Abstract
Background
Pre-Exposure Prophylaxis (PrEP) for HIV prevention is a novel biomedical prevention method. We have previously modeled PrEP during rectal SHIV exposures in macaques and identified that SHIV-specific T cell responses were induced in the presence of antiretroviral drugs, an observation previously termed T cell chemo-vaccination. This report expands those initial findings by examining a larger group of macaques that were given oral or topical PrEP during repeated vaginal virus exposure.
Methods
Thirty-six female pigtail macaques received up to 20 repeat low-dose vaginal inoculations with wild type (WT) SHIVSF162P3 (n=24) or a clonal derivative with the tenofovir K65R drug resistant mutation (n=12). PrEP consisted of oral Truvada (n=6, WT), tenofovir vaginal gel (n=6, K65R), or tenofovir intra-vaginal ring (n=6, WT). The remaining animals were PrEP-inexperienced controls (n=12, WT; n=6, K65R). SHIV-specific T cells were identified and characterized using IFNγ ELISPOT and multi-parameter flow cytometry.
Results
Of nine animals that were on PrEP and remained uninfected during WT SHIV vaginal challenges, eight (88.9%) developed virus-specific T cell responses. T cells were in CD4 and CD8 compartments, reached up to 4,900 IFNγ producing cells per million PBMCs, and primarily pol directed. In contrast, the replication impaired K65R virus did not induce detectable T cell responses, likely reflecting the need for adequate replication.
Conclusion
Virus-specific T cell responses occur frequently in oral or topical PrEP-protected pigtail macaques after vaginal exposure to WT SHIV virus. The contribution of such immune responses to protection from infection during and following PrEP warrants further investigation.
Keywords: SHIV, pre-exposure prophylaxis, T cell immune response, exposed uninfected, pig tail macaques
Introduction
Four human clinical trials (iPrEX, CDC TDF2, Partner PrEP, Bangkok Tenofovir Study) have provided proof of concept for use of Pre-Exposure Prophylaxis (PrEP) against HIV-1 infection by men who have sex with men, heterosexually active men and women and intravenous drug users1–4. And, Truvada, the combination of the antiretroviral drugs Emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF) has been licensed by the US Food and Drug Administration as the first PrEP drug5–7. PrEP continues to be studied to improve adherence and implementation, among other follow up studies8,9.
We have previously examined if immune responses are affected by the use of PrEP10–12, modeling PrEP in rhesus macaques. We showed that rectal SHIV exposure with repeat low-doses (RLD) in the presence of oral PrEP can result in the development of SHIV-specific adaptive T cell immune responses in protected rhesus macaques, an effect previously termed chemo-vaccination10. Our previous report was based on studying four macaques, of which two developed measurable anti-SHIV T cell responses. It is not known if virus-specific T cell priming is a common phenomenon during PrEP. To better understand these T cells and their induction, here we examined a larger cohort of macaques, and asked whether T cell priming occurs frequently, and if it develops in female pigtail macaques during vaginal SHIV exposure with topical or systemic PrEP.
Understanding the mechanism of, and eliciting cell-mediated mucosal immune response against HIV is one focus of current vaccine strategies13–15. For instance, attenuated vaccine strains of SIV have been shown to elicit CD8+ T cell responses that protect and control SIV infection in macaques14,15. Our previous work has shown that T cell responses that develop in macaques during rectal SHIV challenges with oral Truvada use consisted of CD4+ and CD8+ SHIV-specific T cells10. It is not clear whether such immune responses have any consequences for or confer any advantage during subsequent virus exposures. Ideally, immunity would contribute to the efficacy of PrEP by induction of antiviral and cytotoxic CD8+ T cells and helper CD4+ T cells. T cell priming could improve immunity, should breakthrough infections occur, by increasing antiviral cytokines and immune responses against a possibly attenuated virus by the presence of suppressive antiretroviral drugs11,16. It is also possible, however, that T cell priming might create immune activated cells that are highly susceptible to subsequent infection.
The contribution of virus-specific T cell responses to the efficacy of PrEP and the mechanisms by which the T cell responses occur are at present not clear. In this report, we sought to further characterize the responses to learn more about their frequency of occurrence, magnitude, epitope specificity, and immune-phenotypic features. We specifically asked how frequently they occur, using a larger cohort of macaques than previously studied. We also examined whether virus-specific T cell responses on PrEP are induced after vaginal route of virus exposure as opposed to our previous report using rectal virus exposures. Furthermore, we investigated if they occur in pigtail macaques which differ from the rhesus macaque species that we previously studied, and using different PrEP modalities that included oral and topical drug formulations. Finally, because the mechanisms of T cell induction are unknown, but could be dose dependent, could involve antigen-presentation through non-classical pathways that are independent of viral replication, and because persons receiving PrEP may be exposed to HIV variants with drug-resistance mutations, we evaluated the virus-specific responses in animals exposed to a replication-impaired virus with a K65R mutation17, given at a higher dose than the wild type virus.
Methods and Materials
Ethics statement
The Institutional Animal Care and Use Committee (IACUC) of the Center for Disease Control and Prevention (CDC) approved all the macaque procedures for the studies. Macaques were housed at the CDC under the full care of CDC veterinarians in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institute of Health18. All procedures were done under anesthesia using ketamine and efforts were made to minimize suffering, improve housing conditions and to provide enrichment opportunities.
PrEP efficacy studies with RLD vaginal SHIV exposures in pigtails
The Repeat-Low dose (RLD) mucosal virus exposure model used in this study involves repetitive mucosal exposure using low doses of virus (10 to 50 TCID50, equivalent to 105–106 virus particles), which approximates viral loads observed in semen during acute HIV-1 infection19. The RLD model mimics repeated sexual exposures to the virus during high-risk sexual activity19. Thirty-six female pigtail macaques were enrolled in three different studies designed to evaluate the efficacy of PrEP. The intermittent oral Truvada PrEP study has been described20. Briefly, six pigtail macaques were in the PrEP-treated group receiving intermittent oral Truvada (given at 20 mg/kg of FTC and 22 mg/kg of TDF 24 h before and 2 h after vaginal SHIV) and six were in the control group receiving placebo. Virus challenge consisted of RLD vaginal exposures of R5-tropic SHIVSF162P3 virus21, obtained through the NIH AIDS Reagent program, Germantown, MD. It was propagated at CDC in pigtail PBMCs, titrated and given to macaques once weekly for up to 18 challenges at a dose of 50 TCID50. Twelve macaques were in the intra-vaginal ring-tenofovir (IVR-TFV) study: six with silicone IVRs (25 × 5 mm) containing four 15mg TFV pods/ring and six with placebo IVRs with blank pods as described previously22,23. The virus dose in the IVR-tenofovir study was also low (50 TCID50) administered twice weekly for up to 14 challenges. The vaginal tenofovir gel study with SHIVSF162P3-K65R virus included six pigtail macaques in the PrEP-treated and six in the control arms24. SHIVSF162P3-K65R virus has a K65R mutation in the reverse transcriptase enzyme, rendering the virus partially resistant to tenofovir17. SHIVSF162P3-K65R virus has 10-fold less infectivity compared to SHIVSF162P3, based on virus infectivity to particle ratio, described in25. Vaginal tenofovir gel (1%, delivering 30 mg tenofovir in a 3 mL volume) or a placebo gel were administered to the animals 30 minutes prior to vaginal virus challenges at an adjusted dose of 500 TCID50 SHIVSF162P3-K65R to compensate for lower infectivity, twice weekly for up to 20 challenges. SHIV infection was detected and monitored in all three studies using a previously described quantitative RT-PCR method26, with detection limit of the assay being 50 viral RNA copies/mL. Virus challenges were stopped once systemic SHIV infection occurred, here defined as detection of plasma SHIV RNA of at least 50 copies/ mL on two consecutive blood collections.
Detection of SHIV-specific T cells using IFN-γ ELISPOT assays
Freshly isolated peripheral blood mononuclear cells (PBMCs) obtained from the three studies at baseline, during virus challenges and post-challenge follow ups were used in ELISPOT assays. The IFN-γ ELISPOT assay for nonhuman primate PBMCs was described previously10. Briefly, MultiScreen-HA 96-well plates (EMD Millipore, Billerica, MA) were pre-incubated with 5ug/ml of anti-human IFNγ (BD Biosciences, San Diego, CA) overnight at 4°C. Next day, plates were blocked with complete RPMI medium for 1 h at 37°C. Plates were then washed with RPMI and 200,000 PBMCs per well were seeded and incubated with SHIV peptide pools (15-mers with 11 amino acid overlaps) at final concentrations of 1.5ug/ml in the presence of 1.25ng/ml IL-15 (Sigma-Alderich, St. Louis, MO). The following peptide pools obtained through the NIH AIDS Reagent Program (Germantown, MD) and their respective catalogue numbers shown in brackets, were used. Full length gag (#6204), pol (#6443), nef (#8762), vpr (#6449) and vif (#6205) were derived from SIVmac239. The env peptide pools (#7619) were from SHIVSF162P3, which harbors HIV-1 subtype B envelope. Tat (#5138) and rev (#6445) peptide pools were from HIV-1 consensus B peptides. Staphylococcus aureus Enterotoxin B (SEB) peptide and Ebola virus-derived peptides (EB) served as positive and negative (mock peptide) controls, respectively, as previously described27. After 36 h stimulation with the peptide pools, plates were washed and biotinylated anti-human IFN-γ antibody (MABTECH, Mariemont, OH), horse radish peroxidase conjugated streptavidin (BD Biosciences, San Diego, CA) and Nova red color substrate (Vector Labs, Burlingame, CA) were used sequentially to stain and detect IFN-γ secretions. The IFN-γ spot forming units (SFUs) were enumerated using a S5 Core Analyzer (Cellular Technology, Shaker Heights, OH). SHIV-specific T cells responding to each peptide pool were determined as SFUs/106 PBMCS after subtracting the values obtained by mock peptide stimulation.
Multi-parameter flow cytometry
PBMCs archived and available at one-time point from the intermittent oral Truvada study were analyzed by flow cytometry for surface and intracellular markers. For the PrEP-protected group, PBMCs that showed the highest SHIV-specific T cell responses (identified by IFN-γ ELISPOT) were analyzed, and in the control treated-infected group, PBMCs at the time of peak viremia were analyzed. PBMCs were limiting and were unavailable at other time points or from the IVR-tenofovir and vaginal tenofovir gel with SHIVSF162P3-K65R studies. Multi-parameter flow cytometer techniques are described elsewhere14,28. Briefly, frozen PBMCS were thawed and allowed to rest overnight in a cell culture at 37°C with 5% CO2. Next day, cells were washed and transferred to round bottom 96-well plates (Corning Life Sciences, Tewksbury, MA). Cells were stimulated for 6 h at 37°C with peptide pools of gag or pol, using the same peptide pools as used in ELISPOTs at 1.5ug/ml final concentration in the presence of GolgiStop Protein Transport Inhibitor (BD Biosciences, San Diego, CA). EB and SEB peptides served as negative and positive antigenic stimulation controls. Cells were then stained using extracellular surface marker antibodies. Permeabilization and staining for intracellular cytokines followed using the BD Cytofix/Cytoperm kit according to the manufacturer’s instruction (BD Biosciences, San Diego, CA). The following antibodies were purchased from BD Biosciences with clone names indicated in the parenthesis. Anti-CD3 Pacific Blue (SP34-1), anti-CD4-V500 (L200), anti-CD8 APC-Cy7 (SK1), anti-CD28-PE-CF594 (CD28.2), anti-IL-2 APC (MQ1-17H12), anti-TNFα Alexa F700 (MAB11), anti-IFN-γ PE-Cy7 (B27) and MIP-1β PE (D21-1351). Anti-CCR7-FITC (050503) was from R and D Systems (Minneapolis, MN). Data were acquired using LSR II Flow Cytometer (BD Biosciences, San Diego, CA) and analyzed using FlowJo software version 7.6.5 (Tree Star, San Carlos, CA).
Statistical analysis of anti-viral T cell respnses
For our first analysis method, we used the following definition for virus-specific T cell responses during PrEP: SHIV-specific T cell responses that occurred in PrEP-treated macaques without systemic SHIV infection at the time, and that were above a cut-off of all 36 study animals’ mean IFN-γ SFU baseline responses plus three times the standard deviation, as previously done10. This resulted in an IFN-γ SFU cut-off value of 907/106 PBMCs. As a second analysis method, and when indicated, we also studied SHIV-specific T cell responses in PrEP-treated and in untreated animals, even if they did not meet our above cut-off for virus-specific T cell responses during PrEP. These responses were calculated after subtracting the values obtained by mock peptide stimulation on the same day, to exclude non-specific responses. Median SFU values were calculated for each monkey from its multiple measures taken over time and either Mann-Whitney tests were performed to test for statistical differences between groups or paired t-test were performed for comparisons to baseline values using GraphPad software.
Results
Virus-specific T cell responses develop in systemic or topical PrEP-experienced, exposed uninfected pigtails
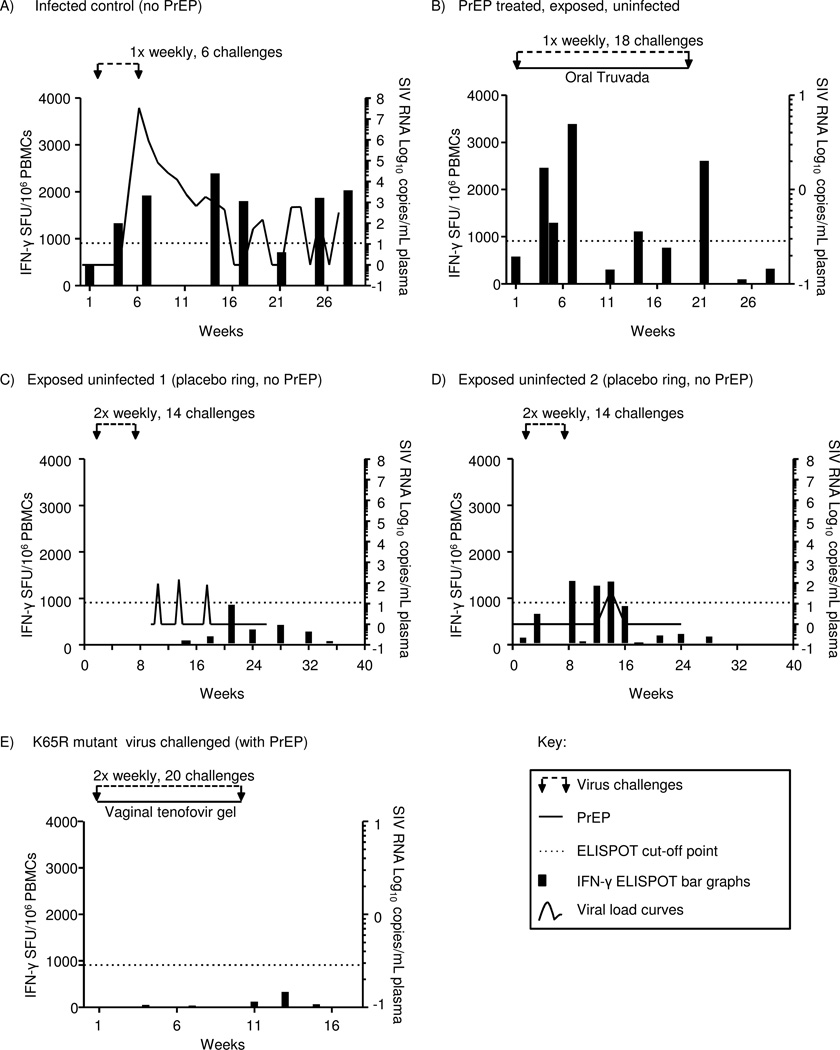
To evaluate whether virus-specific T cell responses are induced, we examined nine macaques that remained uninfected after receiving PrEP in oral form (n=6, oral Truvada)20, or topically delivered (n=3, IVR-tenofovir)22 during 18 or 14 RLD vaginal challenges with wildtype SHIVSF162P3, respectively. Three PrEP-treated animals that became wildtype SHIVSF162P3-infected during the studies and two animals that were not PrEP-experienced and remained uninfected were also evaluated. SHIV-specific T cell responses were determined using IFN-γ ELISPOT assays. Assays were performed on PBMC specimens collected at baseline (before start of virus challenges), weekly during virus challenges and as a follow up after completion of virus challenges. Figure 1 shows representative data of T cell responses in an infected control macaque shown for comparison (Fig. 1A, PrEP-inexperienced), in a PrEP-treated macaque with virus-specific T cell responses (Fig. 1B), and in two PrEP- inexperienced control macaques that remained uninfected despite virus challenges and despite intermittently detected virus blips that did not result in systemic infection (Fig. 1C, D). SHIV-specific T cells were expressed as total SFUs per 106 PBMCs by adding SFU responses obtained with stimulation by individual SHIV peptide pools that spanned the full proteome of SHIV, and are depicted in black bar graphs scaled on the left y-axes. To rigorously distinguish virus-specific T cell responses from low-level anti-SHIV T cell responses in the analyses shown in Figure 1, we applied a strict cut-off value based on mean baseline responses of all 36 macaques involved in this study and three standard deviations (dotted line in Fig. 1). In the examples shown in Fig. 1B, the PrEP-experienced uninfected macaque had evidence of virus-specific T cell responses on five occasions. The first uninfected macaque without PrEP treatment did not have T cell responses above the cut-off, i.e., 907/106 IFN-γ SFU (Fig. 1C), and the other had three (Fig. 1D).
Figure 1. Schematic representation of SHIV-specific T cell responses measured by IFN-γ ELISPOT assay for the intermittent oral Truvada and vaginal tenofovir gel studies with repeated vaginal SHIVSF162P3 and SHIVSF162P3-K65R virus challenges, respectively.
(A) Data of representative control (no PrEP) infected macaque from the intermittent oral Truvada study are shown. Once-weekly virus challenges were stopped after the 6th challenge when the animal was confirmed infected. The line curve depicts viral loads scaled logarithmically on the right Y-axes. (B) Data of a representative monkey that was protected by intermittent oral Truvada and developed virus-specific T cells during 18 once-weekly virus challenges are shown. (C, D) Data from the two exposed, uninfected macaques participating in the intra-vaginal ring study is shown. (E) Data of a representative monkey with lack of SHIV-specific T cell responses during 20 virus challenges with SHIVSF162P3-K65R mutant virus in the presence of vaginal tenofovir gel are shown. The black bar graphs, scaled on the left Y-axes, represent SHIV-specific T cell responses expressed as IFN-γ total SFUs per million PBMCs. X-axes show weeks relative to virus challenges, where week 1 was the start of virus challenges. The dotted horizontal lines on each graph show the rigorous cut-off point for virus-specific T cell responses during PrEP (SFU 907/106 PBMCs).
Virus-specific T cell responses in the representative macaque shown in Fig.1B started after four exposures and were intermittently observed throughout the 18 weeks of virus challenges. They were detectable for up to three weeks following termination of virus challenges, i.e. last in study week 21. T cell responses reached 3,371 SFU/ 10^6 in the example shown in Fig. 1B. The other seven uninfected animals with T cell responses had comparable responses. A response of 4,900 IFNγ producing cells per million PBMCs was the strongest response observed in a PrEP-treated, uninfected animal (data not shown). T cell responses could thus reach the magnitude of T cell responses in infected animals (e.g., in Fig. 1A), although many lower responses continued to occur even after initial virus-specific T cell responses above cut-off were observed (e.g., in Fig. 1B, study week 11).
Eight of the nine PrEP-protected macaques had detectable virus-specific responses (five in the intermittent oral Truvada and three in IVR-tenofovir studies, data summarized Table 1). One PrEP-treated, uninfected animal (in the oral Truvada group) did not meet the criteria for virus-specific T cells because its’ T cell responses remained below our cut-off in this analysis. In summary, we found that virus-specific T cell responses during PrEP develop in a majority of exposed-uninfected monkeys (n=8/9 (88.9%)).
Table 1. Development of virus-specific T cells with oral or topical PrEP modalities.
Pigtail macaques that underwent different PrEP efficacy studies using RLD virus challenge model were assessed for development of virus-specific T cell responses. WT virus (SHIVSF162P3) was used for intermittent oral Truvada and IVR-tenofovir studies, and the K65R mutant (SHIVSF162P3-K65R) for vaginal tenofovir gel study.
| PrEP efficacy studies and number of monkeys tested |
Viruses used for RLD vaginal challenges (dose) |
Uninfected PrEP-treated macaques, number of challenges |
Number of these macaques developing virus-specific T cells |
|---|---|---|---|
| Intermittent oral Truvada (n=6 treated, n=6 controls) reference20 | SHIV 162p3, WT (50 TCID50) | N=6 18 challenges |
5 of 6 |
| IVR-tenofovir (n=6 treated, n=6 controls) reference22 | SHIV 162 p3, WT (50 TCID50) | N=3 14 challenges |
3 of 3 |
| Vaginal tenofovir gel (n=6 treated, n=6 controls), reference24 | SHIV 162 p3, K65R mutant (500 TCID50) | N=5 20 challenges |
0 of 5 |
We also examined the ability of a mutant, replication-impaired virus to induce virus-specific T cell responses during PrEP. We examined induction of SHIV-specific T cell responses in animals on topical PrEP with vaginal tenofovir gel and challenged with a drug-resistant virus containing the K65R point mutation (SHIVSF162P3-K65R)24. This mutation reduces the replicative capacity of the virus17. To adjust for the partial replication impairment, virus exposures were performed at 10 times the original dose for wild type virus (500 TCID50 instead of 50 TCID50). Despite the larger inoculum, none of the five PrEP-treated uninfected macaques developed virus-specific T cell responses (0/5 [0%]). This was in contrast to eight of nine PrEP-treated, uninfected, WT-SHIV exposed macaques (Table 1). We found that the IFN-γ-ELISPOT T cell responses (SFUs) for all the five animals were below cut-off; representative data of one animal are shown in Fig.1E. This indicated that SHIV with K65R mutation, although infectious, is not as efficient as wildtype virus in inducing SHIV-specific T cell responses (P=0.003, Fisher’s exact test to compare the two proportions).
Induction of T cell responses over baseline responses following virus exposures
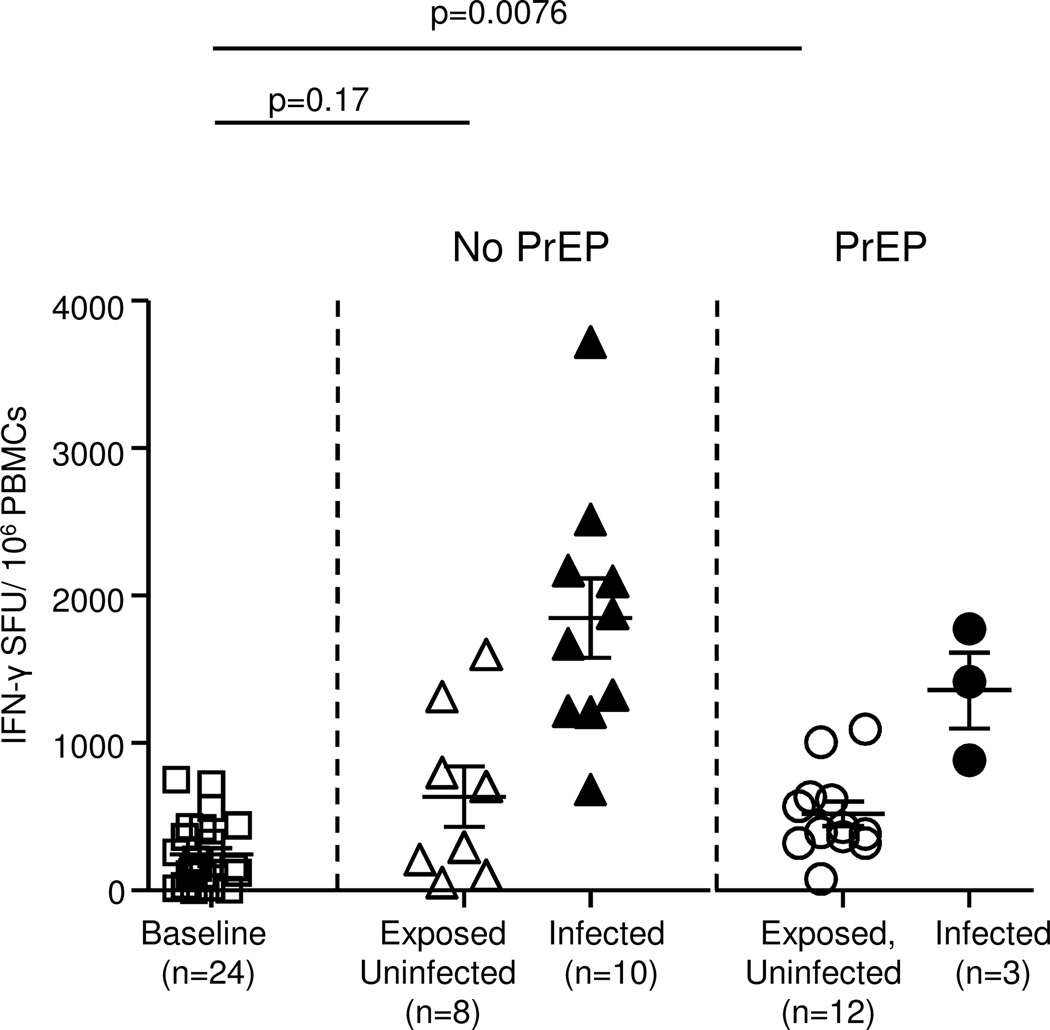
As an additional method to document induction of T cell responses over baseline we examined all anti-SHIV T cell responses after virus exposures, not only responses in PrEP-treated animals that were intermittently above our rigorous cut-off. This was done because application of the rigorous cut-off based on mean group baseline response plus three standard deviations, as done in Fig 1, could obscure small but nevertheless significant T cell response amplifications. The analyses were still done after subtraction of responses to unrelated, mock peptide pools, another safeguard against evaluating non-specific responses. Fig. 2 shows the distributions of median anti-SHIV T cell responses from all longitudinal measures per animal, at baseline and after virus exposure. At baseline, we observed a median 156 SFUs per 106 PBMCs, calculated from all 24 animals that received wildtype virus. In PrEP-treated animals, median SFUs were 411 per 106 PBMCs after virus exposure (Fig.2). The difference over their own baseline value was statistically significant in these animals (p=0.01, paired, two-tailed t-test), indicating induction of T cell immunity due to SHIV exposure. Note that this group included T cell responses of 12 monkeys (nine PrEP-treated and remaining uninfected, and three PrEP-treated prior to developing breakthrough infection). We also examined animals that did not receive PrEP. This group included the two monkeys that remained uninfected and six that had T cell responses prior to developing infection, although the time span for T cell induction was often brief (example, SFU response at week 4 of Fig.1A). Four monkeys had become infected so quickly that no ELISPOTS were performed prior to infection. The median T cell response was 501 SFUs per 106 PBMCs in this group; the difference was not statistically significant over their baseline (p=0.17, paired, two-tailed t-test). Fig. 2 also shows median T cell responses after infection in PrEP-treated and untreated animals. Data were obtained from a minimum of five, and up to 12 IFNgamma-ELISPOT assays performed after infection. As expected, these responses were robust and were a median of 1774 and 1414 SFUs per 106 PBMCs, respectively.
Figure 2. Amplification of SHIV-specific T cell responses in macaques during RLD vaginal virus exposures with intermittent oral Truvada or IVR-tenofovir studies.
IFN-γ total SFUs are shown for all the 24 monkeys enrolled in oral Truvada and IVR-tenofovir studies at baseline and all time points examined. Median SFUs of each monkey are represented by the symbols and median SFUs of each group by horizontal lines, without application of a cutoff value. SFUs of the control group (no PrEP, exposed uninfected) show responses of eight monkeys (two monkeys that remained uninfected and six that had T cell responses prior to developing infection). SFUs of the PrEP-treated exposed uninfected group included responses of 12 monkeys (nine monkeys that were on PrEP and remain uninfected, and three prior to developing breakthrough infection). For the infected group, the SFUs are shown of 10 PrEP-inexperienced (control) monkeys that got infected. Two tailed, paired t-tests were performed to test for statistical significance (p values), using only the baseline values from each group for comparison.
Pol epitope focus of virus-specific T cell responses during PrEP
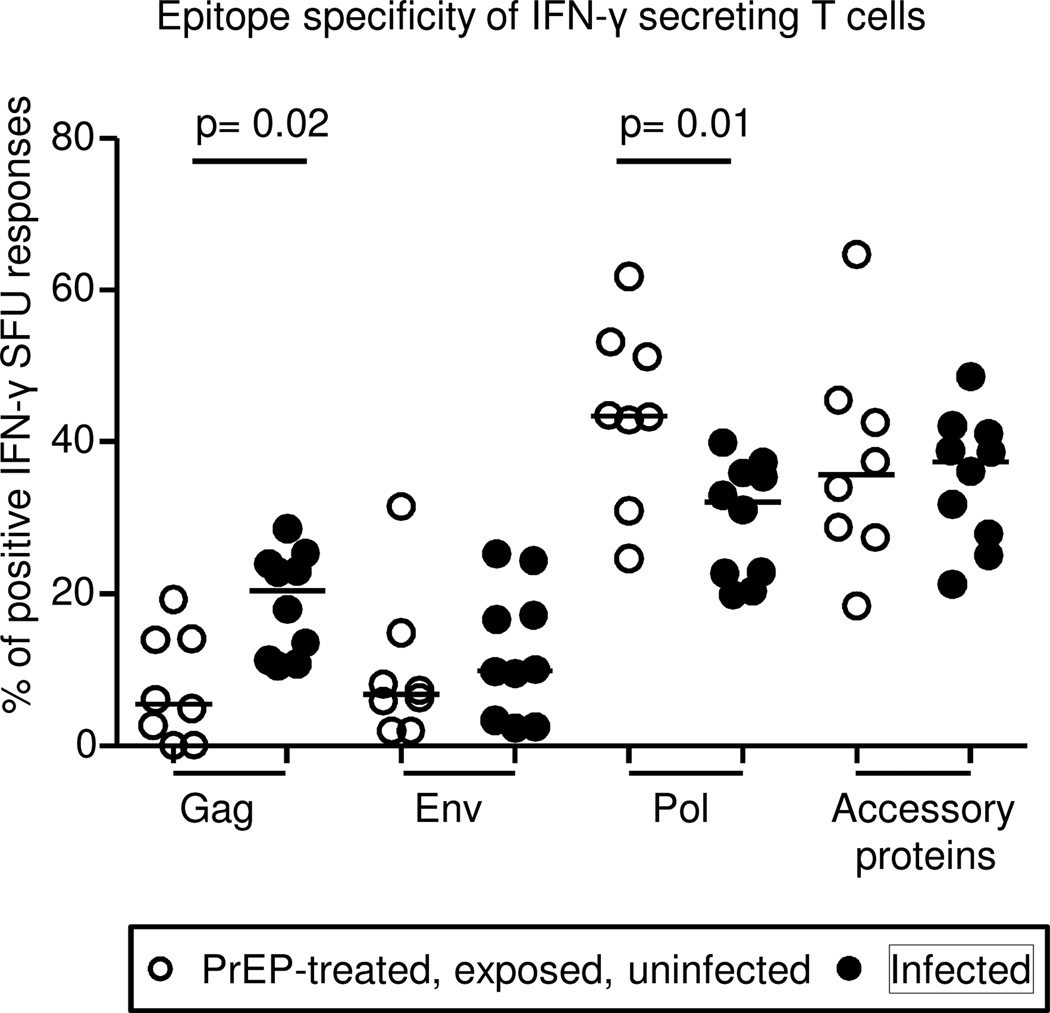
There is a well-documented relationship between virus-specific CD8+ T cell response and control of SIV/SHIV/HIV infection. Presence of MHC class I restricted CD8+ T cell responses has been positively correlated with control of acute viremia and disease progression in monkeys29 and humans30,31. We therefore characterized to which SHIV epitopes virus-specific T cell responses during PrEP were focused (Fig.3). The distributions of env and accessory protein-specific responses were not different in the infected group (filled black circles) relative to the PrEP-treated, uninfected group (open circles). Gag responses were higher in the infected than the PrEP-treated, uninfected group (p=0.02, two-tailed Mann-Whitney test). However, pol-specific responses were substantially more dominant in the PrEP-treated, uninfected group than the infected group (p=0.01, two-tailed Mann-Whitney Test, Fig.3).
Figure 3. Epitope focus of virus-specific T cell responses during PrEP.
Gag, env, pol and accessory protein (nef, vpr, vif, rev and tat combined)-specific IFN-γ SFUs in the intermittent oral Truvada and IVR-tenofovir studies were analyzed for the eight animals with T cell responses during PrEP and 10 infected monkeys, depicted in open and black circles respectively. Only responses above the cut-off were used (907 SFU/106 PBMCs). Percentages of peptide-specific responses were expressed relative to the total summated SFU responses obtained by antigenic stimulation with individual SHIV-specific peptide pools spanning the full SHIV proteome. Each circle represents the median percentage of each animal and the horizontal lines show the percentage median of each group. Two-tailed Mann Whitney test was performed on the percentage medians to test for statistical significance (p values).
Characteristics of T cell responses in uninfected macaques during PrEP
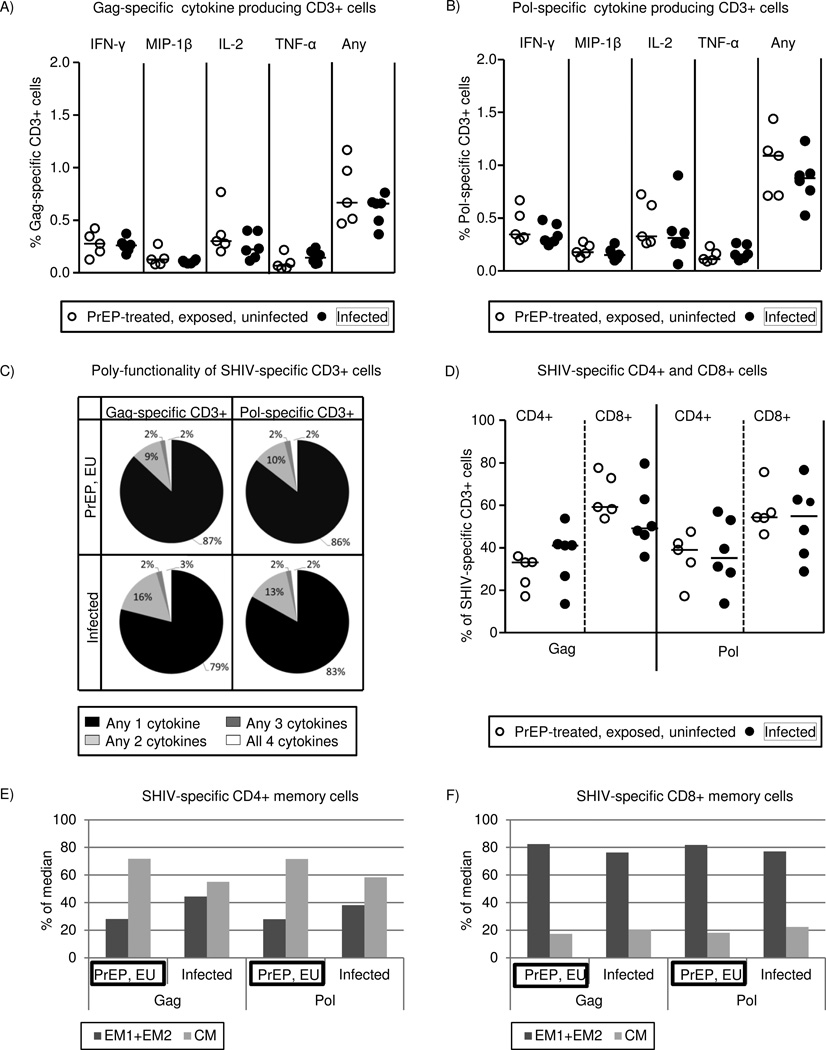
To further examine the nature of virus-specific T cell responses during PrEP, we characterized surface and functional markers by multi-parameter flow cytometry. Sample availability was a limiting factor and only PBMCs from one time of the oral Truvada study could be obtained and analyzed, which was not sufficient for statistical analysis. PBMCs were obtained from the week of highest IFN-γ SFU responses in the intermittent oral Truvada-treated monkeys; and from peak viremia for the infected (PrEP-inexperienced) control group. CD3+ T lymphocytes in the PrEP-treated group had a similar cytokine secretion profile as T cells in infected animals in response to gag or pol peptide stimulations (Fig.4A and Fig.4B, respectively). Poly-functionality of T cells, the ability to secrete more than one cytokine, is an immunological quality that correlates with control of HIV/SIV disease progression32,33. We therefore looked into the poly-functionality of T cells in PrEP-treated, uninfected macaques, and infected controls groups. SHIV-specific T cells producing any two, three or four of the cytokines IFN-γ, MIP-1α, IL-2 and TNF-α were similarly present in the two groups (Fig.4C). The majority of SHIV-specific T cells in both groups were single cytokine producers, followed by two, three and four cytokine producers (Fig.4C).
Figure 4. Characterization of virus-specific T cell responses during PrEP by multi-parameter flow cytometry.
Archived PBMCs from five uninfected macaques in the intermittent oral Truvada study were obtained during the week of highest SHIV-specific T cell ELISPOT response, and for the infected animals (PrEP-inexperienced controls) at the week of peak viremia. (A) and (B) show median percentages of CD3+ T cells that were able to produce any of the cytokines IFN-γ, MIP-1β, IL-2 and TNF-α in response to gag (A) or pol (B) peptide stimulation. “Any” refers to producing any of the four cytokines (IFN-γ or MIP-1β or IL-2 or TNF-α) but not necessarily simultaneously. (C) Poly-functionality of responses. Flow-cytometric data of SHIV-specific T cells to simultaneously produce multiple cytokines were analyzed using Boolean and combined gating strategies. The average percentage of any single, two, three, and all four cytokines producing CD3+ cells following gag or pol antigenic stimulation is shown for the two groups. (D) CD4+ and CD8+ phenotyping of SHIV-specific cytokine producing CD3+ T cells. The median percentages of gag or pol-specific CD3+ T cells that were either CD4+ or CD8+ are shown for the two groups. (E) and (F) show phenotyping of cytokine producing SHIV-specific CD4+ and CD8+ T cells in effector memory (EM1=CCR7− and CD28+ and EM2=CCR7− and CD28−) and central memory (CM= CCR7+ and CD28+) subsets.(E) shows CD4+ and (F) shows CD8+ SHIV specific memory T cells. Medians of percentages are shown for the PrEP-treated, uninfected, and infected control groups.
SHIV-specific cytokine producing CD4+ and CD8+ T cells were present in both groups (Fig. 4D). For instance, 39.0% in the PrEP-treated, uninfected group and 35.3% in the infected group were pol-specific CD4+ T cells (Fig.4D). Pol-specific CD8+ T cells were 54.4% for the PrEP-treated, uninfected group and 55.0% for the infected group. Similar results were obtained for gag-specific CD4+ and CD8+ T cells (Fig.4D).
Finally, SHIV-specific cytokine producing CD4+ and CD8+ T cells in the PrEP-treated, uninfected, and infected groups were analyzed for central memory cells (CM= CCR7+, CD28+) and effector memory cells (EM1+EM2, where EM1= CCR7−, CD28+, and EM2=CCR7−, CD28−) (Fig.4E and Fig4F). Overall, CD4 memory cells were predominantly CM and CD8 memory cells were predominantly EM. Similar antigen-specific effector memory and central memory phenotypes were observed in both groups. For instance, pol-specific CD8+ T cells with effector memory phenotype were 81.8% in the PrEP-treated, uninfected and 77.1% in infected control groups (Fig.4F). Results were similar for central memory phenotype and gag-specific memory cells (Fig.4E and 4F). In sum, the poly-functionality, CD8+, CD4+ and memory phenotypes of SHIV-specific cytokine secreting T cells of PrEP-treated, uninfected macaques were similar to SHIV-specific T cells that develop during infection.
Discussion
We and others previously reported that T cell priming or so-called “chemo-vaccination” occurs during RLD mucosal virus challenges and with the use of oral or topical PrEP in a small number of macaques12,10,34. The current study expands these earlier isolated observations by showing that virus-specific T cell responses can develop in eight of nine exposed uninfected macaques during vaginal virus challenges while on PrEP. Hence, we now affirm that virus-specific T cell responses during PrEP can be a frequent phenomenon in exposed uninfected monkeys. It remains to be determined whether the vaginal route of virus challenge is more effective than the rectal route in inducing the responses. Furthermore, an important question relevant to PrEP development remains unanswered, i.e., if animals with these responses are more or less susceptible to subsequent infection. It will be necessary to design macaque experiments specifically to answer these questions, while our current results stem from left-over samples from studies designed to test PrEP efficacy20–24.
PrEP constitutes a novel usage of antiretroviral drugs for HIV-1 prevention in high-risk groups1–7,35, and ongoing and future clinical trials will address issues like improving the adherence and implementation of PrEP8,9,36. Virus-specific T cell responses during PrEP might also be a common occurrence in humans, and it may enhance protection from infection, alter the course of breakthrough infection, or increase infection risk. So far, very few studies have addressed the effect of PrEP drugs on HIV immunity16,37, perhaps owing to the difficulty of getting adequate and timely samples from exposed uninfected individuals while on PrEP. For instance, a study by Mureithi and colleagues16 showed that in individuals infected despite vaginal tenofovir gel use as PrEP, HIV-1 immunity was modulated by better preservation of gag-specific IFN-γ secreting CD4+ T cells than controls that became infected without vaginal gel use. Melchjorsen and colleagues on the other hand, found that HIV-uninfected human PBMCs treated in vitro with tenofovir have altered secretion of the cytokines IL-10 and IL-12, thereby affecting the overall inflammatory response of treated PBMCs37. Thus, further studies are required to understand if virus-specific T cell responses during PrEP occur in humans and to decipher the immunological effects of tenofovir and other PrEP agents in exposed uninfected PrEP-experienced individuals.
The mechanism by which virus-specific T cell responses during PrEP occur is unknown. The observation is somewhat reminiscent of exposed uninfected humans38,39, 40. T cell responses developing with and without PrEP may be similar in nature, but not in magnitude in our model. Responses in exposed uninfected control macaques remained below the threshold for statistical significance (Fig. 2), or were of lower magnitude than in PrEP-treated exposed uninfected macaques (Fig. 1C, D). It is possible that anti-retroviral drugs affect the responses in ways currently not fully understood; this merits further investigation in our model. It is also possible that PrEP-treatment allows T cells to develop more efficiently than in other exposed uninfected individuals, because individuals on PrEP may experience more initial virus replication (see below), stay uninfected for longer, and experience more virus exposures without infection, and thus have more opportunities for T cell induction. Presumably, the presence of virus at mucosal tissues with low levels of replication owing to inhibiting antiretroviral drugs can lead to activation of antigen presenting cells and immune processing of viral fragments without productive infection, the end result being stimulation of the adaptive virus-specific T cell responses. Interestingly, we noted that despite the use of a larger viral inoculum, virus-specific T cell responses during PrEP were not observed in monkeys repeatedly exposed to a virus containing the K65R mutation in its reverse transcriptase gene. Our earlier works have shown that the K65R mutation results in reduced replication and viral fitness17,25. Induction of virus-specific T cell responses did not occur in this group probably because the virus was replication impaired. Thus, antigenic exposure resulting from some form of viral replication rather than from the viral inoculum might be critical to induce T cells during PrEP. Likewise, the presence of T cells directed to accessory proteins of SHIV (as seen in our study, Figure 3) are consistent with replication playing a role41. Studying tissue resident antigen-presenting cells and mucosal T cells for occult infections might help to understand the mechanism of T cell priming during PrEP. Our experiments were limited by having only specimens of opportunity available for study. The impact of virus dose and replication on T cell priming warrants further investigation in experiments specifically designed to address these questions, and would best be addressed by including different doses of wildtype, replication-competent virus, and by collecting mucosal specimens for analysis.
Both our current and previous studies10 showed that the SHIV-specific T cells in uninfected, PrEP-treated macaques are comprised of polyfunctional CD8+ and CD4+ T cells. Thus, there was potential induction of CD8+ T cells capable of controlling acute HIV or SHIV infection42–45, but also of CD4+ T cells which might create a large pool of target cells for infection46,47. We observed a shift toward more pol-specific T cells in the PrEP-treated, uninfected group than in the infected group. A similar tendency has been reported in our earlier study10. Presence of gag-specific CD8+ T cells correlates with viral control in long-term non-progressors30,31,48. However, pol-specific MHC class I restricted CD8+ T cell responses have also been shown to recognize infected cells in ex vivo co-culture models49. In this regard, future studies of PrEP-mediated immunity might reveal interesting insights as to why pol-specific T cells are preferentially induced, where the exact epitopes recognized map in each animal, and whether they have any beneficial role. As previously reported10, we found no evidence for B cell induction in PrEP-treated, uninfected macaques in our present study.
In summary, our study confirmed that the induction of SHIV-specific T cells in exposed uninfected monkeys during repeated mucosal virus challenges in the presence of PrEP, is a common occurrence. Both oral and topical PrEP modalities were able to induce systemic responses. Replication-impaired virus failed to induce virus-specific T cell responses, suggesting that viral replication is critical for their induction. It remains of interest to examine if virus-specific T cell responses occur in humans taking PrEP. Ultimately, the role virus-specific T cells have on the efficacy of PrEP and antiretroviral therapy should be studied in broader detail.
Acknowledgements
Dr. T. Tsegaye was supported by an ASM (American Society for Microbiology)/CDC postdoctoral research fellowship. This work was funded by CDC, and partially supported by Interagency Agreement Y1-AI-0681-02 between CDC and NIH. We thank CDC veterinary staff, animal care providers and the Preclinical Evaluations Team Animal Model Activity members that made the PrEP studies possible from which specimens for this follow-up study were obtained.
Footnotes
Author Contributions: Designed the study: ENK, TST; Acquired data: TST, KB, WL, RA; Analyzed and interpreted results: TST, KB, WL, RA, EK, JMN; provided samples: PS, JR, SS, CD, GGL, WH; Performed statistical analysis: TST, ENK, DLH; Wrote manuscript: TST and ENK. All authors read and approved the manuscript.
Conflicts of interest: The authors declare no conflicts of interest
Part of the data was presented at the 19th Conference on Retrovirus and Opportunistic Infections (CROI 2012), Seattle, WA, USA.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012 Aug 2;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013 Jun 15;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 5.(CDC) CfDCaP. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR. Morbidity and mortality weekly report. 2011 Jan 28;60(3):65–68. [PubMed] [Google Scholar]
- 6.(CDC) CfDCaP. Update to Interim Guidance for Preexposure Prophylaxis (PrEP) for the Prevention of HIV Infection: PrEP for injecting drug users. MMWR. Morbidity and mortality weekly report. 2013 Jun 14;62(23):463–465. [PMC free article] [PubMed] [Google Scholar]
- 7.FDA. FDA approves first drug for reducing the risk of sexually acquired HIV infection. [Accessed April 6 2013];2012 Jul 16; 2012: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm.
- 8.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure Prophylaxis for HIV Prevention: Where Have We Been and Where Are We Going? Journal of acquired immune deficiency syndromes. 2013 Jul;63(Suppl 2):S122–S129. doi: 10.1097/QAI.0b013e3182986f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnell D, Hughes JP, Wang L, Chen YQ, Fleming TR. Study design considerations for evaluating efficacy of systemic preexposure prophylaxis interventions. Journal of acquired immune deficiency syndromes. 2013 Jul;63(Suppl 2):S130–S134. doi: 10.1097/QAI.0b013e3182986fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kersh EN, Adams DR, Youngpairoj AS, et al. T cell chemo-vaccination effects after repeated mucosal SHIV exposures and oral pre-exposure prophylaxis. PloS one. 2011;6(4):e19295. doi: 10.1371/journal.pone.0019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersh EN, Luo W, Zheng Q, et al. Reduced inflammation and CD4 loss in acute SHIV infection during oral pre-exposure prophylaxis. The Journal of infectious diseases. 2012 Sep 1;206(5):770–779. doi: 10.1093/infdis/jis422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. Journal of virology. 2009 Oct;83(20):10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annual review of medicine. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 14.Hansen SG, Ford JC, Lewis MS, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011 May 26;473(7348):523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen SG, MP, Ventura AB, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013 Sep 11; doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mureithi MW, Poole D, Naranbhai V, et al. Preservation HIV-1-specific IFNgamma+ CD4+ T-cell responses in breakthrough infections after exposure to tenofovir gel in the CAPRISA 004 microbicide trial. Journal of acquired immune deficiency syndromes. 2012 Jun 1;60(2):124–127. doi: 10.1097/QAI.0b013e31824f53a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cong ME, Mitchell J, Sweeney E, et al. Prophylactic Efficacy of Oral Emtricitabine and Tenofovir Disoproxil Fumarate Combination Therapy Against a Tenofovir-Resistant Simian/Human Immunodeficiency Virus Containing the K65R Mutation in Macaques. The Journal of infectious diseases. 2013 Aug;208(3):463–467. doi: 10.1093/infdis/jit189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Council NR. 8 ed. Washington DC: National Academies Press (US); 2011. Guide for the Care and Use of Laboratory Animals. http://www.ncbi.nlm.nih.gov/books/NBK54050/ [PubMed] [Google Scholar]
- 19.Kim CN, Adams DR, Bashirian S, Butera S, Folks TM, Otten RA. Repetitive exposures with simian/human immunodeficiency viruses: strategy to study HIV pre-clinical interventions in non-human primates. Journal of medical primatology. 2006 Aug;35(4–5):210–216. doi: 10.1111/j.1600-0684.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 20.Radzio J, Aung W, Holder A, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PloS one. 2012;7(12):e50632. doi: 10.1371/journal.pone.0050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science (New York, N.Y.) 1999 Apr 30;284(5415):816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan P, Zhang J, Martin A, et al. Tenofovir released from an intravaginal ring reduces viral shedding in infected pigtailed macaques. Paper presented at 29th Annual Symposium on Nonhuman Primate Models for AIDS; 2011; Seattle, Washington. [Google Scholar]
- 23.Gunawardana M, Moss JA, Smith TJ, et al. Microbial biofilms on the surface of intravaginal rings worn in non-human primates. Journal of medical microbiology. 2011 Jun;60(6):828–837. doi: 10.1099/jmm.0.028225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobard C, Sharma S, Cong M, et al. Paper presented at: International Workshop on HIV and Hepatitis Virus Drug Resistance and Curative Strategies. Mexico: Los Cabos; 2011. Vaginal tenofovir (TFV) gel is effective against transmission of TFV-resistant virus in macaques. [Google Scholar]
- 25.Cong ME, Youngpairoj AS, Aung W, et al. Generation and mucosal transmissibility of emtricitabine- and tenofovir-resistant SHIV162P3 mutants in macaques. Virology. 2011 Apr 10;412(2):435–440. doi: 10.1016/j.virol.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. The Journal of infectious diseases. 2006 Oct 1;194(7):904–911. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 27.Kersh EN, Luo W, Adams DR, et al. Repeated rectal SHIVSF162P3 exposures do not consistently induce sustained T cell responses prior to systemic infection in the repeat-low dose preclinical macaque model. AIDS research and human retroviruses. 2009 Sep;25(9):905–917. doi: 10.1089/aid.2008.0287. [DOI] [PubMed] [Google Scholar]
- 28.Donaldson MM, Kao SF, Eslamizar L, et al. Optimization and qualification of an 8-color intracellular cytokine staining assay for quantifying T cell responses in rhesus macaques for pre-clinical vaccine studies. Journal of immunological methods. 2012 Dec 14;386(1–2):10–21. doi: 10.1016/j.jim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mudd PA, Martins MA, Ericsen AJ, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012 Nov 1;491(7422):129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakrabarti LA, Simon V. Immune mechanisms of HIV control. Current opinion in immunology. 2010 Aug;22(4):488–496. doi: 10.1016/j.coi.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migueles SA, Connors M. Long-term nonprogressive disease among untreated HIV-infected individuals: clinical implications of understanding immune control of HIV. JAMA : the journal of the American Medical Association. 2010 Jul 14;304(2):194–201. doi: 10.1001/jama.2010.925. [DOI] [PubMed] [Google Scholar]
- 32.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thobakgale CF, Streeck H, Mkhwanazi N, et al. Short communication: CD8(+) T cell polyfunctionality profiles in progressive and nonprogressive pediatric HIV type 1 infection. AIDS research and human retroviruses. 2011 Sep;27(9):1005–1012. doi: 10.1089/aid.2010.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cranage M, Sharpe S, Herrera C, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS medicine. 2008 Aug 5;5(8):e157. doi: 10.1371/journal.pmed.0050157. discussion e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease C, Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR. Morbidity and mortality weekly report. 2012 Aug 10;61(31):586–589. [PubMed] [Google Scholar]
- 36.Amico KR, Mansoor LE, Corneli A, Torjesen K, van der Straten A. Adherence Support Approaches in Biomedical HIV Prevention Trials: Experiences, Insights and Future Directions from Four Multisite Prevention Trials. AIDS and behavior. 2013 Feb 23; doi: 10.1007/s10461-013-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melchjorsen J, Risor MW, Sogaard OS, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. Journal of acquired immune deficiency syndromes. 2011 Aug 1;57(4):265–275. doi: 10.1097/QAI.0b013e3182185276. [DOI] [PubMed] [Google Scholar]
- 38.Erickson AL, Willberg CB, McMahan V, et al. Potentially exposed but uninfected individuals produce cytotoxic and polyfunctional human immunodeficiency virus type 1-specific CD8(+) T-cell responses which can be defined to the epitope level. Clinical and vaccine immunology : CVI. 2008 Nov;15(11):1745–1748. doi: 10.1128/CVI.00247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farquhar C, Lohman-Payne B, Overbaugh J, et al. Breast milk HIV-1 RNA levels and female sex are associated with HIV-1-specific CD8+ T-cell responses in HIV-1-exposed, uninfected infants in Kenya. The Journal of infectious diseases. 2011 Dec 1;204(11):1806–1810. doi: 10.1093/infdis/jir643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirbod T, Broliden K. Mucosal immune responses in the genital tract of HIV-1-exposed uninfected women. Journal of internal medicine. 2007 Jul;262(1):44–58. doi: 10.1111/j.1365-2796.2007.01822.x. [DOI] [PubMed] [Google Scholar]
- 41.Yu XG, Lichterfeld M, Addo MM, Altfeld M. Regulatory and accessory HIV-1 proteins: potential targets for HIV-1 vaccines? Current medicinal chemistry. 2005;12(6):741–747. doi: 10.2174/0929867053202205. [DOI] [PubMed] [Google Scholar]
- 42.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. Journal of virology. 1994 Sep;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan CA, Ibarrondo FJ, Sugar CA, et al. Early HLA-B*57-restricted CD8+ T lymphocyte responses predict HIV-1 disease progression. Journal of virology. 2012 Oct;86(19):10505–10516. doi: 10.1128/JVI.00102-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendoza D, Migueles SA, Rood JE, et al. Cytotoxic Capacity of SIV-Specific CD8(+) T Cells against Primary Autologous Targets Correlates with Immune Control in SIV-Infected Rhesus Macaques. PLoS pathogens. 2013 Feb;9(2):e1003195. doi: 10.1371/journal.ppat.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science (New York, N.Y.) 1999 Feb 5;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 46.Porichis F, Kaufmann DE. HIV-specific CD4 T cells and immune control of viral replication. Current opinion in HIV and AIDS. 2011 May;6(3):174–180. doi: 10.1097/COH.0b013e3283454058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staprans SI, Barry AP, Silvestri G, et al. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proceedings of the National Academy of Sciences of the United States of America. 2004 Aug 31;101(35):13026–13031. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science (New York, N.Y.) 2010 Dec 10;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacha JB, Chung C, Reed J, et al. Pol-specific CD8+ T cells recognize simian immunodeficiency virus-infected cells prior to Nef-mediated major histocompatibility complex class I downregulation. Journal of virology. 2007 Nov;81(21):11703–11712. doi: 10.1128/JVI.00926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]