Abstract
Understanding the role of predators in food webs can be challenging in highly diverse predator/prey systems composed of small cryptic species. DNA based dietary analysis can supplement predator removal experiments and provide high resolution for prey identification. Here we use a metabarcoding approach to provide initial insights into the diet and functional role of coral-dwelling predatory fish feeding on small invertebrates. Fish were collected in Moorea (French Polynesia) where the BIOCODE project has generated DNA barcodes for numerous coral associated invertebrate species. Pyrosequencing data revealed a total of 292 Operational Taxonomic Units (OTU) in the gut contents of the arc-eye hawkfish (Paracirrhites arcatus), the flame hawkfish (Neocirrhites armatus) and the coral croucher (Caracanthus maculatus). One hundred forty-nine (51%) of them had species-level matches in reference libraries (>98% similarity) while 76 additional OTUs (26%) could be identified to higher taxonomic levels. Decapods that have a mutualistic relationship with Pocillopora and are typically dominant among coral branches, represent a minor contribution of the predators’ diets. Instead, predators mainly consumed transient species including pelagic taxa such as copepods, chaetognaths and siphonophores suggesting non random feeding behavior. We also identified prey species known to have direct negative interactions with stony corals, such as Hapalocarcinus sp, a gall crab considered a coral parasite, as well as species of vermetid snails known for their deleterious effects on coral growth. Pocillopora DNA accounted for 20.8% and 20.1% of total number of sequences in the guts of the flame hawkfish and coral croucher but it was not detected in the guts of the arc-eye hawkfish. Comparison of diets among the three fishes demonstrates remarkable partitioning with nearly 80% of prey items consumed by only one predator. Overall, the taxonomic resolution provided by the metabarcoding approach highlights a highly complex interaction web and demonstrates that levels of trophic partitioning among coral reef fishes have likely been underestimated. Therefore, we strongly encourage further empirical approaches to dietary studies prior to making assumptions of trophic equivalency in food web reconstruction.
Keywords: Coral reefs, Food web, Interactions, Invertebrates, Mutualism, Exosymbionts, Pyrosequencing, COI
Introduction
Anthropogenic stressors are impacting all ecosystems on Earth, causing both drastic changes in the structure of communities and a reduction in biodiversity (Wright, 2005; Hoegh-Guldberg & Bruno, 2010). Predators are among the most vulnerable trophic group, and have long been known to play a crucial role in stabilizing ecosystems by generating top-down forces and trophic cascades (Paine, 1966; Paine, 1969). Yet, because all predator species are not functionally equivalent, understanding how species partition their diet as well as their ecological role in food webs have become a major focus to help predict the consequences of their decline on ecosystem services (Harley, 2011).
A detailed knowledge of a predator’s diet is a key element for deciphering its ecological function. Among the numerous techniques used in the literature to characterize a predator’s diet, PCR-based molecular analysis of gut contents is among the most powerful because species-diagnostic DNA fragments can be detected even after several hours of digestion (Symondson, 2002). Moreover, the availability of versatile PCR primers targeting short hypervariable DNA regions combined with a high-throughput sequencing platform now offer the possibility to characterize the dietary breadth of any predator (Pompanon et al., 2012; Leray et al., 2013a). The ecological influence of a predator may then be inferred from its dietary selectivity as well as the traits and functional role of prey consumed (Chapman et al., 2013). On land, this tool is already proving invaluable for understanding the biological control potential of insect predators (Mollot et al., 2014) and the ecological effects of large herbivores (Kowalczyk et al., 2011) and carnivores (Shehzad et al., 2012). The use of high-throughput sequencing for understanding trophic links in marine systems has been more limited to date (Leray et al., 2013a).
On coral reefs, one of the most diverse and threatened of ecosystems, predatory fish feeding on benthic invertebrates are the dominant trophic category. They often dwell within the reef framework where they feed upon diverse communities of small cryptic species that are known to perform a variety of functions including direct positive or negative interactions with stony corals, the foundation species of the coral reef ecosystem (reviewed by Stella et al., 2011). Some invertebrate taxa promote the survival and growth of corals by slowing the progression of coral diseases (Pollock et al., 2012), protecting corals against corallivores (Glynn, 1980; Glynn, 1983; McKeon & Moore, 2014; Rouzé et al., 2014), removing sediments from their coral host (Stewart et al., 2006; Stier et al., 2012) and alleviating detrimental effects of coral competitors or parasites (Stier et al., 2010). Other invertebrates have deleterious effects on corals as they are known vectors of coral diseases (Sussman et al., 2003; Williams & Miller, 2005), are parasites of stony corals (Humes, 1985; Shima, Osenberg & Stier, 2010) or feed upon coral polyps (Turner, 1994; Rotjan & Lewis, 2008; Rawlinson et al., 2011) sometimes causing extensive and widespread coral mortality (Leray et al., 2012a; Kayal et al., 2012). As a consequence, the feeding behavior of these predatory fish may have significant cascading effects on the dynamics of stony corals and subsequently the dynamics of the whole coral reef ecosystem, but it has proven challenging to understand their ecological role.
The flame hawkfish (Neocirrhites armatus), arc-eye hawkfish (Paracirrhites arcatus) and coral croucher (Caracanthus maculatus) are common predatory fish species on Indo-Pacific coral reefs. They co-occur among the branches of Pocilloporids (genus Stylophora and Pocillopora), one of the most important reef building corals, along with a wide diversity of invertebrates (Patton, 1974; Coles, 1980; Odinetz, 1983; Stella, Jones & Pratchett, 2010). These invertebrates include both coral mutualistic (family: Trapeziidae and some Alpheidae) and parasitic (family: Cryptochiridae) decapod species (Simon-Blecher & Achituv, 1997), which are potential prey for coral dwelling fish. A field manipulation of the two Pocilloporid obligate species, the flame hawkfish and the coral croucher (habitat specialists), highlighted that their presence among the branches of Pocillopora eydouxi reduced total abundance and diversity of decapod recruits by 34% and 20% respectively (Stier & Leray, 2014). These predators modified the composition and abundance of key mutualists (coral crabs, genus: Trapezia), whose benefits to Pocillopora are known to be both density- and diversity-dependent (Stier et al., 2012). Predator removal experiments have also shown that the presence of arc-eye hawkfish decreases the density of coral associated mutualist damselfish (Holbrook, Schmitt & Brooks, 2011). Preliminary molecular dietary analysis using traditional cloning showed the presence of coral mutualists in the gut contents of both hawkfish species (Leray et al., 2013b), but sampling and sequencing effort were too limited to understand their contribution to each species’ diets.
In the present study, we use a high throughput sequencing approach targeting the mitochondrial Cytochrome c. Oxidase subunit I gene (COI) (also referred to as metabarcoding approach, Taberlet et al., 2012) to describe the dietary breadth of these predators. The study was conducted in Moorea, French Polynesia, where an extensive library of COI DNA barcodes, including all Pocillopora associated species, has been built by the BIOCODE project (Leray et al., 2012b). Implications of each predator’s feeding behavior are further discussed in light of our findings.
Methods
Predator and prey collections
Twenty-five adult specimens of each of the three predator fish species were speared after sunset, which corresponds to peak feeding time for all three species (M Leray, pers. obs., 2010), in the lagoon of the North shore of Moorea on the 8th, 10th and 15th of July 2010. We limited our collections to a single site (17°28′40S; 149°50′25W, Fig. 1) where coral populations had been little impacted by the recent outbreak of the corallivorous seastar, Acanthaster planci (Adjeroud et al., 2009; Kayal et al., 2011; Rouzé et al., 2015). Adults of the flame hawkfish and coral croucher always co-occurred among Pocillopora branches, whereas adult arc-eye hawkfish were occasionally present. Fish were individually preserved in cold 50% ethanol in situ after which their digestive track was dissected within 3 h and preserved in eppendorf tubes containing 80% ethanol.
Figure 1. Map of the study location.
Laboratory protocol
The total content of the digestive track of each fish was dissected and used for total genomic DNA extraction using the QIAGEN DNeasy Blood & Tissue kit. Genomic DNA was then purified using the PowerClean DNA clean-up kit (MO BIO) to remove potential PCR inhibitors. We used a single set of versatile PCR primers (mlCOIintF/jgHCO2198, Geller et al., 2013; Leray et al., 2013a) known to perform well across the diversity of marine invertebrates, to amplify a 313bp region of the mitochondrial Cytochrome c. Oxidase subunit I (COI) region from each gut content sample. Moreover, this primer set was recently shown to provide reliable estimates of relative abundance for metabarcoding benthic samples (Leray & Knowlton, 2015). Because predator DNA co-amplification is known to impede prey detection (Vestheim & Jarman, 2008), predator-specific annealing blocking primers were included at ten times the concentration of versatile primers during PCR reactions as in Leray et al. (2013a). All primer sequences are provided in Table 1. The PCR cocktail and touchdown temperature profile used in this study can both be found in Leray et al. (2013a). Three PCR replications per sample were generated, pooled, gel excised to ensure that all primer dimers were screened away, purified using QIAGEN® MinElute columns and eluted in 12 µl of elution buffer. PCR product concentration was measured with the Qubit® Fluorometer (Invitrogen, Carslsbad, California, USA).
Table 1. List of primers used in this study.
| Primer label | Sequence (5′–3′) | Reference |
|---|---|---|
| mlCOIintF | GGWACWGGWTGAACWGTWTAYCCYCC | (Leray et al., 2013a) |
| jgHCO2198 | TAIACYTCIGGRTGICCRAARAAYCA | (Geller et al., 2013) |
| Narmatus_Blocking | CAAAGAATCAAAACAGGTGTTGATAAAGA-C3 | (Leray et al., 2013b) |
| Parcatus_Blocking | CAAAGAATCAGAACAGATGTTGGTAAAGA-C3 | (Leray et al., 2013b) |
| Cmaculatus_Blocking | CAAAGAATCAGAATAGGTGTTGGTACAGA-C3 | Herein |
We pooled equimolar amounts of the combined amplicons per individual gut content for each predator species (e.g., 25 flame hawkfish gut content samples were pooled together) and 500 ng of PCR product was used per species for library preparation for Roche 454 FLX sequencing. Amplicons were end-repaired and dA-tailed using the NEBNext Quick DNA Sample Prep Reagent Set 2 chemistry (New England BioLabs, Ipswitch, Massachusetts, USA). We then performed a ligation of 454 Multiplex Identifiers (a total of three, each one containing a recognizable sequence tag) using the FLX Titanium Rapid Library MID Adaptors Kit (Roche, Basel Switzerland). Finally, the ligated PCR product of each sample was purified using Agencourt AMPure beads (Beckman Coulter Genomics, Danvers, Massachusetts, USA), eluted in 40 µl of TE buffer, and the three samples pooled together for sequencing at the Duke Institute for Genome Sciences and Policy (Duke University, North Carolina, USA). Note that the three samples of the present study were combined with several other samples in the same 454 run.
Analysis of FLX sequencing data
We followed a sequence data analysis pipeline optimized for analyzing large COI datasets. The pipeline detailed in Leray et al. (2013a) takes advantage of the coding properties of the barcoding region to discard all dubious sequences.
The initial step denoised flowgrams using Pyronoise (Quince et al., 2011) implemented in Mothur (Schloss et al., 2009). We then further quality filtered the dataset by removing any reads that met the following criteria: shorter than 200bp; more than two mismatches in the primers sequence; any ambiguous base calls (e.g., “N”); or with any homopolymer regions longer than 8bp. Remaining sequences were subsequently aligned to a high quality reference dataset (Moorea BIOCODE barcode library) based on amino acid translations using the option “enrichAlignment” in MACSE (Ranwez et al., 2011) and all sequences with any of the following were also discarded: stop codon; frame shift; insertion; or more than three deletions. Finally, potential chimeric sequences identified using UCHIME (Edgar et al., 2011) were removed to obtain a high quality sequence dataset for downstream analysis.
To evaluate prey richness and composition, sequences were clustered in Operational Taxonomic Units (OTUs) using a Bayesian algorithm implemented in CROP (Hao, Jiang & Chen, 2011). This program delineates OTUs based on the natural distribution of sequence dissimilarity in the data and within a range of sequence similarity values defined by the user. This approach performs better for clustering sequences obtained from environmental samples than a fixed dissimilarity cutoff (e.g., 5%) because they contain a diversity of phyla that differ in their rate of COI evolution. The lower and upper bound variance were set to 3 and 4 respectively (which corresponds to 6% and 8%) as they were shown to provide the best results for marine invertebrates (Leray et al., 2013a; Leray et al., 2013b). Following OTU delineation, a representative sequence per OTU was used for taxonomic identification using BLAST searches in the local BIOCODE database and in GENBANK. We considered that there was a species level match when sequence similarity was at least 98% (Machida et al., 2009; Plaisance et al., 2009). Whenever sequence similarity was lower than 98%, we used a Bayesian approach implemented in the Statistical Assignment Package (SAP, Munch et al., 2008) to assign OTUs to a higher taxonomic group. SAP conducts assignments by building 10,000 unrooted phylogenetic trees from a collection of homologue sequences retrieved from a sequence database. It then calculates the probability that a query sequence belongs to a monophyletic group within that set of homologues. Here, we allowed SAP to retrieve 50 homologues from GENBANK with >70% sequence similarity to each query sequence (i.e., each OTU representative sequence) and accepted taxonomic assignments at an 80% posterior probability cutoff. Importantly, SAP can only assign sequences to taxonomic groups that are represented in the database, as is also the case with other assignment methods. Therefore, to minimize misidentification at lower taxonomic levels, we only report assignments to the phylum, class and order levels (Appendix S1).
Results
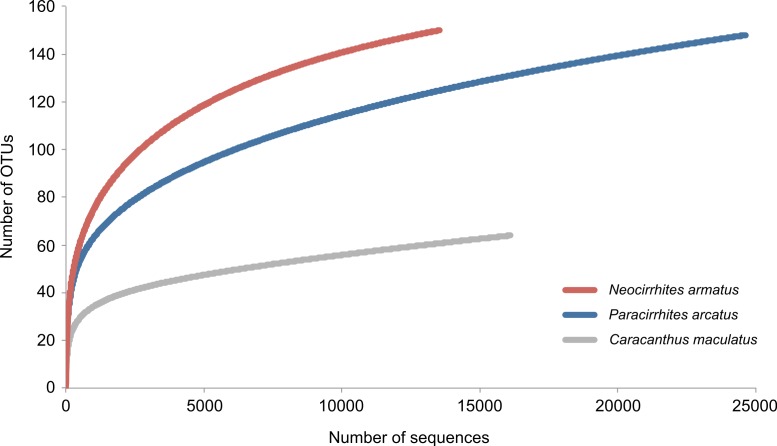
We obtained 69,663 reads of which 54,283 high quality reads were retained for downstream analysis (arc-eye hawkfish: 24,629; flame hawkfish: 13,536; coral croucher: 16,118). The Bayesian clustering algorithm delineated 292 OTUs in the gut contents of the three predatory fish species (Appendix S1). The number of dietary items was much lower in the gut contents of the coral croucher (64 taxa) than in both arc-eye (147 taxa) and flame hawkfish (149 taxa). BLAST searches provided high-resolution taxonomic assignments (>98% similarity) for 149 OTUs (51%) (Appendix S1) and the statistical assignment approach enabled the identification of 76 additional OTUs to a higher taxonomic level (26%). 67 OTUs (22.9%) remained unidentified (labeled as “Unidentified” in Appendix S1). None of the rarefaction curves reached a plateau (Fig. 2) which indicates that further sequencing effort would be necessary for a more exhaustive dietary analysis of these predatory fish.
Figure 2. Rarefaction curves to evaluate the completeness of the sequencing effort at describing the diversity of dietary items in the gut contents of three coral reef fish species.
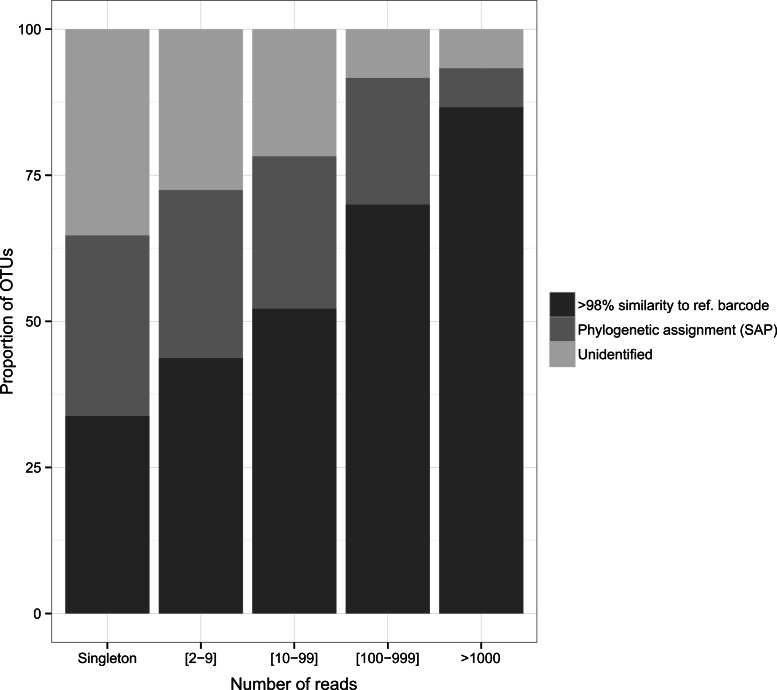
The diversity of dietary items spanned 25 classes belonging to 17 phyla. Malacostraca was the dominant taxonomic prey group (36.7%, 21.5% and 43.7% for the arc-eye hawkfish, the flame hawkfish and the coral croucher, respectively). The arc-eye hawkfish also consumed numerous species of Actinopterygii (17.7% total OTUs) and Maxillopoda (10.9% total OTUs). A significant proportion of the flame hawkfish and coral croucher’s diet was represented by Maxillopoda (12.1% and 6.2% total OTUs, respectively) and Gastropoda (9.4% and 4.7% total OTUs, respectively). Eighteen OTUs (28%) detected in the gut contents of the coral croucher remained unidentified. Direct matches to reference barcodes (>98% similarity) were more prevalent among Actinopterygii (94.1%), Malacostraca (74.1%) and Gastropoda (79.2%) compared to Maxillopoda (40%). Moreover, direct matches were more prevalent for OTUs represented by large numbers of sequences (Fig. 3). Almost nine of ten OTUs (86.7%) matched reference barcodes if they were common in the amplicon libraries (>1,000 sequences), whereas only a third (33.8%) of the single sequences matched a reference sequence. Probability of a match increased as the number of sequences increased (1: 33.8%; [2–9]: 43.7%; [10–99]: 52.2%; [100–999]: 70%; >1,000: 86.7%; Fig. 3).
Figure 3. Proportion of identified OTUs in relation to the number of sequences they represent.
Whenever OTU sequence similarity to a reference barcode was <98%, we used the Phylogenetic Bayesian assignment tool implemented in SAP to assign OTUs to a higher taxonomic group.
Most Malacostraca OTUs were decapods (81.5%, 46.9% and 78.6% for the arc-eye hawkfish, the flame hawkfish and the coral croucher respectively— Appendix S1). All three predatory fish fed upon Pocillopora obligate decapod species, but they represent a minor fraction of the total diversity of the prey they consumed (arc-eye hawkfish: 2%; flame hawkfish: 4%, coral croucher: 9.3%). Among them, we detected five coral crab species of the genus Trapezia that are mutualists of Pocillopora (Trapezia bidentata, T. serenei, T. rufopunctata, T. areolata and T. spp). These mutualists also represented a minor proportion of sequences in the gut contents of the arc-eye and flame hawkfish (proportion of total sequences: 5.6% and 2.4%; proportion of decapod sequences: 9.1% and 12.7%, respectively). Pocillopora mutualists represented a higher proportion of the coral croucher’s diet with 15.3% of the total number of sequences and 47.9% of the total number of decapod sequences.
Additional trophic links involving non-decapod prey are of particular interest for understanding the effect of predators on coral and its associated communities. Predatory fish had fed upon coral associated planktivorous damselfishes of the family Pomacentridae (Dascyllus flavicaudus: 0.02%, 0% and 0.12%, Chromis viridis: 0.01%, 0.69% and 0% of total sequences in the diet of the arc-eye hawkfish, the flame hawkfish and the coral croucher, respectively) that benefit the growth of the coral host (Holbrook et al., 2008). Interestingly, Anthozoa were represented by two OTUs among which the host Pocillopora itself accounted for 20.8% and 20.1% of total number of sequences in the guts of flame hawkfish and coral croucher, but was completely absent from the gut of the arc-eye hawkfish. On the other hand, Hapalocarcinus sp, a gall crab considered a coral parasite, was recovered in the diet of both the arc-eye and flame hawkfish. Both hawkfish had also consumed vermetid snails known for their deleterious effects on coral growth (Shima, Osenberg & Stier, 2010). Harpiliopsis beaupresii, a caridean shrimp associated with Pocillopora but whose function is unknown, was also detected in the gut contents of the coral croucher. Almost 10 percent (8.3%) of the coral croucher’s diet is composed of two snails (Drupa ricinus and Pascula muricata). Finally, predators had also consumed pelagic taxa including members of Maxillipoda, Chaetognatha and Hydrozoa (Appendix S1).
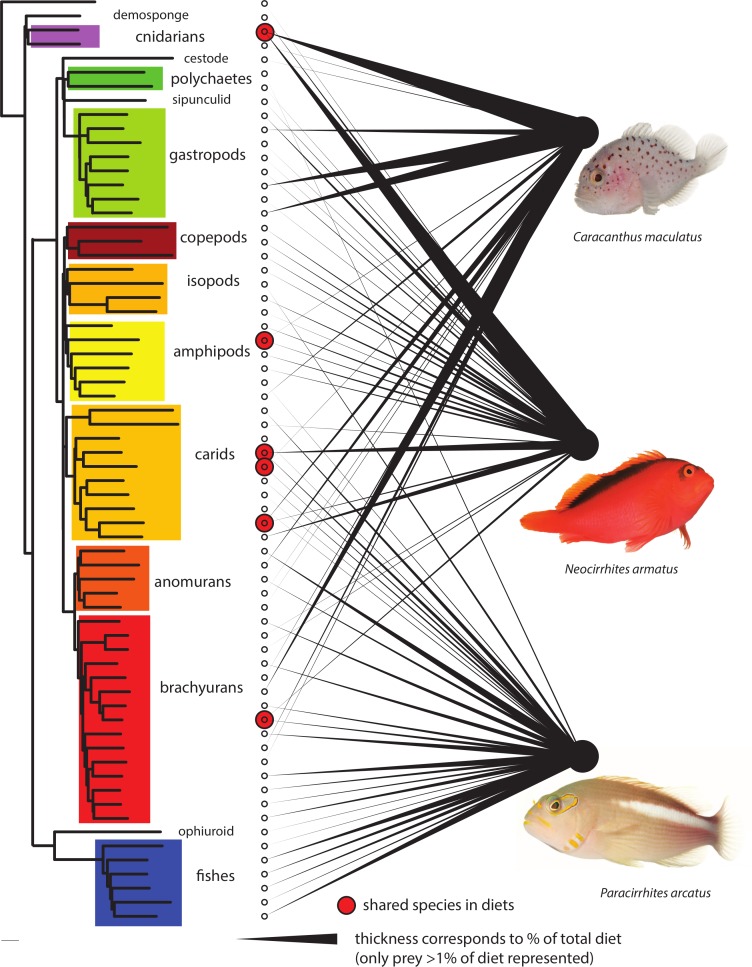
Prey species were remarkably partitioned among predators (Fig. 4). Almost eighty percent (79.5%) of prey species had been consumed by only one predator species (232 of 292). Eighteen percent (N = 52) were found in two predator diets and only eight prey species (>3%) had been ingested by all three predatory fish species analyzed. Of the shared components, the arc-eye hawkfish and the coral croucher had consumed 14 taxa in common among which six were Malacostraca. The arc-eye and flame hawkfish shared 29 prey taxa with a majority of Actinopterygii and Malacostraca. Prey sharing was lowest (nine OTUs; of which six were Malacostraca) between the two species that were always found co-occuring together in the coral host, the flame hawkfish and coral croucher. Analyses that included only prey OTUs consisting of >1% of either of the three species diets according to the relative abundance of reads demonstrate even greater partitioning (Fig. 4). Only six of the sixty-six prey items were shared at a proportion greater than 1% in any two fish species diets, and no prey species were shared among all three. Of the 66 prey items making up at least 1% of any diet, nine out of ten were consumed by only one predator.
Figure 4. Dietary partitioning among the three predatory fish species.
Left neighbor-joining phylogeny using LogDet distance model based on a constraint topology of major clades represents relationship among the 66 prey OTUs that comprise >1% of any one species diet. Thickness of linkages to right represents relative proportion of predatory diets. Six shared species are highlighted with circles. Fish images courtesy of D. Liittschwager. The 66 OTUs are highlighted in Appendix S1.
Discussion
Dietary analysis can be a powerful approach to gain insights into the ecological role of reef-dwelling predatory fish, but low taxonomic resolution in prey identification often obscures the complexity of trophic links (Longenecker, 2007). For example, the diet of the arc-eye hawkfish, flame hawkfish and coral croucher previously described from morphological identification of prey remains in gut contents was considered to be simply composed of small benthic crustaceans (class: Malacostraca) (Bachet, Zysman & Lefevre, 2007). Preliminary DNA analysis using traditional cloning revealed a breadth of prey species in the guts of the arc-eye and flame hawkfish, the majority of which were crustaceans 18 of 24 (75%) and 21 of 31 (68%) respectively (Leray et al., 2013b). This study highlights that a metabarcoding approach significantly increases the taxonomic scope by documenting an even broader taxonomic distribution of species consumed by hawkfishes (Appendix S1). The coral croucher diet also includes a wide spectrum of prey demonstrating that all three predatory fish feed broadly across community diversity. Our results highlight the importance of collecting empirical dietary data to understand processes of species coexistence in this high diversity marine ecosystem.
The ecological influence of a predator is contingent upon the prey it consumes. Their feeding behavior may induce cascading effects that will depend on the type of association that the prey they consume (or interfere with) have with keystone species. For example, in terrestrial ecosystems where up to 90% of flowering plant species use animal pollinators for reproduction (Bushmann & Nabhan, 1996), a predator’s effect on plant reproductive success, growth and survival will depend on its relative consumption of pollinators and phytophageous insects (Dukas, 2005; Knight et al., 2006). Similarly, some coral reef dwelling predatory fish may either disrupt benefits to corals if they derive a significant proportion of their diet from coral mutualists or alternatively alleviate deleterious effects on corals if they consume coral parasites. Invertebrate communities occurring among the branches of live Pocillopora corals in Moorea or elsewhere in the Pacific are typically composed of a preponderance of decapod mutualists (>80% of diversity and abundance in live Pocillopora—see Patton, 1974; Coles, 1980; Odinetz, 1983; Stella, Jones & Pratchett, 2010; Leray et al., 2012a). Based on previous cloning studies (Leray et al., 2013b) only the arc-eye hawkfish consumed functionally important prey (Trapezia tigrina). With increased sequencing depth herein, we demonstrate that while many other mutualist decapod species do occur in the diets of the arc-eye hawkfish, flame hawkfish and coral croucher (5.6%, 2.4% and 15.3% of sequence abundance, respectively; Appendix S1), they represent a much smaller proportion of the diet than would be expected from their density in natural communities. Interestingly, we found evidence of the Pocillopora obligate pontoniid shrimp Harpiliopsis beaupressi but no detection of congeneric H. depressa and H. spinigera in the predators’ gut contents, despite their very high abundance reported on head-size Pocillopora in Moorea (Leray et al., 2012a). It is also surprising not to discover Alpheus lottini in the diets of the three species although this is a common species found in all living Pocillopora observed and known to have beneficial effects on coral survivorship (Stier et al., 2012). Overall, our data indicate a non random pattern of prey consumption atypical of an opportunistic feeding behavior (where prey would be consumed in proportion to their abundance—Heinlein, Stier & Steele, 2010) which suggests the outcome of coevolutionary dynamics between Pocillopora associated predator and prey.
Nevertheless, while our metabarcoding dietary analysis suggests limited predation pressure on mutualists, a four-month recruitment experiment conducted on the North shore of Moorea in 2009 showed a lower abundance of mutualists in corals where the coral croucher and the flame hawkfish occurred (Stier & Leray, 2014), a pattern that may be driven by non-consumptive effects of predators. For example, competent larvae may preferentially settle on corals where predators are absent. Regardless of the mechanisms, such predator effects have important implications for coral performance, because density and composition of mutualist assemblages are known to be important for the quality of the services provided to their host (Stier et al., 2012; Rouzé et al., 2014).
In addition, our metabarcoding analyses of gut contents revealed for the first time predation on a gall crab (Hapalocarcinus sp) and vermetid snails (genus: Dendropoma), which are considered detrimental to the coral host (Simon-Blecher & Achituv, 1997; Shima, Osenberg & Stier, 2010). Vermetid snails are particularly prevalent in Moorea where they can reduce coral growth by up to 81% and survival by up to 52% (Shima, Osenberg & Stier, 2010). Predation on parasites may compensate for the negative effects of the reduction in density of decapod mutualists in corals facing environmental stressors. We also recovered a significant proportion of sequences belonging to Pocillopora from the flame hawkfish and the coral croucher gut contents, which suggest that these predatory fish also feed on mucus released by their biogenic habitat. The absence of Symbiodinium COI sequences from our dataset also supports the consumption of mucus rather than coral polyps. Alternatively, Pocillopora DNA may have been sufficiently abundant and well preserved in the gut contents of mucus feeding prey (e.g., Trapeziidae) to be co-amplified (Harwood et al., 2001; Sheppard et al., 2005). Importantly though, Pocillopora was completely absent from the arc-eye hawkfish diet which also includes Trapeziid species, suggesting minimal secondary consumption or associated eDNA amplification. Overall, high-resolution dietary data are revealing a highly complex interaction web with very specialized functional roles played by each species. This highlights the shortcomings of the functional groups approach commonly used to evaluate redundancy and complementarity among coral reef species (Naeem & Wright, 2003; Micheli & Halpern, 2005).
Fine-scale spatial partitioning commonly occurs among coral reef fish species (Robertson & Lassig, 1980; Waldner & Robertson, 1980; Ebersole, 1985; Bouchonnavaro, 1986; Munday, Jones & Caley, 1997; Depczynski & Bellwood, 2004; Gardiner & Jones, 2005) but the extent of food partitioning remains controversial (Longenecker, 2007). In fact, most early work investigating differences in diet among reef fish species showed high levels of diet overlap (Hiatt & Strasburg, 1960; Randall, 1967; Hobson, 1974; Talbot, Russell & Anderson, 1978; Harmelin-Vivien, 1979; Anderson et al., 1981; Bouchonnavaro, 1986; Ross, 1986; Depczynski & Bellwood, 2003; Kulbicki et al., 2005; Longenecker, 2007; Castellanos-Galindo & Giraldo, 2008) which has led many to the conclusion that trophic partitioning was not a mechanism promoting species coexistence on coral reefs. However, these studies, which rely on morphological identification of semi-digested prey remains in gut contents grouped food items into broad categories therefore impeding accurate measures of partitioning (Longenecker, 2007). Alternative strategies such as field observations of feeding behavior (Pratchett, 2005; Pratchett, 2007; Pratchett & Berumen, 2008) or a combination of gut content and stable isotope analyses (Ho et al., 2007; Nagelkerken et al., 2009) helped describe dietary differences between closely related species, but generalizations about the importance of trophic partitioning for the maintenance of coral reef diversity remain difficult. In the present study, high-resolution molecular data highlight an unexpected level of dietary partitioning among the three study species. While both hawkfish species are from the same family (Cirrhitidae), they share only a single prey item at greater than 1% of either of their diets (Trapezia serenei). There is also a minor dietary overlap between the coral croucher (family Caracanthidae) and the flame hawkfish that were always found co-occurring in Pocillopora and are known to rarely venture outside the branching structure provided by their host coral (Hiatt & Strasburg, 1960; Stier & Leray, 2014). These results demonstrate that levels of trophic partitioning have likely been underestimated. We strongly encourage further empirical approaches to dietary studies prior to making assumptions of trophic equivalency in food web reconstruction (Leibold & McPeek, 2006).
The extent to which secondary prey co-amplification could lead to errors in food web analysis has not been quantified in marine systems (see Sheppard et al., 2005 for an example in a terrestrial system). In the present dataset, numerous prey species identified in fish gut contents are either grazers or detritivores (e.g., isopods, amphipods, decapods, ophiuroids and gastropods) and are therefore unlikely to consume each other. Some fish species detected in the gut contents are higher-level predators (e.g., Caranx melampygus) that could consume benthic grazers and detritivores as adults, but they were most likely fed upon at a younger developmental stage (egg, larva or juvenile) given the size of predators. Demospongiae, Ascidiacea and Gymnolaemata represented by few or a single sequence in the dataset were, however, possibly ingested unintentionally as secondary prey or epiphytes on the carapace of spider crabs (e.g., Menaethius monoceros and Perinia tumida). Parasites of prey (e.g., parasitic isopods of coral crabs of the genus Trapezia, Appendix S1) and parasites of a predator’s digestive track (e.g., Trematoda and Cestoda) may also confound food web reconstructions and care should be taken to consider the targeted roles these fish predators have on various parasites. The recovery of secondary prey may artificially inflate dietary partitioning if those lower levels are also partitioned. However, we expect the amount of DNA that these secondary prey items represent in the guts of our target predators should be minor and highly digested in comparison to primary prey. A recent metabarcoding analysis of benthic samples (Leray & Knowlton, 2015) showed evidence of a correlation between amount of DNA and number of reads. Thus if secondary prey is quickly degraded, those taxa should be represented by one or few reads only. The present dataset shows minor dietary overlap both with and without rare OTUs (<1% of total OTUs, Fig. 4), further supporting our conclusions regarding the extent of trophic partitioning among all three fish species.
Importantly, our analysis shows that in-depth sequencing would enable a more comprehensive representation of trophic links in this multi-faceted ecosystem. Additional reads would provide more OTUs matching reference barcodes (in GENBANK, BOLD or BIOCODE) but also a higher proportion of unidentified OTUs represented by a single sequence (“singleton”, Fig. 3) that are likely to be either (1) small taxa underrepresented in DNA barcode libraries (Leray et al., 2013a), or (2) the product of sequencing artifacts despite our very stringent quality filtering based on amino-acid translation. Further barcoding initiatives aiming to catalogue small life forms (e.g., meiofauna) will be crucial to advance our understanding of food webs. Systematic removal of singletons may also be used as a conservative measure, although most of them likely represent valid taxa (Huse et al., 2010). As coral reef ecosystems decline worldwide, understanding the role of predator species in a dominant, yet largely understudied trophic category, is essential. Our study highlights the tremendous potential of metabarcoding as an approach to provide unprecedented taxonomic resolution in the diet of coral dwelling predatory fish. We encourage that further work should be conducted to understand the ecological role of reef dwelling fish and invertebrates.
Supplemental Information
BIOCODE reference specimen number or GENBANK accession number are indicated only when sequence similarity with reference barcode sequence was >98% (using BLASTn search) (Machida et al., 2009; Plaisance et al., 2009). When sequence similarity to a reference barcode was <98%, we used the Bayesian assignment tool implemented in SAP to assign each OTU to a higher taxonomic group. Photographs and additional information about BIOCODE reference specimens can be obtained at http://biocode.berkeley.edu. The number of sequences for each OTU is provided in the table. * indicates prey items consisting of >1% of either of the three species diets according to the relative abundance of reads.
Acknowledgments
We thank Gustav Paulay, Arthur Anker, Joseph Poupin and the BIOCODE teams who collected both marine and terrestrial specimens, the “Centre de Recherche Insulaire et Observatoire de l’Environnement (CRIOBE) de Moorea” and the Richard B. Gump field station in Moorea for logistical support.
Funding Statement
Funding was provided by the Gordon and Betty Moore Foundation, France American Cultural Exchange program (FACE)/Partner University Fund (PUF), the Smithsonian Institution fellowship program and the Agence National de Recherche, ANR-11-JSV7-012-01 Live and Let Die. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Matthieu Leray is a postdoctoral fellow at the National Museum of Natural History, Smithsonian Institution. Christopher P Meyer is a research zoologist at the Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution.
Author Contributions
Matthieu Leray conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Christopher P. Meyer conceived and designed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, reviewed drafts of the paper.
Suzanne C. Mills conceived and designed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Approval was granted from our institutional animal ethics committee, le Centre National de la Recherche Scientifique (CNRS), for sacrificing and subsequently dissecting fish (Permit Number: 006725). None of the fish species are on the endangered species list and no specific authorization was required from the French Polynesian government for collection.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
An alignment of all OTU representative sequences is provided in Appendix S2 and the denoised sequence dataset is deposited in the Dryad Repository DOI 10.5061/dryad.v0p71).
References
- Adjeroud et al. (2009).Adjeroud M, Michonneau F, Edmunds PJ, Chancerelle Y, de Loma TL, Penin L, Thibaut L, Vidal-Dupiol J, Salvat B, Galzin R. Recurrent disturbances, recovery trajectories, and resilience of coral assemblages on a South Central Pacific reef. Coral Reefs. 2009;28:775–780. doi: 10.1007/s00338-009-0515-7. [DOI] [Google Scholar]
- Anderson et al. (1981).Anderson GRV, Ehrlich AH, Ehrlich PR, Roughgarden JD, Russell BC, Talbot FH. The community structure of coral reef fishes. American Naturalist. 1981;117:476–495. doi: 10.1086/283729. [DOI] [Google Scholar]
- Bachet, Zysman & Lefevre (2007).Bachet P, Zysman T, Lefevre Y. Au vent des iles, collection nature et environnement d’Oceanie. 2007. Guide des poissons de Tahiti et de ses iles. [Google Scholar]
- Bouchonnavaro (1986).Bouchonnavaro Y. Partitioning of food and space resources by chaetodontid fish on coral reefs. Journal of Experimental Marine Biology and Ecology. 1986;103:21–40. doi: 10.1016/0022-0981(86)90130-9. [DOI] [Google Scholar]
- Bushmann & Nabhan (1996).Bushmann S, Nabhan G. The forgotten pollinators. Washington, DC: Island Press; 1996. [Google Scholar]
- Castellanos-Galindo & Giraldo (2008).Castellanos-Galindo GA, Giraldo A. Food resource use in a tropical eastern Pacific tidepool fish assemblage. Marine Biology. 2008;153:1023–1035. doi: 10.1007/s00227-007-0874-y. [DOI] [Google Scholar]
- Chapman et al. (2013).Chapman EG, Schmidt JM, Welch KD, Harwood JD. Molecular evidence for dietary selectivity and pest suppression potential in an epigeal spider community in winter wheat. Biological Control. 2013;65:72–86. doi: 10.1016/j.biocontrol.2012.08.005. [DOI] [Google Scholar]
- Coles (1980).Coles SL. Species diversity of decapods associated with living and dead reef coral Pocillopora meandrina. Marine Ecology-Progress Series. 1980;2:281–291. doi: 10.3354/meps002281. [DOI] [Google Scholar]
- Depczynski & Bellwood (2003).Depczynski M, Bellwood DR. The role of cryptobenthic reef fishes in coral reef trophodynamics. Marine Ecology-Progress Series. 2003;256:183–191. doi: 10.3354/meps256183. [DOI] [Google Scholar]
- Depczynski & Bellwood (2004).Depczynski M, Bellwood DR. Microhabitat utilisation patterns in cryptobenthic coral reef fish communities. Marine Biology. 2004;145:455–463. doi: 10.1007/s00227-004-1342-6. [DOI] [Google Scholar]
- Dukas (2005).Dukas R. Bumble bee predators reduce pollinator density and plant fitness. Ecology. 2005;86:1401–1406. doi: 10.1890/04-1663. [DOI] [Google Scholar]
- Ebersole (1985).Ebersole JP. Niche separation of two damselfish species by aggregation and differential microhabitat utilization. Ecology. 1985;66:14–20. doi: 10.2307/1941302. [DOI] [Google Scholar]
- Edgar et al. (2011).Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner & Jones (2005).Gardiner N, Jones G. Habitat specialisation and overlap in a guild of coral reef cardinalfishes (Apogonidae) Marine Ecology Progress Series. 2005;305:163–175. doi: 10.3354/meps305163. [DOI] [Google Scholar]
- Geller et al. (2013).Geller JB, Meyer CP, Parker M, Hawk H. Redesign of PCR primers for mitochondrial Cytochrome c. oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Molecular Ecology Resources. 2013;13:851–861. doi: 10.1111/1755-0998.12138. [DOI] [PubMed] [Google Scholar]
- Glynn (1980).Glynn PW. Defense by symbiotic crustacea of host corals elicited by chemical cues from predator. Oecologia. 1980;47:287–290. doi: 10.1007/BF00398518. [DOI] [PubMed] [Google Scholar]
- Glynn (1983).Glynn PW. Increased survivorship in corals harboring crustacean symbionts. Marine Biology Letters. 1983;4:105–111. [Google Scholar]
- Hao, Jiang & Chen (2011).Hao X, Jiang R, Chen T. Clustering 16S rRNA for OTU prediction: a method of unsupervised Bayesian clustering. Bioinformatics. 2011;27:611–618. doi: 10.1093/bioinformatics/btq725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley (2011).Harley CDG. Climate change, keystone predation, and biodiversity loss. Science. 2011;334:1124–1127. doi: 10.1126/science.1210199. [DOI] [PubMed] [Google Scholar]
- Harmelin-Vivien (1979).Harmelin-Vivien ML. Ichthyofaune des recifs coralliens de Tulear (Madagascar): ecologie et relations trophiques. Aix-Marseilles, France: University of Aix-Marseilles; 1979. [Google Scholar]
- Harwood et al. (2001).Harwood JD, Phillips SW, Sunderland KD, Symondson WOC. Secondary predation: quantification of food chain errors in an aphid-spider-carabid system using monoclonal antibodies. Molecular Ecology. 2001;10:2049–2057. doi: 10.1046/j.0962-1083.2001.01349.x. [DOI] [PubMed] [Google Scholar]
- Heinlein, Stier & Steele (2010).Heinlein JM, Stier AC, Steele MA. Predators reduce abundance and species richness of coral reef fish recruits via non-selective predation. Coral Reefs. 2010;29:527–532. doi: 10.1007/s00338-010-0592-7. [DOI] [Google Scholar]
- Hiatt & Strasburg (1960).Hiatt RW, Strasburg DW. Ecological relationships of the fish fauna on coral reefs on the Marshall Islands. Ecological Monographs. 1960;30:66–127. doi: 10.2307/1942181. [DOI] [Google Scholar]
- Ho et al. (2007).Ho CT, Kao SJ, Dai CF, Hsieh HL, Shiah FK, Jan RQ. Dietary separation between two blennies and the Pacific gregory in northern Taiwan: evidence from stomach content and stable isotope analyses. Marine Biology. 2007;151:729–736. doi: 10.1007/s00227-006-0517-8. [DOI] [Google Scholar]
- Hobson (1974).Hobson ES. Feeding relationships of teleostean fishes on coral reefs in Kona, Hawaii. Fishery Bulletin. 1974;72:915–1031. [Google Scholar]
- Hoegh-Guldberg & Bruno (2010).Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- Holbrook et al. (2008).Holbrook SJ, Brooks AJ, Schmitt RJ, Stewart HL. Effects of sheltering fish on growth of their host corals. Marine Biology. 2008;155:521–530. doi: 10.1007/s00227-008-1051-7. [DOI] [Google Scholar]
- Holbrook, Schmitt & Brooks (2011).Holbrook SJ, Schmitt RJ, Brooks AJ. Indirect effects of species interactions on habitat provisioning. Oecologia. 2011;166:739–749. doi: 10.1007/s00442-011-1912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes (1985).Humes A. A review of the Xarifiidae (Copepoda, Poecilostomatoida), parasites of scleractinian corals in the Indo-Pacific. Bulletin of Marine Science. 1985;36:467–632. [Google Scholar]
- Huse et al. (2010).Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environmental Microbiology. 2010;12:1889–1898. doi: 10.1111/j.1462-2920.2010.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal et al. (2011).Kayal M, Lenihan HS, Pau C, Penin L, Adjeroud M. Associational refuges among corals mediate impacts of a crown-of-thorns starfish Acanthaster planci outbreak. Coral Reefs. 2011;30:827–837. doi: 10.1007/s00338-011-0763-1. [DOI] [Google Scholar]
- Kayal et al. (2012).Kayal M, Vercelloni J, Lison de Loma T, Bosserelle P, Chancerelle Y, Geoffroy S, Stievenart C, Michonneau F, Penin L, Planes S, Adjeroud M. Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE. 2012;7:e1047. doi: 10.1371/journal.pone.0047363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight et al. (2006).Knight TM, Chase JM, Hillebrand H, Holt RD. Predation on mutualists can reduce the strength of trophic cascades. Ecology Letters. 2006;9:1173–1178. doi: 10.1111/j.1461-0248.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- Kowalczyk et al. (2011).Kowalczyk R, Taberlet P, Coissac E, Valentini A, Miquel C, Kamiński T, Wójcik JM. Influence of management practices on large herbivore diet—Case of European bison in Białowieża Primeval Forest (Poland) Forest Ecology and Management. 2011;261:821–828. doi: 10.1016/j.foreco.2010.11.026. [DOI] [Google Scholar]
- Kulbicki et al. (2005).Kulbicki M, Bozec Y-M, Labrosse P, Letourneur Y, Mou-Tham G, Wantiez L. Diet composition of carnivorous fishes from coral reef lagoons of New Caledonia. Aquatic Living Resources. 2005;18:231–250. doi: 10.1051/alr:2005029. [DOI] [Google Scholar]
- Leibold & McPeek (2006).Leibold MA, McPeek MA. Coexistence of the niche and neutral perspectives in community ecology. Ecology. 2006;87:1399–1410. doi: 10.1890/0012-9658(2006)87[1399:COTNAN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Leray et al. (2013b).Leray M, Agudelo N, Mills SC, Meyer CP. Effectiveness of annealing blocking primers versus restriction enzymes for characterization of generalist diets: unexpected prey revealed in the gut contents of two coral reef fish species. PLoS ONE. 2013b;8:e1047. doi: 10.1371/journal.pone.0058076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leray et al. (2012a).Leray M, Beraud M, Anker A, Chancerelle Y, Mills S. Acanthaster planci outbreak: decline in coral health, coral size structure modification and consequences for obligate decapod assemblages. PLoS ONE. 2012a;7:e1047. doi: 10.1371/journal.pone.0035456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leray et al. (2012b).Leray M, Boehm JT, Mills SC, Meyer CP. Moorea BIOCODE barcode library as a tool for understanding predator–prey interactions: insights into the diet of common predatory coral reef fishes. Coral Reefs. 2012b;31:383–388. doi: 10.1007/s00338-011-0845-0. [DOI] [Google Scholar]
- Leray & Knowlton (2015).Leray M, Knowlton N. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2076–2081. doi: 10.1073/pnas.1424997112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leray et al. (2013a).Leray M, Yang JY, Meyer CP, Mills SC, Agudelo N, Ranwez V, Boehm JT, Machida RJ. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Frontiers in Zoology. 2013a;10:34. doi: 10.1186/1742-9994-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker (2007).Longenecker K. Devil in the details: high-resolution dietary analysis contradicts a basic assumption of reef-fish diversity models. Copeia. 2007;3:543–555. doi: 10.1643/0045-8511(2007)2007[543:DITDHD]2.0.CO;2. [DOI] [Google Scholar]
- Machida et al. (2009).Machida RJ, Hashiguchi Y, Nishida M, Nishida S. Zooplankton diversity analysis through single-gene sequencing of a community sample. BMC Genomics. 2009;10:438. doi: 10.1186/1471-2164-10-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon & Moore (2014).McKeon CS, Moore JM. Species and size diversity in protective services offered by coral guard-crabs. PeerJ. 2014;2:e1047. doi: 10.7717/peerj.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli & Halpern (2005).Micheli F, Halpern BS. Low functional redundancy in coastal marine assemblages. Ecology Letters. 2005;8:391–400. doi: 10.1111/j.1461-0248.2005.00731.x. [DOI] [Google Scholar]
- Mollot et al. (2014).Mollot G, Duyck P-F, Lefeuvre P, Lescourret F, Martin J-F, Piry S, Canard E, Tixier P. Cover cropping alters the diet of arthropods in a banana plantation: a metabarcoding approach. PLoS ONE. 2014;9:e1047. doi: 10.1371/journal.pone.0093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch et al. (2008).Munch K, Boomsma W, Huelsenbeck JP, Willerslev E, Nielsen R. Statistical assignment of DNA sequences using Bayesian phylogenetics. Systematic Biology. 2008;57:750–757. doi: 10.1080/10635150802422316. [DOI] [PubMed] [Google Scholar]
- Munday, Jones & Caley (1997).Munday PL, Jones GP, Caley MJ. Habitat specialisation and the distribution and abundance of coral-dwelling gobies. Marine Ecology-Progress Series. 1997;152:227–239. doi: 10.3354/meps152227. [DOI] [Google Scholar]
- Naeem & Wright (2003).Naeem S, Wright JP. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecology Letters. 2003;6:567–579. doi: 10.1046/j.1461-0248.2003.00471.x. [DOI] [Google Scholar]
- Nagelkerken et al. (2009).Nagelkerken I, Van der Velde G, Wartenbergh SLJ, Nugues MM, Pratchett MS. Cryptic dietary components reduce dietary overlap among sympatric butterflyfishes (Chaetodontidae) Journal of Fish Biology. 2009;75:1123–1143. doi: 10.1111/j.1095-8649.2009.02303.x. [DOI] [PubMed] [Google Scholar]
- Odinetz (1983).Odinetz MO. Ecologie et structure des peuplements de crustaces decapodes associes aux coraux du genre Pocillopora en Polynesie Francaise et en Micronesie (Guam) Paris: Universite Pierre et Marie Curie; 1983. [Google Scholar]
- Paine (1966).Paine RT. Food web complexity and species diversity. American Naturalist. 1966;100:65–75. doi: 10.1086/282400. [DOI] [Google Scholar]
- Paine (1969).Paine RT. A note on trophic complexity and community stability. American Naturalist. 1969;103:91–93. doi: 10.1086/282586. [DOI] [Google Scholar]
- Patton (1974).Patton WK. Community structure among the animals inhabiting the coral Pocillopora damicornis at Heron Island, Australia. In: Vernberg WB, editor. Symbiosis in the sea. Columbia: University of South Carolina Press; 1974. [Google Scholar]
- Plaisance et al. (2009).Plaisance L, Knowlton N, Paulay G, Meyer C. Reef-associated crustacean fauna: biodiversity estimates using semi-quantitative sampling and DNA barcoding. Coral Reefs. 2009;28:977–986. doi: 10.1007/s00338-009-0543-3. [DOI] [Google Scholar]
- Pollock et al. (2012).Pollock FJ, Katz SM, Bourne DG, Willis BL. Cymo melanodactylus crabs slow progression of white syndrome lesions on corals. Coral Reefs. 2012;32:43–48. doi: 10.1007/s00338-012-0978-9. [DOI] [Google Scholar]
- Pompanon et al. (2012).Pompanon F, Deagle BE, Symondson WOC, Brown DS, Jarman SN, Taberlet P. Who is eating what: diet assessment using next generation sequencing. Molecular Ecology. 2012;21:1931–1950. doi: 10.1111/j.1365-294X.2011.05403.x. [DOI] [PubMed] [Google Scholar]
- Pratchett (2005).Pratchett MS. Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at Lizard Island, northern Great Barrier Reef. Marine Biology. 2005;148:373–382. doi: 10.1007/s00227-005-0084-4. [DOI] [Google Scholar]
- Pratchett (2007).Pratchett MS. Dietary selection by coral-feeding butterflyfishes (Chaetodontidae) on the Great Barrier Reef, Australia. Raffles Bulletin of Zoology. 2007;14:171–176. [Google Scholar]
- Pratchett & Berumen (2008).Pratchett MS, Berumen ML. Interspecific variation in distributions and diets of coral reef butterflyfishes (Teleostei: Chaetodontidae) Journal of Fish Biology. 2008;73:1730–1747. doi: 10.1111/j.1095-8649.2008.02062.x. [DOI] [Google Scholar]
- Quince et al. (2011).Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ. Removing noise from pyrosequenced amplicons. BMC Bioinformatics. 2011;12:38. doi: 10.1186/1471-2105-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall (1967).Randall JE. Food habits of reef fishes of the West Indies. Studies in Tropical Oceanography. 1967;5:665–847. [Google Scholar]
- Ranwez et al. (2011).Ranwez V, Harispe S, Delsuc F, Douzery EJP. MACSE: multiple Alignment of Coding SEquences accounting for frameshifts and stop codons. PLoS ONE. 2011;6:e1047. doi: 10.1371/journal.pone.0022594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson et al. (2011).Rawlinson KA, Gillis JA, Billings RE, Borneman EH. Taxonomy and life history of the Acropora-eating flatworm Amakusaplana acroporae nov. sp. (Polycladida: Prosthiostomidae) Coral Reefs. 2011;30:693–705. doi: 10.1007/s00338-011-0745-3. [DOI] [Google Scholar]
- Robertson & Lassig (1980).Robertson DR, Lassig B. Spatial distribution patterns and coexistence of a group of territorial damselfishes from the Great Barrier reef. Bulletin of Marine Science. 1980;30:187–203. [Google Scholar]
- Ross (1986).Ross ST. Resource partitioning in fish assemblages—a review of field studies. Copeia. 1986;2:352–388. doi: 10.2307/1444996. [DOI] [Google Scholar]
- Rotjan & Lewis (2008).Rotjan R, Lewis S. Impact of coral predators on tropical reefs. Marine Ecology Progress Series. 2008;367:73–91. doi: 10.3354/meps07531. [DOI] [Google Scholar]
- Rouzé et al. (2015).Rouzé H, Lecellier G, Langlade MJ, Planes S, Berteaux-Lecellier V. Fringing reefs exposed to different levels of eutrophication and sedimentation can support similar benthic communities. Marine Pollution Bulletin. 2015;92:212–221. doi: 10.1016/j.marpolbul.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Rouzé et al. (2014).Rouzé H, Lecellier G, Mills SC, Planes S, Berteaux-Lecellier V, Stewart H. Juvenile Trapezia spp. crabs can increase juvenile host coral survival by protection from predation. Marine Ecology Progress Series. 2014;515:151–159. doi: 10.3354/meps10970. [DOI] [Google Scholar]
- Schloss et al. (2009).Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad et al. (2012).Shehzad W, McCarthy TM, Pompanon F, Purevjav L, Coissac E, Riaz T, Taberlet P. Prey preference of snow leopard (Panthera uncia) in South Gobi, Mongolia. PLoS ONE. 2012;7:e1047. doi: 10.1371/journal.pone.0032104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard et al. (2005).Sheppard SK, Bell J, Sunderland KD, Fenlon J, Skervin D, Symondson WOC. Detection of secondary predation by PCR analyses of the gut contents of invertebrate generalist predators. Molecular Ecology. 2005;14:4461–4468. doi: 10.1111/j.1365-294X.2005.02742.x. [DOI] [PubMed] [Google Scholar]
- Shima, Osenberg & Stier (2010).Shima JS, Osenberg CW, Stier AC. The vermetid gastropod Dendropoma maximum reduces coral growth and survival. Biology Letters. 2010;6:815–818. doi: 10.1098/rsbl.2010.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Blecher & Achituv (1997).Simon-Blecher N, Achituv Y. Relationship between the coral pit crab Cryptochirus coralliodytes Heller and its host coral. Journal of Experimental Marine Biology and Ecology. 1997;215:93–102. doi: 10.1016/S0022-0981(97)00002-6. [DOI] [Google Scholar]
- Stella, Jones & Pratchett (2010).Stella JS, Jones GP, Pratchett MS. Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral Reefs. 2010;29:957–973. doi: 10.1007/s00338-010-0648-8. [DOI] [Google Scholar]
- Stella et al. (2011).Stella JS, Pratchett MS, Hutchings PA, Jones GP. Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanography and Marine Biology: An Annual Review. 2011;49:43–104. doi: 10.1201/b11009-3. [DOI] [Google Scholar]
- Stewart et al. (2006).Stewart HL, Holbrook SJ, Schmitt RJ, Brooks AJ. Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs. 2006;25:609–615. doi: 10.1007/s00338-006-0132-7. [DOI] [Google Scholar]
- Stier et al. (2012).Stier AC, Gil MA, McKeon CS, Lemer S, Leray M, Mills SC, Osenberg CW. Housekeeping mutualisms: do more symbionts facilitate host performance? PLoS ONE. 2012;7:e1047. doi: 10.1371/journal.pone.0032079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier & Leray (2014).Stier AC, Leray M. Predators alter community organization of coral reef cryptofauna and reduce abundance of coral mutualists. Coral Reefs. 2014;33:181–191. doi: 10.1007/s00338-013-1077-2. [DOI] [Google Scholar]
- Stier et al. (2010).Stier AC, McKeon CS, Osenberg CW, Shima JS. Guard crabs alleviate deleterious effects of vermetid snails on a branching coral. Coral Reefs. 2010;29:1019–1022. doi: 10.1007/s00338-010-0663-9. [DOI] [Google Scholar]
- Sussman et al. (2003).Sussman M, Loya Y, Fine M, Rosenberg E. The marine fireworm Hermodice carunculata is a winter reservoir and spring-summer vector for the coral-bleaching pathogen Vibrio shiloi. Environmental Microbiology. 2003;5:250–255. doi: 10.1046/j.1462-2920.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Symondson (2002).Symondson WOC. Molecular identification of prey in predator diets. Molecular Ecology. 2002;11:627–641. doi: 10.1046/j.1365-294X.2002.01471.x. [DOI] [PubMed] [Google Scholar]
- Taberlet et al. (2012).Taberlet P, Coissac E, Pompanon F, Brochmann C, Willerslev E. Towards next-generation biodiversity assessment using DNA metabarcoding. Molecular Ecology. 2012;21:2045–2050. doi: 10.1111/j.1365-294X.2012.05470.x. [DOI] [PubMed] [Google Scholar]
- Talbot, Russell & Anderson (1978).Talbot FH, Russell BC, Anderson GRV. Coral reef fish communities—unstable, high diversity systems. Ecological Monographs. 1978;48:425–440. doi: 10.2307/2937241. [DOI] [Google Scholar]
- Turner (1994).Turner S. The biology and population outbreaks of the corallivorous gastropod Drupella on Indo-Pacific reefs. Oceanography and Marine Biology. 1994;32:461–530. [Google Scholar]
- Vestheim & Jarman (2008).Vestheim H, Jarman SN. Blocking primers to enhance PCR amplification of rare sequences in mixed samples—a case study on prey DNA in Antarctic krill stomachs. Frontiers in Zoology. 2008;5:12. doi: 10.1186/1742-9994-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldner & Robertson (1980).Waldner RE, Robertson DR. Patterns of habitat partitioning by eight species of territorial Carribbean damselfishes (Pisces: Pomacentridae) Bulletin of Marine Science. 1980;30:171–186. [Google Scholar]
- Williams & Miller (2005).Williams D, Miller M. Coral disease outbreak: pattern, prevalence and transmission in Acropora cervicornis. Marine Ecology Progress Series. 2005;301:119–128. doi: 10.3354/meps301119. [DOI] [Google Scholar]
- Wright (2005).Wright SJ. Tropical forests in a changing environment. Trends in Ecology & Evolution. 2005;20:553–560. doi: 10.1016/j.tree.2005.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BIOCODE reference specimen number or GENBANK accession number are indicated only when sequence similarity with reference barcode sequence was >98% (using BLASTn search) (Machida et al., 2009; Plaisance et al., 2009). When sequence similarity to a reference barcode was <98%, we used the Bayesian assignment tool implemented in SAP to assign each OTU to a higher taxonomic group. Photographs and additional information about BIOCODE reference specimens can be obtained at http://biocode.berkeley.edu. The number of sequences for each OTU is provided in the table. * indicates prey items consisting of >1% of either of the three species diets according to the relative abundance of reads.