Highlights
-
•
We experienced a rare case of lately recurrent granulosa cell tumors.
-
•
Granulosa cell tumors rarely metastasize to the liver.
-
•
Diagnostic laparoscopy is useful for definitive diagnosis.
-
•
Aggressive therapy may contribute of the patient’s long-term prognosis.
Keywords: Granulosa cell tumor, Metastatic liver tumor, Diagnostic laparoscopy, Recurrence
Abstract
Introduction
Granulosa cell tumors (GCTs) are rare functional sex-cord-stromal ovarian neoplasms characterized by low malignancy potential and late relapse, which rarely metastasize to the liver.
Presentation of case
A 43-year-old female, who had undergone surgery to treat a GCT of the left ovary in 1976, complained of abdominal distention in May 2001. Imaging studies demonstrated masses in the right lobe of the liver, together with massive ascites. The patient’s bloody ascites showed no cytological evidence of malignancy. A diagnostic laparoscopy was performed, and the biopsy specimen was histologically proven to be a recurrent granulosa cell tumor. The patient was successfully treated surgery followed by systemic chemotherapy. Her postoperative course was uneventful and systemic chemotherapy was repeated due to the suspicion of a recurrence in the pelvic cavity.
Discussion
GCTs which are rare malignant tumors of the ovary, tend to be associated with late recurrence. Although most recurrences occur within 10 years after the initial diagnosis, there are occasional reports of recurrences after 10 years have been. We experienced the rare case of a patient who relapsed 25 years after the initial diagnosis.
Conclusion
The long natural history of this disease highlights the importance of extended follow up for GCT patients. In addition, aggressive therapy including surgery and chemotherapy may contribute to a patient’s long-term prognosis.
1. Introduction
Granulosa cell tumors (GCTs) are rare functional sex-cord-stromal ovarian neoplasms characterized by low malignancy potential and late relapse, which rarely metastasize to the liver [1]. We describe a patient with a recurrent ovarian GCT with extensive liver metastasis 25 years after her initial diagnosis, for which diagnostic laparoscopy and biopsy were useful.
2. Case presentation
A 43-year-old female, gravida 2, para 2, with a history of left salpingo-oophorectomy in 1976 for GCT, underwent an examination after presenting with abdominal distention in May 2001. Massive ascites and multilocular masses were observed in the right lobe of the liver on ultrasound (US) and computed tomography (CT). Paracentesis revealed bloody ascites with a class II cytology. The diagnosis on referral to our hospital, was a possible cystadenocarcinoma of the liver.
On physical examination, the patient’s abdomen was distended with massive ascites. The laboratory examinations revealed no abnormalities: the level of estradiol and tumor markers including CA546, CA125, CEA, C19-9, and DUPAN-2 were all normal. A US revealed massive ascites. Repeated paracentesis of the abdomen failed to demonstrate malignant cells. An abdominal US and CT demonstrated multilocular cystic masses in the right lobe of the liver measuring 10 cm in diameter, while a 2 cm cyst was detected in the right ovary. On magnetic resonance imaging (MRI), the liver masses were found to have extended superiorly and invaded the diaphragm (Fig. 1). Angiography demonstrated the encasement of the right hepatic artery and the inferior phrenic artery branches and tumor neovascularity (Fig. 2A and B). Although the imaging studies were highly suggestive of cystadenocarcinoma of the liver, the diagnosis of recurrent GCT could not be ruled out.
Fig. 1.
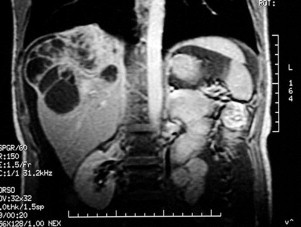
MRI of the abdomen reveals the liver masses invading the diaphragm.
Fig. 2.
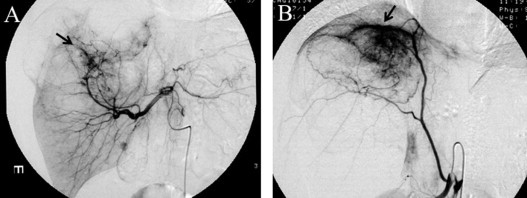
Angiography shows the encasement of the artery branches and neovasculatity (arrows). (A) The common hepatic artery. (B) The right inferior phrenic artery.
A diagnostic laparoscopy was performed in May 2001. After the aspiration of 3400 ml of bloody ascites, multilocular masses were observed in the right lobe of the liver, which had ruptured, resulting in local peritoneal dissemination. A small cyst was also present in the right ovary. Biopsies of the liver, the right ovarian cyst and the areas of peritoneal dissemination revealed a GCT that resembled the previous pathology of the left ovary (Fig. 3).
Fig. 3.
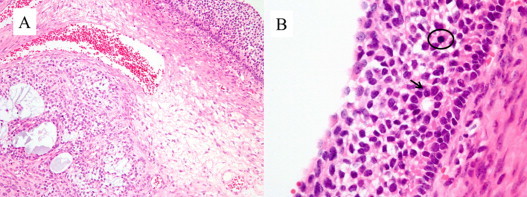
The microscopic features of the original ovarian tumor. (A) The tumor cell is growing with a multiple distributive pattern, including macrofollicular and microfollicular patterns (H and E staining, ×100). (B) The original tumor also shows mitotic figures (circle) and grooves nuclei (arrow). (H and E staining, ×400).
Because of the favorable outcomes associated with platinum-based chemotherapy in patients with recurrent GCTs, we speculated that a mass reduction and postoperative chemotherapy could give her the best chance of cure. We performed an extended right hepatic lobectomy with the combined resection of the diaphragm, cholecystectomy, omentectomy, right salpingo-oophorectomy, and hysterectomy. The final pathologic examination showed metastatic recurrent granulosa cell tumors of the liver and invasion into the diaphragm. A microscopic examination revealed a mostly microfollicular pattern of growth with Call–Exner bodies and occasional macrofollicular pattern areas (Fig. 4A). The nuclei of the tumor cells also showed mitotic figures and grooves resulting in a characteristic “coffee-bean-like” appearance (Fig. 4B). An immunohistochemical staining was performed and was positive for inhibin (Fig. 4C and D).
Fig. 4.
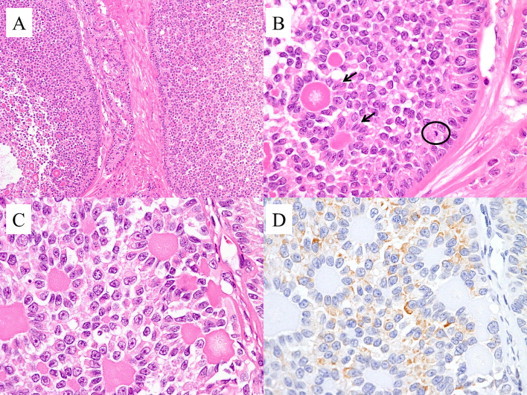
The microscopic features of the liver. (A) A granulosa cell tumor shows macrofollicular and microfollicular patterns. (H and E staining, ×100). (B) A microfollicular pattern with Call–Exner bodies. The nuclei show mitotic figures (circle) and grooves resulting in the characteristic “coffee-bean-like” appearance (arrow). (H and E staining, ×400). (C) A microfollicular pattern of growth with Call–Exner bodies surrounded by granulosa cells (H and E staining, ×400). (D) An immunohistological staining was positive for inhibin (×400).
The patient’s postoperative course was uneventful. After being discharged from the hospital, systemic chemotherapy was administered consisting of carboplatin and cyclophosphamide. The systemic chemotherapy was repeated again due to a suspected tumor recurrence in the pelvic space and liver. She remains alive at 14 years after the surgery.
3. Discussion
GCTs are rare tumors which account for approximately 2–3% of all ovarian malignancies. They are associated with a favorable prognosis, especially when they are detected in the early stages [2–4]. Björkholm and Silfversward reported in 1981 that the 5-year survival rates were over 95% for stage I, 55% for stage II, and 25% for stage III tumors [3]. Lauszus et al., reported that the survival rates for stage I at 5, 10 and 20 years were 94%, 82%, and 62%, respectively [4]. These data suggest that GCTs tend to be associated with late recurrence.
Several series have shown the recurrence rates range between 9 and 35% [3,4]. The recurrence of GCTs is associated with a poor prognosis. Although most recurrences are within 10 years after the initial diagnosis [4,5], there are reports of recurrence after 10 years. In our case, the patient relapsed 25 years after the initial diagnosis. Table 1 shows the reported cases of GCT recurrence after 20 years.
Table 1.
The reports of late recurring granulosa cell tumors (after >20 years).
| Reference | Year | Disease-free interval (years) | Site of recurrence |
|---|---|---|---|
| Shimizu et al. [7] | 1999 | 20 | Lung |
| Spencer et al. [8] | 1999 | 21 | Lung |
| Stenwig et al. [5] | 1979 | 22 | Not specified |
| Li and van der Walt [9] | 1984 | 22 | Paraaortic, extending to kidney |
| Chen et al. [10] | 2012 | 22 | Peritoneum |
| Evans et al. [2] | 1980 | 23 | Not specified |
| Anikwue et al. [11] | 1978 | 24 | Not specified |
| Asschenfeldt and Thind [12] | 1984 | 24 | Peritoneum |
| Piura et al. [13] | 1994 | 24 | Liver, omentum, pelvis, lung |
| Pal and Chowdhury [14] | 1986 | 25 | Vulva |
| Hasiakos et al. [15] | 2008 | 25 | Pelvis |
| Hitchcock et al. [16] | 1989 | 26 | Peritoneum |
| Sommers et al. [17] | 1955 | 26 | Omentum |
| Chew et al. [18] | 2003 | 29 | Spleen |
| Singh-Ranger et al. [19] | 2004 | 30 | Pelvis |
| Hines et al. [1] | 1996 | 37 | Pelvis |
| East et al. [20] | 2005 | 40 | Not specified |
| (Present report) | 2001 | 25 | Liver, peritoneum, other ovary |
GCTs are associated with a low incidence of hepatic metastasis(<5–6%) [6]. On imaging studies, the liver metastases of GCTs are characterized by thickened walls with hypervascularity and nodular excrescences. Our case was initially suspected of having a suspicious cystadenocarcinoma of the liver based on the CT findings, while angiography showed neovascularity of the tumor. However, because we could not rule out recurrent GCTs because of the patient’s past history, we performed a laparoscopic examination. We found the laparoscopic examination to be a safe and useful diagnostic technique.
Case of advanced metastatic GCTs, are typically treated, as our patient was treated, with aggressive surgical resection (when possible), followed by postoperative systemic chemotherapy. Radiation therapy may have some effect in cases with minimal residual disease. Systemic chemotherapy should be prescribed to obtain better tumor control and an improved long-term survival rate. Although our patient experienced another recurrence at 2 years after the second surgery, her prognosis improved by combination chemotherapy and she remains alive after 14 years.
4. Conclusions
The long natural history of this disease highlights the importance of extended follow up for GCT patients. In addition, aggressive therapy including surgery and chemotherapy may contribute to a favorable long-term prognosis.
Conflicts of interest
None.
Funding
None.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Fumihiko Fujita contributed reports retrieval and drafting of this manuscript. Eguchi Susumu and Mitsuhisa Takatsuki contributed surgical procedures of this case report. Kazuma Kobayashi and Kengo Kanetaka contributed acquisition of clinical data. Masahiro Ito and Kuniko Abe contributed pathological analysis. Tamotsu Kuroki contributed critical revision of this manuscript.
Guarantor
Fumihiko Fujita.
Contributor Information
Fumihiko Fujita, Email: ffujita-ngs@umin.ac.jp.
Susumu Eguchi, Email: sueguchi@nagasaki-u.ac.jp.
Mitsuhisa Takatsuki, Email: takapon@nagasaki-u.ac.jp.
Kazuma Kobayashi, Email: bakehasky@eagle.ocn.ne.jp.
Kengo Kanetaka, Email: kkanetaka@kce.biglobe.ne.jp.
Masahiro Ito, Email: itohm@nmc.hosp.go.jp.
Kuniko Abe, Email: abek@nagasaki-u.ac.jp.
Tamotsu Kuroki, Email: tkuroki-gi@umin.ac.jp.
References
- 1.Hines J.F., Khalifa M.A., Moore J.L., Fine K.P., Lage J.M., Barnes W.A. Recurrent granulosa cell tumor of the ovary 37 years after initial diagnosis: a case report and review of the literature. Gynecol. Oncol. 1996;60:484–488. doi: 10.1006/gyno.1996.0078. [DOI] [PubMed] [Google Scholar]
- 2.Evans 3 A.T., 3rd, Gaffey T.A., Malkasian G.D., Jr., Annegers J.F. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet. Gynecol. 1980;55:231–238. [PubMed] [Google Scholar]
- 3.Bjorkholm E., Silfversward C. Prognostic factors in granulosa-cell tumors. Gynecol. Oncol. 1981;11:261–274. doi: 10.1016/0090-8258(81)90040-8. [DOI] [PubMed] [Google Scholar]
- 4.Lauszus F.F., Petersen A.C., Greisen J., Jakobsen A. Granulosa cell tumor of the ovary: a population-based study of 37 women with stage I disease. Gynecol. Oncol. 2001;81:456–460. doi: 10.1006/gyno.2001.6183. [DOI] [PubMed] [Google Scholar]
- 5.Stenwig J.T., Hazekamp J.T., Beecham J.B. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecol. Oncol. 1979;7:136–152. doi: 10.1016/0090-8258(79)90090-8. [DOI] [PubMed] [Google Scholar]
- 6.Simmons R.L., Sciarra J.J. Treatment of late recurrent granulosa cell tumors of the ovary. Surg. Gynecol. Obstet. 1967;124:65–70. [PubMed] [Google Scholar]
- 7.Shimizu K., Yamada T., Ueda Y., Yamaguchi T., Masawa N., Hasegawa T. Cytologic features of ovarian granulosa cell tumor metastatic to the lung. A case roport. Acta cytol. 1999;43:1137–1141. doi: 10.1159/000331367. [DOI] [PubMed] [Google Scholar]
- 8.Spencer H.W., Mullings A.M., Char G., Carpenter R. Granulosa-theca cell tumour of the ovaries. A late metastasizing tumour. W. Indian Med. J. 1999;48:33–35. [PubMed] [Google Scholar]
- 9.Li M.K., van der Walt J.D. Recurrent granulosa cell tumor of the ovary 22 years after primary excision. J. R. Coll. Surg. Edinb. 1984;29:192–194. [PubMed] [Google Scholar]
- 10.Chen Y.C., Chang L.C., Soong R.S. A late recurring and easily forgotten tumor: ovarian granulosa cell tumor. World J. Surg. Oncol. 2012;10:85. doi: 10.1186/1477-7819-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anikwue C., Dawood M.Y., Kramer E. Granulosa and theca cell tumors. Obstet. Gynecol. 1978;51:214–220. [PubMed] [Google Scholar]
- 12.Asschenfeldt P., Thind P. [Granulosa cell tumor. A case with primary peritoneal carcinosis and recurrence after 24 years] Ugeskrift Laeger. 1984;146:1938. [PubMed] [Google Scholar]
- 13.Piura B., Nemet D., Yanai-Inbar I., Cohen Y., Glezerman M. Granulosa cell tumor of the ovary: a study of 18 cases. J. Surg. Oncol. 1994;55:71–77. doi: 10.1002/jso.2930550203. [DOI] [PubMed] [Google Scholar]
- 14.Pal S.K., Chowdhury N.N. Late recurrence of granulosa cell tumour. Asia-Oceania J. Obstet. Gynaecol./AOFOG. 1986;12:21–23. doi: 10.1111/j.1447-0756.1986.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 15.Hasiakos D., Papakonstantinou K., Karvouni E., Fotiou S. Recurrence of granulosa cell tumor 25 years after initial diagnosis. Report of a case and review of the literature. Eur. J. Gynaecol. Oncol. 2008;29:86–88. [PubMed] [Google Scholar]
- 16.Hitchcock C.L., Norris H.J., Khalifa M.A., Wargotz E.S. Flow cytometric analysis of granulosa tumors. Cancer. 1989;64:2127–2132. doi: 10.1002/1097-0142(19891115)64:10<2127::aid-cncr2820641026>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Sommers S.C., Gates O., Goodof I.I. Late recurrence of granulosa cell tumors; report of two cases. Obstet. Gynecol. 1955;6:395–398. [PubMed] [Google Scholar]
- 18.Chew D.K., Schutzer R.W., Domer G.S., Jaloudi M.A., Rogers A.M. Splenic rupture from metastatic granulosa cell tumor 29 years after curative resection: case report and review of the literature. Am. Surg. 2003;69:106–108. [PubMed] [Google Scholar]
- 19.Singh-Ranger G., Sharp A., Crinnion J.N. Recurrence of granulosa cell tumour after thirty years with small bowel obstruction. Int. Semin. Surg. Oncol.: ISSO. 2004;1:4. doi: 10.1186/1477-7800-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.East N., Alobaid A., Goffin F., Ouallouche K., Gauthier P. Granulosa cell tumor: a recurrence 40 years after initial diagnosis. J. Obstet. Gynaecol. Can.: JOGC = J. Obstetrique Gynecologie Can.: JOGC. 2005;27:363–364. doi: 10.1016/s1701-2163(16)30464-9. [DOI] [PubMed] [Google Scholar]


