Abstract
The instauration of an immunosuppressive microenvironment is a key event in cancer development and progression. Here, we discuss increasing evidences of the crosstalk between myeloid-derived suppressor cells (MDSCs) and mast cells (MCs) as a new fuel for the cancer immunosuppressive machinery.
Keyword: CD40:CD40L, colon carcinoma, immunosuppression, mast cells, myeloid derived suppressor cells
The accumulation of MDSCs is a crucial step in the instauration of a tumor-promoting microenvironment, and this heterogeneous mixture of granulocytic polymorphonuclear (CD11b+Ly6G+Ly 6Clow, PMN-MDSC) and monocytic (CD11b+Ly6G−Ly6Chigh, M-MDSC) myeloid cells represents a major component of the cancer immunosuppression machinery.1 An increasing number of reports highlights the existence of an important bidirectional axis between MDSCs and MCs that contributes to the development of both a tumor-inflammatory and a tumor-immunosuppressive microenvironment.
To our knowledge, research by Yang and colleagues2 was the first to hypothesize the existence of an interplay between the two immune populations. In an experimental mouse model of hepatocarcinoma, MC accumulation and activation are mediated by tumor-derived SCF and result in the upregulation of multiple pro-inflammatory factors (such as IL-6, TNF-α, VEGF, CCL2, and IL-17) promoting tumor growth.3 Infiltration and activation of MCs lead to the mobilization of MDSCs, mainly via CCL2 secretion with concomitant exacerbation of IL-17 production in the tumor microenvironment. MDSC-derived IL-17 is indirectly responsible through upregulation of other cytokines and chemokines (i.e. CCL17 and CCL22) for the consequent recruitment and activation of regulatory T cells (Tregs), which produce IL-9 and maintain the survival of MCs.2 This vicious axis among tumor-infiltrating MCs, MDSCs, and Tregs results in a highly immunosuppressive microenvironment and in the escape of tumor from immune response (reviewed in ).4
Cheon's group later demonstrated a similar MC-dependent mobilization of MDSCs in a colon cancer model. They showed a relevant role of 5-lipoxygenase (5-LO), an essential enzyme in the arachidonic acid pathway responsible for the production of leukotriene B4 (LTB4), in MC homing and expansion and in sustaining polyposis. Five-LO-competent MCs, through LTB4 production, directly sustain the proliferation of intestinal cells and, to the same degree, regulate tumor immune response by chemoattracting MDSCs to tumor sites. Of note, in 5-LO-competent mice, infiltrating MDSCs also show an increased arginase activity.5
In a mouse model of melanoma metastasis (through intravenous injection of B16 melanoma cells to recapitulate lung colonization), it has been demonstrated that adoptive transfer of MDSCs induces immunosuppression and increases the average number of lung metastases in wt mice but not in c-KitWsh/Wsh. These data suggest that MCs are required to enhance MDSC-mediated immune suppression and tumor escape from immune response, with, in particular, a role attributed to the monocytic-MDSC subset.6
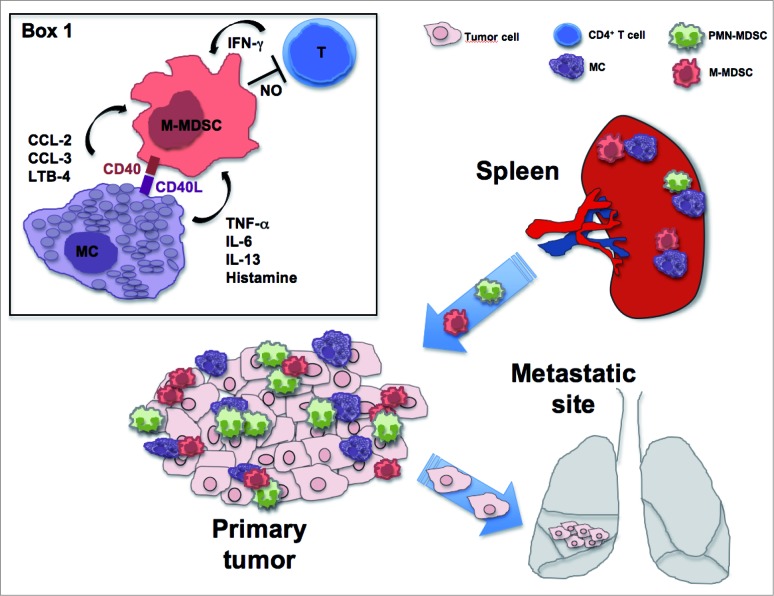
We recently showed cell-to-cell interactions between MDSCs and MCs in the mucosa of colon carcinoma patients and in the colon and spleen of AOM/DSS-treated mice.7 The fact that, in the spleen, MCs can interact with and further amplify the potential of MDSCs to inhibit T cell proliferation could be viewed as a novel mechanism to amplify MDSC-dependent immune tolerance to tumor antigens in the spleen.8 Concordant with a tumor-promoting role of the two populations, ex vivo analyses demonstrated that MCs have the ability to induce the migration (partly through the lipoxygenase pathway) and increase the suppressive properties of spleen-derived monocytic-MDSCs through a mechanism involving IFNγ and nitric oxide production (Fig. 1). In addition, the CD40:CD40L axis was described as responsible for the increase in the production of pro-inflammatory mediators (CCL2, IL-6, TNF-α).7 MDSC ability to selectively increase the production of cytokine (TNF-α, IL-6, IL-13) or chemokine (CCL2, CCL3) by IgE/Ag activated MCs (without a direct production by MDSCs), when co-cultured, is likewise reported and suggested to have an active modulatory effect on MC function.6,9 Not surprisingly, all these mediators could be important pro-inflammatory and pro-tumoral agents as they are linked with MDSC activation and mobilization. Overall, our research further lends support to the role of MCs as bona fide recruiters and activators of MDSCs in a tumor microenvironment and proposes this axis as a potential therapeutical target.
Figure 1.
Mast Cells promote the generation of highly suppressive NO-producing monocytic MDSCs in the tumor microenvironment. MCs produce chemotactic factors for PMN- and M-MDSCs and boost NO-production in the M-MDSC subset, in a process that requires IFNγ availability and drives to T cell proliferation inhibition. On the other side, MDSCs enhance MC cytokine production, partly depending on the CD40:CD40L axis (Box 1). The M-MDSC:MC interaction in the spleen and in the tumor site induces a pro-inflammatory and tumor-promoting microenvironment, resulting in tumor-specific T cell suppression and possible tumor metastasis.
Of note, some aspects of the described bidirectional axis, such as MC promotion of MDSC trafficking and activity or MDSC enhancement of MC responses, are conserved also in other pathological settings such as parasitic infection6 and airway hyper-responsiveness.9 The bidirectional modulation resulting from this interaction highlights the mutual dependence of the two populations to exercise their maximum potential, with a positive (parasite clearance) or negative (cancer, airway hyper-responsiveness) outcome for the host, dependent on the context.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008; 111:4233-44; PMID:; http://dx.doi.org/ 10.1182/blood-2007-07-099226 [DOI] [PubMed] [Google Scholar]
- 2. Yang Z, Zhang B, Li D, Lv M, Huang C, Shen G-X, Huang B. Mast cells mobilize myeloid-derived suppressor cells and treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS ONE 2010; 5:e8922; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0008922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang B, Lei Z, Zhang G-M, Li D, Song C, Li B, Liu Y, Yuan Y, Unkeless J, Xiong H, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood 2008; 112:1269-79; PMID:; http://dx.doi.org/ 10.1182/blood-2008-03-147033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu J, Zhang Y, Zhao J, Yang Z, Li D, Katirai F, Huang B. Mast cell: insight into remodeling a tumor microenvironment. Cancer Metastasis Rev 2011; 30:177-84; PMID:; http://dx.doi.org/ 10.1007/s10555-011-9276-1 [DOI] [PubMed] [Google Scholar]
- 5. Cheon EC, Khazaie K, Khan MW, Strouch MJ, Krantz SB, Phillips J, Blatner NR, Hix LM, Zhang M, Dennis KL, et al. Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APC 468 mice. Cancer Res 2011; 71:1627-36; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-1923 [DOI] [PubMed] [Google Scholar]
- 6. Saleem SJ, Martin RK, Morales JK, Sturgill JL, Gibb DR, Graham L, Bear HD, Manjili MH, Ryan JJ, Conrad DH. Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J Immunol 2012; 189:511-5; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1200647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danelli L, Frossi B, Gri G, Mion F, Guarnotta C, Bongiovanni L, Tripodo C, Mariuzzi L, Marzinotto S, Rigoni A, et al. Mast cells boost myeloid-derived suppressor cells activity and contribute to the development of tumor-favoring microenvironment. Cancer Immunol Res 2014; 3:85-95; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0102 [DOI] [PubMed] [Google Scholar]
- 8. Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2012; 2:1-12; PMID:; http://dx.doi.org/ 10.1016/j.celrep.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 9. Morales JK, Saleem SJ, Martin RK, Saunders BL, Barnstein BO, Faber TW, Pullen NA, Kolawole EM, Brooks KB, Norton SK, et al. Myeloid-derived suppressor cells enhance IgE-mediated mast cell responses. J Leukoc Biol 2014; 95:643-50; PMID:; http://dx.doi.org/ 10.1189/jlb.0913510 [DOI] [PMC free article] [PubMed] [Google Scholar]