Abstract
Cytotoxic T lymphocytes (CTLs) recognize the human leukocyte antigen (HLA) class I and antigenic peptide complex, and they play a crucial role in cancer immunity. Our recent study revealed that HLA class I downregulation is related to poorer prognosis and a low level of intratumoral CTLs is associated with platinum resistance, indicating the significance of immunological surveillance.
Keywords: CTL, HLA class I, ovarian cancer, platinum resistance
Abbreviations: CTL, cytotoxic T lymphocyte; HLA, human leukocyte antigen; MHC, major histocompatibility complex; TAA, tumor-associated antigen
Introduction
Immunotherapy is the fourth-line option in cancer therapy following surgery, chemotherapy, and radiotherapy. Cytotoxic T lymphocytes (CTLs), together with other lymphocyte subsets, play an essential role in cancer eradication. CTLs recognize antigenic peptides with amino acid lengths of 8–10 mer derived from tumor-associated antigens (TAAs), which is presented by the major histocompatibility complex (MHC) class I (i.e., the human leukocyte antigen [HLA] class I). Identification of TAAs and antigenic peptides enables cancer-specific immunotherapy using antigenic peptides.1 The clinical outcomes, however, revealed several problems that have to be overcome for achieving efficient cancer immunotherapy. One major problem was tumor escape from immunological surveillance.2 The mechanisms involved include loss or downregulation of antigens, loss or downregulation of human leukocyte antigen (HLA) class I, and active inhibition of immune responses. In this review, we summarize HLA class I expression and CTL infiltration in cases of human ovarian cancer.3
HLA Class I Expression Is a Predictor of Clinical Outcome in Patients With Ovarian Cancer
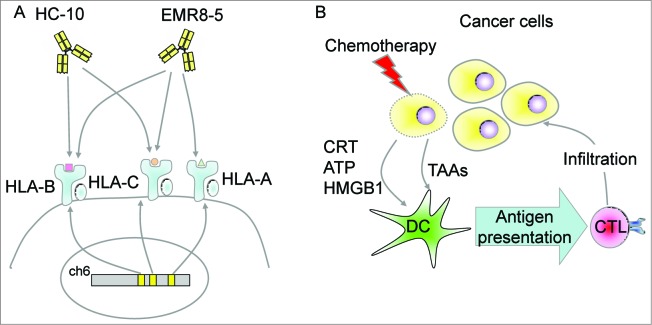
The HLA class I genes are located on chromosome 6p and include HLA-A, -B, and -C. These gene products show extremely high homology with each other. However, the antigenic peptide repertoires that are presented by the HLA class I are different, and each HLA class I gene product is thought to play an important role in total immune responses. In our recent study, we analyzed the expression of the HLA class I in 122 ovarian cancer cases to understand the immunological surveillance of ovarian cancer.4 We used a monoclonal antibody, EMR8–5, that can detect HLA-A, HLA-B, and HLA-C. HLA class I downregulation was related to poorer prognosis in both advanced-stage cases (n = 74) and early-stage cases (n = 48). In several studies, HLA class I expression was analyzed using the monoclonal antibody HC-10, which can detect HLA-B and HLA-C, and the relationship of HLA class I expression with clinical outcome in ovarian cancer cases was examined. Vitale et al. immunostained 51 cases of early-stage ovarian cancer (EOC) using the anti-HLA class I (HC-10) antibody and anti-TAP1 and -2 antibodies, and found that HLA class I downregulation was associated with the disease stage, but not with prognosis.5 Rolland et al. reported a combination staining using HC-10 and β2-microglobulin antibodies for 339 cases of ovarian cancer and suggested that the HC-10+/β2-m+ phenotype is a significant independent prognostic factor; however, there was no correlation between HC-10 positivity and prognosis.6 Han et al. immunostained components of the antigen-processing machinery (APM), including TAP1, TAP2, tapasin, HLA class I (HC-10), β2-microglobulin, CD3+ T cells, and CD8+ T cells.7 They reported that APM component downregulation, lack of intratumoral T-cell infiltrates, and suboptimal cytoreduction were independent prognostic factors in multivariate analysis. In all previous studies, only the HLA class I heavy-chain staining using HC-10 was not an independent prognostic marker of ovarian cancer. CTLs recognize HLA class I molecules including HLA-A, -B, and -C. HC-10 is reactive for HLA-B and -C but not for HLA-A; however, the EMR8–5 is reactive for HLA-A as well as HLA-B and -C. (Fig. 1A). Thus, EMR8–5 might be a better antibody for detection of HLA class I expression as an independent prognostic marker in ovarian cancer. Other types of malignancies should be re-analyzed for HLA class I expression using EMR8–5.
Figure 1.
(A) Monoclonal antibodies specific for HLA Class I. HLA class I genes are located on chromosome 6p and comprise HLA-A, -B, and -C. The monoclonal antibody HC-10 is reactive for HLA-B and -C. The monoclonal antibody EMR8–5 is reactive for HLA-A, -B, and -C. (B) Immunological cell death (ICD) might play a role in chemotherapy sensitivity in human ovarian cancer. Cancer cells undergoing immunologic cell death release calreticulin (CRT), adenosine triphosphate (ATP), and High Mobility Group Box 1 (HMGB1) and activate dendritic cells (DCs) to induce a cancer-specific cytotoxic T lymphocyte (CTL) response.
CD8+ T-Cell Infiltration Is a Predictor of Platinum Sensitivity
In a recent study, we analyzed T-cell infiltration into cancer areas (intratumoral T cells) using anti-CD3, CD4, CD8, and FOXP3 antibodies. CD4+ T-cell infiltration was related to better prognosis in total cases (n = 122), and CD8+ T-cell infiltration was related to better prognosis only in advanced cases. CD3+ T-cell and FOXP3+ cell infiltrations were not related to the prognosis. However, a high CD8+ T cell/FOXP3+ T regulatory (Treg) cell ratio was associated with better prognosis. This result is consistent with a previous report.8 Platinum reagents are key drugs in chemotherapy for ovarian cancer, and platinum resistance is critical for prognosis. Strikingly, our multivariate analysis revealed that a high rate of CD8+ T-cell infiltration was associated with platinum sensitivity. A previous study showed that HLA class I expression detected by HC-10 was associated with platinum sensitivity in ovarian cancer.9 These observations strongly suggest a relationship between chemotherapeutic sensitivity and immunological surveillance. Although the mechanisms are still elusive, immunologic cell death (ICD) might be a dominant explanation. Cancer cells undergoing destruction by chemotherapy release calreticulin, adenosine triphosphate (ATP), and High Mobility Group Box 1 (HMGB1) into the microenvironment and induce immunological response by activating dendritic cells (Fig. 1B).10 The ICD is a well-described phenomenon in mice experimental systems, and recent studies using human tumor samples support the significance of the concept in human cancers.
Conclusion
Our recent study revealed that detection of the HLA class I by the monoclonal antibody EMR8–5 is a good prognostic marker in ovarian cancer and CD8+ T-cell infiltration is a predictor of the outcome of treatment with platinum agents. These observations strongly suggest that immunological surveillance can facilitate control of cancer progression, prognosis, and treatment sensitivity. However, the associations of other types of cells including myeloid-derived suppressor cells (MDSCs), M2 macrophages, and helper T cells (Th1, Th2, and Th17), the dominant antigenic peptide that is recognized by CTLs, and the expression of immune checkpoints (PD-L1 and CTLA-4) have not been characterized as yet. Further analysis should lead to an understanding of the complex immunological surveillance of human cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Noriyuki Sato, Toshihiko Torigoe, and Yoshihiko Hirohashi), the program for developing the supporting system for upgrading education and research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Noriyuki Sato), the Takeda Science Foundation (to Yoshihiko Hirohashi), the Sagawa Foundation for Promotion of Cancer Research (to Yoshihiko Hirohashi), Suharakinennzaidann Co., Ltd. (to Yoshihiko Hirohashi), and the Kobayashi Foundation for Cancer Research (to Yoshihiko Hirohashi).
References
- 1.Aranda F, Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: peptide vaccines in cancer therapy. Oncoimmunology 2013; 2(12):e26621; PMID:; http://dx.doi.org/ 10.4161/onci.26621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol 2000; 74:181-273; PMID:; http://dx.doi.org/ 10.1016/S0065-2776(08)60911-6 [DOI] [PubMed] [Google Scholar]
- 3.Aptsiauri N, Cabrera T, Garcia-Lora A, Lopez-Nevot MA, Ruiz-Cabello F, Garrido F. MHC class I antigens and immune surveillance in transformed cells. Int Rev Cytol 2007; 256:139-89; PMID:; http://dx.doi.org/ 10.1016/S0074-7696(07)56005-5 [DOI] [PubMed] [Google Scholar]
- 4.Mariya T, Hirohashi Y, Torigoe T, Asano T, Kuroda T, Yasuda K, Mizuuchi M, Sonoda T, Saito T, Sato N. Prognostic impact of human leukocyte antigen class I expression and association of platinum resistance with immunologic profiles in epithelial ovarian cancer. Cancer Immunol Res 2014; 2(12):1220-9; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0101 [DOI] [PubMed] [Google Scholar]
- 5.Vitale M, Pelusi G, Taroni B, Gobbi G, Micheloni C, Rezzani R, Donato F, Wang X, Ferrone S. HLA class I antigen down-regulation in primary ovary carcinoma lesions: association with disease stage. Clin Cancer Res 2005; 11(1):67-72; PMID: [PubMed] [Google Scholar]
- 6.Rolland P, Deen S, Scott I, Durrant L, Spendlove I. Human leukocyte antigen class I antigen expression is an independent prognostic factor in ovarian cancer. Clin Cancer Res 2007; 13(12):3591-6; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2087 [DOI] [PubMed] [Google Scholar]
- 7.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT, et al.. HLA class I antigen processing machinery component expression and intratumoral T-cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res 2008; 14(11):3372-9; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al.. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102(51):18538-43; PMID:; http://dx.doi.org/ 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shehata M, Mukherjee A, Deen S, Al-Attar A, Durrant LG, Chan S. Human leukocyte antigen class I expression is an independent prognostic factor in advanced ovarian cancer resistant to first-line platinum chemotherapy. Br J Cancer 2009; 101(8):1321-8; PMID:; http://dx.doi.org/ 10.1038/sj.bjc.6605315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39(1):74-88; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]