Abstract
In this study the phenotype and function of tumor-associated NK cells from peritoneal fluids of a selected cohort of patients with seropapillary ovarian carcinoma were analyzed. In > 50% of these patients, the expression of the activating receptor NKp30 in tumor-associated NK cells was substantially reduced as compared to autologous peripheral blood (PB) NK cells. The impaired expression of this receptor was associated with the presence of one of its cellular ligands (B7-H6), which was detectable as a surface/cytosolic molecule in tumor cells and as a soluble molecule in the peritoneal fluid. NK cells from patients expressing this NKp30low phenotype displayed an impaired interferon-gamma (IFNγ) production and cytolytic function when tested against target cells expressing surface B7-H6. Our data also suggest that in these patients, the defective expression and function of NKp30 may be induced by the chronic engagement of this receptor by soluble B7-H6 or by tumor cells expressing this ligand. The impairment of NK cell functions described herein could represent a novel mechanism by which the tumor microenvironment may contribute to the escape from immune surveillance.
Keywords: B7-H6, mechanism of escape, NK cells, NKp30, ovarian carcinoma
Introduction
Natural Killer (NK) lymphocytes represent one of the most efficient cellular mechanisms by which the immune system can recognize and kill tumor or virally infected cells.1 Human NK cells can be divided into two main functional subsets based on the intensity of CD56 expression.2 Most circulating NK cells (about 90%) belong to the CD56dull subset and are characterized by the CD16 (FcRγIII)+, KIR+, and/or CD94/NKG2A+ phenotype. Conversely, the minor PB NK cell subset (<10%) is CD56bright, CD16−, KIR−, and CD94/NKG2A+. Moreover, the CD56dull CD16+ subset expresses a series of chemokine receptors such as CXCR1, CX3CR1, and ChemR23, whereas the CD56bright CD16− NK cell subset expresses a different set of chemokine receptors, including CCR7 and CXCR3.2-6
The function of NK cells is controlled by different activating and inhibitory receptors that, upon engagement by specific cell ligands on target cells, may either induce or suppress the process of killing and cytokine production. Inhibition is mainly mediated by receptors that upon recognition of HLA class-I molecules on potential target cells deliver negative signals that suppress the process of NK cell activation. These include the Killer Ig-like Receptors (KIR) (also referred to as CD158) that are able to distinguish among different HLA-C, -B and -A allotypes and the CD94/NKG2A (CD159A) heterodimer, specific for HLA-E.7-11 NK activation is induced by a series of triggering receptors,11-13 such as NKG2D, DNAM-1 and the Natural Cytotoxicity Receptors (NCR) that include NKp46 (NCR1, CD335),14,15 NKp44 (NCR2, CD336)16,17 and NKp30 (NCR3, CD337).18
While multiple cell surface ligands for NKG2D and DNAM-1 have been identified,19 tumor cell surface ligands for the NCR family have remained elusive until recently, hindering a complete understanding of their role in tumor surveillance. Thus, although many data suggested a central role of these receptors in tumor recognition and killing, the first NCR ligands to be identified were represented by viral structures such as the influenza hemagglutinin for NKp4620 and the human cytomegalovirus pp65 tegument protein for NKp30.21 Later on additional structures, such as the HLA-B associated transcript 3 (BAT3) protein, now referred to as BAG6, were shown to bind and trigger NKp30.22,23 Recently we identified B7-H6 (NCR3LG1) as a novel cell surface ligand of NKp30.24-26 This molecule appears to be present on a broad panel of hematopoietic and non-hematopoietic tumor cells including lymphoma, leukemia, melanoma, and carcinoma as well as on primary tumor blood cells. On the other hand, B7-H6 transcripts were not detected in normal adult tissues, thus suggesting that its expression could be limited to tumor cells of different histotype and that this molecule may represent a potential new tumor marker.24,27 Interestingly, more recent data indicated that the expression of B7-H6 transcripts as well as B7-H6 cell surface molecules can be upregulated upon TLR stimulation of myeloid cells in inflammatory conditions. Moreover, similar to other members of the B7 family, B7-H6 was also detected in a soluble form capable to inhibit the binding of anti-NKp30 mAbs to NKp30 and to prevent NKp30-mediated NK cell triggering.27,28
In this study, we analyzed the phenotypic and functional characteristics of tumor-associated NK cells isolated from peritoneal/ascitic fluid (PF) from a homogeneous cohort of patients with papillary serous ovarian carcinoma at advanced stages of the disease. Our data indicate that in >50% of the patients, these NK cells display lower expression of the NKp30 receptor and a reduced IFNγ production and cytolytic activity against B7-H6+ tumor target cells, as compared to autologous PB NK cells. Moreover, B7-H6 was expressed in the tumor environment both as a soluble molecule and as a surface/cytoplasmic structure in tumor cells. Taken together, our data reveal a novel escape mechanism from immune surveillance in this type of tumor.
Results
Phenotypic analysis of peripheral blood and peritoneal/ascitic fluid NK cells from patients with ovarian carcinoma of seropapillary histotype
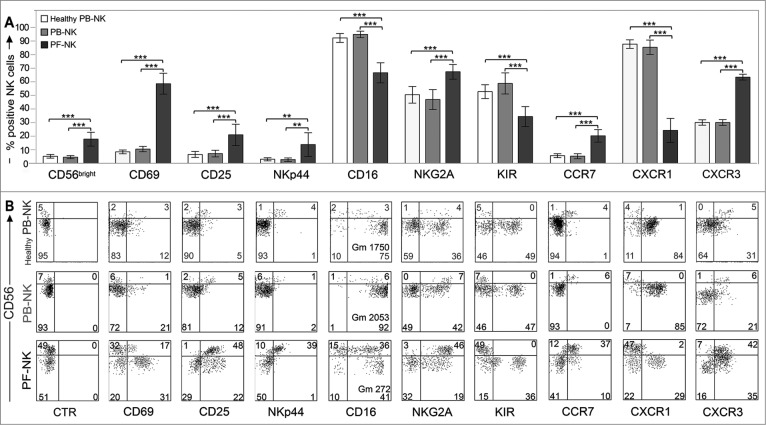
The surface phenotype of NK cells isolated from peritoneal/ascitic fluid (PF-NK) of 50 patients with ovarian carcinoma of seropapillary histotype29-31was compared to that of NK cells from autologous PB-NK and from PB of healthy donor (Healthy PB-NK). As shown in Fig. 1A, PF-NK cells displayed a significant increase in the percent of CD56bright NK cells.
Figure 1.
Surface phenotype of PB- and PF-NK cells derived from patients with ovarian carcinoma of seropapillary histotype. (A) NK cells freshly purified from PF of ovarian carcinoma patients (black bars) were analyzed by cytofluorimetric analysis for the surface expression of a series of NK receptors, and compared with fresh NK cells isolated from autologous PB (gray bars) or from PB of healthy donors (white bars). Histograms indicate the percent of PB-/PF-NK cells positive for the indicated receptors (n = 50). ***p < 0.001, **p < 0.01. (B) The distribution of different receptors on the CD56bright (upper quadrants) and CD56dull (lower quadrants) subsets derived from freshly purified PB- and PF-NK cells is shown for one representative patient. As control, the phenotype of PB-NK cells from one representative healthy donor is shown (Healthy PB-NK). The percentage of cells in each quadrant is reported and, when indicated, also the geo-mean (Gm) is reported. CTR: isotype control.
The CD56bright cell subset on PF-NK cells showed phenotypical features similar to those of classical CD56bright PB-NK cells. In particular, these cells were homogeneously NKG2A+/KIR−, expressed substantial amounts of CD25, NKp44, CCR7, and CXCR3, while displaying heterogeneous/low levels of CD16 (Fig. 1B and Fig. S1). On the other hand, a fraction of the CD56dull PF-NK cell subset expressed the KIR+ and/or NKG2A+ phenotype, while most cells were CD16+, although the geo-mean of this receptor was much lower as compared to that detected in autologous PB-NK cells. Interestingly, a significant fraction of this subset displayed an increased expression of activation markers, such as CD69 and CD25. Moreover CCR7, which is usually absent on CD56dull NK cells,2,5,32 was detected on a small cell fraction.33-35 (Fig. 1B and Fig. S1).
Thus, in agreement with other reports,36,37 these experiments indicated that NK cells derived from tumoral PF are characterized by a significant increase of the CD56bright NK cell subset and by a downregulation of CD16 on the CD56dull NK cell subset. Moreover, these data suggest that a significant fraction of the CD56dull subset is represented by activated NK cells (CD69+, CD25+). In line with this view, the CXCR1 chemokine receptor, which is commonly lost upon NK cell activation, is downmodulated, and CXCR3, usually expressed at low levels in resting CD56dull NK cells, is upregulated.4, 38
Expression of triggering receptors on NK cells derived from PB or PF
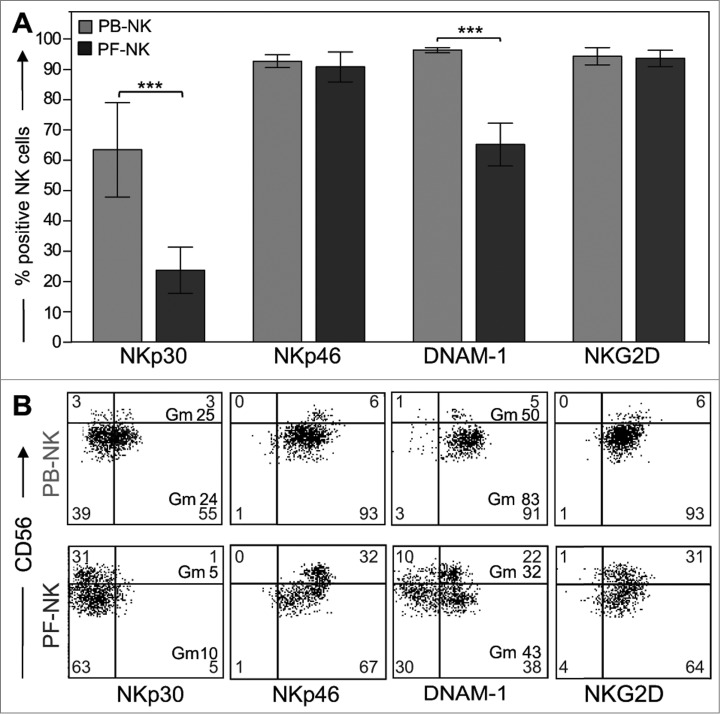
The ability of NK cells to lyse tumor targets correlates with the expression of a series of activating NK receptors including NKp30, NKp46, DNAM-1, and NKG2D.12,18,39 Hence, we analyzed their expression on PF-NK cells as compared with PB-NK cells. Previous data revealed that the expression of the activating receptor DNAM-1 on CD56dull NK cells derived from PF of ovarian carcinoma patients was significantly lower on tumor-associated NK cells than in NK cells isolated from PB.36 In agreement with this study, we found that the geo-mean of expression of this receptor as well as the percentage of DNAM-1+ NK cells were decreased on both CD56bright and CD56dull subsets in most patients analyzed (Fig. 2A, B) .
Figure 2.
Downregulation of activating receptors in NK cells derived from PF of ovarian carcinoma patients. (A) Fresh NK cells isolated from PF of ovarian carcinoma patients (black bars) were analyzed for the surface expression of the main activating NK receptors (NKp30, NKp46, DNAM-1, NKG2D) and compared with NK cells isolated from PB (gray bars) of the same patients. Histograms indicate the percentage of PB-/PF-NK positive cells (n = 50). ***p < 0.001. (B) A representative patient displaying low expression of NKp30 is shown. The percent of cells in each quadrant is reported and, when indicated, also the geo-mean (Gm) is reported.
Remarkably, we observed that in >50% of the patients there was a considerable impairment of NKp30 surface expression both as geo-mean of expression and as percent of NKp30+ NK cells (Figs. 2A, B and 3A). More specifically, we found that the expression of this receptor was strongly reduced in a group of patients (downregulation of NKp30 or NKp30low), but substantially normal in another group (no downregulation of NKp30 or NKp30high) (although some patients displayed intermediate but still significant levels of reduction) (Fig. 3A lower panels). We grouped the patients in these two categories by using a specific criterion. In particular, we calculated the index of NKp30 downregulation as ratio between the percentage of NKp30+ NK cells in the PB (control) and the percentage of NKp30+ NK cells in the PF and included in the category with downregulation of NKp30 the patients with an NKp30 decrease of at least 1.5 times compared to the control (corresponding approximately to 30% of downregulation).
Figure 3.
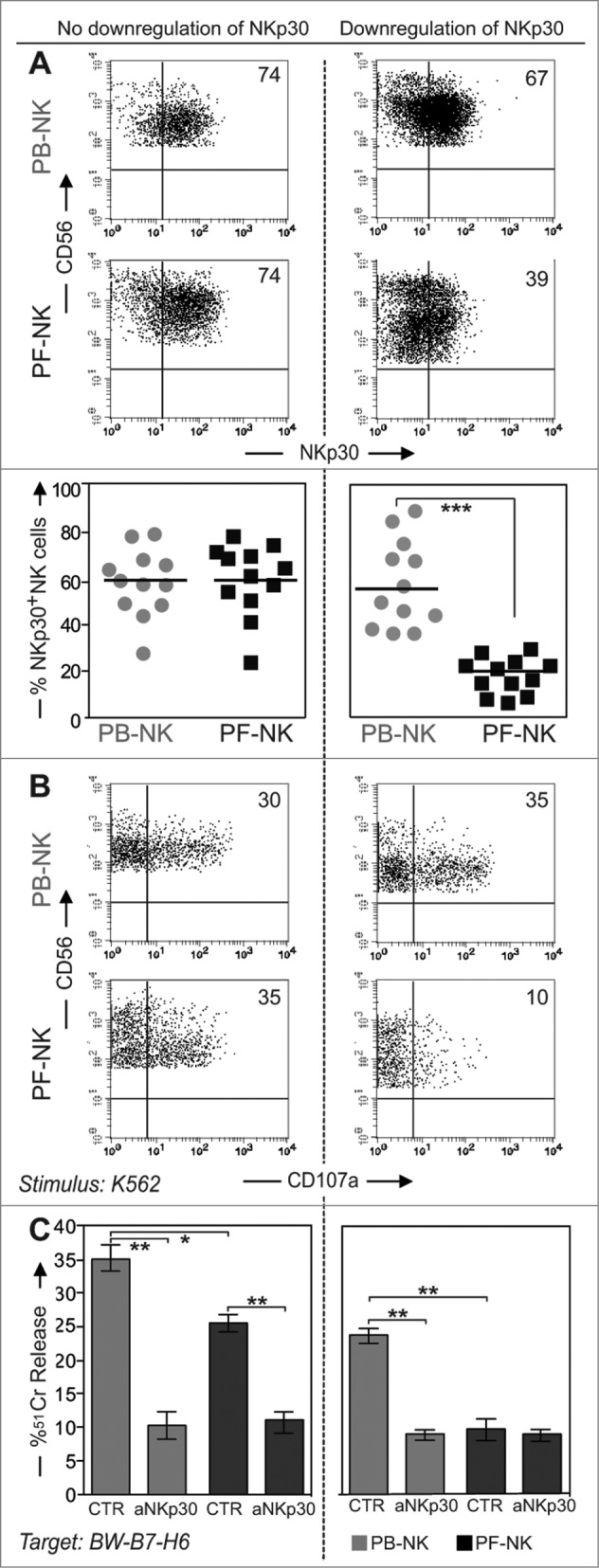
Expression of NKp30 and functional activities of NK cells derived from different groups of patients. Left column: group of ovarian carcinoma patients displaying normal levels of NKp30 expression on PF-NK cells (no downregulation of NKp30). Right column: group of patients displaying reduced levels of NKp30 expression on PF-NK cells (downregulation of NKp30). (A) NKp30 expression evaluated by cytofluorimetric analysis on freshly purified PB- and PF-NK cells from one representative patient for each group. Scatter plots indicate the percent of NKp30+ cells on PB- (gray circle) and PF-NK cells (black square) in 12 different patients belonging to each group. ***p < 0.001. (B) Degranulation assay (CD107a expression) of NK cells from ovarian carcinoma patients after stimulation with the K562 cell line. PB- and PF- lymphocytes were incubated with K562 for 4 h in the presence of anti-CD107a-PE mAb. Then cells were stained with anti-CD56, anti-CD3, anti-CD20, and anti-CD14 mAbs and the CD107a expression was evaluated on gated CD56+ CD3− CD20− and CD14− cells. One representative patient from the two groups is shown. The percent of CD107a+ NK cells is indicated in each upper-right quadrant. (C) Freshly purified PB- (gray bars) and PF- (black bars) NK cells of the two group of patients (n = 12 for each group) were cultured O.N. in the presence of IL2 and then tested for cytolitic activity in a 4-h 51Cr release assay against the BW-B7-H6 cell line, in the absence or in the presence of anti-NKp30 mAb. The E/T ratio used was 40:1. **p < 0.01, *p < 0.05.
The reduction of NKp30 surface density involved both CD56bright and CD56dull NK cell subsets, although in most instances it was more evident in the CD56dull subset (Fig. 3A upper panels).
In contrast, the expression of NKp46 and NKG2D in PF remained unaltered as compared to NK cells from PB (Fig. 2A, B), although the geo-mean of these receptors was frequently increased in PF-NK cells.
PF-NK cells expressing low levels of NKp30 receptor display low degranulation and cytolytic activity against B7-H6+ target cells
Next, NK cells from the various patients were tested in degranulation assays against the K562 cell line. This cell line was previously shown to express high levels of surface B7-H6, a ligand recognized by the NKp30 receptor.24 As shown in Figure 3B, PB-NK cells derived from two different representative patients displayed comparable levels of degranulation following exposure to K562. On the contrary, under the same conditions, PF-NK cells derived from these two patients were characterized by substantial differences in terms of degranulation. In particular, the degranulation of PF-NK cells that displayed decreased expression of NKp30 was significantly lower than that of PF-NK cells expressing normal levels of this receptor. To more directly assess the role of NKp30 receptor in the cytolytic activity of PF-NK cells, a murine cell line (BW) transfected with human B7-H6 was used as target cell in 51Cr release assays. In these experiments, the cytolytic activity of NK cells was evaluated in the absence or in the presence of an anti-NKp30 mAb capable of disrupting the recognition of B7-H6. In line with the degranulation experiments, PF-NK cells from patients showing an impaired expression of NKp30 displayed low ability to kill BW-B7-H6 transfectants, whereas PF-NK cells expressing regular levels of NKp30 displayed higher cytotoxic capacity toward these target cells, and the killing was strongly inhibited in the presence of anti-NKp30 mAb (Fig. 3C). In control experiments no differences in the killing efficacy toward untransfected BW cell lines could be detected between NKp30low and NKp30high PF-NK cells (not shown).
Analysis of perforin and granzyme B in NK cells derived from PB or PF
As shown in Fig. 3C (and not shown), PF-NK cells generally displayed lower capability to kill BW-B7-H6 cell transfectants than autologous PB-NK cells. This difference was not only due to their differential expression of NKp30, since a substantial reduction of cytolytic activity was detected also in patients that expressed comparable levels of this receptor in their PF and PB (Fig. 3C left panel). Although the reduction of DNAM-1 expression, observed in all the patients analyzed, may certainly affect the cytotoxic activity of PF-NK cells against targets expressing ligands for this receptor, it should not be relevant against target cells lacking these ligands, such as the murine BW-B7-H6 transfectants. Since the capability of NK cells to lyse given target cells usually correlates with their content in perforin and granzyme B,40 we next analyzed the expression of these cytoplasmic proteases in PB- and in PF-NK cells. As shown in Fig. 4A, in all patients analyzed we could detect a significant decrease in the geo-mean of expression of both proteases in PF-NK cells as compared to autologous PB-NK cells.41 This phenomenon was more evident after gating on the CD56dull subset (Fig. 4B) .
Figure 4.
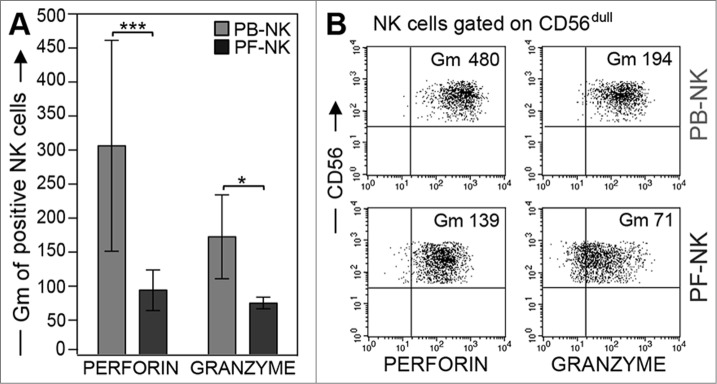
Low perforin and granzyme expression in PF-NK cells. (A) Freshly purified PB- (gray bars) and PF- (black bars) NK cells were analyzed for perforin and granzyme content by cytofluorimetric analysis after fixation and permeabilization. Histograms indicate the geo-mean (Gm) of perforin and granzyme fluorescence (n = 50). ***p < 0.001, *p < 0.05. (B) Expression of the two proteases in freshly purified NK cells derived from PB or PF of one representative patient. Cells were gated on the CD56dull subset. The geo-mean (Gm) of fluorescence of CD56dull NK cells stained for perforin (left) or granzyme (right) is shown.
IFNγ release by NK cells derived from PB or PF
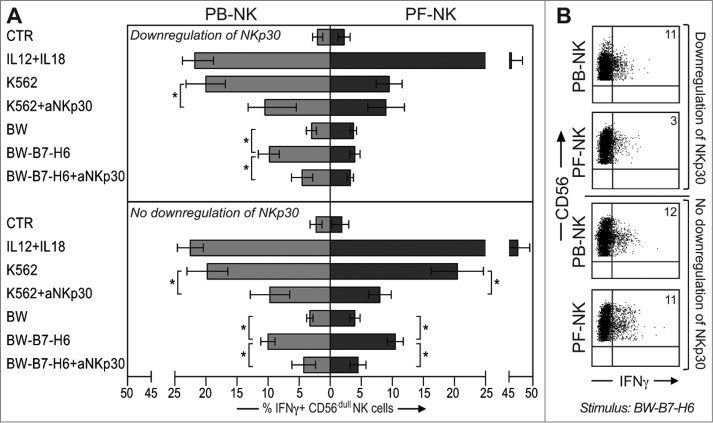
In these experiments, we assessed the ability of PF- and PB-NK cells to release IFNγ in response to exogenous cytokines or following exposure to K562 or to BW either transfected or not with B7-H6. Interestingly, after stimulation with rIL12+rIL18,42 PF-NK cells could release higher IFNγ amounts than PB-NK (Fig. 5A), independent of their NKp30low/high phenotype. On the contrary, in response to K562 (Fig. 5A) or BW-B7-H6 (Fig. 5A, B), PF-NK cells displaying an NKp30low phenotype released lower levels of this cytokine as compared to PB-NK cells. Remarkably, mAb-mediated blocking of the NKp30-B7-H6 interaction inhibited IFNγ release by PB-NK cells in response to K562 or BW-B7-H6 (Fig. 5A).
Figure 5.
PF-NK cells displaying NKp30 downregulation show impaired IFNγ production in response to B7-H6+ target cells. (A) PB- and PF- lymphocytes, after O.N. incubation with low doses of rIL18+rIL15, were washed and then incubated with rIL12+rIL18 or coincubated with different target cells (K562, BW, BW-B7-H6), in the presence or in the absence of anti-NKp30 mAb at an E/T ratio of 1:2. After 4 h NK cells (gated on CD56+ CD3− CD20− and CD14− cells) were assessed for intracellular IFNγ by cytofluorimetric analysis. The top panels show a comparison between IFNγ production by PB- (gray bars, on the left) and PF-NK cells (black bars, on the right) from the group of patients with downregulation of NKp30. The bottom panels show the same comparison performed for the group of patients with no downregulation of NKp30. Histograms indicate the percent of PB-/PF-NK cells positive for intracellular IFNγ staining (n = 4 for each group). *p < 0.05. (B) The IFNγ production of PB-/PF-NK cells after BW-B7-H6 stimulation in one representative patient of each group is shown. The percentages of IFNγ+ NK cells are indicated in the upper-right quadrants.
Thus, the high production of IFNγ by PF-NK cells in response to rIL12+rIL18 suggests that these cells are not strongly compromised in terms of functional activity and that their behavior when exposed to B7-H6+ target cells (reduced production of IFNγ) is secondary to the low expression of the NKp30 receptor rather than to an intrinsic inability to produce this cytokine.
NKp30 downmodulation in NK cells of ovarian carcinoma patients is associated with the presence of a soluble form of B7-H6 in the tumor environment
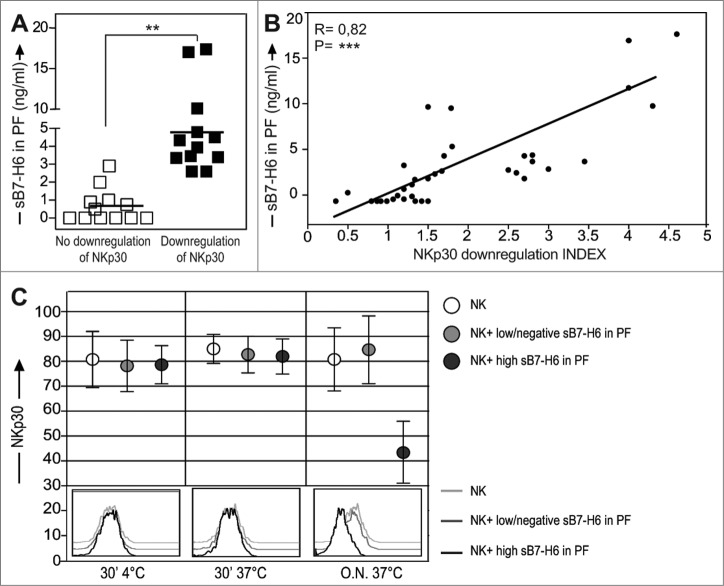
Recently a soluble form of the B7-H6 molecule (sB7-H6) was demonstrated in inflammatory conditions after TLR-mediated activation of monocytes and neutrophils.28 Interestingly we found that this molecule was detectable also in the PF of the patients and that its presence appeared to be associated with the altered expression of NKp30 on the surface of PF-NK cells (Fig. 6A). More specifically, patients displaying an important decrease in the expression of NKp30 exhibited also a high concentration of sB7-H6 in the PF. On the other hand, patients displaying higher levels of surface NKp30 on PF-NK were characterized by low/negative concentration of sB7-H6 in PF (Fig. 6A, B) .
Figure 6.
A soluble form of B7-H6 ligand downmodulates the expression of NKp30 on PF-NK cells. (A) The PF of 24 representative ovarian carcinoma patients belonging to the two groups are showed for the presence of sB7-H6. On the left (white squares): patients with normal NKp30 expression (n = 12). On the right (black squares): patients with downregulated NKp30 expression (n = 12). **p < 0.01. (B) The relationship between the index of NKp30 downregulation (calculated as ratio between the percentage of NKp30+ NK cells in the PB and the percentage of NKp30+ NK cells in the PF) and the concentration of sB7-H6 in 36 representative patients was analyzed according to a linear regression model (R = 0.82) and confirmed by analyzing the Pearson Correlation Coefficient (=0.82) ***p < 0.001. (C) A short pre-incubation (30 min) of NK cells from healthy donors in the presence of PF with high concentration of sB7-H6 (> 3.5ng/mL) (five representative donors) or with low/negative sB7-H6 (< 0.3 ng/mL) (five representative donors) was done on ice. In comparison, the same cells were incubated also at 37°C for a short time or O.N. (top). Then, NK cells were analyzed by flow cytometry with anti-NKp30 mAb. One representative experiment out of ten is shown (bottom). The black line represents the expression of NKp30 in NK cells treated with PF characterized by high sB7-H6.
Statistical analysis indicated that a direct correlation between the level of NKp30 downmodulation on tumor-associated NK cells and sB7-H6 concentration in the PF could be established (Fig. 6B).
Thus, these results, by revealing the existence of an interesting correlation between the reduction of NKp30 expression on tumor-associated NK cells and the detection of sB7-H6 in PF, suggest that the reduction may be due to modulation induced by the soluble form of the NKp30 ligand (Fig. 6B).
To verify whether the NKp30low phenotype in NK cells was due to the receptor modulation induced by the chronic receptor–ligand interaction or to simple sB7-H6-mediated masking of the receptor (that could prevent the binding of anti-NKp30 mAbs), experiments were carried out in which NK cells from healthy donors were stained with anti-NKp30 mAbs after pre-incubation with PF containing high levels of sB7-H6. The pre-incubation was performed on ice or at 37°C for 30 min or overnight (O.N.). As shown in Fig. 6C, short time exposure to sB7-H6 did not prevent binding of anti-NKp30 mAb and the NKp30low phenotype was achieved only after O.N. incubation.
These data support the concept that the acquisition of the NKp30low phenotype is not consequent to masking by sB7-H6, but to chronic receptor–ligand (NKp30-sB7-H6) interactions both in vivo and in vitro.
Interestingly, in most instances a significant increase of TGFβ1 could be detected in the PF from patients displaying an NKp30low phenotype as compared to patients displaying normal levels of this receptor (not shown). Since previous data indicated that TGFβ1 can downmodulate the surface expression of NKp30,43 it cannot be excluded that the downmodulation of NKp30 may be the result of the combined action of sB7-H6 and TGFβ1 that are enriched in the PF of patients with an NKp30low phenotype.
IL18 present in the PF is primarily responsible for additional phenotypic changes observed in PF-NK cells
To further characterize the soluble factor(s) potentially involved in the shaping of the phenotypic/functional characteristics of PF-NK cells, PF were analyzed for the presence of up to 50 soluble mediators using a Multiplex Assay (see Materials an Methods). As shown in Fig. 7A, the PF analyzed contained substantial amounts of MIG/CXCL9 and IP-10/CXCL10 (ligands of CXCR3) involved in the preferential recruitment of CD56bright NK cells,38 as well as growth factors including MCP-1 (CCL2), MIF, and MIP-1 that recruit innate cells, including monocytes, macrophages, granulocytes, and dendritic cells at inflammatory sites. Importantly, this analysis also revealed the presence of various pro-inflammatory cytokines, including IFNγ, IL18, and IL12 (both p40 and p70) (Fig. 7A).
Figure 7.
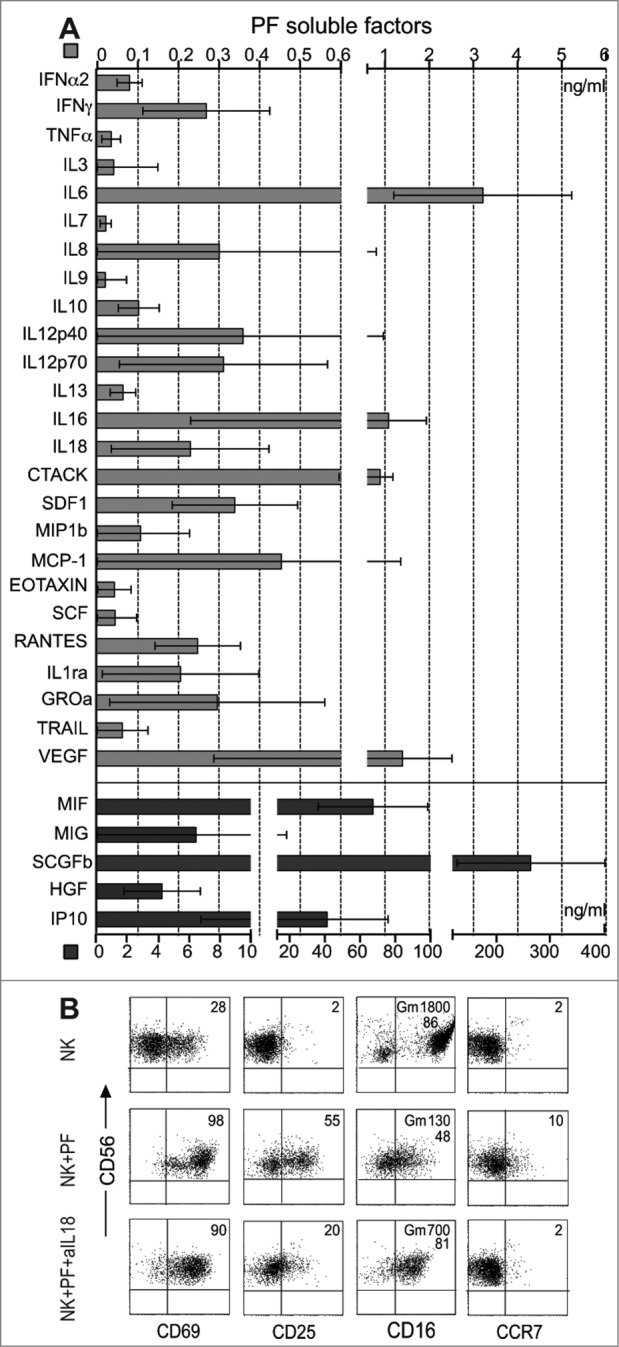
Soluble factors present in PF of ovarian carcinoma patients: evidence that IL18 modulates the expression of additional NK receptors. (A) PF from 50 patients was independently assessed by multiplex analysis for the presence of a series of soluble factors in different concentration. Some of these are shown with a high scale up to 400 ng/mL (black bars), others, present in lower doses, are shown with a low scale up to 6 ng/mL (gray bars). (B) Purified NK cells from healthy donors were cultured in medium or resuspended in PF from ovarian carcinoma patients either in the presence or in the absence of a blocking anti-IL18 mAb. After O.N. incubation, NK cells were analyzed by cytofluorimetric analysis for the expression of CD69, CD25, CD16, and CCR7 receptors in combination with CD56. One representative experiment out of ten is shown. The percentages (and the geo-mean (Gm) when indicated) of expression of the various receptors analyzed are indicated in the upper-right quadrants of each panel.
Interestingly, we also observed that NK cells from healthy donors, cultured in the presence of PF (derived from patients displaying either normal or low expression of NKp30) acquired some of the phenotypic features typical of PF-NK cells (Fig. 7B). These included the expression of activation markers such as CD25 and CD69, the de novo expression of CCR7 on the CD56dull cell subset and the downmodulation of CD16 (and CXCR1, not shown). This phenotype was compatible with the effects mediated by IL18,33-35 and in fact it was substantially reversed by anti-IL18 mAbs (Fig. 7B), thus indicating that this cytokine may play a major role in the acquisition of the PF-NK surface phenotype. In NK cells from healthy donors cultured in the presence of PF we could detect also downmodulation of NKp30 (but not of NKp46). This effect however was only observed with PF derived from patients expressing an NKp30low phenotype that were characterized by high levels of sB7-H6. In this case the anti-IL18 mAbs did not affect the expression of NKp30 and of NKp46 (not shown). On the other hand, no significant NKp30 modulation could be observed after culture of NK cells in the presence of PF derived from patients expressing an NKp30high phenotype (not shown) characterized by low/negative sB7-H6.
Analysis of NK receptor ligands on ovarian carcinoma cells
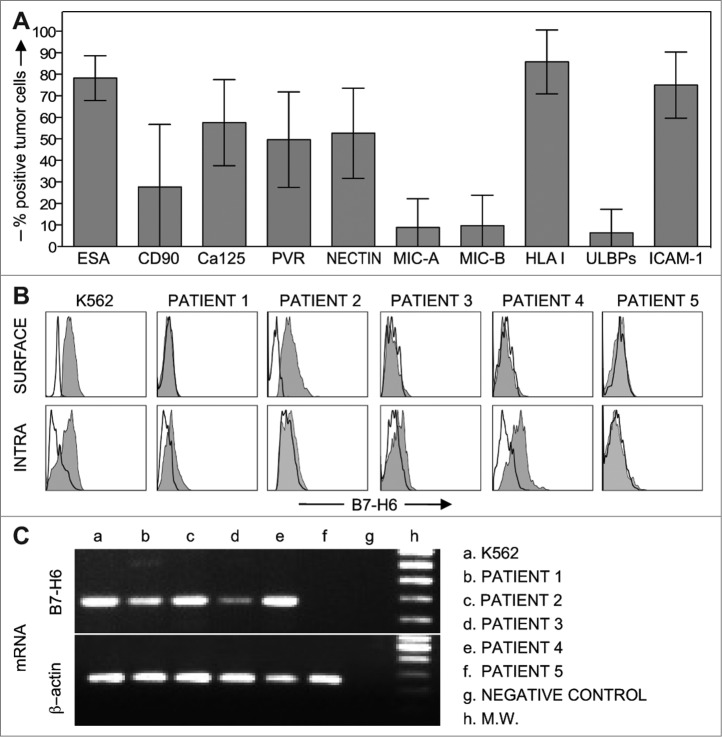
In limited number of cases, we were able to isolate enough tumor cells for subsequent cytofluorimetric and mRNA analysis. In these experiments, tumor cells (expressing an ESA+ and Ca125+ phenotype)37, 44 were analyzed for the expression of ligands specific for different NK cell receptors. As shown in Fig. 8A, these cells expressed HLA class-I molecules and some activating NK receptor ligands including PVR and Nectin-2 (DNAM-1 ligands), while they did not express MIC-A, MIC-B, and ULBPs (NKG2D ligands).19 At least in patients characterized by the NKp30low phenotype, tumor cells expressed also B7-H6 (NKp30 ligand), although, in some of the cases analyzed, this molecule was only detectable in the cytoplasm and not at the cell surface (Fig. 8B). On the other hand, in patients characterized by lack of NKp30 downmodulation, B7-H6 could not be detected neither on the surface nor in the cytoplasm of tumor cells (see patient 5 in Fig. 8B). Finally, the same ovarian carcinoma cells as well as K562 (as control) were analyzed by RT-PCR for B7-H6 mRNA expression (Fig. 8C). Data from these experiments essentially correlated with those obtained by flow cytometric analysis.
Figure 8.
Phenotypic and molecular analysis of ovarian cancer cells. (A) Tumor cells, freshly isolated from PF, were analyzed for the expression of specific tumor markers, such as ESA, CD90, Ca125, and of ligands for different NK cell receptors. Histograms indicate the percent of tumor cells positive for the indicated molecules (n = 30). ULBPs: ULBP1,2,3. (B) Tumor cells, freshly isolated from PF, were analyzed for the surface (top) or the cytosolic (bottom) expression of the NKp30 ligand B7-H6, in comparison with the K562 cell line. Four representative cases of patients characterized by downregulation of NKp30 on PF-NK cells and one patient (patient 5) with no NKp30 downregulation are shown. Solid lines constitute isotype controls. (C) B7-H6 mRNA expression was analyzed by RT-PCR in K562 cell line and in tumor cells freshly isolated from PF. Primers specific for β-actin were utilized as positive control. PCR products were run on a 1.5 % agarose gel and visualized by ethidium bromide staining.
Discussion
In the present study, we analyzed the phenotypic and functional properties of tumor-associated NK cells derived from PFs of a selected cohort of patients with seropapillary ovarian carcinoma. Our data indicate that in a fraction of these patients the expression of the activating receptor NKp3018 is substantially reduced in tumor-associated NK cells as compared to autologous PB-NK cells. Remarkably, the aberrant expression of this activating receptor was correlated with the expression of one of its cellular ligands (B7-H6)24, 27 in tumor cells and in the PF. Patients expressing low levels of NKp30 displayed an impaired NK-mediated antitumor cytolytic activity and low IFNγ production in response to B7-H6+ target cells. These data suggest a possible novel mechanism by which in this type of tumor the microenvironment (e.g., tumors cells and soluble factors) may contribute to the escape from immune surveillance by inducing impairment of the NK-mediated activity.
Ovarian cancer is the fourth leading cause of cancer-related death in women. Its poor prognosis is mainly due to the difficulty in early detection because of the general asymptomatic presentation of the disease.45 This study included 50 patients with ovarian carcinoma of seropapillary histotype that is the most common ovarian cancer subtype.30,31,45 More than 50% (35/50) of these patients showed altered expression of the activating NKp30 receptor on tumor-associated NK cells. Apparently this modulation was frequent in this tumor subtype, while it was rare (3/17) in other ovarian carcinoma subtypes, including serous, clear cell, endometrioid, and mucinous30,46,47 (not shown). We found that the NKp30 downregulation was associated with the downmodulation of another activating receptor, DNAM-1. However, while NKp30 expression was reduced in a portion of the patients, the downmodulation of DNAM-1 was detectable in all patients analyzed, including those affected by other ovarian carcinoma subtypes. These data are in line with those recently reported by Carlsten et al. showing that, in patients with ovarian carcinoma, chronic DNAM-1/CD155 interactions in the tumor environment could induce reduction of DNAM-1 expression on tumor-associated NK cells.36,48
It has been recently shown that certain tumors express a cell surface molecule termed B7-H6, which is a ligand for the NKp30 receptor.24 Thus, in order to establish whether the loss of NKp30 expression could be caused by exposure to this cellular ligand, we analyzed tumor cells from PF for the expression of this molecule. Moreover, based on the fact that a reduced expression of this triggering receptor has been previously demonstrated in vitro after exposure to soluble factors, such as TGFβ1,43 we analyzed the PF for the presence of a panel of different soluble factors including several cytokines and chemokines. We show that B7-H6 was expressed in PF-derived cancer cells either at the cell surface or in the cytoplasmic compartment. In addition, a soluble form of this molecule (sB7-H6) could be detected in the PF of the patients. In this context, a soluble form of this molecule released in membrane vesicles by monocytes and neutrophils after TLR-mediated activation was recently demonstrated in inflammatory/pathological conditions.28 In this study, sB7-H6 was associated with an impairment of NKp30 expression and function. Moreover, it has been recently shown that sB7-H6 was detectable in sera of certain melanoma patients and that the release of this soluble form was regulated by the metalloproteases ADAM-10 and ADAM-17.27 In this case, sB7-H6 generated by ectodomain shedding had no impact on NKp30 expression. Another recent report showed that the expression of B7-H6 in various tumor cell lines was regulated by histone deacetylase inhibitors (HDACi).49 Interestingly, a soluble and exososomal form of another NKp30 ligand, termed BAG6/BAT3,22,23,50 was recently described in the plasma of chronic lymphocytic leukemia (CLL) patients with the highest levels at the advanced disease stages. At this regard, unfortunately the expression of BAG6 on tumor cells and in ascites could not be analyzed due to the unavailability of specific reagents.
Remarkably, we found that the expression of B7-H6 on tumor cells and/or the presence of high concentrations of sB7-H6 in PF were associated with low NKp30 expression on NK cells. The NKp30low phenotype was even more evident in patients who concomitantly exhibited sizeable concentrations of the immunosuppressive TGFβ1 factor in the PF. In order to assess whether the NKp30 downregulation could have any effect on NK cell function, we performed a series of functional assays in which NK cells were stimulated with target cells expressing surface B7-H6 molecules. We show that tumor-associated NK cells displaying an NKp30low phenotype had a reduced ability to recognize and kill target cells expressing the surface ligand for this receptor, including the K562 cell line and BW-B7-H6 cells. Only occasionally sufficient amounts of fresh autologous tumor cells could be collected to perform cytotoxicity experiments. These experiments essentially confirmed the impairment of tumor cell recognition by PF-NK cells (not shown).
Our results suggest that chronic receptor–ligand interaction may cause loss of NKp30 expression on NK cells in the tumor microenvironment, thereby contributing to poor NK cell-mediated elimination of ovarian carcinoma cells. In all patients, however, PF-NK cells were characterized by lower content of perforin and granzyme as compared to PB-NK cells. As a result, the cytolytic activity was reduced also in patients expressing normal levels of NKp30. In this case, however, the ability of PF-NK cells to degranulate in response to B7-H6+ target cells (K562) was approximately the same as the one of PB-NK cells (Fig. 3B).41 Thus, it is presumable that the cumulative effect of the decreased expression of activating receptors (e.g., NKp30 and DNAM-1) and of components of the cytotoxic apparatus (e.g., perforin) could be responsible for a reduced NK cell-mediated detection and destruction of tumor target cells. In this context, we failed to detect reduced responsiveness to NKp46 or NKG2D mAbs in redirected killing assays in which the cytolytic activity of PF-NK cells expressing NKp30 low or NKp30 high was comparatively evaluated (not shown). This is reminiscent of data from Arnon TI et al.21 who reported that the inhibition induced by the interaction between NKp30 and pp65 CMV protein is specific and does not affect other pathways of NK cell activation.
In line with the functional impairment in terms of degranulation and cytolytic activity we show that tumor-associated NK cells displayed also a defect in the ability of releasing IFNγ when stimulated with B7-H6+ target cells (although the ability of releasing this cytokine was not compromised in experiments in which the same NK cells were stimulated in the presence of rIL12+rIL18 (see Fig. 5).
The analysis of soluble factors present in the PF allowed the detection not only of B7-H6 and TGFβ1 that are likely to promote the acquisition of the NKp30low phenotype but also of other important citokines that may modulate NK cell functions. For example, we detected the presence of chemokines such as MIG/CXCL9 and IP-10/CXCL10, that are able to preferentially attract CD56bright NK cells, that express CXCR3.38 This may contribute at least in part to the recruitment of these cells in PF and may explain their increment as compared to CD56dull NK cells at these sites, further compromising the situation since it is known that this cell subset is less active against tumor cells being characterized by a limited cytotoxic activity.
Among the different soluble factors detected in the PF, IL18 may account for a series of additional phenotypic and functional modifications observed on tumor-associated NK cells.33 For example, the phenotypic modifications could include the downregulation of CD16, the upregulation of activation markers (CD25 and CD69) and the de novo expression of CCR7 on a fraction of CD56dull NK cells. It is also likely that IL18 may be responsible, at least in part, for a reduced cytolytic activity of PF-NK cell and for their high IFNγ production.33 In this context, we found that exposure of healthy PB-derived NK cells to peritoneal effusions from patients with NKp30 downregulation could induce not only the down-modulation of NKp30 expression but also the acquisition of the above IL18-induced phenotypic modifications of NK cells that could be inhibited in the presence of blocking anti-IL18 mAbs (Fig. 6).
In conclusion, our study provides a novel molecular mechanism that may contribute to impaired NK cell-mediated tumor rejection in ovarian carcinoma. We have shown that the loss of NKp30 expression on tumor-associated NK cells results in impaired NK cell function caused by the inability to efficiently recognize cells expressing an NKp30 ligand termed B7-H6. This ligand can be expressed by the tumor but can also be released in soluble form in the tumor environment by tumor cells (and likely by other cell types present in the tumor, e.g., monocytes/macrophages).24,28 Thus, the chronic receptor–ligand interactions that in the tumor environment induce loss of NKp30 may be due to continuous perturbation by soluble or surface-bound B7-H6 molecules. The downmodulation of NKp30 expression was detected in >50% of the patients affected by ovarian carcinoma of seropapillary histotype, but was rare in other subtypes of ovarian carcinoma. This may explain why in other studies performed on ovarian carcinomas of various subtypes the impairment of this receptor could not be appreciated. Our present results may help to refine the selection of a subset of patients with ovarian carcinoma and may provide important implications for the design of future protocols of adoptive NK cell- and Ab-based immunotherapies for this subtype of tumor.51,52
Materials and Methods
Patients and samples
This study included 50 patients with ovarian carcinoma of seropapillary histotype, subjected to primary surgery before chemotherapy.4 The clinical stage of the patients was IIIC, according to FIGO (International Federation of Gynecologists & Obstetricians) classifications.29 The study was conducted in accordance with a protocol approved by the Spedali Civili of Brescia institutional ethical board and informed consent was obtained from all patients according to the Declaration of Helsinki.
Buffy-coats (healthy controls) were collected from volunteer blood donors admitted at the blood transfusion center of IRCCS S. Martino-IST. All biological samples were collected after obtaining informed consent and the study was approved by the Ethical committee of IRCCS S. Martino-IST (39/2012).
Monoclonal antibodies
The following mAbs generated in our laboratories (Laboratory of Molecular Immunology, DIMES, University of Genoa and Department of Molecular and Translational Medicine, University of Brescia), were used in this study: BAB281 and KL247 (IgG1 and IgM, anti-NKp46, respectively), AZ20 and F252 (IgG1 and IgM, anti-NKp30, respectively), Z321 (IgG1, anti-NKp44), ON72 (IgG1, anti-NKG2D), SUS142 (IgG2b, anti-CD16), C227 (IgG1, anti-CD69), FS123 and F5 (IgG2a and IgM, anti-DNAM-1, respectively), 11PB6 (IgG1, anti-KIR2DL1/S1), GL183 (IgG1, anti-KIR2DL2/L3/S2), AZ158 (IgG2a, anti-KIR3DL1/S1/L2), Z199 (IgG2b, anti-CD94/NKG2A), 5A10 (IgG1, anti-poliovirus receptor PVR), A6/136 (IgM, anti-HLA-I), L14 (IgG2a, anti-Nectin-2) MAR93 (IgG1, anti-CD25), PP35 (IgG1, anti-2B4), BAM195 (IgG1, anti-MICA), 7E22 (IgG1, anti-ICAM1). Anti-B7-H6 (IgG1) was produced at the Centre d'Immunologie de Marseille-Luminy. The following purchased mAbs were used in this study: anti-CXCR1 (IgG1, Santa Cruz biotechnologies, Santa Cruz, CA; USA), anti-ESA (IgG1, Novocastra Laboratories Ltd), anti-Ca125 (IgG1, anti-MUC16 Novocastra Laboratories Ltd), anti-CD90, anti-CD107a PE-labeled, anti-IFNγ PE-labeled (IgG1, BD-Bioscience, Pharmigen CA, USA), mixture of FITC–labeled CD3 plus PC5-labeled CD56, FITC-labeled CD20 and FITC-labeled CD14 (Beckman Coulter, Immunotech, Marseille, France), anti-hCCR7 IgG2A and anti-CXCR3 IgG1 (R&D Systems, MN, USA), anti-Perforin PE (Ancell, MN, USA), anti-Granzyme PE (Alexis Biochemicals, CA, USA), anti-human MICB (IgG2B, R&D Systems Inc..Abingdon, UK), anti-ULPB1 (clone M295), anti-ULBP2 (clone M310), and anti-ULBP3 (clone M550) (Amgen, Seattle, WA), anti-Muc-1 Glycoprotein (IgG1, Novocastra Laboratories Ltd, Newcastle, UK), anti–TGF-β1 and anti–TGF-β2 (R&D Systems).
Cell preparations and flow cytometry analysis
Mononuclear cells from heparinized PB and from PF were obtained by density gradient centrifugation over Ficoll (Sigma, St. Louis, MO), then resuspended in RPMI 1640 medium, supplemented with 2 mM glutamine, 50 μg/mL penicillin, 50 μg/mL streptomycin and 10% heat-inactivated FCS (Fetal Calf Serum, Sigma, St. Louis, MO).
Pure populations of NK cells were obtained from mononuclear cells using the NK cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instruction. The purity of NK cells was greater than 98% (NK cells defined as CD56+CD3−).
To obtain activated polyclonally expanded NK cells, freshly isolated NK cells were cultured on irradiated feeder cells in the presence of 100 U/mL recombinant human IL-2 (Proleukin; Chiron) and 1.5 ng/mL phytohemagglutinin (PHA) (GIBCO Ltd).
NK cells from PB and PF were first stained with appropriate primary mAbs followed by PE-conjugated isotype-specific goat anti-mouse secondary reagent (Southern Biotechnology, Birmingham, AL) then the cells were stained with a mixture of FITC-labeled CD3 and PC5-labeled CD56, FITC-labeled CD20 and FITC-labeled CD14. NK cells analysis by flow cytometry was performed by gating on CD56+ CD3− CD20− and CD14− cells. Perforin and granzyme B expression analyses in NK cells were performed using purified anti-perforin mAb and purified anti-granzyme B mAb, respectively, after fixation (in 1% paraformaldehyde) and permeabilization. (Cytofix/Cytoperm, BD-Bioscience, Pharmigen CA, USA).53
Ovarian cancer cells were accessible as single-cell suspensions in ascites fluid of patients with advanced disease. To obtain enriched freshly epithelial ovarian carcinoma cells from PF, after depletion of plastic-adherent cells, cell populations from PF were incubated with anti-CD45 and anti-CD90 mAbs followed by goat anti-mouse coated Dynabeads and immunomagnetic depletion (Magnetic Dynabeads Goat anti-Mouse IgG). The purity of epithelial ovarian carcinoma cells (defined as ESA+ cells) was about 80%. ESA+ enriched tumor cell populations were analyzed by flow cytometry without further processing or culturing. Cells acquisition was performed on a FACSCanto flow cytometer or on a FACSCalibur flow cytometer and data analyzed using the Diva software or the CellQuestPro software respectively (Becton Dickinson, Mountain View, CA).
Cell lines and cytotoxicity assays
The following target cells were used in this study: the human erythroleukemia K562 cell line, the murine thymoma cell line BW1547 (BW) and BW-B7-H6 cells. BW-B7-H6 cells were prepared by retrovirus gene transfer. B7-H6 ORF cDNA was obtained by RT-PCR from human myelomonocytic cell line MM6 and subcloned in pMXs-IG (IRES-GFP) retrovirus vector (kindly provided by Dr. Kitamura, Tokyo, Japan). The pMXs-IG-B7-H6 construct was transiently transfected into Plat-E packaging cell line in order to generate viral particles that were used to infect BW cells. B7-H6-positive cells were selected and enriched by staining with soluble NKp30Fc receptor followed by microbeads coated with mouse anti-human IgG (Miltenyi Biotec). Subsequently, B7-H6-positive cells were subcloned by limiting dilution.
Tumor cells from patients were limited in number and not proliferating in culture conditions, so it was not possible to obtain primary lines. All the experiments were performed fresh isolated tumor cells without further processing or culturing.
Purified NK cells or PBMC derived from patient PB and PF and from healthy donor PB were cultured O.N. in the presence of IL2 and then tested for cytolytic activity in a 4-h 51Cr release assay as previously described,53 in the absence or in the presence of various mAbs. MAb concentration for the masking experiments was 10 μg/mL.
Analysis of NK cell degranulation and IFNγ production
To obtain pure (>90%) lymphocyte cell populations, ESA+, and CD90+ cells were depleted (using Magnetic Dynabeads Goat anti-Mouse IgG) from PF.
For degranulation assay, PB and PF lymphocytes were cultured O.N. in the presence of IL2 and then coincubated with K562 target cells at an E/T ratio of 1:2 in a final volume of 200 μL in round-bottomed 96-well plates at 37°C and 5% CO2 for 4 h in culture medium supplemented with anti-CD107a-PE mAb. After 1 h of coincubation, GolgiStop (BD Biosciences PharMingen, San Diego, CA, USA) was added at a 1:100 dilution. Surface staining was done by incubating the cells with anti-CD3, anti-CD56, anti-CD20, and anti-CD14 mAbs for 30 min at 4°C. The cells were washed and analyzed by flow cytometry (FACSVerse, Becton Dickinson). Analysis of NK cells was made on CD56+ CD3− CD20− CD14− gated cells.
To detect intracellular production of IFNγ, PB, and PF lymphocytes were incubated with IL18 0,1 ng/mL+IL15 0,2 ng/mL for O.N. period. Then PB- and PF-lymphocytes were washed and cultured with IL12 1 ng/mL+IL18 100 ng/mL or coincubated with target cells (K562, BW, BW-B7-H6) at an E/T ratio of 1:2 in a final volume of 200 μL in V-bottomed 96-well plates at 37°C and 5% CO2 for 4 h in the presence of GolgiStop. Thereafter cells were washed, stained as described above for CD107a assays and then fixed and permeabilized with BD Cytofix/Cytoperm kit from BD Bioscience PharMingen. IFNγ production was detected by subsequent intracellular staining with anti-IFNγ-PE and cytofluorimetric analysis. The percent of positive cells was calculated subtracting the baseline CD107a or IFNγ expression in control cultures without stimuli from targets.
Cytokine production
We analyzed supernatants obtained from peritoneal fluid for the presence of different soluble factors. In particular, soluble B7-H6 (sB7-H6) was measured in cell supernatants by enzyme-linked immunosorbent assay (ELISA) as previously described.28
To analyze the presence of a panel of different soluble factors we used three different kits (Bio-Plex Pro Human Cytokine 27-plex Assay, Bio-Plex Pro Human Cytokine 21-plex Assay, Bio-Plex Pro TGFβ Premixed Assay) from Bio-Rad (Hercules, CA, USA) and all assays were performed according to Biorad kit procedures. In some cases, results obtained with Bio-Plex assays were confirmed by ELISA. ELISA kits used were: IFNγ, IL-12p40, IL12p70, (BioSource International, Inc.), and IL-18 (Medical Biological Laboratories). For the analysis of soluble factors present in patients ascites, human AB plasma (ICN Biomedical, Inc. Ohio 44202) collected from healthy donors was used as a control. For each soluble factor, the value of human AB plasma was subtracted from the value obtained from patients. Data were collected and analyzed; the results are shown as the mean of the observed concentration and the standard error of the mean.
RT-PCR
Total RNA was extracted using RNAeasy Mini Kit (Qiagen, Hilden, Germany) from K562 cell line and from ovarian carcinoma cells. Oligo(dT)-primed cDNA was prepared by standard technique using a Transcriptor First Strand cDNA Synthesis Kit (Roche diagnostic, Mannheim, Germany). Reverse transcription was performed at 42°C for 10 min and at 55°C for 50 min. PCR amplifications were carried out for 30 or 35 cycles with Platinum TAQ (Invitrogen, Carlsbad, CA) following manufacturer's instructions. Primers used were: β-actin up 5′ ACTCCATCATGAAGTGTGACG and β-actin dw 5′ CATACTCCTGCTTGCTGATCC; H6 for 5′ CAGTTGCCGAAGGGATCTG and H6 rev 5′ GGTAGAACCCACTTGACTCA. PCR products (249 bp fragment for β-actin and 677 bp for B7-H6) were run on a 1.5% agarose gel and visualized by ethidium bromide staining.
Statistical analysis
Mann–Whitney non-parametric test were employed for evaluating quantitative variables. The statistical level of significance (p) is indicated. Graphic representation and statistical analysis were performed using the PASW Statistics, version 20.0 software (formerly SPSS Statistics) (IBM, Milan, Italy) and GraphPad Prism 6 (GraphPad Software La Jolla, CA).
Acknowledgments
We thank Benassi Marzia for technical assistance.
Disclosure of Potential Conflicts of Interest
A.M. and E.V. are a co-founders and shareholders in InnatePharma. The other authors have no conflicting interests.
Funding
Supported by grants awarded by Associazione Italiana Ricerca per la Ricerca sul Cancro (AIRC)-Special Project 5×1000 no. 9962 and IG 2014 Id. 15704 (Alessandro Moretta); PRIN 2010 (Alessandro Moretta); Progetto di Ricerca Fondazione Carige 2013 (Emanuela Marcenaro); Progetto di Ricerca di Ateneo 2014 (Emanuela Marcenaro). Eric Vivier laboratory is supported by the European Research Council (THINK Advanced Grant), by Equipe Labellisée La Ligue and by institutional grants from INSERM, CNRS, and Aix-Marseille University to CIML.
Supplemental Material
Supplemental data for this article can be accessed on http://www.dx.doi.org./10.4161/19420862.2014.976428 the publisher's website.
References
- 1. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:; http://dx.doi.org/ 10.1126/science.1198687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22:633-40; PMID:; http://dx.doi.org/ 10.1016/S1471-4906(01)02060-9 [DOI] [PubMed] [Google Scholar]
- 3. Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol 2001; 2:123-8; PMID:; http://dx.doi.org/ 10.1038/84219 [DOI] [PubMed] [Google Scholar]
- 4. Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007; 109:3625-32; PMID:; http://dx.doi.org/ 10.1182/blood-2006-08-038844 [DOI] [PubMed] [Google Scholar]
- 5. Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol 2002; 71:173-83; PMID: [PubMed] [Google Scholar]
- 6. Marcenaro E, Della Chiesa M, Ferranti B, Moretta A. In the thick of the fray: NK cells in inflamed tissues. Adv Exp Med Biol 2007; 598:12-9; PMID:; http://dx.doi.org/ 10.1007/978-0-387-71767-8_2 [DOI] [PubMed] [Google Scholar]
- 7. Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol 1996; 14:619-48; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.14.1.619 [DOI] [PubMed] [Google Scholar]
- 8. Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol 1999; 17:875-904; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.17.1.875 [DOI] [PubMed] [Google Scholar]
- 9. Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol 2002; 20:217-51; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.20.092501.134942 [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Botet M, Llano M, Navarro F, Bellon T. NK cell recognition of non-classical HLA class I molecules. Semin Immunol 2000; 12:109-19; PMID:; http://dx.doi.org/ 10.1006/smim.2000.0213 [DOI] [PubMed] [Google Scholar]
- 11. Lanier LL. NK cell receptors. Annu Rev Immunol 1998; 16:359-93; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.16.1.359 [DOI] [PubMed] [Google Scholar]
- 12. Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol 2001; 19:197-223; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.19.1.197 [DOI] [PubMed] [Google Scholar]
- 13. Lanier LL. On guard–activating NK cell receptors. Nat Immunol 2001; 2:23-7; PMID:; http://dx.doi.org/ 10.1038/83130 [DOI] [PubMed] [Google Scholar]
- 14. Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med 1997; 186:1129-36; PMID:; http://dx.doi.org/ 10.1084/jem.186.7.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med 1998; 188:953-60; PMID:; http://dx.doi.org/ 10.1084/jem.188.5.953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, Augugliaro R, Moretta L, Moretta A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med 1998; 187:2065-72; PMID:; http://dx.doi.org/ 10.1084/jem.187.12.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, Parolini S, Moretta L, Moretta A, Biassoni R. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med 1999; 189:787-96; PMID:; http://dx.doi.org/ 10.1084/jem.189.5.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med 1999; 190:1505-16; PMID:; http://dx.doi.org/ 10.1084/jem.190.10.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol 2005; 26:221-6; PMID:; http://dx.doi.org/ 10.1016/j.it.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 20. Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 2001; 409:1055-60; PMID:; http://dx.doi.org/ 10.1038/35059110 [DOI] [PubMed] [Google Scholar]
- 21. Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, Gazit R, Gonen-Gross T, Hanna J, Nahari E, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol 2005; 6:515-23; PMID:; http://dx.doi.org/ 10.1038/ni1190 [DOI] [PubMed] [Google Scholar]
- 22. Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Böll B, Simhadri VL, Borchmann P, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 2007; 27:965-74; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 23. Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One 2008; 3:e3377; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0003377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med 2009; 206:1495-503; PMID:; http://dx.doi.org/ 10.1084/jem.20090681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaifu T, Escaliere B, Gastinel LN, Vivier E, Baratin M. B7-H6/NKp30 interaction: a mechanism of alerting NK cells against tumors. Cell Mol Life Sci 2011; 68:3531-9; PMID:; http://dx.doi.org/ 10.1007/s00018-011-0802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, Wang Q, Mariuzza RA. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J Exp Med 2011; 208:703-14; PMID:; http://dx.doi.org/ 10.1084/jem.20102548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schlecker E, Fiegler N, Arnold A, Altevogt P, Rose-John S, Moldenhauer G, Sucker A, Paschen A, von Strandmann EP, Textor S, et al. Metalloprotease-Mediated Tumor Cell Shedding of B7-H6, the Ligand of the Natural Killer Cell-Activating Receptor NKp30. Cancer Res 2014; 74:3429-40; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3017 [DOI] [PubMed] [Google Scholar]
- 28. Matta J, Baratin M, Chiche L, Forel JM, Cognet C, Thomas G, Farnarier C, Piperoglou C, Papazian L, Chaussabel D, et al. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood 2013; 122:394-404; PMID:; http://dx.doi.org/ 10.1182/blood-2013-01-481705 [DOI] [PubMed] [Google Scholar]
- 29. Prat J, Oncology FCoG . Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014; 124:1-5; PMID:; http://dx.doi.org/ 10.1016/j.ijgo.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 30. Torres MP, Ponnusamy MP, Lakshmanan I, Batra SK. Immunopathogenesis of ovarian cancer. Minerva medica 2009; 100:385-400; PMID: [PubMed] [Google Scholar]
- 31. Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. AmJ Surg Pathol 2010; 34:433-43; PMID:; http://dx.doi.org/ 10.1097/PAS.0b013e3181cf3d79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol 2001; 166:6477-82; PMID:; http://dx.doi.org/ 10.4049/jimmunol.166.11.6477 [DOI] [PubMed] [Google Scholar]
- 33. Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med 2005; 202:941-53; PMID:; http://dx.doi.org/ 10.1084/jem.20050128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marcenaro E, Cantoni C, Pesce S, Prato C, Pende D, Agaugue S, Moretta L, Moretta A. Uptake of CCR7 and acquisition of migratory properties by human KIR+ NK cells interacting with monocyte-derived DC or EBV cell lines: regulation by KIR/HLA-class I interaction. Blood 2009; 114:4108-16; PMID:; http://dx.doi.org/ 10.1182/blood-2009-05-222265 [DOI] [PubMed] [Google Scholar]
- 35. Marcenaro E, Pesce S, Sivori S, Carlomagno S, Moretta L, Moretta A. KIR2DS1-dependent acquisition of CCR7 and migratory properties by human NK cells interacting with allogeneic HLA-C2+ DCs or T-cell blasts. Blood 2013; 121:3396-401; PMID:; http://dx.doi.org/ 10.1182/blood-2012-09-458752 [DOI] [PubMed] [Google Scholar]
- 36. Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, Kiessling R, Malmberg KJ. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol 2009; 183:4921-30; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0901226 [DOI] [PubMed] [Google Scholar]
- 37. Belisle JA, Gubbels JA, Raphael CA, Migneault M, Rancourt C, Connor JP, Patankar MS. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125). Immunology 2007; 122:418-29; PMID:; http://dx.doi.org/ 10.1111/j.1365-2567.2007.02660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wendt K, Wilk E, Buyny S, Buer J, Schmidt RE, Jacobs R. Gene and protein characteristics reflect functional diversity of CD56dim and CD56bright NK cells. J Leukoc Biol 2006; 80:1529-41; PMID:; http://dx.doi.org/ 10.1189/jlb.0306191 [DOI] [PubMed] [Google Scholar]
- 39. Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, Moretta L, Moretta A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol 1999; 29:1656-66; PMID:; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199905)29:05%3c1656::AID-IMMU1656%3e3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- 40. Lowrey DM, Hameed A, Lichtenheld M, Podack ER. Isolation and characterization of cytotoxic granules from human lymphokine (interleukin 2) activated killer cells. Cancer Res 1988; 48:4681-8; PMID: [PubMed] [Google Scholar]
- 41. Bryceson YT, Pende D, Maul-Pavicic A, Gilmour KC, Ufheil H, Vraetz T, Chiang SC, Marcenaro S, Meazza R, Bondzio I, et al. A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood 2012; 119:2754-63; PMID:; http://dx.doi.org/ 10.1182/blood-2011-08-374199 [DOI] [PubMed] [Google Scholar]
- 42. Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol 1999; 162:4511-20; PMID: [PubMed] [Google Scholar]
- 43. Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A 2003; 100:4120-5; PMID:; http://dx.doi.org/ 10.1073/pnas.0730640100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP, et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol ancer 2010; 9:11; PMID:; http://dx.doi.org/ 10.1186/1476-4598-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oei AL, Sweep FC, Thomas CM, Boerman OC, Massuger LF. The use of monoclonal antibodies for the treatment of epithelial ovarian cancer (review). Int J Oncol 2008; 32:1145-57; PMID:; http://dx.doi.org/ 10.3892/ijo_32_6_1145 [DOI] [PubMed] [Google Scholar]
- 46. Tabellini G, Benassi M, Marcenaro E, Coltrini D, Patrizi O, Ricotta D, Rampinelli F, Moretta A, Parolini S. Primitive neuroectodermal tumor in an ovarian cystic teratoma: natural killer and neuroblastoma cell analysis. Case Rep Oncol 2014; 7:70-8; PMID:; http://dx.doi.org/ 10.1159/000357802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Funamizu A, Fukui A, Kamoi M, Fuchinoue K, Yokota M, Fukuhara R, Mizunuma H. Expression of natural cytotoxicity receptors on peritoneal fluid natural killer cell and cytokine production by peritoneal fluid natural killer cell in women with endometriosis. Am J Reprod Immunol 2014; 71:359-67; PMID:; http://dx.doi.org/ 10.1111/aji.12206 [DOI] [PubMed] [Google Scholar]
- 48. Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K, Kiessling R, Ljunggren HG, et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res 2007; 67:1317-25; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-2264 [DOI] [PubMed] [Google Scholar]
- 49. Fiegler N, Textor S, Arnold A, Rolle A, Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M, Cerwenka A. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood 2013; 122:684-93; PMID:; http://dx.doi.org/ 10.1182/blood-2013-02-482513 [DOI] [PubMed] [Google Scholar]
- 50. Reiners KS, Dassler J, Coch C, Pogge von Strandmann E. Role of exosomes released by dendritic cells and/or by tumor targets: regulation of NK cell plasticity. Front Immunol 2014; 5:91; PMID:; http://dx.doi.org/ 10.3389/fimmu.2014.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kellner C, Maurer T, Hallack D, Repp R, van de Winkel JG, Parren PW, Valerius T, Humpe A, Gramatzki M, Peipp M. Mimicking an induced self phenotype by coating lymphomas with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J Immunol 2012; 189:5037-46; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1201321 [DOI] [PubMed] [Google Scholar]
- 52. Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754-8; PMID:; http://dx.doi.org/ 10.1182/blood.V99.3.754 [DOI] [PubMed] [Google Scholar]
- 53. Marcenaro E, Della Chiesa M, Bellora F, Parolini S, Millo R, Moretta L, Moretta A. IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J Immunol 2005; 174:3992-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.174.7.3992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.