Abstract
The purpose of the present study was to test the prediction that the unique manifestation of chemotherapeutic-induced peripheral neuropathy (CIPN) would be reflected in a specific pattern of changes in the regulation of the intracellular Ca2+ concentration ([Ca2+]i) in subpopulations of cutaneous neurons. To test this prediction, we characterized the pattern of changes in mechanical nociceptive threshold associated with paclitaxel administration (2 mg/kg, iv, every other day for four days), as well as the impact of target of innervation and paclitaxel treatment on the regulation of [Ca2+]i in subpopulations of putative nociceptive and non-nociceptive neurons. Neurons innervating the glabrous and hairy skin of the hindpaw as well as the thigh were identified with retrograde tracers, and fura-2 was used to assess changes in [Ca2+]i. Paclitaxel was associated with a persistent decrease in mechanical nociceptive threshold in response to stimuli applied to the glabrous skin of the hindpaw, but not the hairy skin of the hindpaw or the thigh. However, in both putative nociceptive and non-nociceptive neurons, resting [Ca2+]i was significantly lower in neurons innervating the thigh after treatment. The magnitude of the depolarization-evoked Ca2+ transient was also lower in putative non-nociceptive thigh neurons. More interestingly, while paclitaxel had no detectable influence on either resting or depolarization-evoked Ca2+ transients in putative non-nociceptive neurons, in putative nociceptive neurons there was a subpopulation- specific decrease in the duration of the evoked Ca2+ transient that was largely restricted to neurons innervating the glabrous skin. These results suggest that peripheral nerve length alone, does not account for the selective distribution of CIPN symptoms. Rather, they suggest the symptoms of CIPN reflect an interaction between the toxic actions of the therapeutic and unique properties of the neurons deleteriously impacted.
Keywords: dorsal root ganglion, neuropathic pain, von Frey test, nociceptor
The impact of peripheral nerve injury on the regulation of the intracellular Ca2+ concentration ([Ca2+]i) in sensory neurons has received considerable attention because of the importance of neural [Ca2+]i to transmitter release (Parnas and Parnas, 2010), gene expression (Fields et al., 2005), excitability (Hogan, 2007), and neurotoxicity (Berridge et al., 2000). These are all neural processes that are not only changed by nerve injury (Lekan et al., 1997, Costigan et al., 2002, Yaksh, 2006, Wilson et al., 2012, Ratte et al., 2014) but appear to contribute to the signs and symptoms of peripheral neuropathy (Siau and Bennett, 2006). For example, in models of traumatic nerve injury, which is often associated with paresthesias, dysesthesias, and ongoing pain, there are changes in the regulation of [Ca2+]i in both putative nociceptive and non-nociceptive neurons in a pattern that appears to depend on whether or not the neurons were injured or were adjacent to those that were injured. That is, the duration of evoked Ca2+ transients were shorter in injured, but longer in adjacent putative nociceptive neurons (Fuchs et al., 2007). Similarly there was a decrease in resting [Ca2+]i in injured putative non-nociceptive neurons with no change in resting [Ca2+]i in adjacent putative nociceptive neurons (Fuchs et al., 2005). In contrast, in a model of diabetic neuropathy, while there is an increase in the duration of the evoked Ca2+ transient in putative nociceptive neurons, there is also an increase in resting [Ca2+]i (Kostyuk et al., 2001). In both models of peripheral neuropathy, there is evidence to suggest that the mechanisms contributing to the changes in the regulation of [Ca2+]i contribute to changes in excitability (Tang et al., 2012). While there may be marked differences between peripheral nerve injury models with respect to the pattern of changes in the regulation of [Ca2+]i, there are only relatively subtle differences in the pain behavior associated with each model, highlighting the fact that the nervous system is able to achieve the same phenotype via multiple mechanisms.
One potential explanation for the differences between models with respect to the pattern of changes in the regulation of [Ca2+]i, is that each model impacts a different subpopulation of sensory neurons and as a result different subpopulations of neurons contribute to the observed pain behavior. For example, spontaneous activity in muscle afferents appears to play a particularly important role in pain behavior associated with traumatic nerve injury (Michaelis et al., 2000), while neurons innervating more superficial targets appear to play a more prominent role on diabetic neuropathy (Johnson et al., 2008). Furthermore, there are differences between subpopulations of sensory neurons with respect to the regulation of [Ca2+]i (Lu et al., 2006). Consequently, the specific pattern of changes in the regulation of [Ca2+]i may be a reflection of an interaction between the unique properties of specific subpopulations of sensory neurons and the relative impact of the nerve injury.
We reasoned that a chemotherapy model of peripheral neuropathy might enable us to begin to test this suggestion. Chemotherapeutic-induced peripheral neuropathy (CIPN) is associated with a unique distribution, in what is referred to as a “stocking-glove” pattern, with signs and symptoms of neuropathy largely restricted to the hands and feet. More interestingly, CIPN also presents with what appears to be a differential impact on subpopulations of sensory neurons. Numbness and tingling appear to be associated with a loss of intra-epidermal nerve fiber density thought to reflect a selective dye-back of low threshold fibers, while the pain and hypersensitivity is thought to reflect the sensitization of nociceptive afferents (Tanner et al., 1998, Dougherty et al., 2004). Thus, we predicted, that this unique manifestation of CIPN would be reflected in a specific pattern of changes in the regulation of [Ca2+]i in subpopulations of cutaneous neurons. To test this prediction, we employed a combination of behavioral analysis and fura-2 based microfluorimetry to study the impact of paclitaxel-induced CIPN on different subpopulations of sensory neurons. An electronic von Frey device was used to assess the pattern of changes in mechanical sensitivity in the hindlimb. Retrograde tracers were used to identify subpopulations of cutaneous neurons innervating the glabrous and hairy skin of the hindpaw and skin of the thigh. Acutely dissociated DRG neurons were used to assess the impact of paclitaxel-induced CIPN on the regulation of [Ca2+]i in subpopulations of neurons defined by target of innervation. Our results support the suggestion that the unique manifestation of CIPN is reflected in a specific pattern of changes in the regulation of [Ca2+]i in subpopulations of cutaneous neurons and argue against a prevailing hypothesis that nerve length can account for the manifestation of CIPN.
EXPERIMENTAL PROCEDURES
Animals
Adult (250–320g) male Sprague-Dawley rats (Harlan, Indianapolis, IN)) were used for all experiments. Rats were housed two per cage in a temperature and humidity controlled, Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited animal housing facility on a 12h:12h light:dark schedule with food and water available ad libitum. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines for the use of laboratory animals in research.
Tissue labeling
1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbo-cyanine perchlorate (DiI) was injected intradermally at three different locations, one per animal, so as to label subpopulations of cutaneous afferents identified based on the target of innervation. These sites included the glabrous skin of the hind paw, the hairy skin on the dorsal side of the hind paw, and the hairy skin of the upper inner thigh. The hair covering the thigh was removed with an electrical shaver before retrograde labeling with DiI. DiI was injected with a 30 g needle under isoflurane (Abbott Laboratories, North Chicago, IL) anesthesia at 3–5 sites per target for a total volume of 10 µL in the dorsal and ventral hindpaw and 20 µL in the thigh.
Paclitaxel treatment
One week following the DiI injection, rats were anesthetized with isofluorane and injected into the tail vein with 2 mg/kg paclitaxel or its vehicle (1:1:23, cremophor EL:ethanol:0.9% saline). The tail vein injection was repeated three more times every other day for a total of four injections.
Behavioral Assessment of Mechanical Hypersensitivity
All behavioral data were collected in the Rodent Behavior Analysis Core of the University of Pittsburgh Schools of Health Sciences. Rats were habituated to the testing procedure, equipment, and the experimenter for two to three days before the collection of baseline data. An electronic von Frey (IITC Plantar Test Analgesia Meter 2390; IITC Life Sciences Inc., Woodland Hills, CA) fitted with a rigid tip (1.0-mm tip diameter) was used to assess changes in mechanical threshold. For assessment of changes in mechanical threshold in the glabrous skin, rats were placed in acrylic clear boxes on an aluminum mesh, which were separated by opaque dividers and, the tip was applied to the center of the middle of the hind paw from below with steady vertical pressure until the paw was lifted off the mesh floor. For assessment of mechanical threshold on the dorsal side of the hindpaw, rats were gently restrained in cotton socks cut so that their hind legs and tail protruded from the back. This enabled application of the mechanical probe in a manner comparable to that used for the glabrous skin of the hindpaw, perpendicular to the plane of the skin. Mechanical threshold was assessed in a similar manner at mid-thigh following removal of the hair covering the thigh with an electrical shaver, and placement of a spot on the target for mechanical probing with a permanent marker, both of which were repeated every 2–3 days until the completion of the experiments. Mechanical sensitivity was assessed at each site three times each day with an inter-stimulus interval of 5 minutes, and the average of the three measures for each paw or thigh was considered the withdrawal threshold. While the investigator collecting behavioral data was blinded to treatment group, the weight loss in the paclitaxel treated animals made blinding difficult through the entire testing period.
Sensory Neuron Isolation
Rats were deeply anesthetized with an intraperitoneal injection (1 ml/kg) of an anesthethic cocktail containing ketamine (55 mg/kg), xylazine (5.5 mg/kg) and acepromazine (1.1 mg/kg). L4 and L5 DRGs were removed bilaterally, enzymatically treated, and mechanically dissociated. DRG neurons were plated on laminin (Invitrogen, 1mg/ml) and poly-L-ornithine (Sigma-Aldrich, 1 mg/ml) coated glass cover slips as previously described (Lu et al., 2006). All subsequent experiments were performed within 8 h of tissue harvest. Only neurons containing the retrograde label DiI were included for further analysis.
Ca2+ Imaging
Prior to fluoremetric analysis, neurons were incubated with 2.5 µM Ca2+ indicator fura-2 AM ester with 0.025 % Pluronic F-127 for 20 min at room temperature. Neurons were then incubated with FITC-conjugated IB4 (10 µg/ml) for 10 min at room temperature, placed in a recording chamber and continuously superfused with a HEPES-buffered bath solution consistent of (in mM): 130 NaCl, 3 KCl, 2.5 CaCl2, 0.6 MgCl2, 10 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES), 10 glucose, pH 7.4, osmolality 325 mOsm. Fluorescence data were acquired on a PC running Metafluor software (Molecular Devices, Sunnyvale, CA) via an EMCCD camera (Photometrics, Tucson, AZ; model QuantEM 512SC). The ratio (R) of fluorescence emission (510 nm) in response to 340/380nm excitation (controlled by a DG-4 (Sutter Instrument, Novato, CA)) was acquired at 1 Hz during application of KCl or capsaicin, which were applied through a computer-controlled, piezo-driven perfusion system (switching time <20 ms; Warner Instruments, Hamden, CT, USA, Fast-Step Model SF-77B). [Ca2+]i was determined from fura-2 ratio according to the equation [Ca2+ ]i (nM) = Kd (Sf2/Sb2) ((R-Rmin)/(Rmax-R)) following in situ calibration as described previously (Scheff et al., 2013), where Kd is the dissociation constant for fura-2 for Ca2+ at room temperature; Sf2/Sb2 is the fluorescence ratio of the emission intensity excited by 380 nm signal in the absence of Ca2+; Rmin and Rmax are the minimal and maximal fluorescence ratios respectively.
Chemicals
Paclitaxel (Sigma-Aldrich, St Louis, MO, USA), was dissolved at 25 mg/mL in 1:1 Cremophor EL: ethanol and freshly diluted 1:12.5 in 0.9% sterile saline prior to injections. The retrograde tracer, DiI (Invitrogen, Carlsbad, CA, USA), was dissolved at 170 mg/mL in dimethylsufoxide (DMSO, Sigma-Aldrich) and diluted 1:10 in 0.9% sterile saline. FITC-conjugated Isolectin B4 (IB4, Sigma-Aldrich) was dissolved in dH20 as a stock solution of 1 mg/ml, and then diluted to a final concentration of 5 µg/ml in HEPES bath solution the day of use. Fura-2 acetoxymethyl (AM) ester (TEF Laboratories, Austin, TX, USA) was dissolved in DMSO as a 2.5 mM stock solution and diluted to a final concentration of 2.5 µM in HEPES bath solution. Pluronic F-127 (TEF Laboratories) was dissolved in DMSO as a 25% stock solution and diluted to 0.025% in HEPES bath solution. Capsaicin (Sigma-Aldrich) was dissolved in ethanol as a 10 mM stock solution and diluted to 500 nM in HEPES bath solution.
Data Analysis
Neurons from a single field were studied on each coverslip. Resting Ca2+ was determined prior to stimulation and was taken as the average of measurements taken over the 30 second prior to evoking a Ca2+ transient with high K+ (30 mM, 4 seconds). The magnitude of the evoked Ca2+ transient was determined as the difference between resting and the peak of the evoked Ca2+ transient. The duration of the evoked Ca2+ transient was determined as the time for the transient to decay to 50% of the peak (T50). For experiments involving the application of test compounds, a vehicle control group was always included. Neurons with a small cell body diameter (< 30 µm), responsive the capsaicin (500 nM), and labeled with the lectin IB4, were referred to as putative nociceptors. Cell body diameter was determined with a calibrated eye-piece reticle. Capsaicin sensitivity was assessed at the end of every experiment and neurons were considered capsaicin sensitive if application of capsaicin resulted in an increase in [Ca2+]i greater than 20% above baseline. IB4 binding was determined under epifluorescence illumination prior to the start of each experiment. Neurons in which the plasma membrane was clearly defined by epifluorescence were considered IB4+. Data are expressed as mean ± s.e.m. One and two-way ANOVA were used for analysis of more than two groups with the Holm-Sidak test used for posthoc analysis. Statistical significance was assessed at p < 0.05.
RESULTS
Paclitaxel-induced mechanical hypersensitivity
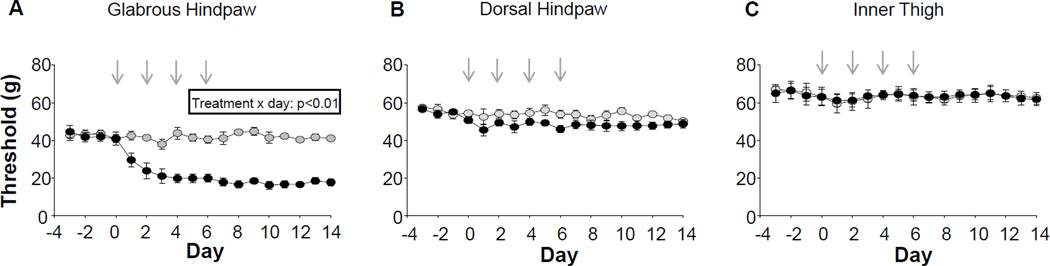
The rat model of CIPN used in the present study has been well described (Polomano et al., 2001, Flatters and Bennett, 2006, Xiao et al., 2008, Boyette-Davis et al., 2011). However, despite the fact that clinical manifestation of CIPN is largely restricted to the feet and hands (Dougherty et al., 2004, Boyette-Davis et al., 2013), and in particular, the glabrous skin of the feet and hands (Dougherty et al., 2004, Pachman et al., 2011), assessment of mechanical and thermal sensitivity has largely be restricted to the hindpaw (Flatters and Bennett, 2006, Xiao et al., 2008, Boyette-Davis et al., 2011). We therefore sought to determine the extent to which the pattern of hypersensitivity in the rat model parallels that observed clinically. Consistent with previous results (Polomano et al., 2001, Flatters and Bennett, 2006), mechanical hypersensitivity was readily detectable on the glabrous skin of the hindpaw with a significant decrease in threshold observed by the second paclitaxel injection (Figure 1A). In contrast, however, no detectable change in threshold was observed on the hairy skin of the hindpaw (Figure 1B) or the inner thigh (Figure 1C).
Figure 1.
Paclitaxel (2mg/kg per treatment, black circles) induced mechanical hypersensitivity in the glabrous skin of the hindpaw (A) but not in the hairy skin of the dorsal hindpaw (B) or inner thigh (C), as assessed with an electronic Von Frey device. No changes in mechanical threshold were observed in vehicle treated (grey circles) animals. Paclitaxel/vehicle were administered at the points indicated with gray arrows n = 7 per group.
The impact of target of innervation on resting and evoked intracellular calcium concentration [Ca2+]i in putative nociceptive cutaneous neurons
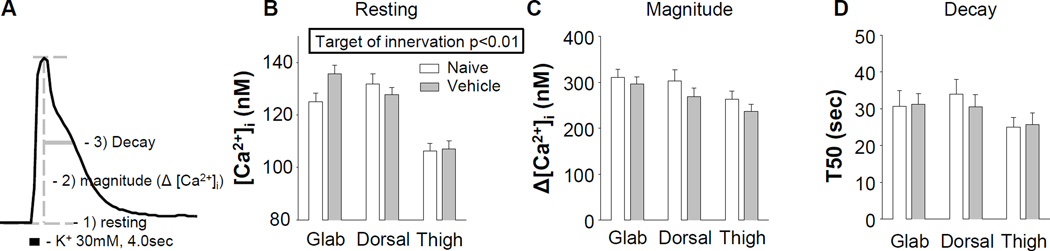
Given evidence that dysregulation of [Ca2+]i in subpopulations of nociceptive neurons may contribute to the manifestation of mechanical hypersensitivity (Kawano et al., 2009), we first sought to determine whether differences among subpopulations of nociceptive neurons defined by target of innervation may contribute to the selective manifestation of paclitaxel-induced hypersensitivity. Putative nociceptive neurons were identified based on cell body size (Lawson, 2002), IB4 binding (Fang et al., 2006) binding, and sensitivity to the algogenic compound, capsaicin (Holzer, 1991). Resting and evoked Ca2+ transients (magnitude and decay) were assessed as illustrated in Figure 2A. In putative nociceptive cutaneous neurons from naïve rats, there was a significant (p < 0.01, One-way ANOVA) difference between subpopulations defined by target of innervation in the resting [Ca2+]i, due to the level of Ca2+ in neurons innervating the thigh, which was significantly lower than that in neurons innervating the hairy (p < 0.01) or glabrous (p < 0.01) skin of the hindpaw (Figure 2B). However, magnitude (Figure 2C) and duration (Figure 2D) of the high K+ evoked transient were comparable between subpopulations (p > 0.05).
Figure 2.
Resting and evoked Ca2+ transients from putative nociceptive DRG neurons defined by target of innervation from naive rats or rats treated with the paclitaxel vehicle (cremophor EL: ethanol 1:1, diluted 1/12.5 with saline). A) Typical Ca2+ transient evoked with 30 mM K+ in a putative nociceptive neuron labeled from the glabrous skin. Features of the Ca2+ transient subsequently analyzed are indicated. Pooled data from naïve and vehicle treated rats were analyzed with a two-way ANOVA. B) While there was no detectable influence of vehicle on resting [Ca2+]i in subpopulations of putative nociceptive neurons defined by target of innervation, there was a main effect of target of innervation on this parameter. There was no detectable influence of either vehicle treatment of target of innervation on either the magnitude (C) or decay (D) of the evoked Ca2+ transient. Glab (glabrous skin of the hindpaw), Dorsal (dorsal skin of the hindpaw) and Thigh (thigh skin of the hindleg) refer to targets of innervation. Numbers of neurons in each group are naïve glabrous = 38, vehicle glabrous = 53, naïve dorsal = 27, vehicle dorsal = 23, naïve thigh = 30, vehicle thigh = 32).
While there was no detectable influence of the paclitaxel vehicle on nociceptive threshold, even relatively simple solvents, such as DMSO can influence a variety of cellular processes. More importantly, there is evidence suggesting that the paclitaxel vehicle may be responsible for some of the side effects of the chemotherapy treatment (Gelderblom et al., 2001, Ahn et al., 2014). We therefore assessed the impact of vehicle on resting [Ca2+]i and evoked transients in subpopulations of putative nociceptive neurons defined by target of innervation. Neurons from vehicle treated animals were harvested 24 days after the first IV administration. Data were analyzed with a two-way ANOVA. While there was a main effect associated with target of innervation (p < 0.01, Figure 2B) on resting [Ca2+]i, there was no detectable influence of vehicle on the resting [Ca2+]i or the magnitude (Figure 2C) and duration (Figure 2D) of the evoked Ca2+ transient among subpopulations of cutaneous neurons. Data from naïve and vehicle treated animals was therefore pooled for subsequent analyses and are referred to as control neurons.
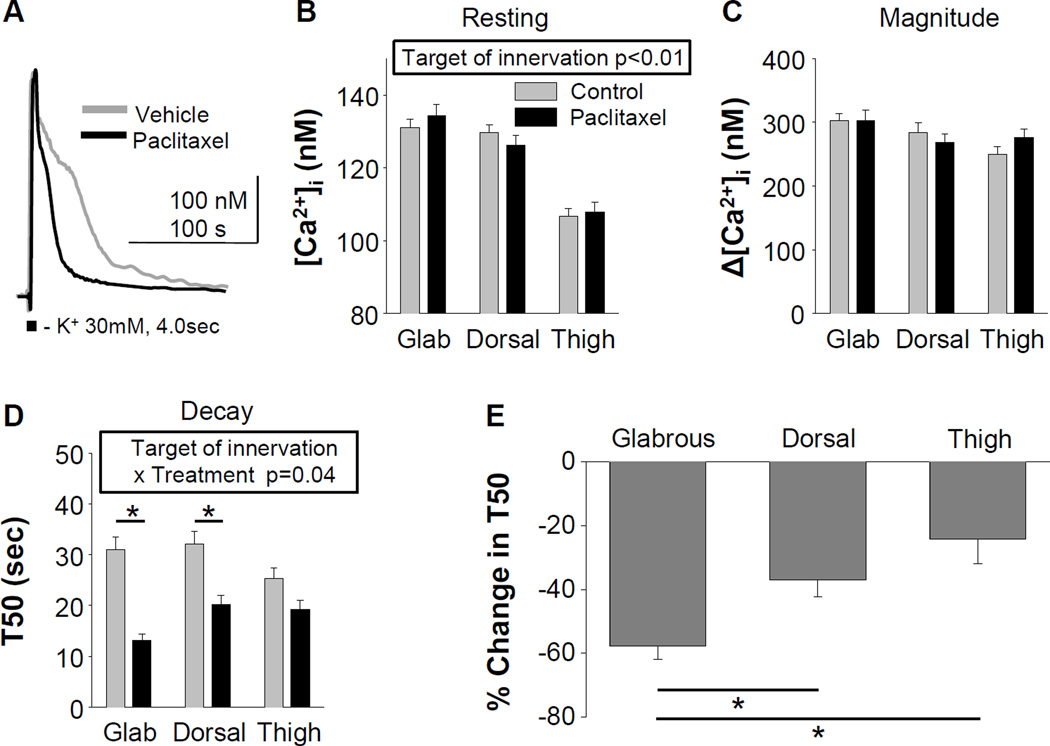
Paclitaxel attenuates the duration of the evoked Ca2+ transient in putative nociceptors
We next sought to determine whether changes in resting [Ca2+]i or evoked Ca2+ transients in putative nociceptive cutaneous neurons could account for the pattern of changes in mechanical sensitivity. Data were again analyzed with a two-way ANOVA, in which the impact of target of innervation and paclitaxel treatment were compared. While the main effect (p < 0.01) associated with target of innervation persisted on resting Ca2+, there was no significant influence of paclitaxel treatment, or a significant interaction between paclitaxel treatment and target of innervation on this parameter (Figure 3A and B). There was no detectable influence of either paclitaxel or target of innervation, or an interaction between the two, on the magnitude of the evoked Ca2+ transient (Figure 3A and C). However, there was a significant interaction between target of innervation and paclitaxel treatment on the duration of the evoked Ca2+ transient (Figure 3A and D). Post-hoc analysis indicated that the duration was significantly shorter in neurons innervating the glabrous (p < 0.01) and hairy skin of the hindpaw (p < 0.05) obtained from paclitaxel compared to control rats (Figure 3D). To determine whether there were differences between groups of putative nociceptive cutaneous neurons defined by target of innervation with respect to the paclitaxel-induced decrease in the duration of the evoked Ca2+ transient, T50 data from paclitaxel treated rats were analyzed as a percent of that in control neurons. Pooled data (Figure 3E) were analyzed with a one-way ANOVA that indicated there was a significant difference between groups (p < 0.01). Post-hoc analysis showed that while there was no difference between hairy hindpaw and thigh skin neurons with respect to the paclitaxel-induced decrease in T50, the decrease in the duration of the evoked transient in putative nociceptive glabrous skin neurons was significantly greater than that in hairy hindpaw or thigh skin neurons (Figure 3E).
Figure 3.
Resting and evoked Ca2+ transients from putative nociceptive DRG neurons defined by target of innervation from control (naïve and vehicle treated) rats or rats treated with paclitaxel. A) Typical high K+ (30 mM) evoked Ca2+ transients from putative nociceptive DRG neurons innervating glabrous skin of the hindpaw from rats treated with either vehicle or paclitaxel. Pooled data were analyzed as in Figure 2. B) While the main effect of target of innervation on resting [Ca2+]i, persisted, there was no detectable influence of paclitaxel on this parameter. C) There was also no detectable influence of paclitaxel on the magnitude of the evoked Ca2+ transient. D) However, there was a significant interaction between target of innervation and paclitaxel treatment on the duration of the evoked Ca2+ transient. Post-hoc analysis confirmed that the decrease in the duration of the evoked Ca2+ transient in both glabrous and hindpaw hairy skin (Dorsal) neurons were significant. To determine whether there was a difference between groups defined by target of innervation with respect to the size of the paclitaxel-induced decrease in duration, data from paclitaxel treated neurons were analyzed as a percent change from control. These data (E), were analyzed with a one-way ANOVA, which confirmed that the difference between groups was significant (p < 0.01). Post-hoc analysis confirmed the decrease in the glabrous neurons was significantly greater than that in neurons from either the dorsal hindpaw or thigh. Numbers of neurons in each group are control glabrous = 91, paclitaxel glabrous = 44, control dorsal = 50, paclitaxel dorsal = 33, control thigh = 62, paclitaxel thigh = 47; * p<0.05).
Paclitaxel does not affect the resting or evoked Ca2+ transient properties in putative non-nociceptive sensory neurons
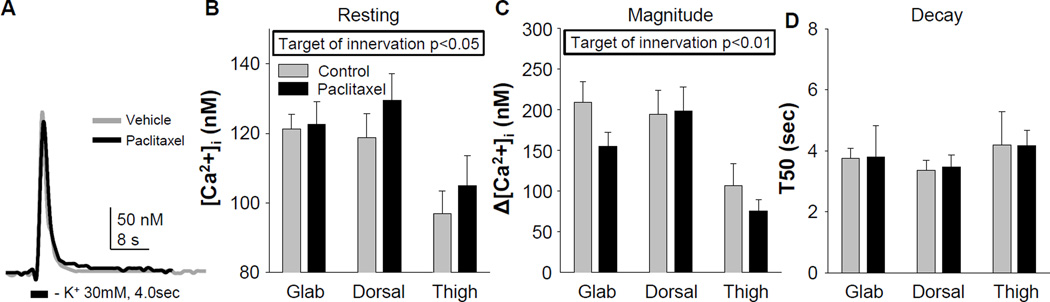
While we were focused on mechanisms that may contribute to the paclitaxel-induced decrease in mechanical threshold, the first sign of CIPN is numbness and tingling, thought to reflect changes in non-nociceptive afferents (Dougherty et al., 2004). We therefore assessed the impact of paclitaxel on resting [Ca2+]i and evoked Ca2+ transients in putative non-nociceptive cutaneous neurons defined by a relatively large cell body diameter, the absence of IB4 binding and insensitivity to capsaicin. Consistent with results from unlabeled DRG neurons (Lu et al., 2006), evoked Ca2+ transients in putative non-nociceptive cutaneous neurons were smaller and decayed more rapidly than those in putative nociceptive neurons (Figure 4A). Nevertheless, two-way ANOVA analysis of pooled data from control and paclitaxel-treated rats indicated that there were no significant interactions between target of innervation and paclitaxel treatment on resting Ca2+ (Figure 4B), the magnitude (Figure 4C), or the duration (Figure 4D) of the evoked transient. There was, however, a main effect of target of innervation on both the resting Ca2+ (Figure 4B) and the magnitude of the evoked Ca2+ transient (Figure 4C). Post-hoc analysis confirmed that both resting Ca2+ and the magnitude of the evoked Ca2+ transient were significantly lower in thigh neurons than neurons innervating hindpaw hairy or glabrous skin.
Figure 4.
Resting and evoked Ca2+ transients from putative non-nociceptive DRG neurons defined by target of innervation from control rats or rats treated with paclitaxel. A) Typical evoked Ca2+ transients from putative non-nociceptive DRG neurons innervating glabrous skin of the hindpaw from rats treated with either vehicle or paclitaxel. Pooled data were analyzed as described in Figure 2. B) While there was no detectable influence of paclitaxel treatment on resting [Ca2+]i, there was a significant main effect of target of innervation on this parameter. Post-hoc analysis indicated that resting Ca2+ in the thigh neurons was significantly lower than that in neurons innervating the hairy hindpaw (p = 0.01) and glabrous (p < 0.05) skin. C) There was also a significant main effect of target of innervation on the magnitude of the high K+-evoked Ca2+ transient in putative non-nociceptive neurons. Post-hoc analysis indicated that the magnitude of the high K+-evoked Ca2+ transient was significant lower in thigh neurons than that in neurons innervating the hairy hindpaw (p = 0.01) and glabrous (p < 0.05), skin. D) However, there was no detectable influence of either treatment or target of innervation on the duration of the high K+ evoked Ca2+ transient in these neurons. Numbers of neurons in each group are control glabrous = 14, paclitaxel glabrous = 10, control dorsal = 15, paclitaxel dorsal = 8, control thigh = 9, paclitaxel thigh = 8).
DISCUSSION
The purpose of the present study was to begin to test the hypothesis that the unique manifestation of CIPN is due to unique properties of afferents defined by target of innervation, in particular, those involved in the regulation of [Ca2+]i. Towards this end, we provided further support for the validity of the rat paclitaxel model of CIPN, which was associated with mechanical hypersensitivity in the glabrous skin of the hindpaw, but not the hairy skin of the hindpaw or mid-thigh. Differences among subpopulations of sensory neurons from naïve animals defined by target of innervation were relatively small and included a lower resting [Ca2+]i in both putative nociceptive and non-nociceptive neurons innervating the thigh, as well as a smaller magnitude of depolarization-evoked Ca2+ transient in putative non-nociceptive thigh skin afferents. And while paclitaxel had no detectable influence on resting [Ca2+]i, or the magnitude or duration of the depolarization-evoked Ca2+ transient in subpopulations of non-nociceptive afferents defined by target of innervation, it was associated with a significant decrease in the duration of the evoked transient in putative nociceptive afferents. This change was significantly larger in neurons innervating glabrous skin than those innervating hairy skin of the hindpaw or thigh. These results suggest that subpopulation and target of innervation are important factors determining the susceptibility and degree of dysregulation of [Ca2+]i, by paclitaxel treatment.
Our behavioral results indicate that the paclitaxel-induced mechanical hypersensitivity is associated with the type of target tissue (glabrous vs. hairy skin) rather than the axonal length. Previously, axonal length was thought to determine the presence of pain symptoms of paclitaxel-induced neuropathy due to the microtubule stabilizing action of paclitaxel presumably disrupting axonal transport and eventually leading to peripheral axon damage (Gornstein and Schwarz, 2014). Arguing against this proposal, however, is the observation that there are relatively minor differences among chemotherapeutics with respect to the resulting CIPN phenotype despite the fact that the antineoplastic efficacy of different chemotherapeutics is due to completely different mechanisms such as proteasome inhibition, DNA alkylation, topoisomerase inhibition (Jaggi and Singh, 2012). Furthermore, our results are consistent with data obtained from chemotherapy patients, in whom painful symptoms of CIPN are confined in the glabrous surfaces and rarely occur in the hairy surfaces of hands and feet of most patients (Dougherty et al., 2004, Pachman et al., 2011). If target of innervation plays a more dominant role in the manifestation of CIPN than axon length, it will be necessary to re-evaluate several of the leading hypotheses concerning the mechanisms of CIPN.
While the differences between subpopulations of neurons defined by target of innervation were relatively small, the presence of differences among subpopulations of cutaneous neurons underscores the impact of target of innervation of afferent properties. Differences based on target of innervation are consistent with our own previous results (Gold and Traub, 2004, Harriott et al., 2006, Harriott and Gold, 2009, Vaughn and Gold, 2010) as well as the results of others (Yoshimura et al., 2003, Beyak et al., 2004, Malin et al., 2011), although differences appear to be most pronounced under pathological conditions, such as the presence of inflammatory mediators (Gold and Traub, 2004, Vaughn and Gold, 2010) or persistent inflammation (Harriott et al., 2006, Zhang et al., 2012b). Minimally, these data suggest that target of innervation must be taken into consideration when analyzing the properties of putative nociceptive and non-nociceptive afferents.
A paclitaxel-induced decrease in the duration of the evoked Ca2+ transient was surprising given the evidence for an increase in the duration of the evoked Ca2+ transient in putative nociceptive neurons from rats with diabetic neuropathy (Kostyuk et al., 2001), a neuropathic pain syndrome that is also manifest in a stocking-glove distribution, as well as changes obtained with models of inflammatory pain, characterized by an increase in magnitude and duration of evoked Ca2+ transient (Lu and Gold, 2008, Scheff et al., 2013, Scheff and Gold, 2014).We predicted changes comparable to those observed in the persistent inflammation model based on previous results indicating that paclitaxel-induced neuropathic pain is associated with upregulation of proinflammatory cytokines and induction of TLR4-mediated signaling cascades (Ledeboer et al., 2007, Li et al., 2014). The difference between our results and the changes described in these other models could be due to the differences between models with respect to the site of release and/or the actions of specific combination of inflammatory mediators released (Ledeboer et al., 2007, Zhang et al., 2012a). More generally, however, the differences between models indicate that the pattern of changes in the regulation of [Ca2+]i in sensory neurons are relatively specific to the site and nature of the nerve injury.
It is possible that the paclitaxel-induced changes in the regulation of [Ca2+]i in putative nociceptive afferents are not directly responsible for the mechanical hypersensitivity observed. For example, the decrease in the duration of the evoked Ca2+ transient could be a compensatory response to the mitotoxic effects of paclitaxel. In this case, facilitating the decrease in the duration of the evoked Ca2+ transient may be beneficial for increasing the health and survival of these neurons. Alternatively, it is reasonable to speculate that a decrease in the evoked Ca2+ transient will increase the excitability of putative nociceptive cutaneous neurons given the importance of Ca2+-dependent K+ channels in the regulation of excitability of this population of afferents (Gold et al., 1996, Chen et al., 2009, Zhang et al., 2010), and the evidence that large, intermediate and small conductance K+ channels are present in small-diameter putative nociceptors (Mongan et al., 2005, Zhang et al., 2010). However, the observation that there was a significant decrease in the duration of the evoked Ca2+ transient in putative nociceptive neurons innervating the hairy skin of the hindpaw, a tissue in which we detected no decrease in mechanical threshold, suggests that if there is a relationship between the decrease in the duration of the evoked Ca2+ transient and nociceptive threshold, it is not so simple. There are at least two potentially complicating factors in this relationship: 1) it is possible that because of the biophysical properties of the Ca2+ dependent K+ channel and/or the spatial distribution of the channel relative to Ca2+ sources in the terminal, the decrease in the evoked Ca2+ transient must reach a threshold before a decrease in Ca2+-dependent K+ channel activity is manifest, and 2) there is a differential distribution of Ca2+-dependent channels in glabrous and hairy skin.
While it will be important to determine the mechanism(s) responsible for the paclitaxel-induced decrease in the duration of the evoked Ca2+, available evidence suggests that this change is likely to be orchestrated by several different Ca2+-regulatory mechanisms rather than a simple change in influx, release, extrusion, or re-uptake. For example, the persistent inflammation-induced increase in the magnitude and duration of the evoked Ca2+ transient (Lu and Gold, 2008), is associated with a decrease in voltage-gated Ca2+ current (Lu et al., 2010), a decrease in sodium/calcium exchanger (NCX) activity, secondary to selective trafficking of NCX to peripheral terminals (Scheff and Gold, 2014), and at least one more mechanism accounting for the increase in the magnitude of the evoked Ca2+ transient. With evidence that Ca2+ influx, reuptake, release and extrusion mechanisms may all be functionally isolated, even in an isolated cell body (Scheff et al., 2013), a simple shift in the coupling of Ca2+ regulatory mechanisms could account for the paclitaxel-induced decrease in the duration of the evoked Ca2+ transient. Ongoing investigations are focused on teasing apart the relative contribution of the various Ca2+ regulatory mechanisms to the paclitaxel effects observed.
In conclusion, our results suggest that axon length alone does not account for the stocking-glove distribution of CIPN. Rather, our results are consistent with our central hypothesis and suggest that the unique distribution of symptoms associated with CIPN are due to an interaction between the toxic effects of paclitaxel and unique properties of subpopulations of afferents defined by target of innervation. Identification of mechanisms responsible for the apparent vulnerability of glabrous skin neurons and/or the resistance of hairy skin neurons to the toxic effects of paclitaxel may suggest novel therapeutic approaches for the treatment, if not prevention of CIPN.
Highlights.
-
-
Paclitaxel-induced mechanical hypersensitivity is specific to the glabrous skin
-
-
Paclitaxel-induced Ca2+ dysregulation is sensory neuron subpopulation-specific
-
-
The largest paclitaxel-induced changes occur in glabrous skin putative nociceptors
-
-
Mechanisms underlying these changes may suggest therapeutic targets for CIPN
Acknowledgements
This research is supported by the Virginia Kaufman Endowment Fund No. 1 (MSG), and grants from the National Institutes of Health T32 NS073548 (EY) and 1R01DE018252 (MSG). We wish the thank Dr. Nicole Scheff for helpful comments on the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn HK, Jung M, Sym SJ, Shin DB, Kang SM, Kyung SY, Park JW, Jeong SH, Cho EK. A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2014;74:277–282. doi: 10.1007/s00280-014-2498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–855. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis JA, Cata JP, Driver LC, Novy DM, Bruel BM, Mooring DL, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol. 2013;71:619–626. doi: 10.1007/s00280-012-2047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Cai YQ, Pan HL. Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J Neurochem. 2009;110:352–362. doi: 10.1111/j.1471-4159.2009.06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26:7281–7292. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Lee PR, Cohen JE. Temporal integration of intracellular Ca2+ signaling networks in regulating gene expression by action potentials. Cell Calcium. 2005;37:433–442. doi: 10.1016/j.ceca.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Lirk P, Stucky C, Abram SE, Hogan QH. Painful nerve injury decreases resting cytosolic calcium concentrations in sensory neurons of rats. Anesthesiology. 2005;102:1217–1225. doi: 10.1097/00000542-200506000-00023. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Rigaud M, Hogan QH. Painful nerve injury shortens the intracellular Ca2+ signal in axotomized sensory neurons of rats. Anesthesiology. 2007;107:106–116. doi: 10.1097/01.anes.0000267538.72900.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Role of a Ca(2+)-dependent slow afterhyperpolarization in prostaglandin E2-induced sensitization of cultured rat sensory neurons. Neurosci Lett. 1996;205:161–164. doi: 10.1016/0304-3940(96)12401-0. [DOI] [PubMed] [Google Scholar]
- Gold MS, Traub RJ. Cutaneous and colonic rat DRG neurons differ with respect to both baseline and PGE2-induced changes in passive and active electrophysiological properties. J Neurophysiol. 2004;91:2524–2531. doi: 10.1152/jn.00866.2003. [DOI] [PubMed] [Google Scholar]
- Gornstein E, Schwarz TL. The paradox of paclitaxel neurotoxicity: Mechanisms and unanswered questions. Neuropharmacology. 2014;76(Pt A):175–183. doi: 10.1016/j.neuropharm.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Harriott AM, Dessem D, Gold MS. Inflammation increases the excitability of masseter muscle afferents. Neuroscience. 2006;141:433–442. doi: 10.1016/j.neuroscience.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Harriott AM, Gold MS. Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. J Neurophysiol. 2009;101:3126–3134. doi: 10.1152/jn.91339.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan QH. Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain. Croat Med J. 2007;48:9–21. [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Capsaicin as a tool for studying sensory neuron functions. Adv Exp Med Biol. 1991;298:3–16. doi: 10.1007/978-1-4899-0744-8_1. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Ryals JM, Wright DE. Early loss of peptidergic intraepidermal nerve fibers in an STZ-induced mouse model of insensate diabetic neuropathy. Pain. 2008;140:35–47. doi: 10.1016/j.pain.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Zoga V, Gemes G, McCallum JB, Wu HE, Pravdic D, Liang MY, Kwok WM, Hogan Q, Sarantopoulos C. Suppressed Ca2+/CaM/CaMKII-dependent K(ATP) channel activity in primary afferent neurons mediates hyperalgesia after axotomy. Proc Natl Acad Sci U S A. 2009;106:8725–8730. doi: 10.1073/pnas.0901815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk E, Voitenko N, Kruglikov I, Shmigol A, Shishkin V, Efimov A, Kostyuk P. Diabetes-induced changes in calcium homeostasis and the effects of calcium channel blockers in rat and mice nociceptive neurons. Diabetologia. 2001;44:1302–1309. doi: 10.1007/s001250100642. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekan HA, Chung K, Yoon YW, Chung JM, Coggeshall RE. Loss of dorsal root ganglion cells concomitant with dorsal root axon sprouting following segmental nerve lesions. Neuroscience. 1997;81:527–534. doi: 10.1016/s0306-4522(97)00173-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. Journal of Pain. 2014;15:712–725. doi: 10.1016/j.jpain.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SG, Gold MS. Inflammation-induced increase in evoked calcium transients in subpopulations of rat dorsal root ganglion neurons. Neuroscience. 2008;153:279–288. doi: 10.1016/j.neuroscience.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol. 2006;577:169–190. doi: 10.1113/jphysiol.2006.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SG, Zhang XL, Luo ZD, Gold MS. Persistent inflammation alters the density and distribution of voltage-activated calcium channels in subpopulations of rat cutaneous DRG neurons. Pain. 2010;151:633–643. doi: 10.1016/j.pain.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci. 2011;31:10516–10528. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Liu X, Janig W. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. J Neurosci. 2000;20:2742–2748. doi: 10.1523/JNEUROSCI.20-07-02742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongan LC, Hill MJ, Chen MX, Tate SN, Collins SD, Buckby L, Grubb BD. The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neuroscience. 2005;131:161–175. doi: 10.1016/j.neuroscience.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90:377–387. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- Parnas I, Parnas H. Control of neurotransmitter release: From Ca2+ to voltage dependent G-protein coupled receptors. Pflugers Arch. 2010;460:975–990. doi: 10.1007/s00424-010-0872-7. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Ratte S, Zhu Y, Lee KY, Prescott SA. Criticality and degeneracy in injury-induced changes in primary afferent excitability and the implications for neuropathic pain. Elife. 2014;3:e02370. doi: 10.7554/eLife.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff NN, Gold MS. Persistent Inflammation-Induced Increase in the Trafficking of Na+/Ca2+ Exchanger Isoform 3 (NCX3) to Peripheral Terminals. International Association for the Study of Pain Meeting Abstracts. 2014 [Google Scholar]
- Scheff NN, Lu SG, Gold MS. Contribution of endoplasmic reticulum Ca2+ regulatory mechanisms to the inflammation-induced increase in the evoked Ca2+ transient in rat cutaneous dorsal root ganglion neurons. Cell Calcium. 2013;54:46–56. doi: 10.1016/j.ceca.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapyevoked painful peripheral neuropathy. Anesth Analg. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Bangaru ML, Kostic S, Pan B, Wu HE, Koopmeiners AS, Yu H, Fischer GJ, McCallum JB, Kwok WM, Hudmon A, Hogan QH. Ca(2)(+)-dependent regulation of Ca(2)(+) currents in rat primary afferent neurons: role of CaMKII and the effect of injury. J Neurosci. 2012;32:11737–11749. doi: 10.1523/JNEUROSCI.0983-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner KD, Reichling DB, Levine JD. Nociceptor hyper-responsiveness during vincristine-induced painful peripheral neuropathy in the rat. J Neurosci. 1998;18:6480–6491. doi: 10.1523/JNEUROSCI.18-16-06480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AH, Gold MS. Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. J Neurosci. 2010;30:7878–7888. doi: 10.1523/JNEUROSCI.6053-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Schmutzler BS, Brittain JM, Dustrude ET, Ripsch MS, Pellman JJ, Yeum TS, Hurley JH, Hingtgen CM, White FA, Khanna R. Inhibition of transmitter release and attenuation of anti-retroviral-associated and tibial nerve injury-related painful peripheral neuropathy by novel synthetic Ca2+ channel peptides. J Biol Chem. 2012;287:35065–35077. doi: 10.1074/jbc.M112.378695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Naso L, Bennett GJ. Experimental studies of potential analgesics for the treatment of chemotherapy-evoked painful peripheral neuropathies. Pain Med. 2008;9:505–517. doi: 10.1111/j.1526-4637.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006;7:S13–30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Erickson KA, Erickson VL, Hancellor MB, de Groat WC. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neurosci. 2003;23:4355–4361. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yoon SY, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain. 2012a;13:293–303. doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Mok LP, Katz EJ, Gold MS. BKCa currents are enriched in a subpopulation of adult rat cutaneous nociceptive dorsal root ganglion neurons. Eur J Neurosci. 2010;31:450–462. doi: 10.1111/j.1460-9568.2009.07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Mok LP, Lee KY, Charbonnet M, Gold MS. Inflammation-induced changes in BK(Ca) currents in cutaneous dorsal root ganglion neurons from the adult rat. Mol Pain. 2012b;8:37. doi: 10.1186/1744-8069-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]