Abstract
Background
Patients with primary hyperoxaluria (PH) overproduce oxalate which is eliminated via the kidneys. If end stage kidney disease develops they are at high risk for systemic oxalosis, unless adequate oxalate is removed during hemodialysis to equal or exceed ongoing oxalate production. The purpose of this study was to validate a method to measure oxalate removal in this unique group of dialysis patients.
Methods
Fourteen stable patients with a confirmed diagnosis of PH on hemodialysis were included in the study. Oxalate was measured serially in hemodialysate and plasma samples in order to calculate rates of oxalate removal. Hemodialysis regimens were adjusted according to a given patient's historical oxalate production, amount of oxalate removal at dialysis, residual renal clearance of oxalate, and plasma oxalate levels.
Results
After a typical session of hemodialysis, plasma oxalate was reduced by 78.4±7.7%. Eight patients performed hemodialysis 6 times a week, two patients 5 times a week and three patients 3 times a week. Combined oxalate removal by hemodialysis and the kidneys was sufficient to match or exceed endogenous oxalate production. After a median period of 9 months, pre-dialysis plasma oxalate was significantly lower than initially (75.1±33.4 mmol/L vs. 54.8±46.6 mmol/L, P=0.02).
Conclusion
This methodology can be used to individualize the dialysis prescription of PH patients to prevent oxalosis during the time they are maintained on hemodialysis, and to reduce risk of oxalate injury to a transplanted kidney.
Keywords: End stage kidney disease, Hemodialysis, Oxalosis, Primary Hyperoxaluria
Introduction
The dicarboxylic acid oxalate is an end product of liver glyoxalate and glycerate metabolism that cannot be further metabolized, and is therefore excreted in the urine [1,2]. Patients with primary hyperoxaluria (PH) have markedly increased oxalate production related to defects in one of at least 3 genes that code for enzymes important in the metabolic pathways. Since oxalate is primarily eliminated via the kidney, PH patients often develop calcium oxalate kidney stones, nephrocalcinosis, and kidney damage. Once kidney failure ensues, oxalate is progressively retained in the body resulting in systemic soft tissue and bone deposition, often leading to early death from oxalosis. Transplantation as soon as possible after the onset of kidney failure is recommended for this patient group. However, waiting times for suitable donor organs often prolong the time until transplantation can be performed, placing the patient at risk of serious complications of systemic oxalosis. In addition, oxalate accumulated in tissues will pose risk to the transplanted kidney as it is slowly mobilized and excreted following transplantation [3]. Therefore, if a patient with PH must be maintained on dialysis it is important to tailor the prescription so that adequate amounts of oxalate are removed [4]. To minimize risk of systemic oxalate deposition, plasma oxalate should be kept below the levels associated with calcium oxalate supersaturation in plasma; that is < 30 μmol/L [2]. However, it is recognized that standard 3 times/week hemodialysis does not achieve sustained levels below this threshold in PH patients unless they have significant residual renal function [5-7]. Peritoneal dialysis is even less effective in oxalate removal [8]. There is little information about how to manage the dialysis of PH patients with end stage kidney disease (ESKD), especially those who await transplantation. In this study we measured plasma and dialysate oxalate in PH patients, and estimated the amount of oxalate removed. Using this data the frequency and/or duration of HD were adjusted as necessary to exceed the estimated daily oxalate production of a given patient.
Subjects and Methods
Patients and sample handling
Patients from the Mayo Clinic Hyperoxaluria Center with a confirmed diagnosis of type 1 PH and ESKD on hemodialysis were considered for hemodialysis oxalate removal studies. This study was approved by Institutional Review Board of Mayo Clinic. Of 17 available patients, 3 were not included in this report because of lack of follow-up data after individualization of the dialysis prescription (N=2), and having undergone a prior liver-only transplantation that corrected the oxalate metabolic defect (N=1). The remaining 14 patients were diagnosed based on hepatic enzyme analysis conforming AGT deficiency (N=3) or genetic testing for AGXT mutations (N=11). Eleven patients receiving pyridoxine remained on stable doses (5-10 mg/kg/d) throughout the study, two patients did not take pyridoxine because of no response to it and none had initiation of pyridoxine during this time. Thirteen patients were on high flux hemodialysis (HD) with Polyflux Revaclear dialyzer (1.4 m2, polyarylethersulfone and polyvinylpyrrolidone membrane, Gambro) which was performed for 3-4 h each session. One female patient was on home hemodialysis with a NxStage System One (NxStage Medical Inc.) performed for 4 h every day. HD prescriptions were adjusted according to the historical oxalate production, mass of oxalate removed, urinary clearance of oxalate and pre-dialysis plasma oxalate.
Blood was sampled at the blood inlet of the dialyzer at the start of dialysis and each hour thereafter. During each one hour period, a constant percentage of the spent hemodialysate was collected and mixed, with one ml sent for oxalate concentration measurement (Oxalatedialysate). Oxalate removal was calculated as Oxalatedialysate × Volumedialysate. KT/V was measured in 9 patients during the dialysis session by an on-line clearance monitor [9]. Previous pharmacokinetic studies of oxalate in man by injecting 14C-oxalate has illustrated 94% of the administered tracer were recovered unchanged in urine in first 24h [10], suggesting renal excretion of oxalate can represent the daily oxalate production. Thus, the historical daily oxalate production was estimated by the 24h urine oxalate determination of a given patient when the GFR was greater than 50 ml/min/1.73m2, since the kidneys can no longer keep up with oxalate production once the GFR declines to less than 40-50 ml/min/1.73m2 [11]. In 4 patients, historical oxalate production was not available due to renal failure at initial presentation. Patients with urine output also collected a 24-hour urine within one week of the dialysis session to calculate renal excretion of oxalate.
Oxalate measurement
Plasma and urine oxalate was measured using an enzymatic oxalate oxidase method (Trinity Biotech, Wicklow, Ireland), as previously described [12]. This enzymatic method for plasma [12] and urine [13] was also validated for dialysate fluid. The test is based upon oxalate reduction by oxalate oxidase yielding hydrogen peroxide, which in the presence of peroxidase reacts with an indamine dye. This colored end point is measured using a sensitive Beckman Coulter DU800 Spectrophotometer at 590 nm.
For assay validation, fresh and waste (exposed to patients) dialysate fluid was obtained and stored for no more than 12 hours at 4°C before testing. To evaluate analyte stability, aliquots were prepared, and the oxalate values obtained immediately at time zero were compared to those under various storage conditions, including multiple freeze thaw cycles.
Data analysis
Analyses were completed using the statistical programs JMP® version 9.0.1 (SAS Institute Inc.) and Microsoft Excel version 2003 (Microsoft Corporation). All values are expressed as the mean and standard error of the mean (M±SEM). Student's t -tests were used to compare changes between two groups. Paired t-tests were used when comparing changes before and after the treatment. The relationship between KT/V and reduction rate of plasma oxalate was estimated by simple linear regression. Trends in urine oxalate excretion following transplant were estimated using slopes derived from within patient linear regression. P values <0.05 were considered to be significant.
Results
Dialysate oxalate validation
Precision estimates (CV) ranged from 1.1% at a dialysate oxalate level of 19 μmol/L to 10% at 2 μmol/L. Accuracy, evaluated by spiking a dialysate sample with standard material, was 94 to 110%. Linearity studies in dialysate fluid yielded measured/expected oxalate signal of 89-109% over a signal range of 2 to 66 μmol/L. Samples could be diluted up to 8-fold with water. Limit of quantification (LOQ) was set to be 2 μmol/L. Proper treatment of dialysate samples after collection is critical, since in certain patient samples oxalate levels increased over the course of 3 days unless samples were acidified to pH 2.5-3.0 within 4 h. After acidification samples are stable for a week of collection when maintained refrigerated (4°C) or frozen at or below -20°C.
Oxalate removal kinetics
The average age of the 13 HD patients was 38±19 years. There were 6 males and 7 females. Five patients had evidence of systemic oxalosis after a full examination of bone, heart and retina. Twelve patients had urine output (2797±1307 mL/24h) at the time of study, with an average urinary oxalate excretion of 1.1±0.8 mmol/24h.
Mean reduction in plasma oxalate was 78.4±7.7% after one HD session, with a mean pre and post dialysis plasma oxalate of 70.6±38.4 μmol/L and 13.3±5.9 μmol/L, respectively. In order to graphically pool data, plasma and dialysate oxalate values were normalized to the pre-dialysis plasma oxalate level. The average profile for all 13 patients (Figure 1A) demonstrates that plasma oxalate fell by approximately 50% during the first hour, and was about 20% of pre-dialysis levels by hour 4. Dialysate oxalate levels were about one-half of the plasma oxalate levels at each time point. Thus, the net rate of oxalate removal progressively fell during dialysis, albeit relatively gradually. As shown in Figure 1B, pooled data from patients at 3 different blood flow rates suggests that higher rates augment the rate of plasma oxalate fall, although the three curves were not statistically different in this small sample (P=0.76).
Figure 1. Plasma and dialysate oxalate kinetics.
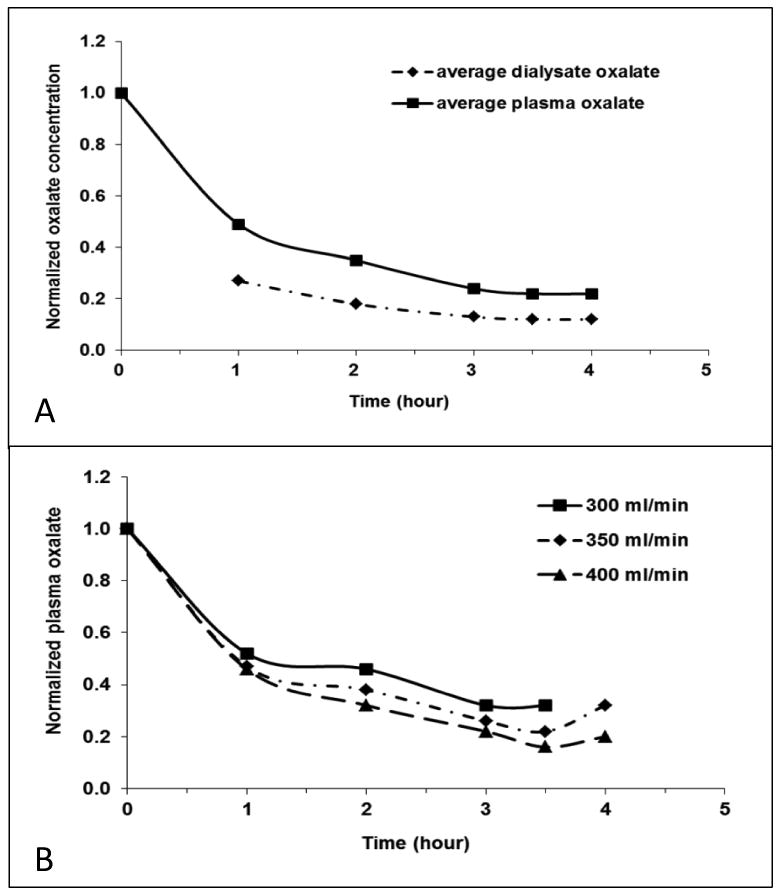
Panel A: The average profile of plasma and dialysate change during one dialysis session. Normalized plasma and dialysate oxalate concentrations were calculated by plasma and dialysate oxalate of each hour in proportion to pre-dialysis plasma oxalate. Panel B: Influence of blood flow rate on oxalate removal. Dialysate flow rate was 800 ml/min for each measurement. The plasma oxalate levels were slightly lower at a blood flow rate of 400 ml/min (n=7) compared to 350 ml/min (n=4) or 300 ml/min (n=1) (P=0.76). The Analysis of Covariance (ANCOVA) was used to compare the slope of different blood flow rate.
There were 9 patients in whom the measured on-line KT/V was 1.52±0.42. Simple linear regression demonstrated a good correlation of KT/V with the final percent reduction in plasma oxalate (R2=0.55, P=0.02). However, there was minimal correlation between KT/V and total oxalate removal by dialysis (R2=0.15, P=0.29).
Modification of Dialysis strategy
A sample dialysis oxalate clearance report from patient A is shown in Table 1. In this patient, urine oxalate excretion at the time of study was 0.6 mmol/24h. The amount removed in three and one-half hours of dialysis was 1.73 mmol. The historical oxalate production rate from this patient (when the kidney function was normal) was 1.75 mmol/day, or 12.25 mmol/wk, so a minimum of 5-6 dialysis sessions per week would be required for its removal. For the 4 patients whose historical urine oxalate excretion was unavailable, one can estimate that oxalate production is in the range of 2.0-2.5 mmol/day for type 1 PH according to published reports [14].
Table 1. Oxalate removal during a dialysis treatment (patient A).
| Pre Dialysis |
1st hr | 2nd hr | 3rd hr | End Dialysis |
|
|---|---|---|---|---|---|
| Time (min) | 0 | 60 min | 120 min | 180 min | 210 min |
| Patient weight (Kg) | 85.6 | X | X | X | 84.2 |
| Arterial Plasma Oxalate (μmol/L) | 54.1 | 22.9 | 13.6 | 10.6 | 11.7 |
| Dialysate Fluid Oxalate (μmol/L) | < 1.0 | 13.7 | 10.9 | 6.3 | 10.3 |
| Dialysate Volume (L) | X | 48 | 48 | 48 | 24 |
| Oxalate removed this time period (mmol) | X | 0.66 | 0.52 | 0.30 | 0.25 |
The blood flow rate was 300 ml/min and dialysate flow rate was 800 ml/min. Oxalate removed through this time interval was calculated by dialysate fluid oxalate × dialysate volume. Total oxalate removed over 3.5 hours was 1.73 mmol.
Based on the method above, eight patients performed HD 6 times a week, two patients 5 times a week and three patients 3 times a week. The blood flow rate was 300∼400 ml/min (average 365±38 ml/min) and dialysate flow rate was 600∼800 ml/min (average 769±75 ml/min). Each patient's total oxalate removal, estimated oxalate production, previous and adjusted HD prescriptions as well as plasma oxalate change are listed in Table 2. For the 9 patients who had historical urine oxalate excretion data, total oxalate removal by HD and the kidneys exceeded or equaled the estimated oxalate production. The average oxalate removed by HD of all patients was 11.13±6.88 mmol/wk. Combined the oxalate removed by HD and urine was 18.34±6.95 mmol/wk. Given the estimated weekly historical oxalate generation of 14.04±4.09 mmol/wk, the current hemodialysis regimen was efficient enough to overcome endogenous oxalate production. After a median period of 9 months (1-29 months) individualized HD, pre-dialysis plasma oxalate was significantly lower than the initial values (75.1±33.4 mmol/L vs. 54.8±46.6 mmol/L, P=0.02).
Table 2. Oxalate removal by HD and urine, historical oxalate production, previous and adjusted HD prescription and plasma oxalate before and after individualized HD.
| patient | Recent urine oxalate excretion (mM/d) |
Oxalate removal by HD/session (mM) |
Previous HD (/wk) |
Adjusted HD (/wk) |
Total oxalate removal (mM/wk) |
historical oxalate production (mM/wk) |
adjusted HD prescription | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pox before (μM/L) |
Pox after (μM/L) |
|||||||
| A | 0.6 | 1.73 | NAa | 6 | 14.58 | 12.25 | 54.1 | 40.2b |
| B | 1.13 | 0.58 | NAa | 5 | 10.79 | 13.72 | 34 | 24.6b |
| C | 1.17 | 4.04 | NAa | 6 | 32.46 | 14.28 | 107 | 47.3b |
| D | 0.71 | 2.97 | NAa | 6 | 22.81 | 7.42 | 142.7 | 97.7b |
| E | 2.06 | 1.16 | NAa | 6 | 21.40 | 10.78 | 63.9 | 32.6b |
| F | 0 | 3.03 | 3 | 6 | 18.20 | NAc | 84.9 | 26.2b |
| G | 0.26 | 2.25 | 3 | 6 | 15.30 | NAc | 108.7 | 49.3b |
| H | 0 | 2.04 | 3 | 6 | 12.21 | 13.23 | 93.5 | 45.2b |
| I | 0.6 | 2.38 | 3 | 5 | 16.08 | 15.19 | 85.4 | 90.2 |
| J | 1.52 | 0.78 | NAa | 3 | 12.97 | NAc | 26.6 | 18.2 |
| K | 3.22 | 1.69 | NAa | 3 | 27.61 | 21.84 | 48.4 | 46 |
| L | 0.95 | 0.97 | NAa | 3 | 9.57 | NAc | 47.2 | 9.6 |
| M | 1.17 | 2.71 | 5 | 6 | 24.43 | 17.64 | 80.2 | 184.8b |
HD was started at the time of study so there was no previous HD prescription.
patients who did transplantation.
historical oxalate production was unavailable due to renal failure at presentation.
Nine patients received transplantation, including one kidney alone transplant in a patient who was fully pyridoxine responsive and eight combined liver-kidney transplant (LKT) after a median period of 6 months (1-27 months) on HD. Except for patient M whose plasma oxalate increased because of a rapid decline in residual renal function (urine oxalate decreased from 1.17 mmol/24h to 0.19mmol/24h before transplantation), the other 8 patients' pre-dialysis plasma oxalate before transplantation was 45.4±23.1 μmol/L, which is significantly lower than the initial pre-dialysis plasma oxalate (before the dialysis prescription was individualized) at 86.1±34.7 μmol/L, P<0.01. In 8 patients that underwent LKT, one patient's renal graft lost function at 3 months after transplantation because of acute tubular necrosis and started hemodialysis again. In the remaining 7 patients, urine oxalate levels dropped gradually and normalized in a median time of 6 months (Figure 2).
Figure 2. Urine oxalate values from 7 PH patients after LKT.
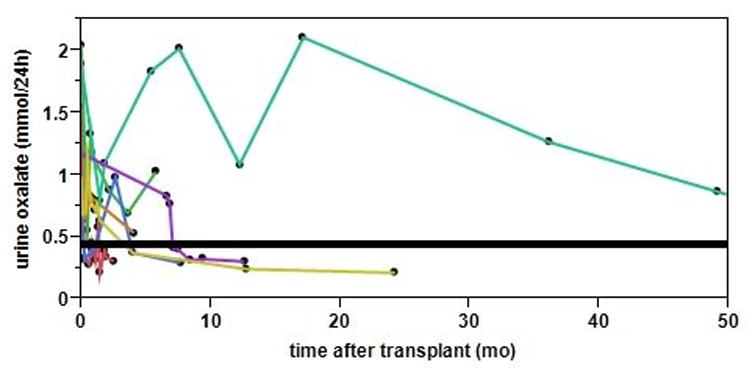
Each line represents one patient. Normal urine oxalate is <0.46 mmol/24h (black solid line). The median time required for normalization of urine oxalate was 6 months.
Results of home daily hemodialysis using NxStage System
One additional female patient (31 years old) was on home hemodialysis. Blood and dialysate flow rates were 390 ml/min and 150 ml/min, respectively. Mean pre-dialysis plasma oxalate was 51.25±10.54 μmol/L with a post-dialysis oxalate of 13.60±1.56 μmol/L averaged over 2 runs (net decrease 82%). Oxalate removed from each session was 1.03±0.20 mmol. A recent 24h urine oxalate was 0.56 mmol. Combining the oxalate removed by HD and urine, total oxalate removal was 1.59±0.20 mmol/day. Her historical estimated oxalate production was 1.45 mmol/day, suggesting that for this patient with moderate oxalate production and residual renal oxalate excretion, daily home HD for 4h was sufficient.
Discussion
Although PH patients produce 3-10 times the normal amount of oxalate, this amount can be efficiently eliminated by well-functioning kidneys. However, since oxalate is largely eliminated by filtration, as glomerular filtration rate (GFR) falls plasma oxalate levels progressively rise. Oxalate distributes throughout the extracellular fluid, and when its concentration exceeds calcium oxalate supersaturation (about 30 μmol/L at normal ionized calcium concentrations), spontaneous crystallization can occur leading to systemic oxalosis [2]. One major site of deposition is bone, and biopsies of the iliac crest have documented localized bone oxalate levels 300X normal in PH patients on dialysis. These levels progressively increase with time on dialysis [15]. Clinical consequences include anemia refractory to erythropoiesis-stimulating agents and osteodystrophy resulting in fractures. Oxalate deposition in the myocardium leads to cardiomyopathy and in the conduction system is a cause of fatal arrhythmias. Involvement of multiple organ systems by oxalosis leads to increasing morbidity and death. As oxalate deposits in tissues, blood levels plateau and no longer increase to accurately reflect the increasing total body oxalate burden [5].
Unfortunately, kidney failure is common in PH. Early studies reported that 50% of PH patients developed ESKD by 15 years of age and 80% by age 30. Recent improvements in the diagnosis and management of PH have increased the median survival without ESKD to 33 years [16]. Our patients developed ESKD at an average age of 37 years, which is in good agreement with these recent reports. Oxalate accumulation in body tissues not only results in significant morbidity or death, gradual mobilization of tissue and bone oxalate stores after kidney transplantation produces hyperoxaluria and places the kidney allograft at risk for oxalate injury over many months or years [17]. Therefore, it is important to develop methods to manage this group of patients when ESKD ensues, including how to effectively dialyze them until transplantation can be performed.
Oxalate is reasonably well-removed by dialysis (clearance about 75% that of urea) [18], as evidenced by the correlation of KT/V with fall in plasma oxalate levels. Even so, the amount removed in a standard 3 day per week hemodialysis regimen cannot keep up with production rates [18], in part because oxalate is only removed from the blood compartment, and equilibration of plasma oxalate with extravascular compartments like bone is slower. These considerations likely explain the poor correlation of KT/V with total oxalate removal. Furthermore, as plasma oxalate levels rapidly decline during a dialysis session, the gradient for oxalate diffusion falls, and the amount removed falls with each time period in a given dialysis session (Figure 1A). A considerable rebound also typically occurs as soon as each session of HD is concluded [6]. Thus, more frequent hemodialysis sessions are more efficient than longer, less frequent dialysis regimens. Our data suggested that higher blood flow rate resulted in a greater reduction of plasma oxalate, consistent with the results of Illies and colleagues [6]. Accordingly, a maximal blood flow rate should be applied.
Oxalate production by the liver is markedly increased in all patients with PH, but the degree of overproduction varies substantially from one PH patient to another, in part related to genotype [16,19]. A method to accurately measure oxalate removal during dialysis is helpful to calculate the appropriate dialysis dose. In this paper we describe a reasonably simple method that employs hourly samples of dialysate and comparison to estimated historical oxalate production of the patient. If unknown, one can estimate that oxalate production is often in the range of 1.5-2.0 mmol/day (types 2 and 3 PH) to 2.0-2.5 mmol/day (type 1 PH) [14]. Pyridoxine responsiveness should also be considered. The genotype G170R for example confers complete or partial response to pyridoxine [19]. A pre-dialysis plasma oxalate well below the predicted calcium oxalate supersaturation threshold of 30 μmol/L would also be reassuring confirmation that the patient is receiving adequate oxalate removal [2].
Although we measured plasma and dialysate oxalate hourly during the treatment, in order to assess the kinetics of oxalate removal, in practice this protocol might be simplified. Determination oxalate in the hourly or total hemodialysate removed and pre- and post-dialysis plasma oxalate could suffice if the only question is how much oxalate was being removed. It is also important to measure 24 hour urine oxalate elimination, since in some patients significant amounts can be eliminated by the kidneys for a long period time on dialysis. It is also necessary to modify the dialysis prescription if GFR declines. In our subgroup of patients with residual urine output, urinary oxalate elimination accounted for a significant amount of total oxalate removal (10%∼66%) that was helpful for managing the patients. Thus all measures should be taken to maintain this urinary excretion, such as avoidance of intravascular volume contraction, including that caused by hypotension and aggressive dialysis ultrafiltration. Indeed, among PH patients who maintain urine output despite ESKD, administration of intravenous fluid during each dialysis session can be helpful.
Based on these measurements, the total amount of oxalate eliminated in this patient group was sufficient to equal estimated endogenous oxalate production. This may be in part due to the highly permeable membrane we used, since previous studies suggested that dialysis oxalate removal may be increased by the use of a dialyzer with a larger surface and a high-flux membrane [7]. After the individualized HD, pre-dialysis plasma oxalate significantly decreased and urine oxalate dropped faster after liver transplantation. In a previous report of PH patients after LKT in 2001 [20], the median time required until urine oxalate reached normal range was 14 months, which was somewhat longer than the time (6 months) in our study (P=0.11 by Wilcoxon test, patients' data obtained from DSM). Thus individualized intense dialysis regimens in PH patients can not only reduce risk for systemic deposition of oxalate during dialysis, but also may shorten the time required for resolution of hyperoxaluria following liver transplantation and improve transplantation outcome. Although dialysis is not an ideal long-term treatment for PH patients with ESKD especially when patients lose urine output, appropriate adjustment of dialysis regimen should be considered in view of liver and kidney transplantation since tissue store of oxalate will expose the new kidney to the damaging effects of hyperoxaluria[3,17].
The NxStage system which uses sterile dialysis fluid in bags and a single extracorporeal circuit cartridge with a polysulfone dialysis is a generally safe and efficient method of dialysis [21,22]. The one patient who used Nxstage System in our study received sufficient oxalate removal for her relatively low production rates, even though oxalate removed by Nxstage was only 1.03±0.20 mmol/L, significantly lower than the patients using a Polyflux Revaclear dialyzer. This individual also had some residual renal clearance of oxalate (0.6 mmol/day). This is probably due to the relatively low dialysate flow rates (150 ml/min) and small volume of dialysate employed in this home system. Whether or not patients with higher oxalate production rates can be safely managed by Nxstage dialysis remains to be assessed.
This study also demonstrates that oxalate can be accurately measured in dialysate using an oxalate oxidase-based assay adapted from a sensitive plasma method. Sample handling is important in order to achieve accurate results. In particular, acidification within 4 hours is essential to prevent non-enzymatic production of oxalate, as has been well-described during measurement of oxalate in plasma [12]. In addition, samples should be maintained frozen (-20°C) or refrigerated (4°C) after acidification. Frozen, non-acidified samples can be transported or stored for 3 days prior acidification with expected signal change below 15%.
In conclusion, we have analytically validated a method to accurately measure oxalate in hemodialysis, and provide an example how this can be applied to patients with hyperoxaluria. Dialysis regimens of PH patients on hemodialysis should be adjusted based on plasma, dialysate oxalate and urine oxalate values to make sure that there is enough oxalate removed to minimize the risk of systemic oxalosis.
Acknowledgments
We would like to thank the Mayo Clinic Renal Function Laboratory staff for their help with sample analysis. This study was supported by the Rare Kidney Stone Consortium (U54KD083908), a member of the NIH Rare Diseases Clinical Research Network (RDCRN), funded by the NIDDK and the National Center For Advancing Translational Sciences (NCATS), the Mayo Hyperoxaluria Center, the Mayo Foundation, and by Shanghai Top Priority Key Clinical Disciplines Construction Project (XT).
Footnotes
Conflict of interest statement: All the authors declared no competing interests.
References
- 1.Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, Fargue S, Groothoff J, Harambat J, Hoppe B, Jamieson NV, Kemper MJ, Mandrile G, Marangella M, Picca S, Rumsby G, Salido E, Straub M, van Woerden CS. Primary hyperoxaluria type 1: Indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant. 2012;27:1729–1736. doi: 10.1093/ndt/gfs078. [DOI] [PubMed] [Google Scholar]
- 2.Hoppe B, Kemper MJ, Bokenkamp A, Portale AA, Cohn RA, Langman CB. Plasma calcium oxalate supersaturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int. 1999;56:268–274. doi: 10.1046/j.1523-1755.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Ruder H, Otto G, Schutgens RB, Querfeld U, Wanders RJ, Herzog KH, Wolfel P, Pomer S, Scharer K, Rose GA. Excessive urinary oxalate excretion after combined renal and hepatic transplantation for correction of hyperoxaluria type 1. Eur J Pediatr. 1990;150:56–58. doi: 10.1007/BF01959482. [DOI] [PubMed] [Google Scholar]
- 4.Canavese C, Petrarulo M, Massarenti P, Berutti S, Fenoglio R, Pauletto D, Lanfranco G, Bergamo D, Sandri L, Marangella M. Long-term, low-dose, intravenous vitamin c leads to plasma calcium oxalate supersaturation in hemodialysis patients. Am J Kidney Dis. 2005;45:540–549. doi: 10.1053/j.ajkd.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Marangella M, Petrarulo M, Cosseddu D, Vitale C, Linari F. Oxalate balance studies in patients on hemodialysis for type i primary hyperoxaluria. Am J Kidney Dis. 1992;19:546–553. doi: 10.1016/s0272-6386(12)80833-x. [DOI] [PubMed] [Google Scholar]
- 6.Illies F, Bonzel KE, Wingen AM, Latta K, Hoyer PF. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 2006;70:1642–1648. doi: 10.1038/sj.ki.5001806. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Quillard M, Takahashi S, Nguyen-Khoa M. Oxalate removal by daily dialysis in a patient with primary hyperoxaluria type 1. Nephrol Dial Transplant. 2001;16:2407–2411. doi: 10.1093/ndt/16.12.2407. [DOI] [PubMed] [Google Scholar]
- 8.Watts RW, Veall N, Purkiss P. Oxalate dynamics and removal rates during haemodialysis and peritoneal dialysis in patients with primary hyperoxaluria and severe renal failure. Clin Sci (Lond) 1984;66:591–597. doi: 10.1042/cs0660591. [DOI] [PubMed] [Google Scholar]
- 9.Kuhlmann U, Goldau R, Samadi N, Graf T, Gross M, Orlandini G, Lange H. Accuracy and safety of online clearance monitoring based on conductivity variation. Nephrol Dial Transplant. 2001;16:1053–1058. doi: 10.1093/ndt/16.5.1053. [DOI] [PubMed] [Google Scholar]
- 10.Hautmann R, Osswald H. Pharmacokinetic studies of oxalate in man. Invest Urol. 1979;16:395–398. [PubMed] [Google Scholar]
- 11.Edvardsson VO, Goldfarb DS, Lieske JC, Beara-Lasic L, Anglani F, Milliner DS, Palsson R. Hereditary causes of kidney stones and chronic kidney disease. Pediatr Nephrol. 2013 doi: 10.1007/s00467-012-2329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladwig PM, Liedtke RR, Larson TS, Lieske JC. Sensitive spectrophotometric assay for plasma oxalate. Clin Chem. 2005;51:2377–2380. doi: 10.1373/clinchem.2005.054353. [DOI] [PubMed] [Google Scholar]
- 13.Koch GH, Strong FM. Determination of oxalate in urine. Anal Biochem. 1969;27:162–171. doi: 10.1016/0003-2697(69)90227-9. [DOI] [PubMed] [Google Scholar]
- 14.Milliner DS, Wilson DM, Smith LH. Phenotypic expression of primary hyperoxaluria: Comparative features of types i and ii. Kidney Int. 2001;59:31–36. doi: 10.1046/j.1523-1755.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- 15.Marangella M, Vitale C, Petrarulo M, Tricerri A, Cerelli E, Cadario A, Barbos MP, Linari F. Bony content of oxalate in patients with primary hyperoxaluria or oxalosis-unrelated renal failure. Kidney Int. 1995;48:182–187. doi: 10.1038/ki.1995.283. [DOI] [PubMed] [Google Scholar]
- 16.Harambat J, Fargue S, Acquaviva C, Gagnadoux MF, Janssen F, Liutkus A, Mourani C, Macher MA, Abramowicz D, Legendre C, Durrbach A, Tsimaratos M, Nivet H, Girardin E, Schott AM, Rolland MO, Cochat P. Genotype-phenotype correlation in primary hyperoxaluria type 1: The p.Gly170arg agxt mutation is associated with a better outcome. Kidney Int. 2010;77:443–449. doi: 10.1038/ki.2009.435. [DOI] [PubMed] [Google Scholar]
- 17.Bergstralh EJ, Monico CG, Lieske JC, Herges RM, Langman CB, Hoppe B, Milliner DS. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10:2493–2501. doi: 10.1111/j.1600-6143.2010.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marangella M, Petrarulo M, Mandolfo S, Vitale C, Cosseddu D, Linari F. Plasma profiles and dialysis kinetics of oxalate in patients receiving hemodialysis. Nephron. 1992;60:74–80. doi: 10.1159/000186708. [DOI] [PubMed] [Google Scholar]
- 19.Monico CG, Rossetti S, Olson JB, Milliner DS. Pyridoxine effect in type i primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 2005;67:1704–1709. doi: 10.1111/j.1523-1755.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 20.Monico CG, Milliner DS. Combined liver-kidney and kidney-alone transplantation in primary hyperoxaluria. Liver Transpl. 2001;7:954–963. doi: 10.1053/jlts.2001.28741. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein SL, Silverstein DM, Leung JC, Feig DI, Soletsky B, Knight C, Warady BA. Frequent hemodialysis with nxstage system in pediatric patients receiving maintenance hemodialysis. Pediatr Nephrol. 2008;23:129–135. doi: 10.1007/s00467-007-0649-1. [DOI] [PubMed] [Google Scholar]
- 22.Kraus M, Burkart J, Hegeman R, Solomon R, Coplon N, Moran J. A comparison of center-based vs. Home-based daily hemodialysis for patients with end-stage renal disease. Hemodial Int. 2007;11:468–477. doi: 10.1111/j.1542-4758.2007.00229.x. [DOI] [PubMed] [Google Scholar]


