Abstract
Previous evidence has shown that ligating the femoral artery for 72 h resulted in an exaggerated exercise pressor reflex. To provide electrophysiological evidence for this finding, we examined in decerebrated rats whose femoral arteries were either freely perfused or ligated for 72 h the responses of thin-fiber (i.e., groups III and IV) afferents to static contraction of the hindlimb muscles. We found that contraction increased the combined activity of group III and IV afferents in both freely perfused (n = 29; baseline: 0.3 ± 0.1 imp/s, contraction: 0.8 ± 0.2 imp/s; P < 0.05) and ligated rats (n = 28; baseline: 0.4 ± 0.1 imp/s, contraction: 1.4 ± 0.1 imp/s; P < 0.05). Most importantly, the contraction-induced increase in afferent activity was greater in ligated rats than it was in freely perfused rats (P = 0.005). In addition, the responses of group III afferents to contraction in ligated rats (n = 15; baseline 0.3 ± 0.1 imp/s, contraction 1.5 ± 0.2 imp/s) were greater (P = 0.024) than the responses to contraction in freely perfused rats (n = 18; baseline 0.3 ± 0.1 imp/s, contraction 0.9 ± 0.2 imp/s). Likewise, the responses of group IV afferents to contraction in ligated rats (n = 13; baseline 0.5 ± 0.1 imp/s, contraction 1.3 ± 0.2 imp/s) were greater (P = 0.048) than the responses of group IV afferents in freely perfused rats (n = 11; baseline 0.3 ± 0.1 imp/s, contraction 0.6 ± 0.2 imp/s). We conclude that both group III and IV afferents contribute to the exaggeration of the exercise pressor reflex induced by femoral artery ligation.
Keywords: peripheral artery disease, group III and IV afferents, sympathetic activity during exercise
the exercise pressor reflex is firmly established to increase the sympathetic outflow to the vascular tree and the heart, as well as to decrease the parasympathetic outflow to the heart in humans and animals (Alam and Smirk 1937; Amann et al. 2010; Coote et al. 1971; McCloskey and Mitchell 1972; McMahon and McWilliam 1992; Mitchell et al. 1983). The afferent arm of the exercise pressor reflex is composed of thinly myelinated group III afferents and unmyelinated group IV afferents (McCloskey and Mitchell 1972). Thickly myelinated afferents, also referred to as muscle spindles and Golgi tendon organs, do not contribute to the exercise pressor reflex (Hodgson and Matthews 1968; Mitchell et al. 1983).
The discharge properties of group III afferents have been found to differ from those of group IV afferents. Specifically, group III afferents are primarily mechanically sensitive, whereas group IV afferents are primarily metabolically sensitive. Group III afferents respond vigorously at the onset of contraction, with the first impulse being discharged within the first 200 ms of contraction. Moreover, the responses of group III afferents to contraction decrease as the muscle fatigues (Kaufman et al. 1983). Nevertheless, these thinly myelinated afferents are thought to have polymodal discharge properties, as they respond to both mechanical and chemical stimuli, some of which are metabolic by-products of contraction (Kaufman et al. 1983, 1984; Kenagy et al. 1997; Kumazawa and Mizumura 1977; Mense and Meyer 1988; Mense and Stahnke 1983; Paintal 1960; Rotto and Kaufman 1988; Rybicki et al. 1985; Sinoway et al. 1993; Thimm and Baum 1987). Group IV afferents respond to metabolic by-products of contraction, many of which are the same as those that stimulate group III afferents (Kaufman et al. 1983).
Peripheral artery disease (PAD) has been found to exaggerate the exercise pressor reflex. For example, when patients with PAD walk slowly on a treadmill, both blood pressure and heart rate increased significantly more than they did in healthy controls (Baccelli et al. 1999). One rodent model of PAD involves unilaterally ligating a femoral artery and then allowing the rat to recover for 72 h. This procedure has been shown to reduce hindlimb blood flow to 10–20% of normal when the rat is exercising, yet it still provides adequate blood flow to meet muscle metabolic demand at rest (Prior et al. 2004). The exercise pressor reflex evoked by statically contracting the hindlimb muscles in which the femoral artery was ligated for 72 h was significantly greater than the reflex evoked by statically contracting hindlimb muscles that were freely perfused (Tsuchimochi et al. 2010). Endoperoxide 4 (EP4) receptors (Yamauchi et al. 2013), purinergic 2X (P2X) receptors (Stone et al. 2014), and acid sensing ion channels (ASIC3) (Tsuchimochi et al. 2011) appear to contribute to the exaggerated exercise pressor reflex in rats whose femoral artery was ligated for 72 h. Although evidence suggests that metabolic stimuli produced by muscle contraction contributes to the exaggerated exercise pressor reflex seen in the rat PAD model, the specific contribution of group III and/or group IV afferents in evoking this exaggerated response remains unknown.
We therefore sought to determine the contribution of group III and IV muscle afferents in evoking the exaggerated exercise pressor reflex in simulated PAD. We hypothesized that the responses of group III and/or IV afferents to contraction would be greater in rats whose femoral artery was ligated for 72 h than the responses of these afferents to contraction in rats whose femoral artery was freely perfused.
MATERIALS AND METHODS
All procedures were approved by the Institutional Care and Use Committee of the Pennsylvania State University College of Medicine. The responses of 57 thin-fiber afferents to muscle contraction were recorded from adult male Sprague-Dawley rats (N = 40, 440 ± 6 g). Seventy-two hours before the experiment, a group of rats underwent surgery to induce unilateral femoral artery ligation. The rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen. The femoral artery was isolated, and 5-0 suture was tied tightly around it ∼3 mm distal to the inguinal ligament. Rats were allowed 72 h to recover following the procedure. Normal cage activity is not affected by femoral artery occlusion (Taylor et al. 2008). Rats that underwent this surgery will be referred to as “ligated,” whereas those who did not have their femoral artery ligated will be referred to as “freely perfused.”
On the day of the experiment, rats were anesthetized with isoflurane (2–3%) in oxygen. The trachea was cannulated, and the lungs mechanically ventilated with the gaseous anesthetic. One carotid artery and one jugular vein were cannulated (PE-50) to measure arterial blood pressure and to administer drugs and fluids, respectively.
Arterial blood gases and pH were measured using an automated blood-gas analyzer (ABL 80, Radiometer). Pco2 and arterial pH were maintained within normal ranges either by adjusting ventilation and oxygen or by an intravenous injection of sodium bicarbonate (8.5%). Body temperature was maintained between 36.5 and 38.0°C by a heat lamp. Arterial blood pressure was measured by attaching one carotid cannula to a Statham P23XL strain gauge. Experiments were discontinued if mean arterial pressure fell below 60 mmHg.
The rats were secured in a Kopf customized spinal frame by clamps placed on the rostral lumbar vertebra and the pelvis. The left ankle was secured in a clamp, and the knee was stabilized so that the leg did not move during contraction of the hindlimb. A precollicular decerebration was performed, and all neural tissue rostral to the superior colliculi was removed (Smith et al. 2001; Tsuchimochi et al. 2010). Bleeding was controlled, and the cranial vault was filled with gauze. Immediately after the decerebration, anesthesia was discontinued.
Using the skin of the hindlimb, we formed space for a pool and then filled it with warm (37°C) mineral oil. The sciatic nerve was isolated, and a stimulating electrode was placed underneath it. The left calcaneal bone was sectioned and then attached to a force transducer (FT-10, Grass) to measure the tension developed by the contracting triceps surae muscles.
Electrophysiological recordings.
The impulse activity from group III and IV afferents with endings in the left triceps surae muscles were recorded from filaments dissected from the sciatic nerve. The afferent signals were passed through a high-impedance probe, amplified and filtered (100-3,000 Hz). The conduction velocity of an afferent was calculated by dividing the conduction distance between the recording electrode and the stimulating electrode by the conduction time (Fig. 1). We injected pancuronium bromide (1 mg/kg) to paralyze the rat before measuring conduction times. Afferents conducting impulses between 1.6 and 10 m/s were classified as group III afferents, and those conducting impulses less than 1.6 m/s were classified as group IV afferents. The afferents were located in the triceps surae muscles by poking or pinching their receptive field in the muscles using the wooden end of a cotton tip applicator and blunt serrated forceps, respectively. The intensities of these stimuli were categorized as noxious or nonnoxious based on the degree of pain perceived by the investigator when the same stimulus was applied to the investigator's forearm. The triceps surae muscles were contracted statically by electrical stimulation (20 Hz; 0.01 ms; 2–3 times motor threshold; for 30 s) of the sciatic nerve. A 30-s contraction was used because almost all of the difference in the exercise pressor reflex between freely perfused and ligated rats occurs within this time period.
Fig. 1.
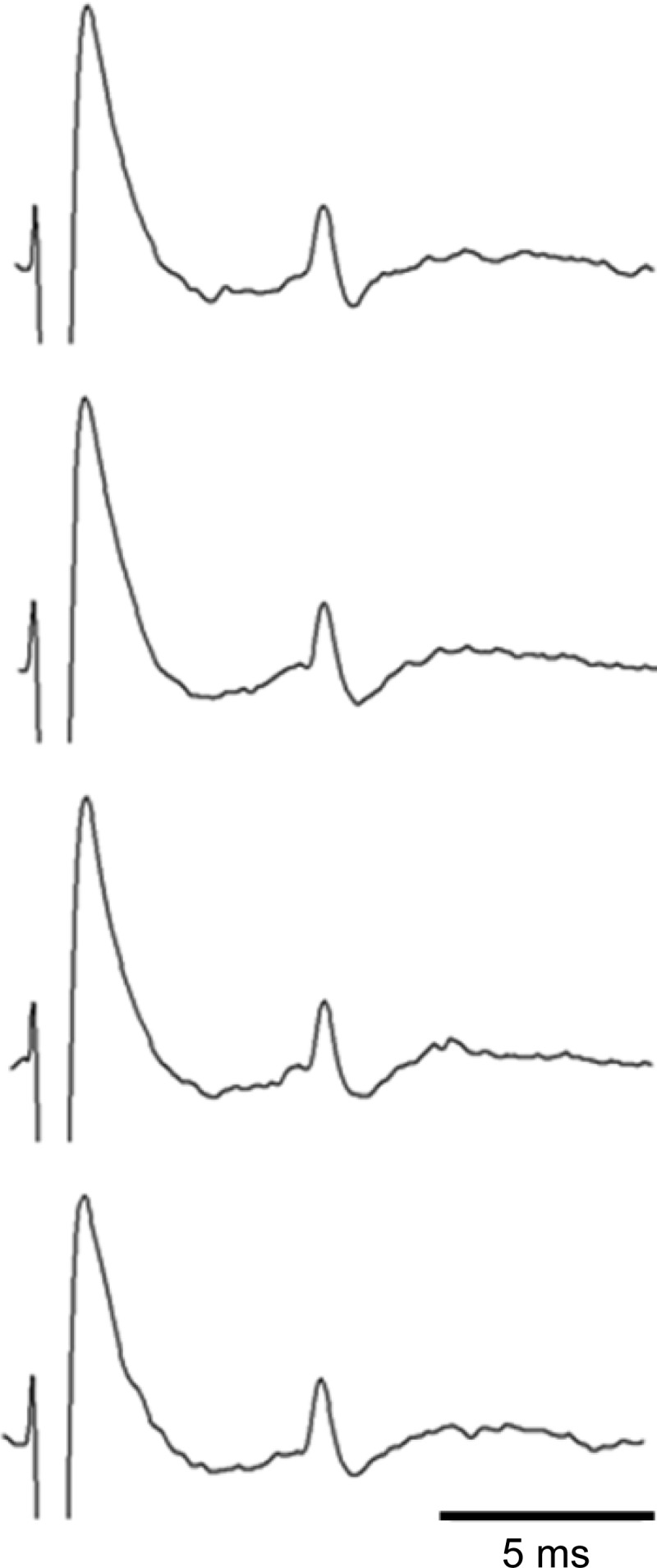
Four consecutive “sweeps” showing the measurement of the conduction time of a group III afferent whose impulse activity was recorded from the sciatic nerve. Its receptive field in the gastrocnemius muscle was stimulated at 0.4 Hz. The conduction time was 7.7 ms, the conduction distance was 42 mm, and the calculated conduction velocity was 5.5 m/s.
Data analysis.
The activity of each afferent was recorded for 30 s immediately preceding contraction and for 30 s during contraction. We defined a response to contraction as a 50% increase or more in afferent activity above baseline activity. Spike2 data acquisition software (Cambridge Electronic Design, Cambridge, UK) was used to record blood pressure (mean arterial pressure), developed tension [tension-time index (TTI)], and afferent activity. Data were analyzed using two-way repeated-measures ANOVAs and Holm-Sidak's multiple-comparison post hoc tests. Data are reported as means ± SE.
RESULTS
We examined the effect of contraction on the discharge of 57 group III and IV afferents whose receptive fields were located in the triceps surae muscles. Of the 57 afferents, 28 were from ligated rats, and 29 were from freely perfused rats. All of the group III afferents from both freely perfused and ligated rats responded to nonnoxious pinching and/or poking of the triceps surae muscles. When locating receptive fields, we found that 6 of 11 group IV afferents from freely perfused rats and 4 of 13 group IV afferents from ligated rats required either noxious pinching or poking to stimulate them. The remaining five group IV afferents from freely perfused rats and nine group IV afferents from ligated rats responded to nonnoxious pinching and/or poking of the muscles. The conduction velocities of group III afferents in freely perfused rats (4.8 ± 0.5 m/s; range: 1.6–6.2 m/s) were not different from those in ligated rats (4.9 ± 0.6 m/s; range: 1.7–9.2 m/s), P > 0.05. Likewise, the conduction velocities of group IV afferents in freely perfused rats (1.0 ± 0.1 m/s; range: 0.4–1.5 m/s) were not different from those in ligated rats (1.0 ± 0.1 m/s; range: 0.4–1.38 m/s), P > 0.05.
We found that, when group III and IV afferents were viewed together, contraction significantly increased their discharge over baseline levels in both the freely perfused and the ligated rats. Nevertheless, the response of afferents to contraction was significantly greater in the ligated rats than that in the freely perfused rats (Fig. 2A). When viewed alone, the responses of the group III afferents to static contraction were greater in the ligated rats compared with those in the freely perfused rats (Fig. 2B). In addition, the responses of group III afferents to contractions in both freely perfused and ligated rats were significantly greater than their baseline levels. When viewed alone, the responses of group IV afferents to static contraction were greater in ligated rats compared with those in freely perfused rats (Fig. 2C). Unlike the response of the group III afferents to contraction, the responses of the group IV afferents were not significantly increased over their baseline levels in freely perfused rats. In ligated rats, however, the response of group IV afferents to contraction was significantly increased (Fig. 2C).
Fig. 2.
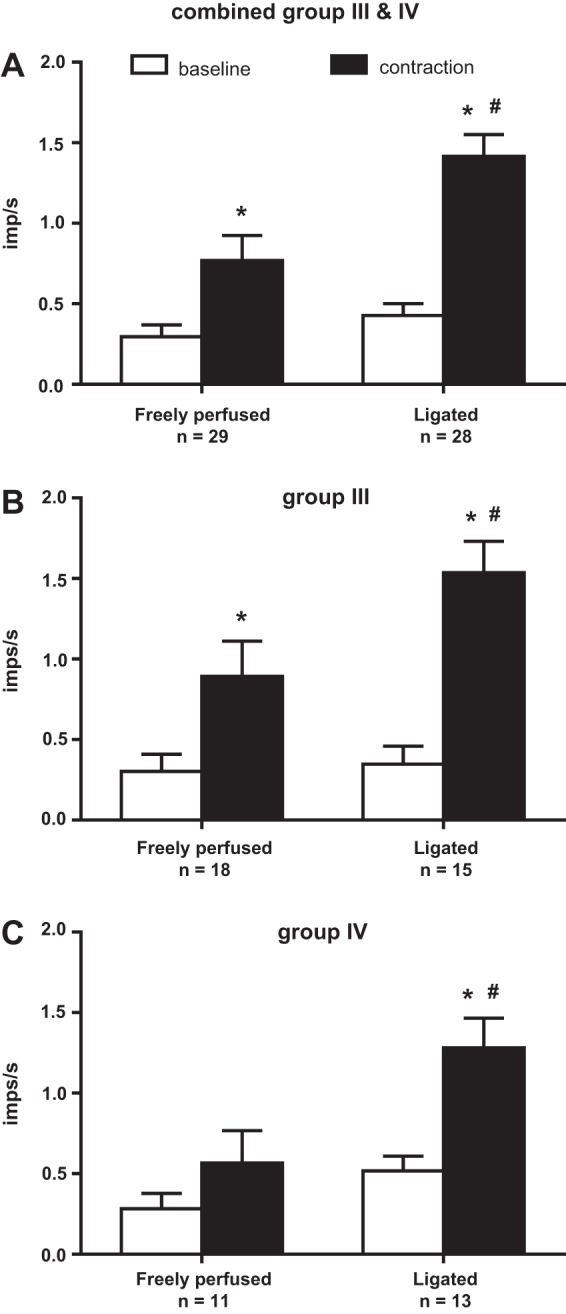
A: femoral artery ligation increased the responses of thin-fiber (i.e., group III plus group IV) afferents to static contraction. B: separately, the responses of group III afferents to static contraction were significantly greater in ligated rats than those in freely perfused rats. C: likewise, the responses of group IV afferents to static contraction were significantly greater in ligated rats than those in freely perfused rats. Values are means ± SE; n, no. of rats. *Contraction value was significantly greater than its corresponding baseline value, P < 0.05. #Difference in afferent activity from baseline to contraction was greater in the ligated rats than it was in the freely perfused rats. The tension-time indexes for the freely perfused and ligated conditions were not significantly different.
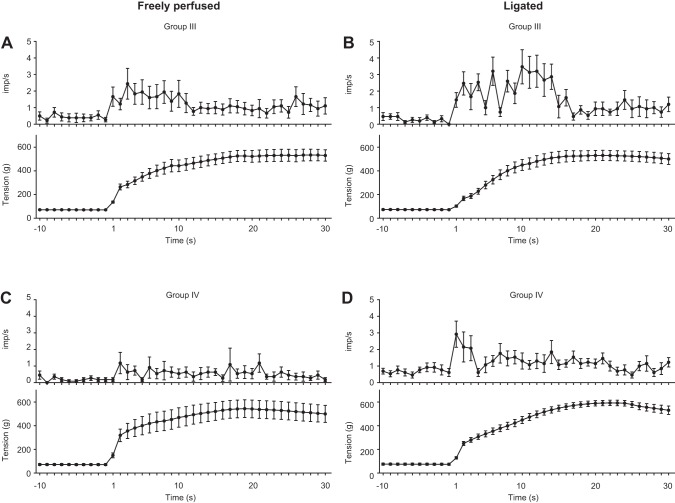
The response topography of group III and IV afferents to static contraction differed between freely perfused and ligated rats. Group III afferents responded vigorously to the first 10 s of contraction in freely perfused rats (Fig. 3A) and to the first 15 s of contraction in ligated rats (Fig. 3B). The TTIs (11 ± 1 and 11 ± 1 kg/s) and peak tensions (555 ± 51 and 522 ± 40 g) were not different between freely perfused and ligated rats, respectively, during the 30-s contractions. On the other hand, group IV afferents in freely perfused rats responded modestly at the onset of contraction and continued to respond modestly for the remainder of contraction (Fig. 3C). In ligated rats, however, group IV afferents responded vigorously to the first 3 s of contraction and then responded moderately for the remainder of contraction (Fig. 3D). The TTIs (12 ± 2 and 13 ± 1 kg/s) and peak tensions (489 ± 50 and 542 ± 19 g) were not different between freely perfused and ligated rats, respectively, during the 30-s contractions. Baseline activities between freely perfused and ligated rats for both group III and IV afferents were not significantly different.
Fig. 3.
Time course of the average activity of group III (A and B) and IV (C and D) afferents before and during 30 s of static contraction of the hindlimb muscles in freely perfused (A and C) and ligated (B and D) rats. Values are means ± SE.
In freely perfused rats, 15 of the 18 group III afferents responded to stretch, and, of those, 13 fibers responded to contraction. In ligated rats, 13 of the 15 group III afferents responded to stretch, and each responded to contraction. In freely perfused rats, 3 of the 11 group IV afferents responded to stretch, and, of those, 2 responded to contraction. In ligated rats, 2 of 13 group IV afferents responded to stretch, and both responded to contraction. Furthermore, we found that, in the freely perfused rats, four group IV afferents did not respond to stretch or contraction, but did respond to moderate to noxious pinching of the triceps surae muscles.
DISCUSSION
We found that the responses of group III and IV afferents to contraction were greater in ligated rats than in freely perfused rats. Our findings provide an electrophysiological basis for the finding that ligating a femoral artery for 72 h exaggerates the exercise pressor reflex in rats (Tsuchimochi et al. 2010). Our findings further indicate that group III afferents play a role in evoking the exercise pressor reflex in both the ligated and freely perfused rats, whereas group IV afferents play a significant role in evoking the exercise pressor reflex in ligated rats, but play only a minimal role in evoking the reflex in freely perfused rats.
Studies using pharmacological blockade indicated that ASIC3 channels (Tsuchimochi et al. 2011), P2X receptors (Stone et al. 2014), and EP4 receptors (Yamauchi et al. 2013) on group III and IV afferents contributed to the exaggerated reflex caused by femoral artery ligation. In addition, studies using immunocytochemical techniques indicated that both ASIC3 and P2X3 receptor numbers were increased in dorsal root ganglion neurons from the hindlimb muscles of rats whose femoral artery was ligated for 72 h (Liu et al. 2010, 2011). Furthermore, protein expression of EP4 receptors was upregulated in dorsal root ganglion cells innervating the hindlimb muscles in rats whose femoral artery was ligated for 72 h compared with the protein expression in rats whose femoral arteries were freely perfused (Yamauchi et al. 2013).
Group III afferents are thought to be mechanically sensitive, whereas group IV afferents are thought to be metabolically sensitive (Kaufman et al. 1983). Specifically, group III afferents discharge vigorously at the onset of contraction and then discharge less frequently as the muscle fatigues, whereas group IV afferents discharge weakly if at all at the onset of contraction and then discharge more frequently as the muscle fatigues. Several studies support the idea that metabolic by-products of muscle contraction, especially those produced during ischemia, sensitize group III afferents to mechanical stimuli (Rotto et al. 1990b; Sinoway et al. 1993). We speculate that this phenomenon could explain our finding that group III afferents in ligated rats responded vigorously for the first 15 s of contraction, whereas those in freely perfused rats responded similarly, but only for the first 10 s of contraction. On the other hand, the typical response of group IV afferents to contraction is to discharge only a few impulses at the onset of contraction, which are most likely elicited by a mechanical stimulus (Kaufman and Rybicki 1987; Rotto et al. 1990a). According to the average group IV afferent responses plotted for baseline and static contraction (see Fig. 3), we speculate that the increased response of group IV afferents in ligated rats at contraction onset was due to a metabolically induced increased sensitivity to the sudden mechanical stimulation.
Tendon stretch is commonly used in human studies as a stimulus to activate mechanosensitive endings on thin-fiber muscle afferents (Fisher et al. 2005; Gladwell and Coote 2002). In those studies, mechanically sensitive afferents that respond to stretch were assumed to be the same as those that respond to contraction. Our findings in rats support the concept that both stretch and contraction stimulate the same afferents. Specifically, we found that, in freely perfused rats, 87% of the group III afferents that responded to stretch responded to contraction. Similarly, we found that, in ligated rats, 100% of the group III afferents that responded to stretch responded to contraction. Contrary to our findings in rats, Hayes et al. (2005) found that, in cats, only 50% of the group III afferents that responded to stretch also responded to contraction. Species differences may account for these conflicting findings.
We identified group III and IV afferents using mechanical stimuli, which included poking, pinching, or stretching the gastrocnemius muscle. Consequently, it is not surprising that most of our fibers responded to contraction, a stimulus which mechanically distorts their receptive fields. Additionally, when probing receptive fields, we found that nonnoxious mechanical stimulation stimulated group III afferents, whereas noxious mechanical stimulation was often needed to stimulate group IV afferents. Our results confirm those found previously in studies using cats and dogs (Kaufman et al. 1983; Kumazawa and Mizumura 1977; Paintal 1960).
In summary, we found that the responses of group III and IV afferents to contraction were significantly greater in ligated rats than were the responses to contraction of these afferents in freely perfused rats. We speculate that the responses of both group III and group IV afferents to contraction are responsible for the exaggerated exercise pressor reflex in ligated rats. We also found that ligating the femoral artery for 72 h appeared to increase the metabosensitivity of group III afferents, as well as to increase the mechanosensitivity of group IV afferents.
GRANTS
This work was supported by National Institutes of Health Grants HL-096570 and AR-059397.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J.S. and M.P.K. conception and design of research; A.J.S. and J.L.M. performed experiments; A.J.S. and J.L.M. analyzed data; A.J.S., S.W.C., and M.P.K. interpreted results of experiments; A.J.S. prepared figures; A.J.S. and M.P.K. drafted manuscript; A.J.S., S.W.C., and M.P.K. edited and revised manuscript; A.J.S., S.W.C., and M.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Joyce Kim for technical expertise.
REFERENCES
- Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Bell MP, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol 90: 773–781, 2005. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol 540: 1095–1102, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005. [DOI] [PubMed] [Google Scholar]
- Hodgson HJF, Matthews PBC. The ineffectiveness of excitation of the primary endings of the muscle spindle by vibration as a respiratory stimulate in the decerebrate cat. J Physiol 194: 555–563, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: 160–165, 1987. [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984. [DOI] [PubMed] [Google Scholar]
- Kenagy J, VanCleave J, Pazdernik L, Orr JA. Stimulation of group III and IV afferent nerves from the hindlimb by thromboxane A2. Brain Res 744: 175–178, 1997. [DOI] [PubMed] [Google Scholar]
- Kumazawa TN, Mizumura K. Thin-fibre receptors responding to mechanical, chemical and thermal stimulation in the skeletal muscle of the dog. J Physiol 273: 179–194, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li JD, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H1070–H1079, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SE, McWilliam PN. Changes in R-R interval at the start of muscle contraction in the decerebrate cat. J Physiol 447: 549–562, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Meyer H. Bradykinin-induced modulation of the response behaviour of different types of feline group III and IV muscle receptors. J Physiol 398: 49–63, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Stahnke M. Responses in muscle afferent fibers of slow conduction velocity to contractions and ischemia in the cat. J Physiol 342: 383–397, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol 152: 250–270, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates the responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol 259: H745–H750, 1990a. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol 68: 861–867, 1990b. [DOI] [PubMed] [Google Scholar]
- Rybicki KJ, Waldrop TG, Kaufman MP. Increasing gracilis interstitial potassium concentrations stimulates group III and IV afferents. J Appl Physiol 58: 936–941, 1985. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol 69: 1053–1059, 1993. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AJ, Yamauchi K, Kaufman MP. Purinergic 2X receptors play a role in evoking the exercise pressor reflex in rats with peripheral artery insufficiency. Am J Physiol Heart Circ Physiol 306: H396–H404, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol 586: 1649–1667, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm F, Baum K. Response of chemosensitive nerve fibers of group III and IV to metabolic changes in rat muscles. Pflügers Arch 410: 143–152, 1987. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol 589: 6173–6189, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Kim JS, Stone AJ, Ruiz-Velasco V, Kaufman MP. Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591: 2949–2962, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]