Abstract
Dendritic cells (DCs) are known to regulate the functions of various immune cells. Reciprocal signaling by these immune cells also “educate” the DCs and determine the quality of the ensuing immune responses. Recently, we demonstrated that human DCs undergo maturation upon interaction with activated B cells to acquire unique abilities to promote polarization of Th2 cells.
Keywords: antibody response, antitumor, B lymphocytes, dendritic cells, Th2, OX-40 ligand
Abbreviations: BAFF-R, B–cell-activating factor receptor; BCR, B-cell receptor; DAMPs, damage-associated molecular patterns; DCs, dendritic cells; OX-40L, OX-40 ligand; PAMPs, pathogen-derived molecular patterns (PAMPs); TACI, transmembrane activator and calcium-modulating cyclophilin ligand interactor; Tregs, regulatory T cells
Introduction
Dendritic cells (DCs) are the specialized antigen-presenting cells with juxtaposition at the site of immune reaction, such as the entry point of pathogens, inflammation, and tumor growth. Signaling by pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and inflammatory cytokines imparts several changes to DC characteristics including maturation, cytokine/chemokine secretion, expression of chemokine receptors, migration to secondary lymphoid organs, and polarization of T-cell responses. Therefore, DCs are critical for the regulation of antigen-specific immune responses through modulation of the functions of other immune cells. However, the interaction between DCs and other cells of the immune system is not a monolog; rather, it is a dialog. Thus, reciprocal signaling by these immune cells also “educate” the DCs and the quality of the ensuing immune responses. Notably, activated T cells, natural killer cells, and neutrophils are reported to induce maturation and activation of DCs. Conversely, regulatory T cells (Treg) restrain DC maturation and induce tolerogenic features.1 Interestingly, in addition to antibody production, B cells are also known to exert both effector as well as regulatory functions, mainly through antigen presentation, polarization of CD4+ T helper (Th) cell responses, and secretion of cytokines and chemokines, as observed in various models of autoimmunity and infections.2,3 However, the modulation of DC functions by B cells through direct interaction is relatively unexplored.
In order to explore the direct effect of B cells on human DC phenotype, we co-cultured immature monocyte-derived DCs with resting or B-cell receptor (BCR)-activated CD19+B cells. We found that activated, but not resting, B cells were capable of inducing the maturation of DCs, characterized by increased expression of the co-stimulatory molecules CD80, CD86, and CD40, the antigen-presenting molecule human leukocyte antigen (HLA)-DR, and the terminal maturation marker CD83 (Fig. 1). Here, the soluble factors secreted by B cells were not involved in this process. Furthermore, trans-well culture and use of blocking antibodies to activation-associated molecules on B cells, that is, to the B–cell-activating factor receptor (BAFF-R), transmembrane activator, and calcium-modulating cyclophilin ligand interactor (TACI), and CD69 revealed that direct contact is, indeed, essential and sufficient for the induction of DC maturation.4
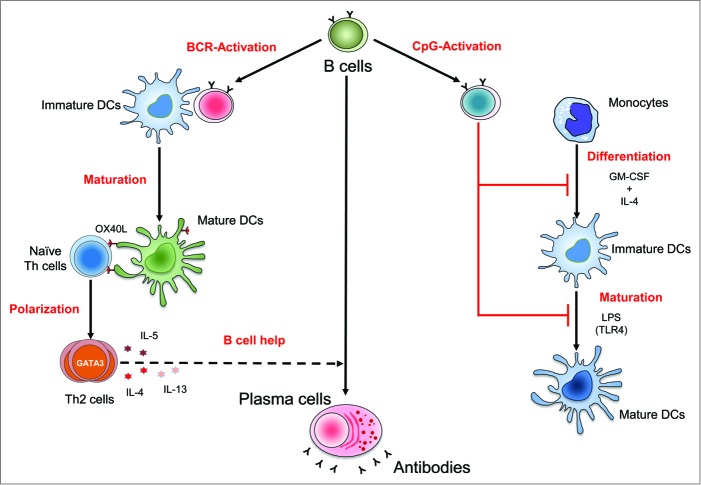
Figure 1.
Differential regulation of human dendritic cells by B cells dependent on activation stimuli. B cells activated by B-cell receptor (BCR)-signaling induce maturation of dendritic cells (DCs) through contact-dependent mechanisms. B–cell-matured DCs are capable of polarizing the naïve CD4+ Th cells to Th2 cells through OX-40 ligand (OX-40L)-signaling. Th2 cells secrete cytokines such as interleukin (IL)-4, IL-5, and IL-13, which could promote development of B cells into antibody-producing plasma cells. Conversely, B cells activated by CpG (Toll-like receptor (TLR) 9 ligand) interfere with the differentiation of monocytes into DCs and inhibit the TLR (lipopolysaccharide [LPS], a TLR4 ligand)-mediated maturation of DCs.
Next, we looked at the DC functions by evaluating the CD4+ T-cell stimulatory and polarization features. In line with the phenotype, B–cell-matured DCs induced significantly increased proliferation and interleukin (IL)-2 secretion in CD4+ T cells. Notably, B–cell-matured DCs induced differentiation of Th2 cells that expressed IL-4, IL-5, and IL-13. These differentiated T cells also showed enhanced expression of the Th2 lineage-specific transcription factor GATA3 and CCR4 (Fig. 1). However, the basal level differentiation of other Th cell subsets Th1 (interferon gamma [IFNγ]), Th17 (IL-17), and Treg (Foxp3) cells were not altered by B–cell-matured DCs.4
To investigate the mechanisms of selective induction of Th2 cells, we analyzed the phenotypic and functional features of B–cell-matured DCs. We found that activated B cells did not induce Th1-triggerring molecules, such as IL-12 and CD70, in DCs. Further, B–cell-matured DCs did not secrete IL-33 – a cytokine that was shown to amplify the Th2-type response.1 Interestingly, the expression of a Th2-inducing molecule OX-40 ligand (OX-40L) was upregulated on B–cell-matured DCs, and blocking of OX-40L significantly reduced the induction of Th2 cells (Fig. 1). Moreover, B–cell-matured DCs secreted significant amounts of IL-6, which could promote Th2-cell differentiation over that of Th1 cells. In addition, higher secretion of chemokines CCL17 and CCL22 by B–cell-matured DCs could recruit Th2 cells. Concisely, our results demonstrate that DCs receiving signals from BCR-activated B cells acquire distinctive features to selectively induce the development and function of Th2 cells.4 Notably, B cells activated by CD40-signaling also induced DC maturation. We believe that, during an immune response, B cells could positively dictate their own effector functions by modulating DCs to induce Th2 cells, which are known to promote humoral (B–cell-mediated) immune responses.
What is the importance of our results for oncoimmunology? In humans, a positive correlation has been observed between survival and tumors with tertiary lymphoid structures containing CD20+ B cells, together with T cells and DCs. It is now evident that the majority of cancer patients mount autoantibody responses to tumor antigens such as c-myc, cyclin B1, Her2/neu, MUC1, NY-ESO-1, and P53. These antibodies might regulate the expression and function of target antigens and promote antitumor immune responses via complement-mediated lysis of tumor cells, antibody-dependent cytotoxicity, or opsonization of tumor antigens.5 Antibodies against MUC1 are expressed in more than 90% of all adenocarcinomas, which include breast, colorectal, pancreatic, and ovarian cancers, and are reported to be associated with a favorable prognosis in patients with early-stage cancer. A similar observation was also made with another tumor antigen Her2/neu. However, based on the current literature, it is difficult to ascertain definitively an either pro- or antitumor role for these antibodies, as antibodies to another tumor-associated antigen p53 were associated with poor disease outcome. Thus, it appears that antibodies to tumor antigens might not have a common functional role in the immune response against tumors. In some cases, antibodies against tumor-associated antigens represent mere markers of prolonged exposure of the immune system to the antigen without functional relevance; this is because, following the surgical removal of tumors, antibodies to tumor antigens decline or may even disappear. The role of antibodies in tumor immunology might depend on the abundance of a particular isotype/subclass of antibodies to tumor-associated antigens (as demonstrated in the case of antifungal antibodies), type of tumor, and the cellular location of the tumor antigens, the pre-tumor level of antibodies to tumor antigens, and the glycosylation pattern of these antibodies.6,7 The underlying cellular mechanism for the appearance of antitumor antibodies is not completely understood. Our results demonstrating the induction of maturation of DCs with Th2-polarizing features by BCR-activated B cells4 might provide a mechanistic insight into the process of autoantibody production by tumor-infiltrating B cells.
B cells could also acquire suppressive “regulatory” features under certain conditions to restrain the immune response and to inhibit the inflammation.3 Current evidences suggest that regulatory B cells that secrete IL-10 inhibit antitumor immunity.8 In fact, toll-like receptor 9 (TLR9; CpG) signaling, alone or in combination with BCR or CD40-stimulation, could render regulatory properties to B cells with a capacity to block the differentiation of monocyte-derived DCs as well as TLR-mediated maturation of DCs (Fig. 1).9,10 Thus, inhibiting the activation of DCs might explain, in part, the detrimental role of regulatory B cells in antitumoral immune responses.
To conclude, B cells modulate human DC functions, and this regulation is dependent on the type of activation stimuli received by the B cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce TH2 and tolerogenic responses. Nat Immunol 2010; 11:647–55; PMID: ; http://dx.doi.org/ 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 2.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood 2008; 112:1570–80; PMID: ; http://dx.doi.org/ 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol 2012; 30:221–41; PMID: ; http://dx.doi.org/ 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 4.Maddur MS, Sharma M, Hegde P, Stephen-Victor E, Pulendran B, Kaveri SV, Bayry J. Human B cells induce dendritic cell maturation and favour Th2 polarization by inducing OX-40 ligand. Nat Commun 2014; 5:4092; PMID: ; http://dx.doi.org/ 10.1038/ncomms5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother 2009; 58:1535–44; PMID: ; http://dx.doi.org/ 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elluru SR, Kaveri SV, Bayry J. The protective role of immunoglobulins in fungal infections and inflammation. Semin Immunopathol 2014; 37:187–197; PMID: ; http://dx.doi.org/ 10.1007/s00281-014-0466-0. [DOI] [PubMed] [Google Scholar]
- 7.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol 2007; 19:239–45; PMID: ; http://dx.doi.org/ 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends Immunol 2013; 34:169–73; PMID: ; http://dx.doi.org/ 10.1016/j.it.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Morva A, Lemoine S, Achour A, Pers JO, Youinou P, Jamin C. Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood 2012; 119:106–14; PMID: ; http://dx.doi.org/ 10.1182/blood-2011-06-360768. [DOI] [PubMed] [Google Scholar]
- 10.Maddur MS, Kaveri SV, Bayry J. Regulation of human dendritic cells by B cells depends on the signals they receive. Blood 2012; 119:3863–64; PMID: ; http://dx.doi.org/ 10.1182/blood-2012-02-408948. [DOI] [PubMed] [Google Scholar]