Abstract
It has become evident that tumor-induced immuno-suppressive factors in the tumor microenvironment play a major role in suppressing normal functions of effector T cells. These factors serve as hurdles that limit the therapeutic potential of cancer immunotherapies. This review focuses on illustrating the molecular mechanisms of immunosuppression in the tumor microenvironment, including evasion of T-cell recognition, interference with T-cell trafficking, metabolism, and functions, induction of resistance to T-cell killing, and apoptosis of T cells. A better understanding of these mechanisms may help in the development of strategies to enhance the effectiveness of cancer immunotherapies.
Keywords: immunosuppression, immunotherapy, immunosuppressive factors, T cells, tumor microenvironment
Abbreviations: 1MT, 1-methyltryptophan; COX2, cyclooxygenase-2; Gal1, galectin-1; GM-CSF, granulocyte macrophage colony-stimulating factor; GPI, glycosylphosphatidylinositol; HDACi, histone deacetylase inhibitor; HLA, human leukocyte antigen; IDO, indoleamine-2,3- dioxygenase; IL-10, interleukin-10; IMC, immature myeloid cell; iNOS, inducible nitric-oxide synthase; MDSC, myeloid-derived suppressor cells; MHC, major histocompatibility; MICA, MHC class I related molecule A; MICB, MHC class I related molecule B; NO, nitric oxide; PD-1, program death receptor-1; PD-L1, programmed death ligand 1; PGE2, prostaglandin E2; SOCS, suppressor of cytokine signaling; STAT3, signal transducer and activator of transcription 3; SVV, survivin; TCR, T-cell receptor; TGF-β, transforming growth factor β; TRAIL, TNF-related apoptosis-inducing ligand; PARP, poly ADP-ribose polymerase; RCAS1, receptor-binding cancer antigen expressed on Siso cells 1; RCC, renal cell carcinoma; VCAM-1, vascular cell adhesion molecule-1; XIAP, X-linked inhibitor of apoptosis protein
Introduction
Significance of studying the tumor microenvironment
Cancer represents a challenging disease for which the development of innovative treatments is desperately needed to improve the quality of life and survival of patients. Immunotherapy, particularly T cell-mediated therapy, has emerged as a promising cancer therapeutic strategy based on its ability to specifically recognize and destroy tumor cells without harming the surrounding normal cells. However, a current limitation of cancer immunotherapy is the presence of various immunosuppressive factors in the tumor microenvironment that pose a formidable barrier to T-cell infiltration and function. The tumor microenvironment contains a network of immunosuppressive factors that are capable of inhibiting T-cell function despite the activated immune response against the tumor achieved through immunotherapy. Tumor cells have the ability to reprogram the tumor microenvironment and form a strong immunosuppressive network to limit the ability of T cells to eradicate tumor cells. Thus, to improve cancer immunotherapy a better understanding of the tumor microenvironment and tumor-induced immunosuppressive mechanisms is essential for the development of molecular interventions that can be used in conjunction with various cancer immunotherapies to enhance therapeutic effects and specificities.
Overview of immune-related aspects of the tumor microenvironment
The tumor microenvironment consists of cellular components of the tumor, the surrounding extracellular matrix, and interstitial fluid. These factors interact with each other, contributing to the hallmarks of cancer,1 and have a significant influence on immune responses against the tumor. The cellular components in the tumor include tumor cells themselves, associated stromal cells such as fibroblasts, endothelial cells, and infiltrating immune cells. The infiltrating immune cells play an essential role in immune responses against cancer. For example, particular subsets of immune cells such as cytotoxic T lymphocytes and natural killer (NK) cells inhibit tumor growth. Other infiltrating immune cells may either assist in tumor growth (e.g., tumor-associated macrophages, neutrophils, and mast cells) or inhibit immune reactions against tumor cells (e.g., regulatory T cells and myeloid-derived suppressor cells [MDSCs]). These tumor and non-tumor cells express molecules on their cell surfaces and secrete extracellular matrix components, growth factors, cytokines, chemokines, proteases, other enzymes, and metabolites that may affect the effectiveness of tumor immunotherapy.2 Other characteristics of the tumor microenvironment may significantly influence T-cell immune responses against cancers. For example, hypoxia has been shown to inhibit T-cell receptor (TCR) and CD28-mediated activation of T lymphocytes, in addition to indirectly recruiting regulatory T cells,3 which may suppress T-cell immune responses. In addition, low extracellular pH,4 low glucose concentration,5 and aberrant vasculature may affect T-cell trafficking, infiltration, and function.5 This is a dynamic relationship in which the tumor shapes its microenvironment, influencing T-cell activity, while a balance between pro- and anti-malignancy factors in the microenvironment regulate the growth of the tumor.
Focus on tumor escape of T-cell attack
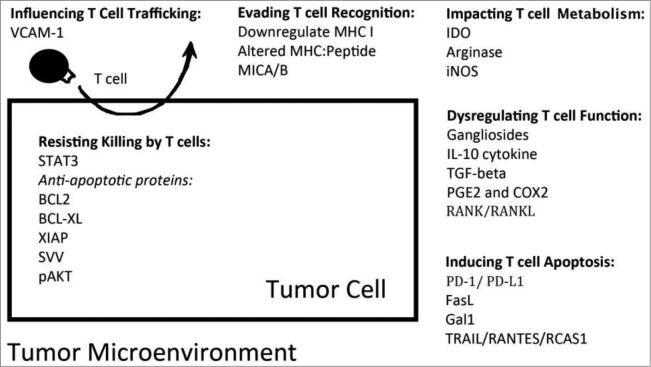
Increasing evidence suggests that despite our ability to effectively generate potent tumor-specific effector T cells through active immunization or adoptive T-cell transfer, cancer cells may possess, or develop over time, several strategies to successfully evade immune attack mediated by T cells. For instance, tumor cells can activate antiapoptotic pathways or inhibit proapoptosis signaling to resist killing mediated by tumor-specific CD8+ T cells.6,7 Alternatively, tumor cells and other cell types involved or recruited in the tumor microenvironment can inhibit T-cell proliferation, cause dysfunction of T cells, and induce apoptosis of T cells.6,8-10 This review will focus on the different mechanisms by which tumor cells manipulate the microenvironment to hinder effector T cell infiltration and function. More specifically, we will summarize several key factors present in the tumor microenvironment that contribute to tumor evasion of T-cell attack (as summarized in Fig. 1) and discuss strategies to block these molecular targets, which will allow for better efficacy of immunotherapeutic treatment for the control of cancer.
Figure 1.
Factors contributing to tumor immune evasion in the tumor microenvironment. Tumor cells can influence T-cell trafficking by upregulating adhesion molecules that prevent T cells from infiltrating the tumor.45 In addition, tumors can evade T-cell recognition through alteration of their MHC Class I/ tumor antigenic peptide complexes and antigen presentation machinery.11 Tumor cells utilize a number of altered metabolic pathways to contribute to an unfavorable environment for T-cell expansion.55,79 Another mechanism used by tumors to dysregulate T-cell function98,118,146,167, 175 or induce T-cell apoptosis10,201,204,209 is the production and secretion of immunosuppressive factors into the microenvironment. Finally, tumors can prolong their survival by overexpressing various antiapoptotic proteins.177
Molecular Mechanisms of Immune Evasion by Tumors
Evading recognition by T cells
MHC class I: antigen complex
Although tumor antigen-specific T cells can access tumors, they are unable to target them for destruction if they cannot recognize the tumor cells as their target. Tumors can evade detection by the immunosurveillance system through alteration of their major histocompatibility complex (MHC) Class I/tumor antigenic peptide complexes and antigen presentation machinery (for review, see ref. 1111). It has been shown that epigenetic silencing and subsequent transcriptional repression of MHC class I genes lead to loss of function of MHC class I molecules or loss of the MHC class I molecules themselves (for review, see ref. 1212). Genetic alterations of human leukocyte antigen (HLA), including mutations leading to HLA total losses, haplotype losses, allelic losses, and downregulation of specific loci, may result in reduced or complete loss of MHC class I molecules on the tumor cell surfaces (for review, see ref. 1313). A variety of altered human MHC class I genes have been identified in human tumors, including ovarian, cervical, breast, skin, esophageal, and colorectal cancers (for review, see ref 12, 1412,14). Studies have shown that mutations at the β-2 microglobulin locus contribute to loss of function of MHC class I molecules on the cell surface.15-18 Tumors may also alter their antigen processing and presentation machinery,19 thus preventing tumor antigens from being presented on surface MHC I molecules. These alterations occur in the transporter associated with antigen processing (TAP20), subunits of the immunoproteosome (LMP-2, LMP-7,20 PA2821), tapasin, calreticulin, and calnexin,22 which have been found in many human cancers (for review, see ref. 1111). Mutations in tumor antigenic peptides may also result in a change in avidity of peptides to their MHC Class I molecules, rendering T cells unable to recognize tumor antigens. For instance, mutations in antigenic epitopes, or antigenic drift, enable the tumor to evade host immunity.23 These antigen presentation machinery deficiencies may be reversed by treatment with interferon gamma (IFN-γ).24
Soluble and exosomal NKG2D ligands
Another way that tumors can evade immune recognition is through the shedding of NKG2D ligands, including major histocompatibility complex class I-related molecules A and B (MICA and MICB) and UL16-binding proteins. NKG2D ligands are glycoproteins that share a common MHC class I-related α1α2 superdomain and bind to the membrane through either transmembrane domains or glycosylphosphatidylinositol (GPI) anchors.25 In their membrane-bound form, NKG2D ligands are upregulated in tumors in response to infection, malignant transformation, or other cellular stresses through various pathways, resulting in enhanced activation of NK cells and CD8+ T cells as well as subsequent cytotoxic effects against NKG2D-ligand expressing cells (for review, see ref. 25-2725-27). However, tumors can shed NKG2D ligand into the tumor microenvironment through tumor cell death, secretion of exosomes, or proteolysis by matrix metalloproteinases.26,28-35 These soluble or exosomal NKG2D ligands can bind to NKG2D receptors on T cells and induce the internalization and degradation of NKG2D in CD8+ T cells infiltrating the tumor.26,33, 36 The degradation of NKG2D in T cells ultimately results in decreased activation of CD8+ T cells upon contact with tumor cells, contributing to tumor immune evasion.34,37 In some human cancers, serum MICA (sMICA) and serum MICB (sMICB) are related to disease stage and survival rates, and thus may be used to predict prognosis (for review, see ref. 3838). Histone deacetylase inhibitors (HDACis) are being tested as treatments to upregulate MICA/MICB (for review, see ref. 2525). Other substances that target the shedding of NKG2DL are also being explored to enhance CD8+ T cell cytotoxic killing of cancer cells.39
Influencing T-cell trafficking
Although cytotoxic T cells are likely to play a role in eliminating tumors, failure to access the target tumor tissue presents a critical obstacle. Tumors may influence T-cell trafficking by downregulating adhesion molecules such as ICAM-1/2, VCAM-1, and CD34 in their vessel component to prevent T cells from infiltrating the tumor.40,41 Tumors may also adversely influence T-cell trafficking by overexpressing vascular cell adhesion molecule-1 (VCAM-1).42 VCAM-1 is widely known as a cell surface glycoprotein expressed in endothelial cells that mediates leukocyte extravasation to inflammatory sites by binding to α4 integrin on T cells43 (for review, see ref. 4444). Recently, overexpression of VCAM-1 in tumors has been proposed as an important mechanism for tumor metastasis and immune escape.45 A study led by Lin et al. demonstrated that introducing VCAM-1 into a murine cancer cell line could render the tumor highly resistant to T-cell infiltration and killing.42 Tumors expressing VCAM-1 with mutated amino acids at sites required for interaction with α4β1 integrins completely lost the immune resistance conferred by VCAM-1.42 This interaction between VCAM-1 and α4 integrin is thought to promote T-cell migration away from the tumor, thus reducing infiltration of CD8+ T cells. Aberrant expression of VCAM-1 on tumors is not only found in preclinical models, but also in human tumors. For example, human renal cell carcinomas (RCCs) are highly positive for VCAM-1 expression.46 VCAM-1 may also be associated with tumor stage and overall survival of patients with RCC.47,48 Interestingly, the only RCC that responded to immunotherapy in one clinical trial was negative for VCAM-1.46 Thus, VCAM-1 expression may serve as an indicator for the outcome of immunotherapy.
Affecting T-cell metabolism
IDO
Tumor cells use a number of altered metabolic pathways to contribute to an unfavorable environment for T-cell expansion. For instance, indoleamine-2,3- dioxygenase (IDO) is a heme-containing enzyme that is overexpressed in tumors49 and overexpression of IDO has been correlated with poor prognosis of several types of cancer50-54(for review, see ref. 5555). In addition, IDO overexpression has been found in stromal immune cells, especially in certain sets of dendritic cells and MDSCs,49,56 where it reduces the levels of tryptophan, an essential nutrient for T cells. This function has multiple effects on T-cell mediated clearance of tumor cells. T cells are very sensitive to tryptophan shortage, therefore deprivation of tryptophan ultimately impairs T-cell proliferation in the tumor microenvironment by causing arrest in the G1 phase of the cell cycle.49,57 Depletion of tryptophan also causes downregulation of TCR ζ-chain in CD8+ T cells, which impairs T-cell function.58 IDO may convert tryptophan into toxic metabolites, such as kynurenine, that are harmful to T-cell function59 (for review, see ref. 6060) and induce IDO-dependent apoptosis of T cells.61-63 In addition, tumor-derived IDO has been shown to recruit regulatory T cells into the tumor microenvironment and promote their differentiation from naïve T cells, thus exerting an immunosuppressive effect.50,58, 64 Although the exact mechanisms that regulate IDO expression in tumor cells remain to be clarified, one study has shown that IDO is under the genetic control of the tumor suppressor gene Bin1.65 Elevated expression of IDO may therefore be related to the loss of Bin1 in tumors.66
Consequently, blocking IDO may allow for effective T-cell immune responses against tumors. Several studies have shown that inhibition of IDO with 1-methyltryptophan (1MT) or other small molecule inhibitors, including thiohydantooin derivatives of tryptophan, or by RNA interference can promote antitumor effects by re-establishing T-cell immunity (for review, see ref. 6767).65, 68 1MT is anticipated to have no serious side effects since it inhibits IDO while sparing tryptophan dioxygenase, a hepatic enzyme that regulates body tryptophan levels.69 Design and development of more effective IDO inhibitors is underway (for review, see ref. 60, 67, 70).60, 67, 70
Arginase and nitric-oxide synthase
Alteration in the pathway involving the catabolism of L-arginine is linked to the suppression of T-cell expansion. Two important enzymes involved in arginine metabolism are arginase and inducible nitric oxide synthase (iNOS).9 Arginine is used by iNOS as a precursor for the production of nitric oxide (NO). Therefore, elevated levels of arginase and iNOS deplete arginine, an essential nutrient of T cells, from the tumor microenvironment.9,71 Various types of tumors exhibit elevated arginase and iNOS levels,72-76 and MDSCs recruited by tumor cells into the tumor microenvironment78,79 have been shown to produce arginase.75, 79, 80 Arginine depletion by increased levels of arginase leads to downregulation of ζ-chains on T-cell receptors80, 81 and is associated with cell cycle arrest of T cells72,82 (for review, see ref. 7979). Increased iNOS expression by MDSCs, and thus higher levels of NO, may also induce cell cycle arrest of T cells83 and has been shown to be related to tumor progression and angiogenesis.84 In addition, increased NO blocks T cell production of IL-2,85,86 a cytokine that stimulates T-cell proliferation. Consequently, the use of inhibitors against arginase/iNOS, such as N(omega)-Hydroxy-nor-L-arginine (nor-NOHA), N(omega)-Hydroxy-L-arginine (NOHA),87-89 or the iNOS inhibitor NG-Monomethyl-L-arginine, monoacetate salt (L-NMMA), has been shown to restore T-cell expansion and block tumor growth in mouse models.80, 90-93 Blocking NO may also allow for effective antitumor effects. One study showed that NO inhibition using nitroaspirin (NCX-4016) combined with a tumor vaccine improved the number and effector function of T cells, leading to reduced tumor growth and improved survival of mice.94 Although arginine analogs that block arginase activity are available for investigating this biological pathway,95,96 none are currently used for clinical studies because of safety concerns associated with disrupting the natural role of arginine in the urea cycle.
Dysregulating the function of T cells
Gangliosides
Tumors are capable of escaping destruction by adopting strategies that impair T-cell function in the microenvironment. One proposed mechanism involves the shedding of gangliosides by tumors. Gangliosides are glycosphingolipids found as clusters on the surface of all mammalian cells that regulate cellular responses such as growth and differentiation (for review, see ref. 97, 9897,98). Many tumors, however, express large quantities of gangliosides that are not expressed in their normal tissue origin or overexpress certain gangliosides specific to the tissue that are often shed into the microenvironment. This phenomenon has been observed in several types of human cancers (for review, see ref. 9898). The soluble gangliosides shed into the tumor microenvironment can dysregulate T-cell function in multiple ways. For instance, there is evidence that these soluble gangliosides inhibit tumor-specific T-cell proliferation99,100 and induce T-cell apoptosis.8,101-103 They may play a role in disrupting cytokine production, including that of IFNγ in T helper 1 cells104,105 and IL-5 in T helper 2 cells.106 In addition, soluble gangliosides may skew the T-cell response against tumor antigen toward a Th2 response, which contributes far less than a Th1 response to tumor clearance.105,107 Furthermore, soluble gangliosides have been shown to disrupt nuclear factor kappa B (NF-B) function in immune cells108,109 as well as lytic granule trafficking and exocytosis in CD8+ T cells.110 Thus, gangliosides that are shed into the microenvironment can disrupt the normal functioning of T cells in numerous ways. Therapies targeting the tumor gangliosides GD2, GM3, and GD3 may potentially prevent gangliosides from inducing T-cell dysfunction. For example, an anti-GM2 monoclonal antibody, DMF10.167.4, has been shown to inhibit tumor growth in vitro and in a preclinical model.111 Antibodies targeting gangliosides GD2, GM3, and GD3 may also serve as promising vaccines,111-115 (for review, see ref. 116, 117116,117). Since gangliosides are expressed on all cells, it is essential that the engineered monoclonal antibodies bind specifically to tumor gangliosides and not to normal tissues (for review, see ref. 116116).
Interleukin-10
Another mechanism utilized by tumors to disrupt T-cell function is the production of interleukin-10 (IL-10) and its secretion into the microenvironment. IL-10 is an important cytokine that displays both immunostimulatory and immunosuppressive activities toward various components of the immune system.118,119 IL-10 mRNA and protein have been found in freshly excised human tumors and cancer cell lines of a variety of origins,120-124 and IL-10 secreted into the tumor microenvironment can be produced by tumor cells120 or tumor-associated macrophages.125 There are several ways in which IL-10 may assist in tumor immune evasion. For instance, IL-10 downregulates HLA class I expression on tumors, thereby facilitating tumor escape from T-cell recognition.126 In addition, IL-10 has been shown to downregulate immune effector mechanisms, such as CD8+ T-cell mediated tumor cell lysis.126,127 Furthermore, IL-10 may block T helper 1 cell differentiation and proliferation and suppress production of Th1 cytokines,128-130 which would negatively impact the proliferation of cytotoxic T cells. IL-10 also serves as an activator of STAT, which in turn inhibits maturation of dendritic cells and immature myeloid cells (IMCs) through STAT3 activation.131-133 These immature myeloid cells then become MDSCs and exert immunosuppressive effects on CD8+ T-cells through various mechanisms.134,135 Considering the immunosuppressive functions of IL-10, administration of neutralizing antibodies may provide a promising strategy to target IL-10.136 In fact, neutralizing antibodies against IL-10 have been shown to significantly restore T-cell proliferation137,138 and enhance antitumor immune responses. However, given the dual function of IL-10, its knockdown may also diminish immune responses (for review, see ref. 119119). Thus, further evaluation of IL-10 blockade as an immunomodulatory approach is required (for review, see ref. 139139). Major efforts have also been made to identify inhibitors or inhibition strategies of STAT3, downstream of the IL-10 pathway. For example, suppressor of cytokine signaling (SOCS) has been shown to negatively regulate STAT3 activation.140,141 In a preclinical study, demethylation followed by pharmacologic inhibitors of SOCS-1 expression resulted in the inhibition of STAT3 activation and cell proliferation and the stimulation of cell apoptosis in a mouse carcinoma cell line.140 Although several STAT3 inhibitors are under development, none of them have made it to clinical stage investigation (for review, see ref. 142142). With recent advancements of technology, small molecule STAT3 inhibitors such as TW-37 represent potential candidates for further development in clinical trials143-145 and STAT3 still serves as a promising target for cancer immunotherapy (for review, see ref. 140, 142140, 142).
TGF-β
Overexpression of transforming growth factor (TGF)-β, a cytokine known to regulate the growth and activity of T cells, profoundly suppresses T-cell responses (for review see ref. 146146). Various types of tumors that produce TGF-β exploit this mechanism to evade immune attack.147 Overexpression of TGF-β by tumors suppresses T-cell responses through numerous TGF-β signaling pathways. In terms of activation and function, TGF-β cytokines may bind to TGF-β receptors on T cells and inhibit IL-2 production, cytotoxic T lymphocyte activation, clonal expansion of memory CD8+ T cells, and expression of perforin, an essential mediator for CD8+ T cell killing of tumor cells148,149 (for review, see ref. 150150). Another equally important immunosuppressive role of TGF-β is altering the differentiation of Th1 and Th2 cells by inhibiting their lineage specification transcription factors.151, 152 TGF-β is also involved in inducing the expression of transcription factor FoxP3, which ultimately promotes growth and differentiation of CD4+ CD25+ T regulatory cells.153-155 These regulatory T cells then secrete TGF-β and other inhibitory cytokines to suppress CD8+ T cell-mediated killing of tumor cells (for review, see ref. 155155). TGF-β can also promote the development and maintenance of T helper 17 (Th17) cells, which have been shown to suppress CD8+ T-cell effector functions.156 The roles of Th17 cells in the tumor microenvironment, however, appear to be double edged. In addition to its role in the procarcinogenic inflammatory response, Th17 cells may also play a role in antitumor immunity, as demonstrated by a number of studies on human cancer and mouse models.157-161 Nevertheless, inhibiting TGF-β-induced inactivation of T cells may provide protection against tumors.147 In fact, several agents are being developed in preclinical and clinical settings with this aim. One potential strategy to block TGF-β is the use of antisense gene therapy, which impedes the translation of TGF-β mRNA.162 Other strategies to inhibit TGF-β include monoclonal anti-TGF-β antibodies, small molecule inhibitors of TGF-β, and Smad inhibitors (for review, see ref. 163, 164163, 164). Thus, we expect that future endeavors will focus on effective ways to block TGF-β to enhance T-cell responses.
PGE2 and COX2
Numerous tumors, particularly colorectal, pancreatic, lung, and breast cancer, overexpress cyclooxygenase-2 (COX2) enzyme and its metabolite prostaglandin E2 (PGE2), which both contribute to T-cell dysfunction165,166 (for review, see ref. 167167). PGE2 induces the accumulation of MDSCs, which inhibit the activation of CD4+ and CD8+ T cells.165 The interaction between PGE2 released by tumors and prostaglandin E2 receptor (EP2) on T cells has been shown to alter the cytokine profile of T cells, fueling the Th2-type while reducing Th1-type cytokine levels.168,169 This cytokine imbalance promotes humoral immune responses that are ineffective in targeting tumors, which requires cellular immune responses established by Th1 cells. In vivo studies have shown that PGE2 overexpression results in production of the immunosuppressive cytokine IL-10 and downregulation of the immunostimulatory cytokines IL-12 and IFNγ.169-172 Further support of the importance of COX2 in inhibiting antitumor immune responses is provided by an in vivo study in which COX2 expression was silenced with antisense oligonucleotides or COX2 activity was blocked with a selective COX2 inhibitor.173 Knockdown/inhibition of COX2 resulted in increased lymphocyte infiltration, increased levels of IL-12 and IFNγ, and decreased levels of IL-10, ultimately reducing tumor burden.171, 173 Thus, inhibition of COX2 and PGE using COX2 inhibitors may stimulate cellular immunity, resulting in potent antitumor effects.172
RANK/RANKL
Receptor activator of NFkB (RANK)8 is a member of the tumor necrosis factor (TNF) receptor molecular subfamily that is associated with immune cell function. It has been shown that RANK is highly expressed in breast and prostate cancers.174 The engagement of RANK with RANK ligand (RANKL) expressed on regulatory T cells leads to the expansion of regulatory T cells, one of the major types of immunosuppressive cells in the tumor microenvironment, ultimately forming an immunosuppressive niche that contributes to cancer bone metastasis and disrupts effector T cell function.175 Since this pathway is important in bone marrow metastasis of prostate cancer in humans and immunosuppression, RANK/RANKL is a clinically validated target.
Resisting killing by T cells
Antiapoptotic proteins
Tumors can prolong their survival by overexpressing antiapoptotic proteins. Well-known antiapoptotic proteins include Bcl-2, Bcl-xL, X-linked inhibitor of apoptosis protein (XIAP), survivin (SVV), and an active form of phospho-Akt (pAkt). Upregulated Bcl-xL has been found in 63% of hepatocellular carcinoma specimens and has been associated with poor survival.176 High expression of SVV or XIAP (for review, see ref. 177177) has also been observed in numerous cancers. Both XIAP and SVV have been shown to be highly expressed in malignant mesotheliomas.178 XIAP overexpression in renal cell carcinomas was associated with worse prognosis 179 and SVV overexpression was associated with tumor progression, tumor cell resistance to chemotherapy, and tumor recurrence180 (for review, see177,181). In one study, tumor expression of antiapoptotic proteins was linked to tumor immune evasion. Kim et al. developed an HPV-16 E7–expressing tumor capable of escaping attack by E7-specific CD8+ T cells through multiple cycles of in vivo immune selection.182 Further characterization of the tumor revealed increased expression of pAkt, which led to upregulation of antiapoptotic proteins Bcl-2, Bcl-xL, and XIAP. Tumors can also express activated signal transducer and activator of transcription 3 (STAT3), which is associated with numerous oncogenic signaling pathways and linked to increased proliferation and inhibited apoptosis of tumor cells (for review, see ref. 183183). This constantly activated STAT3 pathway in tumor cells has multiple effects. For example, STAT3 signaling pathways upregulate the antiapoptotic protein BCL-X, rendering the tumor cell more resistant to T-cell induced apoptosis.
Inducing apoptosis of T cells
Co-inhibitory molecules
Tumors can evade immune attack by inducing apoptosis of T cells. When induced by oncogenic signals or in response to endogenous antitumor immune responses, tumors upregulate a co-inhibitory molecule, programmed death ligand 1 (PD-L1), which functions as an immune checkpoint signal.6, 184-187 Binding of PD-L1 to program death receptor-1 (PD-1) on activated T cells188, 189 results in T-cell anergy or death, thus dampening antitumor activity and promoting tumor growth.188, 190, 191 Studies have also shown high levels of PD-L1 on tumor-associated myeloid cells in the tumor microenvironment of human cancers, serving as an important immune escape mechanism.192,193 These discoveries propelled development of the current PD-1/PD-L1 blockade therapeutic strategies, which have been successful in overcoming this tolerance mechanism. Antibody blockade of PD-L1 can protect CD8+ T cells from apoptosis in vitro and augment the antitumor effects of adoptively transferred T cells194 and tumor cell-based vaccines.195 In addition, clinical trials using anti-PD-1 and anti-PD-L1 antibodies showed durable tumor regression and prolonged stabilization of disease in non-small cell lung cancer, melanoma, and renal cell carcinoma.185, 196-200 Other novel approaches targeting PD-L1, including interference RNA or small molecules (for review, see ref. 201201) and soluble PD-1, which can bind PD-L1 and render it inactive,202 are under development. Encouraging clinical results using single blocking agents against PD-1 have led to trials exploring the combination of anti–PD-L1 antibody or anti–PD-1 treatment with a granulocyte macrophage colony-stimulating factor (GM-CSF)-secreting allogeneic pancreatic tumor cell vaccine (GVAX) in human patients.203
B7-H4 is another co-inhibitory molecule that is expressed on the surface of tumor cells and immunosuppressive tumor-associated macrophages and has been used as a negative prognostic indicator for many human tumors. It has the ability to inhibit T-cell proliferation, cell cycle progression, IL-2 cytokine production, and effector T cell function. The specific receptor of B7-H4 has not yet been identified, necessitating further investigation (for review, see ref. 204204). As B7-H4 molecules play such an important role in inhibiting T-cell function, strategies aimed at blocking their activity are currently being developed to improve the efficacy of cancer immunotherapy. The application of monoclonal antibodies that block B7-H4 has been shown to promote T-cell responses,205 although studies using anti-B7-H4 combined with immunotherapy remain to be tested.
Fas ligand
A wide variety of tumors have been reported to express Fas ligand (FasL/CD95L) (for review, see ref. 1010). Upregulation of FasL on tumor cells might be another mechanism that allows tumors to counterattack T cells. FasL is a transmembrane protein belonging to the TNF superfamily and the death receptor subfamily that can trigger apoptotic cell death when bound to its Fas (CD95) receptor. FasL expression and its association with tumor immune escape have been extensively studied. Activated effector T cells upregulate expression of Fas on their surface upon recognition of tumor antigenic peptides.206 It has been proposed that upregulation of Fas ligand on tumor cells enables the tumors to counterattack T cells.207-213 When FasL on tumors interacts with the Fas receptor on T cells, FasL delivers death signals to Fas-expressing T cells resulting in apoptosis of tumor-specific effector T cells that have infiltrated the tumor207,208,213 However, the role of FasL in tumor immune evasion is not entirely clear10 and several contradictory results have been reported in recent studies.10,214-217 For instance, FasL expression on tumor cells may confer antitumor and proinflammatory effects.207,211,217,219 Researchers have therefore hypothesized that other immunosuppressive factors are required in order for FasL to exert its effect.209,213 As shown in the next section, galectin-1 can help to create an immunosuppressive tumor microenvironment in favor of FasL action. Despite its debatable role in immune counterattack, FasL still presents a potential target for cancer therapy. Strategies to downregulate FasL expression or block FasL on tumors may decrease its binding to Fas on T cells, thus decreasing Fas-mediated apoptosis of T cells. Downregulation of FasL expression using an antisense FasL has been shown to significantly reduce tumor bulk and suppress tumor immune evasion of colon cancers in a preclinical model as a result of increased T-cell infiltration within tumors.220 Further research is encouraged and indeed necessary to unravel the application of FasL inhibition in cancer therapy.
Galectin-1
Galectin-1 (Gal1) overexpression by tumors contributes to immune evasion by promoting T-cell apoptosis. Gal1 is a β-galactoside-binding protein that is involved in cell–cell adhesion,221 cell–matrix interactions,221-223 immune system homeostasis224,225 and cell growth.225 It is also associated with angiogenesis,226,227 transformation,228 and poor prognosis.226, 229-231 Studies have shown that Gal1 is overexpressed on tumor cells and secreted at high levels in a wide variety of cancers (for review, see ref. 232, 233232,233), in which Gal1 may serve as a negative regulator of immune responses.234 Gal1 interacts with a receptor on T cells that is yet to be identified, and can induce apoptosis of activated T cells.215,234 There are contradicting opinions on whether CD45 is the functional Gal1 receptor expressed on T cells.235-241 Gal1 is a determining factor of tumor cell-induced T-cell apoptosis, as demonstrated by in vitro and in vivo Gal1 knockdown experiments.215 Hypoxia, which is commonly present in solid tumors, enhances Gal1 secretion from tumors, further promoting T-cell apoptosis.242,243 Expression and cell surface/extracellular matrix presentation of Gal1 on tumor cells contributes to tumor cell-induced T-cell death,243-245 which requires direct T cell–tumor cell contact.245 High expression levels of Gal1 by tumor-associated stromal cells246-249 and tumor-associated blood vessel endothelium250, 251 enhance this process. Upon encountering its target on T cells, Gal-1 induces the caspase-dependent mitochondrial route of apoptosis involving p56Lck and ZAP70.235, 245 Furthermore, extracellular Gal1 can regulate the survival of tumor-infiltrating T cells by promoting Fas ligand-induced apoptosis in T cells, thus reinforcing the immunoregulatory effect of Fas ligand.252 As mentioned previously, Gal1 can help create an immunosuppressive microenvironment in favor of FasL action. Gal1 may suppress IFNγ production and increase IL-10 release from T cells215 253 or skew the Th1/Th2 cytokine balance toward a more Th2 cytokine profile,253 thereby further decreasing cellular immunity against tumors. Targeting galectin-1 might promote antitumor responses by improving T-cell infiltration into tumors, reducing T-cell death, and enhancing cellular immunity (for review, see ref. 232232). Strategies currently under investigation include Gal-1 neutralizing antibodies,254, 255 competent inhibitors of Gal1-binding,256-259 and metabolic modifiers of N-acetyl-D-Lactosamine (LacNAc).
TRAIL, RANTES, RCAS1
Other mechanisms that tumors employ to outmaneuver immune attack by inducing T-cell apoptosis involve TRAIL, RANTES, and RCAS1. TNF-related apoptosis-inducing ligand (TRAIL) is a type II transmembrane protein of the TNF family that can activate apoptosis through the death-signaling receptors DR4 and DR5 and formation of a death-inducing signaling complex208 (for review, see ref. 209209). TRAIL shares significant homology with FasL, which as mentioned above, also belongs to the TNF family and can induce T-cell apoptosis.207, 211, 214 Moreover, TRAIL seems to suppress cytotoxic T cell responses in a manner similar to that of FasL.216 Both tumor cell membrane-bound and soluble forms of TRAIL can initiate apoptosis in IL-2–secreting T cells, but not in inactivated T cells.210 RANTES (also known as CCL5) is a chemokine that can also activate the apoptotic cell death pathway in T cells. RANTES is a strong chemoattractant for CD8+ T cells and can bind to G protein-coupled receptors CCR1, CCR3, CCR4, and CCR5 on T cells.213 Upon binding to CCR5 on tumor infiltrating T lymphocytes, RANTES activates a CCR5-dependent apoptotic pathway that involves the release of cytochrome-c into the cytosol, activation of caspase-3 and caspase-9 pathways, and cleavage of poly ADP-ribose polymerase (PARP).206, 260, 261 Hypoxia in the tumor microenvironment can induce strong release of RANTES, which promotes tumor migration.262, 263 In addition, RANTES in serum is associated with cancer clinical stage and tumor progression in several cancers (for review, see ref 264264).191, 263, 265-267 One study demonstrated that RANTES may induce Fas-mediated apoptosis of cytotoxic T cells,268 whereas another study found that RANTES is able to enhance regulatory T cell-mediated CD8+ T cell killing.269 Tumors can also enhance T-cell apoptosis by expressing the membrane ligand receptor-binding cancer antigen expressed on Siso cells 1 (RCAS1), which is secreted by ectodomain shedding.218, 270 RCAS1 is expressed in a variety of tumors and is related to poor patient survival (for review, see ref. 270, 271270,271). Soluble RCAS1 can bind to RCAS1 receptors on activated T cells, initiating cell cycle arrest and thereby suppressing clonal expansion and increasing the destruction of RCAS1 receptor-positive T cells via apoptosis.217, 218, 272 One study used shRNA to knock down RCAS1 expression, which reduced T-cell apoptosis and partially reversed T-cell function.273 However, current knowledge of the role of TRAIL, RANTES, and RCAS1 in T-cell apoptosis is very limited and further research is recommended before these 3 factors can be used as immunotherapeutic targets. Future development of new strategies to interfere with their immunosuppressive effects may be helpful as an adjuvant to cancer treatment.
Conclusion
The tumor microenvironment is predominantly infiltrated with immunosuppressive factors that cripple T cell responses against the tumor. These factors are not present in normal tissues, but are components of tumor regulatory pathways in response to inflammatory or infectious etiologies. They are also induced or “hijacked” by tumor cells to act as tumor protectors. Altering these factors may provide effective cancer immunotherapy. For example, PD-1 and PD-L1 have become 2 of the most exciting targets for immune-based cancer therapies.
Despite efforts to understand tumor-induced immunosuppressive factors and their interactions in the tumor microenvironment, our current understanding is insufficient to develop a comprehensive treatment strategy for many cancers. Moreover, targeting one single immunosuppressive factor is often not effective because tumor cells have formed a network of immunosuppressive factors to protect them and have programed the tumor microenvironment to be immune quiescent. Identification of novel and effective molecular targets in the tumor immunosuppressive network and stimulants of T-cell–mediated immunity is desperately needed. Treatment strategies that change the balance of the immune regulatory network and reprogram the tumor microenvironment from an immune quiescent one to an immune active one may render tumors more susceptible to immunotherapies that would be otherwise not be effective as monotherapy. Finally, strategies for targeting the tumor immunosuppressive network as a whole, rather than targeting a single molecular target, should also be established.
Acknowledgments
We thank Benjamin Yang for his helpful comments and critical review of the manuscript. This review is not intended to be an encyclopedic one, and we apologize to those whose work is not cited.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309-22; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 3.Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res 2012; 18:1207-13; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1591 [DOI] [PubMed] [Google Scholar]
- 4.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol 2001; 69:522-30; PMID: [PubMed] [Google Scholar]
- 5.Singer K, Kastenberger M, Gottfried E, Hammerschmied CG, Buttner M, Aigner M, Seliger B, Walter B, Schlösser H, Hartmann A, et al.. Warburg phenotype in renal cell carcinoma: high expression of glucose-transporter 1 (GLUT-1) correlates with low CD8(+) T-cell infiltration in the tumor. Int J Cancer 2011; 128:2085-95; PMID:; http://dx.doi.org/ 10.1002/ijc.25543 [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karni R, Jove R, Levitzki A. Inhibition of pp60c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptors. Oncogene 1999; 18:4654-62; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1202835 [DOI] [PubMed] [Google Scholar]
- 8.Sa G, Das T, Moon C, Hilston CM, Rayman PA, Rini BI, Tannenbaum CS, Finke JH. GD3, an overexpressed tumor-derived ganglioside, mediates the apoptosis of activated but not resting T cells. Cancer Res 2009; 69:3095-104; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 2005; 5:641-54; PMID:; http://dx.doi.org/ 10.1038/nri1668 [DOI] [PubMed] [Google Scholar]
- 10.Igney FH, Krammer PH. Tumor counterattack: fact or fiction? Cancer Immunol Immunother 2005; 54:1127-36; PMID:; http://dx.doi.org/ 10.1007/s00262-005-0680-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leone P, Shin EC, Perosa F, Vacca A, Dammacco F, Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst 2013; 105:1172-87; PMID:; http://dx.doi.org/ 10.1093/jnci/djt184 [DOI] [PubMed] [Google Scholar]
- 12.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene 2008; 27:5869-85; PMID:; http://dx.doi.org/ 10.1038/onc.2008.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido F, Algarra I. MHC antigens and tumor escape from immune surveillance. Adv Cancer Res 2001; 83:117-58; PMID:; http://dx.doi.org/ 10.1016/S0065-230X(01)83005-0 [DOI] [PubMed] [Google Scholar]
- 14.Garcia‐Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol 2003; 195:346-55; PMID:; http://dx.doi.org/ 10.1002/jcp.10290 [DOI] [PubMed] [Google Scholar]
- 15.Romero JM, Jimenez P, Cabrera T, Cozar JM, Pedrinaci S, Tallada M, Garrido F, Ruiz-Cabello F. Coordinated downregulation of the antigen presentation machinery and HLA class I/beta2-microglobulin complex is responsible for HLA-ABC loss in bladder cancer. Int J Cancer 2005; 113:605-10; PMID:; http://dx.doi.org/ 10.1002/ijc.20499 [DOI] [PubMed] [Google Scholar]
- 16.Dierssen JW, de Miranda NF, Ferrone S, van Puijenbroek M, Cornelisse CJ, Fleuren GJ, van Wezel T, Morreau H. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer 2007; 7:33; PMID:; http://dx.doi.org/ 10.1186/1471-2407-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Miranda NF, Nielsen M, Pereira D, van Puijenbroek M, Vasen HF, Hes FJ, van Wezel T, Morreau H. MUTYH-associated polyposis carcinomas frequently lose HLA class I expression - a common event amongst DNA-repair-deficient colorectal cancers. J Pathol 2009; 219:69-76; PMID:; http://dx.doi.org/ 10.1002/path.2569 [DOI] [PubMed] [Google Scholar]
- 18.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol 1996; 6:1695-7; PMID:; http://dx.doi.org/ 10.1016/S0960-9822(02)70795-1 [DOI] [PubMed] [Google Scholar]
- 19.Bennaceur K, Chapman JA, Touraine JL, Portoukalian J. Immunosuppressive networks in the tumour environment and their effect in dendritic cells. Biochim Biophys Acta 2009; 1795:16-24; PMID: [DOI] [PubMed] [Google Scholar]
- 20.Seliger B, Hohne A, Knuth A, Bernhard H, Ehring B, Tampe R, Huber C. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and LMP expression. Clin Cancer Res 1996; 2:1427-33; PMID: [PubMed] [Google Scholar]
- 21.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today 2000; 21:455-64; PMID:; http://dx.doi.org/ 10.1016/S0167-5699(00)01692-3 [DOI] [PubMed] [Google Scholar]
- 22.Belicha-Villanueva A. Regulation of Classical and Non-classical Major Histocompatibility Complex Class I Molecules. ProQuest 2008 [Google Scholar]
- 23.Bai X-F, Liu J, Li O, Zheng P, Liu Y. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J Clin Invest 2003; 111:1487-96; PMID:; http://dx.doi.org/ 10.1172/JCI17656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Coordinate downregulation of multiple MHC class I antigen processing genes in chemical‐induced murine tumor cell lines of distinct origin. Tissue Antigens 2000; 56:327-36; PMID:; http://dx.doi.org/ 10.1034/j.1399-0039.2000.560404.x [DOI] [PubMed] [Google Scholar]
- 25.Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology 2013; 2:e26097; PMID:; http://dx.doi.org/ 10.4161/onci.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419:734-8; PMID:; http://dx.doi.org/ 10.1038/nature01112 [DOI] [PubMed] [Google Scholar]
- 27.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013; 31:413-41; PMID:; http://dx.doi.org/ 10.1146/annurev-immunol-032712-095951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, Dranoff G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A 2008; 105:1285-90; PMID:; http://dx.doi.org/ 10.1073/pnas.0711293105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marten A, von Lilienfeld-Toal M, Buchler MW, Schmidt J. Soluble MIC is elevated in the serum of patients with pancreatic carcinoma diminishing gammadelta T cell cytotoxicity. Int J Cancer 2006; 119:2359-65; PMID:; http://dx.doi.org/ 10.1002/ijc.22186 [DOI] [PubMed] [Google Scholar]
- 30.Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: mechanism and soluble MICB in sera of cancer patients. Hum Immunol 2006; 67:188-95; PMID:; http://dx.doi.org/ 10.1016/j.humimm.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 31.Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res 2008; 68:6368-76; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-6768 [DOI] [PubMed] [Google Scholar]
- 32.Salih HR, Rammensee HG, Steinle A. Cutting Edge: Down-Regulation of MICA on Human Tumors by Proteolytic Shedding. J Immunol 2002; 169:4098-102; PMID:; http://dx.doi.org/ 10.4049/jimmunol.169.8.4098 [DOI] [PubMed] [Google Scholar]
- 33.Clayton A, Mitchell JP, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol 2008; 180:7249-58; PMID:; http://dx.doi.org/ 10.4049/jimmunol.180.11.7249 [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Messina L, Ashiru O, Boutet P, Agüera-González S, Skepper JN, Reyburn HT, Valés-Gómez M. Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J Biol Chem 2010; 285:8543-51; http://dx.doi.org/ 10.1074/jbc.M109.045906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res 2006; 66:2520-6; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2520 [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al.. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 1999; 285:730-2; PMID:; http://dx.doi.org/ 10.1126/science.285.5428.730 [DOI] [PubMed] [Google Scholar]
- 37.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Levreri I, Gambini C, Pende D, Steinle A, Ferrone S, Pistoia V. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 2004; 6:558-68; PMID:; http://dx.doi.org/ 10.1593/neo.04316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huergo-Zapico L, Acebes-Huerta A, Lopez-Soto A, Villa-Alvarez M, Gonzalez-Rodriguez AP, Gonzalez S. Molecular Bases for the Regulation of NKG2D Ligands in Cancer. Front Immunol 2014; 5:106; PMID:; http://dx.doi.org/ 10.3389/fimmu.2014.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groth A, Klöss S, Pogge von Strandmann E, Koehl U, Koch J. Mechanisms of tumor and viral immune escape from natural killer cell-mediated surveillance. J Innate Immun 2011; 3:344-54; PMID:; http://dx.doi.org/ 10.1159/000327014 [DOI] [PubMed] [Google Scholar]
- 40.Hellebrekers DM, Castermans K, Vire E, Dings RP, Hoebers NT, Mayo KH, Oude Egbrink MG, Molema G, Fuks F, van Engeland M, et al.. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res 2006; 66:10770-7; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1609 [DOI] [PubMed] [Google Scholar]
- 41.Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med 1995; 181:811-6; PMID:; http://dx.doi.org/ 10.1084/jem.181.2.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu T. The role of vascular cell adhesion molecule-1 in tumor immune evasion. Cancer Res 2007; 67:6003-6; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose DM, Grabovsky V, Alon R, Ginsberg MH. The Affinity of Integrin 4 1 Governs Lymphocyte Migration. J Immunol 2001; 167:2824-30; PMID:; http://dx.doi.org/ 10.4049/jimmunol.167.5.2824 [DOI] [PubMed] [Google Scholar]
- 44.Rose DM, Han J, Ginsberg MH. α4 integrins and the immune response. Immunol Rev 2002; 186:118-24; PMID:; http://dx.doi.org/ 10.1034/j.1600-065X.2002.18611.x [DOI] [PubMed] [Google Scholar]
- 45.Schlesinger M, Bendas G. Vascular cell adhesion molecule‐1 (VCAM‐1)—An increasing insight into its role in tumorigenicity and metastasis. Int J Cancer 2014 [DOI] [PubMed] [Google Scholar]
- 46.Lin KY, Lu D, Hung CF, Peng S, Huang L, Jie C, Murillo F, Rowley J, Tsai YC, He L, et al.. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer Res 2007; 67:1832-41; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hemmerlein B, Scherbening J, Kugler A, Radzun HJ. Expression of VCAM‐1, ICAM‐1, E‐and P‐selectin and tumour‐associated macrophages in renal cell carcinoma. Histopathology 2000; 37:78-83; PMID:; http://dx.doi.org/ 10.1046/j.1365-2559.2000.00933.x [DOI] [PubMed] [Google Scholar]
- 48.Vasselli JR, Shih JH, Iyengar SR, Maranchie J, Riss J, Worrell R, Torres-Cabala C, Tabios R, Mariotti A, Stearman R, et al.. Predicting survival in patients with metastatic kidney cancer by gene-expression profiling in the primary tumor. Pro Natl Acad Sci 2003; 100:6958-63; http://dx.doi.org/ 10.1073/pnas.1131754100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003; 9:1269-74; PMID:; http://dx.doi.org/ 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 50.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon K-S, Auffinger B, Tobias AL, Han Y, Lesniak MS. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 2012; 18:6110-21; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Astigiano S, Morandi B, Costa R, Mastracci L, D'Agostino A, Ratto GB, Melioli G, Frumento G. Eosinophil granulocytes account for indoleamine 2, 3-dioxygenase-mediated immune escape in human non small cell lung cancer. Neoplasia 2005; 7:390-6; PMID:; http://dx.doi.org/ 10.1593/neo.04658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, et al.. Indoleamine 2, 3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 2005; 11:6030-9; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-2671 [DOI] [PubMed] [Google Scholar]
- 53.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Göbel G, et al.. Prognostic value of indoleamine 2, 3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 2006; 12:1144-51; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1966 [DOI] [PubMed] [Google Scholar]
- 54.Pan K, Wang H, Chen M-s, Zhang H-k, Weng D-s, Zhou J, Huang W, Li JJ, Song HF, Xia JC. Expression and prognosis role of indoleamine 2, 3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol 2008; 134:1247-53; PMID:; http://dx.doi.org/ 10.1007/s00432-008-0395-1 [DOI] [PubMed] [Google Scholar]
- 55.Godin-Ethier J, Hanafi L-A, Piccirillo CA, Lapointe R. Indoleamine 2, 3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res 2011; 17:6985-91; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1331 [DOI] [PubMed] [Google Scholar]
- 56.Harden JL, Egilmez NK. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest 2012; 41:738-64; PMID:; http://dx.doi.org/ 10.3109/08820139.2012.676122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 1999; 189:1363-72; PMID:; http://dx.doi.org/ 10.1084/jem.189.9.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al.. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 2006; 176:6752-61; PMID:; http://dx.doi.org/ 10.4049/jimmunol.176.11.6752 [DOI] [PubMed] [Google Scholar]
- 59.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 2002; 196:459-68; PMID:; http://dx.doi.org/ 10.1084/jem.20020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res 2012; 72:5435-40; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-0569 [DOI] [PubMed] [Google Scholar]
- 61.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ 2002; 9:1069-77; PMID:; http://dx.doi.org/ 10.1038/sj.cdd.4401073 [DOI] [PubMed] [Google Scholar]
- 62.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med 2004; 10:15-8; PMID:; http://dx.doi.org/ 10.1016/j.molmed.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 63.Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of Allogeneic T Cell Proliferation by Indoleamine 2, 3-Dioxygenase-expressing Dendritic Cells Mediation of Suppression by Tryptophan Metabolites. J Exp Med 2002; 196:447-57; PMID:; http://dx.doi.org/ 10.1084/jem.20020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zoso A, Mazza EM, Bicciato S, Mandruzzato S, Bronte V, Serafini P, Inverardi L. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur J Immunol 2014; PMID: [DOI] [PubMed] [Google Scholar]
- 65.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med 2005; 11:312-9; PMID:; http://dx.doi.org/ 10.1038/nm1196 [DOI] [PubMed] [Google Scholar]
- 66.Löb S, Königsrainer A, Rammensee H-G, Opelz G, Terness P. Inhibitors of indoleamine-2, 3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer 2009; 9:445-52; PMID:; http://dx.doi.org/ 10.1038/nrc2639 [DOI] [PubMed] [Google Scholar]
- 67.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 2008; 222:206-21; PMID:; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00610.x [DOI] [PubMed] [Google Scholar]
- 68.Qian F, Villella J, Wallace PK, Mhawech-Fauceglia P, Tario JD, Andrews C, Matsuzaki J, Valmori D, Ayyoub M, Frederick PJ, et al.. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2, 3-dioxygenase-mediated arrest of T-Cell proliferation in human epithelial ovarian cancer. Cancer Res 2009; 69:5498-504; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2106 [DOI] [PubMed] [Google Scholar]
- 69.Suzuki S, Tone S, Takikawa O, Kubo T, Kohno I, Minatogawa Y. Expression of indoleamine 2, 3-dioxygenase and tryptophan 2, 3-dioxygenase in early concepti. Biochem J 2001; 355:425-9; PMID:; http://dx.doi.org/ 10.1042/0264-6021:3550425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2, 3-dioxygenase: is it an immune suppressor? Cancer J (Sudbury, Mass) 2010; 16; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu G, MORRIS JS. Arginine metabolism: nitric oxide and beyond. Biochem J 1998; 336:1-17; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Boniface J, Mao Y, Schmidt-Mende J, Kiessling R, Poschke I. Expression patterns of the immunomodulatory enzyme arginase 1 in blood, lymph nodes and tumor tissue of early-stage breast cancer patients. Oncoimmunology 2012; 1:1305; PMID:; http://dx.doi.org/ 10.4161/onci.21678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 2009; 69:1553-60; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, et al.. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 2005; 65:3044-8; PMID: [DOI] [PubMed] [Google Scholar]
- 75.Vanderstraeten A, Luyten C, Verbist G, Tuyaerts S, Amant F. Mapping the immunosuppressive environment in uterine tumors: implications for immunotherapy. Cancer Immunol, Immunother 2014; 63:545-57; http://dx.doi.org/ 10.1007/s00262-014-1537-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mumenthaler SM, Yu H, Tze S, Cederbaum SD, Pegg AE, Seligson DB, Grody WW. Expression of arginase II in prostate cancer. Int J Oncol 2008; 32:357-65; PMID: [PubMed] [Google Scholar]
- 77.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, et al.. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett 2007; 252:86-92; PMID:; http://dx.doi.org/ 10.1016/j.canlet.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 78.Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron 2013; 6:169-77; PMID:; http://dx.doi.org/ 10.1007/s12307-012-0126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raber P, Ochoa AC, Rodríguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest 2012; 41:614-34; PMID:; http://dx.doi.org/ 10.3109/08820139.2012.680634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al.. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 2004; 64:5839-49; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-0465 [DOI] [PubMed] [Google Scholar]
- 81.Munder M. Arginase: an emerging key player in the mammalian immune system. British J Pharmacol 2009; 158:638-51; PMID:; http://dx.doi.org/ 10.1111/j.1476-5381.2009.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007; 109:1568-73; PMID:; http://dx.doi.org/ 10.1182/blood-2006-06-031856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pervin S, Singh R, Chaudhuri G. Nitric oxide-induced cytostasis and cell cycle arrest of a human breast cancer cell line (MDA-MB-231): potential role of cyclin D1. Proc Natl Acad Sci 2001; 98:3583-8; http://dx.doi.org/ 10.1073/pnas.041603998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kostourou V, Cartwright J, Johnstone A, Boult J, Cullis E, Whitley G, Robinson SP. The role of tumour-derived iNOS in tumour progression and angiogenesis. British J Cancer 2010; 104:83-90; PMID:; http://dx.doi.org/ 10.1038/sj.bjc.6606034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol 2002; 168:689-95; PMID:; http://dx.doi.org/ 10.4049/jimmunol.168.2.689 [DOI] [PubMed] [Google Scholar]
- 86.Blesson S, Thiery J, Gaudin C, Stancou R, Kolb JP, Moreau JL, Theze J, Mami-Chouaib F, Chouaib S. Analysis of the mechanisms of human cytotoxic T lymphocyte response inhibition by NO. Int Immunol 2002; 14:1169-78; PMID:; http://dx.doi.org/ 10.1093/intimm/dxf081 [DOI] [PubMed] [Google Scholar]
- 87.Munder M, Schneider H, Luckner C, Giese T, Langhans C-D, Fuentes JM, Kropf P, Mueller I, Kolb A, Modolell M, et al.. Suppression of T-cell functions by human granulocyte arginase. Blood 2006; 108:1627-34 [DOI] [PubMed] [Google Scholar]
- 88.Tenu J-P, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher J-L. Effects of the New Arginase Inhibitor N ω-Hydroxy-nor-l-Arginine on NO Synthase Activity in Murine Macrophages. Nitric Oxide 1999; 3:427-38; PMID:; http://dx.doi.org/ 10.1006/niox.1999.0255 [DOI] [PubMed] [Google Scholar]
- 89.Kitayama J, Emoto S, Yamaguchi H, Ishigami H, Yamashita H, Seto Y, Matsuzaki K, Watanabe T. CD90 (+) CD45 (−) intraperitoneal mesothelial-like cells inhibit T cell activation by production of arginase I. Cell Immunol 2014; 288:8-14; PMID:; http://dx.doi.org/ 10.1016/j.cellimm.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 90.Wang R. Innate immune regulation and cancer immunotherapy. Springer, 2012. [Google Scholar]
- 91.Zhu X, Pribis JP, Rodriguez PC, Morris SM Jr, Vodovotz Y, Billiar TR, Ochoa JB. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury. Ann Surg 2014; 259:171-8; PMID:; http://dx.doi.org/ 10.1097/SLA.0b013e31828611f8 [DOI] [PubMed] [Google Scholar]
- 92.Kusmartsev SA, Li Y, Chen S-H. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol 2000; 165:779-85; PMID:; http://dx.doi.org/ 10.4049/jimmunol.165.2.779 [DOI] [PubMed] [Google Scholar]
- 93.Zhou R, He PL, Ren YX, Wang WH, Zhou RY, Wan H, Ono S, Fujiwara H, Zuo JP. Myeloid suppressor cell‐associated immune dysfunction in CSA1M fibrosarcoma tumor‐bearing mice. Cancer Sci 2007; 98:882-9; PMID:; http://dx.doi.org/ 10.1111/j.1349-7006.2007.00465.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006; 203:2691-702; PMID:; http://dx.doi.org/ 10.1084/jem.20061104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ibiza S, Serrador J. The role of nitric oxide in the regulation of adaptive immune responses. Inmunología 2008; 27:103-17 [Google Scholar]
- 96.Sosroseno W, Herminajeng E, Bird P, Seymour G. l‐arginine‐dependent nitric oxide production of a murine macrophage‐like RAW 264.7 cell line stimulated with Porphyromonas gingivalis lipopolysaccharide. Oral Microbiol Immunol 2004; 19:65-70; PMID:; http://dx.doi.org/ 10.1046/j.0902-0055.2003.00108.x [DOI] [PubMed] [Google Scholar]
- 97.Spiegel S, Merrill AH Jr. Sphingolipid metabolism and cell growth regulation. Faseb J 1996; 10:1388-97; PMID: [DOI] [PubMed] [Google Scholar]
- 98.Krengel U, Bousquet PA. Molecular recognition of gangliosides and their potential for cancer immunotherapies. Front Immunol 2014; 5:325; PMID:; http://dx.doi.org/ 10.3389/fimmu.2014.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McKallip R, Li R, Ladisch S. Tumor gangliosides inhibit the tumor-specific immune response. J Immunol 1999; 163:3718-26; PMID: [PubMed] [Google Scholar]
- 100.Li R, Villacreses N, Ladisch S. Human tumor gangliosides inhibit murine immune responses in vivo. Cancer Res 1995; 55:211-4; PMID: [PubMed] [Google Scholar]
- 101.Biswas S, Biswas K, Richmond A, Ko J, Ghosh S, Simmons M, Rayman P, Rini B, Gill I, Tannenbaum CS, et al.. Elevated levels of select gangliosides in T cells from renal cell carcinoma patients is associated with T cell dysfunction. J Immunol 2009; 183:5050-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0900259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Das T, Sa G, Paszkiewicz-Kozik E, Hilston C, Molto L, Rayman P, Kudo D, Biswas K, Bukowski RM, Finke JH, et al.. Renal cell carcinoma tumors induce T cell apoptosis through receptor-dependent and receptor-independent pathways. J Immunol 2008; 180:4687-96; PMID:; http://dx.doi.org/ 10.4049/jimmunol.180.7.4687 [DOI] [PubMed] [Google Scholar]
- 103.Raval G, Biswas S, Rayman P, Biswas K, Sa G, Ghosh S, Thornton M, Hilston C, Das T, Bukowski R, et al.. TNF-alpha induction of GM2 expression on renal cell carcinomas promotes T cell dysfunction. J Immunol 2007; 178:6642-52; PMID:; http://dx.doi.org/ 10.4049/jimmunol.178.10.6642 [DOI] [PubMed] [Google Scholar]
- 104.Uzzo RG, Rayman P, Kolenko V, Clark PE, Cathcart MK, Bloom T, Novick AC, Bukowski RM, Hamilton T, Finke JH. Renal cell carcinoma-derived gangliosides suppress nuclear factor-kappaB activation in T cells. J Clin Invest 1999; 104:769-76; PMID:; http://dx.doi.org/ 10.1172/JCI6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rayman P, Wesa AK, Richmond AL, Das T, Biswas K, Raval G, Storkus WJ, Tannenbaum C, Novick A, Bukowski R, et al.. Effect of renal cell carcinomas on the development of type 1 T-cell responses. Clin Cancer Res 2004; 10:6360S-6S; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-050011 [DOI] [PubMed] [Google Scholar]
- 106.Biswas K, Richmond A, Rayman P, Biswas S, Thornton M, Sa G, Das T, Zhang R, Chahlavi A, Tannenbaum CS, et al.. GM2 expression in renal cell carcinoma: potential role in tumor-induced T-cell dysfunction. Cancer Res 2006; 66:6816-25; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-0250 [DOI] [PubMed] [Google Scholar]
- 107.Crespo FA, Sun X, Cripps JG, Fernandez-Botran R. The immunoregulatory effects of gangliosides involve immune deviation favoring type-2 T cell responses. J Leukoc Biol 2006; 79:586-95; PMID:; http://dx.doi.org/ 10.1189/jlb.0705395 [DOI] [PubMed] [Google Scholar]
- 108.Caldwell S, Heitger A, Shen W, Liu Y, Taylor B, Ladisch S. Mechanisms of ganglioside inhibition of APC function. J Immunol 2003; 171:1676-83; PMID:; http://dx.doi.org/ 10.4049/jimmunol.171.4.1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thornton MV, Kudo D, Rayman P, Horton C, Molto L, Cathcart MK, Ng C, Paszkiewicz-Kozik E, Bukowski R, Derweesh I, et al.. Degradation of NF-kappa B in T cells by gangliosides expressed on renal cell carcinomas. J Immunol 2004; 172:3480-90; PMID:; http://dx.doi.org/ 10.4049/jimmunol.172.6.3480 [DOI] [PubMed] [Google Scholar]
- 110.Lee HC, Wondimu A, Liu Y, Ma JS, Radoja S, Ladisch S. Ganglioside inhibition of CD8+ T cell cytotoxicity: interference with lytic granule trafficking and exocytosis. J Immunol 2012; 189:3521-7; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1201256 [DOI] [PubMed] [Google Scholar]
- 111.Retter MW, Johnson JC, Peckham DW, Bannink JE, Bangur CS, Dresser K, Cai F, Foy TM, Fanger NA, Fanger GR, et al.. Characterization of a proapoptotic antiganglioside GM2 monoclonal antibody and evaluation of its therapeutic effect on melanoma and small cell lung carcinoma xenografts. Cancer Res 2005; 65:6425-34; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-0300 [DOI] [PubMed] [Google Scholar]
- 112.Lloyd KO, Gordon CM, Thampoe IJ, DiBenedetto C. Cell surface accessibility of individual gangliosides in malignant melanoma cells to antibodies is influenced by the total ganglioside composition of the cells. Cancer Res 1992; 52:4948-53; PMID: [PubMed] [Google Scholar]
- 113.Mazorra Z, Mesa C, Fernández LE. GM3 ganglioside: a novel target for the therapy against melanoma. Biotecnología Aplicada 2009; 26:256-9 [Google Scholar]
- 114.Houghton AN, Mintzer D, Cordon-Cardo C, Welt S, Fliegel B, Vadhan S, Carswell E, Melamed MR, Oettgen HF, Old LJ. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci U S A 1985; 82:1242-6; PMID:; http://dx.doi.org/ 10.1073/pnas.82.4.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheung NK, Neely JE, Landmeier B, Nelson D, Miraldi F. Targeting of ganglioside GD2 monoclonal antibody to neuroblastoma. J Nucl Med 1987; 28:1577-83; PMID: [PubMed] [Google Scholar]
- 116.Ahmed M, Cheung N-KV. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett 588:288-97; PMID: [DOI] [PubMed] [Google Scholar]
- 117.Fernandez LE, Gabri MR, Guthmann MD, Gomez RE, Gold S, Fainboim L, Gomez DE, Alonso DF. NGcGM3 ganglioside: a privileged target for cancer vaccines. J Immunol Res 2010; 2010:814397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Groux H, Bigler M, de Vries JE, Roncarolo M-G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol 1998; 160:3188-93; PMID: [PubMed] [Google Scholar]
- 119.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukocyte Biol 2005; 78:1043-51; PMID:; http://dx.doi.org/ 10.1189/jlb.0705358 [DOI] [PubMed] [Google Scholar]
- 120.Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol 1995; 155:2240-7; PMID: [PubMed] [Google Scholar]
- 121.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res 1995; 55:3847-53; PMID: [PubMed] [Google Scholar]
- 122.Knoefel B, Nuske K, Steiner T, Junker K, Kosmehl H, Rebstock K, Reinhold D, Junker U. Renal cell carcinomas produce IL-6, IL-10, IL-11, and TGF-beta 1 in primary cultures and modulate T lymphocyte blast transformation. J Interferon Cytokine Res 1997; 17:95-102; PMID:; http://dx.doi.org/ 10.1089/jir.1997.17.95 [DOI] [PubMed] [Google Scholar]
- 123.Venetsanakos E, Beckman I, Bradley J, Skinner JM. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br J Cancer 1997; 75:1826-30; PMID:; http://dx.doi.org/ 10.1038/bjc.1997.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pisa P, Halapi E, Pisa EK, Gerdin E, Hising C, Bucht A, Gerdin B, Kiessling R. Selective expression of interleukin 10, interferon gamma, and granulocyte-macrophage colony-stimulating factor in ovarian cancer biopsies. Proc Natl Acad Sci U S A 1992; 89:7708-12; PMID:; http://dx.doi.org/ 10.1073/pnas.89.16.7708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bolpetti A, Silva JS, Villa LL, Lepique AP. Interleukin-10 production by tumor infiltrating macrophages plays a role in Human Papillomavirus 16 tumor growth. BMC Immunol 2010; 11:27; PMID:; http://dx.doi.org/ 10.1186/1471-2172-11-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yue FY, Dummer R, Geertsen R, Hofbauer G, Laine E, Manolio S, Burg G. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer 1997; 71:630-7; PMID:; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19970516)71:4%3c630::AID-IJC20%3e3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- 127.Fujieda S, Sunaga H, Tsuzuki H, Fan GK, Saito H. IL-10 expression is associated with the expression of platelet-derived endothelial cell growth factor and prognosis in oral and oropharyngeal carcinoma. Cancer Lett 1999; 136:1-9; PMID:; http://dx.doi.org/ 10.1016/S0304-3835(98)00281-X [DOI] [PubMed] [Google Scholar]
- 128.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol 1991; 147:2713-6; PMID: [PubMed] [Google Scholar]
- 129.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991; 174:1209-20; PMID:; http://dx.doi.org/ 10.1084/jem.174.5.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin‐10 and transforming growth factor‐β: the role of T regulatory cells. Immunology 2006; 117:433-42; PMID:; http://dx.doi.org/ 10.1111/j.1365-2567.2006.02321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003; 21:685-711; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.21.120601.141040 [DOI] [PubMed] [Google Scholar]
- 132.Nabarro S, Himoudi N, Papanastasiou A, Gilmour K, Gibson S, Sebire N, Thrasher A, Blundell MP, Hubank M, Canderan G, et al.. Coordinated oncogenic transformation and inhibition of host immune responses by the PAX3-FKHR fusion oncoprotein. J Exp Med 2005; 202:1399-410; PMID:; http://dx.doi.org/ 10.1084/jem.20050730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, et al.. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 2004; 10:48-54; PMID:; http://dx.doi.org/ 10.1038/nm976 [DOI] [PubMed] [Google Scholar]
- 134.Sevko A, Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thieves. J Cancer 2013; 4:3-11; PMID:; http://dx.doi.org/ 10.7150/jca.5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baj-Krzyworzeka M, Baran J, Szatanek R, Stankiewicz D, Siedlar M, Zembala M. Prevention and reversal of tumor cell-induced monocyte deactivation by cytokines, purified protein derivative (PPD), and anti-IL-10 antibody. Cancer immun 2004; 4:8; PMID: [PubMed] [Google Scholar]
- 137.Nishijima K-i, Hisatsune T, Minai Y, Kohyama M, Kaminogawa S. Anti-IL-10 Antibody Enhances the Proliferation of CD8+ T Cell Clones: Autoregulatory Role of Murine IL-10 in CD8+ T Cells. Cell Immunol 1994; 154:193-201; PMID:; http://dx.doi.org/ 10.1006/cimm.1994.1068 [DOI] [PubMed] [Google Scholar]
- 138.Avradopoulos K, Mehta S, Blackinton D, Wanebo HJ. Interleukin-10 as a possible mediator of immunosuppressive effect in patients with squamous cell carcinoma of the head and neck. Ann Surg Oncol 1997; 4:184-90; PMID:; http://dx.doi.org/ 10.1007/BF02303803 [DOI] [PubMed] [Google Scholar]
- 139.Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res 2011; 51:170-82; PMID:; http://dx.doi.org/ 10.1007/s12026-011-8262-6 [DOI] [PubMed] [Google Scholar]
- 140.Lee TL, Yeh J, Van Waes C, Chen Z. Epigenetic modification of SOCS-1 differentially regulates STAT3 activation in response to interleukin-6 receptor and epidermal growth factor receptor signaling through JAK and/or MEK in head and neck squamous cell carcinomas. Mol Cancer Ther 2006; 5:8-19; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-05-0069 [DOI] [PubMed] [Google Scholar]
- 141.Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol 2002; 2:410-6; PMID: [DOI] [PubMed] [Google Scholar]
- 142.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang S, Yang D, Lippman ME. Targeting Bcl-2 and Bcl-X L with nonpeptidic small-molecule antagonists. Semin Oncol, 2003; 30: 133-42; http://dx.doi.org/ 10.1053/j.seminoncol.2003.08.015 [DOI] [PubMed] [Google Scholar]
- 144.Mohammad RM, Wang S, Aboukameel A, Chen B, Wu X, Chen J, Al-Katib A. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL ; (−)-gossypol against diffuse large cell lymphoma. Mol Cancer Ther 2005; 4:13-21; PMID:; http://dx.doi.org/ 10.1186/1476-4598-4-13 [DOI] [PubMed] [Google Scholar]
- 145.Mohammad RM, Goustin AS, Aboukameel A, Chen B, Banerjee S, Wang G, Nikolovska-Coleska Z, Wang S, Al-Katib A. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res 2007; 13:2226-35; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1574 [DOI] [PubMed] [Google Scholar]
- 146.Oh SA, Li MO. TGF-beta: guardian of T cell function. J Immunol 2013; 191:3973-9; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1301843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nat Med 2001; 7:1118-22; PMID:; http://dx.doi.org/ 10.1038/nm1001-1118 [DOI] [PubMed] [Google Scholar]
- 148.Smyth MJ, Strobl S, Young H, Ortaldo J, Ochoa A. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol 1991; 146:3289-97; PMID: [PubMed] [Google Scholar]
- 149.Cheng M-L, Chen H-W, Tsai J-P, Lee Y-P, Shih Y-C, Chang C-M, Ting CC. Clonal restriction of the expansion of antigen-specific CD8+ memory T cells by transforming growth factor-β. J leukocyte Biol 2006; 79:1033-42; PMID:; http://dx.doi.org/ 10.1189/jlb.0805474 [DOI] [PubMed] [Google Scholar]
- 150.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β*. Ann Rev Immunol 1998; 16:137-61; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.16.1.137 [DOI] [PubMed] [Google Scholar]
- 151.Bright JJ, Sriram S. TGF-β inhibits IL-12-induced activation of Jak-STAT pathway in T lymphocytes. J Immunol 1998; 161:1772-7; PMID: [PubMed] [Google Scholar]
- 152.Holter W, Kalthoff F, Pickl W, Ebner C, Majdic O, Kraft D, Knapp W. Transforming growth factor-beta inhibits IL-4 and IFN-gamma production by stimulated human T cells. Int Immunol 1994; 6:469; PMID:; http://dx.doi.org/ 10.1093/intimm/6.3.469 [DOI] [PubMed] [Google Scholar]
- 153.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A 2004; 101:4572-7; PMID:; http://dx.doi.org/ 10.1073/pnas.0400810101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen W, Jin W, Hardegen N, Lei K-j, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 2003; 198:1875-86; PMID:; http://dx.doi.org/ 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+ CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 2004; 172:5149-53; PMID:; http://dx.doi.org/ 10.4049/jimmunol.172.9.5149 [DOI] [PubMed] [Google Scholar]
- 156.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangère V, Vincent J, Masson D, et al.. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 2012; 36:362-73; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2011.12.019 [DOI] [PubMed] [Google Scholar]
- 157.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W. Human TH17 cells are long-lived effector memory cells. Sci Trans Med 2011; 3:104ra0; http://dx.doi.org/ 10.1126/scitranslmed.3002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al.. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009; 114:1141-9; PMID:; http://dx.doi.org/ 10.1182/blood-2009-03-208249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, et al.. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011; 35:972-85; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009; 31:787-98; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, Soria JC, Marty V, Vielh P, Robert C, et al.. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res 2011; 71:661-5; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-1259 [DOI] [PubMed] [Google Scholar]
- 162.Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, Brawanski A, Proescholdt M, Schlaier J, Buchroithner J, et al.. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 2007; 17:201-12; PMID:; http://dx.doi.org/ 10.1089/oli.2006.0053 [DOI] [PubMed] [Google Scholar]
- 163.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression by TGF-beta inhibitors. Invest New Drugs 2003; 21:21-32; PMID:; http://dx.doi.org/ 10.1023/A:1022951824806 [DOI] [PubMed] [Google Scholar]
- 164.Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-β targeted cancer therapy. Int J Biol Sci 2012; 8:964; PMID:; http://dx.doi.org/ 10.7150/ijbs.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res 2007; 67:4507-13; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-4174 [DOI] [PubMed] [Google Scholar]
- 166.Wang M-T, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Meta Rev 2007; 26:525-34; PMID:; http://dx.doi.org/ 10.1007/s10555-007-9096-5 [DOI] [PubMed] [Google Scholar]
- 167.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes&quest. Immunol Cell Biol 2011; 90:579-86; PMID:; http://dx.doi.org/ 10.1038/icb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol 1991; 146:108-13; PMID: [PubMed] [Google Scholar]
- 169.Snijdewint F, Kaliński P, Wierenga E, Bos J, Kapsenberg M. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol 1993; 150:5321-9; PMID: [PubMed] [Google Scholar]
- 170.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res 1998; 58:1208-16; PMID: [PubMed] [Google Scholar]
- 171.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, et al.. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol 2000; 164:361-70; PMID:; http://dx.doi.org/ 10.4049/jimmunol.164.1.361 [DOI] [PubMed] [Google Scholar]
- 172.Luo JS, Kammerer R, von Kleist S. Comparison of the effects of immunosuppressive factors from newly established colon carcinoma cell cultures on human lymphocyte proliferation and cytokine secretion. Tumour Biol 2000; 21:11-20; PMID:; http://dx.doi.org/ 10.1159/000030106 [DOI] [PubMed] [Google Scholar]
- 173.Khan I, Al-Awadi F, Thomas N, Haridas S, Anim J. Cyclooxygenase-2 inhibition and experimental colitis: beneficial effects of phosphorothioated antisense oligonucleotide and meloxicam. Scand J Gastroenterol 2002; 37:1428-36; PMID:; http://dx.doi.org/ 10.1080/003655202762671314 [DOI] [PubMed] [Google Scholar]
- 174.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 2011; 470:548-53; PMID:; http://dx.doi.org/ 10.1038/nature09707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET, et al.. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 2012; 1:152-61; PMID:; http://dx.doi.org/ 10.4161/onci.1.2.18480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Watanabe J, Kushihata F, Honda K, Sugita A, Tateishi N, Mominoki K, Matsuda S, Kobayashi N. Prognostic significance of Bcl-xL in human hepatocellular carcinoma. Surgery 2004; 135:604-12; PMID:; http://dx.doi.org/ 10.1016/j.surg.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 177.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 2003; 22:8581-9; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1207113 [DOI] [PubMed] [Google Scholar]
- 178.Kleinberg L, Lie AK, Florenes VA, Nesland JM, Davidson B. Expression of inhibitor-of-apoptosis protein family members in malignant mesothelioma. Hum Pathol 2007; 38:986-94; PMID:; http://dx.doi.org/ 10.1016/j.humpath.2006.12.013 [DOI] [PubMed] [Google Scholar]
- 179.Mizutani Y, Nakanishi H, Li YN, Matsubara H, Yamamoto K, Sato N, Shiraishi T, Nakamura T, Mikami K, Okihara K, et al.. Overexpression of XIAP expression in renal cell carcinoma predicts a worse prognosis. Int J Oncol 2007; 30:919-25; PMID: [PubMed] [Google Scholar]
- 180.Guan J, Chen J. Survivin-a new tumor-specific anti-apoptosis factor. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2001; 23:532-4; PMID: [PubMed] [Google Scholar]
- 181.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis 2007; 28:1133-9; PMID:; http://dx.doi.org/ 10.1093/carcin/bgm047 [DOI] [PubMed] [Google Scholar]
- 182.Noh KH, Kang TH, Kim JH, Pai SI, Lin KY, Hung C-F, Wu TC, Kim TW. Activation of Akt as a mechanism for tumor immune evasion. Mol Ther 2008; 17:439-47; PMID:; http://dx.doi.org/ 10.1038/mt.2008.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7:41-51; PMID:; http://dx.doi.org/ 10.1038/nri1995 [DOI] [PubMed] [Google Scholar]
- 184.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Trans Med 2012; 4:127ra37; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207-12; PMID:; http://dx.doi.org/ 10.1016/j.coi.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cao Y, Zhang L, Ritprajak P, Tsushima F, Youngnak-Piboonratanakit P, Kamimura Y, Hashiguchi M, Azuma M. Immunoregulatory molecule B7-H1 (CD274) contributes to skin carcinogenesis. Cancer Res 2011; 71:4737-41; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0527 [DOI] [PubMed] [Google Scholar]
- 188.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236:219-42; PMID:; http://dx.doi.org/ 10.1111/j.1600-065X.2010.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al.. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793-800; PMID:; http://dx.doi.org/ 10.1038/nm0902-1039c [DOI] [PubMed] [Google Scholar]
- 191.Sugasawa H, Ichikura T, Tsujimoto H, Kinoshita M, Morita D, Ono S, Chochi K, Tsuda H, Seki S, Mochizuki H. Prognostic significance of expression of CCL5/RANTES receptors in patients with gastric cancer. J Surg Oncol 2008; 97:445-50; PMID:; http://dx.doi.org/ 10.1002/jso.20984 [DOI] [PubMed] [Google Scholar]
- 192.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al.. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9:562-7; PMID:; http://dx.doi.org/ 10.1038/nm863 [DOI] [PubMed] [Google Scholar]
- 193.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al.. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 2006; 203:871-81; PMID:; http://dx.doi.org/ 10.1084/jem.20050930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al.. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res 2003; 63:6501-5; PMID: [PubMed] [Google Scholar]
- 195.Li N, Qin H, Li X, Zhou C, Wang D, Ma W, Lin C, Zhang Y, Wang S, Zhang S. Potent systemic antitumor immunity induced by vaccination with chemotactic-prostate tumor associated antigen gene-modified tumor cell and blockade of B7-H1. J Clin Immunol 2007; 27:117-30; PMID:; http://dx.doi.org/ 10.1007/s10875-006-9053-z [DOI] [PubMed] [Google Scholar]
- 196.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al.. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134-44; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al.. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167-75; PMID:; http://dx.doi.org/ 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Flies DB, Sandler BJ, Sznol M, Chen L. Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. The Yale J Biol Med 2011; 84:409; PMID: [PMC free article] [PubMed] [Google Scholar]
- 200.Ascierto PA, Simeone E, Sznol M, Fu Y-X, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol 2010; 37:508-16; http://dx.doi.org/ 10.1053/j.seminoncol.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 201.Ichikawa M, Chen L. Role of B7-H1 and B7-H4 molecules in down-regulating effector phase of T-cell immunity: novel cancer escaping mechanisms. Front Biosci 2005; 10:2856-60; PMID:; http://dx.doi.org/ 10.2741/1742 [DOI] [PubMed] [Google Scholar]
- 202.Geng H, Zhang GM, Xiao H, Yuan Y, Li D, Zhang H, Qiu H, He YF, Feng ZH. HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD-1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int J Cancer 2006; 118:2657-64; PMID:; http://dx.doi.org/ 10.1002/ijc.21795 [DOI] [PubMed] [Google Scholar]
- 203.Soares KC, Zheng L, Edil B, Jaffee EM. Vaccines for pancreatic cancer. Cancer J (Sudbury, Mass) 2012; 18:642; PMID:; http://dx.doi.org/ 10.1097/PPO.0b013e3182756903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Smith JB, Stashwick C, Powell DJ Jr. B7-H4 as a potential target for immunotherapy for gynecologic cancers: a closer look. Gynecol Oncol 2014; 134:181-9; PMID:; http://dx.doi.org/ 10.1016/j.ygyno.2014.03.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003; 18:849-61; PMID:; http://dx.doi.org/ 10.1016/S1074-7613(03)00152-3 [DOI] [PubMed] [Google Scholar]
- 206.Murooka TT, Wong MM, Rahbar R, Majchrzak-Kita B, Proudfoot AE, Fish EN. CCL5-CCR5-mediated Apoptosis in T cells requirement for glycosaminoglycan binding and CCL5 aggregation. J Biol Chem 2006; 281:25184-94; PMID:; http://dx.doi.org/ 10.1074/jbc.M603912200 [DOI] [PubMed] [Google Scholar]
- 207.Roth W, Reed JC. FLIP protein and TRAIL-induced apoptosis. Vitamins & Hormones 2004; 67:189-206; PMID:; http://dx.doi.org/ 10.1016/S0083-6729(04)67011-7 [DOI] [PubMed] [Google Scholar]
- 208.French LE, Tschopp J. The TRAIL to selective tumor death. Nat Med 1999; 5:146-7; PMID:; http://dx.doi.org/ 10.1038/5505 [DOI] [PubMed] [Google Scholar]
- 209.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003; 22:8628-33; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1207232 [DOI] [PubMed] [Google Scholar]
- 210.Marsters SA, Pitti RM, Donahue CJ, Ruppert S, Bauer KD, Ashkenazi A. Activation of apoptosis by Apo-2 ligand is independent of FADD but blocked by CrmA. Curr Biol 1996; 6:750-2; PMID:; http://dx.doi.org/ 10.1016/S0960-9822(09)00456-4 [DOI] [PubMed] [Google Scholar]
- 211.Jeremias I, Herr I, Boehler T, Debatin KM. TRAIL/Apo‐2‐ligand‐induced apoptosis in human T cells. Euro J Immunol 1998; 28:143-52; PMID:; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199801)28:01%3c143::AID-IMMU143%3e3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 212.Hallermalm K, De Geer A, Kiessling R, Levitsky V, Levitskaya J. Autocrine secretion of Fas ligand shields tumor cells from Fas-mediated killing by cytotoxic lymphocytes. Cancer Res 2004; 64:6775-82; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-0508 [DOI] [PubMed] [Google Scholar]
- 213.Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol 1994; 6:865-73; PMID:; http://dx.doi.org/ 10.1016/0952-7915(94)90006-X [DOI] [PubMed] [Google Scholar]
- 214.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies-integrating mammalian biology. Cell 2001; 104:487-501; PMID:; http://dx.doi.org/ 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 215.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 2004; 5:241-51; PMID:; http://dx.doi.org/ 10.1016/S1535-6108(04)00024-8 [DOI] [PubMed] [Google Scholar]
- 216.Giovarelli M, Musiani P, Garotta G, Ebner R, Di Carlo E, Kim Y, Cappello P, Rigamonti L, Bernabei P, Novelli F, et al.. A “stealth effect”: adenocarcinoma cells engineered to express TRAIL elude tumor-specific and allogeneic T cell reactions. J Immunol 1999; 163:4886-93; PMID: [PubMed] [Google Scholar]
- 217.Nakashima M, Sonoda K, Watanabe T. Inhibition of cell growth and induction of apoptotic cell death by the human tumor-associated antigen RCAS1. Nat Med 1999; 5:938-42; PMID:; http://dx.doi.org/ 10.1038/11383 [DOI] [PubMed] [Google Scholar]
- 218.Umata T, Hirata M, Takahashi T, Ryu F, Shida S, Takahashi Y, Tsuneoka M, Miura Y, Masuda M, Horiguchi Y, et al.. A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J Biol Chem 2001; 276:30475-82; PMID:; http://dx.doi.org/ 10.1074/jbc.M103673200 [DOI] [PubMed] [Google Scholar]
- 219.Igney FH, Behrens CK, Krammer PH. CD95L mediates tumor counterattack in vitro but induces neutrophil-independent tumor rejection in vivo. Int J Cancer 2005; 113:78-87; PMID:; http://dx.doi.org/ 10.1002/ijc.20538 [DOI] [PubMed] [Google Scholar]
- 220.Ryan AE, Shanahan F, O'Connell J, Houston AM. Addressing the “Fas counterattack” controversy: blocking fas ligand expression suppresses tumor immune evasion of colon cancer in vivo. Cancer Res 2005; 65:9817-23; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1462 [DOI] [PubMed] [Google Scholar]
- 221.Aubrey M. Glycans in cell interaction and recognition. CRC Press, 2003. [Google Scholar]
- 222.Elola M, Wolfenstein-Todel C, Troncoso M, Vasta G, Rabinovich G. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci 2007; 64:1679-700; PMID:; http://dx.doi.org/ 10.1007/s00018-007-7044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Moiseeva EP, Spring EL, Baron JH, de Bono D. Galectin 1 modulates attachment, spreading and migration of cultured vascular smooth muscle cells via interactions with cellular receptors and components ofextracellu lar matrix. J Vasc Res 1999; 36:47-58; PMID:; http://dx.doi.org/ 10.1159/000025625 [DOI] [PubMed] [Google Scholar]
- 224.Liu F-T. Galectins: a new family of regulators of inflammation. Clin Immunol 2000; 97:79-88; PMID:; http://dx.doi.org/ 10.1006/clim.2000.4912 [DOI] [PubMed] [Google Scholar]
- 225.Liu F-T. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol 2005; 136:385-400; PMID:; http://dx.doi.org/ 10.1159/000084545 [DOI] [PubMed] [Google Scholar]
- 226.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, et al.. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci 2006; 103:15975-80; http://dx.doi.org/ 10.1073/pnas.0603883103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Thijssen VL, Barkan B, Shoji H, Aries IM, Mathieu V, Deltour L, Hackeng TM, Kiss R, Kloog Y, Poirier F, et al.. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res 2010; 70:6216-24; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-4150 [DOI] [PubMed] [Google Scholar]
- 228.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001; 20:7486-93; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1204950 [DOI] [PubMed] [Google Scholar]
- 229.van den Brule FA, Waltregny D, Castronovo V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J Pathol 2001; 193:80-7; PMID:; http://dx.doi.org/ 10.1002/1096-9896(2000)9999:9999%3c::AID-PATH730%3e3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- 230.Langbein S, Brade J, Badawi JK, Hatzinger M, Kaltner H, Lensch M, Specht K, André S, Brinck U, Alken P, et al.. Gene-expression signature of adhesion/growth-regulatory tissue lectins (galectins) in transitional cell cancer and its prognostic relevance. Histopathology 2007; 51:681-90; PMID:; http://dx.doi.org/ 10.1111/j.1365-2559.2007.02852.x [DOI] [PubMed] [Google Scholar]
- 231.Chen J, Zhou S-J, Zhang Y, Zhang G-Q, Zha T-Z, Feng Y-Z, Zhang K. Clinicopathological and prognostic significance of galectin-1 and vascular endothelial growth factor expression in gastric cancer. World J Gastroenterol 2013; 19:2073; PMID:; http://dx.doi.org/ 10.3748/wjg.v19.i13.2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, Serova M, Neuzillet C, Albert S, Raymond E, Faivre S. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat Rev 2014; 40:307-19; PMID:; http://dx.doi.org/ 10.1016/j.ctrv.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 233.Danguy A, Camby I, Kiss R. Galectins and cancer. Biochimica et Biophysica Acta (BBA)-General Subjects 2002; 1572:285-93; http://dx.doi.org/ 10.1016/S0304-4165(02)00315-X [DOI] [PubMed] [Google Scholar]
- 234.Vespa GN, Lewis LA, Kozak KR, Moran M, Nguyen JT, Baum LG, Miceli MC. Galectin-1 specifically modulates TCR signals to enhance TCR apoptosis but inhibit IL-2 production and proliferation. J Immunol 1999; 162:799-806; PMID: [PubMed] [Google Scholar]
- 235.Blaskó A, Fajka-Boja R, Ion G, Monostori É. How does it act when soluble? Critical evaluation of mechanism of galectin-1 induced T-cell apoptosis. Acta Biologica Hungarica 2011; 62:106-11; http://dx.doi.org/ 10.1556/ABiol.61.2011.1.11 [DOI] [PubMed] [Google Scholar]
- 236.Pang M, He J, Johnson P, Baum LG. CD45-mediated fodrin cleavage during galectin-1 T cell death promotes phagocytic clearance of dying cells. J Immunol 2009; 182:7001-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0804329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature 1995; 378:736-9. [DOI] [PubMed] [Google Scholar]
- 238.Walzel H, Schulz U, Neels P, Brock J. Galectin-1, a natural ligand for the receptor-type protein tyrosine phosphatase CD45. Immunol Lett 1999; 67:193-202; PMID:; http://dx.doi.org/ 10.1016/S0165-2478(99)00012-7 [DOI] [PubMed] [Google Scholar]
- 239.Fajka-Boja R, Szemes M, Ion G, Légrádi Á, Caron M, Monostori É. Receptor tyrosine phosphatase, CD45 binds galectin-1 but does not mediate its apoptotic signal in T cell lines. Immunol Lett 2002; 82:149-54; PMID:; http://dx.doi.org/ 10.1016/S0165-2478(02)00030-5 [DOI] [PubMed] [Google Scholar]
- 240.Nguyen JT, Evans DP, Galvan M, Pace KE, Leitenberg D, Bui TN, Baum LG. CD45 modulates galectin-1-induced T cell death: regulation by expression of core 2 O-glycans. J Immunol 2001; 167:5697-707; PMID:; http://dx.doi.org/ 10.4049/jimmunol.167.10.5697 [DOI] [PubMed] [Google Scholar]
- 241.Pace KE, Hahn HP, Pang M, Nguyen JT, Baum LG. Cutting edge: CD7 delivers a pro-apoptotic signal during galectin-1-induced T cell death. J Immunol 2000; 165:2331-4; PMID:; http://dx.doi.org/ 10.4049/jimmunol.165.5.2331 [DOI] [PubMed] [Google Scholar]
- 242.Le Q-T, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O'Byrne KJ, et al.. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol 2005; 23:8932-41; PMID:; http://dx.doi.org/ 10.1200/JCO.2005.02.0206 [DOI] [PubMed] [Google Scholar]
- 243.Banh A, Zhang J, Cao H, Bouley DM, Kwok S, Kong C, Giaccia AJ, Koong AC, Le QT. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res 2011; 71:4423-31; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.He J, Baum LG. Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem 2004; 279:4705-12; PMID:; http://dx.doi.org/ 10.1074/jbc.M311183200 [DOI] [PubMed] [Google Scholar]
- 245.Kovacs-Solyom F, Blasko A, Fajka-Boja R, Katona RL, Vegh L, Novak J, Szebeni GJ, Krenács L, Uher F, Tubak V, et al.. Mechanism of tumor cell-induced T-cell apoptosis mediated by galectin-1. Immunol Lett 2010; 127:108-18; PMID:; http://dx.doi.org/ 10.1016/j.imlet.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 246.Le Mercier M, Mathieu V, Haibe-Kains B, Bontempi G, Mijatovic T, Decaestecker C, Kiss R, Lefranc F. Knocking down galectin 1 in human hs683 glioblastoma cells impairs both angiogenesis and endoplasmic reticulum stress responses. J Neuropathol Exp Neurol 2008; 67:456-69; PMID:; http://dx.doi.org/ 10.1097/NEN.0b013e318170f892 [DOI] [PubMed] [Google Scholar]
- 247.Jung T-Y, Jung S, Ryu H-H, Jeong Y-I, Jin Y-H, Jin S-G, Kim IY, Kang SS, Kim HS. Role of galectin-1 in migration and invasion of human glioblastoma multiforme cell lines. J Neurosurg 2008; 109:273-84. [DOI] [PubMed] [Google Scholar]
- 248.Saussez S, Decaestecker C, Lorfevre F, Cucu D-R, Mortuaire G, Chevalier D, Wacreniez A, Kaltner H, André S, Toubeau G, et al.. High level of galectin-1 expression is a negative prognostic predictor of recurrence in laryngeal squamous cell carcinomas. Int J Oncol 2007; 30:1109-18; PMID: [PubMed] [Google Scholar]
- 249.Saussez S, Camby I, Toubeau G, Kiss R. Galectins as modulators of tumor progression in head and neck squamous cell carcinomas. Head Neck 2007; 29:874-84; PMID:; http://dx.doi.org/ 10.1002/hed.20559 [DOI] [PubMed] [Google Scholar]
- 250.He J, Baum LG. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab Invest 2006; 86:578-90; PMID: [DOI] [PubMed] [Google Scholar]
- 251.Thijssen VL, Hulsmans S, Griffioen AW. The galectin profile of the endothelium: altered expression and localization in activated and tumor endothelial cells. Am J Pathol 2008; 172:545-53; PMID:; http://dx.doi.org/ 10.2353/ajpath.2008.070938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Matarrese P, Tinari A, Mormone E, Bianco GA, Toscano MA, Ascione B, Rabinovich GA, Malorni W. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J Biol Chem 2005; 280:6969-85; PMID:; http://dx.doi.org/ 10.1074/jbc.M409752200 [DOI] [PubMed] [Google Scholar]
- 253.Cedeno-Laurent F, Opperman M, Barthel SR, Kuchroo VK, Dimitroff CJ. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J Immunol 2012; 188:3127-37; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1103433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, Ilarregui JM, Toscano MA, Domaica CI, et al.. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi's sarcoma. J Exp Med 2012; 209:1985-2000; PMID:; http://dx.doi.org/ 10.1084/jem.20111665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Heslop HE. A less sour sweet; blocking galectin. Blood 2011; 117:4165-6; PMID:; http://dx.doi.org/ 10.1182/blood-2011-03-337683 [DOI] [PubMed] [Google Scholar]
- 256.Ito K, Scott SA, Cutler S, Dong L-F, Neuzil J, Blanchard H, Ralph SJ. Thiodigalactoside inhibits murine cancers by concurrently blocking effects of galectin-1 on immune dysregulation, angiogenesis and protection against oxidative stress. Angiogenesis 2011; 14:293-307; PMID:; http://dx.doi.org/ 10.1007/s10456-011-9213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Delaine T, Cumpstey I, Ingrassia L, Mercier ML, Okechukwu P, Leffler H, Kiss R, Nilsson UJ. Galectin-inhibitory thiodigalactoside ester derivatives have antimigratory effects in cultured lung and prostate cancer cells. J Med Chem 2008; 51:8109-14; PMID:; http://dx.doi.org/ 10.1021/jm801077j [DOI] [PubMed] [Google Scholar]
- 258.Iurisci I, Cumashi A, Sherman AA, Tsvetkov YE, Tinari N, Piccolo E, D'Egidio M, Adamo V, Natoli C, Rabinovich GA, et al.. Synthetic inhibitors of galectin-1 and-3 selectively modulate homotypic cell aggregation and tumor cell apoptosis. Anticancer Res 2009; 29:403-10; PMID: [PubMed] [Google Scholar]
- 259.Rabinovich GA, Cumashi A, Bianco GA, Ciavardelli D, Iurisci I, D'Egidio M, Piccolo E, Tinari N, Nifantiev N, Iacobelli S. Synthetic lactulose amines: novel class of anticancer agents that induce tumor-cell apoptosis and inhibit galectin-mediated homotypic cell aggregation and endothelial cell morphogenesis. Glycobiology 2006; 16:210-20; PMID:; http://dx.doi.org/ 10.1093/glycob/cwj056 [DOI] [PubMed] [Google Scholar]
- 260.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91:479-89; PMID:; http://dx.doi.org/ 10.1016/S0092-8674(00)80434-1 [DOI] [PubMed] [Google Scholar]
- 261.Mellado M, de Ana AMn, Moreno MC, Martínez-A C, Rodríguez-Frade JM. A potential immune escape mechanism by melanoma cells through the activation of chemokine-induced T cell death. Curr Biol 2001; 11:691-6; PMID:; http://dx.doi.org/ 10.1016/S0960-9822(01)00199-3 [DOI] [PubMed] [Google Scholar]
- 262.Lin S, Wan S, Sun L, Hu J, Fang D, Zhao R, Yuan S, Zhang L. Chemokine C‐C motif receptor 5 and C‐C motif ligand 5 promote cancer cell migration under hypoxia. Cancer Sci 2012; 103:904-12; PMID:; http://dx.doi.org/ 10.1111/j.1349-7006.2012.02259.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mrowietz U, Schwenk U, Maune S, Bartels J, Küpper M, Fichtner I, Schröder JM, Schadendorf D. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. British J Cancer 1999; 79:1025; PMID:; http://dx.doi.org/ 10.1038/sj.bjc.6690164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm 2014; 2014; PMID:; http://dx.doi.org/ 10.1155/2014/292376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kato T, Fujita Y, Nakane K, Mizutani K, Terazawa R, Ehara H, Kanimoto Y, Kojima T, Nozawa Y, Deguchi T, et al.. CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine 2013; 64:251-7; PMID:; http://dx.doi.org/ 10.1016/j.cyto.2013.06.313 [DOI] [PubMed] [Google Scholar]
- 266.Yaal-Hahoshen N, Shina S, Leider-Trejo L, Barnea I, Shabtai EL, Azenshtein E, Greenberg I, Keydar I, Ben-Baruch A. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin Cancer Res 2006; 12:4474-80; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0074 [DOI] [PubMed] [Google Scholar]
- 267.Lv D, Zhang Y, Kim H-J, Zhang L, Ma X. CCL5 as a potential immunotherapeutic target in triple-negative breast cancer. Cell Mol Immunol 2013; 10:303-10; PMID:; http://dx.doi.org/ 10.1038/cmi.2012.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sugasawa H, Ichikura T, Kinoshita M, Ono S, Majima T, Tsujimoto H, Chochi K, Hiroi S, Takayama E, Saitoh D, et al.. Gastric cancer cells exploit CD4+ cell‐derived CCL5 for their growth and prevention of CD8+ cell‐involved tumor elimination. Int J Cancer 2008; 122:2535-41; http://dx.doi.org/ 10.1002/ijc.23401 [DOI] [PubMed] [Google Scholar]
- 269.Chang L-Y, Lin Y-C, Mahalingam J, Huang C-T, Chen T-W, Kang C-W, Peng HM, Chu YY, Chiang JM, Dutta A, et al.. Tumor-Derived Chemokine CCL5 Enhances TGF-β-Mediated Killing of CD8+ T Cells in Colon Cancer by T-Regulatory Cells. Cancer Res 2012; 72:1092-102; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2493 [DOI] [PubMed] [Google Scholar]
- 270.Zhang Y, Zhu J, Hong X, Zhou Y, Ren K, Shu X, Wang Q. The membrane molecule RCAS1 induces immune cell apoptosis via the RCAS1-RCAS1R pathway. Int J Mol Med 2013; 31:1319-26; PMID: [DOI] [PubMed] [Google Scholar]
- 271.Matsushima T, Nakashima M, Oshima K, Abe Y, Nishimura J, Nawata H, Watanabe T, Muta K. Receptor binding cancer antigen expressed on SiSo cells, a novel regulator of apoptosis of erythroid progenitor cells. Blood 2001; 98:313-21; PMID:; http://dx.doi.org/ 10.1182/blood.V98.2.313 [DOI] [PubMed] [Google Scholar]
- 272.Okada K, Nakashima M, Komuta K, Hashimoto S, Okudaira S, Baba N, Hishikawa Y, Koji T, Kanematsu T, Watanabe T. Expression of tumor-associated membrane antigen, RCAS1, in human colorectal carcinomas and possible role in apoptosis of tumor-infiltrating lymphocytes. Modern Pathol 2003; 16:679-85; PMID:; http://dx.doi.org/ 10.1097/01.MP.0000074732.17945.6C [DOI] [PubMed] [Google Scholar]
- 273.Han Y, Qin W, Huang G. Knockdown of RCAS1 expression by RNA interference recovers T cell growth and proliferation. Cancer Lett 2007; 257:182-90; PMID:; http://dx.doi.org/ 10.1016/j.canlet.2007.07.016 [DOI] [PubMed] [Google Scholar]