Abstract
Ca2+/Calmodulin-dependent protein kinase II (CaMKII) decodes neuronal activity by translating cytoplasmic Ca2+ signals into kinase activity that regulates neuronal functions including excitability, gene expression, and synaptic transmission. Four genes lead to developmental and differential expression of CaMKII isoforms (α, β, γ, δ). We determined mRNA levels of these isoforms in the dorsal root ganglia (DRG) of adult rats with and without nerve injury in order to determine if differential expression of CaMKII isoforms may contribute to functional differences that follow injury. DRG neurons express mRNA for all four isoforms, and the relative abundance of CaMKII isoforms was γ>α>β=δ, based on the CT values. Following ligation of the 5th lumbar (L5) spinal nerve (SNL), the β isoform did not change, but mRNA levels of both the γ and α isoforms were reduced in the directly injured L5 neurons, and the α isoform was reduced in L4 neurons, compared to their contemporary controls. In contrast, expression of the δ isoform mRNA increased in L5 neurons. CaMKII protein decreased following nerve injury in both L4 and L5 populations. Total CaMKII activity measured under saturating Ca2+/CaM conditions was decreased in both L4 and L5 populations, while autonomous CaMKII activity determined in the absence of Ca2+ was selectively reduced in axotomized L5 neurons 21d after injury. Thus, loss of CaMKII signaling in sensory neurons after peripheral nerve injury may contribute to neuronal dysfunction and pain.
Keywords: CaMKII, neuropathic pain, Ca2+ signaling, nerve injury, sensory neuron
Introduction
Intracellular Ca2+ regulates neuronal gene expression, excitability, and synaptic transmission (Berridge, 2006). Following peripheral nerve injury, Ca2+ signaling in sensory neurons is disrupted at several fundamental levels, including depression of resting Ca2+ concentration, depletion of intracellular Ca2+ stores, and amplification of store-operated Ca2+ entry (Fuchs et al., 2005, Rigaud et al., 2009, Gemes et al., 2011). Most critically, voltage-gated Ca2+ channel function is diminished following injury (Hogan et al., 2000, Abdulla and Smith, 2001), leading to reduced activity-induced Ca2+ spikes (Gemes et al., 2010). An important mediator of downstream functional effects may be the multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII). This multimeric serine-threonine kinase translates dynamic depolarization-related Ca2+ spikes into graded levels of sustained catalytic activity through autophosphorylation at the sites Thr286 or 287 (depending on the specific isoform), after which its kinase activity becomes autonomous and independent of Ca2+ levels. By this process, CaMKII retains a molecular memory that functions as a readout of neuronal activity via integrating Ca2+ spike frequency (De Koninck and Schulman, 1998). CaMKII functions in neurons to regulate neurotransmitter synthesis and release, cytoskeletal and ion channel function, and importantly, is implicated in synaptic plasticity (Lisman et al., 2002, Hell, 2014, Shonesy et al., 2014).
Four distinct genes code for a family of highly conserved CaMKII subunit isoforms in eukaryotes (molecular weights ranging from 50 to 72 kDa), termed α, β, γ, and δ (Hudmon and Schulman, 2002, Tombes et al., 2003). CaMKII isoforms are highly homologous in their catalytic and regulatory domains, however variable insertions in the association domain appear to regulate subcellular targeting and CaM binding affinity (Hudmon and Schulman, 2002). These genes display specific expression patterns in different tissues and at different points in development (Beaman-Hall et al., 1992, Li et al., 2001), with the γ and δ isoforms predominantly expressed early in the developing nervous system (Bayer et al., 1999). Although all four isoforms are expressed in neurons (Takeuchi et al., 2004), the α and β isoforms are highly expressed in CNS neurons, whereas the γ, and δ isoforms are highly enriched in astrocytes and expressed throughout the body (Tobimatsu and Fujisawa, 1989, Takeuchi et al., 2000).
The role of CaMKII in the peripheral nervous system and the function different isoforms may play in sensory neuron function and dysfunction has simply not been as well characterized as its counterparts in the CNS and its role in synaptic plasticity and learning. Previous work shows that application of an isoform-nonspecific CaMKII inhibitor decreases neuropathic pain behavior after intrathecal injection (Dai et al., 2005, Hasegawa et al., 2009) or direct DRG application (Chen et al., 2009), pain behavior after nerve injury is not affected in transgenic mice with a point mutation at Thr286Ala selectively in the αCaMKII isoform (Zeitz et al., 2004). Such isoform-specific functional consequences may emerge from the relative abundance of the particular isoforms, since the isoform composition of the complete multimeric CaMKII molecule dictates the frequency of Ca2+ pulses to which it is maximally sensitive (De Koninck and Schulman, 1998, Bayer et al., 2002). Despite these indications of specific roles for the various CaMKII isoforms, there has been little exploration of their differential expression in sensory pathways under physiological conditions or in models of chronic pain.
The first goal of this study was to identify expression levels of different CaMKII isoforms in normal rat DRGs. The α isoform has previously been identified in peripheral neurons (Carlton, 2002), suggesting that this CaMKII is not unique to the CNS. However, the expression level of α relative to the other CaMKII isoforms has not been characterized in the PNS. Secondly, we examined whether chronic pain due to nerve injury is accompanied by a shift in expression of CaMKII isoforms. Plasticity of the expression of CaMKIIα has been demonstrated during pain from tissue inflammation (Carlton, 2002). In the setting of painful peripheral nerve injury, our previous findings have indicated a loss of CaMKII potentiation of K+ currents through ATP-sensitive channels (IK(Ca)) after spinal nerve ligation (SNL) (Kawano et al., 2009), suggesting that CaMKII signaling (i.e. expression or regulation) is diminished with axotomy. In support of this hypothesis, our immunohistochemical characterization indicated a decrease of autophosphorylated CaMKII (pCaMKII) in sensory neuron somata after SNL (Kojundzic et al., 2010). In the present study we examine the influence of injury upon expression of specific CaMKII isoforms using the SNL model, which has two populations of injured neurons. The directly injured neurons of the fifth lumbar (L5) DRG are axotomized at the point of injury in the spinal nerve. The adjacent L4 DRG neurons have intact distal axons, but these axons share fascicles in the sciatic nerve with degenerating detached distal segments of the L5 population, and are therefore exposed to local inflammation. Since the relative contribution of these two populations to the generation of neuropathic pain is unresolved (Gold, 2000), we have examined CaMKII in both the L4 and L5 DRGs.
Experimental Procedures
All methods and use of animals were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Nerve injury model
Male Sprague-Dawley rats (Taconic Farms Inc., Hudson, NY) weighing 160g to 180g were subjected to spinal nerve ligation (SNL) in a manner modified from the original technique (Kim and Chung, 1992). Specifically, rats were anesthetized with 2% isoflurane in oxygen and the right paravertebral region was exposed. After removal of the L6 transverse process, the L5 and L6 spinal nerves were ligated with 6-0 silk suture and transected distal to the ligatures. The fascia was closed with 4-0 resorbable polyglactin suture and the skin closed with staples. Control animals received anesthesia, skin incision and stapling only. After surgery, the rats were returned to their cages and kept under normal housing conditions with access to pellet food and water ad lib.
Sensory testing
Rats underwent sensory testing for hyperalgesic behavior as previously described (Hogan et al., 2004, Wu et al., 2010). Briefly, noxious mechanical stimulation was produced by touching the right plantar skin with a 22G spinal needle with adequate pressure to indent but not penetrate the skin. Whereas control animals respond with only a brief reflexive withdrawal, rats following SNL may display a complex hyperalgesia response that incorporates sustained elevation of the paw, licking, chewing, and grooming. The frequency of hyperalgesia responses out of 10 touches was tabulated for each test day. Sensory testing was carried out 3 days after surgery for animals from which tissue was harvested that day, on days 6 and 7 for animals from which tissue was harvested on day 7, and days 20 and 21 for animals from which tissue was harvest 21 days after surgery. The animals were allowed to rest for 2–3 hours after sensory testing before the tissue was harvested. Statistical analysis of hyperalgesia rates was performed using repeated measures 2-way ANOVA (factors were Day and Injury). Post hoc comparisons were tested for significance by Bonferroni’s test.
Quantitative real-time PCR analysis
Total RNA from the homogenized DRGs was isolated from the aqueous phase following the manufacturer’s instructions using Trizol reagent (Invitrogen, Carlsbad, CA). After DNase treatment, cDNA was synthesized from equal amounts of RNA with random hexamer primers using Superscript III first strand synthesis kit (Invitrogen). Real time PCR analysis was carried out using IQ Syber Green supermix (Biorad Laboratories, Hercules, CA) and specific primers (Table 1) to quantify the cDNA levels of the CaMKII isoform genes (α, β, γ, and δ). Normalization was carried out using the geometric mean of two reference genes, GAPDH and MAPK6, which were chosen for their stability in the context of SNL injury by screening 7 housekeeping genes using the programs geNorm [http://medgen.ugent.be/~jvdesomp/genorm/] and NormFinder [http://www.mdl.dk/publicationsnormfinder.htm]. For each isoform, the fold differences in expression at each time point and injury condition (control, L4 DRG after SNL, and L5 DRG after SNL) were compared to that of the day 3 control samples using the comparative CT method. Statistical analysis of ΔΔCT values used for CaMKII isoforms, normalized with MAPK6 and GAPDH, was performed initially using 2-way ANOVA (factors were Day and Injury), and thereafter by 1-way ANOVA if no main effect for time was observed. Post hoc comparisons were tested for significance by Dunnett’s test. Graphs were plotted using 2−ΔΔCT values for representation of all the isoforms gene expressions.
Table 1.
Isoform-specific CaMKII and reference gene primers.
| Gene Name | Accession | Forward Primer | Reverse Primer | Product Length | Exon spanning | Company |
|---|---|---|---|---|---|---|
| CaMKIIa | NM_012920 | FP:5′TCCGGA GGG AAG AGT GGA GGA AAC 3′ | RP:5′GCT GTC ATT CCA GGG TCG CAC AT 3′ | 197 bp | No | In house |
| CaMKIIb | NM_001042354 | FP:5′GGG GAC TGT CCA CCT ACA GA 3′ | RP:5′TAA CAA GGT GGG TGG GAG AG 3′ | 223 bp | No | Realtime primers.com |
| CaMKIIg | NM_133605 | FP:5′CCA GCG TGC ACC TAA TGG AGC C 3′ | RP:5′TCG TGT AGG CCT CAA AGT CCC CA 3′ | 189 bp | No | In house |
| CaMKIId | NM_012519 | FP:5′CTG GCA CAC CTGGGT ATC TT 3′ | RP:5′CAC TGT GTC CCA TTC TGG TG 3′ | 200 bp | No | Realtime primers.com |
| GAPDH | NM_017008 | FP:5′ AGA CAG CCG CAT CTT CTT GT 3′ | RP: 5′TGA TGG CAA CAA TGT CCA CT 3′ | 142 bp | Yes (exons 1–2) | In house |
| MAPK6 | NM_031622 | FP:5′TAA AGC CAT TGA CAT GTG GG 3′ | RP: 5′TCG TGC ACA ACA GGG ATA GA 3′ | 129 bp | No | In house |
HEK293T Cell Lysates
Lysates from transfected HEK293T cells expressing CaMKII isoforms (α, β, γ, δ) were maintained in DMEM medium (Gibco 11965-092) supplemented with 10% FBS (Hyclone SH30396.03) and 1x Penicillin-Streptomycin-Glutamine (Gibco 10378-016). CaMKII isoforms were transiently transfected using Lipofectamine 2000 (Invitrogen 11668-019) as instructed for 48 hours. Cells were washed 3X with ice-cold phosphate buffered saline and were lysed by sonication in modified RIPA buffer (50mM Tris, pH 8.0, 150mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1mM sodium orthovanadate, 1mM sodium fluoride, 2mM EDTA, 1mM EGTA) containing 1x protease inhibitors (Calbiochem Cat#539137) plus 1x phosphatase inhibitors (Calbiochem Cat#524624). HEK293T lysates were centrifuged for 5 min at 1000g at 4 °C to generate a low-speed supernatant that was aliquoted and stored at −80°C. Total protein concentration of the supernatants were measured by Pierce bicinchoninic acid protein assay kit using bovine serum albumin as a standard (Thermo Scientific 23227). Lysates from adult rodent forebrain and cerebellum tissue were generated using similar methods and used to confirm that known tissue-expression patterns for CaMKII were resolvable using pan-specific Western blotting.
DRG Lysates
To harvest DRGs without influencing CaMKII activation, cardiac perfusion was initiated in anesthetized rats using cold, Ca2+-free buffer, during which DRGs were excised and then frozen in liquid nitrogen. Lysates were subsequently prepared as follows. From 8 rats subjected to SNL, the L4 DRGs were paired to provide 4 samples, and the L5 DRGs were likewise paired to provide 4 samples. The L4 and ipsilateral L5 DRGs from individual control animals were paired to provide 4 control samples. Harvested ganglia were mechanically disrupted using a glass-on-glass homogenizer using 200μl of modified RIPA buffer (as described above). The RIPA buffer was confirmed not to inhibit CaMKII enzymatic activity when diluted using an in vitro kinase assay (described below). After incubation on ice, samples were centrifuged at 1000g for 5 min at 4°C to remove cell debris and stored at −80°C. Total protein concentration of the supernatants were measured by Pierce bicinchoninic acid protein assay kit using bovine serum albumin as a standard (Thermo Scientific 23227).
Western blot analysis
Protein supernatants with equal amount of protein (30 μg) were separated using SDS-PAGE and transferred onto nitrocellulose membrane. After blocking with 10% milk in TBST (Tris buffered saline + 0.1% Tween 20), blots were sequentially probed with primary antibodies (rabbit anti-CaMKII antibody, Abcam ab52476 and rabbit anti-GAPDH antibody, Cell Signaling Technology #2118S) and corresponding secondary antibodies (HRP(horseradish peroxidase)-conjugated goat anti rabbit IgG (Cell Signaling Technology #7074) and goat anti rabbit IgG labeled with IRDye 800CW antibody (Li-Cor, #926-32211). Western Lighting Plus-ECC (Perkin Elmer, #NEL104001EA) or Odyssey Infrared Imager (Li-cor) were used for the detection of the protein bands, respectively. Intensities of protein bands were accessed using Image Studio ver 2.1 (Li-Cor). Total CaMKII amount was normalized based on the signal intensity of GAPDH. One-way ANOVA with Dunnett’s test was performed for control (SS) and treatment groups (L4 and L5). Primary antibodies used in this study are listed in Table 2.
Table 2.
Antibodies used.
| Antibody | Use | Source | Catalogue No. | Dilution | Confirmed Specificity |
|---|---|---|---|---|---|
| Pan CaMKII | Western, IHC | Millipore | 04-1079 | 1:250 | α, β, γ, δA, δC |
| β3-tubulin | IHC | Santa Cruz | sc80016 | 1:600 | |
| Na+, K+- ATPase | IHC | Santa Cruz | sc48345 | 1:600 | |
| glutamine synthetase | IHC | Santa Cruz | sc6640R | 1:800 | |
| β-tubulin I | Western | Sigma-Aldrich | T7816 | 1:5000 | |
| GAPDH | Western | Cell Signaling | 2118S | 1:2000 |
CaMKII catalytic assay
CaMKII catalytic activity was determined using autocamtide-2 (AC-2; KKALRRQETVDAL) with the following reactants as described previously (Hanson and Schulman 1992 JBC 267:17216): 1mM CaCl2, recombinant calmodulin (CaM), 10 mM MgCl2, 100 μM ATP (Sigma #A7699) and γP32 labeled ATP (PerkinElmer #blu502a001mc) for 10 min at 30°C as described (Ashpole et al., 2012a, Ashpole et al., 2013). For autonomous activity measurements, Ca2+/CaM was omitted and replaced by 5 mM EGTA. Reaction mixtures were spotted onto phospho-cellulose paper (Whatman P81) and excess unincorporated [γP32]ATP, removed with multiple washes in 75mM phosphoric acid. P32 incorporation into AC-2 was quantified using a LS6500 Scintillation Counter (Beckman Coulter). The background readings (no AC-2) for each sample were subtracted from their respective Ca2+/CaM-dependent or autonomous activity determinations. The kinase activity is represented as the amount of peptide phosphorylated by the enzyme per μg of total protein for each sample. One-way ANOVA with Dunnett’s test was performed for control (Sham Surgery) and treatment groups (L4 and L5).
Immunohistochemistry
L5 DRGs (n=3) were dissected, post-fixed in Zinc formalin, and processed for paraffin embedding and sectioning. Standard fluorescent immunohistochemistry were performed as described previously (Yu et al., 2004) using appropriate antibodies (Table 2). Briefly, paraffin sections (5 μm thick) were deparaffinized in xylene, rehydrated through graded alcohols and water. After antigen unmasking by incubating sections in 0.5% Triton X-100 for 1h at room temperature, nonspecific antibody binding sites were blocked by incubating the sections with 3% BSA. Staining for the CaMKII was conducted by incubating overnight at 4°C using a rabbit anti-CaMKII antibody (#04-1079, Millipore, Billerica, MA) that has been shown to detect all CaMKII isoforms (Rose and Hargreaves, 2003). Sections were then washed in PBS and incubated for 1h at RT with Alexa Fluor 549-conjugated donkey anti-rabbit second antibody (1:4,000, Jackson ImmunoResearch, West Grove, PA). For co-localization, the following primary antibodies were used: mouse anti-β3-tubulin (sc80016, 1:600, Santa Cruz Biotechnology), mouse anti- Na+, K+-ATPase (sc48345,1:600, Santa Cruz Biotechnology), and rabbit anti-glutamine synthetase (sc6640R, 1:800, Santa Cruz Biotechnology), and the primary antibodies were revealed by the appropriate 488-conjugated secondary antibodies (1:4,000, Jackson ImmunoResearch). After washing with PBS, slides were mounted with Shur/Mount media and covered slipped. In negative control experiments, the incubations with primary antibody were substituted by an equivalent time of incubation in 3% BSA in PBS. The sections were examined and images captured using either conventional fluorescence microscopy (Nikon TE2000-S, Nikon Instruments, Melville, NY, USA) or a confocal microscope (Eclipse TE U200, Nikon Instruments) equipped with EZ C1 laser scanning software with filters suitable for selectively detecting the fluorescence of 488 (green) and 549 (red). For co-localization, images from the same section but showing different antigen signals were overlaid. For correlating CaMKII staining intensity to neuronal size, the full profiles of tubulin-stained neuronal somata with an evident nucleus were traced to determine somatic area using ImageJ (National Institutes of Health, Bethesda MD), which was converted to average diameter. Then average cytoplasmic pixel intensity of each neuron was then determined by separately tracing the cytoplasm alone.
Electron microscopy
A DRG was harvested from a rat, fixed for 1hr in 2.5% glutaraldehyde in 0.1 M cacodylate buffer and post-fixed with 1% OsO4 in buffer for 1hr, washed with distilled water (2×5 min), dehydrated in methanol (50%, 70%, 85%, 95% and 3× 100%) followed by 2× 10min acetonitrile, infiltrated with Epon 812 resin (Shell Chemical, Houston, TX) in acetonitrile 1:1 for 1hr followed by 100% Epon for 3hrs, moved to fresh 100% Epon and polymerized at 70°C overnight. Sections (70nm thick) were cut and stained with saturated uranyl acetate in 50% ethanol and Reynold’s lead citrate. Images were generated using a transmission electron microscope (JEM2100, Japanese Electron Optics Limited, Tokyo) with a high resolution charge-coupled device digital camera (Ultrascan 1000, Gatan Inc., Pleasanton, CA) at 1,000x magnification.
Results
Sensory behavior
For all the protocols considered together, 42 animals received SNL surgery, and 40 control animals had skin incisions alone. Behavior was evaluated prior to surgery as a baseline, and on the day of tissue harvest (3, 7, and 21d) and on the prior day (except for the 3d harvest animals), as an indication of the generation of hyperalgesia. The time course of hyperalgesia behavior (Fig. 1) shows no changes from baseline in the control animals but progressive development of hyperalgesia in the SNL animals.
Figure 1.
Behavioral responses following spinal nerve ligation (SNL). Hyperalgesia developed in rats subjected to SNL but not in sham surgery control rats. The data are represented as frequency of hyperalgesic responses as a percentage total touches, mean ± sem. * P<0.05
Histological evaluation
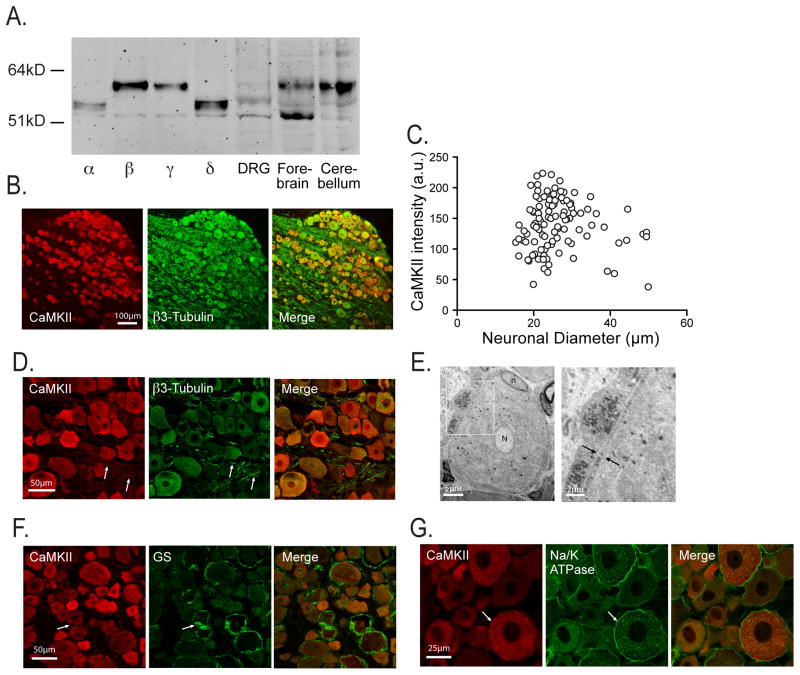
Our examination of enzymatic activity, mRNA levels, and protein levels used entire DRGs, and therefore cannot specify the cellular source of these measures. To address this, we preliminarily examined anatomical distribution of CaMKII in DRG using selective double staining and confocal microscopy. Using a validated pan-isoform CaMKII antibody (Table 2, Fig. 2A), the pattern of CaMKII immunoreactivity (CaMKII-ir) duplicated the profiles of sensory neuron somata identified by β-Tubulin (Fig. 2B). All sizes of neuronal somata showed CaMKII-ir at intensities substantially above background, although generally more intense staining was seen in small neurons (Fig. 2C). Specifically, the average intensity of CaMKII staining for neurons with a diameter less than 35μm (144±4 a.u., n=95) was greater than the average intensity for larger neurons (113±11 a.u., n=12; P<0.05). Neuronal nuclei were variably positive for CaMKII-ir, and there was negligible staining in the fiber tracts (Fig 2D). Satellite glial cells are closely apposed to the neuronal somata and form a very thin layer (Fig. 2E), making fluorescent identification problematic. Selective staining with glutamine synthetase (Weick et al., 2003) (Fig. 2F) showed that CaMKII-ir was not identifiable in the areas positive for this stain. Because of the challenge in resolving the very thin satellite glial cells, we also employed the strategy of staining with an antibody to Na+/K+-ATPase, which identifies the membrane of most DRG somata (Dobretsov et al., 1999). This demonstrated that CaMKII-ir is contained entirely within sensory neurons (Fig. 2G). From these observations, we infer that CaMKII mRNA is preferentially expressed within the DRG neuronal somata in comparison to other DRG cell types.
Figure 2.
Anatomical analysis of CaMKII protein expression. A. A Western immunoblot demonstrates that the pan-CaMKII antibody detects all isoforms in lysates obtained from HEK293T cells over-expressing single recombinant isoforms of CaMKII at distinct molecular weights. Additional lanes show the expression patterns in lysates from rat forebrain and cerebellum. B. Co-staining of DRG sections shows CaMKII immunoreactivity in all neurons identified by β3-tubulin. C. Quantitative analysis shows CaMKII expression in all neurons at levels above background (20 a.u., already subtracted from the plotted cytoplasmic intensities). D. A different section under higher magnification shows variable nuclear CaMKII staining but minimal CaMKII immunoreactivity in fibers (identified by arrows). E. An unstained transmission electron micrograph of a dorsal root ganglion, with outlined area in the left panel shown enlarged in the right panel, demonstrates the very narrow profile of satellite glial cells (between arrows) surrounding neuronal somata. F. Glutamate synthase (GS) immunoreactivity identifies satellite glial cells, which are found not to express CaMKII. E. Sodium/potassium ATPase (Na/K ATPase) identifies the neuronal membrane (arrow), which delimits CaMKII immunoreactivity, demonstrating that CaMKII is expressed predominantly in the cytoplasm of neuronal somata.
Expression of CaMKII isoform mRNAs
In order to confirm primer specificity for quantifying CaMKII mRNA in DRGs, a variety of primer sets were tested against recombinant cDNAs for each individual isoform. The cDNAs for rodent α (Refseq # NM_012920.1), β2 (Refseq # NM_021739.2), δC (Refseq # NM_012519) were used along with the human γb (GenBank: L07044.1) (Nghiem et al., 1993) as representative isoforms for the four CaMKII genes. Primers that proved to be selective for their intended isoform (Table 1) we reused for qPCR analysis of DRG lysates. CT values in control samples indicated that the γ (CT of 24.43±0.15; n=3 for this and other isoforms) and α (Ct: 26.95±0.12) were the most abundant isoforms. Much lower levels were found for β (Ct: 28.99±0.16) and δ (Ct: 28.86±0.20), indicating an approximately 22-fold lower level of these two isoforms compared with γCaMKII.
Comparison of CaMKII isoform mRNA levels across time (Fig. 3) revealed injury-induced depression of the α isoform in both L4 and L5 DRGs after SNL, whereas depression in the γ isoform was seen selectively in the axotomized L5 DRGs. These changes were present as early as day 3 in α and day 7 in γCaMKII, and persisted through day 21. In contrast, β isoform expression was unaffected by injury, while δ levels increased in the L5 ganglia to levels 2.5-fold greater than controls 21 days after SNL. These PCR results complement prior microarray studies that separately identified decreased γ mRNA levels and increased δ levels after nerve injury (Xiao et al., 2002, Valder et al., 2003).
Figure 3.
Expression of different CaMKII gene isoforms after injury. Initial 2-way ANOVA showed no significant time effect for controls. Fold differences of transcript levels in 3, 7 and 21 day control and post-injury samples are calculated by comparison to the respective day controls after normalization with stable reference genes. Samples include control (C), the 4th lumbar dorsal root ganglion after 5th lumbar spinal nerve ligation (SNL L4), and the axotomized 5th lumbar dorsal root ganglion after SNL (SNL L5), at 3, 7 and 21 days after surgery. Isoforms include α (A), β, (B), γ (C), and δ (D). Number in the bars indicate n for DRGs, each in a separate experiment; data are mean ± sem. * P<0.05, ** P<0.01, ***P< 0.001.
Levels of total CaMKII protein
Our finding of mRNA expression of all four CaMKII isoforms predicted that a broad expression profile for isoform in rodent DRGs. We initially tested this using a pan-specific CaMKII antibody selected from several available antibodies by confirming its recognition of all isoforms through testing against lysates obtained from HEK293T cells over-expressing single recombinant isoforms of CaMKII (Fig. 2A). Using this antibody, we observed that DRG lysates contain multiple immunoreactive proteins bands indicative of expression of each transfected CaMKII isoform as well as the endogenous HEK293T cell CaMKII as a tissue control. We also show the expression profile of α versus β isoforms of CaMKII in the forebrain and cerebellum, which demonstrates, as shown previously for mRNA and protein levels, that αCaMKII is the predominant form in forebrain, whereas the βCaMKII isoform is the predominant isoform in cerebellum (Miller and Kennedy, 1985, Beaman-Hall et al., 1992, Brocke et al., 1995). We further attempted to determine the specific protein contribution of each CaMKII isoform in the DRG lysate using isoform-selective antibodies. To this end, we characterized multiple antibodies against HEK lysates selectively expressing single CaMKII isoforms, but we failed to identify commercially available antibodies that were adequately selective for quantification of specific isoform expression levels. We therefore quantified the effect of injury on the total amount of CaMKII available for signaling regardless of isoform by using the pan-CaMKII antibody that we had confirmed as recognizing all four isoforms (Fig. 2A). This revealed a depressed level of CaMKII in both the L4 and L5 sensory neuron population 21 days after SNL (Fig. 4A–C).
Figure 4.
Protein expression of CaMKII in the DRG. A. Western immunoblot shows protein expression using pan-CaMKII antibody to detect total CaMKII expression in DRG neurons. B. Western shows GAPDH expression as a protein loading control for each sample. C. Quantification of CaMKII expression in DRGs (n=4 for each group) normalized to GAPDH signal intensity shows decrease in SNL L4 (4th lumbar dorsal root ganglion after 5th lumbar spinal nerve ligation) and SNL L5, compared to control (C) ** P<0.01.
CaMKII enzymatic activity
CaMKII mRNA levels have been shown previously to reflect isoform expression levels in brain tissue (Beaman-Hall et al., 1992). To directly evaluate the functional consequences of injury-induced changes in CaMKII mRNA and protein, we tested CaMKII enzymatic activity in lysates by quantifying phosphorylation of the CaMKII-specific substrate autocamtide-2 peptide (AC2) (Hanson and Schulman, 1992). Two measures were taken. Maximum recruitable CaMKII activity (total AC2-dependent activity) was determined in the presence of saturating concentrations of Ca2+ and calmodulin, whereas autonomous activity of CaMKII was determined by testing in the absence of Ca2+ and CaM and presence of 5 mM EGTA. Total activity 21 days following SNL (Fig. 5A) showed decreased activity in both the L4 and L5 neurons, mirroring the depressed levels of immunoreactive protein measured by Western blot and decrease in the α–, β–, and γCaMKII mRNA levels seen with qPCR (Fig. 4C). The autonomous fraction of total activity represents the activation state of the CaMKII population, which reflects prior neuronal activity. We found that autonomous activity was significantly reduced in axotomized L5 neurons both in raw data and autonomous activity normalized to total activity (Fig. 5B and 5C), suggesting that mechanisms maintaining autonomous CaMKII activity (e.g. Thr286/287 autophosphorylation, oxidation and glycosylation) are impaired with neuronal injury.
Figure 5.

Effect of injury on CaMKII enzymatic activity. A. Total AC2 phosphorylation by DRG lysates normalized to total μg protein is diminished in SNL L4 (4th lumbar dorsal root ganglion after 5th lumbar spinal nerve ligation) and SNL L5, compared to control (C). B. Autonomous AC2 phosphorylation (EGTA replacing Ca2+/CaM) by DRG lysates is diminished in SNL L5 compared to control (C). C. Percentage of autonomous AC2 phosphorylation generated by normalizing to total AC2 phosphorylation is diminished in SNL L5 compared to control (C). n=4 for each group; * P<0.05, ** P<0.01.
Discussion
Native CaMKII isolated from the brain is composed of homomeric and heteromeric mixed isoform holoenzymes, suggesting that subunit expression may play an important role in regulating CaMKII function in cells (Vallano, 1989). A number of different observations support the concept that CaMKII isoforms play unique functions in the nervous system (Colbran, 2004), including: 1) monocular deprivation leads to a decrease in the expression levels of the α isoform in the visual cortex (Tighilet et al., 1998); 2) βCaMKII regulates neurite extension and morphogenesis through its ability to interact with actin cytoskeleton, whereas, αCaMKII lacks this function (Fink et al., 2003); 3) αCaMKII supports excitatory post-synaptic currents, whereas, βCaMKII accelerates their decay (Thiagarajan et al., 2002); and 4) although less is known about the role of δ and γ isoforms of CaMKII in neurons, γCaMKII was recently shown to play a critical role in cytoplasmic to nuclear shuttling of CaM in superior cervical ganglion neurons (Ma et al., 2014). Our study was undertaken to explore CaMKII isoform expression in sensory pathways under physiological conditions or in models of chronic pain.
In this study, we show that DRGs of adult rats express the full complement of all four CaMKII isoforms. Judged by threshold cycle values in qPCR, γCaMKII is the dominant mRNA isoform, followed by α, with much lower levels of β and δ. Adult expression levels of CaMKII isoform mRNA in DRG have not previously been quantified. Over the relatively short observation interval of our study (between 5 weeks and 8 weeks of life), no changes in expression were observed in control animals. In the central nervous system, the α and β isoforms are predominantly localized in neurons. Further developmental and subcellular distributions of these two isoforms are seen in the brain (Ouimet et al., 1984, Erondu and Kennedy, 1985, McGuinness et al., 1985, Burgin et al., 1990, Ochiishi et al., 1998) and spinal cord (Terashima et al., 1994), which is compatible with the view that different CaMKII isoforms have particular tissue-specific activities. Although β, γ, and δ isoforms can also be expressed in many tissues, including neurons and support cells in the brain (Ouimet et al., 1984, Tobimatsu and Fujisawa, 1989, Sakagami and Kondo, 1993), prior reports of CaMKII expression in DRGs have shown immunohistochemical colocalization with neuronal markers (Bruggemann et al., 2000, Carlton, 2002, Carlton and Hargett, 2002, Kojundzic et al., 2010), and our present observations indicate that all four (α,β,γ,δ) CaMKII isoforms are expressed in DRGs.
Sensory neurons are a heterogeneous population, and neurons transmitting various sensory modalities have different typical firing frequencies (Leem et al., 1993). Activation of CaMKII is sensitive to the frequency of pulsatile Ca2+ elevations (Eshete and Fields, 2001), and the relative mix of different isoforms in CaMKII hetero-multimers tunes the peak sensitivity of the complex to a particular range of Ca2+ pulse frequency (De Koninck and Schulman, 1998, Bayer et al., 2002), so the expression of diverse CaMKII isoforms may equip sensory neurons to customize decoding sensitivity according to their particular firing frequency during natural stimulation. Furthermore, the frequency of neuronal activity may itself regulate relative expression of the different isoforms (Thiagarajan et al., 2002), so bidirectional signaling may optimize the matching of isoform expression to neuronal activity, by which CaMKII-sensitive processes are optimally tuned. Thus, isoforms may not be uniformly distributed among sensory neurons of different functional categories. For instance, prior anatomic studies using antibody to αCaMKII showed minimal staining of large neurons and little of no staining of neuronal nuclei (Carlton, 2002, Carlton and Hargett, 2002), whereas our present findings show cytoplasmic CaMKII expression in all sensory neuronal somata and variable nuclear expression with a pan antibody capable of sampling all four CaMKII isoforms.
Our examination of the effects of neuronal injury on mRNA levels shows that expression in the DRG of the dominant γ and αCaMKII isoforms decrease significantly after SNL injury, while those of the δ isoform are increased. Because of the relatively low overall expression of δCaMKII, judging by transcript level, it would appear that the loss of γCaMKII, and possibly αCaMKII, may be the dominant effect of injury upon CaMKII gene expression at the macroscopic level. This interpretation is supported by our other finding of reduced total CaMKII protein measured both by immunoblot and by total recruitable catalytic activity. However, our findings do not eliminate the possibility of important functional effects of increased δ isoform expression found by observed by us using qPCR and by others in microarray studies (Xiao et al., 2002, Valder et al., 2003), which could contribute to the functional changes in sensory neurons seen with nerve injury if it is expressed in a particular cellular or subcellular pattern. Our observations also indicate that when antibodies are used to evaluate CaMKII expression or anatomy after nerve injury, isoform specificity of the antibody will be a critical factor that affects the experimental results. Additional studies with verified antibodies will be needed to characterize the cell background and subcellular distribution for each CaMKII isoform in DRG tissue.
Calcium-independent activity that persists after the resolution of the initiating intracellular Ca2+ spikes can be maintained by either autophosphorylation (Thr286 in α and Thr287 in β,γ,δ isoforms) or specific oxidation of Met residues in the regulatory domain (Saitoh and Schwartz, 1985, Thiel et al., 1988, Fong et al., 1989). To quantify overall CaMKII activity, we examined catalytic activity directly, using a CaMKII-specific peptide substrate (Hanson and Schulman, 1992). There is no readily available means to identify catalytic activity on an isoform-specific basis using in vitro kinase assays to our knowledge. Accordingly, our findings regarding effects of nerve injury on CaMKII catalytic function represent the combined effect on all isoforms. Calcium-induced total activity was significantly decreased 21 days after nerve injury in both the axotomized L5 SNL neurons and the adjacent L4 neurons, indicating a decreased amount of CaMKII available for activation. It is also conceivable that, in addition to transcriptional regulation of CaMKII activity, there could be post-transcriptional modulation that limits CaMKII activity following nerve injury. Possibilities include enhanced autoregulatory features such as Thr305/306 autophosphorylation (Colbran, 1993), or possibly aggregation/translocation (Churn et al., 1995, Kolb et al., 1995, Morioka et al., 1995, Hudmon et al., 1996) participates in limiting Ca2+/calmodulin-stimulated activity.
The present data are not sufficient to provide a specific mechanism that accounts for the loss of CaMKII expression after peripheral nerve injury. We note, however, that effects were maximal after SNL in the axotomized L5 neuronal population. Nonetheless, CaMKII expression was depressed to a more modest degree in adjacent L4 neurons, as shown by depressed transcript levels of αCaMKII and depressed total CaMKII protein measured by immunoblot and by functional catalytic activity. In the SNL model, DRG neurons are intentionally axotomized at the L5 level, but some unintended neuronal injury takes place in the L4 population after SNL of the L5 spinal nerve, indicated by expression of the marker ATF3 in the L4 DRG (Djouhri et al., 2006), and even after sham exposure of the L5 DRG without nerve transection (Shortland et al., 2006). Such injury responses may reflect axotomy since the dorsal primary rami of the L4 and L5 spinal nerves and their branches are directly traumatized by the paravertebral surgical exposure necessary to reach the DRGs. But additionally, ATF-3 is expressed in sensory neurons when they are exposed to physiological stress and various mediators such as cytokines (Hai et al., 1999), which are a recognized part of the Wallerian degeneration phenomenon that affects L4 neurons following L5 SNL (Wu et al., 2002). So it is possible that our findings in both L4 and L5 neurons after SNL are the result of neuronal injury and the various associated events of loss of target-derived neurotrophins, exposure to inflammatory mediators, and numerous other injury-induced changes. Alternatively, the distinct processes of inflammation affecting the L4 neurons versus axotomy of nearly all L5 neurons may both depress CaMKII expression, but to a different degree. This interpretation is supported by the dissimilar findings in autonomous catalytic activity, which is selectively decreased in the L5 neurons after SNL. Since our tissue harvest technique was designed to avoid stimulation of neuronal activity, we believe this low level of CaMKII activation reflects reduced neuronal firing in vivo prior to anesthesia and perfusion. This would be a feature of essentially all L5 neurons after SNL, which lack a peripheral receptive field and therefore have no natural activity and have minimal ectopic activity (Djouhri et al., 2006), while almost all L4 neurons will continue to receive input from their receptive field, supporting a normal level of pCaMKII.
We have observed that CaMKII function is necessary for full function of voltage-gated Ca2+ channels (Tang et al., 2012, Kostic et al., 2014). In the absence of normal CaMKII signaling, diminished cytoplasmic Ca2+ is available for activation of Ca2+-dependent K+ channels, resulting in neuronal hyperexcitability in PNS and CNS neurons (Ashpole et al., 2012b, Tang et al., 2012). Thus, the depressed levels of CaMKII function we have identified in axotomized sensory neurons may prime them for responding to mechanical stimulation (Wall and Devor, 1983, Tal et al., 1999), or activation by firing of adjacent neuronal somata (Devor and Wall, 1990).
Conclusion
The expression of γCaMKII, the predominant isoform in DRG neurons, is decreased after injury. Total expression of CaMKII protein decreases in both the L4 and L5 neuronal populations, but Ca2+-independent autonomous CaMKII function is decreased selectively in axotomized SNL L5 neurons. Thus, the loss of CaMKII activity induced by nerve injury is consistent with the depressed enzyme protein levels measured with the pan-antibody, which could be contributed by both transcriptional and post-transcriptional mechanisms. These findings are compatible with an important pathophysiological role of CaMKII in producing neuronal dysfunction and pain after peripheral nerve injury.
Acknowledgments
This study was supported by NIH grant NS42150 (to Q. Hogan) and R01NS078171 (to A. Hudmon).
Abbreviations
- SNL
spinal nerve ligation
- CaMKII
Ca2+/Calmodulin-dependent protein kinase II
- CNS
central nervous system
- PNS
peripheral nervous system
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MAPK6
mitogen-activated protein kinase 6
- ATP
adenosine triphosphate
- DRG
dorsal root ganglion
- ANOVA
analysis of variance
- mRAN
messenger ribonucleic acid
- CaMKII-ir
CaMKII immunoreactivity
- qPCR
quantitative polymerase chain reaction
- EGTA
ethylene glycol tetraacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. Journal of neurophysiology. 2001;85:644–658. doi: 10.1152/jn.2001.85.2.644. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Chawla AR, Martin MP, Brustovetsky T, Brustovetsky N, Hudmon A. Loss of calcium/calmodulin-dependent protein kinase II activity in cortical astrocytes decreases glutamate uptake and induces neurotoxic release of ATP. J Biol Chem. 2013;288:14599–14611. doi: 10.1074/jbc.M113.466235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, Bers DM, Hudmon A. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J Biol Chem. 2012a;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Song W, Brustovetsky T, Engleman EA, Brustovetsky N, Cummins TR, Hudmon A. Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J Biol Chem. 2012b;287:8495–8506. doi: 10.1074/jbc.M111.323915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Schulman H. Alternative splicing modulates the frequency-dependent response of CaMKII to Ca(2+) oscillations. Embo J. 2002;21:3590–3597. doi: 10.1093/emboj/cdf360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- Beaman-Hall CM, Hozza MJ, Vallano ML. Detection of mRNAs encoding distinct isoenzymes of type II calcium/calmodulin-dependent protein kinase using the polymerase chain reaction. J Neurochem. 1992;58:1259–1267. doi: 10.1111/j.1471-4159.1992.tb11337.x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Brocke L, Srinivasan M, Schulman H. Developmental and regional expression of multifunctional Ca2+/calmodulin-dependent protein kinase isoforms in rat brain. J Neurosci. 1995;15:6797–6808. doi: 10.1523/JNEUROSCI.15-10-06797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann I, Schulz S, Wiborny D, Hollt V. Colocalization of the mu-opioid receptor and calcium/calmodulin-dependent kinase II in distinct pain-processing brain regions. Brain Res Mol Brain Res. 2000;85:239–250. doi: 10.1016/s0169-328x(00)00265-5. [DOI] [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM. Localization of CaMKIIalpha in rat primary sensory neurons: increase in inflammation. Brain Res. 2002;947:252–259. doi: 10.1016/s0006-8993(02)02932-3. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Stereological analysis of Ca(2+)/calmodulin-dependent protein kinase II alpha -containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;448:102–110. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- Chen Y, Luo F, Yang C, Kirkmire CM, Wang ZJ. Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J Pharmacol Exp Ther. 2009;330:650–659. doi: 10.1124/jpet.109.152165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, Limbrick D, Sombati S, DeLorenzo RJ. Excitotoxic activation of the NMDA receptor results in inhibition of calcium/calmodulin kinase II activity in cultured hippocampal neurons. J Neurosci. 1995;15:3200–3214. doi: 10.1523/JNEUROSCI.15-04-03200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem. 1993;268:7163–7170. [PubMed] [Google Scholar]
- Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wang H, Ogawa A, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Ca2+/calmodulin-dependent protein kinase II in the spinal cord contributes to neuropathic pain in a rat model of mononeuropathy. Eur J Neurosci. 2005;21:2467–2474. doi: 10.1111/j.1460-9568.2005.04091.x. [DOI] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Devor M, Wall PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. Journal of neurophysiology. 1990;64:1733–1746. doi: 10.1152/jn.1990.64.6.1733. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobretsov M, Hastings SL, Stimers JR. Non-uniform expression of alpha subunit isoforms of the Na+/K+ pump in rat dorsal root ganglia neurons. Brain Res. 1999;821:212–217. doi: 10.1016/s0006-8993(98)01361-4. [DOI] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshete F, Fields RD. Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons. J Neurosci. 2001;21:6694–6705. doi: 10.1523/JNEUROSCI.21-17-06694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Fong YL, Taylor WL, Means AR, Soderling TR. Studies of the regulatory mechanism of Ca2+/calmodulin-dependent protein kinase II. Mutation of threonine 286 to alanine and aspartate. J Biol Chem. 1989;264:16759–16763. [PubMed] [Google Scholar]
- Fuchs A, Lirk P, Stucky C, Abram SE, Hogan QH. Painful nerve injury decreases resting cytosolic calcium concentrations in sensory neurons of rats. Anesthesiology. 2005;102:1217–1225. doi: 10.1097/00000542-200506000-00023. [DOI] [PubMed] [Google Scholar]
- Gemes G, Bangaru ML, Wu HE, Tang Q, Weihrauch D, Koopmeiners AS, Cruikshank JM, Kwok WM, Hogan QH. Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury. J Neurosci. 2011;31:3536–3549. doi: 10.1523/JNEUROSCI.5053-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemes G, Rigaud M, Koopmeiners AS, Poroli MJ, Zoga V, Hogan QH. Calcium signaling in intact dorsal root ganglia: new observations and the effect of injury. Anesthesiology. 2010;113:134–146. doi: 10.1097/ALN.0b013e3181e0ef3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS. Spinal nerve ligation: what to blame for the pain and why. [Review] [22 refs] Pain. 2000;84:117–120. doi: 10.1016/s0304-3959(99)00309-7. [DOI] [PubMed] [Google Scholar]
- Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J Biol Chem. 1992;267:17216–17224. [PubMed] [Google Scholar]
- Hasegawa S, Kohro Y, Tsuda M, Inoue K. Activation of cytosolic phospholipase A2 in dorsal root ganglion neurons by Ca2+/calmodulin-dependent protein kinase II after peripheral nerve injury. Mol Pain. 2009;5:22. doi: 10.1186/1744-8069-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Hogan QH, McCallum JB, Sarantopoulos C, Aason M, Mynlieff M, Kwok WM, Bosnjak ZJ. Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain. 2000;86:43–53. doi: 10.1016/s0304-3959(99)00313-9. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Aronowski J, Kolb SJ, Waxham MN. Inactivation and self-association of Ca2+/calmodulin-dependent protein kinase II during autophosphorylation. J Biol Chem. 1996;271:8800–8808. doi: 10.1074/jbc.271.15.8800. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Kawano T, Zoga V, Gemes G, McCallum JB, Wu HE, Pravdic D, Liang MY, Kwok WM, Hogan Q, Sarantopoulos C. Suppressed Ca2+/CaM/CaMKII-dependent K(ATP) channel activity in primary afferent neurons mediates hyperalgesia after axotomy. Proc Natl Acad Sci U S A. 2009;106:8725–8730. doi: 10.1073/pnas.0901815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kojundzic SL, Puljak L, Hogan Q, Sapunar D. Depression of Ca(2+)/calmodulin-dependent protein kinase II in dorsal root ganglion neurons after spinal nerve ligation. J Comp Neurol. 2010;518:64–74. doi: 10.1002/cne.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb SJ, Hudmon A, Waxham MN. Ca2+/calmodulin kinase II translocates in a hippocampal slice model of ischemia. J Neurochem. 1995;64:2147–2156. doi: 10.1046/j.1471-4159.1995.64052147.x. [DOI] [PubMed] [Google Scholar]
- Kostic S, Pan B, Guo Y, Yu H, Sapunar D, Kwok WM, Hudmon A, Wu HE, Hogan QH. Regulation of voltage-gated Ca currents by Ca/calmodulin-dependent protein kinase II in resting sensory neurons. Mol Cell Neurosci. 2014;62C:10–18. doi: 10.1016/j.mcn.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. Journal of neurophysiology. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Li G, Laabich A, Liu LO, Xue J, Cooper NG. Molecular cloning and sequence analyses of calcium/calmodulin-dependent protein kinase II from fetal and adult human brain. Sequence analyses of human brain calciuum/calmodulin-dependent protein kinase II. Mol Biol Rep. 2001;28:35–41. doi: 10.1023/a:1011951814898. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA, Tsien RW. gammaCaMKII shuttles Ca(2)(+)/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell. 2014;159:281–294. doi: 10.1016/j.cell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness TL, Lai Y, Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem. 1985;260:1696–1704. [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem. 1985;260:9039–9046. [PubMed] [Google Scholar]
- Morioka M, Fukunaga K, Nagahiro S, Kurino M, Ushio Y, Miyamoto E. Glutamate-induced loss of Ca2+/calmodulin-dependent protein kinase II activity in cultured rat hippocampal neurons. J Neurochem. 1995;64:2132–2139. doi: 10.1046/j.1471-4159.1995.64052132.x. [DOI] [PubMed] [Google Scholar]
- Nghiem P, Saati SM, Martens CL, Gardner P, Schulman H. Cloning and analysis of two new isoforms of multifunctional Ca2+/calmodulin-dependent protein kinase. Expression in multiple human tissues. J Biol Chem. 1993;268:5471–5479. [PubMed] [Google Scholar]
- Ochiishi T, Yamauchi T, Terashima T. Regional differences between the immunohistochemical distribution of Ca2+/calmodulin-dependent protein kinase II alpha and beta isoforms in the brainstem of the rat. Brain Res. 1998;790:129–140. doi: 10.1016/s0006-8993(98)00058-4. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, McGuinness TL, Greengard P. Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proc Natl Acad Sci U S A. 1984;81:5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Weyker PD, Cruikshank JM, Kawano T, Wu HE, Hogan QH. Axotomy depletes intracellular calcium stores in primary sensory neurons. Anesthesiology. 2009;111:381–392. doi: 10.1097/ALN.0b013e3181ae6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553:303–309. doi: 10.1113/jphysiol.2003.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Schwartz JH. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. The Journal of cell biology. 1985;100:835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami H, Kondo H. Differential expression of mRNAs encoding gamma and delta subunits of Ca2+/calmodulin-dependent protein kinase type II (CaM kinase II) in the mature and postnatally developing rat brain. Brain Res Mol Brain Res. 1993;20:51–63. doi: 10.1016/0169-328x(93)90109-3. [DOI] [PubMed] [Google Scholar]
- Shonesy BC, Jalan-Sakrikar N, Cavener VS, Colbran RJ. CaMKII: a molecular substrate for synaptic plasticity and memory. Prog Mol Biol Transl Sci. 2014;122:61–87. doi: 10.1016/B978-0-12-420170-5.00003-9. [DOI] [PubMed] [Google Scholar]
- Shortland PJ, Baytug B, Krzyzanowska A, McMahon SB, Priestley JV, Averill S. ATF3 expression in L4 dorsal root ganglion neurons after L5 spinal nerve transection. Eur J Neurosci. 2006;23:365–373. doi: 10.1111/j.1460-9568.2005.04568.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Nomura K, Fukunaga K. Differential subcellular distribution of Ca2+/calmodulin-dependent protein kinase II isoforms in the striatum and NG108-15 cells. J Neurosci Res. 2004;75:480–490. doi: 10.1002/jnr.20010. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Yamamoto H, Fukunaga K, Miyakawa T, Miyamoto E. Identification of the isoforms of Ca(2+)/Calmodulin-dependent protein kinase II in rat astrocytes and their subcellular localization. J Neurochem. 2000;74:2557–2567. doi: 10.1046/j.1471-4159.2000.0742557.x. [DOI] [PubMed] [Google Scholar]
- Tal M, Wall PD, Devor M. Myelinated afferent fiber types that become spontaneously active and mechanosensitive following nerve transection in the rat. Brain Research. 1999;824:218–223. doi: 10.1016/s0006-8993(99)01190-7. [DOI] [PubMed] [Google Scholar]
- Tang Q, Bangaru ML, Kostic S, Pan B, Wu HE, Koopmeiners AS, Yu H, Fischer GJ, McCallum JB, Kwok WM, Hudmon A, Hogan QH. Ca(2)(+)-dependent regulation of Ca(2)(+) currents in rat primary afferent neurons: role of CaMKII and the effect of injury. J Neurosci. 2012;32:11737–11749. doi: 10.1523/JNEUROSCI.0983-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima T, Ochiishi T, Yamauchi T. Immunohistochemical detection of calcium/calmodulin-dependent protein kinase II in the spinal cord of the rat and monkey with special reference to the corticospinal tract. J Comp Neurol. 1994;340:469–479. doi: 10.1002/cne.903400403. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Thiel G, Czernik AJ, Gorelick F, Nairn AC, Greengard P. Ca2+/calmodulin-dependent protein kinase II: identification of threonine-286 as the autophosphorylation site in the alpha subunit associated with the generation of Ca2+-independent activity. Proc Natl Acad Sci U S A. 1988;85:6337–6341. doi: 10.1073/pnas.85.17.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighilet B, Hashikawa T, Jones EG. Cell- and lamina-specific expression and activity-dependent regulation of type II calcium/calmodulin-dependent protein kinase isoforms in monkey visual cortex. J Neurosci. 1998;18:2129–2146. doi: 10.1523/JNEUROSCI.18-06-02129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem. 2003;87:560–573. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- Vallano ML. Separation of isozymic forms of type II calcium/calmodulin-dependent protein kinase using cation-exchange chromatography. J Neurosci Methods. 1989;30:1–9. doi: 10.1016/0165-0270(89)90067-8. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Weick M, Cherkas PS, Hartig W, Pannicke T, Uckermann O, Bringmann A, Tal M, Reichenbach A, Hanani M. P2 receptors in satellite glial cells in trigeminal ganglia of mice. Neuroscience. 2003;120:969–977. doi: 10.1016/s0306-4522(03)00388-9. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci. 2002;22:7746–7753. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain. 2010;11:280–286. doi: 10.1016/j.jpain.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wessels A, Chen J, Phelps AL, Oatis J, Tint GS, Patel SB. Late gestational lung hypoplasia in a mouse model of the Smith-Lemli-Opitz syndrome. BMC Dev Biol. 2004;4:1. doi: 10.1186/1471-213X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz KP, Giese KP, Silva AJ, Basbaum AI. The contribution of autophosphorylated alpha-calcium-calmodulin kinase II to injury-induced persistent pain. Neuroscience. 2004;128:889–898. doi: 10.1016/j.neuroscience.2004.07.029. [DOI] [PubMed] [Google Scholar]