Abstract
Background
Epoetin alfa (EPO) and darbepoetin alfa (EPO) are erythropoiesis-stimulating agents that are widely and interchangeably used for the treatment of anemia in patients with advanced chronic kidney disease and end-stage renal disease. No studies have specifically compared the risks of hard study outcomes between EPO and DPO, including mortality.
Methods
We conducted a systematic search of the literature (PubMed, CENTRAL, SCOPUS, and EMBASE, all years) as well as of industry resources to identify all randomized trials comparing EPO versus DPO for the treatment of anemia in adult patients with chronic kidney disease including those requiring dialysis. We then summarized key characteristics and findings of these trials and performed a random effects meta-analysis of trials with at least 3 months duration to identify the summary odds ratio of mortality between patients randomized to DPO versus EPO.
Results
We identified 9 trials that met stated inclusion criteria. Overall, 2024 patients were included in the meta-analysis, of whom 126 died during follow up, which ranged from 20 to 52 weeks. We found no significant difference in mortality between patients randomized to DPO versus EPO (OR=1.33; 95% CI 0.88-2.01). No treatment heterogeneity across studies was detected (Q-statistic = 4.60; P=0.80).
Conclusions
Few trials directly comparing DPO and EPO have been conducted and follow-up was limited. In aggregate, no effect of specific erythropoiesis-stimulating agent on mortality was identified, but the confidence limits were wide and remained compatible with considerable harm from DPO. Absent adequately-powered randomized trials, observational post-marketing comparative effectiveness studies comparing these erythropoiesis-stimulating agents are required to better characterize the long-term safety profiles of these agents.
Keywords: Erythropoesis-stimulating agents, Mortality, Drug Safety
Introduction
Epoetin alfa (EPO) and darbepoetin alfa (DPO) are erythropoiesis-stimulating agents that are widely and interchangeably used for the treatment of anemia in patients with chronic kidney disease including those with end-stage renal disease requiring dialysis. While generally similar to EPO, DPO differs in the number of its carbohydrate side chains, which yields greater receptor affinity and a longer half-life. While longer half-life renders the opportunity to administer drug at extended intervals, choice of erythropoiesis-stimulating agent (EPO vs. DPO) has been associated with increased hemoglobin variability (“cycling”),(1) which itself has been associated with worse outcome.(2, 3) DPO was approved based on the findings of relatively smaller registrational trials that demonstrated similar ability, compared with EPO, to raise or maintain hemoglobin concentrations in patients with CKD. Recent guidelines generally refer to erythropoiesis-stimulating agents (implying a homogenous class) throughout their recommendations and state that: “At present, there is no evidence that any given [erythropoiesis-stimulating agent] brand is superior to another in terms of patient outcomes.”(4) Indeed, no studies have specifically compared the risks of hard study outcomes between EPO and DPO, such as mortality or cardiovascular events. To fill this evidence gap, we conducted a systematic review and meta-analysis of randomized trials that conducted head to head comparisons between EPO and DPO.
Methods
Inclusion and exclusion criteria
We sought to identify from the literature all randomized trials comparing EPO versus DPO for the treatment of anemia in adult patients with chronic kidney disease including those requiring dialysis. The outcome of interest was cumulative all-cause mortality. We excluded trials enrolling children, trials with less than three months of follow up, and trials published in languages other than English.
Search terms and strategy
We searched MEDLINE (on June 1 2014, all years) using the following search algorithm: (darbepoetin OR NESP OR Aranesp) AND (erythropoietin OR rHuEPO OR Epoetin OR Eprex OR Epogen OR Procrit) AND (kidney OR renal OR neph* OR dialysis OR CKD OR CRF OR ESRD); the search results were limited to trials of adults published in English. In addition, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, and EMBASE on October 1, 2014 using the same search terms and limitations with the exception that the EMBASE search included all publication types. Finally, we also searched the website of the manufacturer of EPO and DPO, Amgen Inc. (www.amgen.com), their briefing documents filed to the US Food and Drug Administration (FDA) for a Cardiovascular and Renal Drug Advisory Committee meeting in October 2010,(5) and the FDA Medical Officer Review document for that meeting.
The reports identified through this search were then evaluated by both authors for satisfying stated inclusion criteria. Citations that appeared to meet inclusion criteria were reviewed in further detail, first with a review of the abstract and then, if the citation could not be excluded on that basis, with a review of the complete text. Predefined data from the selected reports were then abstracted and tabulated with resolution of discrepancies via conference.
Analysis
For formal analyses, we used random effects meta-analysis to identify the summary odds ratio of mortality between patients randomized to DPO versus EPO. We tested for effect heterogeneity using the Q statistic. For the primary analysis, we analyzed all trials including those in whom zero deaths were observed in one or both treatment arms. In order to make the odds ratio estimable of trials with zero deaths in one or both treatment arms, we added 0.5 deaths to both arms. In a sensitivity analysis, we excluded trials with zero deaths in one or both treatment arms. All analyses were conducted using Comprehensive Meta-Analysis software (version 2.2.064, Biostat, Englewood, NJ).
Results
Search results
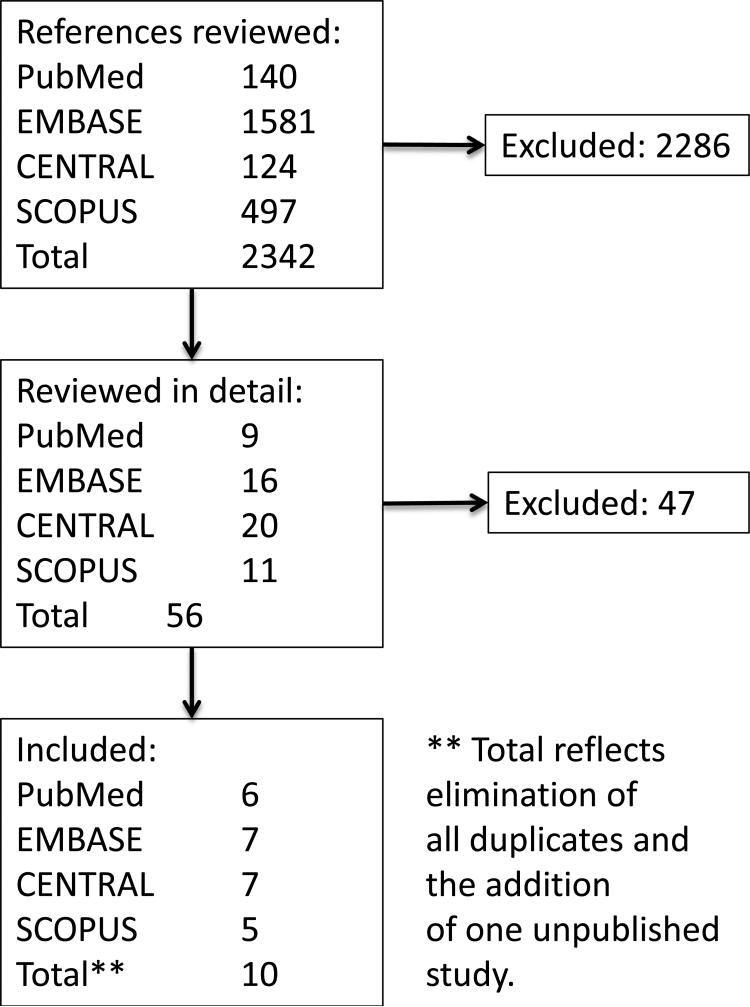
The systematic search yielded a total of non-unique 2342 citations among the four sources (Figure 1). After screening for inclusion based on title alone, 56 were reviewed in detail; of those, 9 trials were identified for potential inclusion (100% concordance between investigators).(6-14) We found one additional unpublished trial on the website of Amgen Inc. (AMGEN #20010125),(15) leading to 10 included trials.
Figure 1.
Flow sheet of search process, study identification, and selection
Of the 10 trials included (Table 1), 8 were conducted in patients with ESRD undergoing dialysis and 2 in patients with advanced CKD not yet requiring dialysis. One study, a cross-over trial with 2 months duration for each of the cross-over exposure periods, was excluded for failing to satisfy the minimum duration requirement of 3 months (and zero deaths were observed in either exposure group during both periods of the cross-over experiment).(14) Study duration for the 9 remaining studies varied from 20 to 52 weeks. Except for one, all studies were industry-sponsored (Amgen Inc., Thousand Oaks, CA or Kirin Pharmaceuticals Co., now Kyowa Hakko Kirin Co., Ltd., Japan). Overall, 2024 patients (1100 in DPO arm and 924 in EPO arm) were included in the meta-analysis, of whom 126 died during follow up (78 in DPO and 48 in EPO).
Table 1.
Description of included studies and key quality measures
| Study (sponsor; study #) | Design and study population | Total N= | Randomized | Double-blinded | Full-text publication | Follow-up period |
|---|---|---|---|---|---|---|
| Coyne, et al., 2000 (AMGEN #980211)(11) | ESRD* and ESA** naïve patients 3:1 randomized to weekly darbepoetin vs. thrice weekly epoetin | 122 | Yes | No | No | 20 weeks |
| Locatelli, et al., 2001 (AMGEN #980202)(6) | Non-ESRD and ESA naïve patients with eGFR° ≤ 30 mL/min, 3:1 randomized to darbepoetin weekly vs. epoetin twice weekly | 166 | Yes | No | Yes | 24 weeks |
| Allon, et al., 2002 (AMGEN #970235)(7) | ESRD patients already on epoetin 1:1:1 randomized to darbepoetin weekly, darbepoetin thrice weekly, or epoetin thrice weekly | 47 | Yes | No | Yes | 52 weeks |
| Nissenson, et al., 2002 (AMGEN #980117)(8) | ESRD patients already on epoetin 2:1 randomized to epoetin thrice weekly vs. darbepoetin weekly | 507 | Yes | Yes | Yes | 28 weeks |
| Vanrenterghem, et al., 2002 (AMGEN #980200)(9) | ESRD patients already on epoetin 2:1 randomized to darbepoetin weekly or every other week vs. epoetin (82% epoetin alfa; 18% epoetin beta) 1-3 times weekly | 522 | Yes | No | Yes | 52 weeks |
| Hori, et al., 2004 (Kirin Pharma Co.)(12) | ESRD patients already on epoetin 2-3 times weekly 1:1 randomized to continue epoetin vs. darbepoetin weekly | 120 | Yes | Yes | Yes | 28 weeks |
| AMGEN #200010125, 2005(15) | African-American ESRD patients already on epoetin thrice weekly 1:1 randomized to continue epoetin vs. darbepoetin weekly | 407 | Yes | Yes | No | 28 weeks |
| Li, et al., 2008 (Kirin Pharma Co.)(10) | ESRD patients on peritoneal dialysis already on epoetin 1:1 randomized to continue epoetin either 5 or 10 times monthly vs. darbepoetin weekly or twice monthly | 45 | Yes | No | No | 24 weeks |
| Jo, et al., 2010 (A)(14) | Non-ESRD patients with eGFR 23 ± 10 mL/min 1:1 randomized to epoetin or darbepoetin weekly for 2 months | 74 | Yes | No | No | 2 months |
| Jo, et al., 2010 (B)(14) | Second trial period for Jo, et al. (A); patients who received epoetin in the initial arm crossed over to darbepoetein after a wash out period; patients who received darbepoetin in the initial arm crossed over to epoetin after a wash out period | 74 | Yes | No | No | 2 months |
| Bernieh, et al., 2014(13) | ESRD patients already on epoetin 1:1 randomized to continue epoetin 1-3 times weekly vs. darbepoetin weekly or every other week | 139 | Yes | No | Yes | 24 weeks |
ESRD = End stage renal disease
ESA = erythropoiesis stimulating agent
eGFR = estimated glomerular filtration rate
The quality of trials was variable. While all trials were randomized (per our inclusion criteria), only 3 were double-blinded and the remaining 6 were open label. Only 5 trials were published in the peer-reviewed literature, whereas 3 were published as abstracts, and the results from one trial were only available on the internet. All trials had a hemoglobin target or maintenance endpoint, but the death counts during follow up for each treatment arm were reported in 100% of trials. Further details of trial design, study population, and quality metrics are listed in Table 1.
Primary analysis
Two small trials observed zero deaths in one of the treatment arms (Li, et al.(10) had no death in the DPO arm; Hori, et al.(12) observed no death in the EPO arm) and were included after adding 0.5 deaths to each treatment arm. Figure 2 shows the study-specific and summary odds ratios and 95% confidence intervals (CI) for mortality. We found no significant difference in mortality between patients randomized to DPO versus EPO (OR=1.33; 95% CI 0.88-2.01). No treatment heterogeneity across studies was detected (Q-statistic = 4.60; P=0.80). In sensitivity analysis, after excluding the 2 trials with zero mortality in one arm, we found a summary odds ratio that was essentially unchanged, OR=1.34 (95% CI 0.88-2.04).
Figure 2.
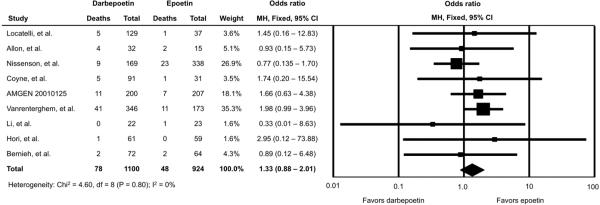
Individual and Summary Odds Ratios for Mortality in Trials of Darbepoetin Alfa versus Epoetin Alfa
Note: CI, confidence interval. For the trials by Li, et al. and Hori et al., a 0.5 death count was added to each of the two trial arms to permit inclusion in this meta-analysis. Sensitivity analyses in which these two trials were excluded yielded almost identical results (odds ratio, 1.34; 95% CI: 0.88-2.04).
Discussion
Our study highlights the paucity of available data on the comparative safety of two widely-used erythropoiesis-stimulating agents, DPO and EPO. Despite conducting a comprehensive search for randomized comparisons of these drugs, including unpublished studies, just over 2000 patients were enrolled in a total of 9 trials and follow up was limited to no more than one year. All studies were focused and powered to compare an anemia treatment parameter, usually hemoglobin. None of the studies were powered for hard safety endpoints such as mortality or nonfatal cardiovascular events. As our study demonstrates, even a synthesis of all available data lacks statistical power to rule out even large effect sizes. While non-significant, the 95% confidence limits cannot rule out a modest 12% reduction, but also a sizeable – more than 2-fold – increase in mortality risk in patients randomized to DPO. However, conclusive evidence on the comparative safety among erythropoiesis-stimulating agents, including EPO and DPO, appears essential. While the current treatment options are limited, more in the U.S. than in other countries due to differences in patent protection of Amgen's branded erythropoiesis-stimulating agents, the current paradigm is to evaluate drugs used to treat anemia mostly on their ability to raise or maintain laboratory target parameters, usually hemoglobin concentrations. Still, even in situations where efficacy outcomes are similar (equivalent or non-inferior) within a drug class, important safety outcomes may differ and the class assumptions so willingly accepted by clinicians need to be challenged since efficacy trials are usually underpowered to identify meaningful differences in relatively rare but important safety outcomes. Notable examples of within-class differences in safety that were identified and subsequently led to restrictions or removals of agents from the market are the classes of cyclooxygenase-2 inhibitors (rofecoxib) and the thiazolidinediones (troglitazone, rosiglitazone). And similarly, peginesatide, another erythropoiesis-stimulating agent that was scrutinized in a large phase III program and powered for hard cardiovascular safety endpoints was found to be unsafe soon after market introduction and use in more than 25,000 patients and subsequently withdrawn.(16)
There are well-documented biologic differences between DPO and EPO.(17-20) Darbepoetin alfa contains five N-linked oligosaccharide chains and up to 22 sialic acids, a molecular weight of 37,100 Da, and a carbohydrate composition of 51%. In contrast, EPO has three N-linked carbohydrate chains, a maximum of 14 sialic acids, a molecular weight of 30,400 Da, and a 40% carbohydrate composition. The additional carbohydrates result in a longer half-life and increased and more sustained biologic activity for DPO compared with EPO. In recent years, other effects of erythropoietin beyond the stimulation of red blood cell production have been described. Its receptors have been identified in several tissues including the brain, heart, uterus, and kidney.(21, 22) Erythropoietin has been ascribed pleiotropic properties, and may induce proliferation, chemotaxis, angiogenesis, and inhibit apoptosis.(23, 24) It seems to play a key role in regulation of vascular repair and even neoangiogenesis. Bahlmann et al. showed that both DPO and EPO enhance mobilization of bone-marrow derived endothelial-progenitor cells in humans,(25, 26) which are critical to vascular reparative processes and endothelial regeneration in ischemia-reperfusion injury.(22, 27, 28) It is unclear, however, whether non-hematopoetic effects of erythropoiesis-stimulating agents are proportional to their relative effectiveness on hematopoiesis. In other words, it is possible that while certain doses of DPO and EPO confer similar hemoglobin response, their effects on other organs or tissues may differ? Carbamylated erythropoietin, a derivative of erythropoietin, confers similar cardioprotection compared to epoetin alfa in an animal model, but without any effect on hematocrit.(29, 30) Hence, it is plausible to consider that safety may differ between DPO and EPO despite their demonstrated similar ability to control anemia in patients with chronic kidney disease.
The strengths of our study include its comprehensive search strategy and the overall high quality of included studies (all were randomized and several were blinded). However, our study does have relevant limitations. First, included studies were generally subject to small enrollment and short follow up. Almost all were funded by industry sponsors and there may be additional studies that were never reported. It would have been desirable to execute our study using the more granular information of patient-level data in which time to death, censoring events, and other important non-death outcomes would have been recorded. Unfortunately, such data are unavailable and our requests to obtain patient-level information were not embraced. While a statistical test failed to reject the homogeneity assumption among trials, power to identify heterogeneity was limited. We focused on mortality as a commonly reported safety outcome; however, studying other hard or patient-reported outcomes would have been informative, but were unavailable from most studies. A recent network meta-analysis on erythropoiesis-stimulating agents conducted by the Cochrane Renal Group investigated a large number of comparison formulations (including placebo) and outcomes and concluded that “the effectiveness of different ESA formulations based on patient-centred outcomes (such as quality of life, fatigue, and functional status) are sparse and poorly reported and current research studies are unable to inform care.”(31) Of note, their mortality comparison between DPO and EPO yielded a result compatible with ours, although fewer studies (6; total N=1205) were analyzed following their search and selection strategy.(31) Finally, we restricted our systematic review and metaanalysis to studies in patients with kidney disease. We felt that including data from the oncology indications for these drugs would not be useful due to the very different populations studied and the different dosing schemes employed. Further, a recent Comparative Effectiveness Review on EPO and DPO use in oncology indications (commissioned by the U.S. Agency for Healthcare Research and Quality) indicated that the currently available data do not support any analyses on comparative long-term survival between DPO and EPO.(32) Of note, a dose-finding study of another long-acting erythropoiesis-stimulating agent currently not available in the U.S., methoxy polyethylene glycol-epoetin beta, was terminated early due to increased mortality compared with another erythropoiesis-stimulating agent,(33) further highlighting the possibility of intraclass differences in safety.
In summary, we did not detect any significant difference in mortality risk between DPO and EPO using the available evidence from randomized head-to-head trials in patients with CKD, but considerable uncertainty remains. Larger (cluster-) randomized or observational post-marketing comparative effectiveness studies comparing these, and other, erythropoiesis-stimulating agents are required to better characterize the long-term safety profiles of these agents.
Acknowledgement
Dr. Winkelmayer had the research idea, defined the study design, and provided mentorship to Dr. Wilhelm-Leen; both Dr. Wilhelm-Leen and Dr. Winkelmayer conducted data acquisition and analysis, interpreted the data, and wrote this report.
Funding:
This study was supported by grant R01DK090181 from the National Institutes of Health, National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) to Dr. Winkelmayer. Dr. Wilhelm-Leen was supported by grant T32DK007357.
Footnotes
Declaration of Interests:
Dr. Winkelmayer reports having served within the past 36 months as a scientific advisor to Amgen, Astellas, Astra-Zeneca, Bayer, Fibrogen, GlaxoSmithKline, Keryx, Merck Sharpe & Dohme, Mitsubishi-Tanabe, and Rockwell Pharma, and on data safety monitoring boards for Medgenics and Medtronic. Dr. Wilhelm-Leen has nothing to disclose.
References
- 1.Portoles JM, de Francisco AL, Gorriz JL, et al. Maintenance of target hemoglobin level in stable hemodialysis patients constitutes a theoretical task: a historical prospective study. Kidney Int. 2008;(Suppl):S82–87. doi: 10.1038/ki.2008.524. [DOI] [PubMed] [Google Scholar]
- 2.Kuragano T, Matsumura O, Matsuda A, et al. Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. 2014;86:845–854. doi: 10.1038/ki.2014.114. [DOI] [PubMed] [Google Scholar]
- 3.Yang W, Israni RK, Brunelli SM, Joffe MM, Fishbane S, Feldman HI. Hemoglobin variability and mortality in ESRD. J Am Soc Nephrol. 2007;18:3164–3170. doi: 10.1681/ASN.2007010058. [DOI] [PubMed] [Google Scholar]
- 4.KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease Chapter 3: Use of ESAs and other agents to treat anemia in CKD. Kidney International Supplements. 2012;2:299–310. doi: 10.1038/kisup.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration [10/25/2010];Briefing Information for the October 18, 2010 Meeting of the Cardiovascular and Renal Drugs Advisory Committee. Available at: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/ucm229325.htm.
- 6.Locatelli F, Olivares J, Walker R, et al. Novel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiency. Kidney Int. 2001;60:741–747. doi: 10.1046/j.1523-1755.2001.060002741.x. [DOI] [PubMed] [Google Scholar]
- 7.Allon M, Kleinman K, Walczyk M, et al. Pharmacokinetics and pharmacodynamics of darbepoetin alfa and epoetin in patients undergoing dialysis. Clin Pharmacol Ther. 2002;72:546–555. doi: 10.1067/mcp.2002.128374. [DOI] [PubMed] [Google Scholar]
- 8.Nissenson AR, Swan SK, Lindberg JS, et al. Randomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patients. Am J Kidney Dis. 2002;40:110–118. doi: 10.1053/ajkd.2002.33919. [DOI] [PubMed] [Google Scholar]
- 9.Vanrenterghem Y, Barany P, Mann JF, et al. Randomized trial of darbepoetin alfa for treatment of renal anemia at a reduced dose frequency compared with rHuEPO in dialysis patients. Kidney Int. 2002;62:2167–2175. doi: 10.1046/j.1523-1755.2002.00657.x. [DOI] [PubMed] [Google Scholar]
- 10.Li WY, Chu TS, Huang JW, Wu MS, Wu KD. Randomized study of darbepoetin alfa and recombinant human erythropoietin for treatment of renal anemia in chronic renal failure patients receiving peritoneal dialysis. Journal of the Formosan Medical Association = Taiwan yi zhi. 2008;107:843–850. doi: 10.1016/S0929-6646(08)60200-4. [DOI] [PubMed] [Google Scholar]
- 11.Coyne DW, Ling BN, Toto R, McDermott-Vitak AD, Trotman ML, Jackson L. Novel erythropoiesis stimulating protein (NESP) corrects anemia in dialysis patients when administered at reduced dose frequency compared with recombinant-human erythropoietin (r-HuEPO) [Abstract]. J Am Soc Nephrol. 2000;11:263A. [Google Scholar]
- 12.Hori K, Tsujimoto Y, Ohmori H, et al. Randomized, double-blind, comparative study of intravenous KRN321 (darbepoetin alfa) compared to intravenous recombinant human erythropoetin alfa (rHuEPO) for treatmet of anemia in subjects with chronic renal failure (CRF) receiving hemodialysis in Japan. [Abstract]. J Am Soc Nephrol. 2004;15 [Google Scholar]
- 13.Bernieh B, Abouchacra S, Boobes Y, et al. Comparison between short- and long-acting erythropoiesis-stimulating agents in hemodialysis patients: target hemoglobin, variability, and outcome. Int Urol Nephrol. 2014;46:453–459. doi: 10.1007/s11255-013-0640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo YI, Yang DH, Shin SK. A randomized, cross-over, multicenter study of biweekly administration of high dose epoetin-A compared with darbepoetin-A in pre-ESRD patients. [Abstract]. Nephrology. 2010;15:39. [Google Scholar]
- 15.Anonymous [1/25/2010];A Randomized, Double-blind Study Comparing Aranesp (darbepoetin alfa) and Recombinant Human Erythropoietin (rHuEPO) in the Treatment of Anemia in African-American Subjects With Chronic Renal Failure (CRF) Receiving Hemodialysis. http://download.veritasmedicine.com/REGFILES/amgen/08D_FDAMA_113_Posting_Summary_33_NESP_20010125.pdf.
- 16.Bennett CL, Jacob S, Hymes J, Usvyat LA, Maddux FW. Anaphylaxis and hypotension after administration of peginesatide. N Engl J Med. 2014;370:2055–2056. doi: 10.1056/NEJMc1400883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdougall IC, Gray SJ, Elston O, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–2395. doi: 10.1681/ASN.V10112392. [DOI] [PubMed] [Google Scholar]
- 18.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP). Nephrol Dial Transplant. 2001;16(Suppl 3):3–13. [PubMed] [Google Scholar]
- 19.Catlin DH, Breidbach A, Elliott S, Glaspy J. Comparison of the isoelectric focusing patterns of darbepoetin alfa, recombinant human erythropoietin, and endogenous erythropoietin from human urine. Clin Chem. 2002;48:2057–2059. [PubMed] [Google Scholar]
- 20.Singh AK. Does TREAT give the boot to ESAs in the treatment of CKD anemia? J Am Soc Nephrol. 2010;21:2–6. doi: 10.1681/ASN.2009111127. [DOI] [PubMed] [Google Scholar]
- 21.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fliser D, Haller H. Erythropoietin and treatment of non-anemic conditions--cardiovascular protection. Semin Hematol. 2007;44:212–217. doi: 10.1053/j.seminhematol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Lappin TR, Maxwell AP, Johnston PG. EPO's alter ego: erythropoietin has multiple actions. Stem Cells. 2002;20:485–492. doi: 10.1634/stemcells.20-6-485. [DOI] [PubMed] [Google Scholar]
- 24.Weiss MJ. New insights into erythropoietin and epoetin alfa: mechanisms of action, target tissues, and clinical applications. Oncologist. 2003;8(Suppl 3):18–29. doi: 10.1634/theoncologist.8-suppl_3-18. [DOI] [PubMed] [Google Scholar]
- 25.Bahlmann FH, DeGroot K, Duckert T, et al. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003;64:1648–1652. doi: 10.1046/j.1523-1755.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 26.Bahlmann FH, De Groot K, Spandau JM, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 27.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 28.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 29.Leist M, Ghezzi P, Grasso G, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 30.Fiordaliso F, Chimenti S, Staszewsky L, et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2005;102:2046–2051. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer SC, Saglimbene V, Mavridis D, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database of Systematic Reviews. 2014 doi: 10.1002/14651858.CD010590.pub2. Issue 12. Art. No.: CD010590. DOI: 10.1002/14651858.CD010590.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epoetin and Darbepoetin for Managing Anemia in Patients Undergoing Cancer Treatment . Comparative Effectiveness Update. Comparative Effectiveness Reviews, No. 113. Blue Cross and Blue Shield Association Technology Evaluation Center; Evidence-based Practice Center. Agency for Healthcare Research and Quality (US); Rockville (MD): Apr, 2013. [12/5/2014]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK143013/?report=reader. [Google Scholar]
- 33.United States Food and Drug Administration [12/11/2014];Drug Approval Package, Mircera (Methoxy Polyethylene Glycol-Epoetin Beta) Injectiion, Office Director Memo. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/125164s000_ODMemo.pdf.