Abstract
Human chronic graft-versus-host disease (cGVHD) is a frequent and disabling complication of allogeneic hematopoietic stem cell transplantation. Regulatory B cells (Bregs) are IL-10–producing B cells that are able to inhibit the innate and adaptive immune responses. We have recently demonstrated a defect in regulatory B cells in human cGVHD.
Keywords: chronic graft-versus-host disease, allogeneic stem cell transplantation, regulatory B cells, interleukin-10
Despite substantial progress in cancer therapy, allogeneic hematopoietic stem cell transplantation (HSCT) remains the only potentially curative approach for many relapsed or refractory hematologic malignancies. Chronic graft-versus-host disease (cGVHD) currently affects more than 50% of allogeneic stem cell transplant recipients. In addition to being the leading cause of non-relapse mortality after allogeneic HSCT, cGVHD severely affects the patient's quality of life. The skin and mucosae (such as the eyes, mouth, gut, and lungs), which interface with the external environment, are the main targets of the graft-versus-host reaction. Although cGVHD frequently occurs in patients with a previous history of acute GVHD, the clinical and pathophysiological features of cGVHD are somewhat different from those of acute GVHD. In cGVHD, the clinical features commonly mimic those seen in autoimmune diseases such as systemic lupus erythematosus, Sjögren's syndrome, rheumatoid arthritis, dermatomyositis, or immune thrombocytopenia.1 As is the case in these diseases, the presence of antibodies against nuclear antigens is common in human cGVHD and disturbance of B-cell homeostasis is a hallmark of the disease.2
Regulatory B cells (Bregs) were first discovered in mouse experimental autoimmune encephalomyelitis by Fillatreau et al. over 10 years ago.3 In 2008, Yanaba et al. identified a unique CD5+CD1dhi B-cell subset highly enriched in Bregs in mice. The adoptive transfer of CD5+CD1dhi regulatory B cells inhibited T-cell mediated inflammation in a mouse model of contact dermatitis.4 The existence of human Bregs was demonstrated 2 years later in our laboratory,5 Tedder's laboratory,6 and by Mauri and colleagues.7 Since then, human Bregs have been shown to be deficient in several autoimmune diseases.6,7 Perhaps due to discrepancies in the stimulation used to detect IL-10 production and in the surface antigens studied, there is still no consensus on the cell surface phenotype of human Bregs. Mauri et al. found that the transitional CD24hiCD38hi B-cell subset was the most highly enriched for Bregs, whereas Tedder et al. showed that Bregs were predominantly found in the CD24hiCD27+ B cell subset. In our experience, human Bregs are not confined to a unique B-cell subpopulation but are enriched in both the CD27+ and CD38+ B-cell subsets. In any case, the ability to produce IL-10 after in vitro stimulation remains the most consensual definition of human Bregs to date. Very interestingly, plasma cells have recently been shown to exhibit regulatory properties in mice.8
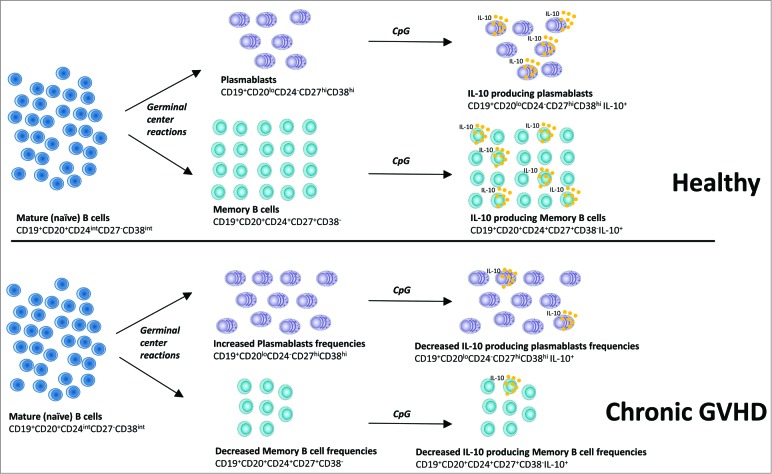
In our study,9 we identified the CD24hiCD27+ subset and the CD24−CD27hiCD38hi plasmablast-like B-cell compartment as the subsets most highly enriched for Bregs in healthy humans and allogeneic transplant recipients. We confirmed that in vitro expanded human plasmablasts and plasma cells were able to produce large amounts of IL-10. Consistent with recently published data by Khoder et al.,10 our analysis of 69 allogeneic transplant recipients confirmed that patients with cGVHD had reduced frequencies of Bregs compared to allogeneic transplant recipients with no history of cGVHD and healthy donors. Importantly, these Breg frequencies were clearly associated with cGVHD activity and cGVHD severity, and not with other baseline characteristics such as the immunosuppressors received by the patients. Patients with active cGVHD had impaired reconstitution of the memory B cell pool and increased plasmablast frequencies compared to the other groups of patients. A defect in STAT3 and Erk signaling, 2 pathways critical for B cell IL-10 production in humans, was also found in patients with cGVHD. These signaling abnormalities might be responsible for the decreased frequency of IL-10–producing B cells observed in cGVHD (Fig. 1). These data from a large number of patients are the first to compare the Breg frequencies in patients with cGVHD to those of allogeneic transplant recipients with no history of cGVHD, which in our opinion is the most appropriate way to explore any Breg cell defect in cGVHD, and offer clues to the in vitro expansion of human Bregs.
Figure 1.
Deficient memory and plasmablast regulatory B cells (Bregs) in human chronic graft-versus-host disease (cGVHD). Stimulation of mature, “naïve” B cells (CD19+CD20+CD24intCD27−CD38int) by antigens and T helper cells results in germinal center formation and their differentiation into memory B cells (CD19+CD20+CD24+CD27+CD38−) and plasmablasts (CD19+CD20loCD24−CD27hiCD38hi). Decreased frequencies of memory B cells and increased frequencies of plasmablasts were observed in the peripheral blood of patients with cGVHD. Memory B cells and plasmablasts were the most highly Breg-enriched subsets in both healthy subjects and patients with cGVHD. However, stimulation with CpG resulted in fewer IL-10–producing B cells in patients with cGVHD than in patients without cGVHD. Finally, patients with cGVHD had fewer IL-10–producing memory B cells and IL-10–producing plasmablasts compared to patients without cGVHD.
Adoptive cell transfer of regulatory T cells for the prevention of GVHD has been shown to be feasible in human clinical trials. In vivo expansion of human regulatory T cells by provision of low-dose IL-2 might be efficient in the treatment of cGVHD. Specific phenotypic markers or transcription factors (such as Foxp3 for natural Treg cells) expressed by human Bregs are yet to be determined in order to consider adoptive transfer of human Bregs in the prevention or treatment of human cGVHD. The potential effect of Bregs on the graft-versus-malignancy reactions must also be taken into account. In any event, in vitro expansion of human Bregs remains a major challenge. Studying the effect of drugs targeting the germinal center reactions (e.g., bortezomib, which has recently been proven to be effective in human cGVHD) on Breg frequencies would be of interest. Unraveling the mechanisms that lead to the induction of Bregs could improve our understanding of B-cell regulation of human immune responses and help further development of cell therapy strategies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Adèle de Masson was supported by the Institut National du Cancer (INCa) – Institut Thématique Multi-Organismes (ITMO) Cancer. This work was funded by a grant from the Société Française de Dermatologie.
References
- 1.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood 2014; 124(3):374-84; http://dx.doi.org/ 10.1182/blood-2014-01-514752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarantopoulos S, Blazar BR, Cutler C, Ritz J. B cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant 2015; 21(1):16-23; PMID:; http://dx.doi.org/ 10.1016/j.bbmt.2014.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 2002; 3(10):944-50; PMID:; http://dx.doi.org/ 10.1038/ni833 [DOI] [PubMed] [Google Scholar]
- 4.Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008; 28(5):639-50; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 5.Bouaziz J-D, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, Musette P. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol 2010; 40(10):2686-91; PMID:; http://dx.doi.org/ 10.1002/eji.201040673 [DOI] [PubMed] [Google Scholar]
- 6.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, et al.. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011; 117(2):530-41; PMID:; http://dx.doi.org/ 10.1182/blood-2010-07-294249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010; 32(1):129-40; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 8.Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, et al.. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014; 507(7492):366-70; PMID:; http://dx.doi.org/ 10.1038/nature12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Masson A, Bouaziz J-D, Le Buanec H, Robin M, O'Meara A, Parquet N, Rybojad M, Hau E, Monfort JB, Branchtein M, et al.. CD24hiCD27+ and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood. 2015; 125(11):1830-9; PMID: ; http://dx.doi.org/ 10.1182/blood-2014-09-599159 [DOI] [PubMed] [Google Scholar]
- 10.Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N, Mielke S, de Lavallade H, Muftuoglu M, Fernandez Curbelo I, et al.. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood 2014; 124(13):2034-45; PMID:; http://dx.doi.org/ 10.1182/blood-2014-04-571125 [DOI] [PMC free article] [PubMed] [Google Scholar]