 24 (49.0) | 57 (41.9) | 81 (43.8) | ||
| Age (Nmiss = 2) | 61.5±17.3 | 68.1±12.7 | 66.3±14.4 | 0.006b | |
| Sex (Nmiss = 2) | Female | 26 (54.2) | 74 (54.8) | 100 (54.6) | 0.9 a |
| Male | 22 (45.8) | 61 (45.2) | 83 (45.4) | ||
| Length of glaucoma diagnosis, years, (Nmiss = 8) | 9.8±9.7 | 12.0±11.0 | 11.5±10.8 | 0.2b | |
| Number of glaucoma medications, (Nmiss = 2) | 2.6±1.2 | 2.4±1.3 | 2.4±1.3 | 0.3b | |
| Number of chronic medical conditions, (Nmiss = 5) | 2.1±1.5 | 2.5±1.7 | 2.3±1.6 | 0.2b | |
| Subjective overall health status (Nmiss = 6) | Excellent | 7 (14.3) | 16 (12.3) | 23 (12.8) | 0.4a |
| Very Good | 19 (38.8) | 42 (32.3) | 61 (34.1) | ||
| Good | 18 (36.7) | 55 (42.3) | 73 (40.8) | ||
| Fair | 4 (8.2) | 17 (13.1) | 21 (11.7) | ||
| Poor | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Very Poor | 1 (2.0) | 0 (0.0) | 1 (0.6) | ||
| Subjective eyesight status (Nmiss = 6) | Excellent | 1 (2.0) | 8 (6.2) | 9 (5.0) | 0.8c |
| Very Good | 11 (22.4) | 31 (23.8) | 42 (23.5) | ||
| Good | 19 (38.8) | 49 (37.7) | 68 (38.0) | ||
| Fair | 12 (24.5) | 33 (25.4) | 45 (25.1) | ||
| Poor | 3 (6.1) | 4 (3.1) | 7 (3.9) | ||
| Very Poor | 3 (6.1) | 4 (3.1) | 7 (3.9) | ||
| Completely Blind | 0 (0.0) | 1 (0.8) | 1 (0.6) | ||
| Education Level (Nmiss = 6) | <High School | 2 (4.1) | 4 (3.1) | 6 (3.4) | 0.3c |
| High School | 20 (40.8) | 45 (34.6) | 65 (36.3) | ||
| College | 17 (34.7) | 37 (28.5) | 54 (30.2) | ||
| Graduate Degree | 10 (20.4) | 44 (33.8) | 54 (30.2) | ||
| Social Support: Patient Lives __ (Nmiss = 5) | Alone | 8 (16.7) | 37 (28.0) | 45 (25.0) | 0.3c |
| With a partner | 23 (47.9) | 61 (46.2) | 84 (46.7) | ||
| With family | 17 (35.4) | 30 (22.7) | 47 (26.1) | ||
| With friends | 0 (0.0) | 3 (2.3) | 3 (1.7) | ||
| Assisted living | 0 (0.0) | 1 (0.8) | 1 (0.6) | ||
| Nursing home | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Chi-square test
Two-sample t-test
Fisher exact test
Barriers to Glaucoma Medication Self-Reported Adherence Among Adherent Subjects
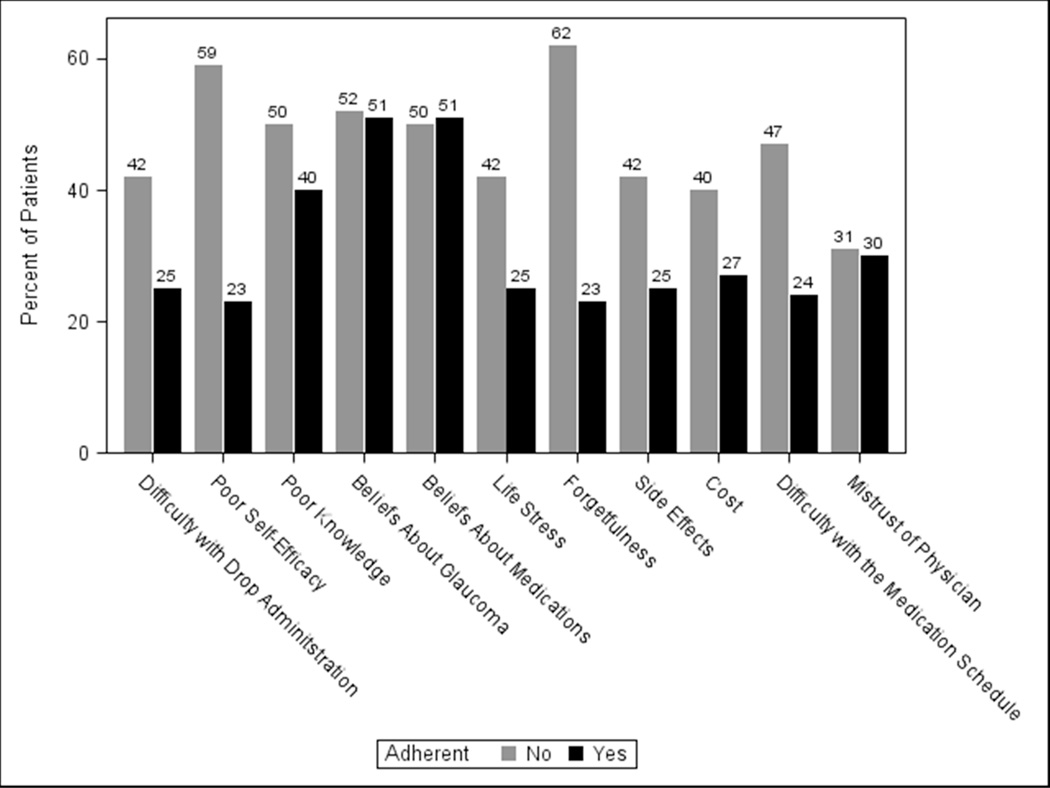
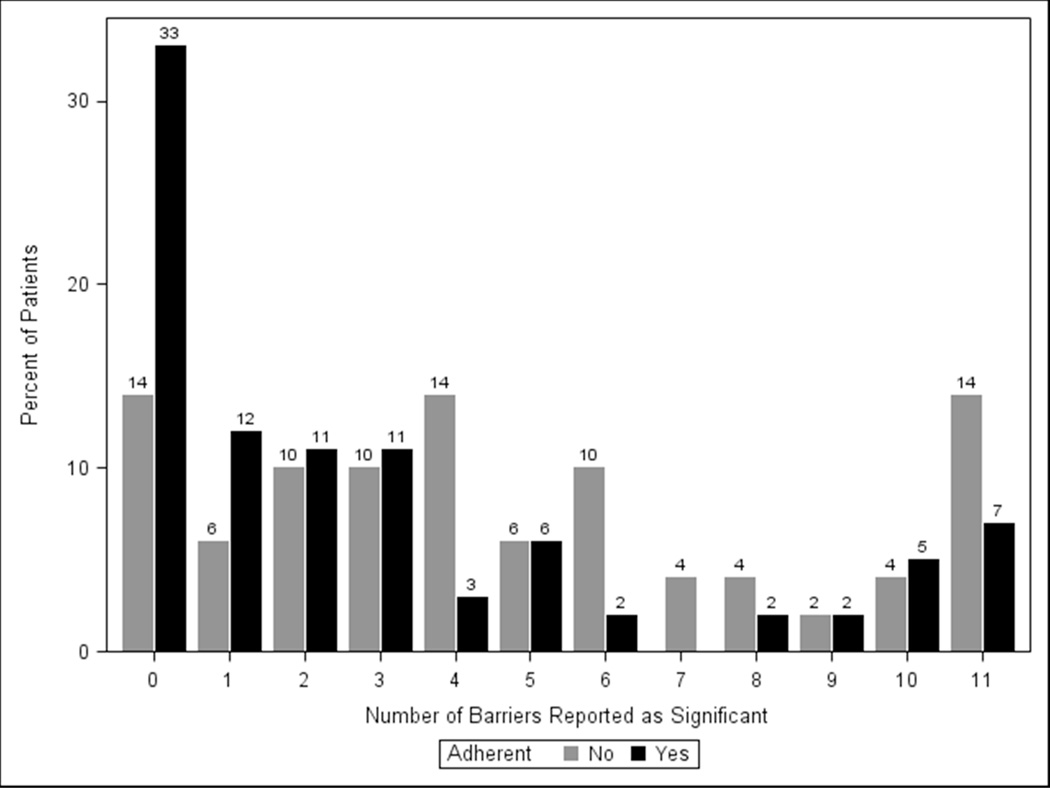
Each of the eleven identified barriers was cited as important by ≥23% of adherent subjects (Figure 1). The most prevalent barriers to optimal adherence were skepticism that glaucoma would lead to vision loss (51% cited as important), skepticism that glaucoma medications would prevent vision loss (51%) and insufficient knowledge about glaucoma (40%) (Figure 1). One-third (33%) of adherent subjects reported having no barriers to adherence, 12% reported having one barrier to adherence, and 55% reported having multiple barriers to adherence (Figure 2).
Figure 1. Prevalence of Important Barriers to Medication Adherence.
The items on the survey describing barriers to medication adherence asked subjects to rate the importance of eleven commonly cited reasons that make it “hard for patients to take glaucoma eye drops.” Subjects rated whether each of these eleven barriers was important to them on a visual analogue scale (VAS) anchored between “strongly agree” and “strongly disagree.” Figure 1 shows the percent of adherent and non-adherent subjects who rated a barrier as ≥ midpoint on the VAS.
Figure 2. Frequency of Number of Barriers.
Percent of subjects, both adherent and non-adherent, who rated different numbers of barriers as important.
Barriers to Glaucoma Medication Adherence Among Self-reported Non-adherent Subjects
Among subjects with poor adherence, each of the 11 barriers was cited as important by ≥31% of subjects. The most prevalent barriers to adherence were forgetfulness (62% cited as important), lack of self-efficacy (59%), skepticism that glaucoma would lead to vision loss (52%), skepticism that glaucoma medications would prevent vision loss (50%) and insufficient knowledge about glaucoma (50%) (Figure 1). Fourteen percent of non-adherent subjects reported no barriers to adherence, 6% reported a single barrier, and 80% reported multiple barriers (Figure 2).
Barrier Ranking
After rating each single barrier as important or not, subjects were asked to rank their top barriers to optimal medication adherence. 87/185 subjects responded to this item for a single-item response rate of 47.0%. Forgetfulness, difficulties instilling eye drops, and difficulties with the medication schedule were the top three barriers listed both by patients who were adherent and by patients who were non-adherent.
Comparing Barriers between Self-reported Adherent and Non-Adherent Subjects
The majority (61%) of subjects cited multiple barriers as important impediments to optimal adherence. Only 10% of patients cited only a single barrier as important. 29% of subjects overall cited no barriers as important; 87% of the subjects who cited no barriers as important reported good adherence.
Univariate logistic regression analysis revealed that the following barriers were associated with higher odds of non-adherence: decreased self-efficacy, OR = 4.7 [95% CI 2.3–9.7, p= < 0.0001]; difficulty instilling drops, OR = 2.1 [95% CI 1.0–4.3, p= 0.04]; forgetfulness, OR = 5.5 [95% CI 2.6–11.4, p= < 0.0001]; side effects, OR = 2.1 [95% CI 1.0–4.3, p= 0.04]; life stress OR = 2.2 [95% CI 1.1–4.4, p= 0.03]; and difficulties with the medication schedule, OR = 2.8 [1.3–5.7, p= 0.006]. For each additional barrier a subject cited as important, there was a 10% increased odds of being non-adherent, OR = 1.1 [95% CI 1.0–1.2, p= 0.007] (Table 3). After adjusting for age, life stress and side effects were no longer significantly associated with non-adherence (Table 3).
Table 3.
Barriers to Medication Adherence
| Barriera | Univariate Analysis Odds Ratio [95% CI] |
P Value | Bivariate Analysisb Odds Ratio [95% CI] |
P Value |
|---|---|---|---|---|
| Difficulty with Drop Administration | 2.1 [1.0 – 4.3] | 0.04 | 2.3 [1.1 – 4.9] | 0.03 |
| Poor Self-Efficacy | 4.7 [2.3 – 9.7] | < 0.0001 | 4.7 [2.2 – 9.7] | <0.0001 |
| Poor Knowledge | 1.5 [0.7 – 2.9] | 0.3 | 1.4 [0.7 – 2.8] | 0.4 |
| Beliefs about Glaucomac | 1.0 [0.5 – 2.1] | 0.9 | 1.1 [0.5 – 2.2] | 0.8 |
| Beliefs about Medicationsd | 0.9 [0.5 – 1.9] | 0.9 | 1.0 [0.5 – 1.9] | 0.9 |
| Life Stress | 2.2 [1.1 – 4.4] | 0.03 | 1.8 [0.9 – 3.8] | 0.1 |
| Forgetfulness | 5.5 [2.6 – 11.4] | < 0.0001 | 5.7 [2.6 – 12.1] | <0.0001 |
| Side Effects | 2.1 [1.0 – 4.3] | 0.04 | 1.9 [0.9 – 4.0] | 0.08 |
| Cost | 1.8 [0.9 – 3.6] | 0.1 | 1.6 [0.8 – 3.3] | 0.2 |
| Difficulty with the Medication Schedule | 2.8 [1.4 – 5.7] | 0.006 | 2.9 [1.4 – 6.0] | 0.006 |
| Mistrust of Physician | 1.0 [0.5 – 2.2] | 0.9 | 0.9 [0.4 – 1.9] | 0.7 |
| Number of Barriers | 1.1 [1.0 – 1.2] | 0.007 | 1.1 [1.0 – 1.2] | 0.01 |
Compared to subjects who did not report each issue as an important barrier
Adjusted for age
Glaucoma beliefs, skepticism that glaucoma will cause vision loss
Medication beliefs, skepticism that glaucoma medications will mitigate vision loss
Of the barriers associated with poor self-reported adherence, forgetfulness and difficulties with the medication schedule were strongly associated with several other barriers (Table 4). Forgetfulness was strongly associated with poor confidence (Spearman correlation coefficient 0.68), stress (Spearman correlation coefficient 0.66) and difficulties with the medication schedule (Spearman correlation coefficient 0.70). Difficulty with the medication schedule was strongly associated with difficulties coping with life stress (Spearman correlation coefficient 0.64), cost of medications (Spearman correlation coefficient 0.70) and difficulties with side effects (Spearman correlation coefficient 0.64).
Table 4.
Correlationsa between Significant Barriers to Glaucoma
| Barriers | Difficulty with Drop Administration |
Poor Self- Efficacy |
Forgetfulness | Difficulty with the Medication Schedule |
|---|---|---|---|---|
| Difficulty with Drop Administration | 1.00 | 0.58 | 0.52 | 0.46 |
| Poor Self-Efficacy | 0.58 | 1.00 | 0.68 | 0.51 |
| Poor Knowledge | 0.44 | 0.56 | 0.49 | 0.41 |
| Beliefs about Glaucomab | 0.36 | 0.42 | 0.41 | 0.37 |
| Beliefs about Medicationsc | 0.40 | 0.35 | 0.34 | 0.40 |
| Life Stress | 0.40 | 0.55 | 0.66 | 0.64 |
| Forgetfulness | 0.52 | 0.68 | 1.00 | 0.70 |
| Side Effects | 0.43 | 0.47 | 0.55 | 0.64 |
| Cost | 0.44 | 0.40 | 0.54 | 0.70 |
| Difficulty with the Medication Schedule | 0.46 | 0.51 | 0.70 | 1.00 |
| Mistrust of Physician | 0.41 | 0.42 | 0.47 | 0.54 |
Spearman correlation coefficients
Glaucoma beliefs, skepticism that glaucoma will cause vision loss
Medication beliefs, skepticism that glaucoma medications will mitigate vision loss
Drop Assist Device
Almost 1/5 of subjects (18%) were interested in utilizing a drop assist device if one were available. Similarly, around 1/5 of the subject population reported various difficulties administering eye drops. Subjects reported the following issues administering eye drops: 24% cited difficulty with aim; 18% cited difficulty controlling the number of drops dispensed; 10% cited difficulty holding steady while squeezing the bottle; 10% cited difficulty with flinching or blinking causing the drops not to enter the eye; and 5% cited difficulty squeezing the bottle. Nearly half of the subjects (46%) reported having no trouble with eye drop administration at all.
Discussion
In this sample of patients taking ≥1 intraocular pressure (IOP) lowering medication in two glaucoma clinics, we found that patients each had their own unique set of barriers to optimal adherence. The majority (62%) of the subject population cited more than one barrier as important. Additionally, the more barriers a subject cited as important, the more likely that subject was to be non-adherent. Compared to adherent patients, non-adherent glaucoma patients were more likely to cite ability to instill eye drops, forgetfulness, poor confidence, and difficulties with their medication schedule as important barriers to medication adherence. Forgetfulness was ranked as the number one barrier to adherence both by adherent and non-adherent patients.
Some barriers, such as forgetfulness, may be perceived as more socially acceptable than other barriers, such as not believing that glaucoma medications are really helping prevent vision loss. Patients want to please their doctor, as evidenced by patient-reported adherence being much higher than electronically measured adherence.36 It may be easier for patients to say that they “forgot” to take their medication than to discuss issues that could be seen as confrontational. Farris and Unni37 found that Medicare patients who had many concerns about their medications were more likely to report forgetting to take their medications. There is a need for physicians and health care providers to probe deeper into the issue of forgetting to take medications, as it is the most common reason cited for poor adherence17 and has been classified as unintentional non-adherence.38 One important component of trying to improve “forgetfulness” is setting an alarm to remind a patient to take the medication,39–41 and automated telephone or text reminders have been found to improve adherence in glaucoma patients.42 However, forgetting may encompass many different issues including concerns about whether a medication is helping. To improve adherence, it will be necessary to address these underlying beliefs along with instituting concrete reminder systems.
It was surprising to note that more non-adherent subjects were not more likely to report skepticism about glaucoma causing vision loss or skepticism about glaucoma medications mitigating that risk compared to adherent subjects, as about half of each group reported these beliefs as important obstacles to optimal adherence. We did not expect such a high prevalence of negative beliefs regarding both the disease process and treatment among adherent subjects. Incorporating discussions of disease and treatment beliefs will likely be an important component both of interventions to improve adherence and interventions to improve patient education and patient satisfaction overall.
Non-adherent patients were more likely to report both poor self-efficacy and difficulty with drop administration compared to adherent patients. Building self-efficacy requires a combination of teaching skills, brainstorming concrete solutions to perceived barriers, exploring resistances to change and building autonomous motivation to change.43 Teaching skills includes teaching how to properly instill glaucoma drops, and about one-fifth of study subjects were interested in using a drop-aid to help them with drop instillation. This illustrates the need for innovation in the area of drop administration and in the arena of teaching patients how to use their drops. We also know that at least 1/3 of patients who report that they know how to instill an eye drop perfectly still miss their eye, instill too many drops or touch the dropper bottle to the ocular surface or adnexae.44 Though 46% of subjects in our study reported no trouble administering their drops, it is likely that some of them do not instill their drops properly.44, 45 Additionally, teaching skills includes teaching people how to utilize reminder devices and how to integrate drop taking into their daily schedule.46, 47
Building self-efficacy also involves overcoming both concrete and psychological barriers to optimal adherence. Brainstorming solutions to barriers involves asking patients to come up with ideas for how to overcome issues that they face in taking their medication and then offering advice that other patients have found helpful. Exploring resistance to change and building autonomous motivation to change are both goals of motivational interviewing, which is a style of counseling whose goal is to improve people’s intrinsic desire to change their behavior.48 Motivational interviewing has been shown to improve medication adherence in different chronic diseases as well as in glaucoma, and is a technique that could be implemented more widely to improve self-management of glaucoma.48–50
In this survey, we found that glaucoma patients varied widely in which barriers to adherence were most salient for them. This suggests that it would be optimal if the information they receive either electronically or in-person was tailored to their needs to ensure that it was relevant. Tailoring refers to creating educational materials that differ for each individual patient based on their personal characteristics, needs, attitudes and beliefs.51, 52 Tailored education has been shown to be more effective than standard health education across many diseases that require people to engage in consistent health behaviors from quitting smoking53 to managing diabetes.54 The information about which barriers each glaucoma patient finds important can be used to create tailored educational materials as well as aid in training providers in how to counsel patients. There is a need to evaluate how tailored glaucoma patient education could play a role in facilitating effective conversations between eye care providers and patients to help patients better manage their glaucoma.
There are several limitations to this study. This was a convenience sample from two glaucoma specialty care clinics in the Midwest and mid-Atlantic states and the results may not be representative of patients followed in comprehensive ophthalmology clinics elsewhere. Though all patients in the waiting room of the glaucoma clinics were approached and asked to complete a survey about how they use their glaucoma medications, patients who were non-adherent may have been less inclined to participate and expose their poor adherence even though no mention of adherence was made in describing the study. Thus, the rate of non-adherence in this study population is likely an underestimate of the rate of non-adherence among the general glaucoma patient population. Furthermore, because patients with worse adherence may not have participated in the study, the frequency with which barriers were identified may not generalize to all patients. Neither race/ethnicity nor health literacy were evaluated in the survey, and so it was not possible to analyze the association between race/ethnicity or health literacy and the various reported barriers to medication adherence. The survey was de-identified, so it was not possible to corroborate patient-reported medical data with a chart review. Though the survey utilized a validated measure of self-reported medication adherence,55 self-reported medication adherence has been shown to be under-reported compared to electronically monitored medication adherence.56
There are also several strengths to this study, including the wide range of barriers that subjects evaluated and the analysis of optimal adherence among both patients who were and were not adherent to their medications. Because this study included many glaucoma patients who had been living with the disease for a longer time (mean number of years 11.5±10.8) and had continued to return to clinic for follow-up care, this study sample is likely more mindful of their disease than the general glaucoma population. To find that even these patients still had many issues managing their disease underscores the need to provide patients with more comprehensive resources for disease self-management.
In conclusion, this study showed that each patient is likely to have his or her own unique set of issues that will need to be addressed to optimize adherence. Further, the greater the number of barriers identified, the greater the likelihood of non-adherence. Interventions focused on improving adherence will need to ensure that they build self efficacy, teach patients proper eye drop instillation, and address issues with forgetfulness and difficulties with the medication schedule. Interventions will also need to individualize, or tailor, information and approaches to address each patient’s unique set of barriers. Future research should evaluate whether tailored education and counseling can increase patients’ motivation to manage their glaucoma and improve their medication adherence.
Supplementary Material
Acknowledgments
Financial Support: Heed Foundation Fellowship, National Eye Institute Michigan Vision Clinician-Scientist Development Program (K12EY022299). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented previously, in part, at the Annual Meeting of the American Glaucoma Society, February 28, 2014 and in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology, May 8, 2014.
Conflict of Interest: No conflicts of interest for PANC, KB, TB, MH. PPL is a consultant for Genentech and Novartis, and has stock in Pfizer, Merck, GSK, Medco Health Solutions,Vital Springs Health Technologies. ALR is a consultant for Merck, Allergan, Cipla Pharma, a medical monitor for Sucampo, on the Scientific Advisory Board for Aerie Pharmaceuticals, and has stock and stock options in Glaukos and Aerie Pharmaceuticals.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Congdon N, O'Colmain B, Klaver CC, Klein R, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Munoz B, West SK, Rubin GS, Schein OD, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–825. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 4.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Heijl A, Hussein M, Bengtsson B, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 6.Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112:953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl1):S57–S68. doi: 10.1016/j.survophthal.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WC, Chorak RP, Hunt HH, Sethuraman G. Factors associated with visual loss in patients with advanced glaucomatous changes in the optic nerve head. Am J Ophthalmol. 1993;116:176–181. doi: 10.1016/s0002-9394(14)71282-6. [DOI] [PubMed] [Google Scholar]
- 9.Rossi GC, Pasinetti GM, Scudeller L, Radaelli R, et al. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21:410–414. doi: 10.5301/EJO.2010.6112. [DOI] [PubMed] [Google Scholar]
- 10.Sleath B, Blalock S, Covert D, Stone JL, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118:2398–2402. doi: 10.1016/j.ophtha.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paula JS, Furtado JM, Santos AS, Coelho Rde M, et al. Risk factors for blindness in patients with open-angle glaucoma followed-up for at least 15 years. Arq Bras Oftalmol. 2012;75:243–246. doi: 10.1590/s0004-27492012000400004. [DOI] [PubMed] [Google Scholar]
- 12.Friedman DS, Hahn SR, Gelb L, Tan J, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115:1320–1327. 7.e1–7.e3. doi: 10.1016/j.ophtha.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Lacey J, Cate H, Broadway DC. Barriers to adherence with glaucoma medications: a qualitative research study. Eye (Lond) 2009;23:924–932. doi: 10.1038/eye.2008.103. [DOI] [PubMed] [Google Scholar]
- 14.Waterman H, Brunton L, Fenerty C, Mottershead J, et al. Adherence to ocular hypotensive therapy: patient health education needs and views on group education. Patient Prefer Adherence. 2013;7:55–63. doi: 10.2147/PPA.S37535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stryker JE, Beck AD, Primo SA, Echt KV, et al. An exploratory study of factors influencing glaucoma treatment adherence. J Glaucoma. 2010;19:66–72. doi: 10.1097/IJG.0b013e31819c4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunnela J, Kaariainen M, Kyngas H. The views of compliant glaucoma patients on counselling and social support. Scand J Caring Sci. 2010;24:490–498. doi: 10.1111/j.1471-6712.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsai JC, McClure CA, Ramos SE, Schlundt DG, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–398. doi: 10.1097/00061198-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Sleath B, Blalock SJ, Stone JL, Skinner AC, et al. Validation of a short version of the glaucoma medication self-efficacy questionnaire. Br J Ophthalmol. 2012;96:258–262. doi: 10.1136/bjo.2010.199851. [DOI] [PubMed] [Google Scholar]
- 19.Taylor SA, Galbraith SM, Mills RP. Causes of non-compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther. 2002;18:401–409. doi: 10.1089/10807680260362687. [DOI] [PubMed] [Google Scholar]
- 20.Safran DG, Neuman P, Schoen C, Kitchman MS, et al. Prescription drug coverage and seniors: findings from a 2003 national survey. Health Aff (Millwood) 2005 doi: 10.1377/hlthaff.w5.152. Suppl Web Exclusives:W5-152-w5-66. [DOI] [PubMed] [Google Scholar]
- 21.Blackstock OJ, Addison DN, Brennan JS, Alao OA. Trust in primary care providers and antiretroviral adherence in an urban HIV clinic. J Health Care Poor Underserved. 2012;23:88–98. doi: 10.1353/hpu.2012.0006. [DOI] [PubMed] [Google Scholar]
- 22.Weng FL, Chandwani S, Kurtyka KM, Zacker C, et al. Prevalence and correlates of medication non-adherence among kidney transplant recipients more than 6 months post-transplant: a cross-sectional study. BMC Nephrol. 2013;14:261. doi: 10.1186/1471-2369-14-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimer BK. Theory at a Glance: A Guide for Health Promotion Practice. 2014 [Google Scholar]
- 24.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Vol. 544. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 25.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman and Co; 1997. [Google Scholar]
- 26.Sleath B, Blalock SJ, Robin A, Hartnett ME, et al. Development of an instrument to measure glaucoma medication self-efficacy and outcome expectations. Eye (Lond) 2010;24:624–631. doi: 10.1038/eye.2009.174. [DOI] [PubMed] [Google Scholar]
- 27.Hall E. Social Ecology of Adherence to Hypertension Treatment in Latino Migrant and Seasonal Farmworkers. J Transcult Nurs. 2014 doi: 10.1177/1043659614524788. [DOI] [PubMed] [Google Scholar]
- 28.Kretchy IA. Mental Health in Hypertension: assessing symptoms of anxiety, depression and stress on anti-hypertensive medication adherence. 2014 doi: 10.1186/1752-4458-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 30.Anderson LA, Dedrick RF. Development of the Trust in Physician scale: a measure to assess interpersonal trust in patient-physician relationships. Psychol Rep. 1990;67:1091–1100. doi: 10.2466/pr0.1990.67.3f.1091. [DOI] [PubMed] [Google Scholar]
- 31.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Lee WY, Ahn J, Kim JH, et al. Reliability and validity of a self-reported measure of medication adherence in patients with type 2 diabetes mellitus in Korea. J Int Med Res. 2013 Aug;41(4):1098–1110. doi: 10.1177/0300060513484433. [DOI] [PubMed] [Google Scholar]
- 33.MA L. Factors Influencing Adherence to Molecular Therapies in Haematology-Oncology Outpatients. Journal of Pharmacy Practice and Research. 2006;36:115–118. [Google Scholar]
- 34.Guilera M, Fuentes M, Grifols M, Ferrer J, et al. Does an educational leaflet improve self-reported adherence to therapy in osteoporosis? The OPTIMA study. Osteoporos Int. 2006;17:664–671. doi: 10.1007/s00198-005-0031-8. [DOI] [PubMed] [Google Scholar]
- 35.DeSalvo KB, Bloser N, Reynolds K, He J, et al. Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med. 2006;21:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kass MA, Meltzer DW, Gordon M, Cooper D, et al. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986;101:515–523. doi: 10.1016/0002-9394(86)90939-6. [DOI] [PubMed] [Google Scholar]
- 37.Unni EJ, Farris KB. Unintentional non-adherence and belief in medicines in older adults. Patient Educ Couns. 2011;83:265–268. doi: 10.1016/j.pec.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Rees G, Leong O, Crowston JG, Lamoureux EL. Intentional and unintentional nonadherence to ocular hypotensive treatment in patients with glaucoma. Ophthalmology. 2010;117:903–908. doi: 10.1016/j.ophtha.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 39.Frick PA, Lavreys L, Mandaliya K, Kreiss JK. Impact of an alarm device on medication compliance in women in Mombasa, Kenya. Int J STD AIDS. 2001;12:329–333. doi: 10.1258/0956462011923048. [DOI] [PubMed] [Google Scholar]
- 40.Charles T, Quinn D, Weatherall M, Aldington S, et al. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. J Allergy Clin Immunol. 2007;119:811–816. doi: 10.1016/j.jaci.2006.11.700. [DOI] [PubMed] [Google Scholar]
- 41.Laster SF, Martin JL, Fleming JB. The effect of a medication alarm device on patient compliance with topical pilocarpine. J Am Optom Assoc. 1996;67:654–658. [PubMed] [Google Scholar]
- 42.Boland MV, Chang DS, Frazier T, Plyler R, et al. Automated telecommunication- based reminders and adherence with once-daily glaucoma medication dosing: the automated dosing reminder study. JAMA Ophthalmol. 2014;132:845–850. doi: 10.1001/jamaophthalmol.2014.857. [DOI] [PubMed] [Google Scholar]
- 43.Ryan MR. Facilitating health behaviour change and its maintenance: Interventions based on Self-Determination Theory. Eur Health Psychol. 2008 [Google Scholar]
- 44.Hennessy AL, Katz J, Covert D, Kelly CA, et al. A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am J Ophthalmol. 2011;152:982–988. doi: 10.1016/j.ajo.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy AL, Katz J, Covert D, Protzko C, et al. Videotaped evaluation of eyedrop instillation in glaucoma patients with visual impairment or moderate to severe visual field loss. Ophthalmology. 2010;117:2345–2352. doi: 10.1016/j.ophtha.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 46.Nadkarni A, Kucukarslan SN, Bagozzi RP, Yates JF, et al. Examining determinants of self management behaviors in patients with diabetes: an application of the Theoretical Model of Effortful Decision Making and Enactment. Patient Educ Couns. 2011;85:148–153. doi: 10.1016/j.pec.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 47.Park DC, Hertzog C, Kidder DP, Morrell RW, et al. Effect of age on event-based and time-based prospective memory. Psychol Aging. 1997;12:314–327. doi: 10.1037//0882-7974.12.2.314. [DOI] [PubMed] [Google Scholar]
- 48.Ng JY. Self-determination theory applied to health contexts: A meta-analysis. Perspect Psychol Sci. 2012:325–340. doi: 10.1177/1745691612447309. [DOI] [PubMed] [Google Scholar]
- 49.Williams GC, Rodin GC, Ryan RM, Grolnick WS, et al. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychol. 1998;17:269–276. doi: 10.1037//0278-6133.17.3.269. [DOI] [PubMed] [Google Scholar]
- 50.Cook PF, Bremer RW, Ayala AJ, Kahook MY. Feasibility of motivational interviewing delivered by a glaucoma educator to improve medication adherence. Clin Ophthalmol. 2010;4:1091–1101. doi: 10.2147/OPTH.S12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreuter MW. What Is Tailored Communication, In: Tailoring Health Messages: Customizing Communication with Computer Technology. Mahwah, NJ: Lawrence Erlbaum Association; 2000. [Google Scholar]
- 52.Hawkins RP, Kreuter M, Resnicow K, Fishbein M, et al. Understanding tailoring in communicating about health. Health Educ Res. 2008;23:454–466. doi: 10.1093/her/cyn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strecher VJ, McClure JB, Alexander GL, Chakraborty B, et al. Web-based smoking-cessation programs: results of a randomized trial. Am J Prev Med. 2008;34:373–381. doi: 10.1016/j.amepre.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spencer MS, Rosland AM, Kieffer EC, Sinco BR, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health. 2011;101:2253–2260. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Nundy S, Dick JJ, Chou CH, Nocon RS, et al. Mobile phone diabetes project led to improved glycemic control and net savings for Chicago plan participants. Health Aff (Millwood) 2014;33:265–272. doi: 10.1377/hlthaff.2013.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.