Abstract
Marburg virus outbreaks are sporadic, infrequent, brief, and relatively small in terms of numbers of subjects affected. In addition, outbreaks most likely will occur in remote regions where clinical trials are not feasible; therefore, definitive, well-controlled human efficacy studies to test the effectiveness of a drug or biologic product are not feasible. Healthy human volunteers cannot ethically be deliberately exposed to a lethal agent such as Marburg virus in order to test the efficacy of a therapy or preventive prior to licensure. When human efficacy studies are neither ethical nor feasible, the US Food and Drug Administration may grant marketing approval of a drug or biologic product under the ‘Animal Rule,’ through which demonstration of the efficacy of a product can be ‘based on adequate and well-controlled animal efficacy studies when the results of those studies establish that the drug is reasonably likely to produce clinical benefit in humans.’ This process requires that the pathogenic determinants of the disease in the animal model are similar to those that have been identified in humans. After reviewing primarily English-language, peer-reviewed journal articles, we here summarize the clinical manifestations of Marburg virus disease and the results of studies in NHP showing the characteristics and progression of the disease. We also include a detailed comparison of the characteristics of the human disease relative to those for NHP. This review reveals that the disease characteristics of Marburg virus disease are generally similar for humans and 3 NHP species: cynomolgus macaques (Macaca fascicularis), rhesus macaques (Macaca mulatta), and African green monkeys (Chlorocebus aethiops).
Abbreviations: HD, high dose; LD, low dose; MARV, Marburg virus; MVD, Marburg virus disease; RAVV, Ravn virus
The genus Marburgvirus, a member of the family Filoviridae, includes a single species, Marburg marburgvirus, with 2 virus members, Marburg virus (MARV) and Ravn virus (RAVV).1,48,50 The family Filoviridae includes one other accepted genus, Ebolavirus. MARV and RAVV differ in nucleotide sequence by approximately 20%,12,84,86 whereas MARV variants (that is, Musoke, Angola, and the still-unnamed variant that caused the outbreaks in 1967) and MARV isolates within a variant (that is, Pop and Ci67) differ by only 7% or less.80,84,85 As would be expected, the nucleotide sequences of the Marburgvirus and Ebolavirus genomes vary considerably. For instance, the N-termini of the nucleoprotein gene, 1 of the 7 genes that that comprise the genomes of Marburgvirus and Ebolavirus, are 55% identical between the MARV Musoke variant and the Ebolavirus Mayinga variant, whereas the C-termini of these viruses are only 23% identical.45,68 The same is true for the glycoprotein genes for Marburgvirus and Ebolavirus, as these differ by at least 55% at the nucleotide level and 67% at the amino acid level when variants are compared across genera.45,69
A likely natural host for MARV and RAVV, and the probable source of the highly infectious viruses that cause Marburg virus disease (MVD) in humans, is the Egyptian fruit bat (Rousettus aegyptiacus). MARV and RAVV RNA transcripts were detected in samples from R. aegyptiacus, with 5 of 32 bats harboring the same variant of live virus (2 bat isolates grouped with the MARV lineage, and the remaining 3 resided within the RAVV lineage).84,86 Of these 5, 4 of the bat isolates were collected in 2007, and the fifth isolate was collected 9 mo later in 2008. This pattern suggests that colonies of the R. aegyptiacus can harbor MARV for extended periods of time. The perpetuation of the virus in the R. aegyptiacus, along with the detection of MARV and RAVV RNA in isolates from these animals, implicates this species as a likely natural host.84
Infections with MARV were reported in hundreds of patients between 1967 and 2008, with mortality as high as 90% in one outbreak6,40,62,65,90 but lower in others.58,59,70,78,92 The origins of cases were largely African equatorial and subSaharan areas; and because of the remoteness of some of these regions and various cultural practices, clinical reports were often incomplete and inaccurate.
When human efficacy studies are neither ethical nor feasible, the pathway to licensure of a drug or biologic product follows the US Food and Drug Administration's ‘Animal Rule’ (see 21 CFR Part 314.600 for drugs; 21 CFR Part 601.90 for biologic products). The Animal Rule states that the effect (that is, efficacy) should be demonstrated in more than one animal species expected to react with a response predictive for humans. However, the rule includes a provision allowing the effect to be demonstrated in a single animal species when that species represents a sufficiently well-characterized animal model for predicting the response in humans (that is, one that has been evaluated sufficiently in regard to its responsiveness). A case can be made for conducting a single pivotal efficacy study in an NHP species to support licensure of a product for MARV under the Animal Rule. First, according to information available in primarily English-language, peer-reviewed publications, NHP (that is, including all NHP genera and species tested to date) are the only known laboratory species that are susceptible to wild-type MARV. Although other filovirus models, including those in mice, hamsters, and guinea pigs,10,38,49,61,87,88 have been developed and are useful for screening, they do not to meet the criteria to serve as a second model for licensure under the Animal Rule. For example, the wild-type virus has to be mutated extensively to be lethal in these models,10,46,61,87,88 whereas the US Food and Drug Administration's draft Guidance for Industry: Product Development Under the Animal Rule (May 2014)25 states that the “challenge agent used in animal studies generally should be the same as the etiologic agent that causes the human disease.”25 Second, the pathophysiologic mechanism underlying the progression of the MVD in NHP is reasonably well understood, and the results of this mechanism have been published extensively. We discuss the results of this published work herein.
To date, no journal article has summarized the clinical manifestations of MVD, detailed the results of studies in NHP showing the characteristics and progression of the disease, and presented a detailed comparison of the characteristics of the human disease relative to those for NHPs. This overview intends to fill that gap with a review of primarily English-language, peer-reviewed journal articles. The animal data focus on the 3 NHP species—cynomolgus macaques (Macaca fascicularis), rhesus macaques (Macaca mulatta), and African green monkeys (Chlorocebus aethiops)—that have been featured in these articles. The intent underlying this comparison is to demonstrate that 1) NHP are valuable animal models for demonstrating the efficacy of a product for MVD and 2) the characteristics of MVD generally are similar across NHP species, such that no single NHP species stands out as being more similar to humans than the others. This information will be useful to researchers and product developers who conduct or sponsor animal studies to evaluate the efficacy of vaccines, therapeutic agents, and postexposure prophylaxis agents against MVD.
Marburg Virus Infection in Humans
Approximately 35 patients in Germany, Yugoslavia, and South Africa have been diagnosed with MARV infection and treated symptomatically at modern medical facilities. These cases provided most of the pathology and clinical laboratory data on MVD in humans. Although mortality was variable across all patient groups, it was noticeably lower (mortality, 20% to 30%) in those receiving intensive care as compared with those restricted to rural African settings (mortality, 80% to 90%).
West Germany (Frankfurt am Main and Marburg) and Yugoslavia, 1967.
5,7,11,22,23,30,42,45,46,49,55,57-59,64,70-72,74,76,78,79,92,93 Three simultaneous outbreaks of MVD, one each in Marburg and Frankfurt am Main, West Germany, and the third in Yugoslavia, occurred in August and September 1967 and were traced to a single source, African green monkeys (Chlorocebus aethiops) imported from Uganda. The monkeys infected technicians and scientists by several possible routes as they obtained and processed tissues for use in the production and testing of biologicals. A total of 31 patients were infected, 7 of whom died within 16 d. Although information on the Yugoslavian patients was limited, detailed data were available on German patients who received prompt and intensive medical care and follow-up, which largely kept the overall lethality to 7 deaths in 29 cases (24%).
Clinical symptoms appeared 3 to 9 d after exposure, were mild for 3 to 4 d, and included malaise, headache, and fever. Symptoms then worsened as patients suffered from diarrhea, nausea and vomiting, and high fever and then exanthema or enanthema, beginning on the trunk and buttocks and spreading to the limbs and the face within 1 wk of onset. Liver enzymes (ALT and AST) increased during this period and peaked at 7 to 8 d after presumed exposure. Lymphopenia and thrombocytopenia were typical findings in this phase. In week 2, the condition of many patients worsened, as hemorrhage, sometimes severe, occurred at various sites (for example, gastrointestinal and nasal). Five of the 7 patients with hemorrhagic syndrome died. The fever subsided in others, and lymphocyte and platelet counts rebounded. Secondary infections occurred in 3 people: a physician after a needle stick, a spouse after sexual intercourse, and a nurse who was exposed to the virus while caring for patients who had the disease.
Autopsies of 5 patients from Marburg, Germany, who died during the 1967 outbreak showed hyperemia of the meninges and edematous brain swelling. Subepicardial and endocardial hemorrhage was observed for all 5 patients, and the heart was dilated in some cases also. In 4 of the 5 patients, the stomach and large sections of the intestines were filled with blood. Hemorrhage of mucous membranes, soft tissues, and parenchymal organs was noted. The gallbladder was always dilated and full (the material that filled the gallbladder was not specified), and lymph nodes were swollen. The spleen was slightly enlarged in 2 patients, and the red pulp was hardened without any visible irregularities. In all 5 cases, kidneys were pale and swollen, and the external genitals near the scrotum or vulva were darkly discolored. Except for the lungs, skeletal muscles, and skeleton, areas of focal necrosis were seen in almost every organ, with large necrotic areas present in the testicles and ovaries.
Microscopic evaluation of tissues from these patients revealed necrosis of the splenic red pulp and medulla of the lymph nodes, as well as monocyte or macrophage infiltration of lymphatic organs and mucous membranes of the stomach and intestines. Parenchymal damage (type of damage not specified) of the kidneys and interstitial edema in the heart were noted also. Panencephalitic glial nodule encephalitis, which expanded to the medulla oblongata and the exiting cranial nerves, and nodular accumulations in the cerebellar and cerebral cortices, medulla, and pons cerebri were detected. Hemorrhage and necrosis surrounding the follicles of the ovaries and hyperemia of the endometrium were reported. An analysis of autopsy samples from 4 patients yielded tissue viral titers values, reported as the amount of virus required to produce a cytopathic effect in 50% of inoculated tissue culture cells per gram of tissue (TCID50/g) and ranged from 3.5 to 7.2 log10 TCID50/g.
The variant that infected the monkeys in Uganda and that responsible for these outbreaks in Europe remain unnamed. However, the isolates Pop and Ci67, mentioned in the following section on MARV infection in NHP, were obtained from Frankfurt and Marburg, Germany, respectively, during those outbreaks.49 The fate of the patients from whom the Pop and Ci67 isolates were collected is unknown currently.
South Africa, 1975.
14,23,27-29,42,45,51,63,64,74,92 Three cases of MVD, each confirmed by virologic studies, occurred in Johannesburg in February 1975. The first case involved a 20-y-old Australian man who apparently acquired the infection while traveling in Rhodesia. The second and third cases affected a 19-y-old woman who traveled with the man and was close to him during his illness and a 20-y-old nurse who cared for the man during his hospitalization, respectively. Patient 1 had myalgia, rash, and severe gastrointestinal upset. Evidence of hepatic (increased liver enzymes) and kidney involvement (increased serum creatinine) were noteworthy. Patient 1 died within 4 d of hospitalization, and the official cause of death was massive hemorrhage in the gastrointestinal tract, with blood reaching the lungs. Increased WBC and low platelet counts were noted on the day of death. Microscopic evaluations revealed hepatocyte necrosis, lymphoid depletion of the spleen and lymph nodes, and basophilic cytoplasmic and extracellular material, thought to be virus, in those tissues. In addition, kidney sections showed pronounced tubular necrosis and some fibrin deposition in the glomeruli.
Patient 2 had symptoms of malaise, headache, and fever, followed by painful joints and muscles and a low WBC count. In addition, a transient rash (days 5 to 7) developed. In light of the disseminated intravascular coagulation that had developed for patient 1, heparin was given intravenously prophylactically starting on day 2 of hospitalization. Intravenous fluids were initiated on day 3 of hospitalization and continued until the patient claimed to feel completely well. Temperature fluctuated between days 3 and 8 of hospitalization, after which it returned to normal.
Patient 3 had symptoms of malaise, backache, and fever on day 1 of hospitalization, followed shortly by muscle ache and tender and slightly enlarged lymph nodes. Abdominal pain and diarrhea were reported on day 3, with diarrhea persisting until day 10. Vomiting presented later (days 6 to 9). Laboratory results revealed leukopenia (days 3 through 8) and thrombocytopenia (days 3 through 10) with an associated increase in PTT (days 3 through 8). Liver enzymes were elevated also (days 4 through 11). She was treated with heparin, Lassa antiserum, plasma, platelets, and intravenous fluids. She had a transient rash (days 5 to 7) but never experienced hemorrhagic events, although there was evidence of possible disseminated intravascular coagulation and some bleeding at needle puncture sites. At 3 mo after recovery, patient 3 presented with anterior uveitis that was culture-positive for MARV. The uveitis resolved, and a specimen collected 1 mo later was negative for the virus.
All 3 patients had antibodies to MARV. Blood viral load titers for these 3 patients were reported as 5.8 log10 TCID50/mL for patient 1 who died, and 4.2 to 4.5 log10 TCID50/mL for patients 2 and 3, both of whom survived. Tissue viral titers for patient 1 were 4.5 and 5.8 log10 TCID50/g for the liver and spleen, respectively; titers in the kidney and brain were below the limit of detection for the endpoint dilution assay.
Democratic Republic of Congo, 1998 to 2000.
4,8,9,15,22,64 Multiple seasonal outbreaks of MVD occurred in a gold-mining region of the Democratic Republic of the Congo (Durba and Watsa Districts) beginning in October 1998 and continuing to September 2000. Epidemiologic studies revealed 118 deaths among 154 patients. The disease was caused by multiple introductions of infection into the population, because at least 9 genetically distinct lineages of the genus Marburgvirus (MARV and RAVV) were circulating during the outbreaks. One-half of the cases involved primary infection of miners, whereas most other patients were housewives, children, and healthcare workers, presumably representing secondary infections from contact with miners. Previous cases in this area reportedly had occurred in 1987, 1990, and 1992. The most frequent symptoms at admission (in descending order) were fever, malaise, headache, nausea or vomiting, anorexia, abdominal pain, diarrhea, myalgia, arthralgia, difficulty breathing, difficulty swallowing or sore throat, hiccups, conjunctival symptoms, lumbar pain, chest pain, cough, and agitation. Hemorrhage was manifest in various ways in most patients (hematemesis, melena, gingival bleeding, and so forth). Petechiae or rashes were reported in a few cases. Pathology, viral culture, and immunologic studies were not performed, except for isolated cases sent to reference laboratories and without published reports.
Angola, 2005.
6,18-21,40,52-54,56,62,65-67,85 In 2005, an outbreak occurred in Uige, Angola, in a major hospital. Suspected cases (unconfirmed as MVD) were first reported in October 2004. Confirmed cases began in early 2005, with the death of a hospital worker in March, and the last case was identified in June 2005.
Investigation of the Angola disease outbreak was hampered by the negative perceptions of the Angolan population in the endemic area. Angolans often resisted medical assistance and epidemiologic studies due to rumors that the foreign teams were responsible for bringing or spreading the virus. Practices such as families hiding sick members, avoiding hospitals, immediately burying the deceased, using native healers, and bringing patients to hospitals soon before death suggest that the true scope of the outbreak and data on morbidity and lethality might never be known. Patient records were kept only at admission and not during hospitalization. At admission, the most frequent complaints were: fever, asthenia, anorexia, myalgia, arthralgia, diarrhea, abdominal pain, nausea, vomiting, headache, dysphagia, conjunctivitis, and dyspnea. Hemorrhage was frequent in later stages of the disease. Lethality was especially high, reported to be 90% of MVD patients. Viral genomics from 15 patient specimens were analyzed, and results suggested that MVD in Angola was not substantially distinct from previous outbreaks in other regions of Africa and that virus was very similar within this outbreak.
Case reports.
Colorado, 2008.
13 A woman from Colorado, USA, visited Python Cave in Uganda on 25 December 2007; on returning home, she experienced symptoms of severe headache, chills, nausea, vomiting, and diarrhea (4 January 2008). She self-treated for traveler's diarrhea with ciprofloxacin, after which she developed a diffuse rash. Results of laboratory tests obtained on 6 and 7 January 2008 while she was an outpatient showed evidence of leukopenia. On 8 January, the patient had persistent diarrhea, abdominal pain, fatigue, generalized weakness, and confusion. Laboratory test results that day also showed evidence of liver (increased AST and ALT) and kidney (increased creatinine) damage. She was admitted to a community hospital for additional management. While she was hospitalized, her symptoms included pancytopenia, coagulopathy, myositis, pancreatitis, and encephalopathy. The patient was discharged on 19 January and experienced a lengthy recovery. Although initial serologic analyses of her serum (sample taken on 10 January) were negative for MARV (antiMARV IgM and IgG ELISA), samples taken on 15 July 2008 and 3 February 2009 tested positive for antiMARV IgG by ELISA. Traditional RT-PCR analysis was negative, and real-time (TaqMan) RT-PCR assay was equivocal; however, nested RT-PCR analysis (modified assay intended to reduce nonspecific binding in samples) confirmed the presence of MARV RNA fragments in the sample taken on 10 January 2008.
Holland, 2008.
83,91 A female Dutch patient visited Uganda, including trips to 2 caves. The first cave she visited was Fort Portal on 16 June 2008; she reported seeing no bats in this cave. She visited the second cave, Python Cave, on 19 June; this cave was known to harbor bat species that have been proven to carry filoviruses. In addition, she reported having had direct contact with one bat. The woman returned home on 28 June and experienced the first signs of illness (fever and chills) on 2 July. Her condition rapidly deteriorated, with liver failure and severe hemorrhaging reported on 7 July. Additional clinical signs of rash, conjunctivitis, and diarrhea, and evidence of kidney failure were reported, but the dates on which signs and symptoms presented were not specified. She died on 11 July of her disease, which was diagnosed by PCR analysis as MVD.
Sweden, 1990 to 1991.
24,44 A male Swedish patient who had just returned from Kenya (and other East and Central African nations) developed fever, headache, and chills, followed a day later by diarrhea, variable high temperature, weakness, and nausea. His condition worsened, with disseminated intravascular coagulation and a macular rash on the face and neck. The patient remained critically ill for 4 wk. With intensive hospital care in Sweden, recovery began slowly in week 5. Filovirus was identified by electron microscopy and MARV by serology.
Kenya, 1980 and 1987.
41,45,49,77 In 1980, 3 cases of Marburg hemorrhagic fever were reported in Kenya: the first patient was a French engineer working in West Kenya; the second was a physician who attended to the first patient; and the third was a nurse who assisted the physician. The engineer developed a sudden febrile illness with headache, myalgia, and malaise, followed by vomiting and diarrhea 3 d later. He experienced severe hematemesis during transport to Nairobi Hospital and on arrival collapsed from massive gastrointestinal hemorrhaging; he also was bleeding from the nose and mouth. The patient died 6 h after admission to the hospital. Autopsy revealed evidence of hepatic necrosis. The presence of virus particles was later confirmed through electron microscopy of formalin-fixed renal (but not liver) tissue. The treating physician became ill with fever, headache, backache, and sore throat 9 d after attempting resuscitation of the engineer. Diarrhea and bloody stool developed on day 4 of illness; liver and kidney functions were reported to be compromised. The physician recovered. In both cases, serology demonstrated increasing titers of antiMARV antibodies. The Musoke variant of MARV, which was used in several of the animal studies described in the following section, was isolated from the physician. In addition, a nurse assisting with the resuscitation of the engineer became ill 11 d later with symptoms of fever, malaise, headache, and backache. She presented with a faint rash on the upper arms 2 wk later and recovered after another 3 wk.
In 1987, a 15-y old boy and his family visited Kitum Cave in Mount Elgon National Park and then traveled to Mombasa. The boy became ill 9 d after exploring the cave and was taken to Aga Khan Hospital in Mombasa. He later was transferred to Nairobi Hospital, where he died. Electron microscopy of tissues revealed a similar pattern of destruction and similar types of lesions as those described for patients in the 1967 outbreak. Immunohistochemical analysis detected virus in circulating and tissue macrophages, fibroblast-like cells, hepatocytes, adrenal cells, neuroendocrine cells of the adrenal medulla, and the α and β cells of the pancreatic islets. In addition, the connective tissue and endothelial cells stained immunopositive multifocally. The virus that was isolated from the boy's clinical sample (Marburg RAVV) was recognized as a new virus within the species Marburg marburgvirus, because it differed genomically from all other known MARV isolates by more than 20% yet maintained high amino-acid conservation, with the exception of the glycoprotein gene.12,49,84,85
Uganda, 2007.
2 Marburg hemorrhagic fever was detected in 4 miners near Kitaka Cave, Kamwenge, Uganda, in 2007. The first patient was admitted to the hospital with a 3-d history of fever, chills, headache, and arthralgia. Fever persisted and was accompanied by vomiting 3 d later, and confusion, seizures, hematemesis, and hematochezia were observed prior to death 7 d after hospitalization. The second patient was admitted to the hospital with fever, arthralgia, vomiting, and headache. Seizure activity was reported on day 2 of hospitalization, and weakness and dizziness developed 7 d later. The second patient recovered and was discharged 18 d after initial hospitalization. The third patient was admitted with fever, arthralgia, and headache and subsequently recovered. The fourth patient was admitted with fever, headache, arthralgia, anorexia, and hematemesis (2 wk after entering the mine) but recovered. The second and third patients tested positive for antiMARV IgG by ELISA.
Marburg Virus Infection in Nonhuman Primates
Studies in cynomolgus macaques.
The first study we summarize involved the exposure of cynomolgus macaques (country of origin unspecified) to aerosolized MARV–Angola variant (isolated from a patient during the 2005 outbreak) at challenge doses of 2 to 14 pfu (low dose [LD], n = 3 animals] or 99 to 705 pfu (high dose [HD], n = 3 animals); the virus was lethal in 100% of the animals.3 The macaque that received the highest dose (705 pfu) succumbed first. Having met the predefined criteria for moribundity for this study, this animal was euthanized at 7 d after infection. The other 2 animals that received high challenge doses (99 and 339 pfu) met the criteria for moribundity on day 8 (for this and all studies, ‘day’ refers to the study day after infection), prompting their euthanasia. All 3 LD macaques met moribundity criteria on day 9 after infection and were euthanized promptly.
Increases in body temperature typically started between days 4 and 5 for macaques in the HD group and between days 5 and 6 for those in the LD group. Anorexia and depression also presented on day 5 to 6, but the authors did not specify whether there was a dose-response relationship with the time-to-onset of anorexia or depression. These signs were followed 1 d later with the onset of a rash on the face, chest, axillary and inguinal regions and along medial aspects of the arms (all 6 macaques). Although lymphopenia was detected only in HD animals (onset on day 3 and persisting until day 5), total WBC counts rebounded for all animals, as exhibited by marked increases in total WBC counts due to increased lymphocytes late in infection (day 6 onward). Granulocyte counts remained at prechallenge levels for 2 HD animals but gradually increased for all other macaques starting on day 5. In addition, all animals exhibited signs of thrombocytopenia (more severe for HD macaques), which typically started by day 3 and was followed by sharp increases in platelet distribution width (day 7 onward). Clinical chemistry analyses were not performed. Increased plasma concentrations of the chemokines CCL2, CCL4, IL8, and IL6 and decreased IL33 levels in pulmonary endothelial cells were noted as well. Viremia was first detected on days 4 to 6, and the time-to-onset was independent of challenge dose. Similarly, peak blood viral load levels were independent of the challenge dose and ranged from 7 to 8 log10 pfu/mL between days 7 and 9.
Immunohistochemistry results showed that MARV was localized in spleen (all 6 macaques), lymph nodes (all 6), esophagus (3), lung (6), and liver (6); however, localization was not noted in the kidney or adrenal gland. Virus did not localize within lymphocytes, dendritic cells, granulocytes, or macrophages in the lymph nodes.
Macroscopic findings were observed in the lung (enlarged lobes with variable congestion and edema, 4 macaques; serosanguinous pericardial fluid, 2 macaques); lymph nodes (enlarged, congested, or hemorrhagic); spleen (enlarged and congestion); liver (enlarged, friable tannish-yellow, all 6 macaques); and intestine (pyloric and duodenal congestion).
Microscopic abnormalities were present in the lungs (alveolar hemorrhage, fibrin, and proteinaceous fluid accumulation, 5 of the 6 macaques); lymph nodes (particularly in the tracheobronchial and mediastinal lymph nodes: lymphoid depletion resulting in partial to complete loss of nodal architecture with cellular debris, hemorrhage, and neutrophils filling the remaining subcapsular and medullary sinuses, as well as fibrinoid vascular necrosis of high endothelial venules, 6 macaques); spleen (red-pulp hemorrhage and congestion, loss of the extramedullary hematopoietic elements, as well as mild expansion of the red pulp by seroproteinaceous and fibrinous material). Additional lesions were noted in the liver (mild to moderate hepatocellular cytoplasmic vacuolation with sinusoidal congestion and increased circulating mononuclear and polymorphonuclear cells, 6 macaques); kidneys (renal tubular degeneration with fibrin deposition, 2 animal); adrenal glands (congestion and degenerate adrenocortical cells, 3 animals); and tonsils (lymphoid depletion, 4 animals). Although amounts varied among animals, prominent fibrin deposition was present in the kidney, lung, spleen, and (rarely) liver.
In the next study we present,31 3 cynomolgus macaques (country of origin unspecified) were injected intramuscularly with 1000 pfu of MARV–Angola variant as untreated controls for a vaccine efficacy study and succumbed to infection on days 8 and 9 after infection. Clinical signs of rash and anorexia were noted for all 3 animals. Lymphopenia and elevated AST or ALT (or both) were present in all animals on day 6 but not at the previous measurement, on day 3. Similarly, viremia (6 to 8 log10 pfu/mL) was detected (by plaque assay) on day 6 but not at the previous measurement (day 3). Body temperature was not mentioned as having been measured. Macroscopic and microscopic pathology results were not reported.
Another 2 cynomolgus macaques (Mauritius origin) served as negative controls in a vaccine efficacy study;81 control animal 1 was challenged with MARV–Musoke, and control animal 2 was challenged with the MARV–Ci67 isolate (which was found to have the same glycoprotein amino-acid sequence as the Pop strain obtained during the same outbreak). The challenge route for both animals was subcutaneous. In both cases, the macaques were vaccinated with an irrelevant, nonspecific vaccine (CAdVax-HC4, a hepatitis C vaccine) prior to viral challenge (1000 pfu). An increase in body temperature (<1 °C) was observed for control animal 1 on day 5 after challenge. An additional spike in body temperature (to approximately 1.5 °C above the baseline value) was present in this animal when body temperature was measured again on day 8 after challenge. A modest increase in temperature (<1 °C change) was observed for control animal 2 on day 5 after challenge and was followed by a sharp increase in temperature on day 8. Sharp increases in ALP and AST, accompanied by more modest increases in ALT and GGT, were present in both control animals 1 (day 8 after challenge) and 2 (day 5 after challenge). Control animal 1 died on day 8 after challenge, and control animal 2 died on day 7 after challenge. Hematology, viral titers, and macroscopic and microscopic pathology results were not reported.
Featured in 2 reports,26,37 18 cynomolgus macaques (country of origin unspecified) were injected intramuscularly on day 1 with 1000 pfu of the MARV–Ci67 isolate and serially euthanized (3 per time point) on days 2, 3, 4, 6, 7, and 8. Cutaneous rashes (axilla or groin or both) and anorexia were observed first on day 5, and fever (>102 °F [>38.9 °C]) was observed starting on day 6. Rashes extended to the thorax, proximal limbs, and face of animals euthanized after day 6. Persistent bleeding at the venipuncture site and bleeding of the gums were observed also on day 6. Severe depression, recumbency, and diarrhea were noted on day 7, and dehydration (according to skinfold retraction) was present on day 8. Lymphopenia and neutrophilia started on day 4 and peaked on day 5. Platelets decreased slightly between days 2 and 5 but remained within the normal range for this species. This decrease was followed by a rebound effect starting on day 6. Increases in D-dimers, PT, and activated PTT time and decreases in activated protein C were noted starting on days 4, 5, 5, and 6, respectively.
Evidence of hepatic damage was observed on day 6, with increases in ALT and AST and lesions in the liver (macroscopic findings: enlarged, congested, or pale yellow friable livers; microscopic findings: hepatocellular swelling and necrosis, hepatocellular cytoplasmic vacuolization, and portal inflammation). Urinary system damage was noted beginning on day 6 to 7 with serum increases in urea nitrogen and creatinine, and lesions of the kidney or urinary bladder (urinary bladder mucosal reddening seen macroscopically and unspecified lesions were observed microscopically). Macroscopic pathologic changes of the lymphoid system included axillary and inguinal lymphadenopathy and congestion, enlarged mandibular lymph nodes, and enlarged and congested spleens. Microscopic changes of lymphoid tissues included sinus histiocytosis, edema, follicular lymphocytolysis, and lymphocyte depletion in the lymph nodes, as well as lymphoid depletion, lymphocytolysis, and red-pulp necrosis of the spleen. Additional macroscopic findings included pyloric and ileocecal reddening (day 4) and pyloric and proximal duodenal mucosal congestion (day 6). Cutaneous petechial rash presented later, on day 7 to 8.
Viremia was first seen on day 3 and peaked on day 8 with a mean titer of 8.2 log10 pfu/mL. Virus was also detected in the liver, lymphoid tissues (spleen and axillary, inguinal and mesenteric lymph nodes), kidney, adrenal gland, lung, pancreas, heart, testis, and bone marrow starting on either day 3 or 4, with mean peak titers ranging from 5.5 to 9.2 log10 pfu/g on day 8. MARV glycoprotein was detected in the circulation (peripheral blood mononuclear cells, day 5 after inoculation) and spleen (day 6 after inoculation, but not on day 4 [previous measurement time point]) with flow cytometry.
Another report82 involved 3 cynomolgus macaques (country of origin unspecified), each of which served as a negative control for MARV–Musoke (control 1), MARV–Ci67 (control 2), and MARV–RAVV (control 3) challenges in a vaccine efficacy study. All 3 were experimentally naïve prior to subcutaneous inoculation with 1000 pfu of the respective virus. Clinical signs (unspecified) were observed on days 8 through 10 after challenge, and controls 1 and 3 were terminally bled and euthanized on days 10 and 8 after challenge, respectively. Control 2 was found dead on day 10; as such, a terminal bleed was not performed to measure clinical pathology endpoints. Evidence of liver damage was seen in all 3 monkeys, with maximal ALT levels approximately 20-fold over the baseline activity levels for controls 1 and 3. Serum from control 2 that was collected at the last measured time point (day 7) showed an ALT increase of 1.3-fold over baseline activity level. As measured by plaque assay, the peak viremia level was approximately 9 log10 pfu/mL for controls 1 and 3 and was greater than 10 log10 pfu/mL for control 2. The day on which peak viremia occurred relative to exposure was not stated. Hematology analysis was performed, but no results were reported. Macroscopic and microscopic pathology evaluations were not performed.
Two cynomolgus macaques (country of origin unspecified) were included as controls in a vaccine efficacy study;16 one was inoculated with MARV–RAVV and the other with MARV–Angola. The challenge dose was 1000 pfu for each animal, but the challenge route of exposure was not stated. Note that these animals were vaccinated with an irrelevant, nonspecific vaccine (VSVΔG/ZEBOVGP) prior to challenge. Clinical signs of fever, macular rash, and depression developed by day 6 for both controls, and both succumbed to infection on day 8. Evidence of liver impairment (elevated ALT and AST) was seen for both on day 6 but not when measured previously (day 3). In addition, lymphopenia and thrombocytopenia after viral challenge were noted for both animals, but the actual day on which these observations occurred was not mentioned. Viremia was first detected for the control animal challenged with MARV–RAVV on day 3 and for that challenged with MARV–Angola on day 6. Peak viremia (>7 log10 pfu/mL) was attained on day 6 for both macaques. Macroscopic and microscopic pathology results were not reported.
Two cynomolgus macaques (country of origin unspecified) served as negative controls for a vaccine efficacy study.33 These animals were infected by aerosol exposure with 1000 pfu of MARV–Musoke. Both of these macaques were vaccinated with a vaccine vector containing the glycoprotein for Zaire ebolavirus (VSVΔG/ZEBOVGP) prior to viral challenge. One animal presented with a small macular rash on one arm on day 6 after challenge, and both showed reduced activity and signs of anorexia and depression on day 9. On day 10, both macaques had macular rashes, lymphopenia, thrombocytopenia, and elevated levels of serum enzyme activities associated with decreased liver function (liver function enzymes were not specified). Plasma viral load, detectable via the plaque assay on day 10 after challenge, was greater than 7 log10 pfu/mL. One animal succumbed to infection on day 10, whereas the other died on day 13. Among the tissues collected and evaluated for viral load, the highest level was in the liver at 9 log10 pfu/g. The lymphoid organs (spleen and axillary, inguinal, and mesenteric lymph nodes), adrenal gland, and bone marrow had the next highest viral load at approximately 7 log10 pfu/g, followed by the lung, kidney, testis, and pancreas at approximately 6 log10 pfu/g. The brain had the lowest viral load (5 log10 pfu/g). Macroscopic and microscopic pathology results were not reported.
Two cynomolgus macaques (country of origin unspecified) served as negative controls for a vaccine efficacy study.43 These animals were infected by intramuscular exposure with 1000 pfu of MARV–Musoke. Prior to viral challenge, these animals were vaccinated with a vaccine vector containing the glycoprotein for Zaire ebolavirus (VSVΔG/ZEBOVGP). The first clinical signs of disease presented on day 4, and both animals died on day 9. No additional information on clinical signs was presented. Plasma viral load was detectable via the plaque assay on day 6 after exposure but not at the previous measurement (on day 3). Peak viremia level was 8 log10 pfu/mL on day 9 for one animal and 6 log10 pfu/mL on day 6 for the other. Organ titers reportedly ranged from 3 to 9 log10 pfu/g, but the organs measured were not specified. Clinical pathology and macroscopic and microscopic pathology results were not reported.
A single cynomolgus macaque (country of origin unspecified) served as a negative control for a vaccine efficacy study.35 The animal was infected intramuscularly with 1000 pfu of MARV–Musoke and had been vaccinated with an irrelevant, nonspecific vaccine (VSVΔG/LASVGPC, a Lassa vaccine) prior to viral challenge. Lymphopenia and thrombocytopenia were reported on day 6 after challenge but not when hematology was evaluated previously on day 3. Clinical signs of anorexia and depression were reported later, presenting on days 8 through 10. In addition, this macaque had a mild rash on day 8 (focal areas of petechiae covering less than 10% of the skin), which progressively worsened (moderate rash, defined as areas of petechiae covering between 10% and 40% of skin) on day 9 to 10. Evidence of hepatobiliary (elevated ALT, AST, GGT, and total bilirubin) and kidney (elevated BUN, creatinine, and uric acid) impairment was seen on day 10 after challenge but not when measured previously on day 6. Plasma viral load levels approximated 6 log10 pfu/mL on days 6 and 10 after challenge. The animal succumbed to MVD on day 10 after challenge. Macroscopic and microscopic pathology results were not reported.
Three cynomolgus macaques (country of origin unspecified) served as negative controls in a vaccine efficacy study.38 All 3 received Venezuelan Equine Encephalitis replicon particles containing an irrelevant antigen (influenza hemagglutinin A) prior to subcutaneous viral challenge with 8000 pfu of MARV–Musoke. Clinical signs were reported on days 7 and 8 after challenge. However, although petechiae, weight loss, reduced activity, and fever were specifically mentioned for the experimental animals, the clinical signs seen in the control animals were not specified. Elevated AST was reported as early as day 5, with an additional increase on day 7. Other clinical chemistry parameters measured (ALT, ALP, and total bilirubin) were not reported as being changed relative to prechallenge levels. Hematology analysis was performed, but no results were reported. Viremia was seen as early as day 3, with peak viremia (7.5 to 8 log10 pfu/mL) on day 7. All 3 macaques died of disease between days 9 and 10 after challenge. Macroscopic and microscopic pathology results were not reported.
Five cynomolgus macaques (country of origin unspecified) served as negative controls for a postexposure therapeutic study with MARV-Musoke challenge.89 Four received a nonspecific therapeutic agent (designed to be effective against Ebola virus challenge) and one was given PBS. All 5 macaques were challenged subcutaneously with 1000 pfu MARV–Musoke. Petechiae, weight loss, fever, and lethargy were reported, but the days of their presentation were not indicated. Decreases in lymphocytes were seen for control groups as early as day 5 after challenge, with nadirs occurring on day 8. Macaques that received the nonspecific treatment showed decreases in platelets on day 8, but platelet counts had recovered for these animals by day 10. Increases in AST (but not ALT), ALP, bilirubin, and GGT were reported for both control groups on day 8, with additional increases on day 10. The PBS control macaque died on day 11, and the 4 other macaques died between days 9 and 12. Peak plasma viremia for all 5 controls was greater than 8 log10 pfu/mL on either day 8 or 10 after challenge. Macroscopic and microscopic pathology results were not reported.
Studies in rhesus macaques.
An initial virulence study was performed with 3 rhesus macaques, and 3 additional rhesus served as controls for a vaccine efficacy study (country of origin unspecified).34 The controls received PBS as a placebo prior to intramuscular injection of 1000 pfu of MARV–Angola. All 6 macaques had a fever (defined as greater than 1.3 °C higher than baseline or at least 0.8 °C higher than baseline and greater than or equal to 39.7 °C) on day 6 after challenge, with 1 of the 6 being febrile when temperature was measured previously (day 3). Five of 6 macaques were anorexic, and all 6 had rashes, mild to severe, between days 6 and 8. Lymphopenia was observed for all 6 macaques on day 3 or 6, followed by a rebound effect on WBC (leukocytosis) for some animals on day 7 or 8. Thrombocytopenia was reported for only 1 of the 6 animals (day 7). Evidence of hepatobiliary damage (elevated ALT, AST, total bilirubin, and GGT) was noted for all macaques as early as day 6. Evidence of coagulopathy (increased D-dimer concentration and decreased protein C activity) was present on day 6 or 7 for all 6 animals, and kidney damage (elevated BUN and creatinine) was seen on day 8 in 50% of the animals. Four macaques died from the infection on day 7, and the remaining 2 died on day 8.
Viremia in plasma (the 3 placebo controls only) was first detected on day 3, with peak viremia levels (>8 log10 pfu/mL) for these animals on day 6; viremia data for virulence study macaques were not provided. Viral titers in the liver and spleen were 9.1 and 8.8 log10 pfu/g, respectively. Viral antigen staining was prominent in liver and splenic red pulp; the adrenal medulla and pancreas also stained positive for antigen. Mean viral load in these and other tissues ranged from 5.3 to 8.4 log10 pfu/g. Immunopositive staining was prominent in hepatocytes and Kupffer cells in liver as well as in monocytes, macrophages, and fibroblasts in most tissues examined. Macroscopic observation showed reticulation and discoloration (pale tan) of the liver. Microscopic evaluation showed hepatocellular degeneration and necrosis with occasional neutrophils and monocytes. Although other tissues (adrenal gland, pancreas, and spleen) were collected and fixed in 10% neutral buffered formalin, histopathology findings were not reported. Phosphotungstic acid–hematoxylin staining revealed sporadic polymerized fibrin in medullary vessels of the kidneys of placebo controls
Three rhesus macaques (country of origin unspecified) were injected intramuscularly with 1000 pfu of MARV–Musoke as controls for a vaccine efficacy study.17 These animals were vaccinated with a nonspecific vaccine (VSVΔG/ZEBOVGP) prior to challenge. One monkey became febrile on day 6 after challenge, whereas the other 2 were febrile when temperature was measured next (day 10). All 3 macaques developed macular rashes on day 10. Viremia was first detected on day 6, with peak levels (6.9 to 7.5 log10 pfu/mL) on day 10 to 11. Leukocytosis with neutrophilia developed in late stages of the disease, and evidence of hepatobiliary (increased ALT, AST, GGT, and total bilirubin) and renal (elevated BUN) damage was reported on day10. Possible pancreatic damage (decreases in amylase) was evident on day 10 for 2 of the 3 controls. Two macaques died on day 11, and the remaining animal died on day 12. Macroscopic and microscopic pathology results were not reported.
Three rhesus macaques (country of origin unspecified) were injected intramuscularly with 1000 pfu of MARV–Musoke as controls for a vaccine efficacy study.32 Two (control animals 1 and 2) were vaccinated with an irrelevant, nonspecific vaccine (VSVΔG/LassaGPC), and the third animal (control animal 3) was not vaccinated. All 3 monkeys developed rashes (moderate to severe) and exhibited signs of anorexia and depression on day 10. In addition, fever was reported for control macaque 1 on day 10. Lymphopenia was noted for control animal 1 on day 10 and for control macaque 2 on day 6, and thrombocytopenia was reported for control animal 3 on day 10. Hepatobiliary (elevated AST, ALT, GGT, and total bilirubin) and renal damage (increased uric acid) was reported on day 10 but not when clinical pathology parameters were previously measured on day 6. Viremia was first detected in plasma on day 6, with a robust increase in viremia levels on day 10 (>7 log10 pfu/mL). Control macaques 1, 2, and 3 died on day 12, 12, and 11, respectively. Macroscopic and microscopic pathology results were not reported.
Two rhesus macaques (country of origin unspecified) were injected intraperitoneally with 200 LD50 of MARV–Pop as controls for a vaccine efficacy study.39 Macaques received PBS as placebo prior to challenge. Daily health-status assessments revealed a rash on arms, chest, and abdomen and around the eyes on day 5 to 6. Decreased body weight and increased ALT developed in both animals, but the days during which these changes were seen were not reported. Viremia was first detected on day 4 and reportedly increased throughout the course of the disease (data not shown). Increases in both IFN (type not specified) and TNFα were noted in the serum of control macaques as compared with surviving vaccinated animals. Cytokine production started as early as day 3 (INF) or day 5 (TNF) and continued to increase until the macaques’ death. Note that this publication predates FACS analysis and the availability of key reagents used in subsequent experiments to evaluate changes in cytokine production. A lymphocyte proliferation assay demonstrated a significant difference in the ability of MARV to induce proliferation in the control macaques as compared with surviving vaccinated animals (approximately 2-fold increase in proliferation in survivors). One animal died on day 10 and the other on day 11. Results of hematology, coagulation assays, macroscopic pathology, and microscopic pathology were not reported.
Studies in African green monkeys.
Nine African green monkeys (country of origin unspecified) were injected intraperitoneally with an unspecified variant or isolate of MARV to evaluate disease pathology.60 In light of the publication date, the variant likely is from one of the 3 outbreaks in 1967 that occurred in West Germany and Yugoslavia. The challenge inoculum was a 10−5 dilution of an infected rhesus macaque liver suspension; the virus used had been isolated from human blood and then passaged 9 times in guinea pigs and 3 times in rhesus macaques. The inoculum volume of 0.2 mL was determined to contain 200 to 260 LD50 according to titration by intraperitoneal inoculation of guinea pigs. As scheduled, one monkey was euthanized daily on days 1 through 9 after challenge, and sections of the lung, liver, and spleen were collected for viral load determination and for microscopic (light and electron) evaluation. Viral titers (expressed as the reciprocal of guinea pig LD50/0.2 mL) ranged from 6.5 to 8.5 for the liver and spleen and 4.5 to 7.5 for the lung; no virus was detected on days 1 and 2 after challenge. Because of the lack of viral titers in the lung, liver, and spleen tissues of the monkey euthanized on day 6, this monkey was thought to not have been infected, and no additional information was reported for this animal. Clinical signs and clinical pathology results were not reported.
Evaluation by light microscopy revealed lesions in the 3 tissues examined. Splenic lesions first appeared on day 1 after challenge, when proliferation of macrophages in the red pulp was observed. These lesions persisted and became more pronounced and widespread as the disease progressed, as demonstrated by the microscopic evaluation of tissues from animals euthanized closer to the usual time of death for this disease. Cellular necrosis in red pulp, necrosis of lymphocytes in white pulp, and accumulation of a large amount of cellular debris were observed in the spleen on day 9. Liver lesions first appeared on day 2 and consisted of swelling of Kupffer cells, necrotic hepatocytes, and increased numbers of macrophages in sinusoids. By day 3, the swollen Kupffer cells were filled with debris, and monocyte infiltration and parenchymatous degeneration were evident. On day 9, the severity of the liver lesions increased to include degeneration and necrosis of hepatocytes and the appearance of small centers of liquefaction of hepatocytes. Lung lesions were apparent on day 3 and were characterized initially by foci of interstitial cellular proliferations. This pattern progressed rapidly to foci of interstitial pneumonitis between days 4 and 5. By days 7 to 9, the lungs were only slightly affected by small foci of interstitial pneumonitis.
With the exception of the spleen on day 1, the presentation of viral antigen in the 3 tissues correlated with the timing that the lesions were first observed in the tissues. By days 7 to 9, viral particles had completely filled necrotic hepatocytes and were present in smaller numbers in the cells lining liver sinusoids. Considerable quantities of viral particles also were present within sinusoids of the spleen, mainly in macrophages, and scattered in moderate amounts in degenerating lymphocytes and in reticuloendothelial cells of the spleen. Furthermore, viral particles were detected in the lung, either inside the endothelial cells within the air sac capillaries or packed within the cytoplasm of macrophages.
Evaluation of the liver by electron microscopy revealed widely scattered foci involving 3 to 4 parenchymal cells and some cellular debris on day 3. In addition, extracellular viral particles were noted on day 3. On day 4, foci of infection in liver were more prevalent than on day 3, distention of endoplasmic reticulum was more marked, and mitochondrial breakdown was evident. At the same time, cellular debris and viral particles were present in sinusoids and pericapillary spaces, and some Kupffer cells contained phagocytosed debris; some of these cells showed normal architecture, whereas others exhibited degenerative changes. At day 5, there was progressive enlargement of individual necrotic foci, and their coalescence involved large areas of the liver and intercellular spaces, including bile canaliculi that contained many viral particles. Massive liver necrosis with the reduction of whole lobules to debris was evident between days 7 and 9. The late events of destruction of hepatic parenchymal cells also included fragmentation of the endoplasmic reticulum in infected cells, rupture of the plasma membrane, and increased lamellation, loss of cristae, and finally, disruption and dissolution of the mitochondria. In the remaining relatively intact areas of the liver, large numbers of viral particles were present between parenchymal cells in sinusoids, bile canaliculi, and pericapillary spaces. In spleen, phagocytosis of cellular debris was seen, starting on day 4 onward. In addition, phagocytosis of cellular debris by alveolar macrophages was present in lung tissue.
In another study involving African green monkeys,47 6 animals (country of origin unspecified) were injected subcutaneously with MARV–Pop as controls for a treatment efficacy study. These animals received human serum albumin as placebo for 5 d after challenge with a liver homogenate suspension from guinea pigs injected with a lethal dose of MARV. Targeted MARV challenge doses of 10, 100, or 1000 guinea pig LD50 were to be administered, according to viral titers of 8.1 log10 LD50/mL measured in guinea pigs by using the end-point dilution assay. Two animals were tested per challenge dose; however, 1 of the 2 animals in the group designated to receive 100 guinea pig LD50 was misdosed and was believed to have received 6 LD50 instead of 100.
Excluding the animal that was likely misdosed, fever (defined as greater than 1.5 °C increase in temperature) was seen in all control animals on day 4 or 6 after challenge. Anorexia was seen on day 4 or 5 as well. Some monkeys in the study also showed apathy or aggressiveness and occasional hemorrhage and bloody diarrhea; however, the group designation(s) for the affected animals were not mentioned. Blood viral titers were measured in guinea pigs by using the end-point dilution assay. Viral titers, first detected on day 4, peaked between days 8 and 10 with levels ranging from 4.75 to 6.00 guinea pig LD50/mL.
Of the 5 remaining control monkeys, 2 died on day 9, 2 died on day 12, and 1 died on day 13; time to death was not indicated for the control animal that was misdosed. Macroscopic findings of enlarged, clay- to sandy-colored liver with necrotic foci at the surface; enlarged dark-red spleen; and ulcers and necrotic foci in the large intestine were reported. However, no distinction was made whether these observations were seen in all groups, or just in some, and if so, which ones. Clinical pathology and microscopic pathology results were not reported.
Studies that included multiple NHP species.
In one study,36 African green monkeys and rhesus macaques (countries of origin unspecified) were infected with blood from either of 2 human patients or with blood from an infected guinea pig. The blood samples from the human patients were reported to have been collected during the acute phase of the disease; however, the fates of the patients were not disclosed. In addition, although not disclosed, the MARV variant(s) used likely originated from 1 or 2 of the 3 outbreaks in West Germany and Yugoslavia, in light of the publication date of the report.
Each NHP was housed in a separate cage, and the isolating partition separating pairs of cages was removed to allow for contact between pairs. Only one monkey of each pair was experimentally infected; the other served as a control. An additional 4 control animals (rhesus macaques) were placed 1 to 2 m from the infected animals but were not inoculated. These served to establish whether the agent could be transmitted over the 1- to 2-m distance via droplet transmission.
Two African green monkeys received human blood from patient 1 (1 mL subcutaneously) and 2 served as their paired controls. Two green monkeys more received human blood from patient 2 (0.5 mL subcutaneously), and another 2 served as their paired controls. An additional 2 monkeys received blood from a MARV-infected guinea pig (1 mL of guinea pig passage 4), and these were paired with their respective noninoculated controls. In addition, rhesus macaques were challenged with either human blood from patient 1 or guinea pig blood. The numbers per group, inoculation route, inoculation volumes, and passage information (guinea pig blood only) were the same as used for the African green monkeys.
Regardless of the inoculation volume or patient of origin, African green monkeys died on day 8 or 9 after experimental exposure to human blood, as did the 2 rhesus monkeys exposed to human blood. All animals experimentally infected with guinea pig blood died on day 7 or 8 after challenge. Paired rhesus controls, which had direct contact with the experimentally infected rhesus monkeys, died 16 d (paired with monkeys injected with guinea pig blood) and 18 d (paired with monkeys injected with blood from patient 1) after pairing. Paired African green monkey controls died 19 to 26 d, 18 to 20 d, and 15 to 36 d after being paired with African green monkeys experimentally challenged with blood from human patient 1, human patient 2, or guinea pig blood, respectively. All 4 rhesus monkeys with no direct contact with experimentally infected animals survived until day 76 of the study, when the experiment ended. These animals exhibited no signs of disease during the study.
Clinical signs for animals that succumbed to the disease included anorexia and lethargy, with only slight responsiveness to their environment. Rashes were not seen. Temperature increases for experimentally infected animals peaked on days 3 and 7 after inoculation, regardless of primate species. Paired rhesus controls had temperature spikes 3, 7, and 13 d after pairing, whereas paired African green controls had temperature spikes at 3, 7, and 15 d.
Viremia was determined as 8 log10 TCID50/mL (median tissue culture infective dose) in primary African green kidney cell culture by using blood collected on day 10 after inoculation from one of the 2 African green monkeys infected with blood from human patient 2. When the same blood (from the African green monkey) was used to infect additional African green monkeys, microscopic lesions developed in the liver, spleen, and lymphoid organs. The types of lesions were not specified. Clinical pathology and macroscopic pathology results were not reported.
In another multispecies study,93 20 rhesus macaques, 12 African green monkeys, and 2 squirrel monkeys (countries of origin unspecified) received an unspecified variant or isolate of MARV at various challenge doses and through various routes (intraperitoneal, intracerebral, and subcutaneous). Given the timing of the publication, the variant is believed to be from 1 of the 3 outbreaks in 1967 that occurred in West Germany and Yugoslavia. The inocula consisted of brain or liver suspensions from previously infected animals; the species from which the brain and liver tissues were obtained was not specified. These findings were limited to macroscopic and microscopic changes only. The authors noted that similar findings were seen in all monkey species.
Macroscopic findings included enlarged, dark, and friable liver that poured blood freely when cut, enlarged and dark bluish-purple to black spleen with soft and mushy pulp, enlarged and congested lymph nodes, presence of nodules and zones of consolidation in the lung, and congestion in the brain with frequent small hemorrhages.
Microscopic lesions affected the liver, spleen, lymph node, lung, brain, and kidneys. Liver lesions consisted of widespread degeneration and necrosis with significant debris and hemosiderin deposits in sinusoids and necrotic areas, swollen Kupffer cells containing cellular debris, and monocyte infiltration in periportal spaces. In the early stages, the spleen was severely congested, with clotting and fibrinous deposits in some sinuses; the red pulp showed proliferation of reticuloendothelial cells, many containing cellular debris; and the white pulp demonstrated lymphocyte depletion with necrosis at the periphery. In late stages, severe necrosis of red pulp, with complete destruction of all lymphoid elements, was seen. Lymph node lesions included coagulative necrosis affecting lymphocytes and reticuloendothelial cells. Lung lesions consisted of foci of interstitial pneumonitis, debris-filled macrophages in air sacs, clotting and endarteritis in the small arterioles, and fibrinous masses that occluded the lumina, with hypertrophied muscular walls in larger vessels. Brain lesions after intraperitoneal and subcutaneous viral inoculation included swollen endothelial cells and the occlusion of some capillaries with coagulated material. When present, kidney lesions included degeneration of the tubular epithelium and capillary swelling in glomeruli. Clinical signs, clinical pathology, and viral titers were not reported.
A third study involved a total of 8 African green monkeys, 22 rhesus macaques, and 2 squirrel monkeys (countries of origin unspecified) that received MARV at various doses and by various routes (intraperitoneal, intracerebral, subcutaneous, and intranasal).73 Various types of inocula were used, including whole blood from human patients, guinea pig blood (passage 3 or 9), blood from infected African green monkeys, or liver suspensions from infected rhesus monkeys (passage 3). The inoculated doses were calculated through intraperitoneal titration in guinea pigs and were expressed as ipLD50 per 0.1 mL. According to the study's publication date, the variant used likely is from one of the 3 outbreaks that occurred in West Germany and Yugoslavia in 1967.
The author noted that similar clinical signs occurred in all monkey species. Elevated temperatures were seen for all animals inoculated intraperitoneally, intranasally, or intracerebrally with primate blood or tissue or human blood, regardless of the primate species or challenge dose. The increase in temperatures for these animals generally started between days 4 and 6. In addition, elevated temperatures were present in rhesus macaques that received rhesus liver suspensions subcutaneously, with the onset generally occurring by day 6 or 7. The onset of temperature increase was delayed, however, occurring between days 8 and 10 for 3 of these animals, which were inoculated subcutaneously with low challenge doses (≤20 guinea pig ipLD50). Additional early clinical signs reported included occasional anorexia and consequent weight loss. Clinical signs reported during late stages of illness (1 or 2 d prior to death) included anorexia, marked weight loss, lethargy or depression, failure to respond to provocation, difficulty breathing, diarrhea, bleeding from rectum or vagina, and enlarged liver that could be palpated. Several animals, especially rhesus macaques, also had a petechial skin rash on arms and thighs, and, to a lesser extent, on the thorax, face, and neck. Blood drawn by venipuncture during terminal disease stages often failed to clot.
Hematologic analysis of blood from rhesus macaques infected with MARV revealed an initial increase in lymphocytes during the first few days after challenge, followed by a decrease between days 4 and 7. Thrombocytopenia, leukocytosis (due to neutrophilia), and an increase in clotting time were observed on day 7. The route of infection and inocula were not specified.
Reported in the same publication,73 African green monkeys were inoculated intraperitoneally with either blood from an infected guinea pig (n = 2) or blood from another infected African green monkey (n = 3). The monkeys inoculated with primate blood succumbed to the disease on day 6 (n = 1) and day 7 (n = 2) after challenge, whereas the 2 that were inoculated with guinea pig blood died on days 7 and 8. In addition, a rhesus macaque infected intraperitoneally with blood from an African green monkey died due to the disease on day 7. Viremia, as determined by titration in guinea pigs, was observed as early as days 1 and 2 after challenge for all animals, regardless of the inoculum or primate species.
Three African green monkeys, 4 rhesus macaques, and 2 squirrel monkeys were inoculated intraperitoneally with various dilutions of rhesus liver suspensions.73 The 3 African green monkeys died between days 7 and 8, the 4 rhesus monkeys died between days 7 and 10, and the 2 squirrel monkeys died on day 6. Although viremia was detected as early as day 1 after challenge for all 3 African green monkeys, it was delayed for rhesus monkeys (detected starting on day 4). Viremia was detected in squirrel monkey blood when first measured on day 4. In addition, virus was detected in the throat and rectal swabs of 3 of the rhesus monkeys on day 6 but from only 1 of the 3 on day 4. MARV was detected postmortem in brain, liver, lung, and spleen.
Eleven rhesus monkeys were inoculated subcutaneously with various dilutions of rhesus liver suspensions.73 The challenge doses were determined to range from 0.02 to 20,000 guinea pig ipLD50. Time to death generally appeared to be related to the challenge dose administered, ranging from day 7 after challenge (for the animal inoculated with the highest dose) to day 13 (for the animal inoculated with the lowest dose). With the exception of the monkey that received the lowest dose, which was viremic on day 9, all animals were viremic by day 6 or 7.
Two rhesus monkeys were inoculated intracerebrally. One received a challenge dose of 15,000 guinea pig ipLD50, and the other received a challenge dose of 1500 guinea pig ipLD50. A third rhesus monkey was inoculated intranasally with a challenge dose of 20,000 guinea pig ipLD50. The inoculum was a diluted suspension of liver tissue harvested from an infected rhesus macaque. All 3 macaques succumbed to the disease on day 7 after challenge. In the 2 animals inoculated intracerebrally, viremia was detected when first measured (day 3). For the animal inoculated intranasally, viremia was not detected on day 2 but was present when next assessed (day 5).
As part of the same study,73 3 rhesus macaques were inoculated intraperitoneally with blood from human patients: 2 received blood from patient 1, and the remaining animal received blood from patient 2. One of the 2 macaques that received blood from patient 1 presented with a petechial rash. Both animals that received blood from patient 1 died from disease on day 6, whereas the macaque that received blood from patient 2 succumbed on day 8.
Two NHP, one African green monkey and one rhesus macaque, that were held in the same room as were the experimentally infected animals became infected and died 7 to 9 d after postmortem examinations of the experimentally infected animals. Clinical chemistry, macroscopic, and microscopic results were not reported.
The final multispecies study we review involved 4 rhesus macaques and 9 African green monkeys (countries of origin unspecified), which all received an unspecified variant or isolate of MARV intraperitoneally to evaluate the pathology of MVD.75 Given the timing of the report's publication, the variant used likely is from one of the 3 outbreaks in 1967 that occurred in West Germany and Yugoslavia. Various types of inoculum (at 0.5 mL/animal) were injected, including human patient whole blood and postmortem brain and kidney tissue suspensions, blood from infected guinea pigs, blood from an infected African green monkey, and liver suspensions from an infected rhesus monkey. Clinical observations were made daily. Hematologic and histologic evaluations were performed. No other assessments were included.
The authors noted that similar findings occurred in all species evaluated. Animals presented with fever (defined as a temperature of at least 40.0 to 40.5 °C) after a 2- to 5-d incubation period, and fever persisted until immediately before death, which occurred on day 6 to 9 after challenge. Weight loss was noted as early as day 4. Lethargy or depression and failure to eat or drink were observed starting the day before death. Petechial rash occasionally developed during the terminal stages of the illness. One animal had diarrhea just before death, and another had rectal bleeding on day 5. On a few occasions, blood taken during the later stages of disease failed to clot.
Similar hematologic changes were seen in rhesus macaques and African green monkeys. Lymphocyte counts increased 1.6-fold by day 3 after challenge and then declined rapidly. Final lymphocyte counts in some animals were 10% of the prechallenge counts. Thrombocytopenia and leukocytosis (due to neutrophilia) were present during the late stages of the disease (approximately day 7). Clinical chemistry and viral titer results were not reported.
Macroscopic findings included enlarged, congested, and friable livers that poured blood freely when cut; moderately enlarged and dark bluish-purple spleen; enlarged mesenteric lymph nodes; and the presence of zones of consolidation in the lung. Macroscopic changes in the brain (blood vessels), gastrointestinal tract, pancreas, adrenals, kidneys, bladder, and testes were limited to congestion. The heart was normal in appearance, except for a small amount of clear pericardial fluid.
Microscopic findings in the liver included widespread degeneration and necrosis of hepatocytes with marked cellular debris, swollen Kupffer cells containing cellular debris, and phagocytic infiltration into sinusoids around the periportal spaces. Lesions of the spleen (examined only in animals with advanced-stage disease) included severe congestion and necrosis in red pulp with prominent cellular debris; lymphocyte depletion or complete destruction of lymphoid elements in white pulp, with evidence of necrosis in the central part of germinal centers; and destroyed or hypertrophied phagocytic cells throughout the tissue. All animals had interstitial pneumonitis, but the degree of lung involvement varied. Lung lesions also included debris- and macrophage-filled air sacs for some animals, hyperplastic arterioles in the muscular layer, and, in some cases, complete obliteration of the lumen as a result of hyperplasia in the small arteries. Lesions of the brain included hyperplastic endothelial cells of small venules and capillaries, with the lumen packed with RBC and occasional macrophages or filled with coagulated material.
Comparison of Marburg Virus Infection in Humans and Nonhuman Primates
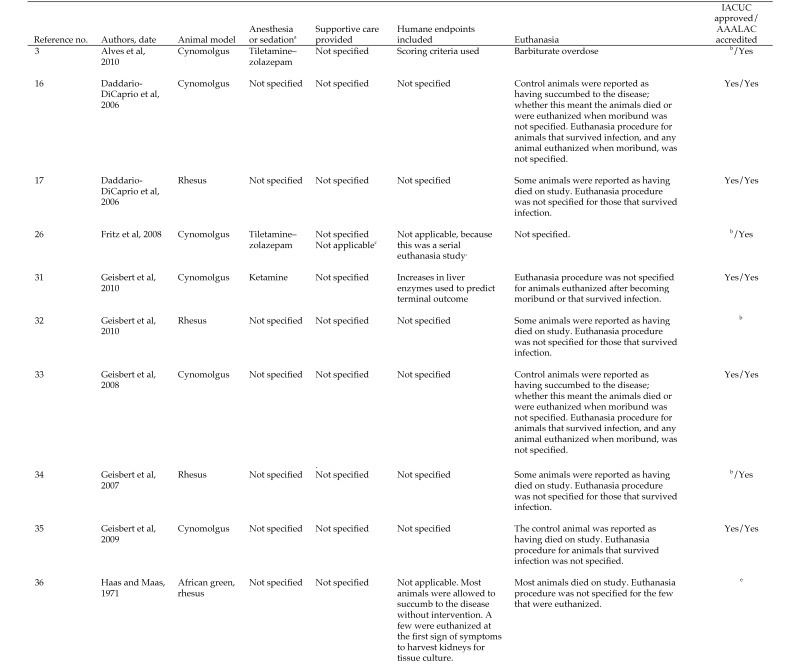
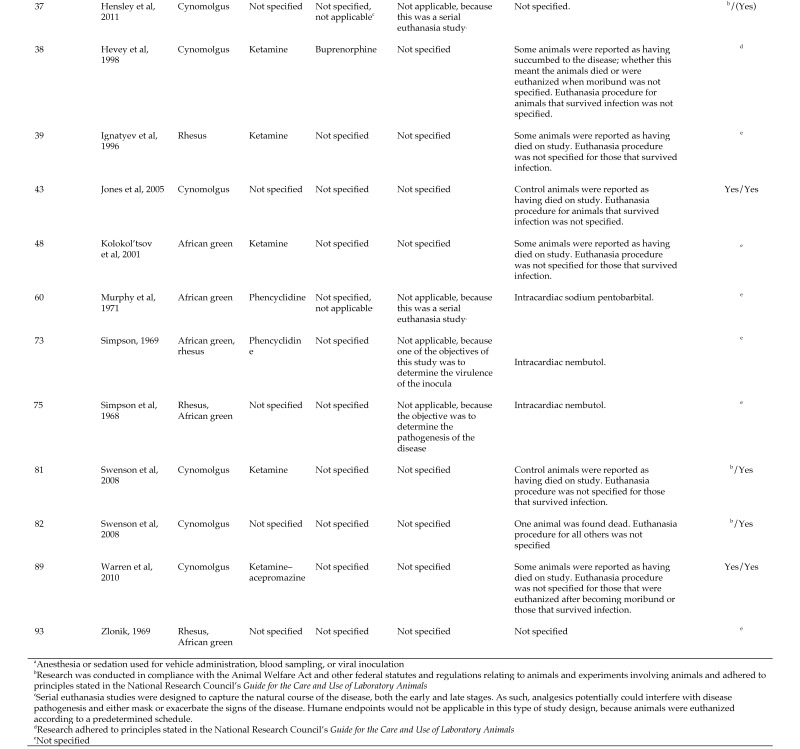
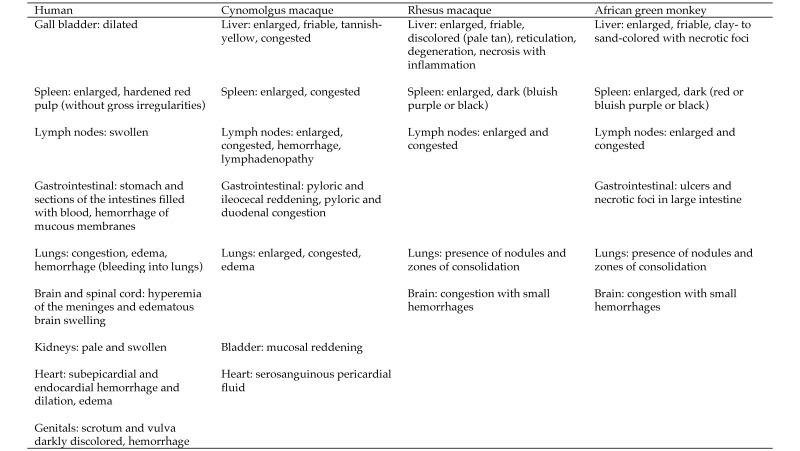
The first objective of this review was to describe the characteristics of MVD in humans, cynomolgus and rhesus macaques, and African green monkeys according to information available primarily in English-language, peer-reviewed journals. For each species, clinical signs, changes in hematology, coagulation, and clinical chemistry parameters, viremia, and macroscopic and microscopic pathology data, whenever available, were presented. In addition and again as available, time-to-onset of clinical signs, changes in clinical pathology parameters, and changes in pathology observations, as well as time-to-death, were reported. Our second objective was to compare (insofar as possible) the characteristics of MVD for humans and the 3 NHP species (Figures 1 through 7). For each NHP study, the anesthesia provided when painful procedures were performed, the analgesics given as supportive care, and any humane endpoints included are provided (data permitting) in Figure 8, which also indicates IACUC compliance and AAALAC accreditation.
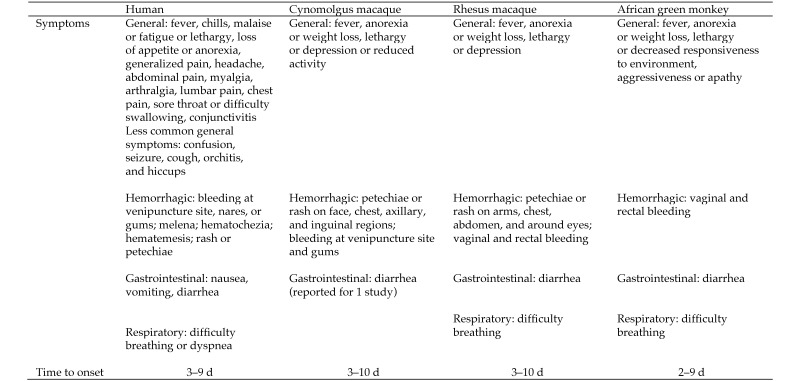
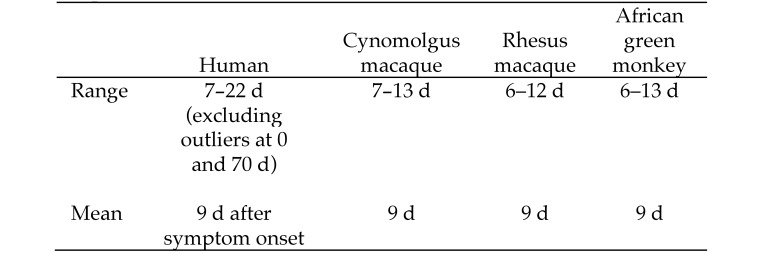
Figure 1.
Symptoms and clinical manifestations associated with MARV. Time to onset of symptoms (human) reported are based on the estimated date of infection and symptomatology in cases from South Africa or Germany. Clinical manifestations in NHP are reported as having occurred x number of days after the animal was challenged with MARV. The range for time to onset of symptoms/clinical manifestations of MVD is similar by comparison between each species of NHP—cynomolgus, rhesus, or African green monkey—and humans. In addition, even though some human symptoms (for example, headache, sore throat) cannot be easily discerned in NHP and although some clinical signs (for example, fever) were not always measured in animal studies, common clinical signs of fever, anorexia or weight loss, lethargy or depression or decreased responsiveness, and rash or petechiae were observed consistently in humans and cynomolgus and rhesus macaques.
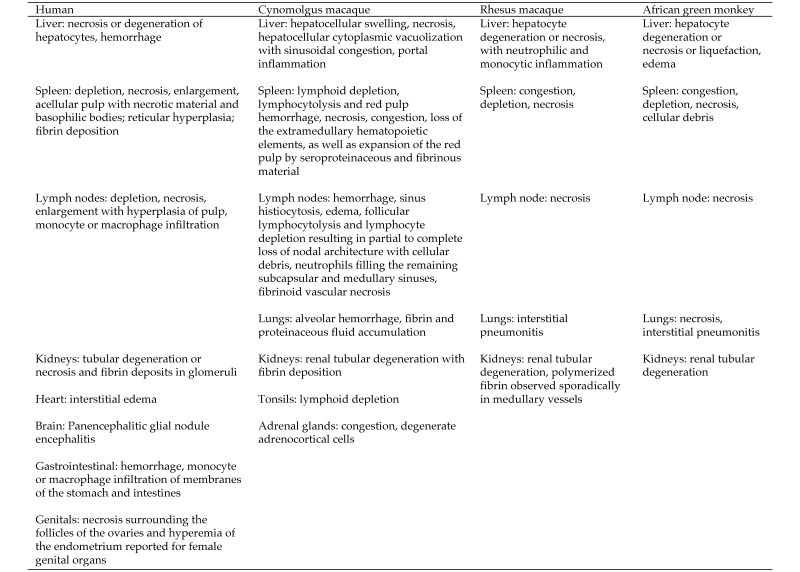
Figure 7.
Microscopic findings associated with MARV. Microscopic lesions of the liver, lymphoid organs, and kidney were common among humans and all 3 NHP species. Adrenal gland involvement was similar between humans and cynomolgus macaques.
Figure 8.
Documentation regarding humane treatment of animals used in studies of MARV.
Figure 2.
Time to death from MARV infection. The time to death for the human cases was based on the estimated date of exposure in cases from South Africa or Germany. The time to death for NHP was always calculated based on the date of challenge with MARV or RAVV. Times to death are similar for MARV or RAVV infections in humans and in each of the 3 NHP species.
Figure 3.
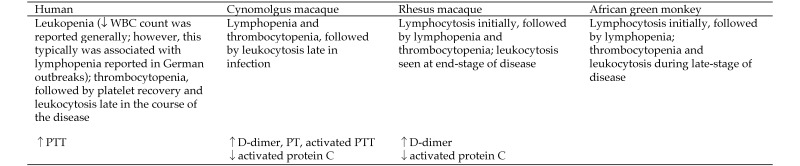
Hematology and coagulation parameters affected by MARV.
Figure 4.
Clinical chemistry parameters affected by MARV. Clinical chemistry data revealed evidence of hepatobiliary and renal damage for humans and cynomolgus and rhesus macaques infected with MARV or RAVV. In addition, an effect on the pancreas was noted for humans and rhesus monkeys; an increase in amylase is presumed, based on the diagnosis of pancreatitis.
Figure 5.
MARV virus detection and measurement. For cynomolgus and rhesus macaques, MARV and RAVV titers were quantitatively measured in blood and tissues by using plaque assays. For African green monkeys, only the viral titers determined by using the TCID50 assay are reported; titers determined in guinea pigs by using the end-point dilution method are not. Viral load data in humans were available only for 4 patients from the 1967 and 1975 outbreaks.91 To confirm active viral infection, human specimens have been tested by using animal inoculation or immunocytochemistry historically or PCR, immunohistochemistry, or ELISA more recently.
Figure 6.
Macroscopic findings associated with MARV.
When comparing the characteristics of MVD in humans with those in NHP, the limitations of the data sets should be considered. First, in the human cases, the time to the onset of clinical signs and symptoms and the time to death were estimated relative to presumptive exposure, the first sign or symptom, or even hospital admission. As a result, the time course of the presentation of signs and symptoms, the onset of laboratory and pathology changes, and the time to death for the course of the human disease all show considerable variability. In contrast, timing in the animal studies was always described relative to the time of exposure. In addition, records for some patients were kept only at admission and others at various hospitals in remote areas were incomplete. These factors contributed to an incomplete or inaccurate characterization of the disease for patients admitted to those hospitals. Moreover, the exposure doses and the route(s) of exposure were not known for the human cases. For NHP, the routes of exposure were always known but not always reported, and in most cases, either the theoretical or actual exposure doses were known as well.
For the NHP studies, clinical symptomatology, such as pain (headache, general, back, muscle, joint, abdominal, and so forth), could not be obtained, thus precluding comparison with the human disease. In addition, many variables emerged across the NHP studies, including differences in inoculation route; inoculum dose; and virus injected, such as viruses within the species Marburg mar burgvirus (MARV and RAVV), different variants (that is, Musoke and Angola), different isolates within a variant (for example, blood samples from different patients during an outbreak, that is, Pop and Ci67); and even whether animals were truly naïve prior to either challenge, PBS administration, vaccination with an irrelevant vaccine, or administration of a nonspecific therapeutic. Furthermore, the parameters measured varied across the NHP studies (that is, hematology parameters were measured for some, but not all, studies, and the tissues collected and evaluated for viral load or histology differed). In some cases, the same parameters were evaluated but were not measured at the same time after exposure. The considerable number of variables and the small number of animals per variable likely contributed to the variability in time to onset of clinical signs, changes in clinical pathology parameters, and time to death. In addition, the numerous variables and few animals per variable precluded drawing any conclusions regarding certain correlations. For example, no conclusions could be made regarding a dose–response association between the inoculum dose and time to death and whether animals that were naïve prior to challenge died sooner than those given an irrelevant vaccine. This difficulty remained even when there were small perceived differences in the time to onset of disease parameters or time to death between studies. There were simply too few animals per group to support definitive conclusions regarding these correlations.
Finally, although the clinical signs, laboratory results, and pathology for African green monkeys with MVD appeared to be similar to those for humans and rhesus and cynomolgus macaques, additional limitations affect the data set for green monkeys. Articles featuring MVD with this primate species either did not specify the variant or isolate used; the inoculum was passaged in guinea pigs prior to infecting monkeys, thereby changing the challenge material; or authors combined the results from multiple primate species, multiple infection routes, or multiple inocula when reporting the results.
Despite the limitations, several consistent patterns emerged regarding the clinical signs; changes in hematology, clinical chemistry, and coagulation parameters; and macroscopic and microscopic lesions associated with MVD. First, the disease characteristics in NHP (clinical signs, changes in hematology and clinical chemistry parameters, or lesions seen grossly or microscopically) did not differ between variants of MARV (Angola, Musoke, and the unnamed variant of which isolates Pop or Ci67 are members) or even between the 2 viral members of the species Marburg marburgvirus (MARV and RAVV). The review also revealed no differences in the clinical signs and symptoms of patients infected with RAVV compared with those infected with MARV. Similarly, the characteristics of the disease in humans did not differ between variants of MARV, even though the outbreaks occurred over several decades. Therefore, the characteristics of MVD caused by the variants of MARV and RAVV are discussed together for humans and for each NHP species. Finally, the review revealed no differences in the disease characteristics in NHP (in terms of the clinical signs, changes in hematology and clinical chemistry parameters, or gross or microscopic lesions reported) according to a particular inoculum route or dose.
In humans, common clinical signs reported for MVD (greater than 50% incidence) included fever, chills, malaise or fatigue or lethargy, anorexia or loss of appetite, pain (headache, general, back, muscle, joint, abdominal), gastrointestinal distress (nausea, vomiting, diarrhea), hemorrhagic symptoms, and rash or petechiae. It is noteworthy that rash or petechiae was documented in all MARV cases from the outbreaks in Marburg, Germany, and Johannesburg, South Africa, as well as from the imported cases in Holland and the United States (Colorado); however, it was uncommon or difficult to detect rash or petechiae on the dark skin of patients in the more recent African outbreaks. Less common signs and symptoms in human cases of MVD included difficulty breathing, pharyngitis or dysphagia, conjunctivitis, enlarged or swollen lymph nodes, and orchitis. Fever, lethargy or depression or decreased responsiveness, weight loss or anorexia, and hemorrhage were common for cynomolgus and rhesus macaques and African green monkeys. In addition, rash or petechiae was common in cynomolgus and rhesus macaques, whereas diarrhea and difficulty breathing were prevalent in some studies that included rhesus macaques and African green monkeys.
The time to onset of disease symptoms or signs was variable, ranging from 3 to 9 d in humans, 3 to 10 d in cynomolgus and rhesus macaques, and 2 to 9 d in African green monkeys. Time to death was estimated to be 7 to 22 d, with a mean of 9 d (on the basis of the presumed time of exposure, when known), in humans. The time to death in MARV- and RAVV-infected NHP was reported as 7 to 13 d (mean, 9 d) for cynomolgus macaques, 6 to 12 d (mean, 9 d) for rhesus macaques, and 6 to 13 d (mean, 9 d) for African green monkeys. As previously mentioned, the variability in time to disease onset and time to death is likely due to the overwhelming interstudy inconsistency in the time points and parameters measured and the few patients or animals for each variable. Notwithstanding, no differences were found among the NHP species or between humans and NHP regarding time to onset of disease and the ranges and means for time to death.
Hematologic changes for humans with MVD include leukopenia early in the disease, which likely was associated with the lymphopenia reported in some clinical cases, and thrombocytopenia. Leukocytosis and platelet recovery occurred late in infection for surviving patients. Lymphopenia and thrombocytopenia followed by leukocytosis and platelet recovery were reported for all 3 NHP species. Prior to lymphopenia, lymphocyte numbers reportedly increased occasionally in some of the studies that included rhesus macaques and African green monkeys. Clinical chemistry evaluations indicated that the liver–biliary system and kidney were targets in humans and cynomolgus and rhesus macaques, given the observed increases in ALT, AST, bilirubin, BUN, and creatinine. Pancreatitis was reported in a single human patient, suggesting an increase in amylase in this patient, whereas decreases in amylase were reported for rhesus macaques in one study. These results showed that clinical chemistry changes were generally similar between humans and rhesus and cynomolgus macaques. Clinical chemistry data were not reported for African green monkeys.
Viral load data for humans were limited to the 1967 and 1975 outbreaks and included only a few patients. Viral titers of 4 to 6 log10 TCID50/mL (n = 2 patients) were reported for blood, whereas titers of 4 to 7 log10 TCID50/g were reported for tissues (n = 4 patients). Blood viral load titers in African green monkeys were reported as 8 log10 TCID50/mL (single time point; day 10 after infection) in one study, and 5 to 6 guinea pig LD50/mL in another study. For cynomolgus and rhesus macaques, blood viral load titers ranged from 6 to 10 log10 pfu/mL and 7 to 8 log10 pfu/mL, respectively, whereas titers in tissues ranged from 5 to 9 log10 pfu/g for both species. The highest tissue titers were consistently found in liver and the lymphoid organs for both macaque species.
Macroscopic lesions developed in the gallbladder, lymphoid system (spleen and lymph nodes), gastrointestinal tract (stomach and sections of the intestines), lung, kidney, heart, brain or spinal cord, and genitals in humans. The liver, lymphoid system (spleen and lymph nodes), gastrointestinal tract (pyloric, ileocecum, and duodenum), lung, urinary bladder, and heart contained macroscopic lesions in cynomolgus macaques. In rhesus and African green monkeys, macroscopic lesions in the liver, lymphoid system (spleen and lymph nodes), lung, and brain were noteworthy. In addition, African green monkeys had additional lesions in the gastrointestinal tract (large intestine). Macroscopic lesions in the liver or gallbladder, lymphoid system and lung were common gross findings among humans and all 3 NHP species, whereas humans and cynomolgus and African green monkeys had the gastrointestinal tract as a common target. Humans and rhesus and African green monkeys shared the brain as a common target.
Microscopic pathology data in the literature were limited for humans and the 3 NHP species. Furthermore, in many cases, whether all tissues were evaluated but lesions occurred only in the tissues presented or whether just the few tissues that were presented were evaluated was unclear. Evaluation of human tissues showed hepatocyte necrosis, lymphoid depletion of the spleen and lymph nodes, and the presence of extracellular material in these tissues (thought to be virus or fibrin deposition). In addition, tubular necrosis with fibrin deposition in the glomeruli of the kidney was noted, as was hemorrhage in the gastrointestinal tract. Cynomolgus macaques demonstrated depletion, hemorrhage, or congestion of lymphoid tissues (lymph nodes, spleen, and tonsils); necrosis, vacuolation, and congestion in the liver; tubular degeneration of the kidneys; and hemorrhage and congestion of the lungs; lesions of the adrenal gland (congestion and degenerate adrenocortical cells) were observed also. Furthermore, significant fibrin deposition was present in the kidney, lung, spleen, and, occasionally, the liver of cynomolgus macaques. Degeneration or necrosis of the liver and kidney, depletion and necrosis of the lymphoid tissues (lymph nodes and spleen), and pneumonitis occurred in rhesus and African green monkeys. According to the microscopic evaluations, the liver, lymphoid tissues, and kidney were common target organs among humans, cynomolgus and rhesus macaques, and African green monkeys; and humans and cynomolgus monkeys had microscopic lesions of the adrenal gland in common.
Discussion
According to available published data, a primate MVD model—either cynomolgus or rhesus macaques—likely is appropriate for demonstrating a response to an antiviral product, vaccine, or postexposure prophylactic agent that would be predictive of the human response. Both of these NHP species are sensitive to wild-type virus; and, as with humans, both species develop lethal disease when untreated. The time-to-death ranges for both animal species were within that for humans. Clinical signs of fever, lethargy or depression, anorexia or weight loss, and rash or petechiae were common between humans and both cynomolgus and rhesus macaques, and these signs are easily measurable in a laboratory setting. Lymphopenia and thrombocytopenia, followed by leukocytosis and thrombocytosis, were common between humans and both of these NHP species, and the liver, spleen, lymph nodes, kidney, and lung were common target organs. As mentioned previously, the many gaps in the African green monkey data set precluded obtaining a complete and accurate description of MVD in this primate species. Furthermore, this data set was devoid of clinical chemistry evaluations to assist in fully characterizing this disease.
Supportive care will likely be a key component of patient care for MVD in developed countries. However in many remote regions, like those in which most MVD outbreaks have occurred and likely will occur in the future, effective supportive care was and likely will continue to be limited at best. Furthermore, the use of analgesics or other types of supportive care was mentioned for only one of the NHP studies reviewed (Figure 8). Depending on the intended use of a potential treatment or prophylaxis agent for which an animal model for MVD may be required, studies that include supportive care might be warranted.
Conclusion
The primate MVD model should be appropriate to demonstrate a response to test articles under development that would be predictive of the human response to MARV infection. As we have shown, the disease characteristics are generally similar for humans and the 3 NHP species that we reviewed. Furthermore, no single NHP species stood out as being more similar to humans than the others, and conducting complete natural history or pathogenesis studies on all 3 species is highly unlikely to result in such a distinction. Therefore, to optimize use of available resources, limit NHP use, and build on available data, either the cynomolgus or rhesus macaque model appears suitable for natural history studies and pivotal challenge or efficacy NHP studies to test MARV vaccine and treatment candidates. In addition, we suggest that because NHP are the only species other than humans that are sensitive to wild-type virus, a single NHP species for pivotal efficacy studies should be sufficient to support licensure under the US Food and Drug Administration's Animal Rule.
References
- 1.Adams MJ, Carstens EB. 2012. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2012). Arch Virol 157:1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjemian J, Farnon EC, Tschioko F, Wamala JF, Byaruhanga E, Bwire GS, Kansiime E, Kagirita A, Ahimbisibwe S, Katunquka F, Jeff B, Lutwama JJ, Downing R, Tappero JW, Formenty P, Amman B, Manning C, Towner J, Nichol ST, Rollin PE. 2011. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J Infect Dis 204:S796–S799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves DA, Glynn AR, Steele KE, Lackemeyer MG, Garza NL, Buck JG, Mech C, Reed DS. 2010. Aerosol exposure to the Angola strain of Marburg virus causes lethal viral hemorrhagic fever in cynomolgus macaques. Vet Pathol 47:831–851. [DOI] [PubMed] [Google Scholar]
- 4.Bausch DG, Nichol ST, Muyembe-Tamfum JJ, Borchert M, Rollin PE, Sleurs H, Campbell P, Tshioko FK, Roth C, Colebunders R, Pirard P, Mardel S, Olinda LA, Zeller H, Tshomba A, Kulidri A, Libande ML, Mulangu S, Formenty P, Grein T, Leirs H, Braack L, Ksiazek T, Zaki S, Bowen MD, Smit SB, Leman PA, Burt FJ, Kemp A, Swanepoel R. 2006. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N Engl J Med 355:909–919. [DOI] [PubMed] [Google Scholar]
- 5.Bechtelsheimer H, Korb G, Gedigk P. 1972. The morphology and pathogenesis of ‘Marburg virus’ hepatitis. Hum Pathol 3:255–264. [DOI] [PubMed] [Google Scholar]
- 6.Bonn D. 2005. Marburg fever in Angola: still a mystery disease. Lancet Infect Dis 5:331. [DOI] [PubMed] [Google Scholar]
- 7.Borchert M, Boelaert M, Sleurs H, Muyembe-Tamfum JJ, Pirard P, Colebunders R, Van der Stuyft P, van der Groen G. 2000. Viewpoint. Filovirus haemorrhagic fever outbreaks: much ado about nothing? Trop Med Int Health 5:318–324. [DOI] [PubMed] [Google Scholar]
- 8.Borchert M, Mulangu S, Swanepoel R, Libande ML, Tshomba A, Kulidri A, Muyembe-Tamfum JJ, Van der Stuyft P. 2006. Serosurvey on household contacts of Marburg hemorrhagic fever patients. Emerg Infect Dis 12:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borchert M, Muyembe-Tamfum JJ, Colebunders R, Libande M, Sabue M, Van Der Stuyft P. 2002. Short communication: a cluster of Marburg virus disease involving an infant. Trop Med Int Health 7:902–906. [DOI] [PubMed] [Google Scholar]
- 10.Bradfute SB, Warfield KL, Bray M. 2012. Mouse models for filovirus infections. Viruses 4:1477–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brauburger K, Hume AJ, Muhlberger E, Olejnik J. 2012. Forty-five years of Marburg virus research. Viruses 4:1878–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll SA, Towner JS, Sealy TK, McMullan LK, Khristova ML, Burt FJ, Swanepoel R, Rollin PE, Nichol ST. 2013. Molecular evolution of viruses of the family Filoviridae based on 97 whole-genome sequences. J Virol 87:2608–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) 2009. Imported case of Marburg hemorrhagic fever—Colorado, 2008. Morb Mortal Wkly Rep 58:1377–1381. [PubMed] [Google Scholar]
- 14.Clausen L, Bothwell TH, Isaacson M, Koornhof HJ, Gear JH, McMurdo J, Payn EM, Miller GB, Sher R. 1978. Isolation and handling of patients with dangerous infectious disease. S Afr Med J 53:238–242. [PubMed] [Google Scholar]
- 15.Colebunders R, Tshomba A, Van Kerkhove MD, Bausch DG, Campbell P, Libande M, Pirard P, Tshioko F, Mardel S, Mulangu S, Sleurs H, Rollin PE, Muyembe-Tamfum JJ, Jeffs B, Borchert M. 2007. Marburg hemorrhagic fever in Durba and Watsa, Democratic Republic of the Congo: clinical documentation, features of illness, and treatment. J Infect Dis 196 Suppl 2:S148–S153. [DOI] [PubMed] [Google Scholar]
- 16.Daddario-DiCaprio KM, Geisbert TW, Geisbert JB, Ströher U, Hensley LE, Grolla A, Fritz EA, Feldmann F, Feldmann H, Jones SM. 2006. Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J Virol 80:9659–9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daddario-DiCaprio KM, Geisbert TW, Ströher U, Geisbert JB, Grolla A, Fritz EA, Fernando L, Kagan E, Jahrling PB, Hensley LE, Jones SM, Feldmann H. 2006. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in nonhuman primates: an efficacy assessment. Lancet 367:1399–1404. [DOI] [PubMed] [Google Scholar]
- 18.Eggertson L. 2005. In the field, Canadians diagnose Marburg. Can Med Assoc J 172:1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enserink M. 2005. Infectious diseases. A puzzling outbreak of Marburg disease. Science 308:31–33. [DOI] [PubMed] [Google Scholar]
- 20.Enserink M. 2005. Infectious diseases. Crisis of confidence hampers Marburg control in Angola. Science 308:489. [DOI] [PubMed] [Google Scholar]
- 21.Feldmann H. 2006. Marburg hemorrhagic fever—the forgotten cousin strikes. N Engl J Med 355:866–869. [DOI] [PubMed] [Google Scholar]
- 22.Feldmann H, Czub M, Jones S, Dick D, Garbutt M, Grolla A, Artsob H. 2002. Emerging and re-emerging infectious diseases. Med Microbiol Immunol (Berl) 191:63–74. [DOI] [PubMed] [Google Scholar]
- 23.Feldmann H, Klenk HD. 1996. Marburg and Ebola viruses. Adv Virus Res 47:1–52. [DOI] [PubMed] [Google Scholar]
- 24.Foberg U, Frydén A, Isaksson B, Jahrling P, Johnson A, McKee K, Niklasson B, Normann B, Peters C, Bengtsson M. 1991. Viral haemorrhagic fever in Sweden: experiences from management of a case. Scand J Infect Dis 23:143–151. [DOI] [PubMed] [Google Scholar]
- 25.Food and Drug Administration (US). [Internet] 2014. Draft Guidance for Industry: Product Development Under the Animal Rule http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf.
- 26.Fritz EA, Geisbert JB, Geisbert TW, Hensley LE, Reed DS. 2008. Cellular immune response to Marburg virus infection in cynomolgus macaques. Viral Immunol 21:355–363. [DOI] [PubMed] [Google Scholar]
- 27.Gear JH. 1979. Hemorrhagic fevers, with special reference to recent outbreaks in southern Africa. Rev Infect Dis 1:571–591. [DOI] [PubMed] [Google Scholar]
- 28.Gear JH, Ryan J, Rossouw E. 1978. A consideration of the diagnosis of dangerous infectious fevers in South Africa. S Afr Med J 53:235–237. [PubMed] [Google Scholar]
- 29.Gear JS, Cassel GA, Gear AJ, Trappler B, Clausen L, Meyers AM, Kew MC, Bothwell TH, Sher R, Miller GB, Schneider J, Koornhof HJ, Gomperts ED, Isaäcson M, Gear JH. 1975. Outbreake of Marburg virus disease in Johannesburg. Br Med J 4:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gedigk P, Bechtelsheimer H, Korb G. 1969. The morbid anatomy of Marburg virus disease. Ger Med Mon 14:68–77, passim. [PubMed] [Google Scholar]
- 31.Geisbert TW, Bailey M, Geisbert JB, Asiedu C, Roederer M, Grazia-Pau M, Custers J, Jahrling P, Goudsmit J, Koup R, Sullivan NJ. 2010. Vector choice determines immunogenicity and potency of genetic vaccines against Angola Marburg virus in nonhuman primates. J Virol 84:10386–10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geisbert TW, Hensley LE, Geisbert JB, Leung A, Johnson JC, Grolla A, Feldmann H. 2010. Postexposure treatment of Marburg virus infection. Emerg Infect Dis 16:1119–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, Ströher U, Fritz EA, Hensley LE, Jones SM, Feldmann H. 2008. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine 26:6894–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geisbert TW, Daddario-DiCaprio KM, Geisbert JB, Young HA, Formenty P, Fritz EA, Larsen T, Hensley LE. 2007. Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein C2. J Infect Dis 196: Suppl 2:S372–S381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, Feldmann H. 2009. Single-injection vaccine protects nonhuman primates against infection with Marburg virus and 3 species of Ebola virus. J Virol 83:7296–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas R, Maass G. 1971. Experimental infection of monkeys with the Marburg virus. p 136–143. In: Martini GA, Siegert R. Marburg virus disease. Berlin (GER): Springer–Verlag Berlin. [Google Scholar]
- 37.Hensley LE, Alves DA, Geisbert JB, Fritz EA, Reed C, Larsen T, Geisbert TW. 2011. Pathogenesis of Marburg hemorrhagic fever in cynomolgus macaques. J Infect Dis 204 Suppl 3:S1021–S1031. [DOI] [PubMed] [Google Scholar]
- 38.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28–37. [DOI] [PubMed] [Google Scholar]
- 39.Ignatyev GM, Agafonov AP, Streltsova MA, Kashentseva EA. 1996. Inactivated Marburg virus elicits a nonprotective immune response in rhesus monkeys. J Biotechnol 44:111–118. [DOI] [PubMed] [Google Scholar]
- 40.Jeffs B, Roddy P, Weatherill D, de la Rosa O, Dorion C, Iscla M, Grovas I, Palma PP, Villa L, Bernal O, Rodriguez-Martinez J, Barcelo B, Pou D, Borchert M. 2007. The medecins sans frontieres intervention in the Marburg hemorrhagic fever epidemic, Uige, Angola, 2005. I. Lessons learned in the hospital. J Infect Dis 196 Suppl 2:S154–S161. [DOI] [PubMed] [Google Scholar]
- 41.Johnson ED, Johnson BK, Silverstein D, Tukei P, Geisbert TW, Sanchez AN, Jahrling PB. 1996. Characterization of a new Marburg virus isolated from a 1987 fatal case in Kenya. Arch Virol Suppl 11:101–114. [DOI] [PubMed] [Google Scholar]
- 42.Johnson KM. 1979. Ebola virus and hemorrhagic fever: andromeda strain or localized pathogen? Ann Intern Med 91:117–119. [DOI] [PubMed] [Google Scholar]
- 43.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 11:786–790. [DOI] [PubMed] [Google Scholar]
- 44.Kenyon RH, Niklasson B, Jahrling PB, Geisbert T, Svensson L, Fryden A, Bengtsson M, Foberg U, Peters CJ. 1994. Virologic investigation of a case of suspected haemorrhagic fever. Res Virol 145:397–406. [DOI] [PubMed] [Google Scholar]
- 45.Khan AS, Sanchez A, Pflieger AK. 1998. Filoviral haemorrhagic fevers. Br Med Bull 54:675–692. [DOI] [PubMed] [Google Scholar]
- 46.Kissling RE, Murphy FA, Henderson BE. 1970. Marburg virus. Ann N Y Acad Sci 174:932–945. [DOI] [PubMed] [Google Scholar]
- 47.Kolokol'tsov AA, Davidovich IA, Strel'tsova MA, Nesterov AE, Agafonova OA, Agafonov AP. 2001. The use of interferon for emergency prophylaxis of Marburg hemorrhagic fever in monkeys. Bull Exp Biol Med 132:686–688. [DOI] [PubMed] [Google Scholar]
- 48.Kuhn JH, Becker S, Ebihara H, Geisbert TW, Jahrling PB, Kawaoka Y, Netesov SV, Nichol ST, Peters CJ, Volchkov VE, Ksiazek TG. 2011. Family Filoviridae. p 665–671. In: King AMQ, Lefkowitz E, Adams MJ, Carstens EB. Virus taxonomy: 9th report of the International Committee on Taxonomy of Viruses. New York (NY): Academic Press. [Google Scholar]
- 49.Kuhn JH. 2008. Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. Vienna, Austria: Springer Verlag; [PubMed] [Google Scholar]
- 50.Kuhn JH, Bao Y, Bavari S, Becker S, Bradfute S, Brister JR, Bukreyev AA, Chandran K, Davey RA, Dolnik O, Dye JM, Enterlein S, Hensley LE, Honko AN, Jahrling PB, Johnson KM, Kobinger G, Leroy EM, Lever MS, Muhlberger E, Netesov SV, Olinger GG, Palacios G, Patterson JL, Paweska JT, Pitt L, Radoshitzky SR, Saphire EO, Smither SJ, Swanepoel R, Towner JS, van der Groen G, Volchkov VE, Wahl-Jensen V, Warren TK, Weidmann M, Nichol ST. 2013. Virus nomenclature below the species level: a standardized nomenclature for natural variants of viruses assigned to the family Filoviridae. Arch Virol 158:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuming BS, Kokoris N. 1977. Uveal involvement in Marburg virus disease. Br J Ophthalmol 61:265–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawrence J, Hill DR. 2005. Largest ever Marburg haemorrhagic fever outbreak, Angola. Euro Surveill 10:E050407.2. [DOI] [PubMed] [Google Scholar]
- 53.Leader A, Snyder A. 2006. Essays in public health and preventive medicine. History and health care in Angola. Mt Sinai J Med 73:567–568. [PubMed] [Google Scholar]
- 54.Ligon BL. 2005. Outbreak of Marburg hemorrhagic fever in Angola: a review of the history of the disease and its biological aspects. Semin Pediatr Infect Dis 16:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luby JP, Sanders CV. 1969. Green monkey disease (‘Marburg virus’ disease): a new zoonosis. Ann Intern Med 71:657–660. [DOI] [PubMed] [Google Scholar]
- 56.Marris E. 2005. Marburg workers battle to win trust of locals. Nature 434:946. [DOI] [PubMed] [Google Scholar]
- 57.Martini GA. 1969. Marburg agent disease: in man. Trans R Soc Trop Med Hyg 63:295–302. [DOI] [PubMed] [Google Scholar]
- 58.Martini GA. 1973. Marburg virus disease. Postgrad Med J 49:542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martini GA, Knauff HG, Schmidt HA, Mayer G, Baltzer G. 1968. A hitherto unknown infectious disease contracted from monkeys. ‘Marburg virus’ disease. Ger Med Mon 13:457–470. [PubMed] [Google Scholar]
- 60.Murphy FA, Simpson DI, Whitfield SG, Zlotnik I, Carter GB. 1971. Marburg virus infection in monkeys. Ultrastructural studies. Lab Invest 24:279–291. [PubMed] [Google Scholar]
- 61.Nakayama E, Saijo M. 2013. Animal models for Ebola and Marburg virus infections. Front Microbiol 4:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ndayimirije N, Kindhauser MK. 2005. Marburg hemorrhagic fever in Angola—fighting fear and a lethal pathogen. N Engl J Med 352:2155–2157. [DOI] [PubMed] [Google Scholar]
- 63.Rippey JJ, Schepers NJ, Gear JH. 1984. The pathology of Marburg virus disease. S Afr Med J 66:50–54. [PubMed] [Google Scholar]
- 64.Roberts A, Kemp C. 2001. Ebola and Marburg hemorrhagic fevers. J Am Acad Nurse Pract 13:291–292. [DOI] [PubMed] [Google Scholar]
- 65.Roddy P, Marchiol A, Jeffs B, Palma PP, Bernal O, de la Rosa O, Borchert M. 2009. Decreased peripheral health service utilisation during an outbreak of Marburg haemorrhagic fever, Uige, Angola, 2005. Trans R Soc Trop Med Hyg 103:200–202. [DOI] [PubMed] [Google Scholar]
- 66.Roddy P, Thomas SL, Jeffs B, Nascimento Folo P, Pablo Palma P, Moco Henrique B, Villa L, Damiao Machado FP, Bernal O, Jones SM, Strong JE, Feldmann H, Borchert M. 2010. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 201:1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roddy P, Weatherill D, Jeffs B, Abaakouk Z, Dorion C, Rodriguez-Martinez J, Palma PP, de la Rosa O, Villa L, Grovas I, Borchert M. 2007. The medecins sans frontieres intervention in the Marburg hemorrhagic fever epidemic, Uige, Angola, 2005. II. Lessons learned in the community. J Infect Dis 196 Suppl 2:S162–S167. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez A, Kiley MP, Klenk HD, Feldmann H. 1992. Sequence analysis of the Marburg virus nucleoprotein gene: comparison to Ebola virus and other nonsegmented negative-strand RNA viruses. J Gen Virol 73:347–357. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. 1996. The virion glycoproteins of Ebola viruses are encoded in 2 reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci USA 93:3602–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shu HL, Siegert R, Slenczka W. 1969. The pathogenesis and epidemiology of the ‘Marburg virus’ infection. Ger Med Mon 14:7–10. [PubMed] [Google Scholar]
- 71.Siegert R, Shu HL, Slenczka W. 1968. Detection of the ‘Marburg virus’ in patients. Ger Med Mon 13:521–524. [PubMed] [Google Scholar]
- 72.Siegert R, Shu HL, Slenczka W. 1968. Isolation and identification of the ‘Marburg virus.’ Ger Med Mon 13:514–518. [PubMed] [Google Scholar]
- 73.Simpson DI. 1969. Marburg agent disease: in monkeys. Trans R Soc Trop Med Hyg 63:303–309. [DOI] [PubMed] [Google Scholar]
- 74.Simpson DI. 1995. The filovirus enigma. Lancet 345:1252–1253. [DOI] [PubMed] [Google Scholar]
- 75.Simpson DI, Zlotnik I, Rutter DA. 1968. Vervet monkey disease. Experiment infection of guinea pigs and monkeys with the causative agent. Br J Exp Pathol 49:458–464. [PMC free article] [PubMed] [Google Scholar]
- 76.Slenczka W, Klenk HD. 2007. Forty years of Marburg virus. J Infect Dis 196 Suppl 2:S131–S135. [DOI] [PubMed] [Google Scholar]
- 77.Smith DH, Johnson BK, Isaacson M, Swanapoel R, Johnson KM, Killey M, Bagshawe A, Siongok T, Keruga WK. 1982. Marburg virus disease in Kenya. Lancet 319:816–820. [DOI] [PubMed] [Google Scholar]
- 78.Stille W, Böhle E, Helm E, van Rey W, Siede W. 1968. An infectious disease transmitted by Cercopithecus aethiops (‘Green monkey disease’). Ger Med Mon 13:470–478. [PubMed] [Google Scholar]
- 79.Strickland-Cholmley M, Malherbe H. 1970. Marburg virus. Lancet 295:476. [DOI] [PubMed] [Google Scholar]
- 80.Swanepoel R, Smit SB, Rollin PE, Formenty P, Leman PA, Kemp A, Burt FJ, Grobbelaar AA, Croft J, Bausch DG, Zeller H, Leirs H, Braack LE, Libande ML, Zaki S, Nichol ST, Ksiazek TG, Paweska JT. 2007. Studies of reservoir hosts for Marburg virus. Emerg Infect Dis 13:1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swenson DL, Wang D, Luo M, Warfield KL, Woraratanadharm J, Holman DH, Dong JY, Pratt WD. 2008. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol 15:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swenson DL, Warfield KL, Larsen T, Alves DA, Coberley SS, Bavari S. 2008. Monovalent virus-like particle vaccine protects guinea pigs and nonhuman primates against infection with multiple Marburg viruses. Expert Rev Vaccines 7:417–429. [DOI] [PubMed] [Google Scholar]
- 83.Timen A, Koopmans MP, Vossen AC, van Doornum GJ, Günther S, van den Berkmortel F, Verduin KM, Dittrich S, Emmerich P, Osterhaus AD, van Dissel JT, Coutinho RA. 2009. Response to imported case of Marburg hemorrhagic fever, the Netherlands. Emerg Infect Dis 15:1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Towner JS, Amman BR, Sealy TK, Carroll SA, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML, Formenty PB, Albarino CG, Miller DM, Reed ZD, Kayiwa JT, Mills JN, Cannon DL, Greer PW, Byaruhanga E, Farnon EC, Atimnedi P, Okware S, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Ksiazek TG, Nichol ST, Rollin PE. 2009. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog 5:e1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Towner JS, Khristova ML, Sealy TK, Vincent MJ, Erickson BR, Bawiec DA, Hartman AL, Comer JA, Zaki SR, Ströher U, Gomes da Silva F, del Castillo F, Rollin PE, Ksiazek TG, Nichol ST. 2006. Marburg virus genomics and association with a large hemorrhagic fever outbreak in Angola. J Virol 80:6497–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Towner JS, Pourrut X, Albariño CG, Nkogue CN, Bird BH, Grard G, Ksiazek TG, Gonzalez JP, Nichol ST, Leroy EM. 2007. Marburg virus infection detected in a common African bat. PLoS ONE 2:e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ursic-Bedoya R, Mire CE, Robbins M, Geisbert JB, Judge A, MacLachlan I, Geisbert TW. 2014. Protection against lethal Marburg virus infection mediated by lipid encapsulated small interfering RNA. J Infect Dis 209:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Warfield KL, Bradfute SB, Wells J, Lofts L, Cooper MT, Alves DA, Reed DK, VanTongeren SA, Mech CA, Bavari S. 2009. Development and characterization of a mouse model for Marburg hemorrhagic fever. J Virol 83:6404–6415 doi: 10.1128/JVI.00126-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, Garza NL, Dong L, Mourich DV, Crumley S, Nichols DK, Iversen PL, Bavari S. 2010. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat Med 16:991–994. [DOI] [PubMed] [Google Scholar]
- 90.World Health Organization. [Internet] 2005. Marburg haemorraghic fever update 25. [Cited 17 December 2006]. Available at: http://www.who.int/csr/don/2005_08_24/en/index.html.
- 91.World Health Organization. [Internet] 2008. Case of Marburg haemorraghic fever imported into the Netherlands from Uganda: fact sheet. [Cited 6 May 2014]. Available at: http://www.who.int/csr/don/2008_07_10/en/
- 92.Wulff H, Slenczka W, Gear JH. 1978. Early detection of antigen and estimation of virus yield in specimens from patients with Marburg virus disease. Bull World Health Organ 56:633–639. [PMC free article] [PubMed] [Google Scholar]
- 93.Zlotnik I. 1969. Marburg agent disease: pathology. Trans R Soc Trop Med Hyg 63:310–327. [DOI] [PubMed] [Google Scholar]