Abstract
Background
Autism is an increasing neurodevelopmental disease that appears by 3 years of age, has genetic and/or environmental etiology, and often shows comorbid situations, such as gastrointestinal (GI) disorders. Autism has also a striking sex-bias, not fully genetically explainable.
Objective
Our goal was to explain how and in which predisposing conditions some compounds can impair neurodevelopment, why this occurs in the first years of age, and, primarily, why more in males than females.
Methods
We reviewed articles regarding the genetic and environmental etiology of autism and toxins effects on animal models selected from PubMed and databases about autism and toxicology.
Discussion
Our hypothesis proposes that in the first year of life, the decreasing of maternal immune protection and child immune-system immaturity create an immune vulnerability to infection diseases that, especially if treated with antibiotics, could facilitate dysbiosis and GI disorders. This condition triggers a vicious circle between immune system impairment and increasing dysbiosis that leads to leaky gut and neurochemical compounds and/or neurotoxic xenobiotics production and absorption. This alteration affects the ‘gut-brain axis’ communication that connects gut with central nervous system via immune system. Thus, metabolic pathways impaired in autistic children can be affected by genetic alterations or by environment–xenobiotics interference. In addition, in animal models many xenobiotics exert their neurotoxicity in a sex-dependent manner.
Conclusions
We integrate fragmented and multi-disciplinary information in a unique hypothesis and first disclose a possible environmental origin for the imbalance of male:female distribution of autism, reinforcing the idea that exogenous factors are related to the recent rise of this disease.
Keywords: Environmental autism, Gut dysbiosis, Immune system, Sex bias, Xenobiotics
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with uncertain etiology, characterized by social deficits, communication difficulties, stereotyped or repetitive behaviors, and in some cases, cognitive delays.1
ASD shows a striking sex bias, with a male:female ratio estimated at 3–4:1, and it usually appears by 3 years of age. Some authors have stated that ASD children often have relatively normal development during the first 12–24 months of life; then, after a period of regression, the full syndrome becomes evident.2
The prevalence of ASD varies among countries and it has increased dramatically in the last decades, reaching 6–7 per 1000 individuals and even more in English-speaking countries. This rise is only partially explained by changes in diagnostic criteria.3
Genetics is considered to play a relevant role in ASD, but only 16–17% of autistics are carriers of a known genetic variant, and only few common alterations have been recognized as candidate genes in linkage and association studies.1 Moreover, the gender distribution of the disease would seem to reflect a genetic X-linked pattern, but very few X-linked forms of autism susceptibility have been found to date. Therefore, in addition to genetics, possible environmental causes such as pathogens, use of antibiotics, heavy metals, chemicals, and toxins have been proposed,4 although in some cases their involvement has been or is still considered controversial. However, none of these factors alone can explain why only few children are sensitive to these environmental effects, why these effects occur in the first 3 years of age, and why males are more sensitive than females.
In many patients, some other diseases, such as epilepsy, metabolic defects, sleep problems, and gastrointestinal (GI) disorders are in co-morbidity with autism and, interestingly, GI disorders have a strong correlation with the severity of the disease.5,6
By collecting data from the literature and organizing fragmented hypotheses, we obtained a unique overview and formulated a hypothesis that tries to explain the sequence of possible causative events for regressive autism development. This hypothesis suggests how, in children not yet immune-competent, GI disorders and alterations of the intestinal ecosystem (gut dysbiosis) can establish, causing the synthesis of neuro-active molecules, leaky gut, and then neurotoxic xenobiotics (compound foreign to a living organism) production and absorption. We also found that, in animal models, some of these substances play a male-dependent neurodevelopment damage, thus reinforcing the environmental hypothesis for regressive autism. The combination of these factors, especially during infancy in which brain's sensitivity to senses and learning must be specifically timed,7 could lead to the development of ASD in vulnerable children. Our hypothesis can be the starting point for the prevention of all the conditions predisposing to dysbiosis and neurotoxic xenobiotics absorption, for the planning of probiotics and/or specific antimicrobial therapies, and to establish the toxin risk assessment and the daily tolerable intake.
Discussion
Gut and microbiota
The role of microbiota in human health
An increasing number of evidences point to the importance of the so-called ‘gut–brain axis’ revealing the central role of the gut microbiota (the new denomination of ‘microflora’), in the post-natal development and maturation of the immune and endocrine systems that, in turn, control the central nervous system (CNS) signaling, brain functions, and behavior.8–10
The gut resident commensal microorganisms consist essentially of prokaryotes, especially bacteria, and of a minor component of archaea, viruses, and eukaryotes such as fungi.
The healthy, balanced gut communities state is called ‘eubiosis’, and in adulthood is dominated by the phyla Firmicutes and Bacteroidetes followed by Actinobacteria and Proteobacteria. These microorganisms have structural and protective functions for humans, such as the intestinal wall formation, the development of both immune and endocrine systems, the increasing resistance to pathogen colonization, the detoxification and degradation of xenobiotics, the production of short-chain fatty acids (acetate, propionate, and butyrate) and vitamins, nutrient absorption, and amino acid synthesis from ammonia or urea.11–13 The fermentation of non-digestible carbohydrates by intestinal microbiota, leading to the production of short-chain fatty acids, has a role in several processes in the gut, such as cell proliferation and differentiation, ions and water adsorption, hormone secretion, and cytokines production (tumor necrosis factor-alpha (TNF-α), interleukin (IL)-2, IL-6, IL-10) that, in turn, activate the immune systems and regulate the leukocyte function. Moreover, by competing for the space and nutrients and by producing antimicrobial substances, commensal bacteria can effectively inhibit the growth and the proliferation of pathogens, contributing to maintain a healthy and well-balanced gut microbiota.13,14
Among the commensal bacteria, Bifidobacteria are generally considered among the most relevant beneficial bacteria and, apart from variations due to diet and age, they represent roughly the 10% of the human adult microbiota. In infants, however, a higher proportion of Bifidobacteria has been reported and strictly associated to an appropriate development of the immune system.15 Together with Lactobacilli, including those derived from breast milk, Bifidobacteria contribute to a healthy infant gut, by modulating both natural and acquired immune response.15 On this regard, it has been reported that Lactobacillus salivarius and Lactobacillus fermentus enhance Type 1 helper T cells cytokines production, such as IL-2 and IL-12 as well as modulate the expression of the inflammatory mediator TNF-α.16 More generally, it is well stated that, especially in animal models, lactic acid bacteria can enhance natural immunity by increasing the non-specific phagocytic cell function as well as cytokines, interferons, and interleukins production.14,17
Furthermore, human milk Lactobacillus strains drastically reduce (up to 46%) GI infections, as well as the general incidence rates of infections in newborns, such as those of the upper respiratory tract. Among Firmicutes, Viridans streptococci, and commensal staphylococci, both supplied by breast milk, help to keep under control the infection of Staphylococcus aureus, representing a high risk for newborns.16,18 In addition, studies carried on mice have been reported that lactic acid bacteria could also inhibit the GI epithelium colonization of Candida albicans, whose overgrowth, if not properly counteracted by commensal bacteria, can trigger a severe infection on mucosal surfaces.19 As by-products of fermentation, Lactobacillus strains produce a large amount of short-chain fatty acids, such as the butyric acid, that can effectively inhibit C. albicans hyphal transformation, the first step for commensal to pathogen switch, leading to hyphal invasion and the systemic infection.19–21
Finally, both Bifidobacteria and Lactobacilli contribute to keep an appropriate microbial homeostasis, by increasing the production of functional metabolites, such a butyrate, the main energy source of colonocytes, and by modulating the breakdown of sugars and proteins, key compounds for the intestinal function.16
Dysbiosis and GI disorders in ASD children
The development of next generation sequencing techniques has permitted to define the entire human microbioma (the genome identification of a microbiota). The pyrosequencing analysis of the 16S bacterial hyper-variable regions has discovered many species of bacteria that have never been cultured nor described previously.22
In this regard, several studies have been carried out in an attempt to profile the ASD microbiota, in comparison with healthy siblings and/or unrelated controls.6,22–24 Despite few studies reported small or no differences in the gut microbiota composition between ASD children and their unaffected siblings,24,25 the imbalance in the microbial gut composition in ASD samples is well stated, especially when compared with unrelated healthy controls.6,22,23,26,27
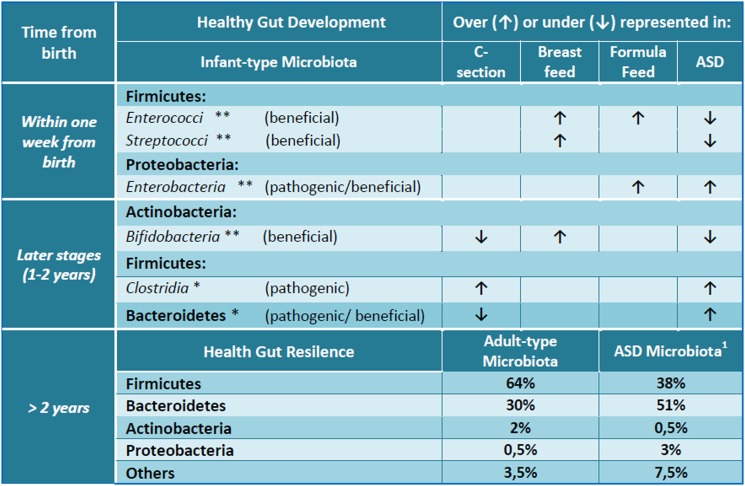
The meta-genomics analyses of stools from ASD patients, in fact, have revealed that ASD microbioma is significantly different from that of controls and consists of over 1000 different species in comparison with the 530 ones of healthy children.22 Moreover, among phyla, ASD patients have under-represented most of Firmicutes and Actinobacteria, especially Bifidobacteria, and over-represented most of Bacteroidetes and Proteobacteria with respect to controls (Fig. 1).22,27,28 Decreasing of Bifidobacteria that help children to develop innate immunity, in ASD microbiota, can explain pathogens overgrowth. Although Firmicutes are under-represented in their complex, the class of Clostridia is more abundant in ASD children with GI disorders. These bacteria are considered the most attractive responsible for the development of autism: many of them are virulent and producing toxins and antimicrobial, oxygen, and drying-resistant spores.23,29 Interestingly, many autistic symptoms ameliorate during oral vancomycin treatment but relapse after the agent is discontinued.26 Oral vancomycin is not absorbed from the intestinal wall and exerts its action exclusively on gut microbiota highlighting the crucial role of the bowel bacterial flora. Indeed, the relapse and persistence of the Clostridia after discontinuation of vancomycin is probably due to germination of spores,29 as well as the relapse of autistic symptoms is imputable to Clostridia re-overgrowth.
Figure 1 .
The gut microbial composition and development in healthy and autistic children. Asterisks refer to a higher (**) or low abundance (*) of microbial flora in an infant healthy gut. Arrows, instead, indicate the increase (↑) or decrease (↓) of gut microbial components according to C-section, breast or formula feed, ASD. ASD, autism spectrum disorder; C-section, cesarean section. 1Data by Finegold et al.27.
Recently, Desulfovibrio, a genus of Gram-negative sulfate-reducing bacteria, was found in 50% of autistic stools and in that of some siblings but not at all in controls. This sulfate-reducing and lipopolysaccharides (LPS)-producing bacterium could be responsible for the aberrant sulfur metabolism and to the high level of serum endotoxins described in ASD subjects. Despite not spore formers, Desulfovibrio can escape antibiotic treatments traveling, by its flagellum, within intestinal biofilm that protect it from peristalsis, antibiotics, and host defenses.27
Interestingly, although Bacteroidetes are over-represented in ASD stools, a significantly lower presence of genus Prevotella, and other fermenters has been described in the gut of ASD children with GI disorders with respect to healthy controls. These microbial differences were more strictly correlated to the severity of autistic symptoms rather than to GI disorders or specific diet regimens.30 Prevotella not only has the ability to synthesize Vitamin B1, which mitigates ASD symptoms,31 but it is also considered a central niche to maintain the community structure of human healthy gut microbiome.32–34 On the other hand, Propionibacterium, as well as Clostridia which are over-represented in ASD intestine, produces propionic acid,35,36 a short-chain fatty acid, able to pass the gut–blood and blood–brain barriers (BBB) altering the neurophysiological processes by binding acetyl-CoA and sequestering acetyl-carnitine involved in mitochondrial lipid transport. Recent experiments demonstrated that administration of propionic acid to young rat models caused the development of mental delay with cognitive impairments, innate neuroinflammation response, and restricted/repetitive behavioral symptoms consistent with human autism.35
Overgrowth of fungi, in particular of Candida, has also been reported as gut infection in some autistic patients,6,37–41 although no eukaryotic microorganism has been described in the most recent publications about autistic microbiota that had molecularly analyzed only the prokaryotic components.5,25,40,42 In the gut of healthy individuals, Candida exist only in small colonies, kept under control by the human immune system and by eubiosis. If it becomes invasive, it produces root-like structures that push through the intestinal walls increasing gut permeability and leading to the so-called leaky-gut syndrome that allows exorphins and toxins to enter the bloodstream. These molecules then trigger food intolerance and allergies. In a recent work, candidiasis and leaky-gut syndrome correlate to a subset (36.7%) of autistic patients.43
GI disorders affect individuals with ASD ranging widely from 9 to 70%, or higher, in different population studies, and show a strong correlation with the severity of the disease.5,6 GI problems of autistic children include abdominal pain, diarrhea, constipation, bloating, increased intestinal permeability, and mucosal dysbiosis.5,42,43 Typically, behavior symptoms and GI disturbances are manifest in parallel.25
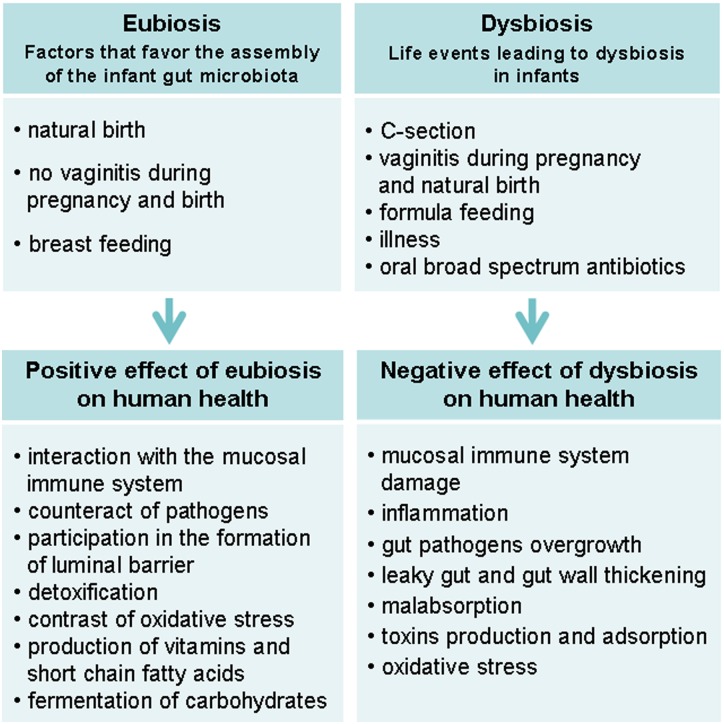
Recent findings have demonstrated that the previously described gut microbiota alterations correlate with these GI disturbances.6,22,25 A series of events that can occur in the very first years of life, 0–36 months, are able to alter the function and balance of gut microbiota, leading to immunological and neurological effects as we show in the following paragraphs (Fig. 2).25,44
Figure 2 .
Differences between eubiosis (a healthy normal microflora) and dysbiosis establishment and consequences.
Gut colonization
Fetal intestine is sterile until birth and its colonization, which occurs during birth, breastfeeding, and weaning, is essential for infant health. The microbial population becomes functional and steady over the time at around 3 years; before this age, infant gut microbiota is dynamic, malleable, and then relatively instable.45 Causes that can hamper or damage eubiosis can be cesarean section, formula-feeding, early weaning, illness, the use of oral broad-spectrum antibiotics, and malnutrition. Many of these situations not only occur in the first 3 years of life, thus disturbing the assembly of a healthy microbiota,45 but are also described as risk factors in ASD: (i) cesarean section (especially if scheduled) has a significantly higher frequency in mother of ASD children (27%) versus mother of healthy children (19.8%);46 (ii) suboptimal or absence of breast-feeding and/or early weaning may increase the occurrence of ASD;47 (iii) infections and the use of oral broad-spectrum antibiotics occur frequently before ASD manifestation.4
Cesarean section deprives the newborn of contact with maternal vaginal microbiota leading to a deficiency of strict anaerobes such Lactobacillus and Prevotella and to an increase of facultative anaerobes from mother skin, especially Staphylococcus, Propionibacterium,48–50 and Clostridium species.48
The gestational age of birth also influences the makeup of bowel flora, since the pattern of gut bacteria in preterm neonates differs from that of full-term newborns. Indeed, preterm newborns are hospitalized in neonatal intensive care units that routinely use formula milk and antibiotics. These practices have a negative impact on gut microbial colonization and could lead to food intolerance, to the development of necrotizing enterocolitis, and long-term neurodevelopment impairment.13,51 Increased prevalence of ASD in children born preterm has also been described.52
Furthermore, in early infancy, gut microbiota is mostly influenced by feeding: the bowel microbial composition is different between breast and formula-fed infants as well as between early and late weaning. Breast milk, containing antimicrobial molecules and prebiotic oligosaccharides, increases the beneficial component of Bifidobacteria and Lactobacilli, whereas formula milk increases the number of Enterococci and Enterobacteria.53–56
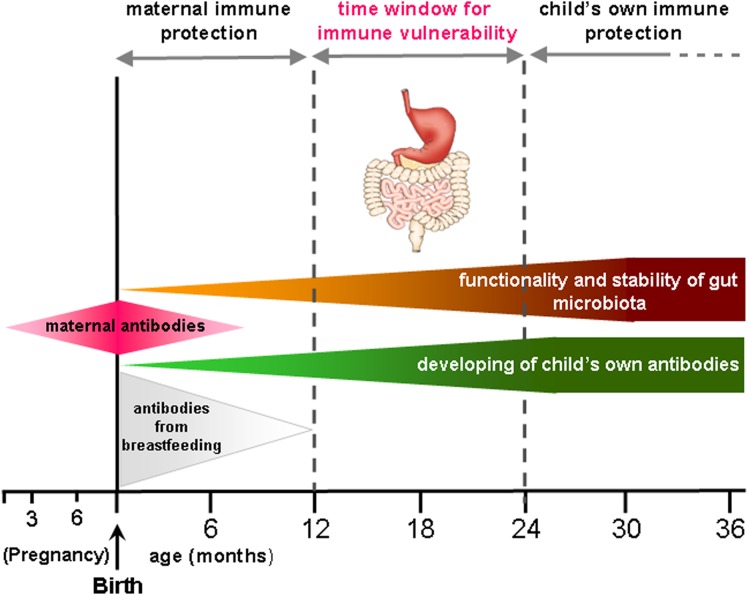
On the other hand, at around 12 months, maternal immune protection decreases while the child's own immune system is not yet competent, resulting in a high susceptibility to infections57,58 (Fig. 3).
Figure 3 .
The critical window for immune vulnerability of children. At about 12 months the maternal protection is disappeared as well as antibodies from breastfeeding (especially in early weaning). On the other hand, the functionality and stability of gut microbiota as well as child's own antibodies formation are not yet reached.
Many infections, especially otitis that are among the most common bacterial infections of childhood,59,60 are treated with oral broad-spectrum antibiotics that can perturb the gut microbiota. Moreover, many parents of autistics patients report episodes of otitis treated with oral broad-spectrum antibiotics before children manifest regressive autism symptoms, as well as some authors find ear canal malformation and/or hearing impairment more frequently in autistic children than in controls; somatic obstructions and malformation, in turn, may favor the onset of otitis and their complications.61 Major microbial alterations were found after antibiotic treatments that lead to a long-term decrease of particular taxa.50,62–64 Even more seriously, the overuse of antibiotics can lead to an increase in antibiotic-resistant pathogens50,65 and these phenomena have been considered as possibly related to the raise up of ASD incidence. For example, the analysis of the clinical history of 206 ASD children with no genetic alteration, birth trauma, or neurological diseases allowed to find that all of them have had recurrent bouts of otitis media treated with a mean number of 12.04 courses of clavulanic acid/amoxicillin.66 Although these data are lacking appropriate aged-matched controls and the author has focused her discussion on the neurotoxic effect of urea, used in drug preparation, on brain tissue, the microbiota damage caused by antibiotics themselves should also be considered. Another factor, possibly related to the recent increase of ASD, is the broad antibiotic prophylaxis against group B streptococcal infections of the newborns from the positive mothers. This clinical practice that has led to a substantial reduction in the incidence of the group B Streptococcus infections,67 it could, however, increase the risk of dysbiosis and then of gut–brain axis alteration.
On the contrary, when ASD and GI disorders occur in parallel,25 the use of dysbiosis-targeted antimicrobials and specific probiotics, to counteract gut pathogens overgrowth, not only ameliorates the GI disorders symptoms, but could also lead to cognitive and behavioral improvement.10,68–73
Moreover, almost 90% of ASD children experience feeding-related concern, especially food selectivity, with a strong preference for starches, processed food, snacks, and strong dislike for almost all vegetables and fruits and/or proteins.47,74 The ability of feeding to regulate the microbiota composition and growth is already well known. Recently, microbiota was found to recognize and produce neuroendocrine hormones such as γ-Aminobutyric acid (GABA) by Lactobacillus and Bifidobacterium, noradrenaline by Escherichia, Bacillus, and Saccaromices, serotonin by Candida, Streptococcus, Escherichia, and Enterococcus, dopamine by Bacillus and Serratia, and acetylcholine by Lactobacillus.75 These compounds are absorbed in the bloodstream and/or interact with the vagus nerve. These findings have lead Norris et al.,76 to propose a positive feedback loop hypothesis involving the host's food preferences and microbiota composition. Through this loop, nutrition influences gut microbiota that, in turn, produces neuroendocrine factors able to influence behavior (e.g. cognition), and food preferences.77 To this purpose, the gut dysbiosis of ASD children can drive their food selectivity in order to ‘feed’ pathogens and perpetuate their overgrowth.
Neurodevelopment
The first periods of life (0–36 months) are particularly delicate for the makeup of the gut complex community, and for immunity and neurodevelopment. Indeed, during this time, acquisition of many sensitive and cognitive processes pass through a ‘critical’ period of elevated neuron plasticity, a specific time window of neurodevelopment during which experiences trig and sculpt the neural circuits involved in that process. Distinct critical periods occur in a hierarchical sequence, starting from primary sensory acquisition up to higher cognition. For example, social relationship and communication are linked to multisensory integration involving visual, auditory, and somatosensory. So, senses (vision, hearing, touch) and intellectual abilities (language, symbols, social relationships) develop, progress, and consolidate in these times of opportunity after which learning anything new becomes very difficult or impossible: if these periods are missed by early disruption or interferences of proper senses and/or social experiences, can no longer be recovered nor senses and skills be acquired.78,79 In some ASD children sensory processes, such as sight and hearing or both of them, are often miswired due to somatic (ear canal malformation), functional, and infectious (otitis) causes, and the severity of sensory stimulation deprivation correlates with the severity of behavior and social relationship deficit.60,61,80,81 Recent findings demonstrated that critical period depends on a perfect balance of cortical excitatory and inhibitory neurotransmission regulated by inhibitor neurotransmitters GABA.82–84 Interestingly, dysfunction in GABA transmitter system has already been described in autism85 and a mistimed in the regulation of these critical periods has been proposed in the developing of ASD.7
Dysbiosis and xenobiotic production
The imbalance of microbiota composition in autistic children with GI disorders can lead to another consequence: the production of xenobiotic substances that can exert a negative systemic effect once entered the bloodstream. Indeed, a change in concentration of mammalian-microbial-cometabolites such as dimethylamine, hippurate, and phenylacetyl-glutamine has been found in the urine of ASD children.86
Such pathogenic linkage was also suggested by Bolte,72 that, starting from the evaluation of the clinical history of a child, speculated that autism could be due to a chronic tetanus infection of the intestinal tract. A possible migration, through a neural route, reaching and persistence of tetanus neurotoxin in the CNS was recently demonstrated by an experiment performed on cats: the transneuronal and transynaptic effect of the toxin injected into the medial rectus muscle of one eye causes a bilateral gaze palsy, revealing the changes in neuron connections due to the action of this neurotoxin on premotor neurons.87
Moreover endotoxins, especially LPS of the outer membrane of Gram-negative bacteria, induce strong inflammation, leaky gut and leaky BBB disrupting the tight junctions of the brain microvascular endothelium, thus increasing paracellular permeability.
LPS endotoxins also inhibit P-glycoprotein, a transmembrane efflux transporter able to extrude a wide range of xenobiotics out of cells, thus causing an intracellular accumulations of these substances. This protein is localized at the luminal epithelial cell surfaces of the gut, liver, proximal tubules of the kidney, hematopoietic cells, and BBB.73,88,89 Many diseases, including ASD, cognitive impairment, schizophrenia, Parkinson's, and Alzheimer's diseases, are associated with increased endotoxins in plasma.89,90
Further support for the microbial metabolite hypothesis and for the key role of the bowel bacteria in autism came from the studies on autistic children treated with antibiotics and probiotics.91 These evidences strongly suggest that autistic symptoms in ASD children with GI disturbances may be related to the production of neurotoxins by the gut microbiota. For example, antifungal drug therapy, such as the oral administration of nystatin, is a potential therapy in ameliorating ASD symptoms.38 Based on the findings of abnormal urinary metabolites, including Krebs cycle metabolites, carbohydrates, and other compounds such as furan compounds, arabinose, tartaric, and citramalic acid, of putative microbial origin in ASD children, it has been reported that antifungal treatment in patients reduces both their clinical symptoms and the abnormal urinary secretion of some chemical compounds suggesting a link between an aberrant fungal or yeast overgrowth in the GI tract and their urinary metabolite profiles.38 Despite any stool testing for yeast or fungi was performed in this study group and due to the small sample size, both elevated arabinose and tartaric acid could likely be associated to GI yeast overgrowth, such as C. albicans.38 Subsequent studies have confirmed that microbial metabolism results in altered metabolite profiles in children with ASD.86,92–94 Recently, using a 1H–13C nuclear magnetic resonance approach, it has been attempted to profile the urine metabolites in ASD, for identifying some discriminating markers between autistics and controls.94 Although the specificity of this new methodology needs to be validated in a larger dataset and on other developmental disorders, an increase in β-alanine, glycine, taurine, and succinate and, on the other hand, a decrease in creatinine and 3-methylhystidine have been reported in the urines of ASD children in comparison with controls.94 High levels of tyrosine analog 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, a bacterial m-tyrosine metabolite that induces characteristic autistic behavioral symptoms in rats, have been found in the urine of some ASD children. Its microbial origin is supported by its decrease in urine after patients were treated for clostridial infections with metronidazole.86,92
Yeast and fungal infections caused by Aspergillus and Candida genus may be related to the production of the gliotoxin, a critical virulence determinant95 of Aspergillus fumigatus, that has an important immunosuppressive activity, facilitating the fungal growth and the host colonization, through the induction of a local or generalized immunosuppression.96 Moreover, Candida itself produces acetaldehyde by glucose fermentation; acetaldehyde acts on central dopaminergic system increasing dopamine levels in the nucleus accumbens, and forms ‘false neurotransmitters’ by Schiff base formation with many aminic neurotransmitters.97 Candida also affects the immune system, inducing higher productions of the interleukins IL-6 and IL-8,98 which were reported to be increased in the blood, brain, or cerebrospinal fluid of autistic subjects.99
Xenobiotics from food
Although there are only small-sized studies, some authors also found food protein exorphins and toxins in the fluids of ASD children.37–39,68,86,92,100–103 In animal models, these xenobiotics interfere with immune, oxidant, and neurological systems suggesting that these substances can interfere also with CNS.35
Exorphin xenobiotics, in particular, casomorphins from bovine β-casein and gliadinomorphines from wheat gluten, are opioid peptides which are formed by the degradation of food proteins, and that bind to opioid receptors inducing interferences in the dopaminergic, serotoninergic, and GABAergic pathways, thus affecting psychomotor development and emotional and motivated behavior.100,104–106 All these effects, known as ‘opioid peptide excess’ theory, are relevant in schizophrenia and autism and confirmed by the improvement in attention and behavior obtained in autistic patients treated with the opioid receptor blocking naltrexone.107 Increasing levels of exorphins also cause a fluctuating dopaminergic hyperfunction that, in animals, induces stereotypy as a typical feature.100
With regards to the diet, it should be pointed out that specific genetic variants of bovine β-casein are associated with higher levels of casomorphines in milk.108 Even if the European Food Safety Authority (EFSA) could not establish the cause–effect relationship between the oral intake of β-casomorphins and the etiology of different human diseases including autism,109 the potential lower production of casomorphins from milk carrying particular β-casein genetic variants or from different dairy species is an intriguing aspect which could be further taken into account in a casein-free diet planning.
Other toxic xenobiotics are mycotoxins, generated by food-contaminating microorganisms, especially fungi, that pollute the 25% of the world crop production.110 The toxicity of some of these mycotoxins, such as ochratoxin A (OTA), fumonisin B1 (FB1), patulin, and gliotoxin, is well known.110–114 Indeed, mycotoxins can increase gut permeability: OTA and patulin alter intestinal functions and intestinal barrier and transport; some experiments have demonstrated that FB1 disturbs the sphingolipid biosynthesis pathway, altering intestinal epithelial cell proliferation and leading to a damage of the barrier function.115 Finally, leaky gut also causes lower adsorption of nutrients, and, indeed, the deficiency of zinc, copper, and B6 vitamin was described in autistic children suffering from GI disorders.116
OTA, produced by Aspergillus ochraceus, Aspergillus carbonarius, and Penicillium verrucosum, is one of the most diffused food-contaminating mycotoxin and it has been found to be implicated in the development of human and animal neurodegenerative diseases and brain dysfunction.113,117
FB1, produced by Fusarium molds and mainly present in corn, causes neuronal tube defects in ex vivo mouse embryos. This effect is related to the folic acid receptor deficiency as a result of the FB1-dependent lipid rafts depletion.114 Among other Fusarium toxins, deoxynivalenol, also known as vomitoxin, is diffused in cereals and, because of its adverse effect of inducing emesis in animals, it has been pointed out as possible serotoninergic and dopaminergic receptor agonist.118–120
Patulin, from Aspergillus and Penicillium, that induces behavioral effects in rats,121 is the most common mycotoxin of apples, pears, and then of baby food such as apple and pear juice and purees,112 thus easily contacts the intestinal tract of infants.
Gliotoxin is a mycotoxin of the epipolythiodioxopiperazine class of fungal toxins produced by a number of Penicillium, Aspergillus, Trichoderma as well as Gliocladium, Thermoascus, and Candida species.122 Through its redox-sensitive transannular disulfide bridge, it exerts toxicity, for example, by conjugation to proteins and inactivation, by inhibiting nuclear factor kappa-light-chain enhancer of activated B-cells (NF-kB) transcription factor, and by generation of reactive oxygen species via redox cycling.122–124
Interestingly, several past and ongoing studies and anecdotal reports point out the positive cognitive and behavioral effects of the gluten-free-casein-free diet on ASD people, especially among children presenting GI disorders, food allergy diagnoses, and suspected food sensitivities.125 However, because of contradictory results, further data are needed to address limitations of current research findings.126 However, the efficacy of this diet could be explained by the fact that it avoids the absorption of both the most important food-derived exorphins (casomorphins and gliadinomorphines) and some mycotoxins, which often contaminate cereals and milk.
Another interesting correlation, still under debate, is between ASD and celiac disease and/or gluten intolerance.127–129 An intriguing correlation comes from the observation that the key molecular player in celiac disease, that is, the tissue transglutaminase enzyme130 has also been reported to be strongly and specifically activated by tetanus and other clostridial toxins.131 Furthermore, patients with ASD were found to be associated with an elevated autoantibody response to tissue transglutaminase.132 A further support for this correlation is the effectiveness of the gluten-free-casein-free diet, whose mechanism of action is still mostly unexplained, for children diagnosed with ASD.125 Finally, it is noteworthy that celiac disease, too, has recently shown a not completely explained increase of prevalence in the last decades.
These findings suggest that the clinical and molecular correlations between celiac and ASD diseases could be at least partially related to the activation of transglutaminase enzyme.
Immune system
The neuro-immune communication, that connects immune system with CNS, is increasingly recognized. The mechanism for humoral-based neuro-immune interaction are numerous and complex and are regulated by the interface of the BBB, thus integrally belonging to the neuro-immune axis. LPS from Gram-negative bacteria and a high level of cytokines can pathologically disrupt BBB interfering with the equilibrium of the two systems.89
Immune aberrations, with increased levels of cytokines, have been reported in autistic children especially in those with a regressive form of the disease.133
Indeed, many proteins that were first discovered in the immune system were subsequently detected in the healthy, uninfected nervous system. For example, the neuronal expression profile of Major histocompatibility complex class 1 (MHC-I) proteins is dynamic during brain development and is spatially restricted throughout life, suggesting that a tight regulation may be necessary for normal neuronal functions. Neuronal MHC-I proteins are in turn modulated by increase and decrease in electrical activity and by the neuronal transcription factors CREB, NPAS4 (in inhibitory neurons), and MECP2 (in the neuron-derived cell line N2A).134 Interestingly, mutation and dysregulation of CREB, NPAS4, and MECP2 have been found in autism.85,135
Furthermore, analysis from post-mortem brains of ASD patients showed that the most consistently shared abnormality in gene expression patterns converge upon immune and enhanced oxidative stress and not upon neurodevelopmental genes, as it would be expected. Dysregulated immune responses accompanied by decreasing ATP production and/or increasing oxidative stress can potentially contribute in determining the onset and the severity of clinical symptoms, especially regression, mental retardation, and stereotypies.136
Recently, an in silico analysis of gene expression profile from ASD involved genes in the healthy developing human brain highlighted a subset of genes mainly expressed in glial cells and strictly involved in the disorder via NF-kB, TNF, and JNK network that converge on central immune-cytokine signaling pathway.137
Interestingly, environmental stimuli such as viruses, bacteria, or mycotoxins could interact with the inflammatory system in ASD via the induction of macrophages and the activation of NF-kB pathway.138 In normal conditions, NF-kB transcription factor mediates the cellular response to exogenic stressors, both by enhancing the expression of inflammatory cytokines/chemokines, and by being induced by them, as in a positive feedback loop.139,140 When NF-kB becomes aberrantly up-regulated, chronic or excessive inflammation is induced.140 An increased expression of NF-kB and an aberrant expression of cytokines, which are, in turn, up-regulated by NF-kB itself, has been found in peripheral blood samples and in neurons, astrocytes, and microglia of ASD donors,141,142 suggesting that ASD children may be unable to turn off the NF-kB stress-induced response.141
Besides modulating inflammatory system, NF-kB is also involved in cell differentiation and proliferation. As for mycotoxins, in pig kidney cell lines, FB1-induced apoptosis is due to the inhibition of protein kinase C activity, and its downstream targets, NF-kB and TNF-α. The long-term NF-kB repression leads to a consequent induction of apoptosis.143 Moreover, NF-kB can be also regulated by oxidative stress.141 OTA, in fact, can induce nitrosative stress in macrophages, brain, kidney, and liver cells, through a NF-kB-dependent induction of inducible nitric oxide synthase (iNOS), an enzyme responsible for the production of nitric oxide. This leads to DNA damage, decrease in DNA repair activity, an increase in lipid peroxidation and, potentially, an impairment in the mitochondrial activity, with a marked toxicity on the nigro-striatal dopaminergic neurons.113,144
Deoxynivalenol from Fusarium has been assessed to have effects on the transcription factors NF-kB, AP-1, and C/EBPβ, which have binding sites in the promoters of numerous immune- and inflammation-related gene,145–147 increasing the synthesis of TNF-α and IL-6 and the induction of COX-2.148 Interestingly, an enhanced oxidative stress in brain, as found in autistic cerebellum tissues, may affect neuronal differentiation, axonal targeting, and synapse formation.149
Sex bias
Sex bias is one of the most reported detection in autism and, clearly, the identification of sex-specific pattern of gene expression can help in understanding the pathophysiology of the disorder.
Recently, Ziats and Rennert150 re-analyzed the sex-specific gene expression profile obtained by a transcriptomic study of normal human brain development, with a combined bioinformatics approach. They found that genes with male-specific pattern of expression are involved in the processes of immune response, cell cytoskeleton, glycoproteins/extracellular matrix, and nucleosome/chromatin that, intriguing, are also implicated in ASD. As for immune response, it was exclusively significant at the expression level, not at the genetic ones, thus suggesting that immune alteration of ASD patients could be more environmental rather than genetics. Male brain development may be naturally more susceptible to environmental adverse events than female ones, as its normal development is more strictly dependent on immune-related pathway.
At environmental level, many toxins and compounds are described to act in a sex-specific manner.
Mycotoxins can have a greater impact on males. For instance, a gender-dependent difference in the incidence of OTA-induced neural tube defects in a mouse model was described.151 It was speculated that these defects were due to the synergistic effect between altered BARX1 and SOX9 gene expressions. SOX9 is a transcription factor essential for skeletal development, but it is also involved in the development of the male phenotype,152 thus contributing to the increased risk of autism in males. Interestingly, a recent study detected up-regulation of SOX9 in autistic cases.153
As for FB1, it significantly affects the humoral immunity of male but not female rats154 and depresses the pig immune response in a sex-specific manner, with males being more susceptible than females.155
Genetic evidences supporting the role of sex steroids in the etiology of ASD have been already presented and explored.156,157 A study showed significant association between autism spectrum quotient and empathy quotient with genes related with sex steroid synthesis and transport functions (e.g. ESR2 and CYP11B1).157 More recently, a new candidate gene for autism, retinoic acid-related orphan receptor-alpha, a hormone-dependent receptor factor, has been introduced: its expression can be regulated by male and female hormones through their respective receptors, and one of its transcriptional targets is CYP19A1 (aromatase), an enzyme responsible for the conversion of testosterone to estrogen.158 In this context, the xenobiotics may interfere on critical functions: zearalenone, for example, a Fusarium toxin commonly found in maize, is a potent estrogen-like toxicant acting as an estrogen agonist in the brain.159
Concerning acetaldehyde, produced by Candida, a study about alcoholism described sex differences in peak acetaldehyde (the first metabolite of ethyl alcohol) concentration, with males showing a higher value than females.160
Very recently, the key role of microbiota on gut–brain axis was investigated and demonstrated using germ-free (GF) mice. Indeed these studies showed that, during early life, GF mice, especially males, manifest significant repetitive behaviors, social avoidance, and deficit that resemble those described in patients with neurodevelopment disorders such as autism. At biochemical level, GF mice presented altered monoamine neurotransmitter levels in the brain, imbalanced neurotrophin levels in the hippocampus and amygdala, increased serotonin level in the hippocampus, and increased neuroendocrine response to stress.9,161 In order to reverse the social deficit, a post-weaning colonization of the gut of GF male mice was carried out and the social behavior was evaluated and compared with that of conventionally colonized mice. The results revealed that GF colonized mice regain the social motivations and interests for social novelty and regulate repetitive behaviors, thus underline the key role of microbiota in neurodevelopment and social behaviors. As these aspects are relevant in autism, that also has a similar male prevalence, they should be important clues for understanding the role of microbiota in the pathogenesis of the disorder and for planning novel therapies for microbial modifications,161 such as probiotics and dietary interventions.
Also, sex-based differences in immunity are known, since human illnesses affect males and females differently. In general, both the proportion of individuals are infected, and the severity of infection are higher in males than females for viral, bacterial, fungal, and parasitic diseases.162,163
Regarding the opioid peptides casomorphins, sex differences in opiate sensitivity have been demonstrated in multiple pre-clinical studies using pain models, and morphine resulted less potent in women compared with men. This is most likely due to differences in opiate receptor density, binding, and localization, as well as sex differences in the anatomy and physiology of opiate-responsive neural circuits.164–166 In animal models, the expression of μ-opioid receptors in the ventro-lateral periaqueductal gray is sexually dimorphic and males have significantly higher levels of μ-opioid receptors compared with females.167
Critical analysis of literature
We have constructed this translational hypothesis for regressive autism pathogenesis assembling, in a complete and logical sequence, many articles and reviews containing different analyses and partial hypotheses for ASD etiology, spanning from genetics to environmental evaluations so as from clinical to pre-clinical studies.
Regarding genetics studies, many and consistent improvements in gene alteration analyses and discovery have been done since the newest technologies have been developed. Indeed, genome wide association has been applied to a great number of patients, thus allowing to find a robust correlation between genetic markers and specific clinical aspects of the disease. On the other hand, the development of next generation sequencing technologies has allowed to perform de novo sequencing, revealing new genes and new alterations causative of ASD. Unfortunately, the power and possibilities offered by these technologies in transcriptome analyses are, in ASD studies, limited to RNA from lymphoblastoid cells, as samples from CNS are, obviously, very rarely available.136,137 However, in spite of the great progress in genetics analyses, only ∼17% of patients carry a genetic alteration unequivocally linked to ASD and very few of them can explain the male excess or the regression to autism that sometimes happen in healthy children between 12 and 36 months.
Many interesting articles about environmental causes of ASD have been published but none explained exhaustively why these agents can impair only few children, which are the predisposing conditions, why some healthy children progressively or suddenly regress to autism and why more males than females are involved. Moreover, some of them are contradictory and this may be due to the sized set and kind of samples analyzed: different age and clinical evaluations and, obviously, an over-representation of males (Table 1).
Table 1 .
Review analysis of literature regarding the most relevant environmental factors or stressors, associated to ASD
| Environmental factors/stressors | Relevant literature | Study type | Association with: |
Main findings | Comments | |
|---|---|---|---|---|---|---|
| ASD | ASD + GI disorders | |||||
| Antibiotics | Sandler et al.69 | Clinical | Yes | Yes | Short-term improvement after low doses of vancomycin and probiotic therapy in a sub-group of regressive ASD children | Small sample size. The reported improvements waned at follow-up. Further investigations are required |
| Atladóttir et al.59 | Clinical | No | – | Population-based cohort study reporting no associations for ASD and mild infections, febrile episodes, or the use of antibiotics during pregnancy | Methodological limitations due to self-reported data/incomplete information | |
| Diet and food xenobiotics | Batista et al.129 | Clinical | No | No | No relevant associations among ASD, celiac disease, and glutein sensitivity | Poor diagnosis for ASD Because of the age of celiac disorder's onset is variable, it is possible that ASD patients would still develop this disease in the future |
| Pennesi and Klein125 | Clinical | Yes | Yes | Effectiveness of glutein/casein free diet in a sub-group of ASD children | Retrospective study Potential diet errors |
|
| Reichelt et al.106 | Clinical | Yes | – | Finding of exorphins in urines of ASD children | Further studies are required to strengthen the role of bio-active peptides in ASD | |
| Critchfield et al.71 | Review | – | – | By comparing GI disorders in ASD and IBD, the supply of probiotics for ameliorating ASD symptoms is strongly encouraged | Need for pre-clinical/clinical trials | |
| Williams et al.42 | Clinical | Yes | Yes | Insights between human gene expression and gut bacterial community composition. | Small sample size. | |
| Genuis et al.128 | Clinical | – | Yes | Glutein-free diet effectiveness in a 5-years-old child with ASD and suspected celiac disease | Case report | |
| Cass et al.126 | Clinical | No | – | Lack of evidence for opioid peptides in urine of male ASD children. No effectiveness for opioids in predicting or monitoring the effects of casein/glutein-free diet | No clinical evaluation for GI disorders in the samples. Further studies are required to validate the effectiveness of casein/glutein-free diet in ASD | |
| Microbial metabolites/end-products | Frye and MacFabe36 | Clinical | Yes | – | Short-chain fatty acids and acetyl-carnitine abnormalities in ASD patients versus controls | Further studies are required to strength the similarity between animal models and ASD and to evaluate the role of microbiota as potential PPA source |
| Mavel et al.94 | Clinical | Yes | – | Aberrant urine metabolic profiling in ASD children versus controls by 1H13C NMR | Needs for larger sample size Needs for testing the methodological specificity |
|
| Kuwabara et al.90 | Clinical | Yes | – | CE-TOFMS revealed aberrant metabolites, associated with oxidative stress and mitochondrial dysfunction, in the plasma of ASD adult males | Only adult males taking into account Needs for testing the methodological specificity and sensitivity |
|
| Ming et al.93 | Clinical | Yes | Yes | Altered gut microbial metabolites in urines of ASD children, with a stronger association in patients with GI disorders, suggesting a link between gut-dysbiosis and metabolic perturbances | No differences between ASD patients and controls have been found, by considering gender, diet, and vitamins supplementations. No evaluation for microbial gut-composition in ASD versus control children |
|
| Kalużna-Czaplińska and Blaszczyk39 | Clinical | – | Yes | High levels of arabinose found in autistic children and positive effects of probiotics in reducing them | No controls evaluated. A possible involvement of Candida is suggested Preliminary study |
|
| MacFabe et al.35 | Pre-clinical | Yes | – | Effects of propionic acid (PPA) in inducing autistic-like behaviors in male adolescent rats | These findings support further evidences on the effects of PPA in young rodents. Further works are required to validate this model in clinical studies | |
| Shaw92 | Clinical | Yes | – | Higher concentrations of HPHPA in the urine of ASD children, in comparison to aged-matched controls, and in one adult affected by C. difficile infection | No clinical evaluation for GI disorders in the samples. No analysis on the ASD stool samples | |
| Yap et al., 2010102 | Clinical | Yes | – | Urinary metabolic alterations in ASD children versus siblings and controls identified by 1H NMR, and potentially associated with gut disorders | No evaluation for gut microbiota in ASD samples. Needs for larger sample of early onset patients to clarify the role of these metabolic differences in the autism etiology |
|
| Shaw et al.38 | Clinical | Yes | – | Positive effect of the antifungal therapy in reducing fungal metabolites in ASD patients | No data reported about GI disorders or the analysis of stool samples | |
| Gut microbiota | Gondalia et al.24 | Clinical | No | No | Pyrosequencing analysis on ASD patients with and without GI disorders and siblings. No evidences for the involvement of gut dysbiosis in ASD | No evaluation for eukaryotic gut microbiota, viruses, or protozoa as well as for dietary differences among participants. Microbial end-products/metabolites should also be studied in the future |
| Adams et al.6 | Clinical | – | Yes | Strong correlation with GI disorders and autism severity; lower levels of Bifidobacteria have been found in ASD patients versus healthy controls | No differences for yeasts was reported. Cultural and biochemical tests for microbiota profiling |
|
| Finegold et al.22 | Clinical | – | Yes | Positive correlation with the highest levels of Bacteroides in ASD children and autism severity. Significantly differences have been also found for Actinobacterium and Proteobacterium | No differences between ASD patients and healthy siblings. No evaluation for the potential diet effects on gut microbiota | |
| Parracho et al.25 | Clinical | Yes | Yes | Association between the high levels of Clostridium spp. and GI disorders in ASD patients versus healthy, unrelated, controls | No differences found between ASD patients and healthy siblings | |
| Song et al., 200423 | Clinical-methodol | Yes | – | Identification with Real-Time PCR of Clostridiales spp. in ASD stools | Small sample size. A comparison group of ASD patients with GI disorders was not included | |
| Finegold et al.40 | Clinical | – | Yes | Higher levels of Clostridium spp. in ASD feces versus healthy controls | Analysis of both fecal flora and gastric and small-bowel specimens. Small sample size | |
ASD, autism spectrum disorder; GI, gastrointestinal; IBD, intestinal bowel disease; PPA, propionic acid; HPHPA, 3-(3-hydroxyphenyl)-3-hydroxypropionic acid; NMR, nuclear magnetic resonance spectroscopy; CE-TOF-MS, capillary electrophoresis time-of-flight mass spectrometry.
Indeed, among the many possible and controversial environmental causes, we focused on the most emerging and convincing evidences and hypothesis: the involvement of gut dysbiosis in the etiology of the disease. Once supported only by poorly powered studies, describing very few cases, this hypothesis is now further supported by subsequent microbioma analyses: indeed, recent next generation sequencing meta-analyses22,91 of stools from autistic patients revealed a microbioma imbalance that might explain the immune system alteration already described in ASD children, although the role of fungi is not taken into account.
On the other hand, the most studied and concordant literature refers to the involvement of immune system in ASD etiology: the involvement of cytokines and other pro-inflammatory compounds in ASD is already well-known.4,99,133
Referring to sex disparity, one of the most typical issues in ASD, neither environmental studies nor those about molecular causes already published try to investigate this sex imbalance, making this issue still incomplete and unexplained. Therefore, in this regard, the studies about this topic are still lacking, making difficult to carry out a comprehensive critical analysis. However, we tried to further analyze how gene expression, immunological susceptibilities, and environmental stressors, such as xenobiotics, could affect mainly males than females, highlighting the role of environment, rather than genetics, in male-predisposing to ASD. Taking all these findings together, our hypothesis organizes partial and multidisciplinary scientific data and hypotheses in a timing sequence and in a logic way. It tries to explain what naturally happens in the first 3 years of life regarding the development and interaction of immune, gut-microbial, and nervous systems and what sequence of events can interfere with this harmonization causing regressive autism especially in males.
Conclusions
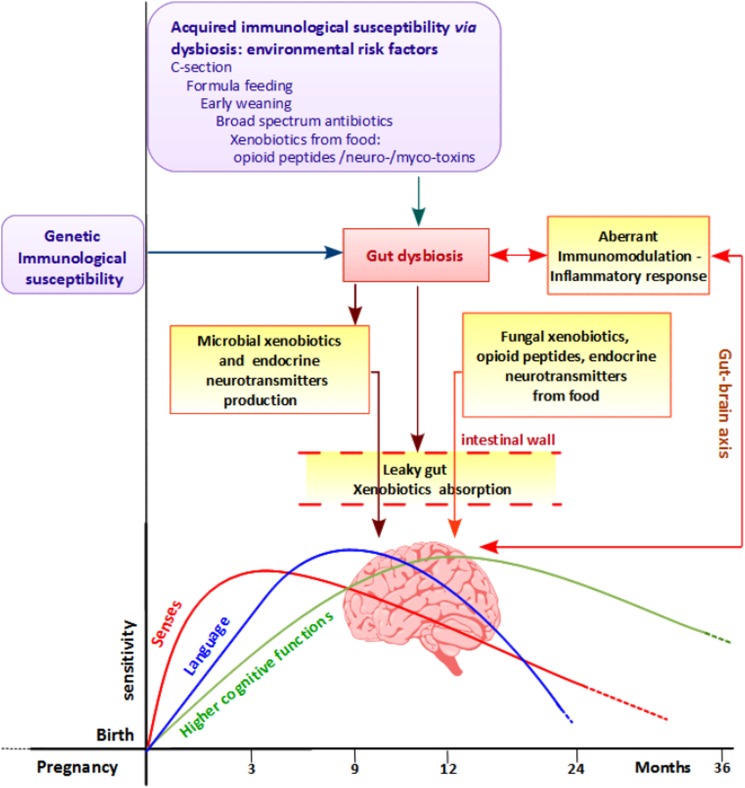
During the first 3 years of life, infant health is particularly vulnerable: at about 1 year the maternal immune protection decreases while the child's own immune system is not yet completely competent.57 Also, the gut microbiota that protects against pathogens and interacts with immune system too is not entirely stable and working until 3 years. This creates a time window of particular vulnerability to infections that, especially if treated with broad-spectrum antibiotics, favors gut dysbiosis and GI disorders. In addition, the published reports highlighted the importance of antibiotic use/overuse and onset of ASD, indicating a 67% of probability of co-occurrence.72 In our opinion, dysbiosis creates a vicious circle leading to a further impairment of the immune system, to the production of microbiota toxins and neurochemical compounds, to the reduction of detoxification, and to the leaky gut causing the adsorption of many xenobiotics (Fig. 4). Some of them promote redox imbalance, intestinal permeability, immunosuppressant, and a sex-specific neurotoxicity, leading to the development of regressive autism, especially in males. This gender-related xenobiotics affect couples with a well-known intrinsic male vulnerability of the immune system.
Figure 4 .
Suggested pathogenesis for autism. Genetic/immunological susceptibility and environmental risk factors could enhance gut dysbiosis, leading to an aberrant inflammatory response, to an abnormal production of microbial end-products, and to leaky gut. The latter can enhance mal-absorption of both microbial and exogenous xenobiotics derived from diet. Once absorbed in the bloodstream, all these compounds can affect the normal brain development and function both directly and impairing the immune system: the latter creates a loop, of aberrant gut–brain axis communication that contributes to enhance these aberrant physiological responses. Finally, endogenous or exogenous stressors might have an impact in the development of senses, language, and higher cognitive functions developing and integrating in the first period of life.
In conclusion: (i) GI disorders should be an expression of an altered intestinal barrier depending on dysbiosis. This could lead to toxins production and adsorption, interfering with normal neurodevelopment in vulnerable children, causing their regression to autism; (ii) the metabolic pathways, altered in ASD children, could be affected by genetic defects or dysregulated by xenobiotics interference; (iii) some enterotoxins and food-xenobiotics promote a male-specific neurotoxicity, thus reinforcing a possible environmental origin for the ASD male excess and the recent rise of the disease prevalence; (iv) the genetic predisposition to microbiota effects and sensitivity to xenobiotics toxicity in males should be investigated, as well as the possible additive and/or synergistic effects of toxins on general population should be defined to establish the risk assessment and the daily tolerable intake.
Acknowledgements
This work has been supported by the Italian Ministry of Health (Targeted Research Funding in Public Health – Young Researchers) GR-2009-1570296. A.M., M.L., and L.M. are supported also by the Italian Ministry of Education and Research (MIUR) through the Flagship project InterOmics (PB05), FIRB ITALBIONET (RBPR05ZK2Z), and HIRMA RBAP11YS7K.
References
- 1.Levy SE, Mandell DS, Schultz RT. Autism. Lancet 2009;374:1627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg WA, Osann K, Filipek PA, Laulhere T, Laulhere T, Jarvis K, et al. Language and other regression: assessment and timing. J Autism Dev Disord 2003;33:607–16. [DOI] [PubMed] [Google Scholar]
- 3.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res 2009;65:591–8. [DOI] [PubMed] [Google Scholar]
- 4.Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol 2013;36:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buie T, Fuchs GJ, Furuta GT, Kooros K, Levy J, Lewis JD, et al. Recommendations for evaluation and treatment of common gastrointestinal problems in children with ASDs. Pediatrics 2010;125Suppl 1:S19–29 [DOI] [PubMed] [Google Scholar]
- 6.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2003;2:255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009;136:2003–14. [DOI] [PubMed] [Google Scholar]
- 9.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 2013;18:666–673. [DOI] [PubMed] [Google Scholar]
- 10.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs S, Sontag G, Stidl R, Ehrlich V, Kundi M, Knasmuller S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem Toxicol 2008;46:1398–407. [DOI] [PubMed] [Google Scholar]
- 12.Aureli P, Capurso L, Castellazzi AM, Clerici M, Giovannini M, Morelli L, et al. Probiotics and health: An evidence-based review. Pharmacol Res 2011;63:366–76. [DOI] [PubMed] [Google Scholar]
- 13.Weber TK, Polanco I. Gastrointestinal microbiota and some children diseases: a review. Gastroenterol Res Pract. 2012;2012:676585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Hara A, O'Regan P, Fanning A, O'Mahony C, MacSharry J, Lyons A. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology 2006;118:202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turroni F, Ribbera A, Foroni E, van Sinderen D, Ventura M. Human gut microbiota and Bifidobacteria: from composition to functionality. Antonie van Leeuwenhoek 2008;94:35–50. [DOI] [PubMed] [Google Scholar]
- 16.El-Ansary A, Shaker GH, Rizk MZ. Role of gut–brain axis in the etiology of neurodevelopmental disorders with reference to autism. J Clin Toxicol 2013;S6:1–8. [Google Scholar]
- 17.Arunachalam K, Gill HS, Chandra RK. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur J Clin Nutr 2000;54:263–7. [DOI] [PubMed] [Google Scholar]
- 18.Uehara Y, Kikuchi K, Nakamura T, Nakama H, Agematsu K, Kawakami Y, et al. Inhibition of methicillin-resistant Staphylococcus aureus colonization of oral cavities in newborns by Viridans group Streptococci. Clin Infect Dis 2001;32:1399–407. [DOI] [PubMed] [Google Scholar]
- 19.Noverr M, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol 2004;12(12):562–8. [DOI] [PubMed] [Google Scholar]
- 20.Böhmig GA, Krieger PM, Säemann MD, Wenhardt C, Pohanka E, Zlabinger GJ. n-Butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology 1997;92(2):234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner RD, Pierson C, Warner T, Dohnalek M, Farmer J, Roberts L, et al. Biotherapeutic effects of probiotic bacteria on candidiasis in immunodeficient mice. Infect Immun 1997;65(10):4165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010;16:444–53. [DOI] [PubMed] [Google Scholar]
- 23.Song Y, Liu C, Finegold SM. Real-time PCR quantitation of Clostridia in feces of autistic children. Appl Environ Microbiol 2004;70(11):6459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, Austin DW. Molecular characterization of gastrointestinal microbiota of children (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res 2012;5(6):419–27. [DOI] [PubMed] [Google Scholar]
- 25.Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 2005;54:987–91. [DOI] [PubMed] [Google Scholar]
- 26.Finegold SM. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe 2011;17:367–8. [DOI] [PubMed] [Google Scholar]
- 27.Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe 2012;18:260–2. [DOI] [PubMed] [Google Scholar]
- 28.Robinson CJ, Bohannan BJ, Young VB. From structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol Rev 2010;74:453–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finegold SM. Therapy and epidemiology of autism-clostridial spores as key elements. Med Hypotheses 2008;70:508–11. [DOI] [PubMed] [Google Scholar]
- 30.Kang DW, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013;8:e68322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lonsdale D, Shamberger RJ, Aduya T. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: a pilot study. Neuro Endocrinol Lett 2002;23:303–8. [PubMed] [Google Scholar]
- 32.Arumugan M, Raes J, Pellettier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of human gut microbiome. Nature 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baquero F, Nombela C. The microbiome as human organ. Clin Microbiol Infect 2012;18(S4):2–4. [DOI] [PubMed] [Google Scholar]
- 35.MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition and neuro inflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res 2011;217:47–54. [DOI] [PubMed] [Google Scholar]
- 36.Fry RE, MacFabe DF. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry 2013;3:e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw W, Kassen E, Chaves E. Increased urinary excretion of analogs of Krebs cycle metabolites and arabinose in two brothers with autistic features. Clin Chem 1995;41:1094–104. [PubMed] [Google Scholar]
- 38.Shaw W, Kassen E, Chaves E. Assessment of antifungal drug therapy in autism by measurement of suspected microbial metabolites in urine with gas chromatography-mass spectrometry. Clin Pract Alter Med 2000;1:15–26. [Google Scholar]
- 39.Kalużna-Czaplińska J, Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 2012;28(2):124–6. [DOI] [PubMed] [Google Scholar]
- 40.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 2002;35Suppl 1:S6–S16. [DOI] [PubMed] [Google Scholar]
- 41.Kidd PM. Autism, an extreme challenge to integrative medicine. Part 2: medical management. Altern Med Rev 2002;7:472–99. [PubMed] [Google Scholar]
- 42.Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 2011;6:e24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 2010;51:418–24. [DOI] [PubMed] [Google Scholar]
- 44.Prakash S, Tomaro-Duchesneau C, Saha S, Cantor A. The gut microbiota and human health with an emphasis on the use of microencapsulated bacterial cells. J Biomed Biotechnol 2011;Article ID 981214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA 2011;108Suppl 1:4578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eriksson MA, Westerlund J, Anderlid BM, Gillberg C, Fernell E. First-degree relatives of young children with autism spectrum disorders: some gender aspects. Res Dev Disabil 2012;33:1642–8. [DOI] [PubMed] [Google Scholar]
- 47.Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Curr Psychiatr Rep 2013;15(2):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Pediatr 2009;98:229–38. [DOI] [PubMed] [Google Scholar]
- 49.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010;107(26):11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clemente JC, Ursell LK, Wegener Parfrey L, Knight R. The impact of gut microbiota on human health: an integrative view. Cell 2013;148:1258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364(3):225–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meldrum SJ, Strunk T, Currie A, Prescott SL, Simmer K, Whitehoue AJO. Autism spectrum disorder in children born preterm-role of exposure to perinatal inflammation. Front Neurosci 2013;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newburg DS. Neonatal protection by innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci 2009;87:26–34. [DOI] [PubMed] [Google Scholar]
- 55.Perez PF, Dorè J, Leclerc M, Levenez F, Benyacoub J, Serrant P, et al. Bacterial imprinting of neonatal immune system: a lesson from maternal cells? Pediatrics 2007;119:e724–32. [DOI] [PubMed] [Google Scholar]
- 56.Hunt KM, Foster JA, Forney LJ, Schütte UM, Beck DL, Abdo Z, et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011;6(6):e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med 2001;345:1331–5. [DOI] [PubMed] [Google Scholar]
- 58.Lainhart JE, Ozonoff S, Coon H, Krasny L, Dinh E, Nice J, et al. Autism, regression, and the broader autism phenotype. Am J Med Genet 2002;113:231–7. [DOI] [PubMed] [Google Scholar]
- 59.Atladóttir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 2012;103(6):e1447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tajima-Pozo K, Zambrano-Enriquez D, De Anta L, Zelmanova J, De Dios Vega JL, Lopez-Ibor J. Otitis and autism spectrum disorders. BMJ Case Rep 2010; 2010: bcr10.2009.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strömland K, Miller M, Sjögreen L, Johansson M, Joelsson BME, Billstedt E, et al. Oculo-auriculo vertebral spectrum: associated anomalies, functional deficits and possible developmental risk factors. Am J Med Genet 2007;143A:1317–25. [DOI] [PubMed] [Google Scholar]
- 62.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008;6(11):e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007;1(1):56–66. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 2001;1(2):101–14. [DOI] [PubMed] [Google Scholar]
- 65.Sommer MO, Dantas G, Church GM. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 2009;325(5944):1128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fallon J. Could one of the most prescribed antibiotics amoxicillin/clavunalate ‘augmentin’™ be a risk factor for autism? Med Hypotheses 2005;64:312–5. [DOI] [PubMed] [Google Scholar]
- 67.Schrag S, Zywicki S, Farley M, Reingold Al, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. New Engl J Med 2000;342:15–20. [DOI] [PubMed] [Google Scholar]
- 68.Reichelt KL, Knivsberg AM. The possibility and probability of a gut-to-brain connection in autism. Ann Clin Psychiatry 2009;21:205–11. [PubMed] [Google Scholar]
- 69.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 2000;15:429–35. [DOI] [PubMed] [Google Scholar]
- 70.Golnik AE, Ireland M. Complementary alternative medicine for children with autism: a physician survey. J Autism Dev Disord 2009;39:996–1005. [DOI] [PubMed] [Google Scholar]
- 71.Critchfield JW, van Hemert S, Ash M, Mulder L, Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract 2011;161358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolte ER. Autism and Clostridium tetani. Med Hypotheses 1998;51:133–44. [DOI] [PubMed] [Google Scholar]
- 73.Bebawy M, Chetty M. Gender differences in p-glycoprotein expression and function: effects on drug disposition and outcome. Curr Drug Metab 2009;10(4):322–8. [DOI] [PubMed] [Google Scholar]
- 74.Sharp WG, Jaquess DL, Lukens CT. Multi-method assessment of feeding problems among children with autism spectrum disorders. Res Autism Spectr Disord 2013;7:56–65. [Google Scholar]
- 75.Roshchina VV. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. In: Lyte M, (ed.). Microbial endocrinology: Interkingdom signaling in infectious disease and health. Freestone PPE editions New York: Springer; 2010. p. 17–52. [Google Scholar]
- 76.Norris V, Molina F, Gewirtz AT. Hypothesis: bacteria control host appetites. J Bacteriol 2013;195(3):411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lyte M. Microbial endocrinology and nutrition: a perspective on new mechanisms by which diet can influence gut-to-brain communication. Pharm Nutr 2013;1:35–9. [Google Scholar]
- 78.LeBlanc JJ, Fagiolini M. Autism: a ‘critical period’ disorder? Neural Plast 2011;2011:921680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bardin J. Unlocking the brain. Nature 2012;487:24–6. [DOI] [PubMed] [Google Scholar]
- 80.Marco EJ, Hinkley LBN, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatric Res 2011;69:48R–54R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vis Res 2009;49:2705–39. [DOI] [PubMed] [Google Scholar]
- 82.Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, et al. Separable futures of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci USA 2003;100(5):2854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fagiolini M, Fritschy JM, Löw K, Möhler H, Rudolph U, Hensch TK. Specific GABBA circuits for visual cortical plasticity. Science 2004;303:1681–3. [DOI] [PubMed] [Google Scholar]
- 84.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABBA circuit control of experience-dependent plasticity in developing visual cortex. Science 1998;282:1504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010;468:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonzalez A, Stombaugh J, Lozupone C, Turnbaugh PJ, Gordon JI, Knight R. The mind-body-microbial continuum. Dialogues Clin Neurosci 2011;13:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Ferero D, Morcuende S, Alvarez FJ, de la Cruz RR, Pastor AM. Transynaptic effects of tetanus neurotoxin in the oculomotor system. Brain 2005;128:2175–88. [DOI] [PubMed] [Google Scholar]
- 88.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis 2010;37:26–32. [DOI] [PubMed] [Google Scholar]
- 89.Bengmark S. Gut microbiota, immune development and function. Pharmacol Res 2013;69:87–113. [DOI] [PubMed] [Google Scholar]
- 90.Kuwabara H, Yamasue H, Koike S, Inoue H, Kawakubo Y, Kuroda M, et al. Altered metabolites in the plasma of autism spectrum disorder: a capillary electrophoresis time of flight mass spectroscopy study. PLoS ONE 2013;8(9):e73814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Louis P. Does the human gut microbiota contribute to the etiology of autism spectrum disorders? Dig Dis Sci 2012;57:1987–9. [DOI] [PubMed] [Google Scholar]
- 92.Shaw W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci 2010;13:135–43. [DOI] [PubMed] [Google Scholar]
- 93.Ming X, Stein TP, Barnes V, Rhodes N, Guo L. Metabolic perturbance in autism spectrum disorders: a metabolomics study. J Proteome 2012;11:5856–62. [DOI] [PubMed] [Google Scholar]
- 94.Mavel S, Nadal-Desbarats L, Blasco H, Bonnet-Brilhault F, Barthelemy C, Montigny F, et al. 1H-13C NMR-based urine metabolic profiling in autism spectrum disorders. Talanta 2013;95–102. [DOI] [PubMed] [Google Scholar]
- 95.Scharf DH, Heinekamp T, Remme N, Hortschansky P, Brakhage AA, Hertweck C. Biosynthesis and function of gliotoxin in Aspergillus fumigatus. Appl Microbiol Biotechnol 2012;93:467–72. [DOI] [PubMed] [Google Scholar]
- 96.Sugui JA, Pardo J, Chang YC, Zarember KA, Nardone G, Galvez EM, et al. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 2007;6:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diana M, Peana AT, Sirca D, Lintas A, Melis E, Enrico P. Crucial role of acetaldehyde in alcohol activation of the mesolimbic dopamine system. Ann NY Acad Sci 2008;1139:307–17. [DOI] [PubMed] [Google Scholar]
- 98.Cheng SC, Chai LY, Joosten LA, Vecchiarelli A, Hube V, Van der Meer JW, et al. Candida albicans releases soluble factors that potentiate cytokine production by human cells through a protease-activated receptor 1- and 2-independent pathway. Infect Immun 2010;78:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol 2009;207:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reichelt KL, Knivsberg AM. Can the pathophysiology of autism be explained by the nature of the discovered urine peptides? Nutr Neurosci 2003;6:19–28. [DOI] [PubMed] [Google Scholar]
- 101.Hopper DG, Bolton VE, Guilford FT, Straus DC. Mycotoxin detection in human samples from patients exposed to environmental molds. Int J Sci 2009;10:1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res 2010;9:2996–3004. [DOI] [PubMed] [Google Scholar]
- 103.Clayton TA. Metabolic differences underlying two distinct rat urinary phenotypes, a suggested role for gut microbial metabolism of phenylalanine and a possible connection to autism. FEBS Lett 2012;586:956–61. [DOI] [PubMed] [Google Scholar]
- 104.Kost NV, Sokolov OY, Kurasova OB, Dmitriev AD, Tarakanova JN, Gabaeva MV, et al. Beta-casomorphins-7 in infants on different type of feeding and different levels of psychomotor development. Peptides 2009;30:1854–60. [DOI] [PubMed] [Google Scholar]
- 105.Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci 2011;31:6362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reichelt KL, Tveiten D, Knivsberg AM, Bronstad G. Peptide's role in autism with emphasis on exorphins. Microbiol Ecol Health Dis 2012;23:18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leboyer M, Bouvard MP, Launay JM, Recasens C, Plumet MH, Waller-Perotte D, et al. Opiate hypothesis in infantile autism? Therapeutic trials with naltrexone. Encephale 1993;19:95–102. [PubMed] [Google Scholar]
- 108.Cieślińska A, Kostyra E, Kostyra H, Oleński K, Fiedorowicz E, Kaminski S. Milk from cows of different β-casein genotypes as a source of β-casomorphin-7. Int J Food Sci Nutr 2012;63:426–30. [DOI] [PubMed] [Google Scholar]
- 109.EFSA. (European Food Safety Authority) Review of the potential health impact of β-casomorphins and related peptides. EFSA Sci Rep 2009;231:1–107. [Google Scholar]
- 110.Bouhet S, Oswald IP. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet Immunol Immunopathol 2005;108:199–209. [DOI] [PubMed] [Google Scholar]
- 111.Belmadani A, Tramu G, Betbeder AM, Steyn PS, Creppy EE. Regional selectivity to ochratoxin A, distribution and cytotoxicity in rat brain. Arch Toxicol 1998;72:656–62. [DOI] [PubMed] [Google Scholar]
- 112.Puel O, Galtier P, Oswald IP. Biosynthesis and toxicological effects of patulin. Toxins 2010;2:613–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sava V, Reunova O, Velasquez A, Harbison R, Sànchez-Ramos J. Acute neurotoxic effects of the fungal metabolite ochratoxin-A. Neurotoxicology 2006;27:82–92. [DOI] [PubMed] [Google Scholar]
- 114.Doi K, Uetsuka K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J Mol Sci 2011;12:5213–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bouhet S, Oswald IP. The intestine as a possible target for fumonisin toxicity. Mol Nutr Food Res 2007;51:925–31. [DOI] [PubMed] [Google Scholar]
- 116.Faber S, Zinn GM, Kern JC, Kingston HM. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers 2009;14:171–80. [DOI] [PubMed] [Google Scholar]
- 117.Razafimanjato H, Garmy N, Guo XJ, Varini K, Di Scala C, Di Pasquale E, et al. The food-associated fungal neurotoxin ochratoxin A inhibits the absorption of glutamate by astrocytes through a decrease in cell surface expression of the excitatory amino-acid transporters GLAST and GLT-1. Neurotoxicology 2010;31:475–84. [DOI] [PubMed] [Google Scholar]
- 118.Fioramonti J, Dupuy C, Dupuy J, Bueno L. The mycotoxin deoxynivalenol delays gastric emptying trough serotonin-3 receptors in rodents. J Pharmacol 1993;266:255–60. [PubMed] [Google Scholar]
- 119.Prelusky DB. The effect of low-level deoxynivalenol on neurotransmitter levels measured in pig cerebral spinal fluid. J Environ Sci Health B 1993;28:731–61. [DOI] [PubMed] [Google Scholar]
- 120.Girish CK, MacDonald EJ, Scheinin M, Smith TK. Effects of feedborne Fusarium mycotoxins on brain regional neurochemistry of Turkeys. Poult Sci 2008;87:1295–302. [DOI] [PubMed] [Google Scholar]
- 121.De Sarro GB, Donato A, Bagetta G, Pujia A, Nisticò G. Behavioural and electrocortical spectrum power changes after intraventricular injection of patulin in rats. Arch Toxicol 1984;7:420–4. [DOI] [PubMed] [Google Scholar]
- 122.Gardiner DM, Waring P, Howlett BJ. The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology 2005;151:1021–32. [DOI] [PubMed] [Google Scholar]
- 123.Lewis RE, Wiederhold NP, Chi J, Han XY, Komanduri KV, Kontoyiannis DP, et al. Detection of gliotoxin in experimental and human aspergillosis. Infect Immun 2005;73:635–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scharf DH, Remme N, Heinekamp T, Hortschansky P, Brakhage AE, Hertweck C. Transannular disulfide formation in gliotoxin biosynthesis and its role in self-resistance of the human pathogen Aspergillus fumigatus. J Am Chem Soc 2010;132:10136–41. [DOI] [PubMed] [Google Scholar]
- 125.Pennesi CM, Klein LC. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: based on parental report. Nutr Neurosci 2012;15:85–91. [DOI] [PubMed] [Google Scholar]
- 126.Cass H, Gringras P, March J, McKendrick I, O'Hare AE, Owen L, et al. Absence of urinary opioid peptides in children with autism. Arch Dis Child 2008;93:745–50. [DOI] [PubMed] [Google Scholar]
- 127.Barcia G, Posar A, Santucci M, Parmeggiani A. Autism and coeliac disease. J Autism Dev Disord 2008;38:407–8. [DOI] [PubMed] [Google Scholar]
- 128.Genuis SJ, Bouchard TP. Celiac disease presenting as autism. J Child Neurol 2010;25:114–9. [DOI] [PubMed] [Google Scholar]
- 129.Batista IC, Gandolfi L, Nobrega YK, Almeida RC, Almeida RM, et al. Autism spectrum disorder and celiac disease: no evidence for a link. Arq Neuropsiquiatr 2012;70:28–33. [DOI] [PubMed] [Google Scholar]
- 130.Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J 2002;368:377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Facchiano F, Deloye F, Doussau F, Innamorati G, Ashton AC, Dolly JO, et al. Transglutaminase participates in the blockade of neurotransmitter release by tetanus toxin: evidence for a novel biological function. Amino Acids 2010;39:257–69. [DOI] [PubMed] [Google Scholar]
- 132.Rosenspire A, Yoo W, Menard S, Torres AR. Autism spectrum disorders are associated with an elevated autoantibody response to tissue transglutaminase-2. Autism Res 2011;4:242–9. [DOI] [PubMed] [Google Scholar]
- 133.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 2011;25:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron 2009;64:93–109. [DOI] [PubMed] [Google Scholar]
- 135.Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M. Npas4: a neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS ONE 2012;7:e46604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lintas C, Sacco R, Persico AM. Genome-wide expression studies in autism spectrum disorder, Rett syndrome, and Down syndrome. Neurobiol Dis 2012;45:57–68. [DOI] [PubMed] [Google Scholar]
- 137.Ziats MN, Rennert OM. Expression profiling of autism candidate genes during human brain development implicates central immune signaling pathways. PLoS ONE 2011;6:e24691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sharif O, Bolshakov NV, Raines S, Newham P, Perkins ND. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol 2007;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999;18:6853–66. [DOI] [PubMed] [Google Scholar]
- 140.Perkins ND. NF-κB: Tumor promoter or suppressor? Trends Cell Biol 2004;14:64–9. [DOI] [PubMed] [Google Scholar]
- 141.Naik US, Gangadharan C, Abbagni K, Nagalla B, Dasari N, Manna SK. A study of nuclear factor-kappaB in childhood autism. PLoS ONE 2011;6:e19488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Young AMH, Campbell E, Lynch S, Suckling J, Powis SJ. Aberrant NF-kappaB expression in autism spectrum condition: a mechanism for neuroinflammation. Front Psychiatry 2011;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gopee NV, He Q, Sharma RP. Fumosin B1-induced apoptosis is associated with delayed inhibition of protein kinase C, nuclear factor-κB and tumor necrosis factor α in LLC-PK1 cells. Chem Biol Interact 2003;146:131–45. [DOI] [PubMed] [Google Scholar]
- 144.Cavin C, Delatour T, Marin-Kuan M, Fenaille F, Holzhauser D, Guignard G, et al. Ochratoxin A-mediated DNA and protein damage: Roles of nitrosative and oxidative stresses. Toxicol Sci 2009;110:84–94. [DOI] [PubMed] [Google Scholar]
- 145.Wong SS, Zhou HR, Pestka JJ. Effects of vomitoxin (deoxynivalenol) on the binding of transcription factors AP-1, NF-kappaB, and NF-IL6 in raw 264.7 macrophage cells. J Toxicol Environ Health A 2002;65:1161–80. [DOI] [PubMed] [Google Scholar]
- 146.Pestka JJ. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit Contam A Chem Anal Control Expo Risk Assess 2008;25:1128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pestka JJ. Deoxynivalenol-induced proinflammatory gene expression: mechanisms and pathological sequelae. Toxin 2010;2:1300–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moon Y, Pestka JJ. Vomitoxin-induced cyclooxygenase-2 gene expression in macrophage mediated by activation of ERK and p38 but not JNK mitogen-activated protein kinases. Toxicol Sci 2002;69:373–82. [DOI] [PubMed] [Google Scholar]
- 149.Sajdel-Sulkowska EM, Xu M, Koibuchi N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum 2009;8:366–72. [DOI] [PubMed] [Google Scholar]
- 150.Ziats NM, Rennert OM. Sex-biased gene expression in the developing brain: implications for autism spectrum disorders. Mol Autism 2013;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ueta E, Kodama M, Sumino Y, Kurome M, Ohta K, Katagiru R, et al. Gender-dependent differences in the incidence of ochratoxin A-induced neural tube defects in the Pdn/Pdn mouse. Congenit Anom (Kyoto) 2010;50:29–39. [DOI] [PubMed] [Google Scholar]
- 152.Barrionuevo F, Scherer G. SOX E genes: SOX9 and SOX8 in mammalian testis development. Int J Biochem Cell Biol 2010;42:433–6. [DOI] [PubMed] [Google Scholar]
- 153.Ghahramani Seno MM, Hu P, Gwadry FG, Pinto D, Marshall CR, et al. Gene and miRNA expression profiles in autism spectrum disorders. Brain Res 2011;1380:85–97. [DOI] [PubMed] [Google Scholar]
- 154.Tryphonas H, Bondy G, Miller JD, Lacroix F, Hodgen M, Mcguire P, et al. Effects of fumonisin B1 on the immune system of Sprague-Dawley rats following a 14-day oral (gavage) exposure. Fundam Appl Toxicol 1997;39:53–9. [PubMed] [Google Scholar]
- 155.Marin DE, Taranu I, Pascale F, Lionide A, Burlacu R, Bailly JD, et al. Sex-related differences in the immune response of weanling piglets exposed to low doses of fumonisin extract. Br J Nutr 2006;95:1185–92. [DOI] [PubMed] [Google Scholar]
- 156.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science 2005;310:819–23. [DOI] [PubMed] [Google Scholar]
- 157.Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-Cawthorne G, Allison C, et al. Genes related to sex steroids, neural growth, and social–emotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res 2009;2:157–77. [DOI] [PubMed] [Google Scholar]
- 158.Sarachana T, Xu M, Wu RC, Hu VW. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS ONE 2011;6:e17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fink-Gremmels J, Malekinejad H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Animal Feed Sci Technol 2007;137:326–41. [Google Scholar]
- 160.Zeiner AR, Kegg PS, Blackburn M, Stratton R. Gender differences in peak acetaldehyde concentration after an acute dose of ethanol. Neurobehav Toxicol Teratol 1983;5:201–4. [PubMed] [Google Scholar]
- 161.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry 2013;65:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008;8:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Exp (Warsz) 2011;59:203–13. [DOI] [PubMed] [Google Scholar]
- 164.Loyd DR, Murphy AZ. Androgen and estrogen (alpha) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. J Chem Neuroanat 2008;36:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray–rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience 2007;147:456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray–rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci 2008;27:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Loyd DR, Wang X, Murphy AZ. Sex differences in μ-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci 2008;28:14007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]