Abstract
A number of studies point to an aberrant differentiation and accumulation of CD14+ PD-L1+ M2-macrophage-like cells in the microenvironment of cervical cancer, which promote immunosuppressive conditions and are associated with tumor invasion, angiogenesis and metastasis. Therapeutic targeting of these macrophages may tip the balance in favor of antitumor immunity. Cervical cancer is the fourth most common cancer among women worldwide and is caused by a persistent infection and subsequent integration of high-risk types of the human papillomavirus. Continuous expression of the viral oncoproteins E6 and E7 has been shown essential to maintain the transformed state of infected keratinocytes. As these non-self oncoproteins are immunogenic, cervical cancer requires a highly immune suppressed tumor microenvironment to metastasize through lymphovascular space invasion (LVSI) to the pelvic tumor-draining lymph nodes (TDLN). Unraveling the mechanisms underlying this immune suppression may uncover novel therapeutic targets aimed at loco-regional control of cervical cancer.
Keywords: cervical cancer, dendritic cells, immunotherapy, macrophages, PD-L1, tumor-draining lymph nodes, tumor microenvironment
Abbreviations: COX, cyclooxygenase; DC, dendritic cell; IL, interleukin; LVSI, lymphovascular space invasion; MDSC, myeloid-derived suppressor cells; PD-L1, programmed death-ligand 1; PGE2, prostaglandin-E2; TDLN, tumor-draining lymph node; Treg, regulatory T cell
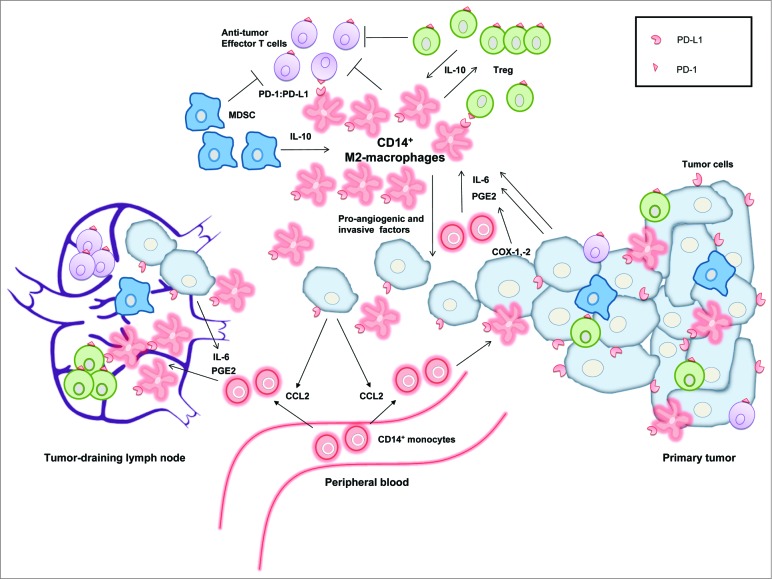
Recently we uncovered a strong association in cervical TDLN between metastatic involvement and high rates of a CD14+ CD163+ M2-macrophage-like subset with high programmed death-ligand 1 (PD-L1) expression and low levels of the co-stimulatory molecules CD80 and CD86.1 As these M2-like cells were virtually absent from uninvolved TDLN and phenotypically differed considerably from the CD14+ dendritic cell (DC) LN-resident subset, we hypothesized that they had originated from monocytes that were both attracted and conditioned by tumor-derived factors. Indeed, we and others have previously shown that monocyte-to-DC differentiation can be diverted to the development of M2-like cells in the presence of primary tumor supernatants, mostly due to high levels of prostaglandin-E2 (PGE2) and interleukin (IL)-6.2,3 In addition, maturation of DC in the presence of IL-10 can lead to their conversion into CD14+ M2-like cells with very similar phenotypic and functional features, i.e., upregulated PD-L1 and CD163 expression, low levels of co-stimulatory molecules, reduced IL-12p70 and increased IL-10 release (upon CD40L and IFNγ stimulation), poor CD8+ effector T cell priming, and induction of CD25hi FoxP3+ regulatory T cells (Treg). Additionally, these M2-like cells expressed pro-angiogenic and pro-tumor invasive factors (Fig. 1).4
Figure 1.
Model showing the central role of CD14+ M2-macrophage-like cells in the immune suppression in cervical cancer. CD14+ monocytes are recruited from the peripheral blood by tumor-derived CCL2, produced by metastasizing tumor cells. In the presence of interleukin (IL)-6 and prostaglandin-E2 (PGE2), these monocytes become converted in the microenvironment of the tumor-draining lymph nodes and the primary tumor into CD14+ M2-macrophages expressing programmed death-ligand 1 (PD-L1). These suppressive CD14+ M2-macrophages promote progression and early metastasis of the primary tumor by producing pro-angiogenic and tumor-invasive factors. In addition, they can bind to PD-1 on antitumor effector T cells, thereby inhibiting an immune response against the primary and metastatic tumor cells. This immunosuppressive cycle is further amplified by the interplay and crosstalk of regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC).
In primary cervical tumors, CD14+ cells are present in large quantities5, and can actually adopt a T cell-stimulatory M1-phenotype, which, in conjunction with CD8+ T cell infiltration, resulted in a prognostically favorable association with overall survival.5 Interestingly, we observed a reduced frequency of CD8+ effector T cells in metastasis-free TDLN of patients with LVSI.1 This is proof of early immune suppression in TDLN preceding actual metastatic spread and indicates the importance for tumor invasion of sabotaging effector T cells. PD-L1 is an inhibitory molecule also expressed on cervical tumor cells, which can bind to PD-1 on activated T cells, thus blocking their antitumor effector functions (Fig. 1). The strong association between metastasis and the presence of PD-L1+ M2-macrophages in TDLN suggests that metastasizing tumor cells have the ability to recruit and convert monocytes to M2-macrophages, likely through the release of chemo-attractants like CCL2 and of M2-inducing factors like PGE2, IL-6, IL-10, IL-4, and IL-13 (Fig. 1).2-4,6,7 These M2-macrophages may aid tumor invasion not only through effector T cell inhibition, but also physically through the release of matrix metalloproteinases.4 In cervical TDLN, we also found a clear and significant correlation between frequencies of Tregs and rates of CD14+ M2-like cells as well as their expression levels of PD-L1.1 Tregs are numerous at both primary and metastatic tumor sites,1,8,9 and in TDLN were previously shown to be highly T cell suppressive.9 Their expansion and activation may be induced by interactions with M2-macrophages, and vice versa, Tregs can promote differentiation of monocytes to immunosuppressive M2-macrophages. Thus, a picture is emerging in metastatic TDLN of a concerted increase in M2-macrophages and Tregs, associated with high IL-10 and IL-6 release levels (Fig. 1).1 We propose that metastasizing tumor cells condition the microenvironment through PGE2 and IL-6 release to engender PD-L1+ M2 skewing, leading to collateral Treg expansion and activation. Cross-talk with co-mobilized myeloid-derived suppressor cells (MDSC)1 may further amplify this vicious cycle of immune suppression, thus enabling immune escape (Fig. 1).
How can we therapeutically target M2-macrophages to lift the immunosuppressive barriers in the microenvironment of cervical tumors and TDLN? A first option would be to interrupt the immunosuppressive cycle through PD-1/PD-L1 checkpoint inhibition. This intervention is already used to great clinical effect in other types of cancer, and is likely to be effective also in cervical cancer. This notion is supported by our observation of increased PD-1 levels on T cells in tumor-involved TDLN.1 Aberrant myeloid differentiation has been linked to JAK2/STAT3 signaling. A promising in vitro study has shown combined inhibition of the STAT3 and p38-MAPK signaling pathways to facilitate CD1a+ DC differentiation in primary tumor suspensions while reducing frequencies of CD14+ cells and inducing a Th1 response.6 Another approach is to block upstream-acting IL-6 or cyclooxygenase (COX) −1/−2 (i.e., the catalytic enzymes controlling PGE2 production),2,3,7 possibly in combination with conventional chemotherapy. Indeed, combined in vitro treatment of tumor cell lines with cytostatic drugs and COX-1/-2 and/or IL-6R inhibitors prevented subsequent M2-skewing.7 A recent publication suggested CSF1R blockade as a means to decrease the number of cervical tumor-associated M2-macrophages through interrupted recruitment, differentiation and turnover rates.10 Alternatively, M2-macrophages may be re-programmed to M1-macrophages through effector molecules associated with Th1 cells such as CD40L and IFNγ, or by the use of TLR ligands.3 However, our own results suggest that this may not suffice.1,2,4,6 Ideally, a combinatorial immunotherapy should be pursued by blocking immune suppression (e.g., through blockade of PD-L1 or STAT3/p38 signaling), thereby minimizing the negative impact of CD14+ M2-macrophage-like cells, while promoting an antitumor response in the TDLN, e.g., by immune potentiation or vaccination. Such a “push and pull” approach could be applied locally to minimize unwanted side effects and might result in effective loco-regional control of cervical cancer. This promising therapeutic approach certainly deserves further (pre-)clinical exploration.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG, Jordanova ES, de Gruijl TD. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical Cancer. Cancer Immunol Res 2014; 3:48-58 PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0149 [DOI] [PubMed] [Google Scholar]
- 2.Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, Pinedo HM, van den Eerthwegh AJ, Scheper RJ, de Gruijl TD. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol 2002; 168:4333-43; PMID:; http://dx.doi.org/ 10.4049/jimmunol.168.9.4333 [DOI] [PubMed] [Google Scholar]
- 3.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoube TH, Gorter A, Welters MJ, van Hall T, van der Burg SH. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 2011; 187:1157-65; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1100889 [DOI] [PubMed] [Google Scholar]
- 4. Lindenberg JJ, van de Ven R, Lougheed SM, Zomer A, Santegoets SJ, Griffioen AW, Hooijberg E, van den Eertwegh AJ, Thijssen VL, Scheper RJ, et al. Functional characterization of a STAT3-dependent dendritic cell-derived CD14 cell population arising upon IL-10-driven maturation. Oncoimmunology 2013; 2:e23837; PMID:; http://dx.doi.org/ 10.4161/onci.23837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Vos van Steenwijk PJ, Ramwadhdoebe TH, Goedemans R, Doorduijn M, van Ham JJ, Gorter A, van Hall T, Kuijjer ML, van Poelgeest MIE, van der Burg SH, et al. Tumor-infiltrating CD14-positive myeloid cells and CD8-positive T-cells prolong survival in patients with cervical carcinoma. Int J Cancer 2013; 133:2884-94; PMID: [DOI] [PubMed] [Google Scholar]
- 6.Oosterhoff D, Lougheed S, van de Ven R, Lindenberg JJ, van Cruijsen H, Hiddingh L, Kroon J, van den Eertwegh AJ, Hangalapura B, Scheper RJ et al. Tumor-mediated inhibition of human dendritic cell differentiation and function is consistently counteracted by combined p38 MAPK and STAT3 inhibition. Oncoimmunology 2012; 1:649-58; PMID:; http://dx.doi.org/ 10.4161/onci.20365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res 2013; 73:2480-92; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3542 [DOI] [PubMed] [Google Scholar]
- 8.Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, van der Burg SH, Fleuren GJ. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res 2008; 14:2028-35; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4554 [DOI] [PubMed] [Google Scholar]
- 9.Battaglia A, Buzzonetti A, Baranello C, Ferrandina G, Martinelli E, Fanfani F, Scambia G, Fattorossi A. Metastatic tumour cells favour the generation of a tolerogenic milieu in tumour draining lymph node in patients with early cervical cancer. Cancer Immunol Immunother 2009; 58:1363-73; PMID:; http://dx.doi.org/ 10.1007/s00262-008-0646-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology 2013; 2:e26968; PMID:; http://dx.doi.org/ 10.4161/onci.26968 [DOI] [PMC free article] [PubMed] [Google Scholar]