Abstract
Myc is a pleiotropic basic helix–loop–helix leucine zipper transcription factor that coordinates expression of the diverse intracellular and extracellular programs that together are necessary for growth and expansion of somatic cells1. In principle, this makes inhibition of Myc an attractive pharmacological approach for treating diverse types of cancer. However, enthusiasm has been muted by lack of direct evidence that Myc inhibition would be therapeutically efficacious, concerns that it would induce serious side effects by inhibiting proliferation of normal tissues, and practical difficulties in designing Myc inhibitory drugs. We have modelled genetically both the therapeutic impact and the side effects of systemic Myc inhibition in a preclinical mouse model of Ras-induced lung adenocarcinoma by reversible, systemic expression of a dominant-interfering Myc mutant. We show that Myc inhibition triggers rapid regression of incipient and established lung tumours, defining an unexpected role for endogenous Myc function in the maintenance of Ras-dependent tumours in vivo. Systemic Myc inhibition also exerts profound effects on normal regenerating tissues. However, these effects are well tolerated over extended periods and rapidly and completely reversible. Our data demonstrate the feasibility of targeting Myc, a common downstream conduit for many oncogenic signals, as an effective, efficient and tumour-specific cancer therapy.
Myc is deregulated and overexpressed in most cancer cells, where it hijacks the diverse intracellular and extracellular regenerative programs that drive normal cell expansion. Consistent with this, de-activation of Myc in established, Myc-induced transgenic tumours triggers proliferative arrest and re-differentiation of tumour cells, and collapse of the tumour microenvironment and vasculature, usually resulting in rapid tumour regression2–7. Although this indicates that Myc might be a good therapeutic target, there are caveats. First, Myc exerts its biological influence through protein–protein and protein–DNA interactions that have proven difficult to disrupt with small molecules. Second, aberrant Myc expression in most human cancers is usually not due to mutation in the Myc gene itself but a consequence of its induction by ‘upstream’ oncogenic signals. The therapeutic utility of inhibiting Myc when its aberrant expression is a consequence, not a cause, of oncogenesis is unclear. Finally, Myc is essential for proliferation and stem cell compartment maintenance of regenerative adult tissues such as the gastrointestinal tract, skin and bone marrow. Hence, blocking Myc function systemically might trigger devastating and irreversible side effects. Together, such concerns greatly undermine the credibility of Myc inhibition as an anticancer strategy.
Myc-dependent transactivation requires heterodimerization with its bHLHZip partner protein Max8–10. Dimerization with Max is also essential for Myc proliferative and oncogenic functions8,11 and its inhibition has been shown to have potential therapeutic value12,13. To model both the therapeutic impact and side effects of Myc inhibition in vivo, we constructed a mouse in which endogenous Myc function may be systemically and reversibly inhibited in tissues of adult animals through inducible expression of the dominant interfering Myc bHLHZip dimerization domain mutant Omomyc. Omomyc has four designed amino acid substitutions that facilitate homodimerization with all three oncogenic Myc proteins (c-Myc, N-Myc and L-Myc) but result in little, if any, interaction with Mad proteins. Omomyc dimerization denies Max access to Myc14 and, because Myc–Omomyc heterodimers cannot bind Myc–Max E-box consensus recognition elements, it efficiently blocks Myc-dependent transcriptional activation15. Omomyc expression reverses Myc-induced transformation in vitro15 and Myc-driven tumorigenesis in vivo16.
We conditionally expressed Omomyc using the highly promiscuous cytomegalovirus (CMV) early promoter, which drives relatively high levels of expression in multiple tissue types17–19. The Omomyc coding sequence was placed downstream of a tetracycline-responsive promoter element (TRE) and mice harbouring the TRE-Omomyc transgene were then crossed into a background expressing the rtTA transactivator from the CMV early promoter20. Omomyc expression was then induced in TRE-Omomyc;CMVrtTA double transgenic animals by administration of doxycycline in their drinking water. Real-time polymerase chain reaction with reverse transcription (RT–PCR) confirmed that Omomyc expression was detectable in all tested tissues of TRE-Omomyc;CMVrtTA double transgenic mice on doxycycline treatment, and was absent from both TRE-Omomyc and CMVrtTA single transgenic mice, as well as TREOmomyc; CMVrtTA double transgenic mice not treated with doxycycline (data not shown). Despite some variation between different tissues, steady-state Omomyc mRNA levels were demonstrably higher than those of endogenous c-myc in most tissues (intestine, kidney, pancreas, heart and lung) and comparable in the rest (for example, spleen and skin) (Supplementary Fig. 1).
To ascertain the therapeutic potential of inhibiting endogenous Myc function in cancer, we chose a well-characterized and validated lung cancer mouse model in which tumorigenesis is initiated by oncogenic activation of endogenous Kras21,22, a common mutation in human non-small-cell lung cancers. Kras is activated in this LSLKrasG12D model by inhalation of adenovirus expressing Cre recombinase, which sporadically excises a transcriptional stop element in bronchioalveolar duct junction (BADJ) epithelial cells, triggering expression of oncogenic KrasG12D driven from the endogenous Kras promoter22. Tumorigenesis involves development of hyperplasia/ adenoma 4–6 weeks after Kras activation followed by sporadic progression of some lesions to adenocarcinoma. The resulting mouse lung tumours closely resemble their human counterparts22,23. To assess the contribution of endogenous Myc to the initiation, early and late phases of Kras-induced lung tumorigenesis, we crossed switchable TRE-Omomyc;CMVrtTA mice into the heterozygous LSL-KrasG12D lung cancer model. To ascertain whether inhibition of Myc prevents initiation of KrasG12D-induced lung tumours, 5 × 107 plaque-forming units (p.f.u.) of Cre-recombinase-expressing adenovirus was administered intranasally to LSL-Kras;TRE-Omomyc; CMVrtTA mice and Omomyc induced after 24 h and thereafter maintained for 4 weeks. Mice were then killed and lung morphology, cell proliferation and apoptosis assessed immunohistochemically. For simplicity, only representative controls are shown: doxycycline-treated CMVrtTA mice exemplify all strains lacking an activatable KrasG12D allele, whereas untreated LSLKras; TRE-Omomyc;CMVrtTA mice represent all strains of mice with activated KrasG12D but no Omomyc. All mice expressing KrasG12D without Omomyc (Fig. 1a, LSL-Kras;TRE-Omomyc;CMVrtTA –doxycycline) developed multiple hyperplasias and atypical adenomatous hyperplasias (present in 33±5.2% of total scored BADJs). By contrast, lesions were absent from all mice expressing KrasG12D together with Omomyc (LSL-Kras;TRE-Omomyc; CMVrtTA+doxycycline). Overall, Omomyc expression significantly suppressed the fraction of cells proliferating (Ki67-positive) at the BADJs (Fig. 1b), consistent with the notion that endogenous Myc is required for proliferation of KrasG12D-induced early-stage tumour cells. No apoptotic (TdT-mediated dUTP nick end labelling (TUNEL)-positive) cells were present in the BADJs of lungs from mice in any of the test or control groups (data not shown).
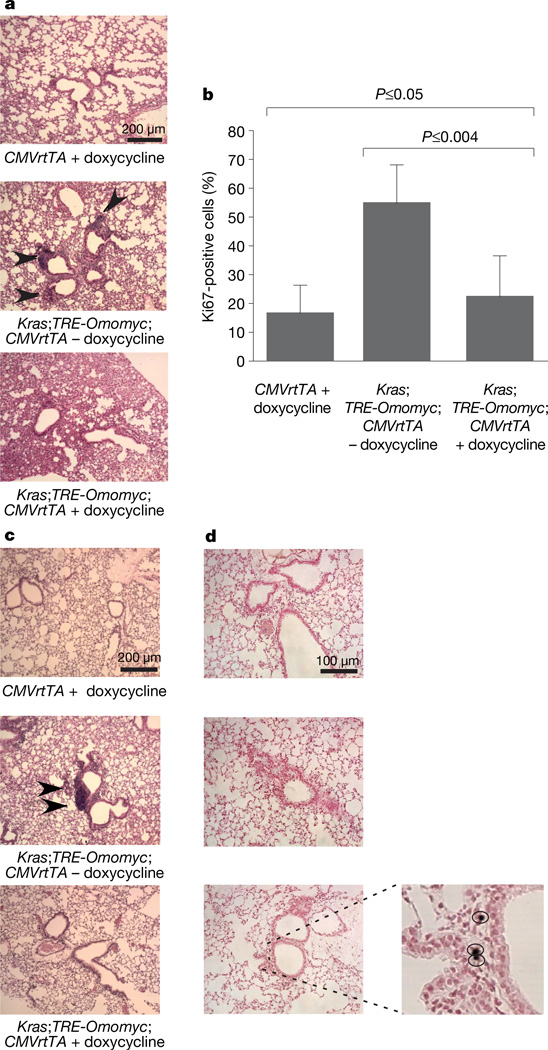
Figure 1. Endogenous Myc function is required for formation and maintenance of early-stage Kras-induced lung hyperplasias/adenomas.
a, Representative haematoxylin-and-eosin-stained sections of lungs from CMVrtTA single transgenic mice (CMVrtTA+doxycycline), untreated Kras;TRE-Omomyc;CMVrtTA triple transgenic mice (Kras;TRE-Omomyc; CMVrtTA − doxycycline) and doxycycline-treated LSL-Kras;TRE-Omomyc;CMVrtTA triple transgenic mice (Kras;TRE-Omomyc;CMVrtTA+doxycycline), 4 weeks after infection with adenoviral Cre. Hyperplastic lesions are indicated by black arrowheads. b, Graphical representation of total BADJ cells scored as proliferating (Ki67-positive). Error bars represent standard deviation derived from approximately 100 BADJs per mouse. At least three mice were used per series. c, Myc inhibition triggers regression of early-stage lung adenomas. Haematoxylin and eosin staining of lungs from mice treated or not with doxycycline for 1 week, starting 6 weeks after Cre-recombinase-expressing adenovirus infection, is shown. A small adenoma is indicated by black arrowheads. d, TUNEL staining reveals positive cells in Omomyc and Ras co-expressing samples (Kras;TRE-Omomyc;CMVrtTA+doxycycline), but not in untreated or single transgenic controls (Kras;TRE-Omomyc;CMVrtTA −doxycycline and CMVrtTA +doxycycline). Higher magnification of positive cells is shown in the insert on the right.
To assess the impact of blocking endogenous Myc on maintenance of early-stage Ras-induced lung adenomas, KrasG12D was activated in LSL-Kras;TRE-Omomyc;CMVrtTA mice and nascent tumours allowed to develop for 6 weeks. Representative mice from cohorts of all strains were then killed and examined histologically. All mice harbouring activated KrasG12D exhibited multiple bronchiolar hyperplasias and adenomatous hyperplasias (present in 48.7 ± 6% of total BADJs). Doxycycline was then administered to one-half of the remaining mice for 1 week. Myc inhibition elicited marked shrinkage of lesions and reduction in their multiplicity (identifiable lesions were observed only in 1.7 ± 0.8% of BADJs) (Fig. 1c). Consistent with the involution of adenomas, we noted significant apoptosis in the residual lesions of mice expressing Omomyc (5.7±2.5% of total cells). Apoptosis was negligible in control LSL-Kras;TRE-Omomyc; CMVrtTA mice in which Omomyc expression had not been induced (0.001%; Fig. 1d). As in the above prevention study, Myc inhibition profoundly reduced the overall numbers of proliferating cells at the BADJs (data not shown).
The induction of apoptosis by Myc inhibition in early-stage Kras-induced lung adenomas suggests that, in addition to proliferation, endogenous Myc is required to maintain survival of early-stage KrasG12D-induced lung tumours. To ascertain whether the same holds true for ‘advanced’ Kras-induced tumours, KrasG12D was sporadically activated in lungs of LSL-Kras;TRE-Omomyc;CMVrtTA mice and tumours allowed to evolve for 18 weeks. At this time, all representative animals exhibited many neoplastic lesions (present in 89±6.2% of total BADJs), with some large and highly vascularized adenocarcinomas, as described22. Endogenous Myc function was then inhibited in one-half of each cohort and groups of three mice each killed 3, 6 and 28 days later. After only 3 days of sustained Myc inhibition, histological analysis of lungs revealed marked shrinkage of tumours (Fig. 2a). By 6 days we noted a significant reduction in the proliferation of bronchioalveolar epithelial cells (1.7±0.7% 5-bromodeoxyuridine (BrdU)-positive cells in BADJs of mice co-expressing Omomyc and KrasG12D versus 4.8±2.2% BrdU-positive cells in mice expressing activated KrasG12D alone, and 0.9±0.3% BrdU-positive cells in KrasG12D-negative mice; Fig. 2b) accompanied by a significant increase in expression of the senescence marker SA-β-galactosidase (data not shown). In addition, we noted significant apoptosis in regressing tumours of Omomyc-expressing mice (5.8±1.3% TUNEL-positive cells versus, 0.001% in Omomyc negative controls; Fig. 2c). By 28 days of sustained Myc blockade, lungs of animals appeared to be free of obvious tumours with only scattered foci of atypical cells at the BADJs (Fig. 2d). Moreover, during this extended period of Omomyc expression we did not detect any occasional tumours that failed to regress and/or continued to grow, even though each animal developed multiple (hundreds) discrete tumours. Thus, endogenous Myc function is continuously required for proliferation and survival of incipient, early and advanced KrasG12D-driven lung tumours in vivo. We do not yet know if Myc inhibition eradicates all the tumours. However, experiments are underway to monitor for relapse and, should some or all re-grow, for the susceptibility of resurgent tumours to further rounds of Myc inhibition.
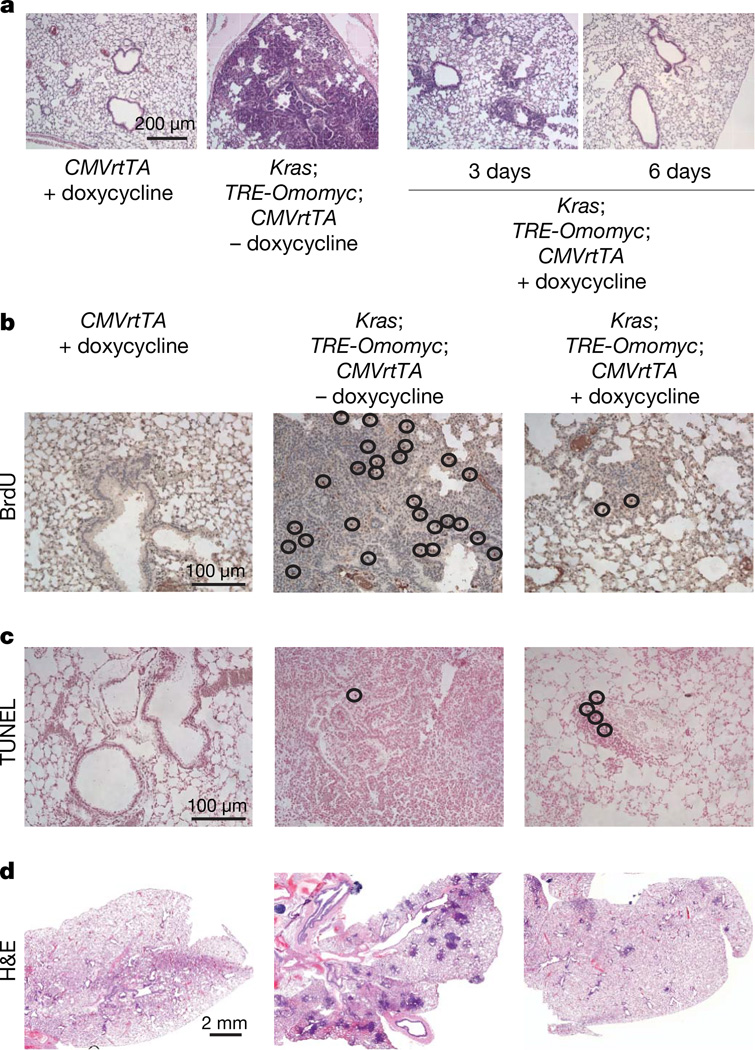
Figure 2. Myc inhibition elicits regression of established lung tumours.
a, Haematoxylin-and-eosin-stained lungs from control CMVrtTA single transgenic mice (CMVrtTA+doxycycline), KrasG12D-expressing mice (Kras;TRE-Omomyc;CMVrtTA − doxycycline) and 3- or 6-day-treated KrasG12D and Omomyc co-expressing mice (Kras;TRE-Omomyc;CMVrtTA +doxycycline) 18 weeks after infection with adenovirus expressing Cre recombinase. An example of a frank tumour is shown from a mouse expressing KrasG12D only (Kras;TRE-Omomyc;CMVrtTA −doxycycline). b, BrdU staining shows a considerable reduction in BrdU-positive cells in KrasG12D and Omomyc co-expressing mice (Kras;TRE-Omomyc;CMVrtTA +doxycycline) compared with tissues from mice expressing KrasG12D only (Kras;TRE-Omomyc;CMVrtTA −doxycycline). c, TUNEL staining indicates the presence of apoptotic cells in Omomyc and KrasG12D co-expressing sections (Kras;TRE-Omomyc;CMVrtTA + doxycycline), but not in untreated or single transgenic control tissues (Kras;TRE-Omomyc; CMVrtTA −doxycycline and CMVrtTA +doxycycline). d, Haematoxylin and eosin staining of lung tissue from mice treated for 4 weeks with doxycycline shows clearance of tumour lesions as a consequence of Omomyc expression (compare Kras;TRE-Omomyc; CMVrtTA +doxycycline with Kras;TRE-Omomyc;CMVrtTA −doxycycline).
Myc has an essential role in the proliferation of all normal cells, raising the possibility that systemic Myc inhibition would trigger severe side effects, especially in continuously proliferating tissues. Therefore, to explore the impact of systemic Myc inhibition on normal tissues, Omomyc-inducible double transgenic (TREOmomyc; CMVrtTA) mice were treated continuously with doxycycline for 4 weeks and monitored throughout. Over this extended period, general health and activity of doxycycline-treated TREOmomyc; CMVrtTA mice appeared indistinguishable from that of non-Omomyc-expressing controls (Omomyc-negative TREOmomyc and CMVrtTA single transgenic mice), with no significant changes in body weight, general activity or blood chemistry (Supplementary Fig. 2 and Supplementary Table 3). After 4 weeks, animals were killed and organs examined. We observed no discernible histological changes in any adult organs with low proliferative indices (for example, pancreas, kidney, liver, heart or lung) (data not shown). However, tissues exhibiting rapid turnover were significantly affected. The basal layer of skin epidermis exhibited marked reduction of proliferating cells (12.6±3.7% of total cells in Omomyc-expressing skin versus 28.5±5.5% of basal layer cells in controls) and thinning of the epidermis (Fig. 3a). Furthermore, hair re-growth after shaving was completely inhibited (Supplementary Fig. 3). Nonetheless, Myc inhibition induced no apoptosis, aberrant differentiation or loss of tissue integrity and mice exhibited no signs of skin tearing, ulceration or evident discomfort, even after 8 weeks of sustained Omomyc expression. In testis, Myc inhibition induced dramatic atrophy marked by loss of spermatogonia and spermatocytes (Fig. 3b) and some inhibition of cell proliferation (32.7±1.8% of total luminal cells in cycle in Omomyc-expressing mice versus 42.3%±3.7% Ki67-positive cells in control mice). We also observed a significant reduction in proliferation in the intestinal crypts (not shown) and striking attrition of villi in the small intestine (Fig. 3c). As with skin, Myc inhibition induced no increase in apoptosis or perturbation of differentiation, and integrity of the epithelium was maintained. Remarkably, all animals retained normal weight, hydration and blood chemistry, confirming maintenance of adequate intestinal absorption and barrier function against bacterial incursion (Supplementary Fig. 2 and Supplementary Table 1).
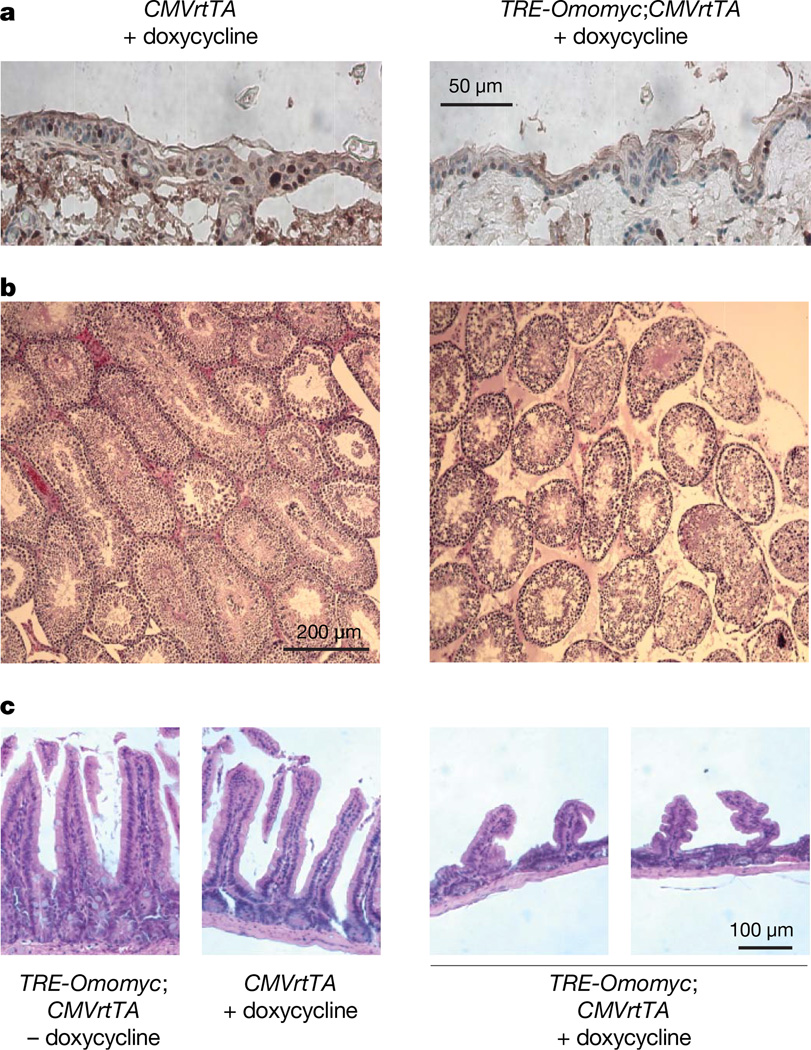
Figure 3. Inhibition of endogenous Myc suppresses proliferation in skin, testis and GI tract.
a, Anti-Ki67-stained sections of epidermis from 4-week doxycycline-treated TRE-Omomyc;CMVrtTA mice and CMVrtTA single transgenic controls. b, Anti-Ki67-stained sections of testis from 4-week doxycycline-treated TRE-Omomyc;CMVrtTA mice show reduced proliferation in seminiferous tubules of doxycycline-treated TRE-Omomyc; CMVrtTA mice compared with Omomyc-negative controls. c, Haematoxylin-and-eosin-stained small intestine sections show blunted villi in TRE-Omomyc;CMVrtTA mice treated for 4 weeks with doxycycline (TRE-Omomyc;CMVrtTA +doxycycline) compared with intestine from untreated TRE-Omomyc;CMVrtTA and doxycycline-treated CMVrtTA single transgenic (CMVrtTA+doxycycline) controls.
Despite its widespread activity in most adult tissues, the CMV early promoter functions only weakly in many haematopoietic lineages20, precluding its use in modelling the collateral impact of Myc inhibition on bone marrow and lymphoid function. We therefore made use of a complementary model in which doxycycline-dependent expression of Omomyc is driven from the β-actin promoter, which is ubiquitously active in haematopoietic lineages and the stem cell compartments of most tissues, including skin and bone marrow24–27. Induction of Omomyc in TRE-Omomyc;β-actin-rtTA mice recapitulated the Omomyc phenotypes in skin, gut and testis (Supplementary Fig. 4 and data not shown) and induced profound inhibition of proliferation in bone marrow (50% reduction of BrdU incorporation after 1 week) plus rapid onset of anaemia and leucopenia (Supplementary Table 2). However, by 2 weeks of sustained Myc inhibition, blood counts had returned close to normal levels, aside from mild polycythaemia (Supplementary Table 2). This recovery coincided with onset of extramedullary haematopoiesis in the spleen, primarily in the erythroid and megakaryocytic lineages (Supplementary Fig. 4). Throughout, all animals showed no overt signs of ill health or distress.
Myc is crucial for the maintenance of stem cell compartments and the balance between self-renewal and differentiation in multiple tissues, including the skin and intestinal crypts28,29. This raised the issue of whether even transient Myc inhibition might trigger irreversible changes in sensitive tissues, undercutting the therapeutic practicability of Myc inhibition therapy. Therefore, to assess the reversibility of Omomyc-induced tissue attrition, TRE-Omomyc;CMVrtTA mice were treated with doxycycline for 4 weeks. Doxycycline was then withdrawn to restore endogenous Myc function, and the status of affected tissues was followed over time. Within only 1 week of doxycycline withdrawal, cell proliferation had returned to pre-Omomyc levels in the basal layer of skin epithelium (Fig. 4a), hair re-growth reinitiated, and thereafter mice revealed no discernible deficits in any aspect of skin biology (data not shown). Likewise, testis rapidly recovered its full complement of spermatogenic cells including mature sperm (Fig. 4b). In the small intestine, villus length was restored within only 4–5 days (Fig. 4c). Long-term (,1 yr) observation of such mice has revealed no discernible pathology in any tissue. Thus, although systemic suppression of Myc activity has profound effects on proliferating somatic tissues, these effects are well tolerated, without any discernible negative impact on animal wellbeing and, moreover, they are completely reversible.
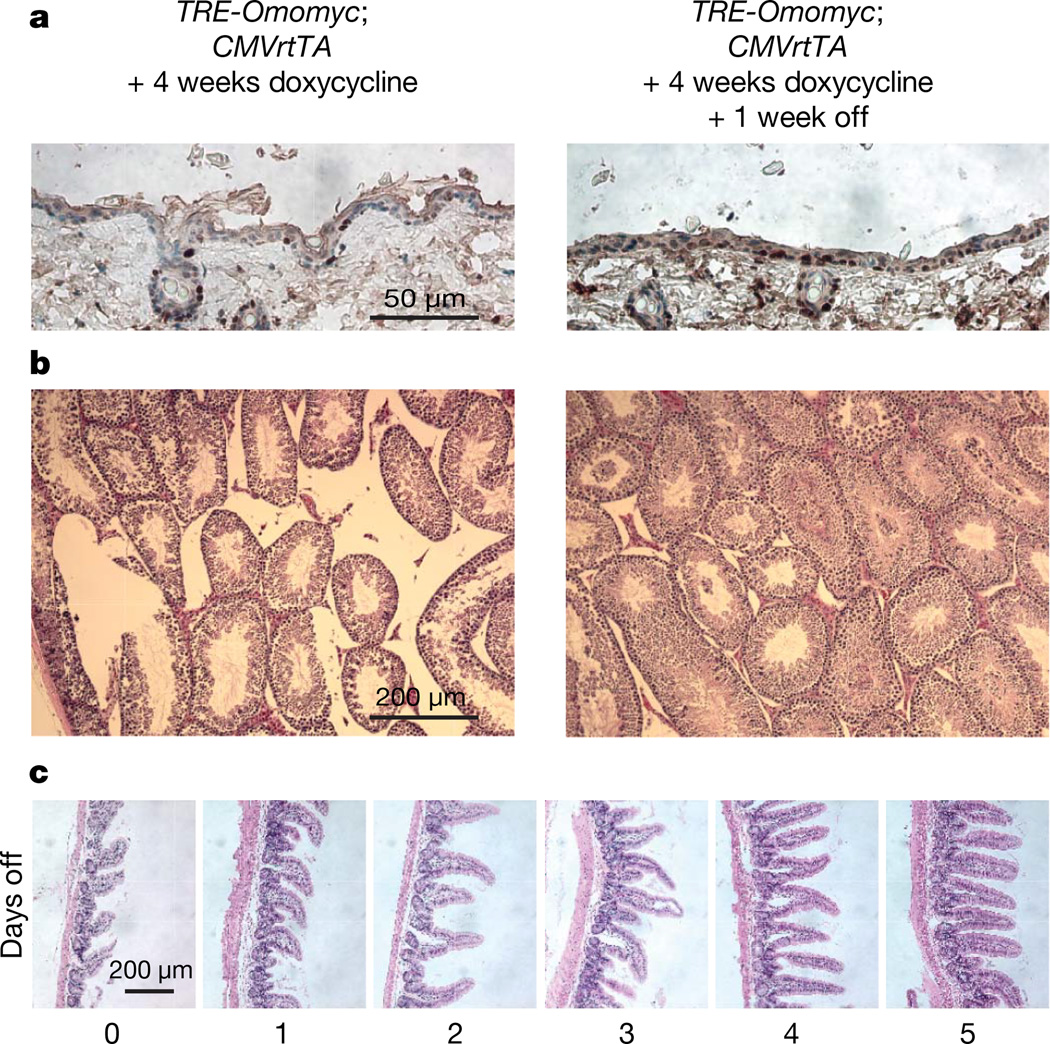
Figure 4. The degenerative phenotypes induced by systemic Myc inhibition are rapidly and completely reversible on restoration of Myc function.
Myc transactivation function was blocked for 4 weeks by sustained Omomyc expression. Doxycycline was then withdrawn for 1 week. a, Ki67 staining indicates rapid recovery of cell proliferation in skin. b, Rapid recovery of spermatogenesis in seminiferous tubules. Haematoxylin-and-eosin-stained sections from Omomyc-expressing testis (left panel) and the same tissue 1 week after discontinuing Omomyc expression (right panel) are shown. c, Rapid recovery of intestinal villus architecture in TRE-Omomyc;CMVrtTA mice after restoration of Myc function. After 4 weeks of sustained Omomyc expression, doxycycline treatment was discontinued and cohorts of mice killed each day for 5 days after doxycycline withdrawal. Representative haematoxylin-and-eosin-stained sections are shown for each time point.
Why Myc inhibition should trigger viable arrest in normal tissues but apoptosis in tumours is unclear. Perhaps sustained flux through endogenous Myc is needed to support the increased metabolic and biosynthetic demands made by oncogenic Ras signalling. Endogenous Myc may, like its oncogenic counterpart, also be essential for maintenance of the tumour microenvironment30. Whatever the underlying mechanism, our studies suggest that pharmacological inhibition of Myc offers both specificity and efficacy in the treatment of neoplastic disease.
METHODS SUMMARY
Transgene construction and generation of mice
Omomyc cDNA was cloned into the pTRE2 plasmid (Clontech). The TRE-Omomyc transgene was microinjected into the male pronucleus of day-1-fertilized (CBA × C57BL/6) F1 embryos, which were transferred into day-1-plugged pseudopregnant foster mice. CMVrtTA mice were obtained from the Jackson Laboratory (Tg(rtTAhCMV)4Bjd/J). LSL-KrasG12D mice were gifts from T. Jacks and the β-actin-rtTA+ mice from S. Artandi. Excision of the STOP element was triggered by infection with a Cre-recombinase-expressing adenovirus (5 × 107 p.f.u.)22. Doxycycline (2 mg ml−1 plus 5% sucrose) was added to drinking water. All mice were treated in accordance with protocols approved by the Institutional Animal Care and Use Committee at UCSF (IACUC approval number AN076148).
Histology and immunohistochemistry
Tissues were stained with haematoxylin and eosin. For antigen retrieval, sections were boiled for 1 min in 0.01M citrate buffer (pH 6.0). Primary antibody (anti-Ki67 (Neomarkers)) was applied in blocking buffer for 2 h. Incorporated BrdU and apoptotic cells were identified with the BrdU Detection kit II (Roche) and Apoptag kit (Chemicon). HRP-conjugated secondary antibodies (Dako and Molecular Probes) were applied for 30 min and visualized with DAB (Vector Laboratories). Cell proliferation was quantified by scoring Ki67-positive cells as a proportion of total cells at the BADJs in lung tissue (approximately 100 BADJs per mouse), as a percentage of total basal cells in skin (at least 600 total cells per mouse), and as a proportion of total cells in each seminiferous tubule in testis (approximately 80 tubules per section). At least three mice were used for every experimental condition and genotype (Supplementary Table 3).
Determination of mRNA levels using real-time PCR
Real-time quantitative RT–PCR (TaqMan) was used to quantify mRNA levels. Total RNA was isolated using Trizol reagent (Gibco). Total RNA (1 µg) was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). The Gus gene was used as an internal amplification control.
Supplementary Material
Acknowledgements
We thank T. Jacks and S. Artandi for their gifts of LSL-KrasG12D and β-actin-rtTA+mice, respectively. We thank F. Rostker for technical assistance and Y. Yaron and L. Johnson for advice on the LSL-KrasG12D model and adenovirus inhalation. We thank our laboratory colleagues for their comments and feedback. This study was supported by grant 2R01 CA98018 from the National Cancer Institute (to G.I.E.). S.N. acknowledges support from AIRC, ASI, CNR, MIUR FIRB and FIRS. C.P.M. is a Leukemia and Lymphoma Society Fellow. J.W. acknowledges support from Human Frontier Science Program.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Oster SK, Ho CS, Soucie EL, Penn LZ. The myc oncogene: MarvelouslY Complex. Adv. Cancer Res. 2002;84:81–154. doi: 10.1016/s0065-230x(02)84004-0. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitis C, Felsher DW. Conditionally MYC: insights from novel transgenic models. Cancer Lett. 2005;226:95–99. doi: 10.1016/j.canlet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 4.Flores I, et al. Defining the temporal requirements for Myc in the progression and maintenance of skin neoplasia. Oncogene. 2004;23:5923–5930. doi: 10.1038/sj.onc.1207796. [DOI] [PubMed] [Google Scholar]
- 5.Jain M, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 6.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 7.Pelengaris S, et al. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell. 1999;3:565–577. doi: 10.1016/s1097-2765(00)80350-0. [DOI] [PubMed] [Google Scholar]
- 8.Amati B, Littlewood TD, Evan GI, Land H. The c-Myc protein induces cell cycle progression and apoptosis through dimerization with Max. EMBO J. 1993;12:5083–5087. doi: 10.1002/j.1460-2075.1993.tb06202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferre-D’Amare AR, Prendergast GC, Ziff EB, Burley SK. Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature. 1993;363:38–45. doi: 10.1038/363038a0. [DOI] [PubMed] [Google Scholar]
- 10.Nair SK, Burley SK. Structural aspects of interactions within the Myc/Max/Mad network. Curr. Top. Microbiol. Immunol. 2006;302:123–143. doi: 10.1007/3-540-32952-8_5. [DOI] [PubMed] [Google Scholar]
- 11.Amati B, et al. Oncogenic activity of the c-Myc protein requires dimerisation with Max. Cell. 1993;72:233–245. doi: 10.1016/0092-8674(93)90663-b. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, et al. Effects of the MYC oncogene antagonist, MAD, on proliferation, cell cycling and the malignant phenotype of human brain tumour cells. Nature Med. 1995;1:638–643. doi: 10.1038/nm0795-638. [DOI] [PubMed] [Google Scholar]
- 13.Prochownik EV. c-Myc as a therapeutic target in cancer. Expert Rev. Anticancer Ther. 2004;4:289–302. doi: 10.1586/14737140.4.2.289. [DOI] [PubMed] [Google Scholar]
- 14.Soucek L, et al. Design and properties of a Myc derivative that efficiently homodimerizes. Oncogene. 1998;17:2463–2472. doi: 10.1038/sj.onc.1202199. [DOI] [PubMed] [Google Scholar]
- 15.Soucek L, et al. Omomyc, a potential Myc dominant negative, enhances Myc-induced apoptosis. Cancer Res. 2002;62:3507–3510. [PubMed] [Google Scholar]
- 16.Soucek L, Nasi S, Evan GI. Omomyc expression in skin prevents Myc-induced papillomatosis. Cell Death Differ. 2004;11:1038–1045. doi: 10.1038/sj.cdd.4401443. [DOI] [PubMed] [Google Scholar]
- 17.Baskar JF, et al. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J. Virol. 1996;70:3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furth PA, et al. The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Res. 1991;19:6205–6208. doi: 10.1093/nar/19.22.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kothary R, et al. Unusual cell specific expression of a major human cytomegalovirus immediate early gene promoter-lacZ hybrid gene in transgenic mouse embryos. Mech. Dev. 1991;35:25–31. doi: 10.1016/0925-4773(91)90038-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhan Y, Brady JL, Johnston AM, Lew AM. Predominant transgene expression in exocrine pancreas directed by the CMV promoter. DNA Cell Biol. 2000;19:639–645. doi: 10.1089/10445490050199045. [DOI] [PubMed] [Google Scholar]
- 21.Jackson EL, et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- 22.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweet-Cordero A, et al. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nature Genet. 2005;37:48–55. doi: 10.1038/ng1490. [DOI] [PubMed] [Google Scholar]
- 24.Okabe M, et al. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 25.Sarin KY, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawamura D, et al. Promoter/enhancer cassettes for keratinocyte gene therapy. J. Invest. Dermatol. 1999;112:828–830. doi: 10.1046/j.1523-1747.1999.00583.x. [DOI] [PubMed] [Google Scholar]
- 27.Wright DE, et al. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood. 2001;97:2278–2285. doi: 10.1182/blood.v97.8.2278. [DOI] [PubMed] [Google Scholar]
- 28.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 29.Murphy MJ, Wilson A, Trumpp A. More than just proliferation: Myc function in stem cells. Trends Cell Biol. 2005;15:128–137. doi: 10.1016/j.tcb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Evan GI. Can’t kick that oncogene habit. Cancer Cell. 2006;10:345–347. doi: 10.1016/j.ccr.2006.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.