Abstract
We have previously reported a novel phenotype of myeloid suppressors in lymphoma patients characterized by a loss of HLA-DR expression on monocytes, CD14+HLA-DRlow/neg. These cells were directly immunosuppressive and were associated with poor clinical outcome. In this study, we found that lymphoma tumors could have more than 30% of their tumor occupied by CD14+ cells. This intimate spatial connection suggested substantial cell–cell communication. We examined cross talk between monocytes from healthy volunteers (normal) and lymphoma cells in co-culture to identify the mechanisms and consequences of these interactions. Normal CD14+HLA-DR+ monocytes lost their HLA-DR expression after co-culture with lymphoma cells. Lymphoma-converted CD14+HLA-DRlow/neg cells exhibited similar immunosuppressive functions as CD14+HLA-DRlow/neg monocytes from lymphoma patients. Unexpectedly monocyte additions to lymphoma cell cultures protected lymphoma from cytotoxic killing by chemotherapy drug doxorubicin (DOX). Monocyte mediated resistance to DOX killing was associated with decreased Caspase-3 activity and increased anti-apoptotic heat shock protein-27 (Hsp27) expression. Soluble Hsp27 was detected in supernatant and patient plasma. Increased Hsp27 in plasma correlated with increased proportion of CD14+HLA-DRlow/neg monocytes in patient blood and was associated with lack of clinical response to DOX. This is the first report to describe a non-immune function of CD14+HLA-DRlow/neg monocytes: enhanced lymphoma resistance to chemotherapy. It is also the first report in lymphoma of Hsp27 as a potential mediator of lymphoma and monocyte crosstalk and chemotherapy resistance. Together with previous reports of the prevalence of these myeloid suppressors in other cancers, our findings identify this pathway and these interactions as a potential novel therapeutic target.
Keywords: chemoresistance, heat shock protein, lymphoma, monocyte, myeloid derived suppressor cells
Introduction
Non-Hodgkin lymphoma (NHL) is the seventh most common type of cancer and one of the top 10 causes of cancer deaths in United States (seer.cancer.gov). Aggressive B-cell NHL such as diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) have high relapse and refractory rates after first-line therapy with even poorer response to salvage treatments.1,2 Alteration of host immunity is an integral part of lymphoma pathogenesis.3,4 Previously, we have reported a population of immunosuppressive CD14+HLA-DRlow/neg monocytes as the major phenotype of myeloid suppressor cells in lymphoma.5 These cells exist as a major leukocyte component in blood and are phenotypically distinct from other myeloid-derived suppressor cells (MDSC) which are CD14neg 6,7 (Fig. S1). Similar to studies in other cancer types,8-12 we found that increased presence of CD14+HLA-DRlow/neg monocytes in lymphoma correlated with increased disease burden, worse treatment response and decreased survival.5,13 In addition, decreased presence of these cells is associated with improved clinical response to treatments.12,14
In this study, we examined lymphoma and monocyte crosstalk that promote lymphoma survival. Using mouse models to mimic our previous findings is difficult. We found these suppressive monocytes by characterizing the blood of humans and by using CD14 as the key identifiable marker. There is no definitive equivalent mouse population of these cells, as mouse MDSC are defined by CD11b and Gr-1, among other markers6,15 (Fig. S1). Therefore, we focused our experiments on developing a human model of this interaction using an in vitro model consisting of primary human monocytes and human lymphoma cell lines. We found that normal monocytes were converted to CD14+HLA-DRlow/neg by co-incubation with lymphoma cells. More interestingly, we found that, beyond the immune suppression, these cells enable lymphoma resistance to cytotoxic killing from DOX, one of the main chemotherapy drugs in front-line treatment for NHL. Our data provide evidence that chemoresistance is mediated by anti-apoptotic mechanisms including heat shock protein (Hsp27). To our knowledge, this is the first report demonstrating CD14+HLA-DRlow/neg monocytic myeloid suppressor cells induced chemoresistance via direct promotion of anti-apoptotic and immune independent mechanisms.
Results
Lymphoma cells convert monocytes to an immune suppressive phenotype
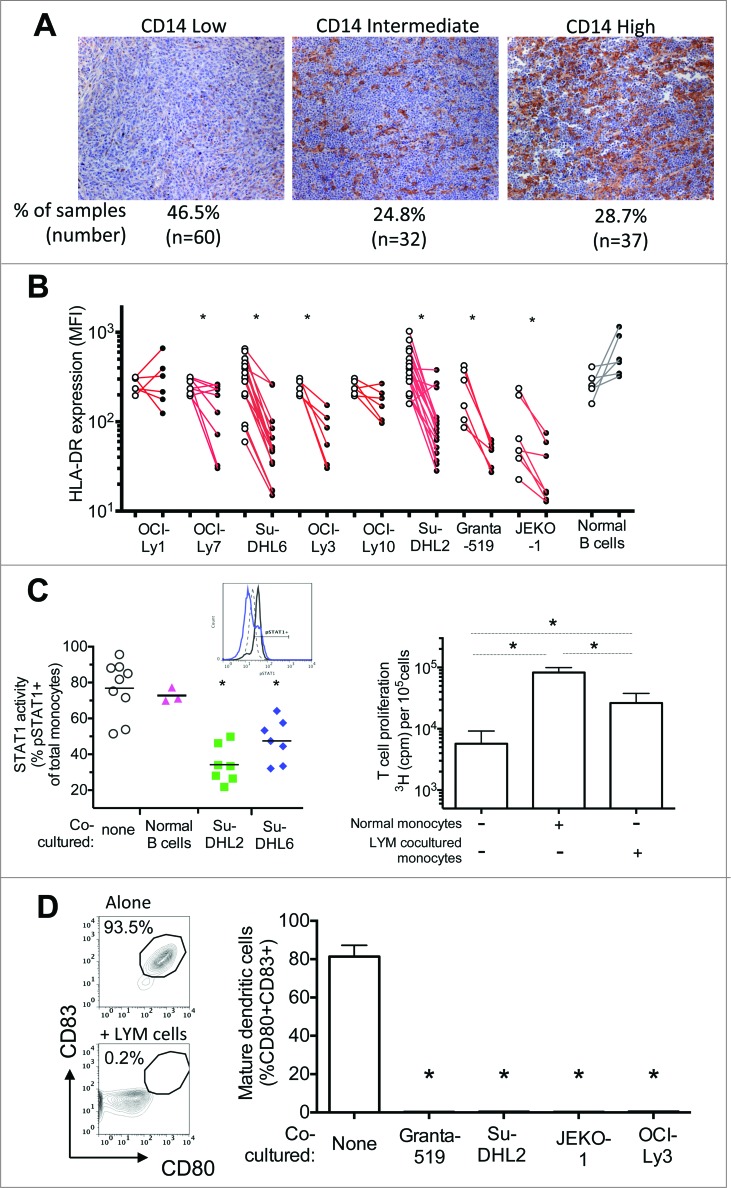
We, and others, have reported on increased proportion of CD14+HLA-DRlow/neg monocytes in a number of malignancies including lymphoma and the association of these cells with suppressed systemic immunity and poor clinical outcome.5,8-10,12,14 We hypothesized that CD14+HLA-DRlow/neg monocytes adopt this phenotype through tumor-mediated factors. We found that lymphoma tumors are frequently heavily infiltrated with CD14+ cells (Fig. 1A). Of the 129 DLBCL samples examined, 53.5% (n = 69) have more than 10% infiltration of CD14+ cells, and 28.7% (n = 37) of the tumors have more than 30% infiltration of these cells. In this study, we co-cultured monocytes from healthy donors with lymphoma cell lines or normal B cells as controls. Six of the eight lymphoma cell lines tested, but not the normal B cells, were capable of inducing HLA-DR loss (Fig. 1B). The reduction in HLA-DR expression on monocytes did not correlate with culture supernatant concentration of IL-10, a known mediator of HLA-DR expression (Fig. S2), suggesting that other lymphoma-derived factors mediate this effect. This was consistent with our previous report of a lack of correlation between this monocyte phenotype with many cytokines known to affect HLA-DR expression, such as VEGF, IL-6 and IL-10.5 In addition, similar to the immunosuppressive functions of the CD14+HLA-DRlow/neg monocytes isolated directly from lymphoma patients, these lymphoma-converted CD14+HLA-DRlo/neg cells had decreased STAT1 phosphorylation to IFNγ stimulation, decreased stimulation of T cell recall response to Influenza vaccine, and decreased capacity to differentiate into mature dendritic cells (Fig. 1C–D, Fig. S3).
Figure 1.
Lymphoma (LYM) cells convert normal monocytes to an immune suppressive phenotype. (A) Lymphoma tumors can have high numbers of CD14+ cells. Representative lymphoma tumors with low, intermediate, or high CD14+ cells are shown (CD14, brown). (B) Co-culture with lymphoma cells, but not normal B cells, induced decreased HLA-DR expression on normal CD14+HLA-DR+ monocytes. Paired data is shown for HLA-DR expression (mean fluorescent intensity, MFI) on monocytes from individual healthy control donors cultured either in media alone or in co-culture with designated cell. (C) LYM-converted CD14+HLA-DRlow/neg monocytes have decreased capacity for immune stimulation. Compared to normal CD14+HLA-DR+ monocytes, LYM-converted CD14+HLA-DRlow/neg monocytes have decreased signaling response of STAT1 phosphorylation to IFNγ stimulation (Left panel). Representative histogram overlay of intracellular phosphorylated STAT1 expression is shown (Left panel inset: dotted line, normal monocytes without IFNγ stimulation; black line, normal monocytes with IFNγ stimulation; blue line: monocytes co-cultured with Su-DHL6 and stimulated with IFNγ). LYM-converted CD14+HLA-DRlow/neg monocytes also have decreased capacity to stimulate T cell recall response to Influenza (Right panel, n = 4). (D) LYM-converted CD14+HLA-DRlow/neg monocytes have decreased capacity to differentiate into mature dendritic cells. Representative dot plots for mature dendritic cell phenotype (CD80+CD83+) is shown for differentiation of monocytes under designated conditions (left panel, LYM = Granta-519). Quantitative analysis of mature dendritic cells purity for designated monocyte conditions is shown in the right panel (mean ± SD, n = 6). * p < 0.05.
Co-culture with monocytes protected lymphoma cells from cytotoxic killing of doxorubicin
Having shown that tumors alter monocyte phenotype, we hypothesized that monocytes were potentially altering the nature of the tumors. The sheer numbers of monocytes in the tumors suggested that they may effect antitumor therapy. Therefore, we examined lymphoma susceptibility to cytotoxic killing using one of the main backbone drugs in the lymphoma chemotherapy regimen: doxorubicin, DOX. Lymphoma cells alone or co-cultured with monocytes were exposed to DOX and their viability was measured by flow cytometry for Annexin-V and 7-AAD. Compared to lymphoma cells alone treated with DOX, cells co-cultured with monocytes and DOX had improved viability and total cell recovery (Fig. 2). Improved lymphoma survival was not a general property of leukocytes as co-culturing with similar numbers of T cells did not exhibit the same effect (Fig. S4).
Figure 2.
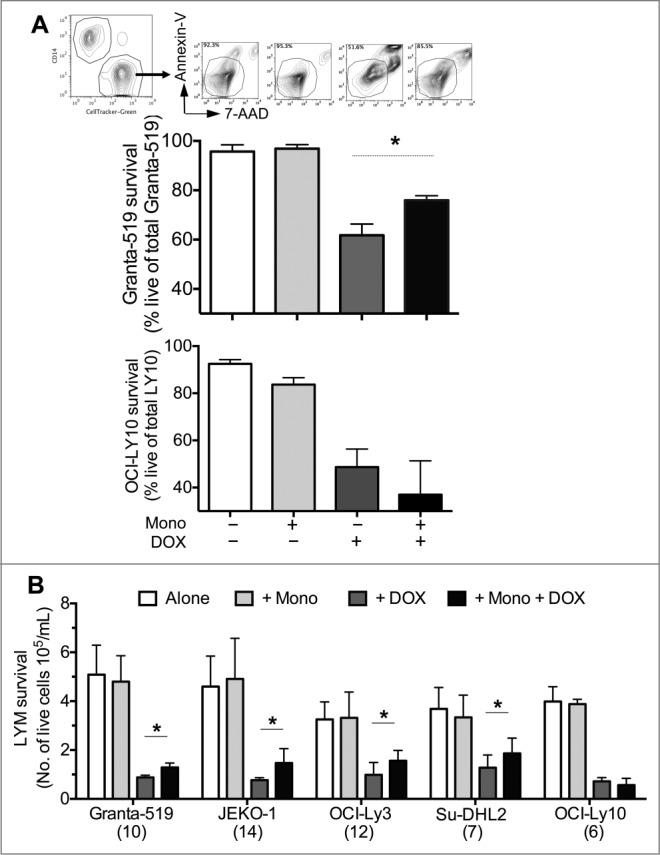
CD14+HLA-DRlow/neg monocytes promote lymphoma cell survival from chemotherapy. (A) Compared to LYM cultured alone with doxorubicin (DOX), co-culture with monocytes increased the viability of LYM cells. Quantitative analysis of live LYM cells for designated conditions are shown for Granta-519 (n = 10) and OCI-Ly10 (n = 6; mean ± SD). Representative dot plots are shown above each condition for Granta-519. (B) Co-culturing with CD14+HLA-DRlow/neg monocytes in the presence of DOX increased the number of live LYM cells compared to LYM alone. Quantitative analysis of number of live LYM cells under designated culture conditions are shown for 5 LYM cell lines (mean ± SD, n under each cell lines). OCI-Ly10, which did not induce decreased HLA-DR expression on monocytes, did not have increased survival from DOX. *p < 0.05.
Monocyte-mediated reduction in doxorubicin cytotoxicity was associated with decreased apoptosis and increased HSP27
DOX killing by inducing apoptosis is well documented.16,17 We identified an increase in cleaved Caspases-3 without an increase in total pro-Caspase-3 protein levels in lymphoma cells, indicating DOX-induced apoptosis. We confirmed DOX-induced Caspase-3 cleavage by intracellular flow cytometry. Treatment with DOX induced increased intracellular cleaved Caspase-3 protein that corresponded to decreased lymphoma cell viability and recovery. Lymphoma cells treated identically, but with added monocytes had less intracellular cleaved Caspase-3 proteins and a decreased ratio of cleaved to total Caspase-3 proteins (Fig. 3). This cytoprotective effect was confirmed with multiple lymphoma cell lines (Fig. 3). Interestingly, lymphoma cell line OCI-Ly1 and OCI-Ly10, which did not induce HLA-DR decrease in monocytes, remained sensitive to DOX killing when co-cultured with monocytes as evidenced by no increase in cell count recovery (Fig. 2B, Fig. S5) and no decrease in cleaved Caspase-3 proteins (Fig. 3D, Fig. S5). Thus, cross-talk by which lymphoma cells induce phenotype changes in monocytes also allows monocytes to mediate drug resistance in lymphoma.
Figure 3.
Improved LYM survival from DOX by monocyte co-culture correlated with decreased LYM apoptosis. (A) Representative fluorescent and light microscopy images are shown for monocyte and LYM co-culture (left two panels. LYM cells are labeled with CD20-PE. Monocytes are labeled by arrows.) Compared to LYM alone with DOX, LYM co-cultured with monocytes has decreased active, cleaved Caspase-3. Representative fluorescent microscopy images are shown for DOX-treated LYM alone and LYM co-cultured with monocytes (right 2 panels, red = CD20, green = cleaved Caspase-3). LYM = Granta-519. Scale bar = 50 μm. (B) Apoptosis activity (ratio of cleaved to pro-Caspase-3 measured by immunoblot) are shown for LYM cells in the designated culture conditions (mean ± SD, n = 3, Granta-519). Representative images of the immunoblot are shown above each condition. (C) Immunoblot results are confirmed by flow cytometry analysis for LYM intracellular cleaved Caspase-3 expression. Representative histograms of LYM (Granta-519) intracellular cleaved Caspase-3 expression are shown for each culture condition (shaded region = isotype control; line = cleaved Caspase-3). (D) Quantitative analysis of intracellular cleaved Caspase-3 expression by flow cytometry is shown for 4 LYM cell lines (mean ± SD, n under designated cell lines). Consistent with LYM survival, LYM co-cultured with CD14+HLA-DRlow/neg monocytes had less cleaved Caspase-3 expression compared to LYM cultured alone with DOX. This decreased apoptosis activity was not seen in OCI-Ly10 cells that did not induce a HLA-DR decrease in co-cultured monocytes. * p < 0.05.
A review of an array of apoptosis regulating proteins by immunoblots revealed heat shock protein 27 (Hsp27) as one of the proteins whose changes in expression correlated with changes in cleaved Caspase-3 levels and lymphoma cell viability (Fig. 4A). Interestingly, even without DOX treatment, contact with monocytes increased Hsp27 expression in lymphoma cells, although the magnitude of the increase is more pronounced following DOX (Fig. 4B). This indicates that the intracellular increase of Hsp27 in lymphoma cells is specifically mediated by monocytes and can be adapted in response to stress. Interestingly, OCI-Ly1 and OCI-Ly10, which did not induce HLA-DR decrease in monocytes and remained sensitive to DOX killing by apoptosis, did not have significant baseline Hsp27 expression and had minimal increase with DOX treatment and no increase from co-culture with monocytes (Fig. 4B, Fig. S5). This suggests that this monocyte-mediated effect may be predominantly with HLA-DRlow/neg cells.
Figure 4.
Heat-shock protein 27 (Hsp27) is associated with LYM resistance to DOX and decreased HLA-DR expression on monocytes. (A) LYM co-cultured with monocytes with and without DOX have increased intracellular Hsp27 expression. Quantitative analysis of LYM (Granta-519) intracellular Hsp27 expression by immunoblot is shown (mean ± SD, n = 3). Representative immunoblot images are shown above each condition. (B) Quantitative analysis of LYM intracellular Hsp27 expression by flow cytometry is shown for OCI-Ly3 (n = 4) and OCI-Ly10 (n = 6; mean ± SD). Representative histogram of OCI-Ly3 intracellular Hsp27 expression are shown above the corresponding condition (shaded = isotype control; line = Hsp27). (C) Hsp27 can directly induce decreased HLA-DR expression on monocytes in a dose-dependent manner (left panel, n = 4). LYM cells can secrete Hsp27. Supernatants from LYM cells and monocyte co-culture with and without DOX have significantly higher soluble Hsp27 compared to media (right panel, white bars, left axis). Culture supernatant from LYM co-culture high in Hsp27, but not media or supernatant from normal B cell co-culture, induced decreased HLA-DR expression on normal CD14+HLA-DR+ monocytes (right panel, black bars, right axis: LYM = Granta-519; n = 6; * p < 0.05 compared to control media; Δ p < 0.05 compared to normal B cell and monocyte co-culture supernatant.) (D) More importantly, Hsp27 is detectable in plasma of patients with lymphoma. Increased plasma level of Hsp27 correlated with increased percentage of CD14+HLA-DRlow/neg monocytes in blood (left panel). Patients who were refractory to DOX treatments had higher plasma Hsp27 levels than those who responded to treatments (right panel).
Monocyte decreased HLA-DR expression is associated with increased soluble Hsp27
Hsp27 is known to suppress immunity by inhibiting monocyte stimulation of T cells and differentiation to dendritic cell, polarizing monocyte differentiation to immunosuppressive M2 macrophage, and altering monocytes’ cytokine production to an increase in IL-10 and decrease in TNFα.18-20 In breast cancer, tumor cells secrete Hsp27 and the secreted, soluble Hsp27 induced decreased HLA-DR expression on CD14+ monocytes.21 We found that addition of recombinant human Hsp27 to monocytes directly induced a decrease in monocyte HLA-DR expression in a dose-dependent manner (Fig. 4C). We found that lymphoma cells were also capable of secreting Hsp27. Lymphoma and monocyte co-culture supernatants had increased soluble Hsp27 (Fig. 4C). Normal CD14+HLA-DR+ monocytes cultured in supernatant with higher soluble Hsp27 had a bigger decrease in HLA-DR expression. This effect was not seen with control media nor supernatant from co-culture with B cells (Fig. 4C). We measured Hsp27 in the plasma of 36 lymphoma patients and found that increased plasma Hsp27 correlated with increased proportion of CD14+HLA-DRlow/neg monocytes (Fig. 4D). Plasma Hsp27 has been correlated with treatment resistance and decreased overall survival in patients with breast cancer.22,23 In our lymphoma cohort, 22 patients had DOX-containing chemotherapy prior to blood sample. Those who were refractory to DOX treatment, as defined by Cheson criteria,24,25, had higher levels of plasma Hsp27 than those who responded to DOX treatments (non-responders: 1049 ± 169 pg/mL, n = 8; responders: 411 ± 70 pg/mL, n = 14; p = 0.0006). This data suggests further study in lymphoma patients to determine the prognostic value of intra-tumor Hsp27 and CD14 cells to predict treatment resistance.
Discussion
We report a novel, non-immune crosstalk between monocytic myeloid suppressor CD14+HLA-DRlow/neg cells and lymphoma that directly promote resistance to chemotherapy. This decrease in lymphoma apoptosis is associated with at least one factor: increased Hsp27. Hsp27 expression de novo has been found in Reed–Sternberg cells of Hodgkin lymphoma.26 Potential association with intra-tumor CD14 was not examined in that report, but could be considered for further study given the known high infiltration of immune cells in the Hodgkin tumors. In a small number of NHL tumors examined in the same report, Hsp27 expression was not identified.26 Interestingly, as Hsp27 expression has been shown to increase in response to treatment,22 we have found increased plasma levels of Hsp27 in lymphoma patients who were refractory to DOX treatments. More definitive study is needed to examine primary NHL tumors for Hsp27 expression and association with intra-tumor CD14 cells.
Even though the importance of tumor microenvironment in lymphoma is well recognized, the prognostic value of stromal immune phenotype remains to be defined and require better understanding of its biologic functions. For example, while the gene signature of non-malignant cells in DLBCL indicates that a myeloid-rich signature is a positive prognostic factor,27 the total number of intra-tumor macrophages did not correlate with prognosis.28 However, evaluation of macrophage phenotype indicated that M2 (CD163+) macrophage predict worse prognosis.29 These studies highlight the heterogeneity of myeloid cell functions. Identification of the phenotype of myeloid cells that predict poor clinical outcome is crucial for use of these cells as predictive biomarker in personalized therapy and for development of targeted therapies to improve lymphoma treatments. Our data suggest that increased intra-tumor CD14 cells may promote lymphoma resistance to chemotherapy.
Hsp27 has been found to mediate anti-apoptotic, pro-survival signaling and resistance to treatments with cisplatin30 and radiation31 in solid tumors and bortezomib in lymphoma.26,32 Blockade of Hsp27 reversed this treatment resistance effect. This is the first report of increased expression of Hsp27 in lymphoma in association with any immune cell. Our data suggest that Hsp27 inhibition may have clinical benefit in lymphoma by re-sensitizing tumors to chemotherapy and improving patient immunity. Preventing CD14+ cell exposure to tumors by inhibiting monocyte trafficking or functions could be another strategy to improve treatment response by interfering with the monocyte lymphoma crosstalk.
In summary, we demonstrate that lymphoma cells and CD14+ monocytes participate in a bi-directional cross talk. Lymphoma cells directly convert normal CD14+HLA-DR+ monocytes to CD14+HLA-DRlow/neg cells that are known major mediators of systemic immune suppression. In addition, these monocytes directly protect lymphoma cells from killing by chemotherapy. We have identified Hsp27 as a potential mediator of both chemotherapy resistance and immune modulation. To the best of our knowledge, this is the first report of CD14+ derived myeloid cells directly facilitating chemotherapy resistance via Hsp27 signaling. Taken together, these findings suggest increased presence of CD14+HLA-DRlow/neg monocytes may be a predictive biomarker of treatment resistance and used to facilitate targeted therapy to improve treatment response. Future studies in the mechanisms regulating lymphoma/monocyte crosstalk can reveal novel strategies in the treatment of this disease. In addition, as these monocytes have been found in a number of cancer types, understanding the mechanism of this monocyte-mediated treatment resistance and identification of novel targeted therapy can have broad implications.
Materials and Methods
Study subjects
This study was approved by the Mayo Clinic Institutional Review Board. All patients provided signed informed consent to provide blood and or tumor samples and to review medical records for research purposes. Samples were collected from patients with biopsy-proven B-cell NHL who had not received treatments for at least 8 weeks prior to sample collection. Blood phenotypes were analyzed by flow cytometry as previously described.5
Tissue immunohistochemistry
Lymphoma tumor tissue microarray was stained by immunohistochemistry with anti-human CD14 rabbit monoclonal antibody (clone EPR3653, Cell Marque, Rocklin, CA). Tumors were graded low, intermediate, and high based on number of CD14+ cells, with low containing less than 10%, intermediate with 10–30%, and high with more than 30% CD14+ cells.
Cell isolation and culture
Monocytes, T cells, and B cells were isolated from peripheral blood of healthy controls by density gradient centrifugation and immunomagnetic bead selection as previously reported.5 All selected cells were found to be more than 95% pure by flow cytometry analysis.
DLBCL cell lines Su-DHL2, Su-DHL6, OCI-Ly1, OCI-Ly7, OCI-Ly3, OCI-Ly10, and MCL cell lines JEKO-1 and Granta-519 cells were gifts from Dr. Mamta Gupta and Dr. Thomas Witzig (Mayo Clinic, Rochester, MN) and maintained in culture as previously described (33,Table S2).
For lymphoma and monocyte co-cultures, lymphoma cell lines were either labeled with CellTracker-green (Molecular Probes, Eugene OR) per manufacturers’ protocol or identified by CD20 expression via flow cytometry. Co-culture experiments were conducted in either CellGenix or RPMI-1640 media (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum. Monocyte alone cultures were supplemented with GM-CSF (2800 U/mL, SANOFI, Bridgewater, NJ) with or without IL-4 (1000 U/mL). When specified, stock solution of DOX (SelleckChem, Houston, TX) in DMSO was diluted into desired concentrations with culture medium.
For culture with recombinant human Hsp27 (R&D Systems) at specified concentration, monocytes were cultured in RPMI-1640 media with 10% fetal bovine serum and M-CSF (10 ng/mL) for 3 d.
Dendritic cell differentiation
To differentiate monocytes into dendritic cells, TNFα (1100 U/mL) and PGE2 (1 μg/mL) were added after 3 d of culture for additional 2 d as previously described.5,8
Cell viability and phenotype
To assess cell viability, cultured cells were collected without further isolation, immediately stained with Annexin-V, 7-AAD, monoclonal antibodies (mAb) and analyzed by flow cytometry. Flow events over one minute interval were used to count cells.
To assess monocyte phenotype, cultured cells were collected as described above and stained immediately with specified mAb and analyzed by flow cytometry. See Table S1 for list of reagents. Data were acquired on a BD FACSCalibur flow cytometer (BD Bioscience) and analyzed with FlowJo v7.6 software (TreeStar, Ashland, OR).
Intracellular protein expression assays
For monocyte intracellular expression of phosphorylated STAT1 (pSTAT1), cells are stimulated with interferon-γ and analyzed as previously described.5 For flow cytometry assessment of lymphoma intracellular expression of cleaved Caspase-3 and Hsp27, cultured cells were collected without further isolation, stained for surface phenotype to distinguish cell populations, permeabilized, and stained with antibodies for specified intracellular proteins following manufacturing protocol (BD Bioscience).
Flu recall response
T cells from healthy controls (105 cells/well) were cultured in 96-well round-bottom plates (Corning Life Sciences) in RPMI-1640 with 10% fetal bovine serum (Mediatech) with or without monocytes at 10:1 cell ratio and stimulated with Fluzone vaccine (0.5 μL/well, 2013-2014 season, Sanofi-Pasteur) for 72 h. Titrated thymidine was added 18 h before collection to assess T cell proliferation.
Apoptosis protein immunoblot
Cell lysate was generated from immunomagnetic bead-isolated lymphoma cells after culture and assayed for apoptotic pathway protein expression by immunoblot per manufacturer protocol (Proteome Profiler® human apoptosis array, R&D Systems). Immunoblot membranes were imaged by ChemiDoc MP (Bio-Rad Laboratories) and integrated pixel intensity of the protein expressions were analyzed using ImageJ software. Pixel intensities were normalized over negative reference spots and per 500μg protein per membrane.
ELISA
IL-10 concentrations in culture supernatant and Hsp27 levels in human plasma were analyzed by ELISA per manufacturer's protocol (R&D Systems).
Fluorescent microscopy
Cells stained with surface and intracellular antibodies as described above were placed on glass slides and imaged using OLYMPUS DP71 fluorescent CCD digital camera (Tokyo, Japan) with UPLanFL 20X or 40X lens (Tokyo, Japan). Images were captured by DP Controller software (v.3.2.1.276) and analyzed to create two-channel overlap images with ImageJ software.
Statistical analysis
Values between groups of data were tested for statistical significance using the two-tailed Student's t test; paired two-tailed Student's t test was used for paired samples. Correlation analysis was performed using Pearson correlation with the coefficient of correlation (r2) calculated using Prism v6.0 (GraphPad software). Statistically significance was set at p < 0.05.
Acknowledgments
We thank Casey N. Aitken and Julianne Lunde for their administrative assistance with this study. Most importantly, we thank the patients for their participation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Funding
This work was supported by Mayo Clinic Cancer Center Paul Calebresi Program in Clinical-Translational Research, NCI CA90628, Henry J Predolin Foundation, Center for Individualized Medicine, and the University of Iowa/Mayo Clinic Lymphoma SPORE.
Supplemental Material
Supplemental data for this article can be accessed on http://dx.doi.org/10.1080/2162402X.2014.996470 the publisher's website.
References
- 1. Habermann TM. New developments in the management of diffuse large B-cell lymphoma. Hematology 2012; 17 Suppl 1:S93-7; PMID:; http://dx.doi.org/ 10.1179/102453312X13336169156014. [DOI] [PubMed] [Google Scholar]
- 2. Zaja F, Federico M, Vitolo U, Zinzani PL. Management of relapsed/refractory mantle cell lymphoma: a review of current therapeutic strategies. Leuk Lymphoma 2014; 55:988-98; PMID:; http://dx.doi.org/ 10.3109/10428194.2013.825903 [DOI] [PubMed] [Google Scholar]
- 3. Rachinel N, Salles G. The host-tumor interface in B-cell non-Hodgkin lymphoma: a new world to investigate. Curr Hematol Malig Rep 2009; 4:196-201; PMID:; http://dx.doi.org/ 10.1007/s11899-009-0026-1 [DOI] [PubMed] [Google Scholar]
- 4. Nogai H, Dorken B, Lenz G. Pathogenesis of non-Hodgkin's lymphoma. J Clin Oncol 2011; 29:1803-11; PMID:; http://dx.doi.org/ 10.1200/JCO.2010.33.3252 [DOI] [PubMed] [Google Scholar]
- 5. Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood 2011; 117:872-81; PMID:; http://dx.doi.org/ 10.1182/blood-2010-05-283820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer 2013; 13:739-52; PMID:; http://dx.doi.org/ 10.1038/nrc3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunol Res 2013; 57:172-84; PMID:; http://dx.doi.org/ 10.1007/s12026-013-8455-2 [DOI] [PubMed] [Google Scholar]
- 8. Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol 2010; 12:631-44; PMID:; http://dx.doi.org/ 10.1093/neuonc/noq001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate 2010; 70:443-55; PMID:; http://dx.doi.org/ 10.1002/pros.21078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 2013; 62:1421-30; PMID:; http://dx.doi.org/ 10.1007/s00262-013-1447-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudolph BM, Loquai C, Gerwe A, Bacher N, Steinbrink K, Grabbe S, Tuettenberg A. Increased frequencies of CD11b(+) CD33(+) CD14(+) HLA-DR(low) myeloid-derived suppressor cells are an early event in melanoma patients. Exp Dermatol 2014; 23:202-4; PMID:; http://dx.doi.org/ 10.1111/exd.12336 [DOI] [PubMed] [Google Scholar]
- 12. Gustafson MP, Abraham RS, Lin Y, Wu W, Gastineau DA, Zent CS, Dietz AB. Association of an increased frequency of CD14+ HLA-DR lo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br J Haematol 2012; 156:674-6; PMID:; http://dx.doi.org/ 10.1111/j.1365-2141.2011.08902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gustafson MP, Lin Y, LaPlant B, Liwski CJ, Maas ML, League SC, Bauer PR, Abraham RS, Tollefson MK, Kwon ED, et al. Immune monitoring using the predictive power of immune profiles. J Immunother Cancer 2013; 1:7; PMID:; http://dx.doi.org/ 10.1186/2051-1426-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khalifa KA, Badawy HM, Radwan WM, Shehata MA, Bassuoni MA. CD14 HLA-DR low/ monocytes as indicator of disease aggressiveness in B-cell non-Hodgkin lymphoma. Int J Lab Hematol 2014; 36:350-55; PMID:; http://dx.doi.org/ 10.1111/ijlh.12203 [DOI] [PubMed] [Google Scholar]
- 15. Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 2010; 40:22-35; PMID:; http://dx.doi.org/ 10.1002/eji.200939903 [DOI] [PubMed] [Google Scholar]
- 16. Drakos E, Rassidakis GZ, Lai R, Herling M, O'Connor SL, Schmitt-Graeff A, McDonnell TJ, Medeiros LJ. Caspase-3 activation in systemic anaplastic large-cell lymphoma. Mod Pathol 2004; 17:109-16; PMID:; http://dx.doi.org/ 10.1038/modpathol.3800039 [DOI] [PubMed] [Google Scholar]
- 17. Eischen CM, Kottke TJ, Martins LM, Basi GS, Tung JS, Earnshaw WC, Leibson PJ, Kaufmann SH. Comparison of apoptosis in wild-type and Fas-resistant cells: chemotherapy-induced apoptosis is not dependent on Fas/Fas ligand interactions. Blood 1997; 90: 935-43; PMID:; http://dx.doi.org/0006-4971/97/9003-0142 [PubMed] [Google Scholar]
- 18. De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol 2000; 165:3951-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.165.7.3951 [DOI] [PubMed] [Google Scholar]
- 19. Kaiser F, Steptoe A, Thompson S, Henderson B. Monocyte cytokine synthesis in response to extracellular cell stress proteins suggests these proteins exhibit network behaviour. Cell Stress Chaperones 2014; 19:135-44; PMID:; http://dx.doi.org/ 10.1007/s12192-013-0440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laudanski K, De A, Miller-Graziano C. Exogenous heat shock protein 27 uniquely blocks differentiation of monocytes to dendritic cells. Eur J Immunol 2007; 37:2812-24; PMID:; http://dx.doi.org/ 10.1002/eji.200636993 [DOI] [PubMed] [Google Scholar]
- 21. Banerjee S, Lin CF, Skinner KA, Schiffhauer LM, Peacock J, Hicks DG, Redmond EM, Morrow D, Huston A, Shayne M, et al. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res 2011; 71:318-27; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-1778 [DOI] [PubMed] [Google Scholar]
- 22. Ciocca DR, Vargas-Roig LM. Hsp27 as a prognostic and predictive factor in cancer. Prog Mol Subcell Biol 2002; 28:205-18; PMID:; http://dx.doi.org/ 10.1007/978-3-642-56348-5_11 [DOI] [PubMed] [Google Scholar]
- 23. Thanner F, Sutterlin MW, Kapp M, Rieger L, Morr AK, Kristen P, Dietl J, Gassel AM, Müller T. Heat shock protein 27 is associated with decreased survival in node-negative breast cancer patients. Anticancer Res 2005; 25:1649-53; PMID: [PubMed] [Google Scholar]
- 24. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol 2014; 32:3059-68; PMID:; http://dx.doi.org/ 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25:579-86; PMID:; http://dx.doi.org/ 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 26. Fuchs D, Berges C, Opelz G, Daniel V, Naujokat C. Increased expression and altered subunit composition of proteasomes induced by continuous proteasome inhibition establish apoptosis resistance and hyperproliferation of Burkitt lymphoma cells. J Cell Biochem 2008; 103:270-83; PMID:; http://dx.doi.org/ 10.1002/jcb.21405 [DOI] [PubMed] [Google Scholar]
- 27. Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008; 359:2313-23; PMID:; http://dx.doi.org/ 10.1056/NEJMoa0802885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasselblom S, Hansson U, Sigurdardottir M, Nilsson-Ehle H, Ridell B, Andersson PO. Expression of CD68+ tumor-associated macrophages in patients with diffuse large B-cell lymphoma and its relation to prognosis. Pathol Int 2008; 58:529-32; PMID:; http://dx.doi.org/ 10.1111/j.1440-1827.2008.02268.x [DOI] [PubMed] [Google Scholar]
- 29. Wada N, Zaki MA, Hori Y, Hashimoto K, Tsukaguchi M, Tatsumi Y, Ishikawa J, Tominaga N, Sakoda H, Take H, et al. Tumour-associated macrophages in diffuse large B-cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology 2012; 60:313-9; PMID:; http://dx.doi.org/ 10.1111/j.1365-2559.2011.04096.x [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Shen X. Heat shock protein 27 protects L929 cells from cisplatin-induced apoptosis by enhancing Akt activation and abating suppression of thioredoxin reductase activity. Clin Cancer Res 2007; 13:2855-64; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2090 [DOI] [PubMed] [Google Scholar]
- 31. Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, Arrigo AP, Rodriguez-Lafrasse C. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys 2008; 70:543-53; PMID:; http://dx.doi.org/ 10.1016/j.ijrobp.2007.08.061 [DOI] [PubMed] [Google Scholar]
- 32. Chauhan D, Li G, Shringarpure R, Podar K, Ohtake Y, Hideshima T, Anderson KC. Blockade of Hsp27 overcomes Bortezomib/proteasome inhibitor PS-341 resistance in lymphoma cells. Cancer Res 2003; 63:6174-7; PMID:; http://dx.doi.org/ 10.1182/blood-2003-08-2873 [DOI] [PubMed] [Google Scholar]
- 33. Gupta M, Han JJ, Stenson M, Maurer M, Wellik L, Hu G, Ziesmer S, Dogan A, Witzig TE. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood 2012; 119:2844-53; PMID:; http://dx.doi.org/ 10.1182/blood-2011-10-388538 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.