Abstract
Background
miR-15b is significantly and consistently downregulated in different clinical phenotypes of myasthenia gravis (MG). However, its role in pathogenesis of MG is still not clear. This study aimed to explore the function of miR-15b in MG.
Material/Methods
Blood samples from early-onset MG, late-onset MG, thymoma patients, and healthy participants were collected. The expression pattern of IL-15 and miR-15b was identified by qRT-PCR and ELISA in patient serum and mouse tissue samples. The regulative role of miR-15b on IL-15 expression was verified in an experimental autoimmune myasthenia gravis (EAMG) mice model.
Results
qRT-PCR and ELISA showed that miR-15b expression was significantly lower and IL-15 expression was significantly higher in all EMG, LMG, and thymoma cases compared to healthy controls. Based on mouse model, we confirmed that miR-15b knockdown could increase IL-15 expression in healthy mice, while miR-15b overexpression could inhibit IL-15 expression in EAMG mice. Through searching in bioinformatics databases, we identified a highly conserved consequential pairing between IL-15 and miR-15b. Subsequent dual luciferase assay further verified this match.
Conclusions
This study is the first to report the miR-15b-IL-15 axis can directly regulate IL15 expression, which helps to further explain the abnormal IL-15 expression in MG patients and the pathogenesis of MG.
MeSH Keywords: Interleukin-15, MicroRNAs, Myasthenia Gravis
Background
Myasthenia gravis (MG) is an autoimmune disease characterized by muscle weakness and fatigability. It is a T cell-dependent and B cell-mediated autoimmune disease closely related to autoantibodies directed against the nicotinic acetylcholine receptor (AChR) of neuromuscular junctions [1]. The pathogenesis of MG is very complicated. The ectopic expression of cytokines probably participates in the development of MG due to their important roles in immune responses in inflammatory diseases [2,3]. Interleukin (IL)-15 is one of the cytokines upregulated in MG patients [4]. IL-15 is produced by muscle cells and has been widely studied in the process of muscle growth, differentiation, and immune responses [5]. Functionally, IL-15 can activate NK cells and T cells producing IFN-γ. In addition, it also can stimulate and enhance antibody synthesis in activated B cells [6]. Increased IL-15 expression in muscle might lead to further activation of immune cells moving around the muscle cells, releasing more cytokines and thereby worsening the clinical course of MG. Therefore, it is assumed that IL-15 is involved in the pathogenesis of MG. However, how its expression is regulated is not well understood.
MicroRNAs (miRNAs) are a group of small and non-coding RNAs silencing or degrading target genes by binding to their 3′-untranslated region (3′-UTR) [7]. In fact, abnormal expression of miRNAs has been reported in MG patients. One recent study found decreased let-7 expression in MG patients and demonstrated its direct regulation over IL-10 expression [8]. Another recent study reported miR-320a is downregulated in MG patients and can modulate inflammatory cytokines production by targeting MAPK1 [9]. miR-15b is also an miRNA significantly and consistently downregulated in different clinical phenotypes of MG [9,10]. However, its role in the pathogenesis of MG is still not clear. In this study, we explored the downstream target of miR-15b in experimental autoimmune myasthenia gravis (EAMG) mice and demonstrated that miR-15b can directly target 3′-UTR of IL-15 and regulate its expression.
Material and Methods
Human samples
We recruited 57 MG patients and 20 healthy controls without history of autoimmune disease from the Fifth Affiliated Hospital of Zhengzhou University. The 57 MG cases include 20 early-onset MG (EMG), 22 late-onset MG (LMG), and 15 thymoma patients. Informed consent was obtained from each participant. We collected 5-ml blood samples from each participant. After coagulation, the samples were centrifuged at 3500 rpm for 10 min in a refrigerated centrifuge to isolate serum.
Animals and induction of EAMG
We maintained 8- to 10-week-old C57BL/6 female mice in a specific pathogen-free facility. Animal-based experiments strictly followed the Guide for the Care and Use of Laboratory Animals (8th ed., National Research Council, National Academy Press, Washington, DC, 2011). EAMG was induced in the mice by injection of Torpedo AChR and the level of weakness was assessed by a blind evaluator according to the method described in a previous study [11]. Generally, mice with grade 2 weakness, which is defined as weakness at rest, were used in this study [11]. 6 EAMG and healthy mice were sacrificed following analysis. We collected 150 μL of blood from each mouse through a tail vein. After collection, blood samples were left to clot by leaving them undisturbed at room temperature. Then the samples were centrifuged at 3500 rpm for 10 min in a refrigerated centrifuge to isolate serum.
Cell cultures
HEK293T cell and mouse myoblast cell line C2C12 were obtained from the American Type Culture Collection (ATCC). Primary extensor digitorum longus (EDL) cells were isolated from healthy and EAMG mice, respectively, according to methods previously described [12]. Generally, individual EDL muscles were cut into small fragments and then smashed and soaked for 15 min at 37°C in a 60-mm culture dish (Corning) containing 0.25% trypsin with 1 mM of EDTA. Cells dislodged were collected by using centrifugation at 100 × g for 10 min at 4°C. Then, the cells were incubated at 37°C for 30 min in 2 ml of DMEM with 10% FBS in 6-well plates at a density of 1×107 cells/ml. The muscle cells were then selected by differential adhesion. The supernatants containing myocytes, which were not bound to the plate within the 30 min, were collected and further cultured for 4–5 days. Then the EDL muscles cells were identified by immunostaining with antibody against of Neural Cell Adhesion Molecule (NCAM) (ab9018, Abcam, 1:300), an adhesion marker also expressed on the surface of skeletal muscle. The cells were cultured in Dulbecco’s modified Eagle’s medium (DEME) medium supplemented with 10% v/v fetal calf serum, 100U/mL penicillin, 100 mg/mL streptomycin, and 2 mM glutamine in humidified air with 5% CO2 at 37°C.
qRT-PCR analysis of miR-15 and IL-15 expression
Total miRNA from cells was extracted by using the mirVana PARIS kit according to the manufacturer’s protocol (Applied Biosystems). The TaqMan MicroRNA Reverse Transcription Kit was used to synthesize miRNA-specific cDNA. The expression of miR-15b was quantified by using Taqman miRNA Assays (Applied Biosystems) according to recommended protocol. RNU6B served as the internal control. To determine IL-15 expression at mRNA level, total mRNA were first extracted from cells by using TRIzol Reagent (Invitrogen). The first-strand cDNA was synthesized by using RevertAid first strand cDNA synthesis kit (Fermentas) according to the manufacturer’s instructions. The primers for IL-15 were: F: 5′-ATGTTCATCAACACGTCCTGACT-3′ AND R: 5′-GCAGCAGGTGGAGGTACCTTAA -3′. qRT-PCR was performed by using SYBR® Premix Ex Taq™ II (Takara). Data are presented as the fold difference in gene expression normalized to GAPDH. Relative quantification was performed using 2−ΔΔCt method.
ELISA of IL-15 expression
Il-15 concentration in human serum and in mouse serum and EDL muscle tissue were measured by using an ELISA kit (USCN Life Science, SEA061Hu and SEA061Mu) according to the manufacturer’s instructions. Serum IL-15 and muscle IL-15 are presented as pg/mL and pg/mg of tissue protein, respectively.
Cell transfection
Lentiviral vector encoding miR-15b (pLenti-miR-15b) was purchased from PrimCells and MISSION® Lenti mouse miR-15b inhibitor was purchased from Sigma-Aldrich. To generate miR-15b overexpression lentiviral particles, pLenti-miR-15b and LentiPower Lentiviral Packaging Mix (PrimCells) were co-transfected to HEK293T cells by using Lipofectamine 2000 (Invitrogen). To generate miR-15b inhibitor lentiviral particles, Lenti miR-15b inhibitor and the packaging plasmids (Sigma-Aldrich) were co-transfected to HEK293T cells by using Lipofectamine 2000 (Invitrogen). At 48 h post-transfection, viral titers of the culture supernatants were measured and the supernatants were harvested if viral titer was 106–107 TU/mL. The primary muscle cells were then treated with the viral supernatants containing 5 μg/ml of Polybrene (Sigma-Aldrich). Chemically synthesized miR-15b mimics and negative control were purchased from Ribo Life Science (China). HEK-293T and C2C12 cells were transfected with miR-15b mimics or the negative control by using lipofectamine 2000 (Invitrogen) according to recommended procedure.
Western blot analysis of IL-15 expression
Primary muscle cells were treated with lysis butter to extract proteins. The concentration of protein samples were measured by BCA protein assay (Pierce, Thermo Scientific). Samples were separated on a 12% Tris-glycine gel and then transferred to a nitrocellulose membrane. The membrane was blocked with 4% BSA in Tris buffer with Tween-20 (TBST) for 1 h and then incubated with primary anti-IL15 (Abcam, ab85010, 1:1000) overnight at 4°C. After being washed with TBST, the membrane was incubated with HRP-conjugated anti-rabbit secondary (Abcam, ab97051, 1:5000) for 2 h at room temperature. The signals were visualized by using enhanced chemiluminescence-plus WB detection reagents (Pierce).
Luciferase reporter assay
The putative binding sites between miR-15b and IL-15 were predicted through comparison in online bioinformatics databases. Based on the predicted results, 2 pairs of double-stranded oligonucleotides containing the wild-type (WT) and mutant (MUT) 3′-UTR of IL-15 and with flanking SacI and SalI site in 2 ends were chemically synthesized. The sequence details are: WT: (F) 5′-cCTGTTATTAAGGTACCTCCACCTGCTGCTCAGAGGCAGCAg-3′, (R) 5′-tcgacTGCTGCCTCTGAGCAGCAGGTGGAGGTACCTTAATAACAGgagct-3′; MUT: (F) 5′-cCTGTTATTAAGGTACCTCCACCGATGATCCAGAGGCAGCAg-3′, (R) 5′-tcgacTGCTGCCTCTGGATCATCGGTGGAGGTACCTTAATAACAGgagct-3′. These 2 pairs of sequences were annealed and then inserted into the sites between SacI and SalI of the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega), respectively, to construct recombinant luciferase reporters. The 2 expression vectors were designated as Luc-IL-15-WT and Luc-IL-15-MUT, respectively. The sequence was verified by sequencing. To verify the putative binding site, HEK293T cells or C2C12 cells were co-transfected with either 100nM miR-15b mimics or miR-NC mimics and 200 ng of plasmid. Cells were lysed 48 h after transfection to measure the relative firefly luciferase activity (normalized with Renilla luciferase) by using the Dual-Light luminescent reporter gene assay (Applied Biosystems).
Statistical analysis
Results are given as mean ±SD. Group comparison was performed by using the unpaired T test. A p value <0.05 was considered as a significant difference. *, **, and *** donates significance at 0.05, 0.01, and 0.001 level, respectively.
Results
MiR-15b is significantly lower, while IL-15 expression is significantly higher in MG patients and EAMG mice
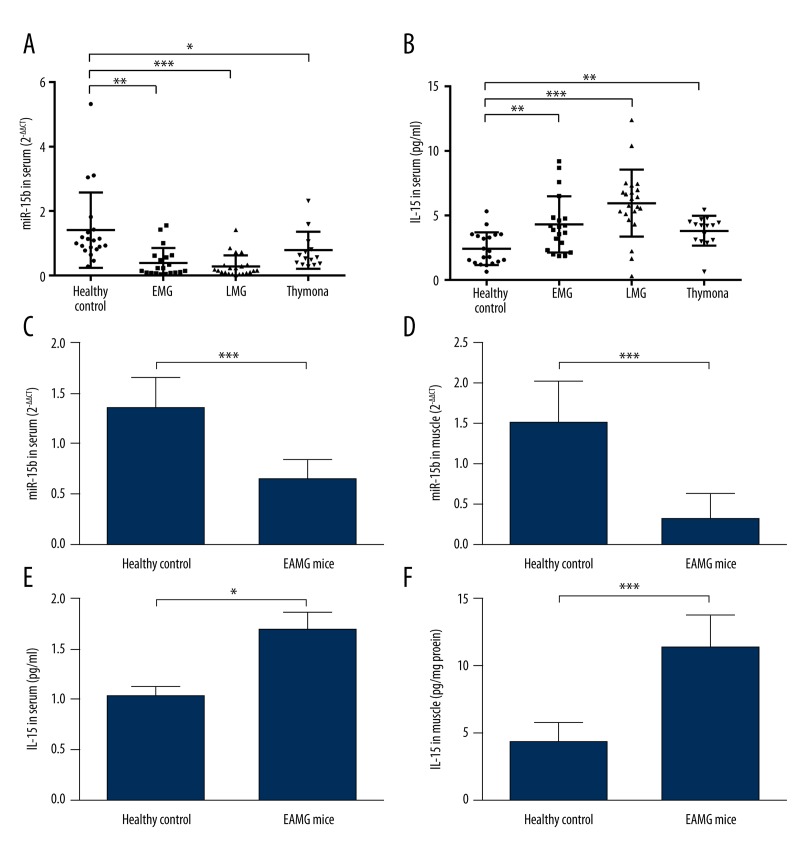
Based on qRT-PCR analysis, miR-15b expression was significantly lower in EMG, LMG, and thymoma cases than in healthy controls (Figure 1A). Interestingly, expression of IL-15 showed an inverse trend. Compared with healthy participants, MG patients, including EMG, LMG, and thymoma, all had significantly higher IL-15 expression (Figure 1B). To further explore the association between IL-15 and miR-15b in MG, EAMG mice were used. In both serum and EDL muscle level, miR-15b was significantly lower in EAMG mice than in healthy controls (Figure 1C, 1D). For IL-15 expression, the average concentration (mean ±SD) in serum (pg/ml) and muscle (pg/mg tissue protein) of EAMG mice were 431.6±36.1 and 11.4±2.4, respectively, which were significantly higher than in healthy mice (264.0±15.4 and 4.4±1.3, respectively) (Figure 1E, 1F). Therefore, these results suggest a reverse expression trend between IL-15 and miR-15b.
Figure 1.
MiR-15b expression is significantly lower, while IL-15 is significantly higher in MG patients and EAMG rats. (A, B) qRT-PCR anlysis of miR-15b and ELISA of serum IL-15. Compared with healthy controls (n=20), expression of miR-15b (A) was significantly lower, while IL-15 (B) was significantly higher in EMG (n=20), LMG (n=22), and thymoma cases (n=15) than in healthy controls. (C, D) qRT-PCR anlysis of miR-15b in serum (C) and EDL muscle (D) of mice. Compared with healthy mice, EAMG mice had signficiantly lower miR-15b in both serum and muscle. (E, F) ELISA of IL-15 in serum (C) and EDL muscle (D) of mice. Compared with healthy mice, EAMG mice had signficiantly higher IL-15 in both serum and muscle. Error bars indicates standard deviation (SD). * P<0.05, ** P<0.01, *** P<0.001.
miR-15b can regulate IL-15 expression
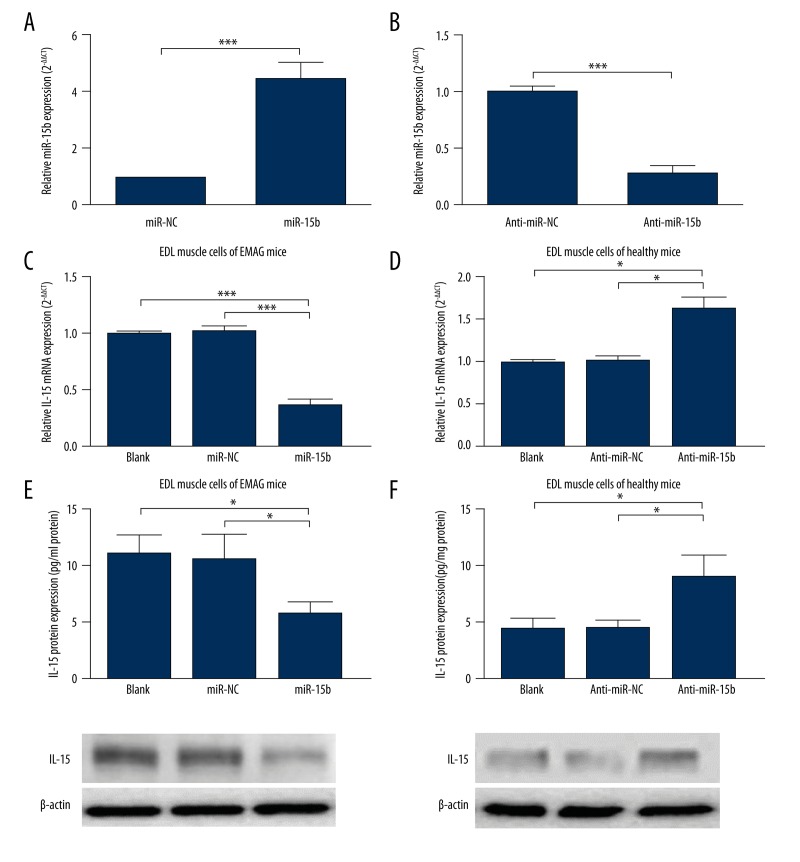
To further explore whether miR-15b can module IL-15 expression, primary EDL muscle cells were used for analysis. First, primary EDL muscle cells from EMAG mice and healthy mice were transfected for overexpression and knockdown of miR-15b, respectively. qRT-PCR results confirmed successful overexpression and knockdown (Figure 2A, 2B). In primary EMAG mouse, EDL muscle cells, miR-15b overexpression led to significantly decreased IL-15 expression at both mRNA (Figure 2C) and protein (Figure 2E) level. In contrast, miR-15b knockdown in primary healthy mouse EDL muscle cells resulted in significantly increased IL-15 expression at mRNA (Figure 2D) and protein level (Figure 2F). Therefore, these results suggest that miR-15b can modulate IL-15 expression.
Figure 2.
miR-15b can regulate IL-15 expression. (A, B) primary EDL muscle cells from EMAG mice (A) and healthy mice (B) were transfected for overexpression (A) and knockdown (B) of miR-15b, respectively. (B–E) In primary EMAG EDL muscle cells, miR-15b overexpression led to significantly decreased IL-15 expression at both mRNA (C) and protein (E) level. In contrast, miR-15b knockdown in primary healthy EDL muscle cells resulted in significantly increased IL-15 expression at mRNA (D) and protein (F) level. Error bars indicates SD. * P<0.05, ** P<0.01, *** P<0.001.
miR-15b regulates IL-15 expression by directly targeting its 3′-UTR
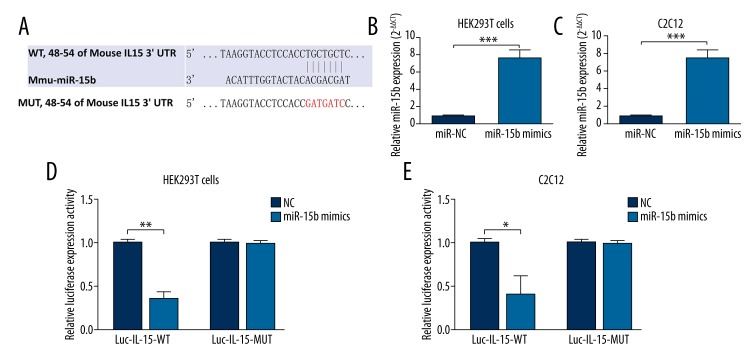
Since the regulative role of miR-15b on IL-15 expression was verified, we further explored whether IL-15 is a direct regulation target of miR-15b by dual luciferase assay. Online comparison in bioinformation databases identified a putative binding site between miR-15b and IL-15 (Figure 3A). To verify the binding site, 2 luciferase reporters containing the wild-type and mutant sequence of the binding site were constructed. MiR-15b overexpression through miR-15b mimics were confirmed in HEK293T cells (Figure 3B) and C2C12 cells (Figure 3C). Dual luciferase assay showed that miR-15b could significantly abrogate luciferase activity of the reporter with wild-type sequence, but had no inhibitive effect on the vector with mutant sequence in both HEK293T and C2C12 cells (Figure 3D, 3E). These results thus confirmed the direct binding and regulating role of miR-15b in IL-15 expression.
Figure 3.
miR-15b regulates IL-15 expression by directly targeting its 3′-UTR. (A) Predicted binding site between miR-15b and 3′-UTR of IL-15. The mutant 3′-UTR of IL-15 without specific binding site is also given. (B, C) (B) HEK293T cells or (C) C2C12 cells transfected with 100nM miR-15b mimics had significantly increased miR-15b. (D, E) (D) HEK293T cells or (E) C2C12 cells were co-transfected with 100 nM of miR-15b mimics or NC oligos and 200 ng plasmids (Luc-IL-15-WT or Luc-IL-15-MUT). miR-15b could significantly abrogate the luciferase activity of Luc-IL-15-WT, but had no inhibitive effect on Luc-IL-15-MUT. Error bars indicate SD. * P<0.05, ** P<0.01, *** P<0.001.
Discussion
Previous studies have reported diverse biological functions of miR-15b in different diseases. In glioma, it acts as a tumor suppressor gene suppressing tumor cell proliferation by targeting cyclin D1, NRP-2, and MMP-3 [13,14]. It can also regulate multidrug resistance in human gastric cancer cells by targeting BCL2 [15] and affect the chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1 [16]. Due to the complex regulative network of miRNAs, it is highly possible that the regulative role of miR-15b might be disease-specific. In MG cases, previous studies based on miRNA microarray or qRT-PCR analysis showed that miR-15b is downregulated compared with healthy controls [9,10]. The current study based on 57 MG cases and 20 healthy controls also demonstrated that miR-15b expression is significantly lower in all EMG, LMG, and thymoma cases compared to healthy controls. However, the exact regulative role and the downstream target of miR-15b in MG are not clear. In fact, this study also observed an inverse expression trend between miR-15b and IL-15 in MG patients. Thus, we further explored the association between them.
Based on a mouse model, we confirmed that miR-15b knockdown can increase IL-15 expression in skeletal muscle of healthy mice, while miR-15b overexpression can inhibit IL-15 expression in EAMG mice. Through searching in bioinformatics databases, we identified a highly conserved consequential pairing between IL-15 and miR-15b. Subsequent dual luciferase assay further verified this match. These results prove that miR-15b can directly target IL-15 and regulate its expression in EAMG mice. Several previous studies also reported significantly higher muscle and serum IL-15 level in MG patients [4,12,17]. However, few studies have explored the underlying regulative network. One study found the increase is a response to IL-4 and IFN-γ [17], while another study found that upregulated IL-4 receptor in muscle cell enhanced the response [12]. Although abnormal miRNAs expression was observed in MG patients, no previous study has reported the regulative role of miRNAs in IL-15 expression. The current study is the first to demonstrate the regulation of miR-15b in IL-15 expression, which helps to further explain the abnormal IL-15 expression in MG patients.
As a multifunctional proinflammatory cytokine, IL-15 is involved in a range of inflammatory diseases, including rheumatoid arthritis, psoriasis, and pulmonary inflammatory diseases [6]. IL-15 neutralization by using IL-15–specific antibodies contributed to reduced severity of psoriasis in a human psoriasis xenograft model [18]. In an in vivo model, blocking IL-15 also prevented the induction of allergen-specific T cells and associated allergic inflammation [19]. Therefore, lower IL-15 expression might be a therapeutic strategy for some autoimmune inflammatory diseases. Our current understanding of the role of IL-15 in EAMG pathogenesis is still quite limited. The only available evidence is all about its inflammation-enhancing effect. One possible hypothesis is that increased IL-15 expression from myocytes causes heightened activation of immune cells travelling around them. The activated immune cells, especially the muscle-associated leukocytes, might further promote activation of the autoimmune response or activation of myocytes to produce more immune mediators, including IL-15. This might generate a vicious cycle of autoimmune response. However, the details of IL-15 function in MG should be further studied.
Conclusions
This study is the first to report that the miR-15b-IL-15 axis can directly regulate IL15 expression, which helps to further explain the abnormal IL-15 expression in MG patients and the pathogenesis of MG.
Footnotes
Source of support: Departmental sources
Reference
- 1.Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2:797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- 2.Aarli JA. Role of cytokines in neurological disorders. Curr Med Chem. 2003;10(19):1931–37. doi: 10.2174/0929867033456918. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 4.Uzawa A, Kawaguchi N, Himuro K, et al. Serum cytokine and chemokine profiles in patients with myasthenia gravis. Clin Exp Immunol. 2014;176(2):232–37. doi: 10.1111/cei.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pistilli EE, Siu PM, Alway SE. Interleukin-15 responses to aging and unloading-induced skeletal muscle atrophy. Am J Physiol Cell Physiol. 2007;292(4):C1298–304. doi: 10.1152/ajpcell.00496.2006. [DOI] [PubMed] [Google Scholar]
- 6.McInnes IB, Gracie JA. Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr Opn Pharmacol. 2004;4:392–97. doi: 10.1016/j.coph.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Jiang L, Cheng Z, Qiu S, et al. Altered let-7 expression in Myasthenia gravis and let-7c mediated regulation of IL-10 by directly targeting IL-10 in Jurkat cells. Int Immunopharmacol. 2012;14:217–23. doi: 10.1016/j.intimp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Z, Qiu S, Jiang L, et al. MiR-320a is downregulated in patients with myasthenia gravis and modulates inflammatory cytokines production by targeting mitogen-activated protein kinase 1. J Clin Immunol. 2013;33(3):567–76. doi: 10.1007/s10875-012-9834-5. [DOI] [PubMed] [Google Scholar]
- 10.Nogales-Gadea G, Ramos-Fransi A, Suarez-Calvet X, et al. Analysis of serum miRNA profiles of myasthenia gravis patients. PloS One. 2014;9(3):e91927. doi: 10.1371/journal.pone.0091927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu B, Goluszko E, Huda R, et al. Experimental autoimmune myasthenia gravis in the mouse. In: Coligan JE, et al., editors. Current protocols in immunology. Unit 15 18. Chapter 15. 2013. [DOI] [PubMed] [Google Scholar]
- 12.Shandley S, Martinez S, Krolick K. IL-4 receptor as a bridge between the immune system and muscle in experimental myasthenia gravis I: up-regulation of muscle IL-15 by IL-4. Clin Immunol. 2009;132(2):246–56. doi: 10.1016/j.clim.2009.03.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun G, Shi L, Yan S, et al. MiR-15b targets cyclin D1 to regulate proliferation and apoptosis in glioma cells. Biomed Res Int. 2014;2014:687826. doi: 10.1155/2014/687826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng X, Chopp M, Lu Y, et al. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329(2):146–54. doi: 10.1016/j.canlet.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia L, Zhang D, Du R, et al. Fan, miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123(2):372–79. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Yao Y, Liu B, et al. MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene. 2012;31(4):432–45. doi: 10.1038/onc.2011.263. [DOI] [PubMed] [Google Scholar]
- 17.Stegall T, Krolick KA. Myocytes respond to both interleukin-4 and interferon-gamma: cytokine responsiveness with the potential to influence the severity and course of experimental myasthenia gravis. Clin Immunol. 2000;94(2):133–39. doi: 10.1006/clim.1999.4822. [DOI] [PubMed] [Google Scholar]
- 18.Villadsen LS, Schuurman J, Beurskens F, et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J Clin Invest. 2003;112(10):1571–80. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruckert R, Brandt K, Braun A, et al. Blocking IL-15 prevents the induction of allergen-specific T cells and allergic inflammation in vivo. J Immunol. 2005;174(9):5507–15. doi: 10.4049/jimmunol.174.9.5507. [DOI] [PubMed] [Google Scholar]