Abstract
Whereas preclinical investigations and clinical studies have established that CD8+ T cells can profoundly affect cancer progression, the underlying mechanisms are still elusive. Challenging the prevalent view that the beneficial effect of CD8+ T cells in cancer is solely attributable to their cytotoxic activity, several reports have indicated that the ability of CD8+ T cells to promote tumor regression is dependent on their cytokine secretion profile and their ability to self-renew. Evidence has also shown that the tumor microenvironment can disarm CD8+ T cell immunity, leading to the emergence of dysfunctional CD8+ T cells. The existence of different types of CD8+ T cells in cancer calls for a more precise definition of the CD8+ T cell immune phenotypes in cancer and the abandonment of the generic terms “pro-tumor” and “antitumor.” Based on recent studies investigating the functions of CD8+ T cells in cancer, we here propose some guidelines to precisely define the functional states of CD8+ T cells in cancer.
Keywords: anergy, anticancer immunity, CD8+ T cells, cytotoxicity, exhaustion, effector, IFNγ, senescence, stemness
Introduction: The Relevance of CD8+ T Cells in Cancer
CD8+ T cells are essential for clearing viral, protozoan, and intracellular bacterial infections.1 Multiple lines of evidence show that CD8+ T cells are also a key component of antitumor immunity. Initial studies in preclinical cancer models showed that CD8+ T cells have a role in the prevention of tumor growth. Uyttenhove et al. showed that escape of P815 mastocytoma was due to loss of distinct CD8+ T cell specificities2 and Nakayama and Uenaka showed that antibodies against CD8+ effectively blocked the spontaneous rejection of transplantable tumors.3 Shankaran et al. and Smyth et al. later showed that adaptive immune responses were essential to prevent growth of mutagen-induced spontaneous tumors.4,5 Interestingly, Shankaran et al. further reported that TAP1-transfected transplantable sarcomas were eliminated in wild-type mice in a CD8+ T cell dependent-manner, suggesting that high expression of tumor antigens could drive activation of anticancer CD8+ T cell responses.4 Subsequent work from Koebel et al. showed that during the equilibrium phase of cancer growth, where cancer cells persist but are kept in check by the immune system,6 depletion of CD8+ T cells drives cancer cell growth, underscoring the importance of CD8+ T cells in controlling cancer growth over long time periods.7
CD8+ T cells have also been shown to be essential effector cells in the context of anticancer therapies. Depletion of CD8+ T cells has been shown to abrogate the anticancer efficacy of oxaliplatin and doxorubicin against EL4 thymoma and MCA2 fibrosarcoma tumors, respectively.8,9 Similarly, the therapeutic effect of local radiotherapy in melanoma, of interferon therapy in leukemia, and of bacille Calmette-Guerin therapy in bladder cancer is abrogated in the absence of CD8+ T cells.10-12 Altogether, these results establish that CD8+ T cells can control spontaneous and carcinogen-induced tumor growth, invasiveness of transplantable cell lines as well as the therapeutic efficacy of some anticancer treatments.
In line with this substantial amount of preclinical work, it has been established in human cancers that CD8+ T cell infiltrates can predict patients’ survival. While in kidney cancer CD8+ T cell infiltrates have been associated with worse outcome and with higher tumor grade,13,14 they are linked to a better clinical outcome in the vast majority of other cancer types. In ovarian cancer, the presence of CD3 T cell infiltrates have been shown to correlate with improved survival rates.15 In colon cancer, tumors without signs of metastatic invasion exhibit increased numbers of effector-memory CD8+ T cells, thereby indicating that the presence of effector-memory CD8+ T cells in the tumor microenvironment correlates with a better prognosis.16 These findings were subsequently confirmed in other cohorts and an international consortium is currently evaluating the possible utilization of immune infiltrate data to predict patient survival in routine clinical settings.17,18 The favorable prognostic value of CD8+ T cell infiltrates has also been documented in other cancer types, such as breast cancer and epithelial ovarian cancer.19,20 These findings suggest that, in humans, even in situations when tumors are detectable, CD8+ T cells can control tumor progression. The clinical relevance of CD8+ T cells in human cancer is further underscored by recent studies in breast cancer patients showing that the combination of high CD8+ and low FOXP3 cell infiltrates after chemotherapy was significantly associated with favorable clinical responses.21 These results were confirmed in two other studies, where CD8+ tumor infiltrating T cells were found to be an independent predictive factor for pathological complete response after anthracycline or anthracycline-taxane-based chemotherapy.9,22 Collectively, these preclinical and clinical observations indicate that CD8+ T cells should not only be contemplated as a putative therapeutic tool but also as a biomarker to monitor the efficacy of cytotoxic chemotherapy. However, recent data also indicate that intra-tumoral CD8+ T cells often lose their effector functions and exhibit a dysfunctional state. Accordingly, the terms “antitumor” and “pro-tumor” have been used in the literature to describe CD8+ T cells in cancer. Given the advances in our knowledge of CD8+ T cell phenotypes in cancer, these terms are clearly an oversimplification. Here, we discuss the different functional states of CD8+ T cells in cancer and propose some guidelines for more accurate designation of CD8+ T cells that exhibit different functional phenotypes.
CD8+ Effector T Cells in Cancer
Naïve CD8+ T cells that undergo priming in vivo in the presence of helper factors produced by CD4+ T cells differentiate into effector T cells that express high levels of perforin and granzymes.23,24 The coordinated delivery of these cytotoxic molecules to cancer cells can drive caspase activation and ultimately cell death23,25-27 (Fig. 1a). Given the demonstrated potential of CD8+ T cells to kill cancer cells, CD8+ T cells are often refered to as cytotoxic T lymphocytes (CTLs). Several different methods can be employed to assess CD8+ T cell cytotoxicity: direct measurement of target cell killing (for example by the chromium 51 release (51Cr) assay28), flow cytometry based or ELISPOT measurement of granzyme B, a component of lytic granules in CD8+ T cells,29,30 and detection of the expression of CD107a, which is present on the cell surface of degranulating CD8+ T cells. While the individual merits of these different methods have been debated, they have all been used to demonstrate CTL activity in cancer. Using quantification of CD107a, Rubio et al. showed that tumor-cytolytic T cells could be elicited in patients after vaccination and that tumor cell killing is associated with the ability of CD8+ T cells to recognize their targets.31 Using a 51Cr release assay, Takeshima et al. showed that in tumor-bearing mice local radiotherapy could elicit cytotoxic tumor-specific CD8+ T cells that prevent tumor growth.32 Importantly, they further demonstrated the importance of CD8+ T cells in mediating tumor regression following radiotherapy in vivo by using a neutralizing CD8+ antibody. This key experiment, which was replicated in other studies,10 was essential because the detection of activated or even antigen-specific cytotoxic T cells in ex vivo/in vitro assays does not necessarily ensure that CD8+ T cells drive tumor regression in vivo.
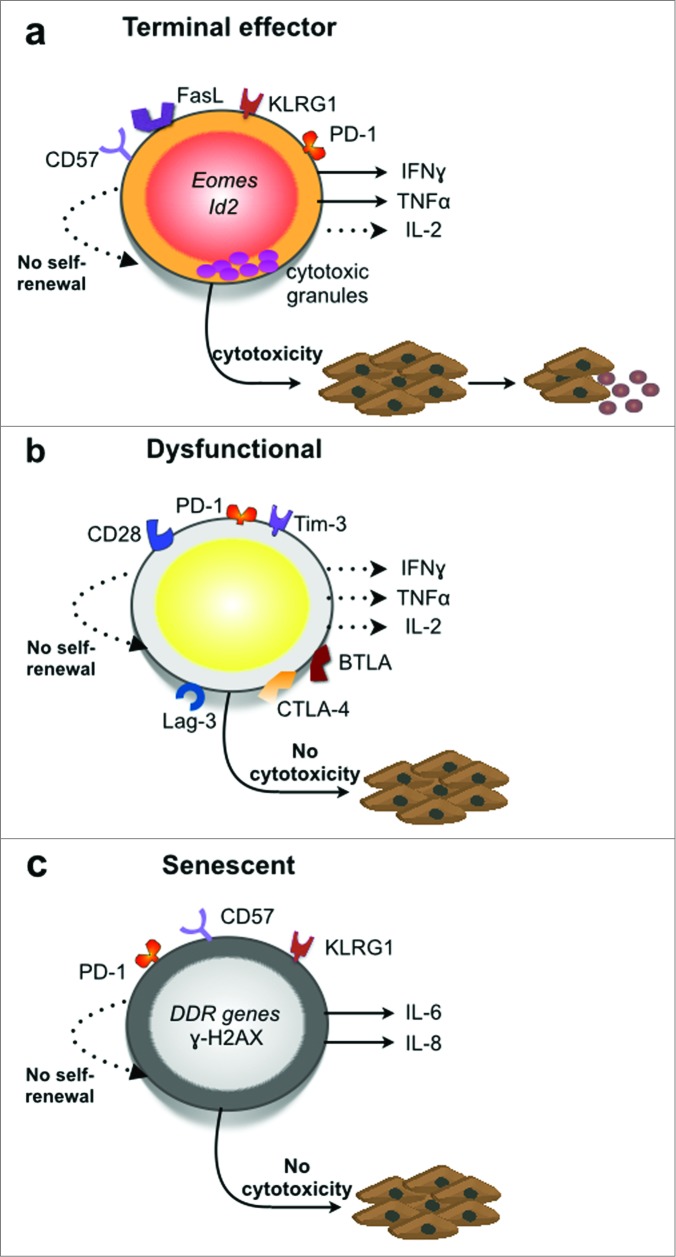
Figure 1.
CD8+ T cell phenotypes in the tumor microenvironment. (a) Effector CD8+ T cells that undergo terminal differentiation are characterized by low IL-2, strong IFNγ and TNFα release as well as high expression levels of the transcription factors Eomes and Id2.83–85 They do not express the surface markers CD62L, CCR7, CD27 but express killer cell lectin-like receptor G1 (KLRG-1) and PD-1.63,86–89 While terminal effector CD8+ T cells exhibit strong cytolytic functions in vitro, their anticancer activity in vivo is limited because of their inability to self-renew compared to stem-cell like memory CD8+ T cells.78,90,91 (b) Dysfunctional CD8+ T cells are characterized by cocomittant expression of two or more inhibitory receptors such as CTLA-4, PD-1, Lag-3, Tim-3, and BTLA.65,92,93 These cells exhibit defects in cytotoxicity, proliferative capacity, and secretion of pro-inflammatory cyotkines: IL-2, TNFα and IFNγ.55,56,94 (c) Senescent CD8+ T cells express killer cell lectin-like receptor G1 (KLRG-1) and CD57 but not CD27 or CD28.87,95 They are characterized by short telomeres, poor proliferative capacity and activation of DNA damage response (DDR) genes.66,68,95,96 These cells were also shown to express PD-1 in chronic lymphocytic leukemia patients.95 Senescent CD8+ T cells lack cytotoxicity,96 and were shown to express the proinflammatory mediators Il6 and Il8 in lung cancer tissue.68
CD8+ T cells can also kill tumors via the Fas/Fas ligand pathway. Indeed, it has been proposed that FasL-driven CD8+ T cell killing could be essential for the elimination of large and/or disseminated tumors.33-35 However, it should be noted that tumors can lose Fas expression or develop mutations in the cell death pathway engaged by FasL, thus developing resistance to FasL/Fas-mediated CD8+ T cell cytotoxicity. Other mechanisms by which tumors can resist CD8+ T cell cytotoxicity are increased expression of anti-apoptotic molecules such as Bcl-2, Bcl-xl, and Mcl-1 and changes in components of the cytoskeleton that impair the formation of stable immunological synapses between cytotoxic CD8+ T cells and tumor cells.36,37
Strategies have also been developed to assess CTL activity in vivo. For this, the selective elimination of adoptively transferred carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled target cells bearing a specific CD8+ T cell peptide has been examined in preclinical models.38 While this technique has the major advantage of assessing CD8+ T cell cytotoxicity in vivo, it does not inform as to the killing mechanism employed nor does it allow for visualization of the killing process. In regards to the latter, the development of intra-vital imaging represents a major advance in monitoring T cell anticancer functions in vivo in mice at the single-cell level. Using this technology, the group of Amigorena has found that activated cytotoxic CD8+ T cells can infiltrate tumors and arrest in close contact to and kill tumor cells provided that the tumor cells express cognate antigen.39 Using a similar methodology, Breart et al. found that in contrast to in vitro cytotoxic assays where tumor cell death occurs within minutes after incubation with cytotoxic T cells, the in vivo destruction of one tumor cell by a cytotoxic T lymphocyte in the tumor bed took on average 6 h, possibly explaining the limited ability of CD8+ T cells to eradicate established tumors.40
While the cytotoxicity of CD8+ T cells against tumor cells has been a major focus, it is important to note that some studies suggest that direct tumor cell killing may not be the major or only mechanism responsible for tumor regression. It has been shown that CD8+ T cells can also recognize tumor antigens processed by the stroma41 and studies using longitudinal confocal microscopy imaging have shown that vessel regression occurs immediately following CD8+ T cell entry from the blood stream into the tumor.42 Thus, cytotoxicity against tumor stroma may also be a major mechanism of tumor regression.
Although much attention has been given to the cytotoxic function of CD8+ T cells, it is not the sole mechanism responsible for the anticancer activity of CD8+ T cells. Activated CD8+ T cells also secrete cytokines like TNFα and IFNγ, which can induce cancer cell senescence and play essential roles in the control of anticancer immune responses and tumor growth43 (Fig. 1a). IFNγ has indeed been shown to be critical for cancer immunosurveillance and its secretion by CD8+ T cells can enhance antigen presentation, the antitumor functions of macrophages, and limit tumor angiogenesis.44-46 CD8+ T cell-derived IFNγ was further shown to be critical for the anticancer efficacy of chemotherapeutic drugs such as doxorubicin and oxaliplatin.8 Importantly, the ability of these drugs to prevent tumor outgrowth was not compromised in perforin-deficient mice, suggesting that in this system CD8+ T cells do not prevent tumor growth through direct cytotoxic activity. Accordingly, immunization of mice with chemotherapy-treated dying tumor cells failed to elicit CD8+ T cell cytotoxicity but instead induced their secretion of IFNγ. Thus, in some contexts the ability of CD8 T cells to produce IFNγ may be more critical than their cytolytic function for antitumor efficacy.8 These observations are in line with previous studies that identified IFNγ-dependent anti-angiogenesis as a general mechanism involved in tumor rejection by CD8+ T cell effectors.47
Altogether, these observations underscore that the “antitumor” activity of effector CD8+ T cells in tumor tissue can be ascribed to both their direct cytolytic activity and their cytokine secretion. Indeed, poly-functional CD8+ T cells that exhibit cytotoxicity along with production of TNFα and IFNγ may be the most robust antitumor effectors. In this regard, it is also important to note that the efficacy of effector CD8+ T cells in the tumor microenvironment may be limited as they undergo terminal differentiation and lose their ability to self-renew. The “antitumor” potential of CD8+ T cells that retain self-renewing capacity is discussed below.
Cancer-Driven CD8+ T Cell Dysfunction
Although effector CD8+ T cells can be found in the tumor microenvironment, it is also well established that tumors can drive CD8+ T cell dysfunction. In the literature, the terms “anergic” and “exhausted” have both been used to describe dysfunctional CD8+ T cells. Whether the CD8+ T cells in cancer are anergic or exhausted has been a matter of debate. Here, we will discuss the use of these terms to describe dysfunctional CD8+ T cells in cancer.
Anergy typically refers to a general state of diminished function of a given immune response. In the 1980s, the term was applied to T cells induced into a state of non-responsiveness in vitro upon engagement of the T cell receptor (Signal 1) in the absence of a costimulatory signal (Signal 2). In a number of in vivo settings, such as tolerance induction by i.v. injection of antigens without adjuvants, it was hypothesized that T cell unresponsiveness was similarly induced by antigen recognition without appropriate co-stimulatory signals.48 Anergic T cells fail to proliferate and produce effector cytokines in response to subsequent stimulation. T cell anergy is believed to be operative in cancer given that tumors often poorly express co-stimulatory molecules such as B7-1/B7-2, that dendritic cells present in tumor tissue express low MHC and low B7-1/B7-2 but high PD-L1 (B7-H1),49 and that myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM) contribute to sub-optimal antigen presentation in the tumor environment.50 Moreover, MDSCs and TAMs can produce arginase-1 and TGF-β and drive oxidative stress, all of which drive suppression of CD8+ T cell responses.51
The term “exhaustion” comes from the study of the CD8+ T cell response to chronic viral infections in mouse models where antigen is not cleared despite ongoing stimulation. There is also an evidence for virus-specific T cell “exhaustion” in humans in the setting of chronic HCV and HIV. Similar to anergic T cells, “exhausted” T cells exhibit defective responses to antigen stimulation; however, unlike anergy which develops as a result of a sub-optimal first encounter of T cells with cognate antigen, exhaustion develops progressively as a result of chronic stimulation of T cells in the face of high antigen burden.52 Indeed, the T cells that develop an “exhausted” phenotype are those that undergo robust activation in the acute phase of the anti-viral response.
“Exhausted” CD8+ T cells express high levels of co-inhibitory receptors such as PD-1, Lag-3, CD244, CD160, and Tim-3, and it has been shown that interfering with the signaling through one or more of these receptors can improve anti-virus CD8+ T cell responses.53,54 CD8+ T cells that express inhibitory molecules and exhibit severe functional deficits have also been described in cancer55-59 (Fig. 1b). These observations have led to the widespread use of the term “exhaustion” to describe the dysfunctional CD8+ T cells in cancer. However, whether the dysfunctional CD8+ T cells observed in cancer are truly analogous to those that arise in chronic viral infection is an open question. Resolution of this issue awaits elucidation of the molecular programs specifically associated with dysfunctional T cells in cancer. These studies are currently at an early stage. An initial study of the dysfunctional CD8+ T cells from the tumor-infiltrated lymph nodes of melanoma patients indicates that the gene profile of these cells is significantly enriched for genes identified in exhausted LCMV-specific murine CD8+ T cells; however, these cells fail to upregulate Batf, a key driver of T cell exhaustion in HIV infection.57
Moreover, it has recently been suggested that “exhaustion” is a misnomer as “exhausted” cells are not completely devoid of function as the term “exhaustion” implies. Rather these cells exhibit an attenuated response that is optimized for minimizing tissue damage while still preserving some level of response against abnormal cells (virally infected or cancerous).60 Indeed, a key function of co-inhibitory receptor expression on highly active T cells is to contract ongoing T cell responses in order to restore immune homeostasis and prevent immunopathology. Unfortunately, tumors have taken advantage of this mechanism to dampen antitumor T cell responses.
At this juncture, we recommend against ascribing the CD8+ T cells in cancer as either “anergic” or “exhausted." This terminology is not useful as these states have been defined and largely studied in other T cell types, such as CD4+ T cells, or in disease conditions that differ significantly from cancer, namely chronic viral infection. We recommend that the term dysfunctional instead be used to describe the poorly functional CD8+ T cells in cancer.61 We further caution against ascribing cells as dysfunctional based on expression of co-inhibitory receptors alone as these molecules are also found on effector T cells that retain functional properties.62 Indeed, expression of these inhibitory receptors could also reflect a state of previous activation of CD8+ T cells indicating that expression of these receptors may identify antitumor specific T cells associated with a good prognosis63,64 . Dysfunctional CD8+ T cells should be defined as cells that exhibit defects in proliferation, lack of inflammatory cytokine production and/or cytotoxic functions, together with expression of one or more co-inhibitory receptors (Fig. 1b).
It is important to note that CD8+ T cell dysfunction in the tumor microenvironment is believed to be reversible, at least to some extent. In pre-clinical cancer models, blockade of signaling through CTLA-4, PD-1, Tim-3, and Lag-3 have been shown to improve CD8+ T cell responses (reviewed in 65). Accordingly, the current success of strategies that interfere with signaling through the PD-1 inhibitory receptor in the clinic is believed, at least in part, to be due to the ability of PD-1 blockade to re-invigorate CD8+ T cell responses.
CD8+ T Cell Senescence in Cancer
Senescent CD8+ T cell phenotypes can also arise in the tumor microenvironment. Senescence refers to an irreversible state of growth arrest that develops in cells upon repeated cellular division, termed replicative senescence, or in response to DNA damage. General characteristics of senescent cells include: short telomeres, irreversible cell cycle-arrest, activation of DNA damage response (DDR) genes, robust secretion of factors that constitute the senescence-associated secretory phenotype (SASP), and accumulation of senescence associated heterochromatin foci (SAHF).66 Specific cell surface markers ascribed to senescent T cells are loss of CD28 and CD27 and high expression of CD57 and KLRG-1 (Fig. 1c).
While senescence has historically been associated with aging, it is now recognized that replicative senescence also develops in the context of chronic antigen stimulation, such as that which occurs in cancer. Indeed, it has been shown that culture of tumor cells with normal healthy human T cells in low tumor to T cell ratios can induce a phenotype consistent with T cell senescence in vitro.67 These cells exhibit decreased CD28 and CD27 expression along with concomitant up-regulation of γH2AX (H2A histone family member X) and ATM (ataxia telangiectasia mutated), both of which are induced as part of the DDR to double strand DNA breaks (Fig. 1c). A recent study further reported the presence of CD8+ T cells that exhibit characteristics of senescence in vivo in human lung cancer tissue.68 These cells are CD28−CD57+ and exhibit accumulation of heterochromatin protein-1 gamma foci, a component of SAHF.
Although senescent T cells are irreversibly cell-cycle arrested, it is important to note that they are not completely devoid of function. The senescent CD8+ T cells found in lung cancer tissue produce IL-6 and IL-8, two hallmark SASP factors.68 These two features distinguish senescent CD8+ T cells from dysfunctional CD8+ T cells (Fig. 1b and c) as the dysfunctional T cells are not irreversibly cell-cycle arrested they exhibit severely impaired production of pro-inflammatory cytokines and other effector molecules.
The two SASP factors that are reported to be expressed by senescent CD8+ T cells, IL-6 and IL-8, are both pro-inflammatory cytokines. IL-6 can suppress regulatory T cell function (Treg)69 and promote the differentiation of IL-17-producing Th17 cells.70 The dampening of Treg function could benefit antitumor immunity by relieving an important mechanism of immune suppression in tumor tissue. However, the outcome of promotion of Th17 cells in tumor tissue is less clear as both “pro-tumor” and “antitumor” properties for Th17 cells have been described.71 Notwithstanding how IL-6 may shape antitumor T cell responses, IL-6 can promote tumorigenesis through its effects in driving cellular proliferation, promoting cell survival by delivering anti-apoptotic signals, and augmenting MDSC suppressive functions.72,73 Indeed, high levels of IL-6 have been associated with multiple cancers and are associated with poor prognosis.74 IL-8 also exhibits pleiotropic tumor promoting effects. It can promote angiogenesis, cancer cell survival, proliferation, migration, and resistance to chemotherapy.75 Thus, by virtue of their production of IL-6 and IL-8 senescent CD8+ T cells could be considered “pro-tumor.”
It is also important to note that terminal effector CD8+ T cells can also exhibit loss of CD28 and upregulation of KLRG-1. Moreover, vaccine-induced CD8+ T cells with optimal antitumor effector function have been noted to express high levels of KLRG-1.76 Thus, senescent phenotype cannot be ascribed solely on the basis of loss of CD28 and expression of KLRG1. Senescent cells must further exhibit SAHF and activation of DDR genes (Fig. 1c).
Stem-Cell Like Memory CD8+ T Cells
Terminal effector CD8+ T cells limit tumor outgrowth. However, these cells can become dysfunctional or senescent in the tumor microenvironment. Recent studies that examine the efficacy of ex vivo generated CD8+ T cells on tumor clearance after adoptive transfer into tumor-bearing hosts show that terminally differentiated effector CD8+ T cells are ineffective at eliminating tumors in vivo compared to less differentiated T cells77,78 (Figs. 1a and 2). This occurs in spite of their higher secretion of IFNγ and cytolytic activity. Instead, it has been suggested that CD8+ T cells that share properties with naïve T cells such as CCR7 and CD62L expression and have the ability to self-renew are more potent for fighting tumors (Fig. 2). Because of their ability to self-renew and persist for long periods of time, these CD8+ T cells have been termed stem-cell like memory T cells. Unfortunately, in contrast to terminal effector CD8+ T cells, stem-cell like memory CD8+ T cells are predominantly found in lymphoid tissue and not in the tumor microenvironment.
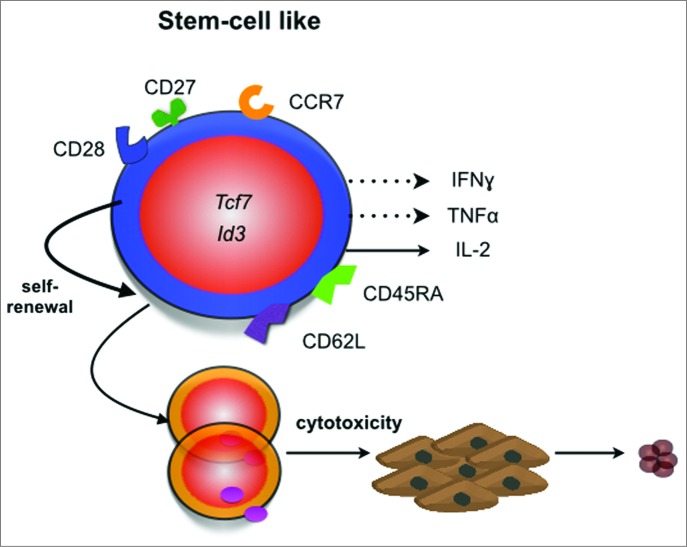
Figure 2.
Features of stem-cell like CD8+ T cells. Stem-cell like memory CD8+ T cells share many phenotypic features with naïve T cells (reviewed in 97). They typically express the CD45RA phosphatase, the lymph node homing molecules CCR7 and CD62L as well as the costimulatory receptors CD27 and CD28.77,98 These cells express the transcription factors Id377 and Tcf7,91 secrete IL-2 and low levels of TNFα or IFNγ. These cells also have the ability to self-renew and exhibit potent anticancer responses in vivo.78,90
It is well known that the omnipotency of naïve T cells is progressively lost with T cell differentiation to memory and effector T cells. Among antigen-experienced T cells, the stem-cell like memory T cells are the ones with the highest potency, producing progeny for both immediate immunity and its long-term maintenance, based on self-renewal. It is likely that tumor-antigen specific effector T cells depend on continuous differentiation from self-renewing memory T cells. Therefore, it remains a major aim to develop methods inducing self-renewing T cells in vitro for adoptive transfer, or in vivo by active immunization. In this regard, the recent identification of IL-7 and IL-15 as molecular signals guiding human naive T lymphocytes to differentiate into stem-cell like memory CD8+ T cells in vitro provides impetus to investigate their anticancer potential in clinical trials.79,80 Progress in basic research, bioengineering, and therapy development will likely further exploit the potential of T cell stemness, as a fundamental basis of robust and long-term T cell responses including the capability to home to tumors and exert effector functions therein.
CD8+ T Cells as Regulatory Cells in Cancer?
The existence of several types of CD8+ T cells with regulatory or suppressive properties in cancer has been proposed. These include: CD8+ CD28−, CD8+ CD25+, CD8+ CD122+, and CD8+ IL-10+ T cells.81,82 At present, there seems to be no consensus in the field as to whether these are overlapping or dissimilar subsets and, moreover, whether these are truly distinct from other cell types that express some of the same surface markers. For these reasons, this potential class of CD8+ T cells will not be further discussed here.
Conclusion
Our understanding of the CD8+ phenotypes that arise in cancer necessitates that we move beyond the simplified nomenclature of “pro-tumor” vs. “antitumor” T cells. We, and others, have now identified stem cell-like, terminal effector, dysfunctional, or senescent CD8+ T cells that are functionally and in many cases molecularly distinct. Currently, there are no unique surface markers that allow for easy discrimination between these CD8+ phenotypes. Thus, accurate identification requires a more in depth analysis that includes examination of cell surface phenotype, functional phenotype, and expression of intracellular markers. Here, we have summarized the current knowledge of CD8+ phenotypes in cancer (Box 1). We recommend avoiding the use of broad terms like “pro-tumor” or “antitumor” CD8+ T cells without providing information on their functional state. We further propose that the term CTL should only be employed when corresponding cytotoxic functions have been experimentally demonstrated and that the term “dysfunctional” rather than “anergic” or “exhausted” be used to describe CD8+ T that exhibit functional deficits in cancer. Importantly, we caution against ascribing CD8+ T cells as dysfunctional based on the expression of co-inhibitory receptors alone. Future studies incorporating T cell analyses should include appropriate markers and functional assays to better define the phenotypes of T cells in peripheral blood, peripheral lymphoid tissues, and tumor biopsy samples.
Financial and Competing Interests Disclosure
MB is currently working as a scientific director of Institut Mérieux, a private company implementing in vitro diagnostics and immunotherapeutic approaches in oncology and infectious diseases. CM is Chief Scientific Officer of ISA Pharmaceuticals, a private biotech company developing synthetic therapeutic vaccines against cancer.
The other authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
Box 1. Distinguishing Features of CD8+ T cell phenotypes
Terminal effector T cells- Surface features are expression of KLRG-1 and loss of CD62L, CCR7, CD27, and CD28. Important molecular features include high expression of the transcription factors eomes and Id2. Important cellular features are low IL-2 production, strong TNFα, IFNγ, and cytotoxic function but poor ability to self-renew.
Dysfunctional T cells- Surface features are expression of multiple co-inhibitory receptors such as CTLA-4, PD-1, Tim-3, and Lag-3. Important cellular features are defects in various effector functions: proliferative response to antigen stimulation, cytotoxicity, and secretion of pro-inflammatory cytokines (IL-2, TNFα, and IFNγ). It is important to note that dysfunctional T cells may not exhibit defects in all effector functions and thus dysfunctional phenotypes exist across a spectrum of weak to severe dysfunction.
Senescent T cells- Surface features are expression of KLRG-1 and CD57 and lack of CD27 and CD28. Important distinguishing cellular features are short telomeres, irreversible cell-cycle arrest, activation of DNA damage response (DDR) genes such as ATM and γH2AX, the presence of senescence-associated secretory phenotype (IL-6 and IL-8), and the presence of senescence-associated heterochromatin foci (SAHF). SAHF are foci of facultative or repressed heterochromatin associated with gene-silencing.
Stem-like T cells- Surface features are expression of CCR7 and expression of CD62L, CD45RA, CD27, and CD28. Important molecular features are expression of the transcription factors Id2 and Tcf7. Important cellular features are potent cytotoxicity in vivo and ability to self-renew.
Acknowledgments
The authors of this manuscript support the guidelines described herein. L.A. and A.C.A. extend their sincere apologies to researchers in the field of CD8+ T cells whose studies were not cited due to space restrictions.
Funding
Work in the author's laboratories is supported by grants from the American Cancer Society (RSG-11-057-02-LIB to A.C.A.), the National Health and Medical Research Council of Australia (628623 to MJS), and the French National Research Agency (ANR-13-JSV3-0001 to L.A.). Due to space and other limitations, it is not possible to include all other sources of financial support.
References
- 1. Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Ann Rev Immunol 2000; 18:275-308; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.18.1.275 [DOI] [PubMed] [Google Scholar]
- 2. Uyttenhove C, Maryanski J, Boon T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J Exp Med 1983; 157:1040-52; PMID:; http://dx.doi.org/ 10.1084/jem.157.3.1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakayama E, Uenaka A. Effect of in vivo administration of Lyt antibodies. Lyt phenotype of T cells in lymphoid tissues and blocking of tumor rejection. J Exp Med 1985; 161:345-55; PMID:; http://dx.doi.org/ 10.1084/jem.161.2.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410:1107-11; PMID:; http://dx.doi.org/ 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- 5. Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 2000; 192:755-60; PMID:; http://dx.doi.org/ 10.1084/jem.192.5.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991-8; PMID:; http://dx.doi.org/ 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 7. Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007; 450:903-7; PMID:; http://dx.doi.org/ 10.1038/nature06309 [DOI] [PubMed] [Google Scholar]
- 8. Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170-8; PMID:; http://dx.doi.org/ 10.1038/nm.2028 [DOI] [PubMed] [Google Scholar]
- 9. Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 2011; 71:4809-20; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0753 [DOI] [PubMed] [Google Scholar]
- 10. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114:589-95; PMID:; http://dx.doi.org/ 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol 1993; 150:1018-23; PMID: [DOI] [PubMed] [Google Scholar]
- 12. Gresser I, Maury C, Carnaud C, De Maeyer E, Maunoury MT, Belardelli F. Anti-tumor effects of interferon in mice injected with interferon-sensitive and interferon-resistant Friend erythroleukemia cells. VIII. Role of the immune system in the inhibition of visceral metastases. Int J Cancer 1990; 46:468-74; PMID:; http://dx.doi.org/ 10.1002/ijc.2910460324 [DOI] [PubMed] [Google Scholar]
- 13. Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 2013; 19:4079-91; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-3847 [DOI] [PubMed] [Google Scholar]
- 14. Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001; 61:5132-6; PMID: [PubMed] [Google Scholar]
- 15. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203-13; PMID:; http://dx.doi.org/ 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 16. Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654-66; PMID:; http://dx.doi.org/ 10.1056/NEJMoa051424 [DOI] [PubMed] [Google Scholar]
- 17. Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 2014; 232:199-209; PMID:; http://dx.doi.org/ 10.1002/path.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koelzer VH, Lugli A, Dawson H, Hadrich M, Berger MD, Borner M, Mallaev M, Galván JA, Amsler J, Schnüriger B, et al. CD8/CD45RO T-cell infiltration in endoscopic biopsies of colorectal cancer predicts nodal metastasis and survival. J Transl Med 2014; 12:81; PMID:; http://dx.doi.org/ 10.1186/1479-5876-12-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949-55; PMID:; http://dx.doi.org/ 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 20. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102:18538-43; PMID:; http://dx.doi.org/ 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rebe C, Coudert B, Martin F, Bizollon MH, Vanoli A, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol 2011; 224:389-400; PMID:; http://dx.doi.org/ 10.1002/path.2866 [DOI] [PubMed] [Google Scholar]
- 22. Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, Kim YJ, Kim JH, Park SY. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 2013; 109:2705-13; PMID:; http://dx.doi.org/ 10.1038/bjc.2013.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol 2002; 2:401-9; PMID:; http://dx.doi.org/10.1038/nri819 [DOI] [PubMed] [Google Scholar]
- 24. Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998; 393:480-3; PMID:; http://dx.doi.org/ 10.1038/31002 [DOI] [PubMed] [Google Scholar]
- 25. Masson D, Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell 1987; 49:679-85; PMID:; http://dx.doi.org/ 10.1016/0092-8674(87)90544-7 [DOI] [PubMed] [Google Scholar]
- 26. Pasternack MS, Verret CR, Liu MA, Eisen HN. Serine esterase in cytolytic T lymphocytes. Nature 1986; 322:740-3; PMID:; http://dx.doi.org/ 10.1038/322740a0 [DOI] [PubMed] [Google Scholar]
- 27. Podack ER, Konigsberg PJ. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med 1984; 160:695-710; PMID:; http://dx.doi.org/ 10.1084/jem.160.3.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brunner KT, Mauel J, Cerottini JC, Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology 1968; 14:181-96; PMID: [PMC free article] [PubMed] [Google Scholar]
- 29. Griffiths GM, Isaaz S. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. J Cell Biol 1993; 120:885-96; PMID:; http://dx.doi.org/ 10.1083/jcb.120.4.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wever PC, Van Der Vliet HJ, Spaeny LH, Wolbink AM, Van Diepen FN, Froelich CJ, Hack CE, ten Berge IJ. The CD8+ granzyme B+ T-cell subset in peripheral blood from healthy individuals contains activated and apoptosis-prone cells. Immunology 1998; 93:383-9; PMID:; http://dx.doi.org/ 10.1046/j.1365-2567.1998.00448.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med 2003; 9:1377-82; PMID:; http://dx.doi.org/ 10.1038/nm942 [DOI] [PubMed] [Google Scholar]
- 32. Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, Kitamura H, Nishimura T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res 2010; 70:2697-706; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-2982 [DOI] [PubMed] [Google Scholar]
- 33. Caldwell SA, Ryan MH, McDuffie E, Abrams SI. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol 2003; 171:2402-12; PMID:; http://dx.doi.org/ 10.4049/jimmunol.171.5.2402 [DOI] [PubMed] [Google Scholar]
- 34. Seki N, Brooks AD, Carter CR, Back TC, Parsoneault EM, Smyth MJ, Wiltrout RH, Sayers TJ. Tumor-specific CTL kill murine renal cancer cells using both perforin and Fas ligand-mediated lysis in vitro, but cause tumor regression in vivo in the absence of perforin. J Immunol 2002; 168:3484-92; PMID:; http://dx.doi.org/ 10.4049/jimmunol.168.7.3484 [DOI] [PubMed] [Google Scholar]
- 35. Afshar-Sterle S, Zotos D, Bernard NJ, Scherger AK, Rodling L, Alsop AE, Walker J, Masson F, Belz GT, Corcoran LM, et al. Fas ligand-mediated immune surveillance by T cells is essential for the control of spontaneous B cell lymphomas. Nat Med 2014; 20:283-90; PMID:; http://dx.doi.org/ 10.1038/nm.3442 [DOI] [PubMed] [Google Scholar]
- 36. Jazirehi AR, Economou JS. Proteasome inhibition blocks NF-kappaB and ERK1/2 pathways, restores antigen expression, and sensitizes resistant human melanoma to TCR-engineered CTLs. Mol Cancer Ther 2012; 11:1332-41; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0814 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Abouzahr S, Bismuth G, Gaudin C, Caroll O, Van Endert P, Jalil A, Dausset J, Vergnon I, Richon C, Kauffmann A, et al. Identification of target actin content and polymerization status as a mechanism of tumor resistance after cytolytic T lymphocyte pressure. Proc Natl Acad Sci U S A 2006; 103:1428-33; PMID:; http://dx.doi.org/ 10.1073/pnas.0510454103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Durward M, Harms J, Splitter G. Antigen specific killing assay using CFSE labeled target cells. J Vis Exp 2010; 45:pii. 2250; PMID:; http://dx.doi.org/10.3791/2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med 2007; 204:345-56; PMID:; http://dx.doi.org/ 10.1084/jem.20061890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest 2008; 118:1390-7; PMID:; http://dx.doi.org/ 10.1172/JCI34388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med 2004; 10:294-8; PMID:; http://dx.doi.org/ 10.1038/nm999 [DOI] [PubMed] [Google Scholar]
- 42. Schietinger A, Arina A, Liu RB, Wells S, Huang J, Engels B, Bindokas V, Bartkowiak T, Lee D, Herrmann A, et al. Longitudinal confocal microscopy imaging of solid tumor destruction following adoptive T cell transfer. Oncoimmunology 2013; 2:e26677; PMID:; http://dx.doi.org/ 10.4161/onci.26677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361-5; PMID:; http://dx.doi.org/ 10.1038/nature11824 [DOI] [PubMed] [Google Scholar]
- 44. Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001; 97:192-7; PMID:; http://dx.doi.org/ 10.1182/blood.V97.1.192 [DOI] [PubMed] [Google Scholar]
- 45. Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med 2002; 196:129-34; PMID:; http://dx.doi.org/ 10.1084/jem.20020063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sato N, Nariuchi H, Tsuruoka N, Nishihara T, Beitz JG, Calabresi P, Frackelton AR, Jr. Actions of TNF and IFN-gamma on angiogenesis in vitro. J Invest Dermatol 1990; 95:85S-9S; PMID:; http://dx.doi.org/ 10.1111/1523-1747.ep12874809 [DOI] [PubMed] [Google Scholar]
- 47. Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res 2003; 63:4095-100; PMID: [PubMed] [Google Scholar]
- 48. Schwartz RH. T cell anergy. Ann Rev Immunol 2003; 21:305-34; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.21.120601.141110 [DOI] [PubMed] [Google Scholar]
- 49. Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9:562-7; PMID:; http://dx.doi.org/ 10.1038/nm863 [DOI] [PubMed] [Google Scholar]
- 50. Melief CJ. Cancer immunotherapy by dendritic cells. Immunity 2008; 29:372-83; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 51. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492-9; PMID:; http://dx.doi.org/ 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- 53. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682-7; PMID:; http://dx.doi.org/ 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 54. Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10:29-37; PMID:; http://dx.doi.org/ 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207:2187-94; PMID:; http://dx.doi.org/ 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010; 207:2175-86; PMID:; http://dx.doi.org/ 10.1084/jem.20100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest 2011; 121:2350-60; PMID:; http://dx.doi.org/ 10.1172/JCI46102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011; 117:4501-10; PMID:; http://dx.doi.org/ 10.1182/blood-2010-10-310425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest 2012; 122:1271-82; PMID:; http://dx.doi.org/ 10.1172/JCI59806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Utzschneider DT, Legat A, Fuertes Marraco SA, Carrie L, Luescher I, Speiser DE, Zehn D. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol 2013; 14:603-10; PMID:; http://dx.doi.org/ 10.1038/ni.2606 [DOI] [PubMed] [Google Scholar]
- 61. Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med 2013; 19:465-72; PMID:; http://dx.doi.org/ 10.1038/nm.3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory receptor expression depends more dominantly on differentiation and activation than "exhaustion" of human CD8 T cells. Front Immunol 2013; 4:455; PMID:; http://dx.doi.org/ 10.3389/fimmu.2013.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124:2246-59; PMID:; http://dx.doi.org/ 10.1172/JCI73639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73:128-38; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2606 [DOI] [PubMed] [Google Scholar]
- 65. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011; 192:547-56; PMID:; http://dx.doi.org/ 10.1083/jcb.201009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Montes CL, Chapoval AI, Nelson J, Orhue V, Zhang X, Schulze DH, Strome SE, Gastman BR. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res 2008; 68:870-9; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2282 [DOI] [PubMed] [Google Scholar]
- 68. Mondal AM, Horikawa I, Pine SR, Fujita K, Morgan KM, Vera E, Mazur SJ, Appella E, Vojtesek B, Blasco MA, et al. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J Clin Invest 2013; 123:5247-57; PMID:; http://dx.doi.org/ 10.1172/JCI70355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 2003; 299:1033-6; PMID:; http://dx.doi.org/ 10.1126/science.1078231 [DOI] [PubMed] [Google Scholar]
- 70. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235-8; PMID:; http://dx.doi.org/ 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 71. Martin F, Apetoh L, Ghiringhelli F. Controversies on the role of Th17 in cancer: a TGF-beta-dependent immunosuppressive activity? Trends Mol Med 2012; 18:742-9; PMID:; http://dx.doi.org/ 10.1016/j.molmed.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 72. Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med 2008; 14:109-19; PMID:; http://dx.doi.org/ 10.1016/j.molmed.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 73. Apetoh L, Vegran F, Ladoire S, Ghiringhelli F. Restoration of antitumor immunity through selective inhibition of myeloid derived suppressor cells by anticancer therapies. Curr Mol Med 2011; 11:365-72; PMID:; http://dx.doi.org/ 10.2174/156652411795976574 [DOI] [PubMed] [Google Scholar]
- 74. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013; 14:e218-28; PMID:; http://dx.doi.org/ 10.1016/S1470-2045(12)70582-X [DOI] [PubMed] [Google Scholar]
- 75. Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008; 14:6735-41; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4843 [DOI] [PubMed] [Google Scholar]
- 76. van Duikeren S, Fransen MF, Redeker A, Wieles B, Platenburg G, Krebber WJ, Ossendorp F, Melief CJ, Arens R. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol 2012; 189:3397-403; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1201540 [DOI] [PubMed] [Google Scholar]
- 77. Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 2012; 12:671-84; PMID:; http://dx.doi.org/ 10.1038/nrc3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nat Med 2011; 17:1290-7; PMID:; http://dx.doi.org/ 10.1038/nm.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013; 121:573-84; PMID:; http://dx.doi.org/ 10.1182/blood-2012-05-431718 [DOI] [PubMed] [Google Scholar]
- 80. Gattinoni L, Restifo NP. Moving T memory stem cells to the clinic. Blood 2013; 121:567-8; PMID:; http://dx.doi.org/ 10.1182/blood-2012-11-468660 [DOI] [PubMed] [Google Scholar]
- 81. Wang RF. CD8+ regulatory T cells, their suppressive mechanisms, and regulation in cancer. Hum Immunol 2008; 69:811-4; PMID:; http://dx.doi.org/ 10.1016/j.humimm.2008.08.276 [DOI] [PubMed] [Google Scholar]
- 82. Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res 2005; 65:5020-6; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-4043 [DOI] [PubMed] [Google Scholar]
- 83. Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 2003; 302:1041-3; PMID:; http://dx.doi.org/ 10.1126/science.1090148 [DOI] [PubMed] [Google Scholar]
- 84. Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol 2011; 12:1230-7; PMID:; http://dx.doi.org/ 10.1038/ni.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D'Cruz LM, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol 2011; 12:1221-9; PMID:; http://dx.doi.org/ 10.1038/ni.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths SJ, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood 2009; 113:6619-28; PMID:; http://dx.doi.org/ 10.1182/blood-2009-01-199588 [DOI] [PubMed] [Google Scholar]
- 87. Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 2002; 100:3698-702; PMID:; http://dx.doi.org/ 10.1182/blood-2002-02-0657 [DOI] [PubMed] [Google Scholar]
- 88. Gothert JR, Eisele L, Klein-Hitpass L, Weber S, Zesewitz ML, Sellmann L, Röth A, Pircher H, Dührsen U, Dürig J. Expanded CD8+ T cells of murine and human CLL are driven into a senescent KLRG1+ effector memory phenotype. Cancer Immunol Immunother 2013; 62:1697-709; PMID:; http://dx.doi.org/ 10.1007/s00262-013-1473-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol 2009; 182:5240-9; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0803245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 2005; 115:1616-26; PMID:; http://dx.doi.org/ 10.1172/JCI24480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 2013; 123:594-9; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res 2012; 72:887-96; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mittal R, Wagener M, Breed ER, Liang Z, Yoseph BP, Burd EM, Farris AB, 3rd, Coopersmith CM, Ford ML. Phenotypic T cell exhaustion in a murine model of bacterial infection in the setting of pre-existing malignancy. PLoS One 2014; 9:e93523; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0093523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S, et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res 2014; 74:1045-55; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nunes C, Wong R, Mason M, Fegan C, Man S, Pepper C. Expansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clin Cancer Res 2012; 18:678-87; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-2630 [DOI] [PubMed] [Google Scholar]
- 96. Beatty GL, Smith JS, Reshef R, Patel KP, Colligon TA, Vance BA, Frey NV, Johnson FB, Porter DL, Vonderheide RH. Functional unresponsiveness and replicative senescence of myeloid leukemia antigen-specific CD8+ T cells after allogeneic stem cell transplantation. Clin Cancer Res 2009; 15:4944-53; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 2013; 43:2797-809; PMID:; http://dx.doi.org/ 10.1002/eji.201343751 [DOI] [PubMed] [Google Scholar]
- 98. De Rosa SC, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med 2001; 7:245-8; PMID:; http://dx.doi.org/10.1038/84701 [DOI] [PubMed] [Google Scholar]