Abstract
Adenoviruses are excellent immunotherapeutic agents with a unique ability to prime and boost immune responses. Recombinant adenoviruses cause immunogenic cancer cell death and subsequent release of tumor antigens for antigen presenting cells, resulting in the priming of potent tumor-specific immunity. This effect may be further enhanced by immune-stimulating transgenes expressed by the virus. We report a case of a 38-year-old female with Stage 3 metastatic micropapillary serous carcinoma of the ovary. She was treated in a Phase I study with a granulocyte-macrophage colony stimulating factor (GMCSF)-expressing oncolytic adenovirus, Ad5/3-D24-GMCSF (ONCOS-102). The treatment resulted in progressive infiltration of CD8+ lymphocytes into the tumor and concomitant systemic induction of several tumor-specific CD8+ T-cell populations. The patient was alive at the latest follow up more than 20 months after initiation of the study.
Keywords: in situ vaccine, viral immunotherapy, anti-tumor immunity, Th1 polarization, anti-tumor CD8+ T cell, tumor infiltrating lymphocyte
Advanced metastatic tumors are often immunosuppressive and therefore challenging to treat with immunotherapy. This is the case with ovarian cancer of epithelial origin (epithelial ovarian carcinoma; EOC), the seventh most common cancer in women. The location of the primary tumor is commonly deep in the pelvis, which frequently leads to delayed diagnosis. At the time of diagnosis the disease has often metastasized to other locations such as regional lymph nodes and the peritoneal cavity. Despite initial response to first-line therapy, the majority of patients relapse and die from their disease.
Over recent years, it has been shown that immune therapy can induce antitumor immunity in several solid tumor types such as melanoma, renal cell carcinoma, and ovarian cancer.1-5 Moreover, this has resulted in clinical efficacy and there are now several immune-based therapies that have been either approved for therapy in melanoma or are in late-stage clinical development.6 The first evidence for the role of immunosurveillance against ovarian cancer was a study showing strong positive correlation between the presence of CD3+ tumor-infiltrating lymphocytes (TILs) and patient survival.1 In the same study, immunohistochemical staining also revealed that intratumoral CD4+ and CD8+ cells are either both present or both absent. Thereafter, numerous studies have confirmed the prognostic power of intraepithelial CD3+, CD4+ and CD8+ TILs in EOC.7,8 In addition to a strong correlation between TIL infiltration and patient survival, there are also other important factors indicating ovarian cancer as a potential target for immunotherapy. Expression of multiple tumor-associated antigens provides targets for humoral and cellular immune responses.9,10 The expression of tumor antigen peptide/MHC complexes can be recognized by CD8+ T cells.3 Of note, CD3-positive tumor-infiltrating T cells are also associated with the clinical responsiveness of ovarian serous carcinoma to chemotherapy, suggesting that the efficacy of this modality may also be partially mediated immunologically.11
Unlike therapies targeted against specific tumor antigens, oncolytic viruses lyse cancer cells and prime a response against the entire pool of tumor epitopes, making them a true in situ vaccine. The remarkable potential of adenovirus for both priming and boosting immune responses sets it apart from other oncolytic viruses.12-14 This, combined with an excellent safety record, makes adenovirus a versatile vector for various types of combination treatment.
ONCOS-102 is an oncolytic serotype 5 adenovirus that features a 5/3-chimeric capsid for enhanced gene delivery to cancer cells. In addition, it is armed with a granulocyte macrophage colony-stimulating factor (GM-CSF) transgene.15 GM-CSF functions by directly recruiting antigen presenting cells (APCs) and natural killer cells, as well as by activating and maturing APCs at the tumor site.16,17 Having completed a Phase I trial (NCT01598129) with ONCOS-102 for the treatment of solid injectable tumors, we report a 38-year-old woman with Stage 3 metastatic micropapillary serous carcinoma of the ovary. We hypothesize that oncolysis and local expression of GMCSF worked together to initiate an innate immune reaction that primed the antitumor immune response for subsequent infiltration of lymphocytes. The patient had previously been treated with surgery, followed by 7 different chemotherapy regimens (paclitaxel/cisplatin, docetaxel/cisplatin, topotecan, gemcitabine, etoposide, and 2 regimens with paclitaxel/carboplatin). These treatments led to varied responses of limited duration. In the trial this chemotherapy refractory patient was treated with ultrasound-guided intratumoral injections of ONCOS-102. Two tumors were chosen for injection based on their size and accessibility, one located in the lesser pelvis and the other between the spleen and colon. The treatment dose was 3 × 1011 virus particles, of which one-fifth was given intravenously and four-fifths intratumorally at the first treatment (day 1). Subsequent treatments were given at the same dose intratumorally on days 4, 8, 15, 29 and monthly thereafter, for a total of 9 treatments. The design of the treatment schedule aimed to achieve efficient priming through oncolysis and subsequent tumor antigen presentation. We believe that continuing the therapy after the initial intense priming was a requisite for a regular immune system boost and update of the tumor antigen profile. Since regulatory cells are known to contribute to the tolerogenic microenvironment that compromises antitumor immune responses,3,4,18 concomitant low-dose (50 mg daily) cyclophosphamide was included to downregulate tumor-promoting T regulatory cells.19,20 This chemoimmunomodulation was given orally, starting 1 day after the first virus injection and continuing up until day 169.
Our data suggest local, rather than systemic, GMCSF production, as there were signs of viral activity but no elevation of GMCSF in the serum. Levels of proinflammatory cytokines IL-6 and IL-8 and anti-inflammatory IL-10 were temporarily elevated after each treatment. According to standard terminology criteria for adverse events (CTCAE), only grade 1–2 adverse events were observed. Anti-adenoviral neutralizing antibodies (NAbs) were present at baseline, increased further after treatment, and remained elevated throughout the trial. This did not seem to reduce the effect of repeated local administration of the virus as viral DNA was consistently detected in blood, suggesting ongoing viral replication despite NAb induction.
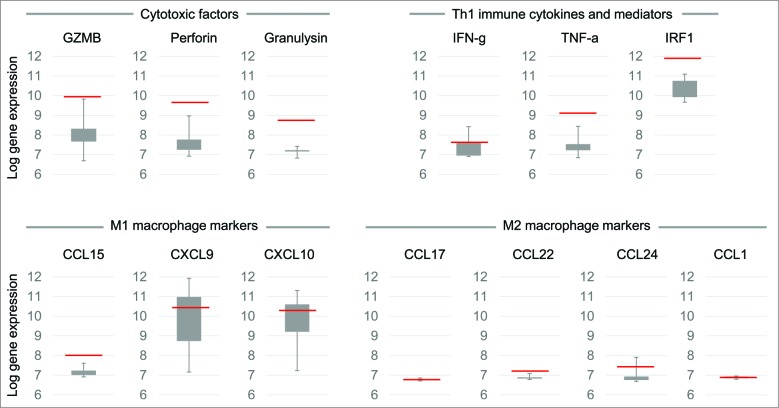
To measure antitumor activity, the 2 injected tumors and 1 non-injected lesion located in the mesenterium were followed by computed tomography (CT) and positron emission tomography (PET). One of the 2 injected tumors, the one located between the spleen and colon, was biopsied at baseline and 1 and 2 months after treatment initiation and immunohistochemical staining for TILs was performed. The baseline biopsy collected at initiation of the immunotherapy lacked TILs. We believe that the heavily immunosuppressed tumor microenvironment, surgery, and several rounds of chemotherapy may all have contributed to the scarcity of TILs.21 A dramatic increase in peritumoral cytotoxic CD8+ T cells and CD4+ helper T cells (Fig. 1) was seen in the biopsies collected 1 and 2 months after treatment initiation. Microarray data from the post-treatment biopsy further confirmed immune activation at the tumor site. High expression levels of genes encoding cytotoxic factors granulysin, granzyme B, and perforin suggest that tumor infiltrating CD8+ T cells displayed an effector cell phenotype (Fig. 2). Furthermore, relatively high expression of a favorable Th1 type22,23 and immune cytokines and mediators (IFN-γ, TNF-α, IRF1) were detected. Antitumor M1-type macrophage markers (CCL15, CXCL9, CXCL10) were present after treatment whereas there was relatively low expression of tumor-promoting M2 macrophage markers CCL17, CCL22, CCL24, and CCL1 (Fig. 2). We think that oral low-dose CPO potentially worked together with ONCOS-102 to reduce tumor-induced immune tolerance.19,20 Such a therapy-induced change to the microenvironment could make tumors susceptible to other immunotherapies.
Figure 1.
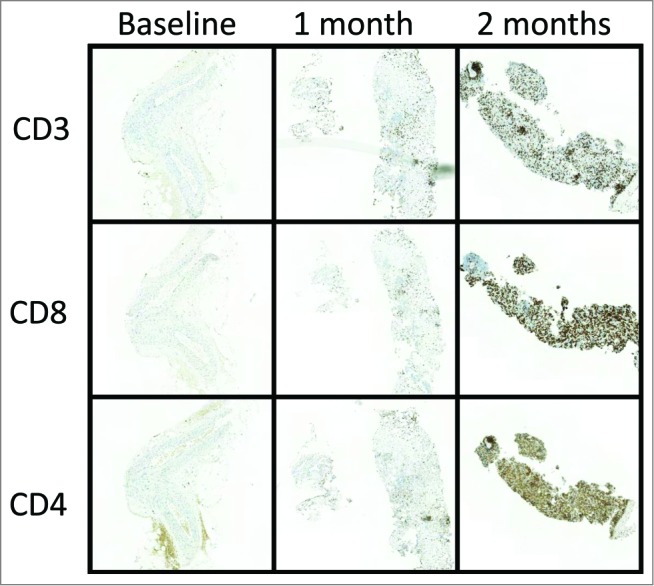
ONCOS-102 treatment initiated infiltration of CD3+, CD4+, and CD8+ T cells to the tumor. Biopsies collected at baseline (A), 29 d (B), and 57 d (C) after treatment initiation were stained for CD3+, CD4+, and CD8+ T cells. Briefly, 3-µm sections were cut from formalin-fixed and paraffin-embedded tissues and processed for immunohistochemical staining with a Ventana BenchMark XT immunostainer (Ventana Medical Systems, Tucson, AZ). For CD3 and CD8, anti-CD3 rabbit monoclonal antibody (clone 2GV6, ready to use; Ventana, Roche), and anti-CD8 rabbit monoclonal antibody (clone SP57, ready to use; Ventana) were used, and visualization was performed using UltraViewDabv3 with amplification (Ventana). For CD4, a 1:100 dilution of CD4 rabbit monoclonal antibody (clone SP35, Cell Marque) was used and visualization was performed using OptiView DAB IHCv3 (Ventana). The specimens were counterstained with hematoxylin and post counterstained with bluing reagent. Positive staining is shown in brown.
Figure 2.
Gene expression levels in tumors 2 months after treatment initiation. Total RNA was extracted from snap-frozen core needle tumor biopsies taken 2 months after treatment initiation and gene expression profiling was performed using HumanHT-12 Illumina microbead chips (Illumina Inc., San Diego, CA). The average signal values were quantile-normalized, log2-transformed, and annotated using the package lumi (Bioconductor open source software). Chip-dependent batch effects were removed using empirical Bayes methods. Whisker box-plots present gene expression estimates for 8 reference patients (not subjects of this report) treated in the same Phase I trial. The red line in each box plot indicates the gene expression estimate of the given molecule for the ovarian cancer patient that was the subject of this report. In comparison to reference patients, markedly elevated expression levels of genes encoding cytotoxic factors (granzyme B, perforin, and granulysin) and genes related to Th1 response (IFN-gamma, TNF-α, and interferon regulatory factor 1 [IRF1]) suggest that CD8+ TILs had an effector phenotype and that the tumor microenvironment was polarized toward a Th1 immune signature. Furthermore, genes encoding markers of cytotoxic M1 macrophages (CCL15, CXCL9, CXCL10) showed higher expression levels than genes encoding markers of tumor-promoting M2 macrophages (CCL17, CCL22, CCL24, CCL1), indicating an immunologically active tumor condition.
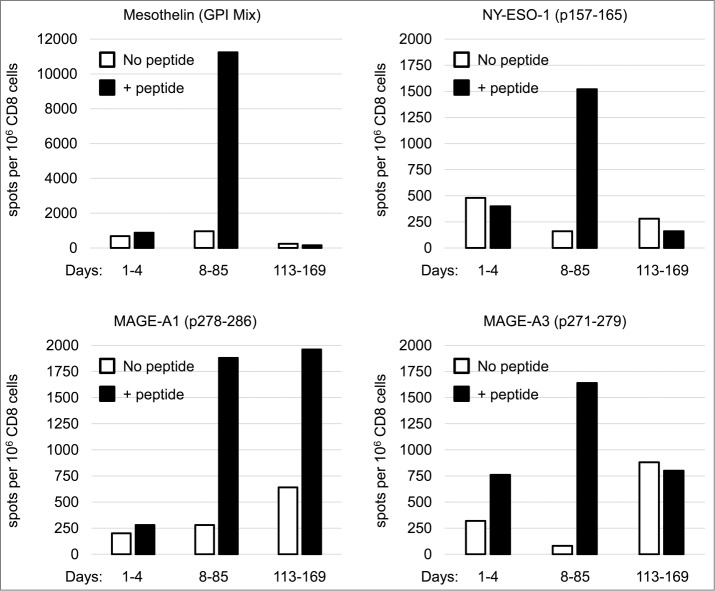
To further investigate the nature of the induced immune activation, CD8+ cells from peripheral blood mononuclear cells were analyzed with IFN-γ ELISPOT for recognition of the cancer-testis antigens NY-ESO-1, MAGE-A1, MAGE-A3 and a differentiation antigen mesothelin. Mesothelin is reported to be widely expressed in ovarian carcinoma.24 Samples from different time points were combined to represent a pool of baseline, early, and late immune responses. Before treatment, antigen-specific CD8+ T cells were not detectable.
Cellular immune responses against all antigens tested were detected among the CD8+ T cells that had been collected on days 8–85. In this early pool, mesothelin-recognizing CD8+ T cells were the most numerous and lower levels of cells recognizing NY-ESO-1, MAGE-A1, and MAGE-A3 were also detected (Fig. 3). Thus, the treatment was able to break tumor tolerance and initiate a tumor-specific immune response, most probably by priming the immune system and facilitating tumor-antigen presentation to cytotoxic cells. In the late pool of CD8+ T cells collected 113–169 days after starting the treatment, levels of antigen-specific T cells had decreased and only MAGE-A1–specific CD8+ T cells could be detected. One can speculate that either concomitant or sequential treatment with an immune checkpoint inhibitor might be beneficial for potentiation of the tumor-targeted T-cell response initiated by ONCOS-102 and could result in a more significant clinical response. Importantly, the presence of CD8+ T cells that are negatively regulated by PD-1/PD-L1 interactions within the tumor has been shown to be required for successful PD-1 therapeutic blockade.25
Figure 3.
IFN-γ enzyme linked immunospot assay (ELISPOT) for mesothelin-specific CD8+ T cells. Purified CD8+ cells were pre-sensitized with peptide-pulsed, irradiated autologous PBMCs depleted of CD4 and CD8 T cells and tested on day 10 by an IFN-γ ELISPOT assay for recognition of the peptide pulsed on autologous antigen-presenting cells. The number of cytokine-producing antigen-specific T cells was evaluated using AID EliSpot Reader Classic ELR 07 (Autoimmun Diagnostika GmbH, Strassberg, Germany). Spot counts per 106 CD8+ T cells without peptide stimulation (no peptide) or with peptide stimulation (+ peptide) are shown for samples obtained at baseline, 8–85 d after treatment initiation, and 113–169 d after treatment initiation. GPI, pooled peptide mix covering the second portion of mesothelin.
Disease stabilization was achieved in both injected tumors, as evaluated by CT (+12% and +8% change in diameter) and PET (−8% and 0% change in activity) at 3 months. PET performed at the non-treated tumor lesion also showed a 10% reduction in activity, which we believe is a strong indication of systemic immune activation targeting lesions with the same epitopes as the injected tumor lesions. This remains to be confirmed in future studies. The patient's performance status has remained good since the treatment (WHO1), and survival has now exceeded 20 months, which is unusually long for chemotherapy-refractory ovarian cancer.
To summarize, intratumoral administration of ONCOS-102 in this 38-year-old patient with chemotherapy refractory ovarian cancer initiated strong trafficking of TILs into a previously TIL-negative tumor, accompanied by a favorable cytotoxic T-cell response on a transcriptional level. Importantly, tumor specific CD8+ T-cell activation was seen in peripheral blood. These results are evidence of systemic tumor-specific activity and suggest the clinical potential of locally administered immune therapy with ONCOS-102.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al.. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203-13; PMID:; http://dx.doi.org/ 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 2.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol 2008; 108:415-20; PMID:; http://dx.doi.org/ 10.1016/j.ygyno.2007.10.016 [DOI] [PubMed] [Google Scholar]
- 3.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al.. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005; 102:18538-43; PMID:; http://dx.doi.org/ 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al.. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:; http://dx.doi.org/ 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- 5.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res 2013; 73:6900-12; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascierto PA. Immunotherapies and novel combinations: the focus of advances in the treatment of melanoma. Cancer Immunol Immunother 2014; 64:271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavoué V, Thédrez A, Levêque J, Foucher F, Henno S, Jauffret V, Belaud-Rotureau MA, Catros V, Cabillic F. Immunity of human epithelial ovarian carcinoma: the paradigm of immune suppression in cancer. J Transl Med 2013; 11:147; PMID:; http://dx.doi.org/ 10.1186/1479-5876-11-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb JR, Milne K, Nelson BH. Location, location, location: CD103 demarcates intraepithelial, prognostically favorable CD8+ tumor-infiltrating lymphocytes in ovarian cancer. Oncoimmunol 2014; 3:e27668; PMID:; http://dx.doi.org/ 10.4161/onci.27668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daudi S, Eng KH, Mhawech-Fauceglia P, Morrison C, Miliotto A, Beck A, Matsuzaki J, Tsuji T, Groman A, Gnjatic S, et al.. Expression and immune responses to MAGE antigens predict survival in epithelial ovarian cancer. PloS One 2014; 9:e104099; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayser S, Watermann I, Rentzsch C, Weinschenk T, Wallwiener D, Guckel B. Tumor-associated antigen profiling in breast and ovarian cancer: mRNA, protein or T cell recognition? J Cancer Res Clin Oncol 2003; 129:397-409; PMID:; http://dx.doi.org/ 10.1007/s00432-003-0445-7 [DOI] [PubMed] [Google Scholar]
- 11.Raspollini MR, Castiglione F, Rossi Degl'innocenti D, Amunni G, Villanucci A, Garbini F, Baroni G, Taddei GL. Tumour-infiltrating gamma/delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann Oncol 2005; 16(4):590-6; PMID:; http://dx.doi.org/ 10.1093/annonc/mdi112 [DOI] [PubMed] [Google Scholar]
- 12.Shiver JW, Fu T-M, Chen L, Casimiro DR, Davies M-E, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, et al.. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002; 415:331-5; PMID:; http://dx.doi.org/ 10.1038/415331a [DOI] [PubMed] [Google Scholar]
- 13.Draper S, Heeney J. Viruses as vaccine vectors for infectious diseases and cancer. Nature Rev Microbiol 2010; 8:62-73; PMID:; http://dx.doi.org/ 10.1038/nrmicro2240 [DOI] [PubMed] [Google Scholar]
- 14.Bart P-A, Huang Y, Karuna S, Chappuis S, Gaillard J, Kochar N, Shen X, Allen MA, Ding S, Hural J, et al.. HIV-specific humoral responses benefit from stronger prime in phase Ib clinical trial. J Clin Invest 2014; 124(11):4843-56; PMID:; http://dx.doi.org/ 10.1172/JCI75894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M, Guse K, et al.. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther 2010; 18:1874-84; PMID:; http://dx.doi.org/ 10.1038/mt.2010.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dranoff G. GM-CSF based cancer vaccines. Immunol Rev 2002; 188:147-154; PMID:; http://dx.doi.org/ 10.1034/j.1600-065X.2002.18813.x [DOI] [PubMed] [Google Scholar]
- 17.van der Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood 2012; 119:3383-3393; PMID:; http://dx.doi.org/ 10.1182/blood-2011-11-370130 [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014; 27:1-7; PMID:; http://dx.doi.org/ 10.1016/j.coi.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 19.Ghiringelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 2007;56:641-648; PMID:; http://dx.doi.org/ 10.1007/s00262-006-0225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, Romano V, Rouvinen N, Tuuminen T, Laasonen L, et al.. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther 2011; 19:1737-46; PMID:; http://dx.doi.org/ 10.1038/mt.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brune IB, Wilke W, Hensler T, Holzmann B, Siewert JR. Downregulation of T helper type 1 immune response and altered pro-inflammatory and anti-inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. Am J Surg 1999; 177:55-60; PMID:; http://dx.doi.org/ 10.1016/S0002-9610(98)00299-2 [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 23.Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Rranslat Med 2012; 10:1-4; PMID:; http://dx.doi.org/ 10.1186/1479-5876-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frierson HF, Moskaluk CA, Powell SM, Zhang H, Cerilli LA, Stoler MH, Cathro H, Hampton GM. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol 2003; 34:605-609; PMID:; http://dx.doi.org/ 10.1016/S0046-8177(03)00177-1 [DOI] [PubMed] [Google Scholar]
- 25.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-571; PMID:; http://dx.doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]