Abstract
Postpartum depression occurs in 14.5% of women in the first three months after birth. This study was an 8 week acute phase randomized trial with three cells (transdermal estradiol (E2), sertraline, and placebo) for the treatment of postpartum major depressive disorder. However, the study was stopped after batch analysis revealed that the E2 serum concentrations were lower than pre-study projections. This paper explores our experiences that will inform future investigations of therapeutic E2 use. Explanations for the low E2 concentrations were: 1) Study patch non-adhesion, which did not explain the low concentrations across the entire sample. 2) Ineffective transdermal patch preparations, although two different patch preparations were used and no significant main effect of patch type on E2 concentrations was found. 3) Obesity, at study entry, E2-treated women had mean ± SD BMI=32.9 ±7.4. No pharmacokinetic data comparing E2 concentrations from transdermal patches in obese women vs. normal weight controls are available. 4) Induction of Cytochrome P450 (CYP450) 3A4 and other E2 elimination pathways in pregnancy. CYP4503A4 is induced in pregnancy and is a pathway for the metabolism of E2. Conversion to estrone and Phase II metabolism via glucuronidation and sulfation, which also increase in pregnancy, are routes of E2 elimination. The time required for these pathways to normalize after delivery has not been elucidated. The observation that transdermal E2 doses greater than 100 mcg/day did not increase serum concentrations was unexpected. Another hypothesis consistent with this observation is suppression of endogenous E2 secretion with increasing exogenous E2 dosing.
Introduction
Epidemiological research has shown that females have an increased risk of major depressive disorder during the childbearing years and reduced risk during periods of hypothalamic-pituitary-gonadal axis quiescence [1-3]. Hormonal fluctuation (as occurs during the menstrual cycle, pregnancy, postpartum, and perimenopause) are associated with depression [4, 5]. Since depression occurs in 14.5% of women in the first three months postpartum [6] it is one of the most common major medical complications of childbearing. Postpartum Major Depressive Disorder (PPMD) has long-lasting adverse effects on children's cognitive and emotional development [7, 8]. PPMD is a disorder that is particularly well-suited for tests of E2 efficacy due to its occurrence in the context of large-scale estrogen withdrawal after childbirth.
A wealth of preclinical data suggests that key neural systems implicated in mood are targets of sex steroids. This is evidenced by the wide distribution of estrogen receptors throughout the brain with concentrated localization in the limbic system [9]. During pregnancy, the brain is exposed to 100-fold increases in ambient E2 concentrations, which abruptly decrease in the first postpartum week. Investigators have hypothesized that neuronal changes during this massive steroid withdrawal event predispose to PPMD. Bloch et al [10] demonstrated that women with PPMD were differentially sensitive to the mood-destabilizing effects of withdrawal from gonadal steroids.
Among available estrogenic treatments, E2 has garnered the greatest interest because it is the bioidentical estrogen with the highest estrogen receptor affinity and also because it has greater brain penetration relative to the commonly prescribed synthetic estrogen, ethinyl estradiol. Transdermal compared to oral E2 delivery confers an advantage because it resembles ovarian E2 production and bypasses the enterohepatic circulation, with consequent reduction in venothromboembolic risk [11].
There is a substantial literature on the widespread neural impact of E2 that supports its efficacy as a treatment for PPMD. E2 has far-reaching effects across monoaminergic neurotransmitter systems [12] [13-15], and brain circuits for emotion processing through direct genomic, indirect genomic, and cell-membrane mediated mechanisms at traditional intracellular ERα and ERβ receptors, cell-membrane ERα, ERβ, and ER-X receptors [16]. Rapid onset cell membrane E2 effects, mediated via second messenger signaling pathways, are vitally important in neural plasticity through dendritic spine morphologic changes within hippocampal [17] and dorsal raphe brain regions [18] that regulate mood and cognitive function. E2 is neuroprotective not only in models of brain injury and stroke, but also in the dorsal raphe nuclei (the primary site of origin for CNS serotonergic neurons) of healthy non-human primates [19]. E2 also mitigates against oxidative stress, glutamateric excitotoxicity, β-amyloid tocixity [20] and microglial cytokine release [21]. Evidence for a relationship between ESR1 polymorphisms, the gene which encodes ERα, and severe depression and anxiety is accumulating [22]. Preclinical experiments have shown that action at both ERα and ERβ are involved in the antidepressant effects of E2 [23], with evidence of the same in a rodent model of postpartum affective disorders [24]. Finally, neuroimaging studies during naturalistic and experimental reproductive hormone fluctuations confirm that cellular-level E2 effects are translated into cognitive [25] [26], reward [27, 28], and other emotion processing functions [29] [30]; [31] in women.
Two trials of E2 for the treatment of PPMD have been published. Gregoire et al [32] randomized 61 women to placebo (PL) or E2 (200 mcg/day) delivered by transdermal patch for 6 months. The mean E2 concentration of actively treated women was 185 pg/ml. The outcome measure was the Edinburgh Postnatal Depression Screening Scale (EPDS) [33]. Significant benefit of E2 compared to PL was evident by 1 month of treatment. By 3 months, 80% of the E2-treated group compared to 31% of the PL-treated group had EPDS scores <14. In this study, women who developed PPMD by 3 months but presented for treatment up to 18 months after birth were included. This time frame is distant from the E2 withdrawal at delivery that theoretically contributes to PPMD risk and is the primary rationale for E2 treatment.
In an open trial, Ahokas et al. [34] treated 23 postpartum, severely depressed inpatient women who presented within 12 months of birth. They were treated openly with sublingual estradiol (mean dose=4.8 mg/day) for 8 weeks. The subjects' mean E2 concentrations rose from a mean of 22 to 130 pg/ml by study completion. Within a week of treatment, 21 of 23 subjects had 50% symptom score reductions. By 2 weeks, 19 of 23 subjects achieved remission. E2 treatment was well-tolerated in both studies as judged by low attrition.
A convergence of epidemiological, preclinical, and clinical research compels further evaluation of E2 as a treatment for PPMD because: 1) an open and a randomized controlled trial have shown robust and rapid response in women with PPMD, 2) many women welcome an alternative to antidepressant agents, 3) major side effects of transdermal E2 are rare within the dose range studied, 4) E2 is a mechanistically appropriate treatment, since women develop depressive symptoms in conjunction with hormone withdrawal, 5) passage of E2 to the infant through breastmilk [35] is minimal, and 6) the treatment is convenient in transdermal patch form.
Methods
Design
The aim of this investigation was to test the efficacy of E2 compared to placebo (PL) for the treatment of PPMD, with sertraline as an antidepressant comparator. The hypothesis was that both E2 and SERT would be significantly more effective than PL in reducing the symptoms of PMDD. However, the study was stopped after E2 batch analysis revealed that the serum concentrations were not in the range expected based upon pre-study projections. Additional pharmacokinetic studies are planned and this paper explores our experiences that will inform future investigations of the use of E2 therapeutically.
The design was an 8 week acute phase randomized trial with three cells (transdermal E2), sertraline (SERT) and placebo (PL) for the treatment of PPMD, which by definition requires 2 weeks of symptoms (post-birth) and onset of symptoms within 4 weeks of delivery. Therefore, study subjects could present for treatment between 2 to 13 weeks postpartum. Each week for 8 weeks, the patient was seen for an in-person clinic assessment (even weeks) or contacted for a phone interview (odd weeks). At each assessment, depression, mania and side effect rating scales were administered.
Subjects
The investigation was conducted at the University of Pittsburgh within an academic psychiatry specialty program in women's health. Women with PPMD were between 18-40 years of age. The subjects had to be planning to use or using a non-estrogen containing birth control regimen. We considered the possibility of making previous nonresponse to SERT an exclusion criterion; however, we decided against this because it is often difficult to be certain that a woman had an adequate therapeutic trial in the past. Women were without major medical problems and had normal lipid profiles (to reduce the risk of thromboembolic events) relative to the postpartum period [36]. Women were not using other therapies for depression, including antidepressants, psychotherapy, light therapy, or herbal remedies. Women with bipolar disorder, a previous psychotic episode, or substance abuse within the last 6 months were not eligible. Women with heavy smoking (>10 cigarettes/day), a thromboembolic event, hypercoagulability, current or past history of breast, uterine, or ovarian cancer; or first degree relatives with thromboembolic events were excluded. A negative urine drug screen was also required at baseline.
Breastfeeding women were included in this investigation. Breastfeeding women were excluded in the Gregoire et al study, but 13 of the 23 women reported by Ahokas et al were breastfeeding their infants. The mean E2 concentration achieved in both studies was in the middle physiologic range of the normal menstrual cycle; therefore, infant E2 exposure through lactation would be similar to that of a regularly cycling woman. Little is known about E2 into breast milk; however, Perheentupa et al [37] did not detect E2 (limit of detection=7 pg/ml) in the breast milk of 18 lactating women who used transdermal E2 50-100 μg/day. While high doses of E2 can reduce breastmilk production [38], breastfeeding frequency was no different between women randomized to treatment with transdermal E2 (50–100 μg/d) or PL for 12 weeks [37].
Randomization Procedures
Randomization into the three arms of the study was stratified by breastfeeding status (2 levels: <50% vs. ≥ 50% of feedings were breastmilk) and infant age (≤ 41 days, > 42 days and ≤ 69 days, and > 70 days. The infant ages were adjusted for gestational age at birth. Separate randomization tables were created for each of the six strata, using the UNIFORM (seed) function in SAS© Base Software (SAS Institute, Cary, NC). Within each stratum, assignments to the three arms were blocked in a random 3- and 6-block design to ensure equal distribution.
Randomization occurred after consented subjects were screened, found to be eligible for enrollment and all baseline data were completed and reviewed by a study physician. Once the physician recorded approval in the study database, a randomization assignment was made within the system, based on entered patient data that defined the appropriate stratum. The randomization assignment was stored in a password protected area of the database that was only available to the study principal investigator (KLW) in the event of an emergency, or at the time a patient completed (or otherwise exited) the study.
Outcome Measures
The primary outcome measures were assessed with the Structured Interview Guide for the Hamilton Depression Rating Scale—Atypical Depression Symptoms Version (SIGH-ADS29); [39]. The scale incorporates the commonly used 17 and 21-item Hamilton Rating Scales for Depression (HRSD) as well as 8 atypical symptoms of depression. The primary outcomes were response (reduction of baseline SIGH-ADS29 score by ≥50%) and remission (Exit SIGH-ADS29 score ≤ 8). A SIGH-ADS29 score of 18 was required for study entry in addition to diagnostic criteria for major depressive disorder according to the Structured Clinical Interview for DSM4. The primary study staff (nurse, interviewers, and research assistants) were blinded to treatment assignment. The medication monitoring by the study psychiatrist was separate from (and blind to) mood symptom monitoring. The Asberg Side Effects scale was used to assess adverse emergent symptoms [40]. All evaluators remained blind to the subject's drug assignment until the trial was completed.
Study Medications
SERT (or PL) were delivered in opaque gelcaps. The E2 (or PL) was delivered in the form of transdermal patches. For women randomized to PL, neither the capsule nor patch contained active agent. Each subject took 2 capsules daily and wore the required number of patches weekly or twice weekly depending upon dose and preparation. For the first portion of the study (07/2008--03/2010), Vivelle Dot® and matching placebo patches were provided by Novartis (Mechanicsburg, PA). Patches were applied twice weekly. The second half of the study (08/2010—8-2013) was conducted with purchased Mylan (Cecil Township, PA) generic E2 patches which were applied weekly and placebo patches with packaging constructed to match the active patches. All patches were latex-free. Instructions included placement on clean, dry skin on the abdomen or upper thigh which was free from lotions or powders; no patch placement near breasts, stretch marks or scars; and rotation of the location of the patches. If a patch did not adhere, subjects were asked to put adhesive tape around the edges of the patch and contact the study team.
We used a parallel dose escalation strategy for E2 with doses ranging from 50 mcg to 200 mcg (the dose used in the Gregoire et al postpartum trial), which is a relatively high dose by today's medical practice [11]. The doses were advanced according to the plan in Supplemental Table A unless side effects were prohibitive or the subject met remission criteria. To reduce the risk of endometrial hyperplasia in study participants who were not taking a progesterone-containing oral contraceptive or intrauterine device, a 14-day administration of medroxyprogesterone (10 mg/day) was given with E2 during weeks 9-10 to women who did not have spontaneous menses. The doses of SERT were tested successfully in our previous study of women with women with PMDD [41].
Study medications were packaged by the study pharmacist and labeled with dose level and assignment arm “A”, “B”, or “C”, and were assigned unique control numbers. Research assistants obtained the medication packets from the pharmacist, logged them into a medications module of the database system, and stored them in a locked cabinet in the clinic. Medications were distributed by order of study physicians, who determined the dose to be provided and entered that into the database. This enabled the medications module of the database system to identify the patient's study arm from its internal randomization table, and present the research assistant with the appropriate packet control number, its dose, and its assignment arm (“A”, “B”, or “C”).
The research assistant obtained the correct packet from the locked cabinet, and using the medications module of the database system, recorded the study ID of the person receiving the packet, confirmed the dose and assignment arm/letter, and then recorded her initials and the date. A second person (another RA or the study coordinator, never a clinician making assessments of the patient) accompanied the research assistant in this task, and recorded her initials in the medications module as well, to confirm that the correct packet was obtained and disbursed. A report of medication disbursements was reviewed weekly at meetings of the data team, the study coordinator, and the research assistants (clinical personnel were excluded), to monitor for unexpected errors and to maintain inventories. Assignment arms were always referred to by letter rather than actual treatment, and additional packets were requested of the study pharmacist by letter and dose only.
Estradiol Assays
All samples were frozen and analyzed at the same time in the same laboratory. E2 was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using the Agilent 6490 with Agilent 1290 series pumps and MassHunter Workstation Software. The internal standard used was 17β-Estradiol-2,4,16,16,17-d5 from CDN isotopes. Standards and serum samples were extracted with methylene chloride and extracts were derivatized with dansyl chloride. Agilent Zorbax XDB-C18 2.1 × 30mm 3.5-micron was used as the loading column and Agilent Poroshell 120 EC-C18; 2.1 × 50mm 2.7-micron was used as the analytical column. Eluting/loading mobile phase A was water + 5mM ammonium formate and eluting/mobile phase B was 98:2 acetonitrile:water + 5mM ammonium formate. The MS settings were as follows: Delta EMV (+) = 600, Fragmentor = 380, Cell Accelerator Voltage = 3, Dwell = 100, Gas temp = 150°C, Gas Flow = 17 l/min, Nebulizer = 45, Shealth Gas Temp = 400°C. The m/z monitored for estradiol and estradiol-d4 were 506.3/171.1 and 511.3/171.1 respectively. The lower limit of sensitivity for the E2 asssay was 0.3 pg/mL. Intra-assay C.V.'s were 11.8%, 7.3%, 6.0%, 1.6%, 1.5% and 1.4% at 0.23, 0.50, 0.74, 35, 151 and 405 pg/mL respectively. Inter-assay C.V.'s were 10.8%, 8.5%, 6.9%, 5.1%, 4.6% and 4.8% at 0.29, 0.50, 0.77, 32, 140 and 382 pg/ml respectively.
Statistical Methods
Descriptive statistics are presented as means and standard deviations or frequencies and percentages for continuous and categorical measures respectively. Group comparisons were conducted with Student's t tests or ANOVA when the dependent measure was normally distributed. Mann-Whitney U or Kruskal-Wallis tests were used when the dependent measure had a non-normal distribution. If the dependent measure was categorical, the Chi-Square test was used to compare groups, or Fisher's exact test when observed cell sizes were smaller than expected. Intent–to-treat analyses were conducted with all available data and last observation carried forward for the primary outcomes of response and remission, A repeated measures mixed linear model was fit to the Structured Interview Guide for the Hamilton Rating Scale for Depression with the Atypical Depressive Supplement (SIGH-ADS29) including terms for treatment, time, and their interaction. An unstructured covariance matrix was assumed and a random intercept included.
Results
Sample Characteristics
The flow of subjects through the study is shown in Supplemental Figure A. Of 85 patients randomized, 62 (73%) completed week 8; 41 were responders and 21 were non-responders.
The study sample was predominantly white, educated, married and the postpartum episode was usually within a recurrent major depressive disorder. The equivalency of randomization was evaluated and no significant differences in demographics were observed across the 3 groups (Supplemental Table B). The randomization assignments were balanced according to stratification variables when enrollment ended at 85 patients. Additionally, differences in demographics were evaluated between completers and non-completers of the 8 week protocol (Data not shown). White women were more likely than women from minority groups to complete the protocol (p<.02).
Outcomes
As expected due to being stopped and therefore underpowered, the study showed no significant differences in either rates of response (58.6%, 42.3% and 63.3% for PL, E2 and SERT, respectively; p=.26) or remission (31%, 26.9%, and 30%; respectively, p=.94) across the three groups (Table 1).
Table 1. Outcome Measures by Treatment.
| Measure | Total (N=85) |
Treatment | Analysis | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Placebo (N=29) |
Estradiol (N=26) |
Sertraline (N=30) |
Test statistic | p | ||
| Exit SIGH-ADS29 | 13.2 ± 7.84 | 13.1 ± 8.41 | 14.7 ± 8.78 | 12.1 ± 6.36 | H(2) = 0.95 | 0.62 |
| % SIGH-ADS29 change | 45 ± 29.3 | 48 ± 29.8 | 38 ± 33.7 | 49 ± 24.1 | F(2,0) =1.19 | 0.31 |
| Response (%SIGH-ADS29 change ≥50) | 47 (55.3) | 17 (58.6) | 11 (42.3) | 19 (63.3) | χ2(2) = 2.69 | 0.26 |
| Remission (Exit SIGH-ADS29 ≤ 8) | 25 (29.4) | 9 (31.0) | 7 (26.9) | 9 (30.0) | χ2(2) = 0.12 | 0.94 |
Abbreviations: SIGH-ADS=Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement; H=the equivalent of the Mann-Whitney U test for 3 or more groups.
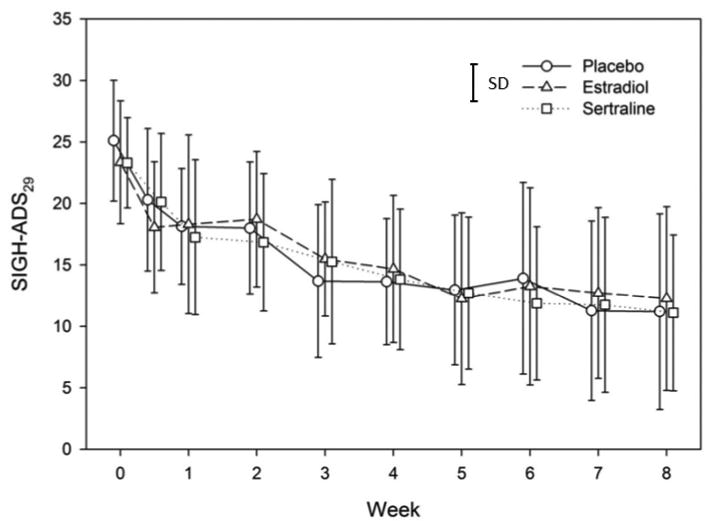
The course of depressive symptoms across the trial is displayed in Figure 1. The repeated measures mixed linear model revealed a significant time effect but a non-significant main effect for treatment in addition to a non-significant effect for the treatment by time interaction.
Figure 1. Course of Depressive Symptoms by Treatment Assignment.
Dose
For the completers, doses at the endpoint of the 8-week acute phase randomized trial were: E2 (mean ± SD =161 ± 54 mcg/day, median=200 mcg/day, range 50-200 mcg/day); SERT (mean ± SD 145 ±55 mg/day, median=150 mg/day, range 50-200 mg/day); Placebo (mean ± SD pill equivalent dose=162 ± 47, median=200; range 50-200).
Few differences in rates of side effects were observed across groups. The Asberg scale contains 27 items of individual side effects, and one question to quantify “distress from side effects.” Among the 27 items, we compared items rated 2 (moderate intensity), and the only significant difference was for headaches, which were more common in the placebo (75%) group than the E2 (46%) or SERT (43%) group (p=.03). For items rated 3 (severe), no significant differences between treatment groups were observed.
Four women developed hypomania and one developed psychosis. Two women (one randomized to SERT and one to PL) became hypomanic within days of initiating the study medication. At week 7, one woman randomized to SERT slept 3 hours and complained of feeling overly energetic, “…like I drank a big pot of coffee.” Another participant randomized to SERT developed hypomania at week 8, and a final woman (randomized to SERT) developed a psychotic depression with cognitive disorganization and visual hallucinations at week 8. These women were withdrawn from the study drug and antimanic agents were administered. An interesting observation is that no women randomized to E2 developed hypomania or psychosis.
Serum E2 Concentrations
In the intent to treat analysis (with all serum concentrations from women who provided them regardless of whether they continued the study drug), the mean ± SD E2 concentrations at exit were as follows in the three treatment groups: PL, 52.3 ± 50.0 pg/ml; E2, 63.8 ± 55.2 pg/ml; SERT, 60.4 ± 69.2 pg/ml; H(2)=0.04, p=.80.
The serum concentrations from only women who completed the 8-week trial on the study drug to which they were assigned were also evaluated (Table 2). The mean E2 ± SD concentrations in the E2-treated group at pretreatment were non-significantly different (32.9 ± 30.9 pg/ml) than SERT (57.4 ± 93.9 pg/ml) and PL groups (36.1 ± 38.3 pg/ml); F(2,39)=0.66, p=.52. Similarly, the group means did not demonstrate a statistically significant difference at week 4 or 8. An unexpected observation was that the mean serum level of E2 at week 8 in the same group of subjects actually decreased from 74.0 pg/ml to 68.5 pg/ml despite a mean transdermal E2 dose increase.
Table 2. Estradiol Levels (pg/ml) Across Time in Completers Only.
| Mean | Std. Dev | Min | Max | ||
|---|---|---|---|---|---|
| Week 0 [E2] |
PL (n=14) | 36.1 | 38.3 | 5.8 | 141.0 |
| E2 (n=14) | 32.9 | 30.9 | 2.8 | 123.0 | |
| SERT(n=14) | 57.4 | 93.9 | 6.9 | 372.0 | |
| Week 4 [E2] |
PL (n=13) | 33.6 | 25.2 | 1.83 | 76.0 |
| E2 (n=13) | 74.0 | 84.1 | 0.6 | 240.0 | |
| SERT(n=13) | 63.8 | 86.9 | 0.6 | 301.0 | |
| Week 8 [E2] |
PL (n=14) | 45.7 | 47.2 | 2.2 | 136.0 |
| E2 (n=16) | 68.5 | 66.7 | 0.4 | 197.0 | |
| SERT (n=14) | 53.8 | 83.6 | 0.5 | 316.0 | |
The concentrations in the group of E2-treated women were dichotomized by responder status to examine their relationship to response and the mean level ± SD was non-significantly higher (93.6 ± 65.8 pg/ml) in responders than nonresponders (56.1 ± 56.5 pg/ml)(p=0.24).
Discussion
The aim of this investigation was to test the efficacy of E2 compared to placebo (PL) for the treatment of PPMD; however, this hypothesis could not be adequately evaluated due to the non-significant (statistically and clinically) E2 concentration differences between the women receiving exogenous E2 compared to the other two treatment groups. Although the serum concentration that constitutes a therapeutic target range to treat MDD at any point in the female life cycle has not been determined, the average concentrations that we observed in our subjects were substantially lower than those observed in the two studies showing efficacy of E2 for PPMD (130 and 185 pg/ml). We evaluated the concentrations in the group of E2-treated women according to responder status, and responders had non-significantly higher mean ± SD (93.6 ± 65.8 pg/ml) concentrations compared to nonresponders (56.1 ± 56.5 pg/ml), which suggests the possibility that concentrations closer to those reported might result in greater efficacy. The mean E2 concentrations achieved in our subjects were less than the mean concentration across the menstrual cycle. The focus of this discussion is an analysis of the results from this study that will inform a definitive investigation.
Prior to the original study, we estimated the range of E2 concentrations that women would develop with E2 patch treatment. We reasoned that the total E2 concentration would equal the endogenously produced E2 plus exogenous E2 absorbed from transdermal patches, with the caveat that endogenous E2 secretion may be suppressed by negative feedback from E2 treatment on gonadotropins. Berlex-sponsored pharmacokinetic studies of the Climara® E2 transdermal system demonstrated an approximate 1:1 ratio of [E2 in mcg] delivered to [serum E2 pg/ml concentration] in menopausal women) [11]. Therefore, we anticipated that E2 doses of 50, 100, 150 and 200 mcg/day would produce 50, 100, 150, and 200 pg/ml serum concentrations. To explain our lower than expected E2 concentrations, we considered the following possible reasons:
Patch non-adhesion
We provided detailed verbal and written instructions for patch placement and management of patch non-adhesion, and compliance data were collected at each assessment. Two women who were randomized to E2 reported patch non-adhesion during the week before serum sampling at week 8. Their serum concentrations were 28 and 29 pg/ml and both were nonresponders. However, three women who reported complete patch adherence had similar low concentrations and they were also nonresponders. Patch non-adhesion may have contributed to the low concentrations of some women at week 8 but cannot explain the low concentrations across the entire sample of women.
Ineffective transdermal patch preparations
At the initiation of the trial, the E2 and matched placebo patches were manufactured by Novartis (Vivelle Dot) and were applied twice weekly. In the second half of the trial the Vivelle-matched placebo patches became unavailable. E2 patches were purchased from Mylan Labs, and were applied once weekly; placebo patches were similar appearing non-hormone containing patches in packaging designed to match the active patches. This situation provided a natural experiment to evaluate the E2 concentrations. At week 8 of the trial, women treated with the Vivelle Dot patch had a serum concentration mean ± SD of 61 ± 59 (range=12-171) pg/ml, which was non-significantly lower than that of women receiving the Mylan patch (88 ± 66 (range=12-197) pg/ml). There was no main effect of patch type on E2 concentrations, F(1,14)=0.71, p=0.41.
Obesity may impact efficacy
Depression is associated with obesity [42]. At study entry, E2-treated women had mean ± SD BMI=32.9 ±7.4 and weight=196.7±48.2 lbs. Could the fat tissue in the abdomen or thigh upon which the transdermal patches were placed reduce absorption of E2? Is E2 metabolism altered in obese women? No pharmacokinetic data comparing E2 concentrations from transdermal patches in obese women vs. normal weight controls are available. However, transdermally delivered hormonal contraception may be less effective in women with body weight of ≥90 kg (≥198 lb) [43]. In transdermal ethinyl estradiol (EE) treated women with low body weight (54.5 kg), EE clearance was 21% lower and concentrations 26% higher than in women at the median weight=84.9 kg. In obese women (122.9 kg), EE clearance was 17% higher and the associated EE level was 15% lower [44]. Ovarian function was sufficiently suppressed in obese women to provide contraception; however, the pharmacodynamic target in our study was PPMD, which may require higher E2 concentrations. Finally, noncompliance is associated with poverty and obesity [45] and depression [46], and may have contributed to low serum concentrations or patch failure (despite claims of adhesion).
Induction of Cytochrome P 450 (CYP 450) 3A4 in pregnancy/early postpartum
The postpartum period is physiologically different from the perimenopause, from which most data regarding E2 concentrations derived from patches have been generated. The serum concentrations developed by newly postpartum women may be lower, especially in breastfeeding women [47]. Additionally, CYP3A4 is induced in pregnancy. E2 is a substrate of CYP3A4, and multiple drugs metabolized through this pathway have increased clearances during pregnancy and require higher doses to maintain efficacy [48-50]. Phase I oxidative metabolism of E2 by CYP3A4, and to a lesser extent other CYPs, play an important role in E2 clearance. E2 is converted to estrone and Phase II metabolism via glucuronidation and sulfation, which are also increased in pregnancy [51-55]. With strong evidence to show increased CYP3A4 activity in pregnancy, induction of glucuronidation enzymes and probable increases in sulfation, it is highly likely that E2 clearance is increased in pregnancy. Glucuronidation returns to its pre-pregnancy baseline by 3-4 weeks postpartum; however, the timing of postpartum normalization of sulfotransferase activity is not known.
The time required for CYP3A4 induction to normalize after delivery has not been elucidated. Most studies have sampled women between 6-15 weeks postpartum to represent the nonpregnant state [50]. Whether this reflects the true nonpregnant CYP3A4 baseline and how rapidly its activity declines after birth is not known. The only study to evaluate CYP3A4 activity near delivery [56] showed that its activity normalized at some point between 1 week and 3 months after birth. The women in our original study were enrolled between 2 weeks and 3 months postpartum; therefore, the concentrations of E2 may have been low due to residual elevated CYP450 3A4 activity. As a result, our findings of unexpectedly low E2 concentrations may reflect residual postpartum activation of CYP3A4 and other pathways.
The observation that transdermal E2 doses >100 mcg/d did not increase serum concentrations from week 4 to week 8 was unexpected. A hypothesis consistent with this observation is suppression of endogenous E2 secretion with increasing exogenous E2 dosing via the transdermal patch.
Although the mechanism(s) responsible for lower than anticipated serum concentrations of E2 require additional exploration, our goal was to determine whether E2 was efficacious for treating PPMD. We established that serum concentrations developed with the 200 mcg/d E2 patch were variable across postpartum women and that the majority did not reach the serum concentrations we anticipated in planning the investigation. Pharmacokinetic studies are planned to establish serum E2 concentrations developed in individual postpartum women from transdermal dosing. The doses required to consistently develop concentrations that have been shown to be efficacious in previous studies (130-185 pg/ml) and predictors of level to dose ratios would advance clinical research in this area, which may provide an alternative treatment for depression in women with PPMD.
Supplementary Material
Acknowledgments
Funding Source: R01 MH057102, K.L. Wisner, PI.
The Department of Psychiatry at Northwestern University receives contractual fees for Dr. Wisner's consultation to Quinn Emanuel Urquhart & Sullivan, LLP (New York City), who represent Pfizer Pharmaceutical Company. Dr. Driscoll serves as the Medical Advisor for development of Postpartum Depression Program at EMMI Solutions. Dr. Wisniewski has current grant funding from Johnson and Johnson. Dr. Moses-Kolko has current grant funding from NICHD: HD067185.
Footnotes
Conflicts of Interest: The remaining authors have no conflicts to report.
References
- 1.Angold A, Worthman C. Puberty onsent of gender differences in rates of depression: a developmental, epidemiological, and neuroendocrine perspective. Journal of Affective Disorders. 1993;29:145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 2.Bebbington P, Dunn G, Jenkins R, et al. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Psychol Med. 1998;28:9–19. doi: 10.1017/s0033291797006077. [DOI] [PubMed] [Google Scholar]
- 3.Lokuge S, Frey BN, Foster JA, et al. Depression in women: windows of vulnerability and new insights into the link between estrogen and serotonin. J Clin Psychiatry. 2011;72:e1563–1569. doi: 10.4088/JCP.11com07089. [DOI] [PubMed] [Google Scholar]
- 4.Rapkin AJ, Mikacich JA, Moatakef-Imani B, et al. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep. 2002;4:419–428. doi: 10.1007/s11920-002-0069-7. [DOI] [PubMed] [Google Scholar]
- 5.Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- 6.Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. 2013;70:1312–1319. doi: 10.1001/jamapsychiatry.2013.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- 9.Gundlah C, Kohama SG, Mirkes SJ, et al. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- 10.Bloch M, Schmidt PJ, Danaceau M, et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 11.Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52:516–529. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS, Alves SE. Estrogen Actions in the central Nervous System. Endocrine Review. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 13.Bethea CL, Mirkes SJ, Shively CA, et al. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biological Psychiatry. 2000;47:562–576. doi: 10.1016/s0006-3223(99)00156-0. [DOI] [PubMed] [Google Scholar]
- 14.Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- 15.Osterlund MK, Halldin C, Hurd YL. Effects of Chronic 17B-Estradiol treatment on the serotonin 5-HT1A receptor mRNA and Binding Levels in the Rat Brain. Synapse. 2000;35:39–44. doi: 10.1002/(SICI)1098-2396(200001)35:1<39::AID-SYN5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS, Akama KT, Spencer-Segal JL, et al. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera HM, Bethea CL. Ovarian steroids increase PSD-95 expression and dendritic spines in the dorsal raphe of ovariectomized macaques. Synapse. 2013;67:897–908. doi: 10.1002/syn.21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bethea CL, Reddy AP, Tokuyama Y, et al. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30:212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amantea D, Russo R, Bagetta G, et al. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52:119–132. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Wu WF, Tan XJ, Dai YB, et al. Targeting estrogen receptor beta in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2013;110:3543–3548. doi: 10.1073/pnas.1300313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan J, Ancelin ML. Polymorphisms of estrogen receptors and risk of depression: therapeutic implications. Drugs. 2012;72:1725–1738. doi: 10.2165/11635960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Calmarza-Font I, Lagunas N, Garcia-Segura LM. Antidepressive and anxiolytic activity of selective estrogen receptor modulators in ovariectomized mice submitted to chronic unpredictable stress. Behav Brain Res. 2012;227:287–290. doi: 10.1016/j.bbr.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 24.Furuta M, Numakawa T, Chiba S, et al. Estrogen, predominantly via estrogen receptor alpha, attenuates postpartum-induced anxiety- and depression-like behaviors in female rats. Endocrinology. 2013;154:3807–3816. doi: 10.1210/en.2012-2136. [DOI] [PubMed] [Google Scholar]
- 25.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs E, D'Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreher JC, Schmidt PJ, Kohn P, et al. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CT, Sierra Y, Oppler SH, et al. Ovarian cycle effects on immediate reward selection bias in humans: a role for estradiol. J Neurosci. 2014;34:5468–5476. doi: 10.1523/JNEUROSCI.0014-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Protopopescu X, Pan H, Altemus M, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Wingen GA, Ossewaarde L, Backstrom T, et al. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 31.Shafir T, Love T, Berent-Spillson A, et al. Postmenopausal hormone use impact on emotion processing circuitry. Behav Brain Res. 2012;226:147–153. doi: 10.1016/j.bbr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347:930–933. doi: 10.1016/s0140-6736(96)91414-2. [DOI] [PubMed] [Google Scholar]
- 33.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 34.Ahokas A, Kaukoranta J, Wahlbeck K, et al. Estrogen Deficiency in Severe Postpartum Depression: Successful Treatment With Sublingual Physiological 17B-Estradiol: A Preliminary Study. Journal of Clinical Psychiatry. 2001;62:332–336. doi: 10.4088/jcp.v62n0504. [DOI] [PubMed] [Google Scholar]
- 35.Perheentupa A, Ruokonen A, Tapanainen JS. Transdermal estradiol treatment suppresses serum gonadotropins during lactation without transfer into breast milk. Fertil Steril. 2004;82:903–907. doi: 10.1016/j.fertnstert.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 36.Prairie BA, Wisniewski SR, Luther JF, et al. Postpartum lipid levels in women with major depression. J Womens Health (Larchmt) 2012;21:534–538. doi: 10.1089/jwh.2011.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perheentupa A, Ruokonen A, Tapanainen JS. Transdermal estradiol treatment suppresses serum gonadotropins during lactation without transfer into breast milk. Fertility & Sterility. 2004;82:903–907. doi: 10.1016/j.fertnstert.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 38.Booker DE, Pahyl IR. Control of postpartum breast engorgement with oral contraceptives. Am J Obst Gyn. 1967;98:1099–1101. doi: 10.1016/0002-9378(67)90034-8. [DOI] [PubMed] [Google Scholar]
- 39.Williams JBW, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS) New York State Psychiatric Institute; New York: 2003. [Google Scholar]
- 40.Asberg M, Cronholm B, Sjoqvist F, et al. Correlation of subjective side effects with plasma concentrations of nortriptyline. Br Med J. 1970;4:18–21. doi: 10.1136/bmj.4.5726.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26:353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- 42.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 43.Zieman M, Guillebaud J, Weisberg E, et al. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77:S13–18. doi: 10.1016/s0015-0282(01)03275-7. [DOI] [PubMed] [Google Scholar]
- 44.Westhoff CL, Reinecke I, Bangerter K, et al. Impact of body mass index on suppression of follicular development and ovulation using a transdermal patch containing 0.55-mg ethinyl estradiol/2.1-mg gestodene: a multicenter, open-label, uncontrolled study over three treatment cycles. Contraception. 2014;90:272–279. doi: 10.1016/j.contraception.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Westhoff CL, Torgal AT, Mayeda ER, et al. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception. 2012;85:465–469. doi: 10.1016/j.contraception.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 46.Wade AG, Haring J. A review of the costs associated with depression and treatment noncompliance: the potential benefits of online support. Int Clin Psychopharmacol. 2010;25:288–296. doi: 10.1097/yic.0b013e328339fbcf. [DOI] [PubMed] [Google Scholar]
- 47.Lawrie TA, Hofmeyr GJ, De Jager M, et al. A double-blind randomised placebo controlled trial of postnatal norethisterone enanthate: the effect on postnatal depression and serum hormones. Br J Obstet Gynaecol. 1998;105:1082–1090. doi: 10.1111/j.1471-0528.1998.tb09940.x. [DOI] [PubMed] [Google Scholar]
- 48.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Choi SY, Koh KH, Jeong H. Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos. 2013;41:263–269. doi: 10.1124/dmd.112.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hebert MF, Easterling TR, Kirby B, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84:248–253. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
- 51.Chen H, Yang K, Choi S, et al. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metabolism and Disposition. 2009;37:1841–1847. doi: 10.1124/dmd.109.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong H, Choi S, Song JW, et al. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38:62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen X, Donepudi AC, Thomas PE, et al. Regulation of hepatic phase II metabolism in pregnant mice. Journal of Pharmacology and Experimental Therapeutics. 2013;344:244–252. doi: 10.1124/jpet.112.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kodama S, Negishi M. Sulfotransferase genes: regulation by nuclear receptors in response to xeno/endo-biotics. Drug Metabolism Reviews. 2013;45:441–449. doi: 10.3109/03602532.2013.835630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itaaho K, Mackenzie PI, Ikushiro S, et al. The configuration of the 17-hydroxy group variably influences the glucuronidation of beta-estradiol and epiestradiol by human UDP-glucuronosyltransferases. Drug Metabolism and Disposition. 2008;36:2307–2315. doi: 10.1124/dmd.108.022731. [DOI] [PubMed] [Google Scholar]
- 56.Ohkita C, Goto M. Increased 6-hydroxycortisol excretion in pregnant women: implication of drug-metabolizing enzyme induction. Dicp. 1990;24:814–816. doi: 10.1177/106002809002400902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


