Abstract
Antitumor immune responses against solid malignancies correlate with improved patient survival. We conducted a comprehensive investigation of immune responses in tumor and tumor-associated stroma in epithelioid malignant pleural mesothelioma with the goal of characterizing the tumor immune microenvironment and identifying prognostic immune markers. We investigated 8 types of tumor-infiltrating immune cells within the tumor nest and tumor-associated stroma, as well as tumor expression of 5 cytokine/chemokine receptors in 230 patients. According to univariate analyses, high densities of tumoral CD4- and CD20-expressing lymphocytes were associated with better outcomes. High expression of tumor interleukin-7 (IL-7) receptor was associated with worse outcomes. According to multivariate analyses, stage and tumoral CD20 detection were independently associated with survival. Analysis of single immune cell infiltration for CD163+ tumor-associated macrophages did not correlate with survival. However, analysis of immunologically relevant cell combinations identified that: (1) high CD163+ tumor-associated macrophages and low CD8+ lymphocyte infiltration had worse prognosis than other groups and (2) low CD163+ tumor associated macrophages and high CD20+ lymphocyte infiltration had better prognosis than other groups. Multivariate analyses demonstrated that CD163/CD8 and CD163/CD20 were independent prognostic factors of survival. With a recent increase in immunotherapy investigations and clinical trials for malignant pleural mesothelioma patients, our observations that CD20+ B lymphocytes and tumor-associated macrophages are prognostic markers provide important information about the tumor microenvironment of malignant pleural mesothelioma.
Keywords: B lymphocyte, immune responses to cancer, pleural malignancy, tumor-infiltrating lymphocytes, T lymphocytes
Abbreviations: CI, confidence interval; FoxP3, forkhead box P3; HR, hazard ratio; IL-7R, interleukin-7 receptor; MDSCs, myeloid-derived suppressor cells; MPM, malignant pleural mesothelioma; OS, overall survival; TAMs, tumor-associated macrophages; TILs, tumor-infiltrating lymphocytes; Tregs, regulatory T cells
Introduction
Malignant pleural mesothelioma (MPM) is a highly aggressive and rare primary pleural malignancy with median survival of 9–12 months.1 In epithelioid MPM, which is the most common histological type of MPM, median survival is only 17 months, even with trimodality therapy—chemotherapy, surgical resection, and thoracic radiation.2,3 Despite poor prognosis, few studies have reported that MPM patients with antitumor immune responses survived longer and that their improved survival was associated with increased CD8+ tumor-infiltrating lymphocytes (TILs).4,5 Immunosuppressive cytokines and regulatory T cells (Tregs) are also hypothesized to infiltrate the tumor microenvironment, dampening antitumor immune function and promoting MPM tumor growth.6 With recent successes, both preclinical7 and clinical,8 in immunotherapy for MPM, understanding the interplay between protumorigenic and antitumorigenic immune factors in the MPM tumor microenvironment is vital to developing novel therapies for MPM patients.
Although the prognostic utility of tumor-infiltrating immune cells for MPM has been previously investigated,4-6,9 these study cohorts were heterogeneous in histologic subtypes and included only a small number of patients. In our study here, we sought to elucidate the prognostic significance of the tumor immune microenvironment, so we qualitatively and quantitatively investigated immune cells infiltrating the tumor nest and tumor-associated stroma, as well as tumoral cytokine (receptor) expression, in a large cohort of patients with epithelioid MPM.
Results
Clinicopathologic variables
Demographical, clinical, and histopathological variables for 230 patients are shown in Table 1. Median follow-up time for survivors was 37.3 months (range, 0.5–92.5 months). Median overall survival (OS) was 16.3 months (95% confidence interval [CI]: 14.6–19.3 months), with 2-year OS of 33.2%, and 5-year OS of 11.6%. Univariate analysis revealed that male sex (P = 0.032), advanced stage (III, IV vs. I, II; P = 0.001), lymphatic invasion (P = 0.038), vascular invasion (P = 0.009), and pleomorphic histology (P = 0.015) were significantly associated with worse OS (Table 1).
Table 1.
Univariate analysis of overall survival and clinicopathologic factors of epithelioid MPM patient cohort
| Variables | N (%) | Median OS | 95% CI | P-value |
|---|---|---|---|---|
| All Patients | 230 | 16.3 | (14.6, 19.3) | |
| Age | ||||
| ≤65 | 136 (59) | 16.3 | (14.1, 22.0) | 0.27 |
| >65 | 94 (41) | 16.3 | (14.5, 19.4) | |
| Gender | ||||
| Female | 63 (27) | 22.4 | (17.4, 28.8) | 0.032 |
| Male | 167 (73) | 15.0 | (12.4, 17.5) | |
| Asbestos | ||||
| Yes | 100 (43) | 16.3 | (14.3, 21.0) | 0.71 |
| No | 66 (29) | 19.4 | (15.0, 23.8) | |
| Unknown | 64 (28) | 14.7 | (10.3, 19.9) | |
| Smoking | ||||
| Yes | 137 (60) | 16.8 | (15.0, 20.7) | 0.29 |
| No | 45 (20) | 19.1 | (14.1, 33.3) | |
| Unknown | 48 (21) | 12.3 | (8.9, 19.3) | |
| Laterality | ||||
| Right | 129 (56) | 16.8 | (13.8, 19.9) | 0.79 |
| Left | 100 (43) | 16.1 | (14.1, 22.0) | |
| T stage | ||||
| T1,2 | 116 (50) | 19.2 | (16.3, 23.8) | 0.025 |
| T3,4 | 114 (49) | 14.3 | (9.5, 17.4) | |
| N stage | ||||
| N0 | 162 (70) | 18.9 | (16.3, 22.4) | 0.077 |
| N1, 2 | 68 (28) | 9.8 | (7.4, 17.5) | |
| Stage | ||||
| I, II | 75 (33) | 23.4 | (19.1, 32.6) | 0.001 |
| II, IV | 155 (67) | 14.1 | (9.8, 17.0) | |
| Procedure | ||||
| EPP | 124 (54) | 15.0 | (12.4, 20.7) | 0.59 |
| P/D | 90 (39) | 18.1 | (15.1, 21.9) | |
| Others (Surgical biopsy) | 16 (7) | 12.9 | (4.8, 38.0) | |
| Lymphatic invasion | ||||
| Absent | 121 (53) | 19.3 | (16.2, 23.8) | 0.038 |
| Present | 109 (47) | 14.5 | (11.4, 16.9) | |
| Vascular invasion | ||||
| Absent | 177 (77) | 17.6 | (15.4, 21.0) | 0.009 |
| Present | 53 (23) | 11.4 | (8.2, 16.2) | |
| Induction Chemotherapy | ||||
| Yes | 63 (28) | 20.1 | (15.1, 27.5) | 0.15* |
| No | 164 (71) | 15.2 | (12.4, 18.1) | |
| Unknown | 3 (1) | 25.0 | (4.1, NA) | |
| Morphology | ||||
| Pleomorphic | 38 (17) | 14.6 | (8.1, 17.5) | 0.015 |
| Non-pleomorphic | 192 (83) | 17.5 | (14.9, 20.9) |
Abbreviations: CI = confidence interval; EPP = extrapleural pneumonectomy; OS = overall survival (months); P/D = pleurectomy/decortication.
Statistical analysis was performed by log-rank test; significant P-values (<0.05) are shown in bold type.
Excludes unknown.
Epithelioid MPM tumor immune microenvironment
The tumor microenvironment of MPM was characterized by examining tumor-associated expression of CD4, CD68, and CD163, as well as stromal-associated expression of CD163, CXCR4, and IL-12Rβ2. Immune and stromal markers were evaluated as described below to detect prognostic markers for MPM.
Survival analysis for tumor-infiltrating lymphocytes and cytokine (receptor) expression
Each immune parameter in the tumor nest and tumor-associated stroma was independently assessed for its associations with survival (Table 2). A high density of CD4-expressing cells in tumors was significantly associated with favorable survival (median OS, 15.2 months for low vs. 17.0 months for high level; P = 0.04; Fig. 1A), whereas a high density of CD8+ lymphoctyes in tumors reflected tendency for longer survival (median OS, 14.7 months for low levels vs. 17.0 months for high level; P = 0.061; Fig. 1B). Elevated levels of the B cell marker CD20 in tumors were also significantly associated with favorable survival (median OS, 14.5 months for low vs. 20.7 months for high level; P = 0.003; Fig. 1C).
Table 2.
Univariate analysis of overall survival and immune parameters
| Marker | Tumor | Stroma | ||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Median OS | 95%CI | P-value* | N (%) | Median OS | 95%CI | P-value* | |
| CD3 | ||||||||
| Low | 54 (25) | 14.3 | (9.4, 24.9) | 0.57 | 143 (67) | 16.2 | (13.8, 19.4) | 0.52 |
| High | 162 (75) | 16.8 | (15.0, 19.8) | 72 (33) | 17.4 | (15.0, 23.3) | ||
| CD4 | ||||||||
| Low | 113 (52) | 15.2 | (10.6, 19.2) | 0.04 | 28 (13) | 15.0 | (7.4, 21.9) | 0.26 |
| High | 105 (48) | 17.0 | (14.5, 24.8) | 188 (87) | 17.0 | (14.9, 20.1) | ||
| CD8 | ||||||||
| Low | 86 (40) | 14.7 | (12.0, 19.8) | 0.061 | 94 (44) | 18.9 | (14.7, 23.0) | 0.68 |
| High | 131 (60) | 17.0 | (14.5, 21.0) | 120 (56) | 15.3 | (12.5, 18.1) | ||
| CD45RO | ||||||||
| Low | 93 (61) | 16.3 | (14.5, 23.8) | 0.98 | 133 (86) | 16.2 | (14.6, 19.4) | 0.72 |
| High | 60 (39) | 15.0 | (9.8, 20.9) | 21 (14) | 17.4 | (10.1, 38.9) | ||
| CD20 | ||||||||
| Low | 128 (59) | 14.5 | (10.5, 16.8) | 0.003 | 94 (44) | 15.0 | (11.4, 18.9) | 0.21 |
| High | 89 (41) | 20.7 | (16.3, 26.9) | 119 (56) | 18.1 | (15.2, 23.3) | ||
| FoxP3 | ||||||||
| Low | 142 (65) | 16.1 | (14.0, 19.9) | 0.58 | 140 (66) | 16.2 | (14.3, 20.1) | 0.87 |
| High | 75 (35) | 16.8 | (11.4, 21.1) | 72 (34) | 17.0 | (15.0, 21.1) | ||
| CD68 | ||||||||
| Low | 172 (79) | 16.3 | (14.3, 19.8) | 0.87 | 22 (10) | 22.0 | (16.2, 61.1) | 0.067 |
| High | 47 (21) | 16.9 | (10.9, 23.4) | 193 (90) | 16.3 | (14.5, 19.2) | ||
| CD163 | ||||||||
| Low | 112 (53) | 19.1 | (15.1, 23.4) | 0.49 | 133 (67) | 18.1 | (15.1, 22.4) | 0.12 |
| High | 98 (47) | 15.0 | (10.6, 18.1) | 66 (33) | 14.5 | (10.3, 17.0) | ||
| IL-7R | ||||||||
| Low | 116 (53) | 19.3 | (16.3, 26.4) | 0.007 | † | |||
| High | 103 (47) | 14.0 | (10.6, 18.1) | |||||
| IL-12Rβ2 | ||||||||
| Low | 112 (75) | 15.4 | (13.8, 19.1) | 0.067 | † | |||
| High | 38 (25) | 19.9 | (14.5, 41.6) | |||||
| CCR7 | ||||||||
| Low | 123 (79) | 15.2 | (14.0, 18.1) | 0.16 | † | |||
| High | 33 (21) | 28.1 | (15.0, 45.5) | |||||
| CXCL12 | ||||||||
| Low | 55 (36) | 15.4 | (11.4, 18.1) | 0.49 | † | |||
| High | 99 (64) | 16.8 | (15.0, 23.4) | |||||
| CXCR4 | ||||||||
| Low | 104 (69) | 15.0 | (12.5, 17.6) | 0.081 | † | |||
| High | 46 (31) | 21.9 | (12.4, 35.2) | |||||
Abbreviations: CI = confidence interval; FoxP3 = forkhead box P3; IL-7R = interleukin-7 receptor; IL-12Rβ2 = interleukin-12 receptor β2; OS = overall survival (months).
Statistical analysis was performed by log-rank test; significant P-values (<0.05) are shown in bold type.
Stroma not applicable for this marker.
Adjusted for search of optimal cut-point.
Figure 1.
Survival analysis of MPM epithelioid patients according to the presence of tumor-infiltrating lymphocytes. Kaplan-Meier survival analysis of of malignant pleural mesothelioma (MPM) patients (n = 230) according to the indicated tumor-associated immune markers. Overall survival (OS) by the presence of cells expressing (A) tumoral CD4, (B) tumoral CD8, (C) tumoral CD20 and (D) IL-7R.
Of the 5 cytokines and cytokine receptors, interleukin-7 receptor (IL-7R) was determined to be a prognostic factor (Table 2). Higher-level expression of IL-7R was associated with increased risk of death (median OS, 19.3 months for low level vs. 14.0 months for high level; P = 0.007; Fig. 1D).
Multivariate analyses were performed for the 3 prognostic immune markers—tumor CD4, tumor CD20, and IL-7R—and these models adjusted for the prognostic clinicopathologic factors from univariate analysis, including sex, disease stage (III, IV vs. I, II), vascular invasion, and pleomorphic morphology. The final multivariate model confirmed that stage (hazard ratio [HR] 1.72, 95% CI 1.26–2.35; P < 0.001) and tumor CD20 (HR 0.69, 95% CI 0.51–0.93; P = 0.015) remained independently associated with survival. Higher-level expression of IL-7R reflected a tendency for increased risk of death (HR 1.34, 95% CI 1.00–1.81; P = 0.052) (Table 3A).
Table 3.
Multivariate analysis of overall survival
| A. Multivariate analysis for single immune marker | |||
|---|---|---|---|
| Factors | HR | 95% CI | P-value |
| Gender (Male vs. Female) | 1.37 | (0.98, 1.90) | 0.062 |
| Stage (III/IV vs. I/II) | 1.72 | (1.26, 2.35) | <0.001 |
| Vascular invasion (Yes vs. No) | 1.20 | (0.85, 1.70) | 0.29 |
| Morphology (Pleomorphic vs. Non-pleomorphic) | 1.34 | (0.9, 2.00) | 0.15 |
| CD20 tumor (High vs. Low) | 0.69 | (0.51, 0.93) | 0.015 |
| IL-7R (High vs. Low) | 1.34 | (1.00, 1.81) | 0.052 |
| B. Multivariate analysis for CD163 combination | |||
| Factors | HR | 95% CI | P-value |
| Gender (Male vs. Female) | 1.39 | (1.00, 1.93) | 0.053 |
| Stage (III/IV vs. I/II) | 1.57 | (1.15, 2.14) | 0.005 |
| Vascular invasion (Yes vs. No) | 1.21 | (0.86, 1.71) | 0.28 |
| Morphology (Pleomorphic vs. Non-pleomorphic) | 1.29 | (0.86, 1.92) | 0.21 |
| CD163/CD8 tumor (High risk vs. Low risk) | 1.64 | (1.01, 2.66) | 0.044 |
| CD163/CD20 tumor (High risk vs. Low risk) | 1.64 | (1.10, 2.44) | 0.015 |
Abbreviations: CI = confidence interval; HR, = hazard ratio; IL-7R = interleukin-7 receptor.
Statistical analysis was performed by Cox proportional hazards regression model; significant P-values (<0.05) are shown in bold type.
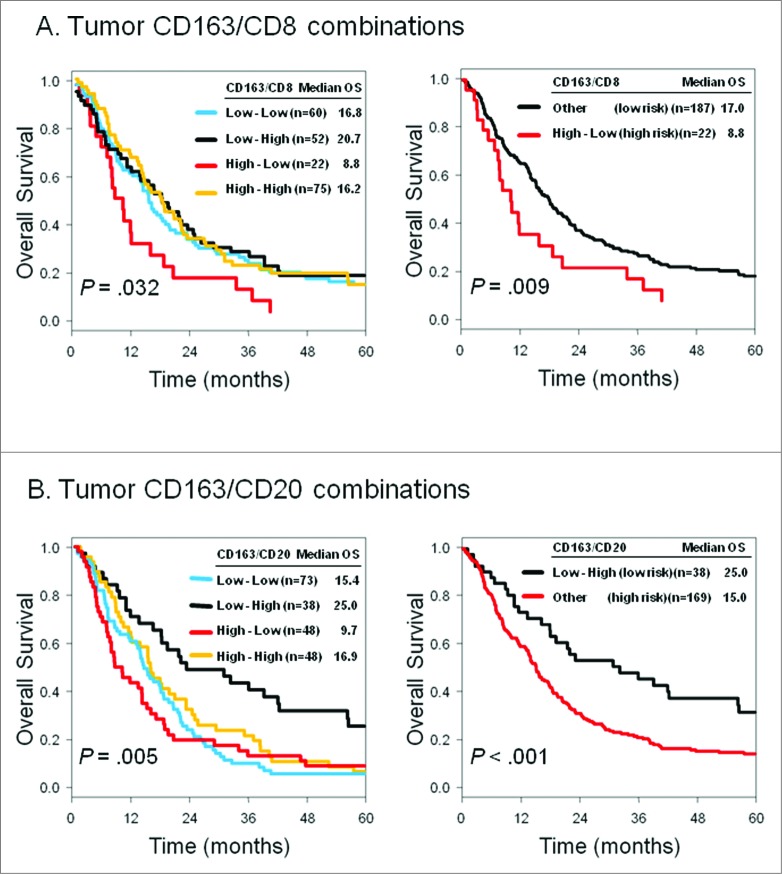
Survival analysis for tumor-associated macrophages levels and ratio to tumor-infiltrating lymphocytes
Analysis of single immune cell infiltration revealed that CD163+ M2-polarized tumor-associated macrophages (TAMs) were not correlated with OS (tumor: P = 0.49, stroma: P = 0.12). Next, we investigated immunologically relevant combinations—the relative proportion of protumorigenic CD163+ TAMs to antitumorigenic tumor-infiltrating lymphocytes (TILs) (Fig. 2). On the basis of this observation, patients with high CD163+ TAMs and low CD8+ lymphocyte infiltration had worse prognosis (median OS, 8.8 months for high risk index) than other groups (median OS, 17.0 months for low risk index; P = 0.009; Fig. 2A) and, conversely, patients with low CD163+ TAMs and high CD20+ lymphocyte infiltration had better prognosis (median OS, 25.0 months for low risk index) than other groups (median OS, 15.0 months for high risk index; P < 0.001; Fig. 2B). In the final multivariate model, stage (HR 1.57; 95% CI 1.15–2.14; P = 0.005), tumoral CD163/CD8 (HR 1.64; 95% CI 1.01–2.66; P = 0.044), and CD163/CD20 (HR 1.64; 95% CI 1.10–2.44 P = 0.015) expression remained independent predictors of worse OS (Table 3B).
Figure 2.
Survival analysis of MPM epithelioid patients according to the presence of tumor-associated macrophages and lymphocytes. Kaplan-Meier survival analysis of of malignant pleural mesothelioma (MPM) patients (n = 230) according to the indicated tumor-associated macrophage marker (CD163) and T and B lymphocytes (CD8 and CD20, respectively). Overall survival (OS) by (A) tumoral CD163/CD8 combinations, and (B) tumoral CD163/CD20 combinations.
Subgroup analysis of non-induction chemotherapy cases
In our cohort, there were 63 (27%) cisplatin-based induction chemotherapy cases and 164 (71%) non-induction chemotherapy cases. We performed subgroup analyses of immune markers for the non-induction chemotherapy cases. In this subgroup, higher-level expression of IL-7R was associated with increased risk of death (median OS, 12.3 months for high vs. 19.1 months for low level; P = 0.006). Low density of CD68 in the stroma was significantly associated with better survival (median OS, 23.4 months for low vs. 15.0 months for high level; P = 0.003). Low density of CD163 in the stroma was also significantly associated with better survival (median OS, 17.5 months for low vs. 11.0 months for high level; P = 0.041) (Table 4). The sample size of induction therapy patients was insufficient to analyze separately. However, immunohistochemical scoring information for the expression of each immune marker in all epitheloid MPM patients-(both with and without induction chemotherapy)-is provided in Supplemental Table 2.
Table 4.
Univariate analysis of overall survival and immune parameter in non-induction chemotherapy patients (N = 164)
| Marker | Tumor | Stroma | ||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Median OS | 95%CI | P- value* | N (%) | Median OS | 95%CI | P-value* | |
| CD3 | 0.24 | |||||||
| Low | 38 (25) | 14.4 | (9.4, 28.8) | 101 (66) | 15.4 | (12.3, 19.3) | 0.65 | |
| High | 115 (75) | 15.4 | (12.3, 19.2) | 52 (34) | 15.2 | (11.4, 20.9) | ||
| CD4 | 0.13 | |||||||
| Low | 87 (56) | 14.7 | (9.8, 18.9) | 19 (12) | 15.0 | (7.4, 22.0) | 0.14 | |
| High | 68 (44) | 16.1 | (12.4, 23.8) | 135 (88) | 16.1 | (12.5, 19.2) | ||
| CD8 | 0.17 | |||||||
| Low | 63 (41) | 14.7 | (12.0, 19.8) | 63 (41) | 18.9 | (14.7, 22.0) | 0.24 | |
| High | 91 (59) | 16.1 | (11.4, 19.3) | 91 (59) | 14.5 | (11.4, 17.0) | ||
| CD45RO | 0.9 | |||||||
| Low | 70 (60) | 15.4 | (12.4, 23.4) | 103 (87) | 15.0 | (12.4, 18.9) | 0.71 | |
| High | 46 (40) | 15.0 | (9.5, 20.9) | 15 (13) | 16.3 | (8.2, 26.6) | ||
| CD20 | 0.18 | |||||||
| Low | 97 (63) | 15.0 | (10.6, 18.1) | 67 (44) | 15.0 | (10.6, 18.9) | 0.22 | |
| High | 57 (37) | 16.9 | (11.4, 23.8) | 85 (56) | 16.3 | (12.4, 20.9) | ||
| FoxP3 | 0.75 | |||||||
| Low | 97 (63) | 16.1 | (12.5, 19.9) | 101 (65) | 15.0 | (12.3, 19.1) | 0.51 | |
| High | 58 (37) | 14.5 | (9.4, 19.4) | 54 (35) | 15.4 | (10.5, 20.9) | ||
| CD68 | 0.79 | |||||||
| Low | 125 (81) | 15.2 | (12.3, 19.2) | 15 (10) | 23.4 | (16.2, 113.8) | 0.03 | |
| High | 30 (19) | 15.0 | (8.1, 23.4) | 139 (90) | 15.0 | (12.3, 17.5) | ||
| CD163 | 0.26 | |||||||
| Low | 81 (54) | 18.9 | (14.7, 22.0) | 94 (67) | 17.5 | (14.5, 22.0) | 0.041 | |
| High | 70 (46) | 12.0 | (8.8, 16.9) | 46 (33) | 11.0 | (8.7, 16.1) | ||
| IL-7R | 0.006 | |||||||
| Low | 80 (52) | 19.1 | (15.2, 28.1) | † | ||||
| High | 75 (48) | 12.3 | (9.0, 16.2) | |||||
| IL-12Rβ2 | 0.27 | |||||||
| Low | 90 (78) | 15.2 | (12.3, 19.1) | † | ||||
| High | 26 (22) | 14.7 | (8.2, 38.0) | |||||
| CCR7 | 0.19 | |||||||
| Low | 94 (80) | 14.9 | (12.0, 17.6) | † | ||||
| High | 24 (20) | 28.1 | (9.5, 45.5) | |||||
| CXCL12 | 0.68 | |||||||
| Low | 40 (34) | 14.0 | (9.8, 18.1) | † | ||||
| High | 77 (66) | 16.3 | (14.5, 22.0) | |||||
| CXCR4 | 0.67 | |||||||
| Low | 82 (72) | 15.0 | (12.3, 19.1) | † | ||||
| High | 32 (28) | 16.2 | (8.8, 28.8) | |||||
Abbreviations: CI = confidence interval; FoxP3 = forkhead box P3; IL-7R = interleukin-7 receptor; IL-12Rβ2 = interleukin-12 receptor β2; OS = overall survival (months).
Statistical analysis was performed by log-rank test; significant P-values (<0.05) are shown in bold type.
Stroma not applicable for this marker.
Adjusted for search of optimal cut-point.
Discussion
In our investigation of TILs and expression of cytokine receptors on tumor cells in MPM, we found that tumor CD20, IL-7R, CD163/CD8, and CD163/CD20 were significant prognostic factors of epithelioid MPM.
It has been demonstrated that the presence of tumor-infiltrating immune cells correlate with the clinical outcome of multiple solid tumors, and with outcomes predicated on type, density, and location of immune cell infiltrates.10-14 While our initial investigations supported the prognostic role of inflammatory responses as independent predictors of survival in 175 epithelioid MPM patients,15 detailed investigations in this study using specific cellular markers highlights the influence of protumor and antitumor immune responses within the tumor and associated stroma.
Other investigators previously reported correlations between the presence of CD8+ TILs and MPM patient survival in a smaller cohort of 32 patients, as demonstrated by immunohistochemical analysis of extrapleural pneumonectomy specimens.4,5 In our study, patients with a high density of CD8+ TILs in tumors tended to exhibit improved survival; although, these results were not statistically significant (P = 0.061; Fig. 1B). Furthermore, Anraku et al.4 demonstrated protective association of CD8+ TILs in patients who received induction chemotherapy and a high density of CD8+ TILs was found to be an independent predictor of prolonged survival and correlated with reduced frequency of mediastinal lymph node metastases. These results suggested that strategies promoting tumor-infiltrating CD8+ T cells may clinically benefit MPM patients.
Various prior studies have investigated the potential effects of the presence of distinct T-cell subpopulations in MPM.4,5 In a preclinical orthotopic mouse model of MPM, we have shown that adoptive T-cell therapy-induced immune responses are predominantly mediated by CD4-expressing T cells that provide beneficial and durable immunity.7 By contrast, there was a distinct lack of data regarding B cell infiltrate in MPM. B lymphocytes are effector cells that have humoral immunity and can terminally differentiate into antibody secreting plasma cells upon stimulation. Moreover, B cells contributed to cellular immunity by serving as antigen-presenting cells and/or by providing stimulatory signals to T cells.16 The role of B lymphocytes during tumor immunity remains controversial. On one hand, antigen-presenting B cells were found to induce tumor-specific cytotoxic T-cell activation,17 and B cell deficient mice exhibited significantly reduced tumor-specific, T-cell immunity.18 On the other hand, B cell antibody response may potentiate chronic inflammation that could enhance tumor development.19 Tumor-infiltrating CD20+ B lymphocytes were found to associate with improved patient survival in primary breast cancer,20 non-small cell lung cancer,21 epithelial ovarian cancer,22 and pancreatic cancer.23 Our study provides the first evidence that B cells, perhaps as part of the humoral immune response, may have a role in constraining epithelioid MPM.
In our study, M2-polarized TAMs (i.e., CD163+) and their ratio with biologically relevant TILs (i.e., CD8+ and CD20+ lymphocytes) were independent predictors of survival in epithelioid MPM. In the non-induction chemotherapy group, high stromal CD163+ TAMs were associated with poor survival. Emerging evidence has pointed to the clinical significance of TAMs in several malignant tumors.24–26 To our knowledge, the association between CD163+ TAMs and clinical outcome has not been fully investigated in patients with MPM. In various solid tumors, while in the presence of appropriate cytokines or ligands, macrophages polarized into 2 types—M1 and M2 TAMs.27 A hallmark of macrophages is their plasticity—the ability to either aid or fight tumors depending on the tumor microenvironment—which has given them the reputation in tumor biology of being a “double-edged sword.”28 M1 TAMs demonstrated immunostimulatory properties and conferred enhanced tumor resistance and cytotoxicity,25 while M2 TAMs (CD163) secreted immunosuppressive cytokines, promoted angiogenesis, and supported tumor progression, invasion, and metastasis.27 Interaction with MPM cells appeared to shift mature macrophages toward the M2 phenotype, which was characterized by poor antigen presentation and increased immunosuppressive activity.29 Upon co-cultivation with MPM cells, macrophages released significant amounts of prostaglandin E2, an arachidonic acid metabolite with considerable anti-inflammatory properties.29 Production of this prostaglandin has been shown to stimulate the development of regulatory T cells, promoting an immunosuppressive tumor microenvironment.30 Tumor-associated macrophages have also been reported to upregulate interleukin-10 and B7-H3 on tumor cells which, in turn, inhibit antitumor T-cell responses.31 Worse prognosis correlating with elevated CD163+ TAMs in the current non-induction chemotherapy cohort supports the premise that the adaptive humoral immune response may play a crucial role in disease progression.
In our study, high expression levels of tumoral IL-7R were associated with unfavorable prognosis, even after excluding the induction chemotherapy group. We previously reported that IL-7R was a poor prognostic marker in early-stage lung adenocarcinoma patients.32,33 The role of IL-7R expression in MPM remains unknown, although in lung34 and breast35 cancers, IL-7R was shown to induce tumor growth and lymphangiogenesis via upregulation of vascular endothelial growth factor D. The ligand of IL-7R, IL-7, is produced by bone marrow and thymic stromal and epithelial cells, as well as a variety of tumor cells. Most human FoxP3+ Tregs express low levels of IL-7R as IL-7 signaling plays a role in the development and function of these cells.36 Therefore, IL-7 may promote tumor progression via the activation of IL-7R on both tumor cells and Tregs. Therefore, therapeutic strategies that target the IL-7/IL-7R axis may decrease tumor growth and lymphangiogenesis while limiting the immunosuppressive effects of Tregs.33
Based on our observation that higher levels of tumor-infiltrating lymphocytes were associated with improved survival among MPM patients, we investigated the therapeutic utility of chimeric antigen receptor (CAR)-directed adoptive T-cell therapy in clinically relevant MPM mouse models, work now being translated into a Phase I clinical trial.7
A side note, as the number of patients receiving neoadjuvant therapy in our cohort was low (n = 63) and the chemotherapy regimen and cycles varied among patients, we did not conduct a separate analysis of this cohort. Similarly, since the number of mesothelioma patients with biphasic (n = 26) and sarcomatoid (n = 23) histologies were low, both subsets of patients displayed shorter survival (median survival was 7.6 months in biphasic patients and 4 months in sarcomatoid patients) and inadequate tissue was available from patients due to lack of surgical resections, such that we also did not perform a separate analysis on this cohort.
Conclusion
Our findings shed light on the complex tumor immune microenvironment in epithelioid MPM. First, we demonstrated the association of CD4-, CD8-, and CD20-expressing lymphocytes with favorable prognosis. Second, we demonstrated that M2-polarized TAMs (CD163+) and their ratio to biologically relevant TILs (CD8+ T cells and CD20+ B cells) were independent markers of prognosis. Third, IL-7R expression on tumor cells was associated with worse patient survival.
To our knowledge, we are among the first to identify prognostic immune factors dictating the tumor immune microenvironment in a large-scale study of epithelioid MPM patients. Our findings provide the foundation for future investigations into immunomodulatory therapies for epithelioid MPM.
Materials and Methods
Patients
Clinical and pathological data for 620 patients diagnosed with MPM between 1989 and 2010 at Memorial Sloan Kettering Cancer Center (MSK) were obtained from the prospectively maintained Thoracic Surgery Mesothelioma Database upon MSK's Internal Review Board approval.15,37-40
From this cohort, we reviewed 395 MPM cases with available hematoxylin and eosin-stained slides. All slides were re-reviewed by 2 pathologists (K.K. and W.D.T.) yielding 301 epithelioid, 59 biphasic, and 35 sarcomatoid MPMs. All slides were evaluated for lymphatic and vascular invasion.37,38 This study focused on epithelioid MPM cases with tissue available for the construction of tissue microarray (TMA). A total of 230 epithelioid MPM cases with TMAs were included in the analysis. Staging was based on the seventh edition of the American Joint Committee on Cancer TNM Cancer Staging Manual.41 All patients were observed until death or end of study (January 2014).
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded tumor specimens were used for TMA construction. For each tumor, the area with the most severe inflammatory reaction was chosen. From each tumor, 9 representative cores with the most abundant inflammatory reaction, 0.6 mm in size, were marked—6 from the tumor nest and 3 from the tumor-associated stroma.42 We constructed TMAs from 230 epithelioid MPM cases. Standard avidin-biotin-peroxidase complex technique was used for immunohistochemical staining of human-specific antibodies (Table 5).32
Table 5.
Antibodies used for immunohistochemistry
| Marker | Source | Type | Manufacturer | Dilution |
|---|---|---|---|---|
| CD3 | Mouse | Monoclonal | Dako (Glostrup, Denmark) | 1:1,600 |
| CD4 | Goat | Polyclonal | R&D Systems (Minneapolis, MN) | 1:100 |
| CD8 | Mouse | Monoclonal | Dako | 1:200 |
| CD20 | Mouse | Monoclonal | Dako | 1:4,000 |
| CD45RO | Mouse | Monoclonal | Dako | 1:4,000 |
| FoxP3 | Mouse | Monoclonal | Abcam (Cambridge, United Kingdom) | 1:2,000 |
| CD68 | Mouse | Monoclonal | Dako | 1:2,000 |
| CD163 | Mouse | Monoclonal | Vector (Burlingame, CA) | 1:100 |
| CCR7 | Rabbit | Monoclonal | Epitomics (Burlingame, CA) | 1:100 |
| CXCL12 | Mouse | Monoclonal | R&D Systems | 1:1,000 |
| CXCR4 | Mouse | Monoclonal | R&D Systems | 1:6,000 |
| IL-7R | Mouse | Polyclonal | Santa Cruz Biotechnology (Santa Cruz, CA) | 1:2,000 |
| IL12R-β2 | Goat | Polyclonal | Santa Cruz Biotechnology | 1:100 |
Abbreviations: FoxP3 = forkhead box P3; IL-7R = interleukin-7 receptor; IL-12Rβ2 = interleukin-12 receptor β2.
Scoring of immunohistochemistry
Representative images and immunohistochemical scoring are shown in Supplemental Figure 1. Under a high-power field (magnification, ×200), each core was scored semi-quantitatively for the degree of immune cell infiltration into the tumor nest and tumor-associated stroma. Scores for each core were averaged to give a single score for each patient. For expression of cytokine and chemokine receptors, we scored tumor stains on the basis of intensity and distribution, as previously described.32,43
Statistical analysis
Associations between variables were analyzed using the Fisher's exact test for categorical variables and the Wilcoxon test for continuous variables.44 CD marker expressions were dichotomized into high versus low using optimal cut-points, which were found using a maximally selected log-rank statistic (Table S1). Overall survival was estimated using the Kaplan-Meier method starting at time of surgery. Patients who did not die during study follow-up were censored at last time they were known to be alive. Differences in OS between patient subgroups were compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards regression model to estimate the effect of immune markers of interest on OS, with adjustments for clinicopathologic factors that were found to be significantly associated with OS on univariate analyses. A separate multivariate model was built for each immune marker that was significant on univariate analyses. The final multivariate model included immune markers that were significantly associated with OS in their separate multivariate models. All significance tests were 2-sided and used a 5% level of significance. Statistical analyses were conducted using the “survival” and “maxstat” packages in R version 3.0.1 (R Development Core Team).
Acknowledgments
We thank Joe Dycoco of the MSK Thoracic Surgery Service for his help with the Thoracic Service Mesothelioma Database; Alex Torres of the MSK Thoracic Surgery Service for his editorial assistance; and Irina Linkov of the MSK Department of Pathology for performing all immunohistochemical staining.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This author's laboratory work is supported by grants from the National Institutes of Health (R21 CA164568-01A1, R21 CA164585-01A1, R01 CA136705-06, U54 CA137788, P30 CA008748-48, and P50 CA086438-13), the US Department of Defense (PR101053 and LC110202), and the Mr. William H. Goodwin and Mrs. Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet 2005; 366: 397–408; PMID:; http://dx.doi.org/ 10.1016/S0140-6736(05)67025-0 [DOI] [PubMed] [Google Scholar]
- 2.Krug LM, Pass HI, Rusch VW, Kindler HL, Sugarbaker DJ, Rosenzweig KE, Flores R, Friedberg JS, Pisters K, Monberg M, et al.. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009; 27: 3007–13; PMID:; http://dx.doi.org/ 10.1200/JCO.2008.20.3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores RM, Krug LM, Rosenzweig KE, Venkatraman E, Vincent A, Heelan R, Akhurst T, Rusch VW. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: a phase II trial. J Thorac Oncol 2006; 1: 289–95; PMID:; http://dx.doi.org/ 10.1097/01243894-200605000-00004 [DOI] [PubMed] [Google Scholar]
- 4.Anraku M, Cunningham KS, Yun Z, Tsao MS, Zhang L, Keshavjee S, Johnston MR, de Perrot M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2008; 135: 823–9; PMID:; http://dx.doi.org/ 10.1016/j.jtcvs.2007.10.026 [DOI] [PubMed] [Google Scholar]
- 5.Yamada N, Oizumi S, Kikuchi E, Shinagawa N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T, Torigoe T, et al.. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother 2010; 59: 1543–9; PMID:; http://dx.doi.org/ 10.1007/s00262-010-0881-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegmans JP, Hemmes A, Hammad H, Boon L, Hoogsteden HC, Lambrecht BN. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur Respir J 2006; 27: 1086–95; PMID:; http://dx.doi.org/ 10.1183/09031936.06.00135305 [DOI] [PubMed] [Google Scholar]
- 7.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014; 6: 261ra151; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3010162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, Kreitman RJ, Miettinen MM, Steinberg SM, Fowler DH, et al.. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med 2013; 5: 208ra147; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3006941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao SC, Klebe S, Henderson DW, Reid G, Chatfield M, Armstrong NJ, Yan TD, Vardy J, Clarke S, van Zandwijk N, et al.. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 2011; 6: 1923–9; PMID:; http://dx.doi.org/ 10.1097/JTO.0b013e31822a3740 [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–4; PMID:; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 11.Bograd AJ, Suzuki K, Vertes E, Colovos C, Morales EA, Sadelain M, Adusumilli PS. Immune responses and immunotherapeutic interventions in malignant pleural mesothelioma. Cancer Immunol Immunother 2011; 60: 1509–27; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al.. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353: 2654–66; PMID:; http://dx.doi.org/ 10.1056/NEJMoa051424 [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al.. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348: 203–13; PMID:; http://dx.doi.org/ 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29: 1949–55; PMID:; http://dx.doi.org/ 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Kadota K, Sima CS, Sadelain M, Rusch VW, Travis WD, Adusumilli PS. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother 2011; 60: 1721–8; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A 2007; 104: 20878–83; PMID:; http://dx.doi.org/ 10.1073/pnas.0709205105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood 2004; 103: 2046–54; PMID:; http://dx.doi.org/ 10.1182/blood-2003-07-2379 [DOI] [PubMed] [Google Scholar]
- 18.Schultz KR, Klarnet JP, Gieni RS, HayGlass KT, Greenberg PD. The role of B cells for in vivo T cell responses to a Friend virus-induced leukemia. Science 1990; 249: 921–3; PMID:; http://dx.doi.org/ 10.1126/science.2118273 [DOI] [PubMed] [Google Scholar]
- 19.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005; 7: 411–23; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 20.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat 2012; 132: 545–53; PMID:; http://dx.doi.org/ 10.1007/s10549-011-1620-1 [DOI] [PubMed] [Google Scholar]
- 21.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008; 14: 5220–7; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0133 [DOI] [PubMed] [Google Scholar]
- 22.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009; 4: e6412; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0006412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tewari N, Zaitoun AM, Arora A, Madhusudan S, Ilyas M, Lobo DN. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer 2013; 13: 436; PMID:; http://dx.doi.org/ 10.1186/1471-2407-13-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 2000; 17: 445–51; PMID: [DOI] [PubMed] [Google Scholar]
- 25.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009; 86: 1065–73; PMID:; http://dx.doi.org/ 10.1189/jlb.0609385 [DOI] [PubMed] [Google Scholar]
- 26.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002; 196: 254–65; PMID:; http://dx.doi.org/ 10.1002/path.1027 [DOI] [PubMed] [Google Scholar]
- 27.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol 2008; 18: 349–55; PMID:; http://dx.doi.org/ 10.1016/j.semcancer.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 28.Brower V. Macrophages: cancer therapy's double-edged sword. J Natl Cancer Inst 2012; 104: 649–52; PMID:; http://dx.doi.org/ 10.1093/jnci/djs235 [DOI] [PubMed] [Google Scholar]
- 29.Izzi V, Chiurchiu V, D'Aquilio F, Palumbo C, Tresoldi I, Modesti A, Baldini PM. Differential effects of malignant mesothelioma cells on THP-1 monocytes and macrophages. Int J Oncol 2009; 34: 543–50; PMID: [PubMed] [Google Scholar]
- 30.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc'h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 2005; 175: 1483–90; PMID:; http://dx.doi.org/ 10.4049/jimmunol.175.3.1483 [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Shen Y, Qu QX, Chen XQ, Zhang XG, Huang JA. Induced expression of B7-H3 on the lung cancer cells and macrophages suppresses T-cell mediating anti-tumor immune response. Exp Cell Res 2013; 319: 96–102; PMID:; http://dx.doi.org/ 10.1016/j.yexcr.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, Sadelain M, Adusumilli PS. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol 2013; 31: 490–8; PMID:; http://dx.doi.org/ 10.1200/JCO.2012.45.2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadota K, Nitadori JI, Adusumilli PS. Prognostic value of the immune microenvironment in lung adenocarcinoma. Oncoimmunology 2013; 2: e24036; PMID:; http://dx.doi.org/ 10.4161/onci.24036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ming J, Zhang Q, Qiu X, Wang E. Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jun-dependent vascular endothelial growth factor-D up-regulation: a mechanism of lymphangiogenesis in lung cancer. Eur J Cancer 2009; 45: 866–73; PMID:; http://dx.doi.org/ 10.1016/j.ejca.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 35.Al-Rawi MA, Watkins G, Mansel RE, Jiang WG. Interleukin 7 upregulates vascular endothelial growth factor D in breast cancer cells and induces lymphangiogenesis in vivo. Br J Surg 2005; 92: 305–10; PMID:; http://dx.doi.org/ 10.1002/bjs.4832 [DOI] [PubMed] [Google Scholar]
- 36.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nature Reviews Immunology 2009; 9: 480–90; PMID:; http://dx.doi.org/ 10.1038/nri2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadota K, Suzuki K, Colovos C, Sima CS, Rusch VW, Travis WD, Adusumilli PS. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol 2012; 25: 260–71; PMID:; http://dx.doi.org/ 10.1038/modpathol.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadota K, Suzuki K, Sima CS, Rusch VW, Adusumilli PS, Travis WD. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol 2011; 6: 896–904; PMID:; http://dx.doi.org/ 10.1097/JTO.0b013e318211127a [DOI] [PubMed] [Google Scholar]
- 39.Servais EL, Colovos C, Rodriguez L, Bograd AJ, Nitadori J, Sima C, Rusch VW, Sadelain M, Adusumilli PS. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res 2012; 18: 2478–89; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flores RM, Zakowski M, Venkatraman E, Krug L, Rosenzweig K, Dycoco J, Lee C, Yeoh C, Bains M, Rusch V. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007; 2: 957–65; PMID:; http://dx.doi.org/ 10.1097/JTO.0b013e31815608d9 [DOI] [PubMed] [Google Scholar]
- 41.Edge SB, American . Joint Committee on Cancer AJCC cancer staging manual. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 42.Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, Chou J, Sima CS, Vertes E, Rusch VW, et al.. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res 2014; 20: 1020–8; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshizawa A, Fukuoka J, Shimizu S, Shilo K, Franks TJ, Hewitt SM, Fujii T, Cordon-Cardo C, Jen J, Travis WD. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clin Cancer Res 2010; 16: 240–8; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med 2000; 19: 113–32; PMID:; http://dx.doi.org/ 10.1002/(SICI)1097-0258(20000115)19:1%3c113::AID-SIM245%3e3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.