Summary
Background
To establish computed tomography (CT) staging of middle ear cholesteatoma and assess its impact on the selection of the surgical procedure.
Material/Methods
Prospective study was conducted on 61 consecutive patients (mean age 26.8 years) with middle ear cholesteatoma. CT scan of the temporal bone and surgery were performed in all patients. CT staging classified cholesteatoma according to its location in the tympanic cavity (T); extension into the mastoid (M); and associated complications (C). Cholesteatoma was staged as stage I (T1, T2), stage II (T3, M1, M2, C1), and stage III (C2).
Results
The overall sensitivity of CT staging of cholesteatoma compared to surgery was 88% with excellent agreement and correlation between CT findings and intra-operative findings (K=0.863, r=0.86, P=0.001). There was excellent agreement and correlation of CT staging with surgical findings for T location (K=0.811, r=0.89, P=0.001), good for M extension (K=0.734, r=0.88, P=0.001), and excellent for associated C complications (K=1.00, r=1.0, P=0.001). Atticotympanotomy was carried out in stage I (n=14), intact canal wall surgery was performed in stage II (n=38), and canal wall down surgery was done in stage III (n=5) and stage II (n=4).
Conclusions
We established CT staging of middle ear cholesteatoma that helps surgeons to select an appropriate surgery.
MeSH Keywords: Adenovirus Infections, Human; Anatomy, Comparative; Imaging, Three-Dimensional; Pain
Background
Middle ear cholesteatomas consist of ectopic keratinized epithelial tissue that grows inside the mucosa-lined middle ear cavity and desquamates, accumulating keratin and epithelial debris. They have erosive potential along the ossicles and bony walls of the middle ear cavity, mostly by means of an inflammatory response that activates osteoclastic activity. Most middle ear cholesteatomas (98%) are acquired. These are usually related to chronic inflammatory middle ear diseases combined with disturbed ventilation of the middle ear [1–4]. Annual incidence of cholesteatomas ranges around 3 in 100,000 in children and 9 in 100,00 in adults, and they are more predominant in men. To date, several pathogenic mechanisms have been proposed to explain the pathogenesis of cholesteatomas [4–8]. Imaging plays an important role in the assessment of patients with cholesteatoma of the middle ear. Owing to its high sensitivity, CT is a valuable tool for extension of cholesteatoma. The hallmarks of cholesteatoma on CT are a soft tissue mass-like opacity in the middle ear cavity and mastoid antrum associated with erosion of the ossicles and pressure erosion of adjacent structures [9–14].
A few studies have attempted to classify cholesteatoma of the middle ear on the basis of clinical or pathological data [14–17]. Cholesteatomas are classified as congenital and acquired, and they are subdivided into primary and secondary. Also, cholesteatomas may be classified based on the site of origin of cholesteatoma (attic, tympanic sinus and pars tensa) [18–20]. The proposed clinical staging classified cholesteatomas according to the site of cholesteatoma, its extension, and associated complications [21]. However, these classifications failed to gain clinical acceptance because they did not propose a management strategy for cholesteatoma [4]. CT has been used for staging of cholesteatoma of the external ear [22,23]. There is difficulty with interpretation of the published data concerning imaging of cholesteatoma. Also, there is an ongoing debate among otologists about the best appropriate surgery; the selected type of surgery for middle ear cholesteatoma is based mainly on intra-operative findings [24–28].
The aim of this work was to establish CT staging of middle ear cholesteatoma and to assess its impact on the selection of the surgical procedure.
Material and Methods
Consent of the editorial review board, as well as informed consent from patients were obtained. This prospective study was conducted in 63 consecutive untreated patients with middle ear cholesteatoma. The inclusion criteria were patients with clinically suspected middle ear cholesteatoma that planned to undergo surgery. Two patients were excluded because they refused surgery. The final patients included in this work were 61 patients with their age range from 5 to 57 years (mean, 26.8±14.5 years). They presented with otorrhea (n=61), hearing loss (n=56), aural polyp (n=13), post-auricular swelling (n=6) and vertigo (n=3). All patients underwent high-resolution CT of the temporal bone.
All imaging examinations were performed with a 16-section multi–detectorrow CT scanner (Light Speed; GE Healthcare, Milwaukee, WI). Helical transverse scans of the temporal bone were acquired in a plane parallel to the orbitomeatal plane with a section thickness of 0.5 mm, spacing of 0.3 mm with overlap, mA of 250 ms, kV of 120 ms, helical pitch of 0.625, rotation time of 0.8 second, and field of view of 240 mm. The raw data were reconstructed by using a bone algorithm to provide optimal visualization of the bony anatomy of the temporal bone. The images of the temporal bone were displayed at a window center of 400 HU and a window width of 4000 HU. Intravenous injection of 100 mL of nonionic contrast medium (nonionic iopromide; Ultravist 370, Schering, Berlin, Germany) at a rate of 2 mL per second was carried out in 5 patients suspected to have intracranial complications. Contrast medium was injected through an 18-gauge needle placed in the antecubital vein.
Image analysis was performed by one radiologist (AA) expert in head and neck imaging for 25 years and another head and neck surgeon with 10-year experience (AB), who were blinded to the clinical presentation and surgical findings. Any disagreement between both reviewers was solved in consensus. The CT staging classified cholesteatoma according to its location within the tympanic cavity (T), extent of mastoid involvement (M), and associated complications (C) (Table 1). The tympanic cavity involvement (T) was classified into attic cholesteatoma (T1), tympanic cholesteatoma (T2), atticotympanic cholesteatoma (T3). The T1 appeared as nondependent soft tissue mass opacity located in the attic region lateral to the ossicles, T2 occupied the tympanic space medial to the ossicles with involvement of the facial recess and sinus tympani, and T3 cholesteatoma occupied the attic and tympanic region with filling of almost entire middle ear cavity. The reporting system classified mastoid extension (M) of cholesteatoma into no mastoid involvement (M0), cholesteatoma extending into the mastoid antrum only (M1), and cholesteatoma extending into the mastoid air cells (M2). Finally, the reporting system classified complications (C) into uncomplicated cholesteatoma (C0), cholesteatoma with extracranial (intratemporal) complications (C1) such as mastoid abscess and labyrinthine fistula, and cholesteatoma with intracranial complications (C2) such as brain abscess and sinus thrombosis. Then, we staged cholesteatoma into 3 stages. Stage (I) where cholesteatoma was limited to one region of the middle ear cavity (T1 and T2), stage (II) where cholesteatoma extended into more than one compartment of the middle ear cavity (T3) or into the mastoid cavity (M1 and M2) and was associated with extracranial complications (C1), and stage (III) where cholesteatoma was associated with intracranial complications (C2).
Table 1.
Sensitivity of CT staging of middle ear cholesteama with surgical and CT findings.
| Classification | CT | Surgery | Sensitivity of CT staging |
|---|---|---|---|
| Tympanic cavity involvement (T): | |||
| T1: Attic cholesteatoma | 11 | 11 | |
| T2: Tympanic cholesteatoma | 13 | 12 | 85% |
| T3: Atticotympanic cholesteatoma | 37 | 38 | |
|
| |||
| Mastoid cavity involvement (M): | |||
| M0: No mastoid cavity involvement | 16 | 14 | |
| M1: Cholesteatoma extending into the mastoid antrum | 25 | 25 | 79% |
| M2: Cholesteatoma extending into the mastoid air cells | 20 | 22 | |
|
| |||
| Complications (C): | |||
| C0: Uncomplicated Cholesteatoma | 50 | 50 | |
| C1: Extracranial complications | 6 | 6 | 100% |
| C2: Intracranial complications | 5 | 5 | |
The time delay between CT and surgery was 5–10 days. Surgery was planned according to the CT staging of cholesteatoma for all patients. Postauricular atticotympanotomy was performed in patients with stage I; intact canal wall tympanomastoidectomy was carried out in patients with stage II cholesteatoma, and canal wall down mastoidectomy was done in patients with stage III cholesteatoma and some patients with stage II.
All statistical analyses were performed with a statistical package for social science (SPSS, version 16.0; SPSS, Chicago, Ill). The Kolmogorov-Smirnov Z-test was used to test the normality of continuous variable groups. The sensitivity of the CT system of cholesteatoma was calculated. The kappa statistic (K) including 95% confidence interval (CI) with an intra-class correlation (r) was conducted to estimate the proportion of agreement for CT and surgery for overall CT staging as well as for staging of T, M and C. The K values were interpreted as follows: k values between 0.00 and 0.20 represented poor; k values between 0.21 and 0.40 represented fair; k values between 0.41 and 0.60 represented moderate; k values between 0.61 and 0.80 represented good; k values between 0.81 and 1.00 represented excellent. A (P) value of less than 0.05 was considered to indicate a statistically significant difference.
Results
Table 1 shows the sensitivity of CT staging of middle ear cholesteatoma with surgical findings. Table 2 shows agreement and correlation of CT staging for middle ear cholesteatoma with surgical findings. The overall sensitivity of CT staging of middle ear cholesteatoma in comparison with surgery was 88% with underestimation in 3% of patients and overestimation in 9% of patients. There was an excellent agreement and correlation between CT staging of cholesteatoma and intra-operative findings (K=863; CI=0.687–0.951, r=0.86, P=0.001).
Table 2.
Sensitivity, agreement, and correlation of CT staging of middle ear cholesteatoma with surgical findings.
| Parameter | Sensitivity | K | 95% CI | R | P | |
|---|---|---|---|---|---|---|
| CT reporting | 88% | 0.863 | 0.687 | 0.951 | 0.86 | 0.001 |
| Tympanic | 85% | 0.811 | 0.669 | 0.932 | 0.89 | 0.001 |
| Mastoid | 79% | 0.734 | 0.573 | 0.912 | 0.88 | 0.001 |
| Complication | 100% | 1 | 1 | 1 | 1.00 | 0.001 |
Attic cholesteatoma (T1) (n=11) appeared as nondependent soft tissue mass opacity located in the attic region lateral to the ossicles. Tympanic cholesteatoma (T2) (n=13) occupied the tympanic space medial to the ossicles with involvement of the facial recess and sinus tympani. Atticotympanic cholesteatoma (T3) (n=37) occupied the attic and tympanic region with filling of almost entire middle ear cavity (Figure 1). The sensitivity of CT staging for diagnosis of tympanic cavity involvement of cholesteatoma was 85%. CT underestimated tympanic involvement of cholesteatoma in 5 patients and overestimated its location in 3 patients. There was an excellent agreement and correlation between CT staging and surgical findings (K=0.811, 95% CI=0.669–0.932, r=0.89, P=0.001) in T classification of cholesteatoma.
Figure 1.
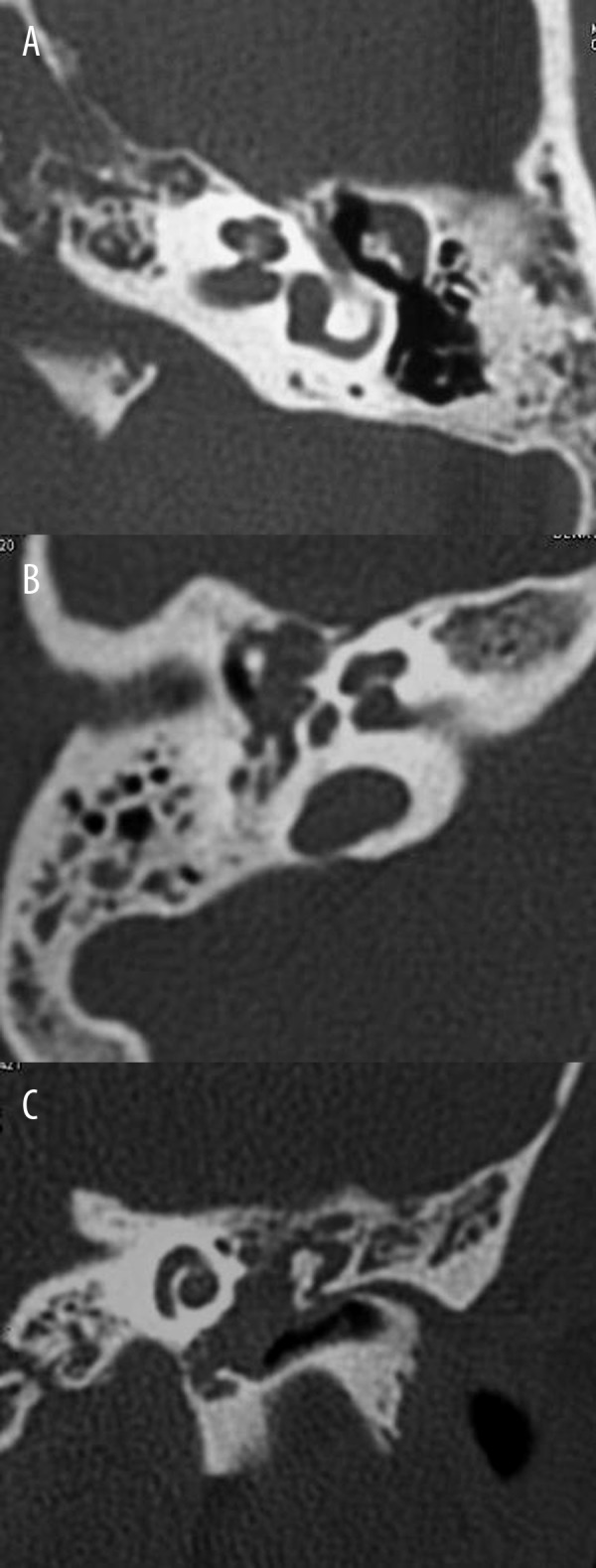
Location of cholesteatoma within the tympanic cavity (T): (A) Axial CT scan of the petrous bone shows attic cholesteatoma located in the attic region lateral to the ossicles (T1). (B) Axial CT scan shows tympanic cholesteatoma located in the tympanic region medial to the ossicles (T2). (C) Coronal CT scan shows atticotympanic cholesteatoma filling the whole middle ear cavity (T3).
According to CT staging, the mastoid air cells were clear (M0) in 16 patients. Cholesteatoma extended into the mastoid antrum (M1) in 25 patients and into the mastoid air cells (M2) in 20 patients (Figure 2). At surgery, the mastoid cavity was clear in 14 patients, the antrum only was involved by cholesteatoma in 25 patients, and the mastoid air cells with antrum were affected in 22 patients. The sensitivity of the CT reporting system for detection of mastoid extension of cholesteatoma was 79%. CT underestimated the extension of cholesteatoma into mastoid in 12 patients (16%). Also, CT overestimated the extension of cholesteatoma into the mastoid cavity in 3 patients (5%) with M2. There was good agreement and correlation between CT staging and intra-operative findings in M classification (K=0.734, 95% CI=0.573–0.912, r=0.88, P=0.001).
Figure 2.
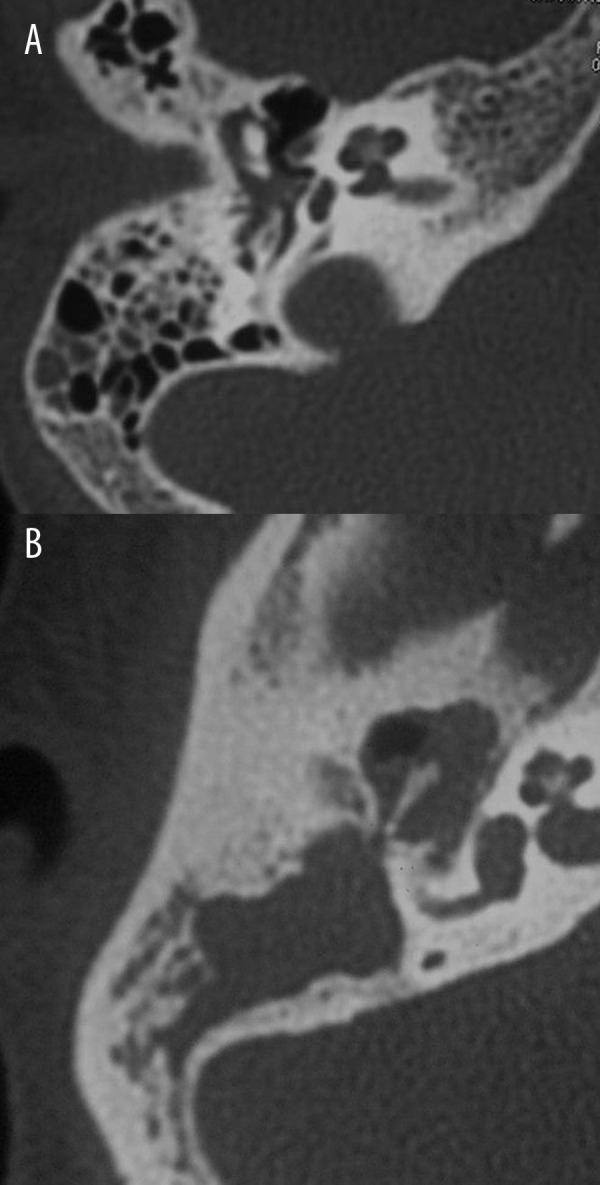
Extension of cholesteatoma into the mastoid (M): (A) Axial CT scan of the petrous bone shows cholesteatoma extending into the mastoid antrum (M1). (B) Axial CT scan shows cholesteatoma extending into the mastoid air cells and mastoid cavity (M2).
No associated complications (C0) were detected in 50 patients. At CT, extracranial complications (C1) of cholesteatoma were observed in 6 patients in the form of mastoid abscess (n=3), postauricular abscess (n=2) and labyrinthine fistula (n=1). Intracranial complications (C2) were detected in 5 patients in the form of brain abscess (n=3) (Figure 3), and sinus venous thrombosis (n=2). Surgery showed similar findings. The sensitivity of CT in detection of complications was 100%. There was an excellent agreement and correlation between CT staging and intra-operative findings regarding C classification (K=1, 95% CI=1–1, r=1.0, P=0.001).
Figure 3.
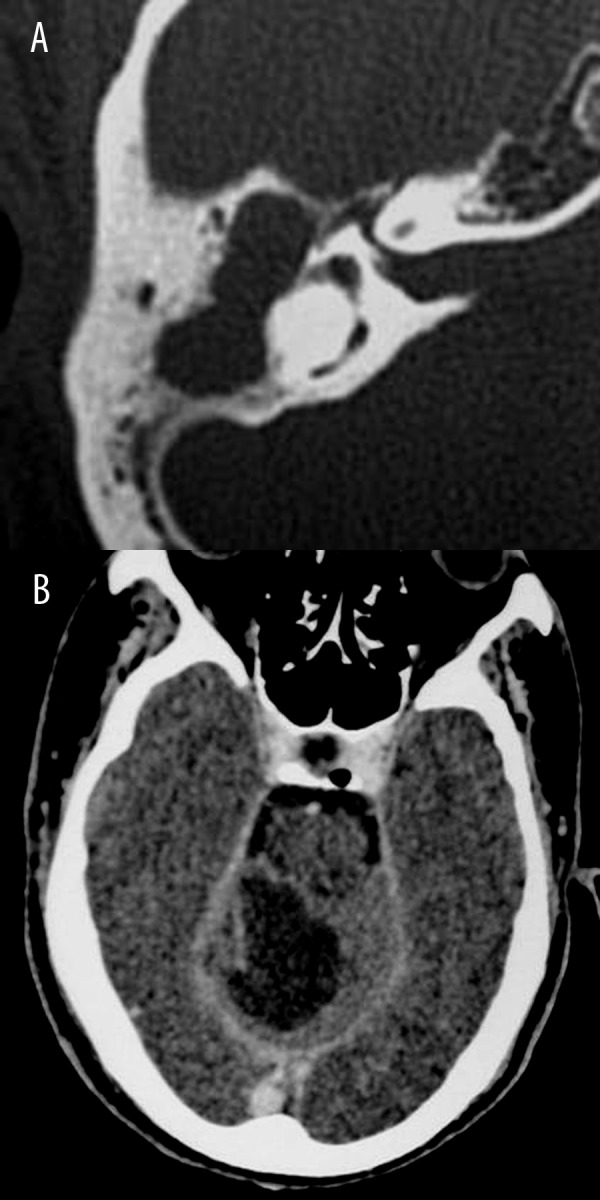
Acquired cholesteatoma of the middle ear with intracranial abscess (C2): (A) Axial CT scan of the petrous bone shows cholesteatoma of the right middle ear and right mastoid. (B) Axial contrast CT scan of the same patient shows marginally enhanced right cerebellar abscess.
The stages of cholesteatoma were as follows: I in 14 patients, II in 42 patients, and III in 5 patients. Atticotympanotomy was performed in patients with stage I (n=14). Intact canal wall tympanomastoidectomy was carried out in 38 patients with stage II. Canal wall down mastoidectomy was done in patients with stage III (n=5) in addition to 4 patients at stage II (dead ear in 2 patients, inadequate access with a contracted mastoid in another 2 patients). No under- or overestimation affected the decision on surgery except for one case of attic cholesteatoma (T1) on CT that revealed associated mastoid involvement (M1) at surgery so the decision was changed from atticotympanotomy to intact canal wall tympanomastoidectomy.
Discussion
In this study, the sensitivity of CT staging for middle ear cholesteatoma is 88% with excellent agreement and correlation with surgical findings. The overestimation (9%) of the extent of choesteatoma at CT may be attributed to cholesteatoma sac, associated granulation tissue, mucosal edema and effusion that may be indistinguishable in CT scans. Although cholesteatoma revealed a lower attenuation value than granulation tissue, the difference is subtle on CT and the differentiation of cholesteatoma using attenuation values is impossible [29,30]. Differentiation between cholesteatoma and scar tissue requires non-echoplanar diffusion weighted MR imaging [1–3]. The cause of underestimation (3%) may be attributed to difficult sharp anatomical demarcation between the mastoid antrum and the surrounding air cells on CT. Nag et al. [16] reported good to excellent radiosurgical agreement in the assessment of the status of various middle ear structures.
In this study, attic cholesteatoma was present in 18% of patients, tympanic in 21.3%, and atticotymapnic in 60.7%. On CT, attic cholesteatoma appears as nondependent soft tissue mass opacity located in Prussak’s space lateral to the ossicles, while tympanic cholesteatoma occupies the tympanic space medial to the ossicles, usually with typical involvement of the facial recess and sinus tympani. In atticotymapnic cholesteatoma, cholesteatoma fills most of the tympanic cavity [31,32]. Attic cholesteatoma was seen in 41% of patients, tympanic cholesteatoma in 45%, and combined attic and tympanic cholesteatoma in 14% [4].
Cholesteatoma is primarily a middle ear pathology and not a mastoid pathology. Therefore, surgery for cholesteatoma should be limited to the middle ear and then follow the cholesteatoma into the mastoid cavity [5–7]. The superoposterior communication between the attic and the mastoid antrum is called the aditus ad antrum. When cholesteatoma is present, it may erode its wall and widen the “waistline” (aditus) resulting in the loss of the “figure of 8” [20–24]. The extent of mastoid involvement in patients with cholesteatoma has an impact on patient management. CT is important to determine the extent of the mastoid air cells to be exenterated to avoid disease recurrence [1–3].
In this work, the sensitivity of CT for detection of mastoid involvement was 79%.
Extracranial and intracranial complications may be seen in patients with cholesteatoma. The presence of a complication and its location helps in the choice of treatment [1–3]. Labyrinthine fistula with involvement of the lateral semicircular canal was reported in 7% of patients with cholesteatoma. The presence of a pneumolabyrinth is a definite sign of fistulas, but its detection is unusual [2–4]. Subperiosteal abscess is the most common extratemporal complication caused by the spread of infection from the mastoid towards the periosteal space by erosion of the mastoid cortex [1–4]. Brain abscess especially in the temporal lobe and cerebellum is the most common intracranial complication of cholesteatoma [3–8]. Lateral or sigmoid sinus thrombosis makes up 20% of intracranial complications of cholesteatoma. The mechanism is either bone erosion exposing the perisinus space, or spread of mastoid emissary vein thrombophlebitis [5–7]. In this study, extracranial complications were detected in 6 patients and intracranial complications were reported in 5 patients with cholesteatoma.
The decision to perform an intact canal wall, canal wall down, or transcanal atticotomy for cholesteatoma is a problem for otologists. This staging system may help with a correct and possibly less extensive surgical procedure, as well as better clinical results, as it helps the surgeon to select the appropriate surgery of cholesteatoma according to its location, extension into the mastoid cavity and presence of associated complications. Further studies are recommended to correlate this staging with patient-reported outcomes.
There are a few limitations of this study. First, the study included a small number of patients; multicenter studies with validation on a large number of patients enhance the application of CT staging for cholesteatoma. Second; this study was conducted on 16 multi-detector CT scanners. Application of higher multi-detector CT scanners, such as 64 or 256, dual energy CT and cone beam CT will improve the image quality [33–35]. Moreover, comparing CT results with diffusion MR imaging and contrast MR imaging in complicated cases will improve the results.
Conclusions
We established a new non-invasive CT staging of middle ear cholesteatoma that helps the surgeons to select the appropriate type of surgery.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Barath K, Huber A, Stampfli P, et al. Neuroradiology of cholesteatomas. Am J Neuroradiol. 2011;32:221–29. doi: 10.3174/ajnr.A2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juliano AF, Ginat DT, Moonis G. Imaging review of the temporal bone: part I. Anatomy and inflammatory and neoplastic processes. Radiology. 2013;269:17–33. doi: 10.1148/radiol.13120733. [DOI] [PubMed] [Google Scholar]
- 3.Más-Estellés F, Mateos-Fernández M, Carrascosa-Bisquert B, et al. Contemporary non-echo-planar diffusion-weighted imaging of middle ear cholesteatomas. Radiographics. 2012;32:1197–213. doi: 10.1148/rg.324115109. [DOI] [PubMed] [Google Scholar]
- 4.Lemmerling M, De Foer B, Verbist B, VandeVyver V. Imaging of inflammatory and infectious diseases in the temporal bone. Neuroimag Clin North Am. 2009;19:321–37. doi: 10.1016/j.nic.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Bruce B, Ian G. Acquired cholesteatoma: classification and outcomes. Otol Neurotol. 2011;32:992–95. doi: 10.1097/MAO.0b013e3182255874. [DOI] [PubMed] [Google Scholar]
- 6.Trojanowska A, Drop A, Trojanowski P, et al. External and middle ear diseases: radiological diagnosis based on clinical signs and symptoms. Insights Imaging. 2012;3:33–48. doi: 10.1007/s13244-011-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Razek A, Huang B. Lesions of the Petrous Apex: Classification and Findings at CT and MR Imaging. Radiographics. 2012;32:151–73. doi: 10.1148/rg.321105758. [DOI] [PubMed] [Google Scholar]
- 8.Yildirim-Baylan M, Ozmen C, Gun R, et al. An evaluation of preoperative computed tomography on patients with chronic otitis media. Indian J Otolaryngol Head Neck Surg. 2012;64:67–70. doi: 10.1007/s12070-011-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majithia A, Lingam R, Nash R, et al. Staging primary middle ear cholesteatoma with non-echoplanar (half-Fourier-acquisition single-shot turbo-spin-echo) diffusion-weighted magnetic resonance imaging helps plan surgery in 22 patients: Our experience. Clin Otolaryngol. 2012;37:325–30. doi: 10.1111/j.1749-4486.2012.02502.x. [DOI] [PubMed] [Google Scholar]
- 10.Ayache D, Darrouzet V, Dubrulle F, et al. Imaging of non-operated cholesteatoma: Clinical practice guidelines. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:148–52. doi: 10.1016/j.anorl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Eshetu T, Aygun N. Imaging of the temporal bone: A symptom-based approach. Semin Roentgenol. 2013;48:52–64. doi: 10.1053/j.ro.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita K, Yoshiura T, Hiwatashi A, et al. Contributing factors in the pathogenesis of acquired cholesteatoma: Size analysis based on MDCT. Am J Roentgenol. 2011;196:1172–75. doi: 10.2214/AJR.10.5414. [DOI] [PubMed] [Google Scholar]
- 13.Stasolla A, Magliulo G, Cortese A, et al. Preoperative imaging assessment of chronic otitis media: what does the otologist need to know? Radiol Med. 2011;116:114–24. doi: 10.1007/s11547-010-0589-x. [DOI] [PubMed] [Google Scholar]
- 14.Lemmerling M, De Foer B, VandeVyver V, et al. Imaging of the opacified middle ear. Eur J Radiol. 2008;66:363–71. doi: 10.1016/j.ejrad.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Schmerber S, Lefournier V, Karkas A. What the surgeon cannot see and needs to see before middle ear surgery. J Otorhinolaryngol Relat Spec. 2010;72:145–57. doi: 10.1159/000272136. [DOI] [PubMed] [Google Scholar]
- 16.Ng JH, Zhang E, Soon S, et al. Pre-operative high resolution computed tomography scans for cholesteatoma: Has anything changed? Am J Otolaryngol Head and Neck Med and Surg. 2014;35:508–15. doi: 10.1016/j.amjoto.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Corrales CE, Blevins NH. Imaging for evaluation of cholesteatoma: current concepts and future directions. Curr Opin Otolaryngol Head Neck Surg. 2013;21:461–67. doi: 10.1097/MOO.0b013e328364b473. [DOI] [PubMed] [Google Scholar]
- 18.Manasawala M, Cunnane ME, Curtin HD, Moonis G. Imaging findings in auto-atticotomy. Am J Neuroradiol. 2014;35:182–85. doi: 10.3174/ajnr.A3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleh HA, Mills RP. Classification and staging of cholesteatoma. Clin Otolaryngol Allied Sci. 1999;24:355–59. doi: 10.1046/j.1365-2273.1999.00272.x. [DOI] [PubMed] [Google Scholar]
- 20.Tos M. Cartilage tympanoplasty methods: proposal of a classification. Otolaryngol Head Neck Surg. 2008;139:747–58. doi: 10.1016/j.otohns.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Belal A, Reda M, Mehana A, Belal Y. TMC: A new staging system for tympano-mastoid cholesteatoma. J Int Adv Otol. 2012;8:63–68. [Google Scholar]
- 22.Shin SH, Shim JH, Lee HK. Classification of external auditory canal cholesteatoma by computed tomography. Clin Exp Otorhinolaryngol. 2010;3:24–26. doi: 10.3342/ceo.2010.3.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inokuchi G, Okuno T, Hata Y, et al. Congenital cholesteatoma: posterior lesions and the staging system. Ann Otol Rhinol Laryngol. 2010;119:490–94. doi: 10.1177/000348941011900711. [DOI] [PubMed] [Google Scholar]
- 24.Tomlin J, Chang D, McCutcheon B, Harris J. Surgical technique and recurrence in cholesteatoma: A meta-analysis. Audiol Neurootol. 2013;18:135–42. doi: 10.1159/000346140. [DOI] [PubMed] [Google Scholar]
- 25.Galm T, Martin TP, Raut V. Open and closed cavity mastoid operations: comparing early hearing results. Eur Arch Otorhinolaryngol. 2013;270:77–80. doi: 10.1007/s00405-011-1914-2. [DOI] [PubMed] [Google Scholar]
- 26.Gaillardin L, Lescanne E, Morinière S, et al. Residual cholesteatoma: Prevalence and location. Follow-up strategy in adults. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:136–40. doi: 10.1016/j.anorl.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 27.lvarez F, Gómez J, Bernardo M, Suárez C. Management of petrous bone cholesteatoma: Open versus obliterative techniques. Eur Arch Otorhinolaryngol. 2011;268:67–72. doi: 10.1007/s00405-010-1349-1. [DOI] [PubMed] [Google Scholar]
- 28.Carlson ML, Latuska RF, Pelosi S, et al. Evolving considerations in the surgical management of cholesteatoma in the only hearing ear. Otol Neurotol. 2014;35:84–90. doi: 10.1097/MAO.0b013e3182a00495. [DOI] [PubMed] [Google Scholar]
- 29.Park M, Rah Y, Kim Y, Kim J. Usefulness of computed tomography Hounsfield unit density in preoperative detection of cholesteatoma in mastoid ad antrum. Am J Otolaryngol Head Neck Med Surg. 2011;32:194–97. doi: 10.1016/j.amjoto.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Marchioni D, Mattioli F, Cobelli M, et al. CT morphological evaluation of anterior epitympanic recess in patients with attic cholesteatoma. Eur Arch Otorhinolaryngol. 2009;266:1183–89. doi: 10.1007/s00405-008-0871-x. [DOI] [PubMed] [Google Scholar]
- 31.Rogha M, Hashemi SM, Mokhtarinejad F, et al. Comparison of preoperative temporal bone CT with intraoperative findings in patients with cholesteatoma. Iran J Otorhinolaryngol. 2014;26:7–12. [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips G, LoGerfo S, Richardson M, Anzai Y. Interactive web-based learning module on CT of the temporal bone: Anatomy and pathology. Radiographics. 2012;32:E85–105. doi: 10.1148/rg.323115117. [DOI] [PubMed] [Google Scholar]
- 33.Tawfik AM, Kerl JM, Razek AA, et al. Image quality and radiation dose of dual-energy CT of the head and neck compared with a standard 120-kVp acquisition. Am J Neuroradiol. 2011;32:1994–99. doi: 10.3174/ajnr.A2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theunisse HJ, Joemai RM, Maal TJ, et al. Cone-beam CT versus multi-slice CT systems for postoperative imaging of cochlear implantation – a phantom study on image quality and radiation exposure using human temporal bones. Otol Neurotol. 2015;36:592–99. doi: 10.1097/MAO.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 35.Agacayak KS, Gulsun B, Koparal M, et al. Alterations in Maxillary Sinus Volume among Oral and Nasal Breathers. Med Sci Monit. 2015;21:18–26. doi: 10.12659/MSM.891371. [DOI] [PMC free article] [PubMed] [Google Scholar]


