Supplemental digital content is available in the text.
Key Words: Ovarian cancer, Platinum refractory, Platinum resistant, Chemotherapy
Abstract
Objective
Primary platinum-resistant epithelial ovarian cancer (EOC) is an area of unmet medical need. There is limited evidence from small studies that platinum-based combinations can overcome “resistance” in a proportion of patients. We investigated the efficacy and toxicity of platinum-based combination chemotherapy in the platinum-resistant and platinum-refractory setting.
Methods
Epirubicin, cisplatin, and capecitabine (ECX) combination chemotherapy was used at our institution for the treatment of relapsed EOC. From the institutional database, we identified all patients with primary platinum-refractory or platinum-resistant relapse treated with ECX as second-line therapy between 2001 and 2012. We extracted demographic, clinical, treatment, and toxicity data and outcomes. We used logistic and Cox regression models to identify predictors of response and survival respectively.
Results
Thirty-four 34 patients (8 refractory, 26 resistant) were treated with ECX. Response Evaluation Criteria In Solid Tumors (RECIST) response rate was 45%, median progression-free survival (PFS) was 6.4 months, and overall survival (OS) was 10.6 months. Platinum-resistant patients had better outcomes than did platinum-refractory patients (response rate, 54% vs 0%, P = 0.047; PFS 7.2 vs 1.8 months, P < 0.0001; OS 14.4 vs 3 months, P < 0.001). In regression models, time to progression after first-line treatment and platinum-refractory status were the strongest predictors of response and PFS or OS, respectively. Patients with time to progression after first-line treatment longer than 3 months showed PFS and OS of 7.9 and 14.7 months, respectively. Toxicity was manageable, with only 13% of cycles administered at reduced doses.
Conclusions
Epirubicin, cisplatin, and capecitabine seems to be active in platinum-resistant relapsed EOC with manageable toxicity. Further prospective investigation of platinum-anthracycline combinations is warranted in patients who relapse 3 to 6 months after first-line platinum-taxane treatment.
Epithelial ovarian cancer (EOC) is a significant cause of morbidity and the commonest cause of death from gynecologic cancer.1 For most patients presenting with stage III-IV disease, first-line treatment consists of debulking surgery and chemotherapy with a platinum taxane doublet, most commonly carboplatin and paclitaxel.
Despite frequent complete responses (CRs) to first-line treatment, relapse occurs in up to 85% of patients with stage III-IV disease, with a median time to relapse of 18 months.2 Approximately a third of these relapses occur within 6 months of first-line treatment,3 and the disease is then considered primary platinum resistant or refractory. Single-agent treatment with a nonplatinum drug is the preferred treatment option in this setting4,5; commonly used drugs are pegylated liposomal doxorubicin (PLD), topotecan, and, more recently, weekly paclitaxel. However, outcomes are poor, with response rates (RRs) ranging from 6% to 12% for PLD and topotecan6,7 and 27% to 35% for weekly paclitaxel.8,9 Median progression-free survival (PFS) and overall survival (OS) typically range from 3 to 6 months and from 10 to 13 months, respectively, with some improvement in the latter in more recent studies, particularly those incorporating weekly paclitaxel.6–10
However, platinum resistance is generally not absolute. Response rates of 29% to 39% have been reported in small studies of platinum-based regimens such as cisplatin etoposide, cisplatin gemcitabine, or oxaliplatin 5-fluorouracil in platinum-resistant patients, suggesting that combination cisplatin or oxaliplatin containing chemotherapy can overcome “platinum resistance” in some patients.11–13 Consequently, efforts are being made to try new platinum-based combinations both in the platinum sensitive and the “platinum-resistant” setting. Previous work by Ahmed et al14 demonstrated an RR of 41% for recurrent ovarian cancer, relapsing within 12 months of prior platinum treatment, using the 3-drug combination of epirubicin, cisplatin, and continuous infusional 5-fluoruracil. Because the delivery of infusional 5-fluorouracil requires an indwelling central venous catheter, the same group investigated the combination of epirubicin, carboplatin, and the oral fluoropyrimidine capecitabine (ECarboX) in patients with disease relapsing 6 months or more after prior platinum treatment.15 Eleven (61%) of 18 patients demonstrated either complete or partial radiologic response, implying considerable activity of this combination. However, hematologic toxicity was significant, necessitating frequent dose reductions and interruptions.
These encouraging reports of preliminary activity have led to the frequent use of a modified ECarboX regimen in our institution for fit patients with platinum-refractory or resistant relapse. Specifically, we resubstituted cisplatin for carboplatin to ameliorate haematologic toxicity, leading to a regimen of epirubicin, cisplatin, and capecitabine (ECX). We report here our institutional experience with this regimen, over more than a decade, in patients with first platinum-refractory or resistant relapse.
METHODS
This is a retrospective single-center case series of patients with ovarian cancer receiving ECX as first relapse therapy at Addenbrooke’s Hospital, Cambridge, UK, between January 2001 and September 2012. To be included in the series, patients required histologically confirmed EOC with platinum-refractory or platinum-resistant relapse after first-line treatment with platinum-based chemotherapy. Platinum-refractory relapse was defined as radiologic progression on treatment or within 1 month of the final cycle of first-line platinum treatment (day 1). Platinum-resistant relapse was defined similarly, except that time to progression was between 1 and 6 months after first-line treatment. Patients were excluded if they had platinum-sensitive disease (relapse >6 months from prior platinum treatment), nonepithelial ovarian malignancy, had received more than 1 line of previous chemotherapy in the relapse setting, or received ECX after suboptimal debulking surgery without documented disease progression.
Treatment Administration
Epirubicin was administered as a bolus dose of 50 mg/m2 on day 1, followed by cisplatin 60 mg/m2 as a 3-hour infusion with conventional preinfusion and postinfusion hydration. Capecitabine was administered orally at a dose of 650 mg/m2 twice a day for 14 days, starting on the evening of day 1. Standard antiemetic prophylaxis consisted of ondansentron and dexamethasone, with aprepitant added in selected patients in more recent years. Cycles were repeated every 3 weeks for a maximum of 6 cycles of treatment. The decision to proceed with each subsequent cycle of treatment rested with the reviewing clinician, although, generally, a neutrophil count greater than 1.0 × 109/L and a platelet count greater than 100 × 109/L were required. Growth factor support was not routinely administered.
Data Collection
Demographic information, histologic details, details of first-line treatment, and any previous surgery were extracted from the clinical records. Epirubicin, cisplatin, and capecitabine administration details, including actual administered doses, dose reductions, and delays, were extracted from the pharmacy database and, since 2011, from the computerized chemotherapy prescription platform (Aria). Nonhematologic toxicity grading was extracted from prospectively recorded toxicity charts in the clinical records. Hematologic and biochemical toxicities were extracted from the hospital electronic database and graded according to National Cancer Institute-Common Toxicity Criteria Version 4.0.
Assessment of Response
Patients were assessed clinically and with serum cancer antigen 125 (CA-125) measurements before each cycle of treatment. Disease burden was measured with computed tomography at baseline, approximately every 9 weeks while receiving ECX and every 12 weeks after completion of chemotherapy. All scans were retrospectively evaluated according to Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 by consultant radiologists specializing in gynecologic cancer, and overall response was reported. Repeat imaging was not carried out to confirm response.
Outcome Measures
Best radiologic responses and CA-125 responses were recorded according to both RECIST and GCIG criteria, respectively.16,17 Clinical benefit rate was defined as the proportion of patients achieving RECIST response or stable disease (SD) for at least 8 weeks. Progression-free survival was calculated as the interval from the first day of ECX administration to unequivocal clinical or radiologic (but not CA-125) disease progression or death from any cause, whichever occurred first. Overall survival was calculated as the interval from the first day of ECX administration to death. All patients included in this series have died, and therefore, there are no censored observations. Feasibility and tolerability of the regimen were recorded as manifested by dose reductions, treatment delays, and grade 3/4 toxicities.
Statistics
Descriptive statistics are presented as mean ± SD or as median with interquartile range (IQR), as appropriate. Proportions were compared with the Fisher exact test. Survival curves were constructed using the Kaplan-Meier method. Univariate and multivariate logistic regressions were performed to identify modifiers of response. Survival curves were compared with the log-rank test, and Cox regression was performed to identify modifiers of PFS and OS. Variables entered in the logistic and Cox regressions were age (in years), histologic subtype (clear cell vs others), International Federation of Gynecology and Obstetrics stage (IV vs III), first-line treatment (carboplatin paclitaxel vs carboplatin), resistance type (resistant vs refractory), and time to progression after first-line treatment (TTP1; in days as a continuous variable). Variables with P < 0.2 were entered in multivariate models that were constructed using backward selection. All analyses were performed using GraphPad Prism v6 (San Diego, CA) and MedCalc v13.2.2.0 (Ostend, Belgium). P < 0.05 indicates statistical significance.
RESULTS
Patient Characteristics
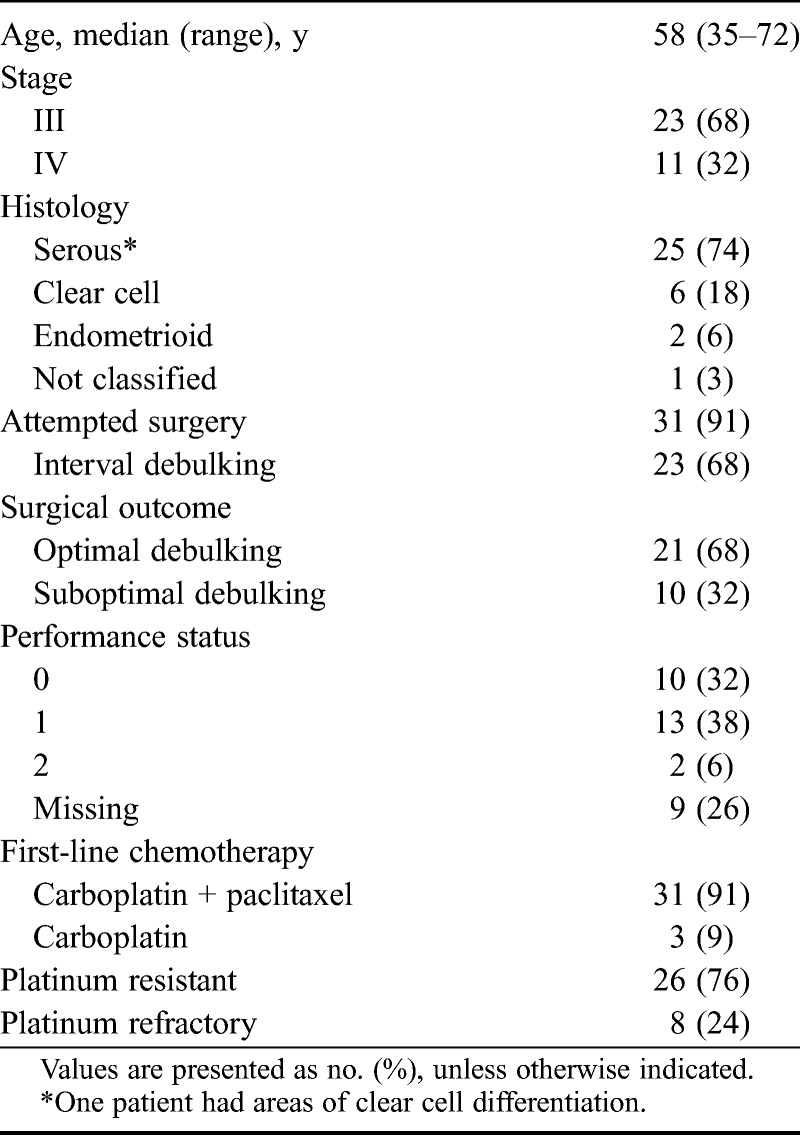
Thirty-four patients commenced treatment with second line ECX between June 2004 and February 2011. Patient characteristics are summarized in Table 1. Median age was 58 years (range, 35–72 years), and all patients presented with stage III or IV disease. The predominant histology was high-grade serous EOC (68% of cases). Twenty-one (62%) patients underwent optimal debulking surgery (<1 cm of residual), and 11 (32%) had no macroscopic residual at the end of their operation. Eight (24%) patients progressed on or within 1 month of first-line chemotherapy and were considered platinum refractory, and 6 (75%) of these 8 patients had clear cell histology. All patients had progressed within 6 months after first-line treatment and were therefore considered platinum resistant. Median TTP1 was 115 days (IQR, 64.5–161.3), and median treatment-free interval was 136.5 days (IQR, 78.75–183.8).
TABLE 1.
Patient characteristics
Treatment Administration
The median number of ECX cycles administered per patient was 6, for a total of 168 cycles. The proportion of cycles with at least 90% of the planned dose administered was 93% for epirubicin, 79% for cisplatin, and 81% for capecitabine. Median dose delivered was 50 and 60 mg/m2 for epirubicin and cisplatin, respectively. The median delivered dose of capecitabine was 650 mg/m2 twice a day for 14 of 21 days per cycle. It should be noted that the first 7 patients were started on continuous capecitabine; all required a change to a 2-week on/1-week off schedule due to toxicity. Overall, 19 (56%) of patients completed 6 cycles of ECX. The most common reason for stopping early was disease progression, occurring in 10 (29%) patients.
Twelve (35%) patients had a dose reduction for at least 1 drug in the regimen, most commonly capecitabine. Capecitabine was discontinued in 3 cases (2 due to chest pain and 1 for diarrhea and palpitations) and cisplatin in 1 (due to ototoxicity), with no other modifications to the rest of the regimen. In total, 22 (13%) cycles were delayed, with neutropenia being the predominant reason (Table S1, available online as Supplemental Digital Content at http://links.lww.com/IGC/A286).
Efficacy
Twenty-nine patients were evaluable by RECIST criteria (Table 2). For 5 patients, treated in the earlier years of this series, scans were not available for review. None of these patients had documented early clinical progression before the first assessment performed after approximately 9 weeks of treatment. The best radiologic responses were CR in 2 (7%) patients, partial response (PR) in 11 (38%), SD in 9 (31%), and progressive disease (PD) in 7 (24%), for a best overall RR (ORR) of 45%. Responses tended to occur early, with 8 of the 13 documented at the first interim scan. There were no responses in the platinum refractory group. Two of the 5 assessable refractory patients had SD and 3 had PD. The ORR was significantly higher in platinum-resistant compared with platinum-refractory patients (P = 0.047). In univariate logistic regression (Table 3), only TTP1 was a significant predictor of CR/PR (P = 0.015), and therefore, multivariate regression was not performed. It should be noted that because there were no responses in patients with platinum-refractory or clear cell disease, logistic regression results for these parameters were nonestimable.
TABLE 2.
Best response to ECX by RECIST criteria
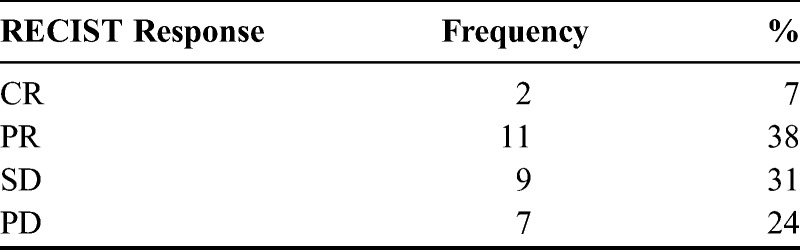
TABLE 3.
Logistic regression of response variables
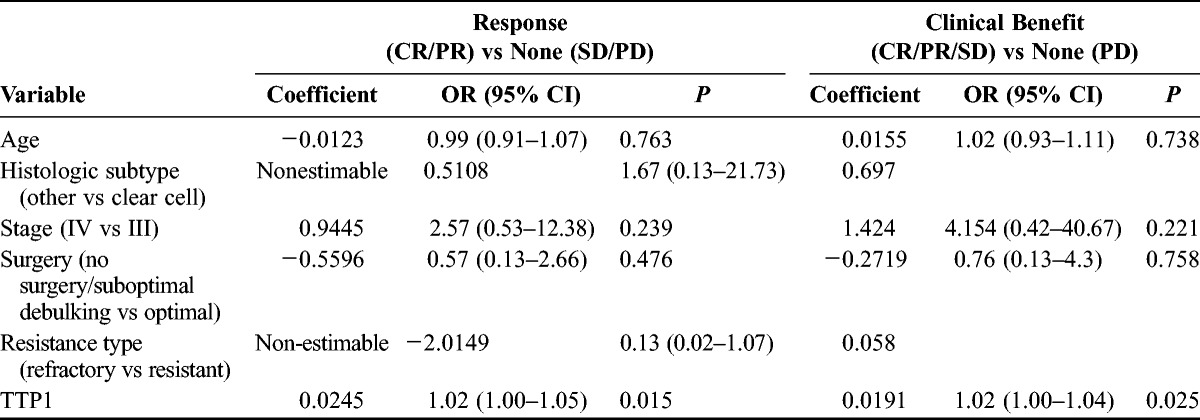
The clinical benefit rate was 83% in platinum-resistant and 40% in platinum-refractory patients (P = 0.07). In univariate logistic regression (Table 3), platinum-refractory patients were less likely to exhibit clinical benefit (odds ratio [OR], 0.13; 95% confidence interval [CI], 0.02–1.07; P = 0.058), as were patients with shorter TTP1 (P = 0.025). Time to progression after first-line treatment was the only variable retained as significant in a multivariate model (P = 0.03). Twenty-six patients were evaluable for CA-125 response evaluation, with 18 (69%) achieving a response by GCIG criteria (7 CR, 11 PR). Significantly more patients with platinum-refractory compared with resistant disease showed CA-125 progression as their best marker response (4/6 vs 2/20, P = 0.013). Of note, 5 patients were CA-125 but not RECIST evaluable; 2 (40%) showed a CA-125 PR and 3 (60%) showed PD.
Median PFS for the 34 patients was 6.4 months. It was 7.2 and 1.8 months for platinum-resistant and platinum-refractory patients, respectively (P < 0.0001; Fig. 1). Similarly, median OS was 10.6 months. Patients with platinum-refractory disease showed a very short median OS of 3 months compared with 14.4 months for platinum-resistant patients (P < 0.0001; Fig. S1, available online as Supplemental Digital Content at http://links.lww.com/IGC/A286).
FIGURE 1.
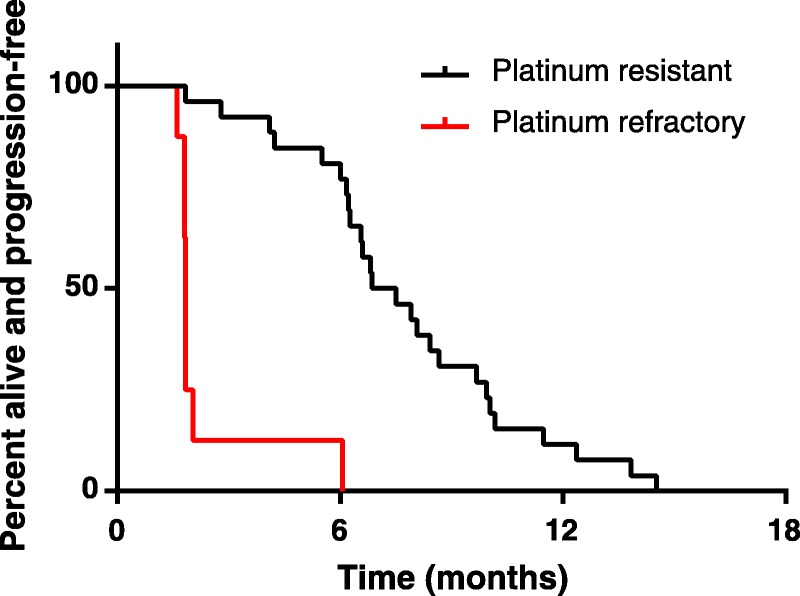
PFS in platinum-refractory and platinum-resistant patients treated with ECX.
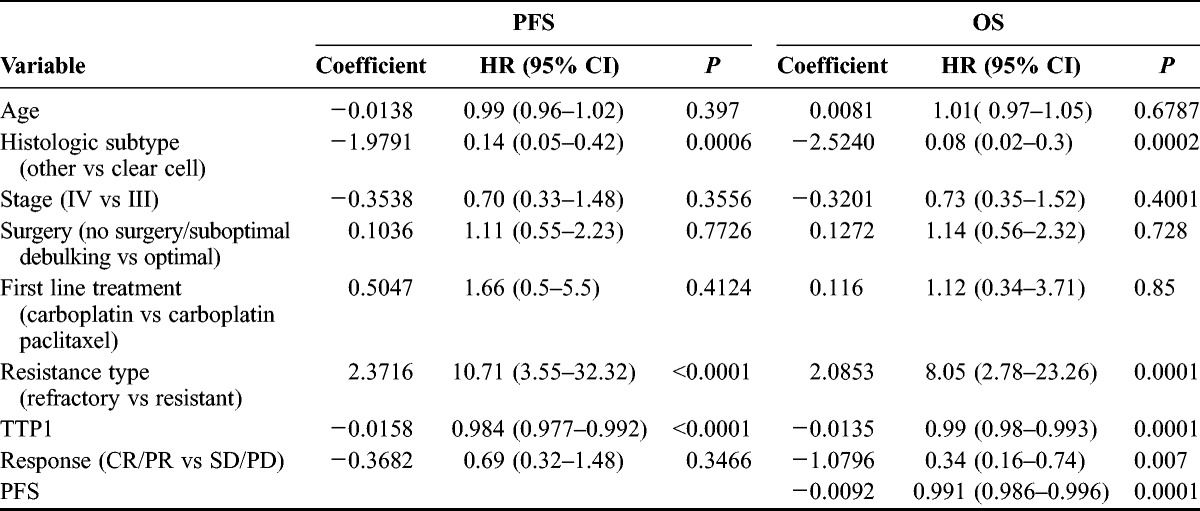
In univariate Cox regression, platinum-refractory status increased the risk of a PFS event by a factor of 10 (hazard ratio [HR], 10.71; P < 0.0001). Clear cell histologic subtype (P = 0.0006) and shorter TTP1 (P ≤ 0.0001) also predicted shorter PFS (Table 4). In a multivariate model, platinum-refractory status (P = 0.0025) and TTP1 (P = 0.021) were retained as independent predictors of PFS. Platinum-refractory status (HR, 8.05; P = 0.0001), clear cell histology (HR, 12.5; P = 0.0002), shorter TTP1 (P = 0.0001), shorter PFS (P = 0.0001), and nonresponse to ECX (P = 0.007) were significant predictors of shorter survival in univariate analysis (Table 4). When all 5 variables were entered in a multivariate model, platinum-refractory status was the single most significant predictor of shorter OS (HR, 6.85; 95% CI, 1.95–23.99; P = 0.003). Complete response/PR to ECX was also retained in the model as a predictor of longer OS (HR, 0.42; 95% CI, 0.18–0.98; P = 0.046).
TABLE 4.
Univariate analysis of PFS and OS predictors
In a further analysis, we looked at the outcomes in patients with TTP1 > 3 months. Median PFS and OS in the 23 patients with TTP1 > 3 months were 7.9 and 14.7 months compared with 1.8 and 3.2 months in the 11 patients with TTP1 < 3 months (P = 0.006 and P < 0.0001, respectively; Fig. 2). This remained the case when patients with clear cell histology were excluded (PFS 1.8 vs 7.5 months, P < 0.0001: OS 6.8 vs 14.7 months, P = 0.0002). Interestingly, patients with CR/PR at first interim assessment had significantly prolonged survival compared with patients with SD (median OS 23.2 vs 10.2 months, respectively; P = 0.02); survival of the latter was very similar to that of patients with PD at first assessment (median, 8.3 months; P = 0.88).
FIGURE 2.
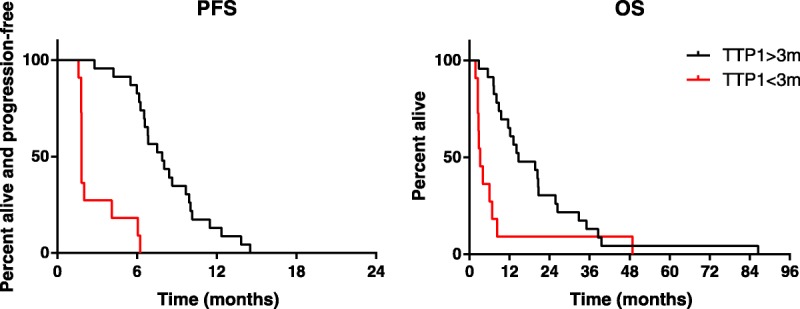
PFS and OS according to TTP1.
Toxicity
Hematologic toxicity was common, with 17 (50%) of patients experiencing grade 3 or 4 neutropenia (Table S2, available online as Supplemental Digital Content at http://links.lww.com/IGC/A286) of whom 6 (18%) had G4 neutropenia. One (3%) patient experienced grade 4 thrombocytopenia, without bleeding. Twenty (59%) patients experienced grade 2 anemia with no recorded grade 3/4 toxicity. Growth factor support was infrequent, with only 1 patient receiving secondary granulocyte colony-stimulating factor prophylaxis for 2 cycles. Of the nonhematologic toxicities (Table S3, available online as Supplemental Digital Content at http://links.lww.com/IGC/A286), nausea, vomiting, and fatigue were most common. Ten (30%) patients) patients had grade 3 or 4 vomiting; 13 (38%) and 6 (18%) patients had grade 3 nausea and fatigue, respectively. There were a total of 17 hospital admissions, affecting 13 patients during treatment. One patient with known generalized atherosclerotic disease had 2 hospital admissions related to lower limb ischaemia, judged unrelated to the chemotherapy, and eventually underwent amputation. There were 9 infection-related admissions, with neutropenia documented in 4 patients, to give an overall 12% rate for patients experiencing febrile neutropenia. The remaining admissions were predominantly for symptom control, although 2 patients were admitted with chest pain attributed to capecitabine.
DISCUSSION
Platinum-refractory or resistant ovarian cancer represents an area of unmet medical need. Despite intensive research efforts over the last 2 decades, standard treatment options remain limited to single-agent treatment with nonplatinum compounds such as PLD, topotecan, or paclitaxel.4 Here, we report substantial clinical activity of cisplatin-based combination chemotherapy in this difficult-to-treat patient group. In our single-institution series of 34 patients with primary “platinum resistance,” median PFS and OS were 6.4 and 10.6 months, respectively, with 45% of patients achieving radiologic responses by RECIST criteria.
Treatment with ECX was feasible and, despite the relatively high rates of neutropenia and infection, treatment discontinuations for reasons other than disease progression were infrequent. Cycle delays were uncommon, affecting 13% of cycles, and 80% to 90% of patients received at least 90% of the planned dose for each of the 3 drugs. The problems with prolonged neutropenia seen with ECarboX15 seem to have been significantly improved by resubstituting cisplatin for carboplatin in the ECX regimen used in the current study. The overall toxicity profile of the regimen is similar to the ECX regimen used in gastroesophageal cancer, with the exception of nausea and vomiting, which seem significantly more common in the ovarian population (30%-40% vs <10%).18 The higher rates seen in our study could be related to different disease distribution, affecting the peritoneum, and patient demographics. Toxicity rates are similar to those seen with other platinum-based combination regimens including dose-dense regimens, although with less G3/4 neurotoxicity.
Our results are similar to those reported in multiple single arm phase II studies for patients with platinum-resistant disease with regimens such as cisplatin-gemcitabine,11 cisplatin-etoposide,13 cisplatin-ifosfamide,19 dose-dense carboplatin paclitaxel,20 oxaliplatin-5FU,12 or alternating PLD/gemcitabine and cisplatin/cyclophosphamide.21 In these studies, RR ranged from 29% to 51% and PFS from 4.9% to 8 months. Most of these studies reported lower RR in patients with PFI < 3 months,11,13,19 a finding also seen in our series—patients with TTP1 < 3 months had particularly poor outcomes with median PFS and OS of 1.8 and 3.2 months, respectively. Consistent with this, in multivariate models, platinum-refractory status and short TTP1 were the most significant predictors of short OS and PFS. However, it should be noted that these observations may be confounded by histology because 6 of the 8 platinum refractory statuses were of clear cell histotype. Although the regression models suggest that platinum-refractory status is a stronger predictor of poor outcomes than clear cell histology, examination of a larger data set is needed to increase confidence in this conclusion. In our evaluation, ECX showed considerable activity in patients with TTP1 > 3 months: median PFS and OS were 7.9 and 14.7 months, respectively, and ORR was 60% in this group. The high response in this group may explain our interesting observation that early response to ECX, compared with disease stabilization, was associated with significantly prolonged OS, contrary to previous reports in which outcomes in patients achieving SD were similar to those achieving PR/CR.22
A strength of our series is that we restricted eligibility to patients with primary platinum resistance only, whereas most previously published studies have included a mix of patients with primary and secondary (ie, developing after ≥2 platinum courses) resistance. Little is known at present about specific differences in resistance pathways between patients with primary and secondary resistance; however, tumors with adverse characteristics such as CCNE1 amplification and increased proliferation seem to be enriched in the first group.23 Data from our series support the notion that platinum-based combinations can overcome primary platinum resistance in EOC. Limitations of this study include incomplete information on potential confounders such as performance status, residual disease postsurgery, and response assessments. Retrospective collection of adverse event grading from the medical records may either underestimate their frequency in our series, especially for low-grade toxicities, or overestimate because causality may be over attributed. Our OS results may have been affected by variations in the availability of other treatments over the time span of this series. Because there was no randomization, it cannot be established that similar outcomes could not have been achieved with non–platinum-based regimens; however, any selection bias operating would equally apply to a prospective single-arm study.
What is the way forward for platinum-based chemotherapy in platinum-resistant relapse? Evidence from a number of studies now suggests that patients with primary platinum resistance and TTP1 < 3 months seem to have very poor outcomes and should be prioritized for entry into clinical trials of novel agents or best supportive care, as appropriate. Considering our results in the context of other studies of platinum-based chemotherapy in patients with platinum-resistant relapse and TTP1 > 3 months, it seems that outcomes with platinum-based combinations are at least as good as those achieved with the best non–platinum-containing regimens (weekly paclitaxel plus bevacizumab or trebananib as per the AURELIA10 and TRINOVA-124 studies, respectively). The response to platinum seen in this and other studies challenges the dogma that the RRs in this population are 10%,25 a figure derived in an era when platinum resistance was defined by clinical progression. An explanation for the discrepancy is that with modern surveillance practice and earlier detection of relapse, this RR may more closely reflect what would now be considered the platinum refractory population.
Given the limited activity of single-agent capecitabine26 or 5FU27 in EOC, and the substantial additive benefit of PLD to platinum in both in the first-line28 and relapse setting,29 it is not clear whether capecitabine contributes to the activity of the cisplatin-epirubicin backbone in ECX. It is tempting to speculate that similar efficacy with less toxicity could be achieved with the combination of a more tolerable platinum and anthracycline such as carboplatin and PLD alone in the >3-month TTP1 group.
Furthermore, given the significant improvement in PFS from the addition of bevacizumab30 or cediranib31 to platinum-based chemotherapy in patients with platinum-sensitive relapse, it will be interesting to study whether the same benefits are seen from these agents when added to platinum-based chemotherapy in platinum-resistant EOC. Therefore, a prospective comparison of a platinum-anthracycline chemotherapy regimen against weekly paclitaxel (with or without an angiogenesis inhibitor in both arms) in patients with primary platinum resistance and TTP1 > 3 months would be recommended. That ours (RECIST ORR, 45%; median PFS, 6.4 months) and others’ results with platinum-based chemotherapy without an antiangiogenesis agent11–13,19–21 are very similar to those achieved with the current standard of care of nonplatinum chemotherapy plus bevacizumab in AURELIA (ORR, 31%; median PFS, 6.7 months) in platinum-resistant patients provides support for such a study. Should that happen, biospecimen collection will be extremely important in trying to define molecular biomarkers of response to treatment to supplement clinical ones such as TTP1.
CONCLUSIONS
We report significant activity with manageable toxicity of second-line cisplatin-epirubicin-capecitabine chemotherapy in EOC patients with primary platinum resistance and time to progression greater than 3 months. Platinum-based chemotherapy remains an option for these patients and needs to be prospectively compared with standard-of-care treatments in this setting.
Supplementary Material
Footnotes
Ioannis Gounaris has received honoraria and support to attend conferences from PharmaMar which is unrelated to this work. The remaining authors declare no relevant conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
K. Sayal and I. Gounaris share joint first authorship.
H. Earl and C. Parkinson share joint last authorship.
REFERENCES
- 1. Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64: 9– 29. [DOI] [PubMed] [Google Scholar]
- 2. Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. Lancet. 2014; 384: 1376– 1388. 0.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3. Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012; 30: 2654– 2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology [Internet]. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site Accessed June 29, 2014.
- 5.NICE. Paclitaxel, pegylated liposomal doxorubicin hydrochloride and topotecan for second-line or subsequent treatment of advanced ovarian cancer: review of Technology Appraisal Guidance 28, 45 and 55. Available at: https://www.nice.org.uk/guidance/ta91. Accessed April 8, 2015. [Google Scholar]
- 6. Gordon BAN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma : a randomized phase III study of pegylated liposomal doxorubicin. J Clin Oncol. 2001; 19: 3312– 3322. [DOI] [PubMed] [Google Scholar]
- 7. Mutch DG, Orlando M, Goss T, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007; 25: 2811– 2818. [DOI] [PubMed] [Google Scholar]
- 8. Lortholary a, Largillier R, Weber B, et al. Weekly paclitaxel as a single agent or in combination with carboplatin or weekly topotecan in patients with resistant ovarian cancer: the CARTAXHY randomized phase II trial from Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens (GINECO). Ann Oncol. 2012; 23: 346– 352. [DOI] [PubMed] [Google Scholar]
- 9. Karlan BY, Oza AM, Richardson GE, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol. 2012; 30: 362– 371. [DOI] [PubMed] [Google Scholar]
- 10. Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA Open-Label Randomized Phase III Trial. J Clin Oncol. 2014; 32: 1302– 1308. [DOI] [PubMed] [Google Scholar]
- 11. Bozas G, Bamias A, Koutsoukou V, et al. Biweekly gemcitabine and cisplatin in platinum-resistant/refractory, paclitaxel-pretreated, ovarian and peritoneal carcinoma. Gynecol Oncol. 2007; 104: 580– 585. [DOI] [PubMed] [Google Scholar]
- 12. Pectasides D, Pectasides M, Farmakis D, et al. Oxaliplatin plus high-dose leucovorin and 5-fluorouracil (FOLFOX 4) in platinum-resistant and taxane-pretreated ovarian cancer: a phase II study. Gynecol Oncol. 2004; 95: 165– 172. [DOI] [PubMed] [Google Scholar]
- 13. Van der Burg MEL, de Wit R, van Putten WLJ, et al. Weekly cisplatin and daily oral etoposide is highly effective in platinum pretreated ovarian cancer. Br J Cancer. 2002; 86: 19– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmed F, King D, Nicol B, et al. eds. Preliminary results of infusional chemotherapy (cisplatin, epirubicin and 5-fluorouracil, ECF) for refractory and relapsed epithelial ovarian cancer. ASCO Annual Meeting; 1995. [Google Scholar]
- 15. Rothermundt C, Hubner R, Ahmad T, et al. Combination chemotherapy with carboplatin, capecitabine and epirubicin (ECarboX) as second- or third-line treatment in patients with relapsed ovarian cancer : a phase I/II trial clinical studies. Br J Cancer. 2006; 94: 74– 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228– 247. [DOI] [PubMed] [Google Scholar]
- 17. Rustin GJS. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003; 21 (suppl 10): 187s– 193s. [DOI] [PubMed] [Google Scholar]
- 18. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008; 358: 36– 46. [DOI] [PubMed] [Google Scholar]
- 19. Song T, Kim MK, Lee Y-Y, et al. Phase II study of ifosfamide and cisplatin for the treatment of recurrent ovarian cancer. Cancer Chemother Pharmacol. 2013; 72: 653– 660. [DOI] [PubMed] [Google Scholar]
- 20. Van der Burg MEL, Vergote I, Onstenk W, et al. Long-term results of weekly paclitaxel carboplatin induction therapy: an effective and well-tolerated treatment in patients with platinum-resistant ovarian cancer. Eur J Cancer. 2013; 49: 1254– 1263. [DOI] [PubMed] [Google Scholar]
- 21. Pectasides D, Xiros N, Papaxronis G, et al. Gemcitabine and pegylated liposomal doxorubicin alternating with cisplatin plus cyclophosphamide in platinum refractory/resistant, paclitaxel-pretreated, ovarian carcinoma. Gynecol Oncol. 2008; 108: 47– 52. [DOI] [PubMed] [Google Scholar]
- 22. Gronlund B, Høgdall C, Christensen IJ, et al. Is stabilization of disease a useful indicator for survival in second-line treatment of ovarian carcinoma pre-treated with paclitaxel-platinum? Gynecol Oncol. 2004; 94: 409– 415. [DOI] [PubMed] [Google Scholar]
- 23. Etemadmoghadam D, deFazio A, Beroukhim R, et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin Cancer Res. 2009; 15: 1417– 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014; 15: 799– 808. [DOI] [PubMed] [Google Scholar]
- 25. Blackledge G, Lawton F, Redman C, et al. Response of patients in phase II studies of chemotherapy in ovarian cancer: implications for patient treatment and the design of phase II trials. Br J Cancer. 1989; 59: 650– 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pisano C, Morabito A, Soror R, et al. A phase II study of capecitabine in the treatment of ovarian cancer resistant or refractory to platinum therapy: a multicentre Italian trial in ovarian cancer (MITO-6) trial. Cancer Chemother Pharmacol. 2009; 64: 1021– 1027. [DOI] [PubMed] [Google Scholar]
- 27. Kamphuis JT, Huider MC, Ras GJ, et al. High-dose 5-fluorouracil and leucovorin as second-line chemotherapy in patients with platinum-resistant epithelial ovarian cancer. Cancer Chemother Pharmacol. 1995; 37: 190– 192. [DOI] [PubMed] [Google Scholar]
- 28. Pignata S, Scambia G, Ferrandina G, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized phase III trial. J Clin Oncol. 2011; 29: 3628– 3635. [DOI] [PubMed] [Google Scholar]
- 29. Gladieff L, Ferrero a, De Rauglaudre G, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Ann Oncol. 2012; 23: 1185– 1189. [DOI] [PubMed] [Google Scholar]
- 30. Aghajanian C, Blank SV, Goff B, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012; 30: 2039– 2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ledermann J, Perren T, Raja F, et al. Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: results of the ICON6 trial. Presented at: the 38th Congress of the European Society for Medical Oncology (ESMO); Amsterdam, the Netherlands; September 27–October 1, 2013. Abstract No. 10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


