Abstract
Human γδ T cells contribute to tissue homeostasis under normal conditions and participate in lymphoid stress surveillance against infection and tumors. However, the molecular mechanisms underlying the recognition of complex cell stress signatures by γδ T cells are still unclear. Tumor cells and human cytomegalovirus (HCMV)-infected cells are known targets of γδ T cells. We show here that many tumor and CMV-infected cells express caspase-1 inflammasomes and release interleukin (IL)-18. Engagement of the T-cell receptor (TCR) on Vδ2neg γδ T cells controlled the direct innate immune sensing of IL-18 that enhanced cytotoxicity and interferon gamma (IFNγ) production. This TCR-dependent sensitization to IL-18 was mediated by the upregulation of the innate IL-18 receptor β chain (IL-18Rβ) expression. These findings shed light on inflammasomes as a unified stress signal of tumor and infected cells to alert γδ T cells. Moreover, uncovering the TCR-mediated sensitization of γδ T cells to inflammatory mediators establishes a molecular link between the innate and adaptive immune functions of γδ T cells that could fine tune the commitment of antigen-experienced γδ T cells to inflammatory responses.
Keywords: cancer, cytomegalovirus, caspase-1, γδ T cells, IL-18, immunology, inflammasome, TCR
Introduction
The presence of γδ T cells, along with αβ T cells and B cells, in all higher vertebrates suggest that each contributes uniquely to host immune competence and is essential for its maintenance. Human γδ T cells are a relatively rare immune population in peripheral blood, mostly composed of cells expressing the Vγ9Vδ2 variable regions. Epithelial tissues are substantially enriched in other γδ T-cell subsets (collectively called Vδ2neg γδ T cells) that mediate first-line host response to a wide variety of cellular insults triggered by malignancy or bacterial/viral infection, and contribute to tissue homeostasis.1,2 The γδ T cells have been shown to recognize stress-associated proteins, metabolites, and lipids in a classical major histocompatibility complex (MHC)-unrestricted manner.1 A critical protective role against tumors and infections has been demonstrated in vivo using γδ T–cell-deficient mice 3,4 as well as in the context of chemotherapy.5 Human γδ T cells can infiltrate tumors and infected tissues, and their expansion in blood correlates with better clinical outcome in both malignancies and infectious diseases.6,7 Notably, they can also regulate αβ T cells 8,9 and maintain tissue integrity.10 In vitro, human γδ T cells can kill transformed cells, infected cells, and microorganisms. To date, phase 1 and 2 clinical trials have been conducted to evaluate the use and efficacy of Vγ9Vδ2 γδ T–cell-based immunotherapy. Trials against solid tumors revealed mixed results, as they were hampered by fluctuating responses to ex vivo stimulation and a strong susceptibility of this population for activation-induced cell death (AICD).11
Interestingly, AICD seems to be reduced for epithelial Vδ2neg γδ T cells, and increasing evidence supports an important role of this subset for tumor and infection immunosurveillance.12 Human Vδ2neg γδ T cells expand in the periphery of individuals during CMV infection in various pathophysiological contexts, including solid-organ and stem cell transplantation,13–17 where they develop cytotoxic function and produce proinflammatory cytokines such as tumor necrosis factor α (TNFα) and IFNγ.18 Importantly, CMV-induced expansion of Vδ2neg γδ T cells correlates with decreased susceptibility to post-transplant cancers, suggesting a role in tumor immunosurveillance in vivo.19 In line with this, such cells display a TCR-dependent cross-reactivity to several solid tumor cell lines and CMV-infected cells, which can be explained by the recognition of common, specific, stress-induced γδ TCR ligands (e.g., EPCR) expressed by cellular targets in both conditions.18 However, a complex array of co-stimulatory signals along with TCR engagement seem to be required to fine tune appropriate Vδ2neg γδ T-cell responses to tissue stress.18
Inflammasomes are multi-protein complexes that stimulate caspase-1, and initiate inflammatory signaling in response to molecular determinants produced by pathogens and cell stress.20,21 Two types of inflammasomes have been described, involving either nucleotide-binding domain leucine-rich repeat-containing receptors (NOD-like receptor [NLRs] family proteins) or the PYHIN family protein (absent in melanoma 2 [AIM2]-like receptors). These serve as anchoring scaffolds onto which protein-complex assembly occurs to process procaspase-1 into active caspase-1. Subsequently, active caspase-1, composed of the hetero-tetramer (p10)2/(p20)2, facilitates maturation of pro–IL-1β and pro–IL-18 into active cytokines to be secreted and prime the inflammatory response. While playing critical roles in host defense against pathogens, NLR functions have also been associated with the progression of metabolic syndrome,22,23 thereby contributing to their definition as danger sensors of metabolic perturbations. Interestingly, inflammasome assembly seems to occur in response to a metabolic reprogramming similar to the one triggered by both cellular transformation and viral infection (including CMV).24-26 IL-18 is crucially involved in tissue homeostasis.27-29 In vivo studies have shown IL-18 expression during late stages of tumorigenesis in tumor tissues and the serum of patients with various types of cancer 30,31 together with an immunoablative role of natural killer (NK) cells.32 Various epithelial cells express NLRs 33,34; however, the role of NLRs in the activation of inflammasomes within tissue-derived malignant and infected cells, as well as their direct role in controlling effector functions of intraepithelial lymphocytes (IEL), remains to be defined. We hypothesized that inflammasome activation may represent a unified stress signal triggered by both CMV infection and cellular transformation, which in turn could modulate human Vδ2neg γδ T-cell response through the secretion of soluble signaling molecules including IL-1β and IL-18 cytokines. Such a mechanism may represent an additional stress signal recognized by γδ T cells to sense disturbed tissue integrity.
Results
Tissue-derived cellular targets of human Vδ2neg γδ T cells secrete mature IL-18
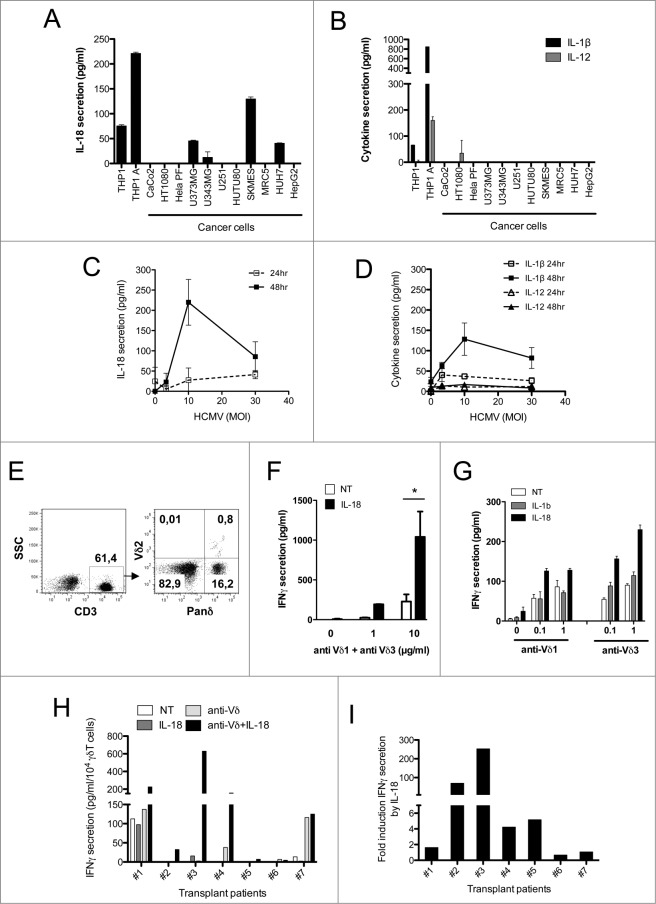
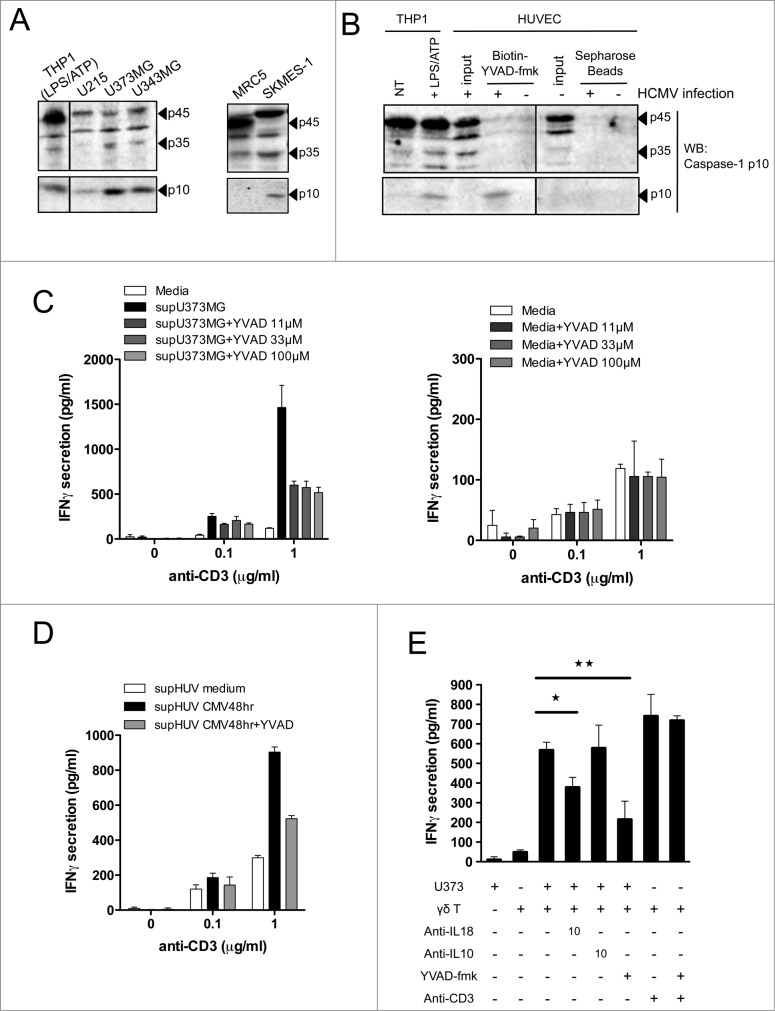
Human Vδ2neg γδ T cells recognize a wide range of cancer cells as well as CMV-infected endothelial cells through a γδ–TCR-dependent mechanism.35 We first evaluated whether these cancer cells may secrete inflammasome-dependent inflammatory cytokines including IL-1β and IL-18, as well as products of antigen-presenting cells (APCs) such as IL-12. We screened several human cancer cell lines and noticed the secretion of mature IL-18, from glioblastoma U373MG and U343MG, lung adenocarcinoma SKMES-1, and hepatocarcinoma HUH7, as measured by ELISA (ranging from ˜50 to 200 pg/mL) (Fig. 1A). In contrast to IL-18, mature IL-1β and IL-12 were not detected from the supernatants of tested cell lines (except minor amounts of IL-12 for HT1080), although both were readily detectable in culture supernatants of the lipopolysaccharide (LPS)/adenosine triphosphate (ATP)-activated monocytic THP-1 cell line used as a positive control (Fig. 1B). We also observed a significant increase of IL-18 secretion from human umbilical vein endothelial cells (HUVECs) following HCMV infection with increased doses of virus (Fig. 1C). Secretion of mature IL-1β followed that of IL-18 but to a lesser extent, and IL-12 secretion was barely detected from HCMV-infected HUVEC cultures (Fig. 1D). Therefore, both human targets of Vδ2neg γδ T cells tested here (cancer cells and HCMV-infected cells) secrete caspase–1-dependent cytokines.
Figure 1.
IL-18 is secreted by cancer cells and HCMV-infected cells, and enhances IFNγ production by human Vδ2neg γδ T cells within PBMCs. (A) IL-18 or (B) IL-1β and IL-12 secretion by cancer cell lines. Cancer cell lines were cultured for 48 h and the secretion of cytokines was measured by ELISA from cell culture supernatants. Results are normalized by the same amount of cells used for each cell line. HUVEC endothelial cells were infected with HCMV at various multiplicities of infection (MOIs), and cell culture supernatant at 24 and 48 h post-infection was used to monitor (C) IL-18 or (D) IL-1β and IL-12 secretion by ELISA. Data are expressed as concentration of cytokines (pg/mL; mean ± SD; n = 3). (E) Example of Panδ immunotyping from whole blood. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of a kidney transplant patient and Panδ populations were quantified using anti-panδ and anti-Vδ2 antibodies within the CD3+ population by flow cytometry. (F) PBMCs isolated from a patient with expanded Vδ2neg population (>12% of CD3+) were incubated with various concentrations of anti-Vδ1 and anti-Vδ3 antibodies in the presence or absence of recombinant IL-18 (50 ng/mL) for 24 h at 37°C, then IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (G) Same as in (F) but PBMCs from another patient were treated with either anti-Vδ1 or anti-Vδ3 antibodies at various concentrations in the presence or absence of recombinant IL-18 or IL-1β (50 ng/mL) for 24 h at 37°C; then, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (H) IFNγ secretion obtained from several kidney transplant patient's PBMCs was normalized by the same number of Vδ2neg γδ T cells and plotted as raw data. Anti-Vδ indicates treatment with combined anti-Vδ1+anti-Vδ3 antibodies (10 μg/mL each). (I) IL-18 response is represented as fold induction of IFNγ secretion after IL-18 treatment (anti-Vδ2neg +IL-18/anti-Vδ2neg). ★, P < 0.05.
γδ TCR signaling sensitizes Vδ2neg γδ T cells to soluble IL-18, thereby enhancing IFNγ production
To address whether non-myeloid IL-18 could participate in γδ T-cell activation by tumor and CMV-infected cells, it was first important to assess the γδ T-cell response to recombinant IL-18. Therefore, we investigated the direct effect of IL-18 ex vivo on fresh peripheral blood mononuclear cells (PBMCs) isolated from CMV-infected kidney transplant recipients that contained at least 15% of Vδ2neg γδ T cells (mostly expressing Vδ1 and Vδ3) among CD3+ T cells (Fig. 1E; Fig. S1). 13 Among PBMCs, the Vδ2neg γδ T cells were specifically stimulated through the TCR (by using anti-Vδ1+ anti-Vδ3 Abs), and left untreated or treated with IL-18 or IL-1β. IL-18, but not IL-1β, strongly induced IFNγ secretion from bulk PBMCs, but only in the presence of TCR stimulation mediated by both anti-Vδ1 and anti-Vδ3 Abs used either combined (Fig. 1F) or separately (Fig. 1G). Among PBMCs from 7 patients that were tested, none showed an increase of IFNγ secretion after IL-18 treatment alone, conversely to that with co-treatment with IL-18 and γδ TCR agonists (Fig. 1H). When normalized by the level of IFNγ secreted in the presence of TCR agonists, 4 patients showed a >fold4-increase of IFNγ secretion upon IL-18 stimulation versus no IL-18 (Fig. 1I). Therefore, IL-18 treatment of patient PBMCs acts synergistically with TCR stimulation to enhance IFNγ secretion.
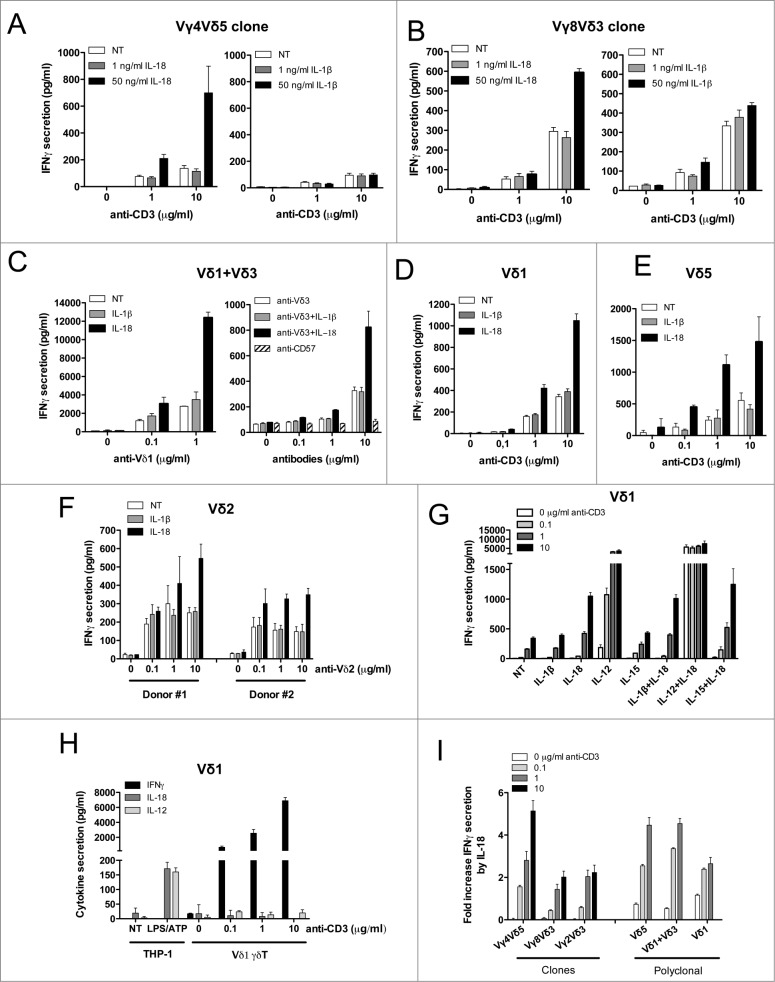
As IL–18-mediated IFNγ secretion from PBMCs might be attributed to indirect effects on other cells among PBMCs (αβ T and NK cells), we investigated whether purified Vδ2neg γδ T cell clones and short-term polyclonal cell lines would behave similarly. We used purified Vδ2neg γδ T-cell clones (expressing Vγ4Vδ5, Vγ8Vδ3, or Vγ2Vδ3 TCRs) and short-term polyclonal cell lines (Vδ1, Vδ1+ Vδ3, and Vδ5) generated from the blood of CMV-infected patients as described in Halary et al. 35 First, γδ T cells were stimulated with increased concentrations of anti-CD3 in the presence or absence of recombinant cytokines IL-18 or IL-1β (Fig. 2A and B). For all clones tested, the amount of IFNγ secreted was significantly higher in the presence of IL-18, regardless of IL-1β. These results were confirmed on short-term polyclonal cell lines (Vδ1, Vδ1+ Vδ3, and Vδ5) activated with either anti-Vδ1 monoclonal antibody (mAb) or anti-CD3 (Fig. 2C–E). TNFα secretion was also measured, with the same result as for IFNγ secretion (Fig. S2A). The effects of IL-18 were not restricted to Vδ2neg γδ T cells because they were also observed on purified and expanded polyclonal Vγ9Vδ2 T cells isolated from 2 different healthy donors, upon TCR stimulation using anti-Vδ2 antibody (Ab) (Fig. 2F). Therefore, TCR-stimulated γδ T cells respond directly to IL-18 and likely contribute to the IFNγ production observed in PBMCs.
Figure 2.
For figure legend, see next page. Figure 2 (see previous page). IL-18 alone enhances IFNγ production by purified Vδ2neg γδ T cells in a TCR-dependent manner. Human Vδ2neg γδ T-cell clones including (A) Vγ4Vδ5 or (B) Vγ8Vδ3 purified from human PBMCs were cultured through polyclonal activation and incubated with various concentrations of anti-CD3 in the presence or absence of IL-18 or IL-1β for 24 h at 37°C; then, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). The same procedure was applied to purified Vδ2neg γδ T polyclonal cell lines isolated from kidney transplant patients, including (C) Vδ1+Vδ3, or (D) Vδ1, or (E) Vδ5 γδ T cells. Polyclonal cell lines were incubated with various concentrations of γδ TCR agonists (anti-Vδ1, or anti-Vδ3, or anti-CD3 antibodies) in the presence or absence of either IL-18 or IL-1β for 24 h at 37°C. Anti-CD57 was used as negative control for TCR stimulation. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (F) Vδ2 γδ T polyclonal cell lines were isolated from PBMCs of 2 healthy donors and cultured through polyclonal activation using 4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) and IL-2, then incubated with various concentrations of γδ TCR agonist anti-Vδ2 in the presence or absence of either IL-18 or IL-1β for 24 h at 37°C. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (G) A Vδ1 γδ T polyclonal cell line was treated with anti-CD3 in the presence or absence of various cytokines alone or in combination for 24 h at 37°C. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (H) A Vδ1 γδ T polyclonal cell line treated with anti-CD3 and cell culture supernatant was used to monitor IFNγ, IL-18, or IL-12 secretion by ELISA. LPS/ATP-treated THP-1 cells were used as positive control for cytokine secretion (mean ± SD; n = 3). (I) The various Vδ2neg γδ T-cell clone and polyclonal cell line responses to IL-18 are represented as fold induction of IFNγ secretion by IL-18 (IL-18 + TCR agonist/TCR agonist).
We next tested whether IL-18 could synergize with others cytokines to increase IFNγ production. IFNγ secretion was measured in the presence of cytokines alone (IL-1β, IL-18, IL-12, and IL-15) or in combination. When used alone, only IL-12 or IL18 increased IFNγ secretion following anti-CD3 treatment (using either Vδ1 or Vδ5 polyclonal cell lines; Fig. 2G; Fig. S2B, respectively). The combination of IL-18 and IL-12 had a synergistic effect even in the absence of TCR stimulation (as already observed for αβ T cells).36 The TCR-mediated effect on the IL-18 response could not be attributed to IL-12 secretion by γδ T cells themselves because anti-CD3 treatment of Vδ1 polyclonal cell line did not induce any detectable IL-12 in cell culture supernatant (neither IL-18), while inducing IFNγ (Fig. 2H). In conclusion, the TCR-dependent response to IL-18 is a general mechanism among all human Vδ2neg γδ T cells tested (clones and polyclonal cell lines) that mediates at least a fold2-induction of IFNγ secretion in comparison to TCR signaling alone (Fig. 2I). Vδ2neg γδ T cells do not require any other co-factor provided by APCs such as IL-12 to respond to IL-18, although this combination can lead to a better response. Strikingly, the γδ T-cell responsiveness to IL-18 is highly dependent on the strength of γδ TCR signaling.
γδ TCR signaling induces the expression of IL-18Rβ chain
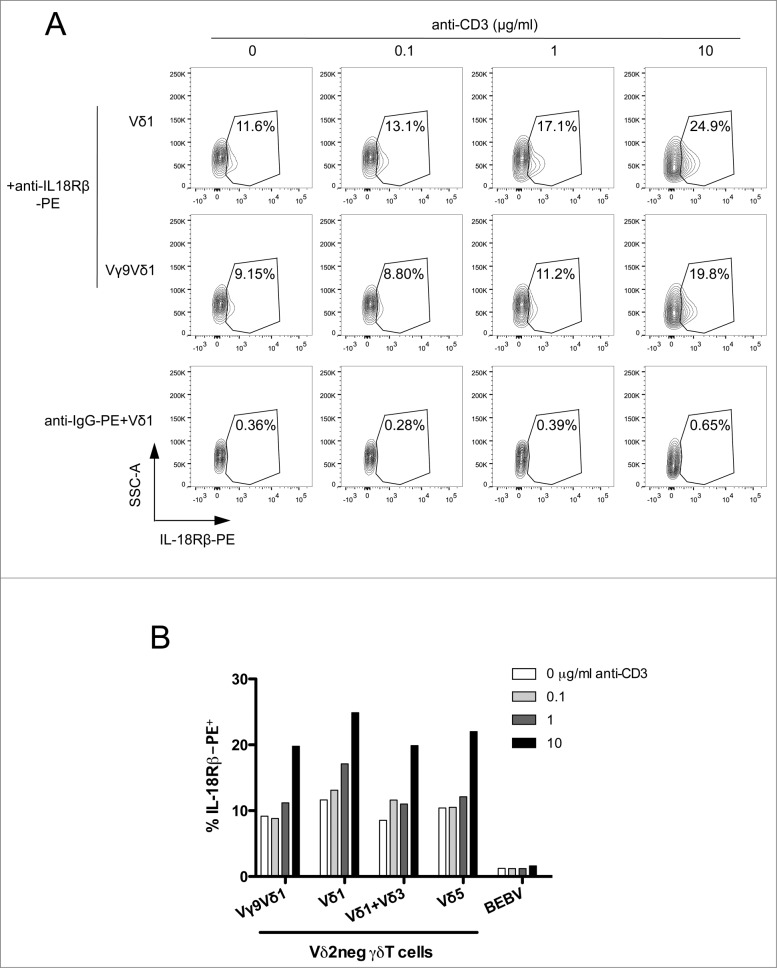
We next tested whether γδ TCR engagement would increase IL-18 sensing by upregulating expression of the cell membrane receptor IL-18Rβ (which controls the number of high-affinity IL-18 receptors).37 This was tested on various Vδ2neg γδ T cells (clones and polyclonal cell lines), upon γδ TCR stimulation by anti-CD3 agonist Ab. We detected basal expression of IL-18Rβ in approximately 10% of γδ T-cell clones (Vγ9Vδ1) and polyclonal cell lines (Vδ1, Vδ1+3, Vδ5) (Fig. 3A). Upon anti-CD3 stimulation, we noticed a dose-dependent increase of the number of IL–18Rβ-positive cells by at least fold2-(Fig. 3B). These results suggest 1 a molecular link between γδ TCR signaling and the expression of innate IL-18Rβ, and 2 that TCR-mediated responses to IL-18 might be a specific characteristic of γδ T cells that allows them to enhance IFNγ production and cytotoxicity.
Figure 3.
γδ TCR signaling increases membrane IL-18Rβ chain expression. (A) A Vδ1 γδ T polyclonal cell line, Vγ9Vδ1 cell clone, or B-cell lymphoma BEBV cell line were incubated with various concentrations of anti-CD3 for 24 h at 37°C, and immunostained with PE-conjugated anti-IL-18Rβ or IgG-PE control (for the Vδ1 γδ T polyclonal cell line). The expression of membrane IL-18Rβ chain was determined by flow cytometry within the healthy cell population. IL-18Rβ-PE+ cells are shown in the gate with the percentage of positive cells as indicated. (B) Bar graph summarizing IL-18Rβ-PE+ cells (%) from various Vδ2neg γδ T-cell clones (Vγ9Vδ1) and polyclonal cell lines (Vδ1, Vδ1+3, Vδ5) after incubation with various concentrations of anti-CD3. Data are representative of at least 3 independent experiments with similar results (mean of at least 5.103 cells).
Soluble IL-18 secreted from tumor and infected target cells enhances IFNγ production by Vδ2neg γδ T cells
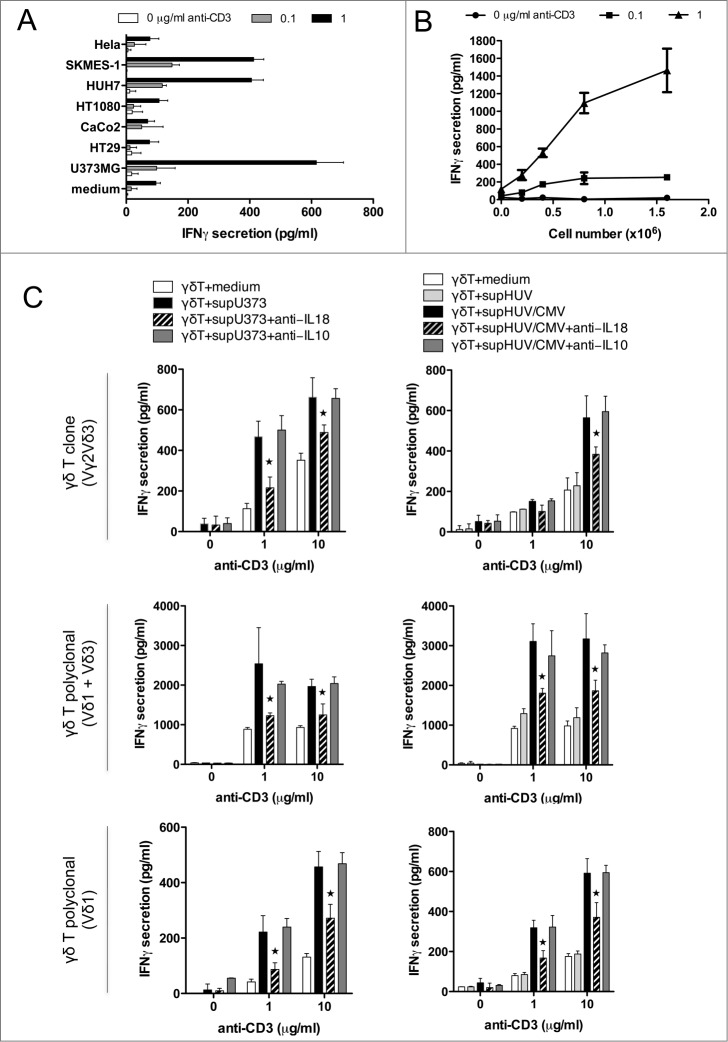
Cancer cells and HCMV-infected endothelial cells are both targets of Vδ2neg γδ T cells, and both can secrete IL-18. Therefore, we tested if conditioned culture supernatant of target cells can promote IFNγ secretion from Vδ2neg γδ T cells in the presence of TCR signaling. Polyclonal Vδ2neg γδ T-cell lines were incubated with plate-coated anti-CD3 and the conditioned culture supernatants (CS) of either cancer cells or HCMV-infected HUVEC cells. We first screened various CS with the Vδ1 polyclonal cell line and observed that glioblastoma U373MG, lung adenocarcinoma SKMES-1, and hepatocarcinoma HUH7 were potently able to enhance IFNγ secretion, which is consistent with the level of IL-18 secreted by these cells (Fig. 1A). At least a fold4-increase was observed at 1 μg/mL of anti-CD3 stimulation (Fig. 4A). γδ T-cell responsiveness to CS followed the amounts of U373MG cancer cells used to generate the CS as shown in Figure 4B. Importantly, no effect of CS was observed without TCR stimulation.
Figure 4.
Soluble IL-18 secreted by cancer cells and HCMV-infected cells enhances IFNγ production by human Vδ2neg γδ T cells, in a TCR-dependent manner. (A) Conditioned culture supernatants of various cancer cell lines, or fresh media, were isolated after 48 h at 37°C, cleared by centrifugation, and incubated with a Vδ1 γδ T polyclonal cell line in the presence of various concentrations of coated anti-CD3 for 24 h at 37°C. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). Results are normalized by the same amount of cells used for each cancer cell line. (B) Conditioned culture supernatants from various amounts of U373MG cancer cells, or fresh media, were isolated after 48 h at 37°C, cleared by centrifugation, and incubated with a Vδ1 γδ T polyclonal cell line in the presence of anti-CD3 for 24 h at 37°C. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (C) Conditioned culture supernatants of U373MG cancer cells, or HUVECs infected or not with HCMV (MOI 10), or fresh media, were isolated after 48 h at 37°C, cleared by centrifugation, and incubated with Vδ2neg γδT cells in the presence of anti-CD3 with or without anti-IL-18 or control anti-IL-10 antibodies (10 μg/mL). After 24 h at 37°C, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). As indicated, Vγ2Vδ3 T cell clone, Vδ1, and Vδ1+3 polyclonal cell lines were tested. ★, P < 0.05.
Altogether these results suggest that IL-18 secreted by cancer cells could enhance IFNγ production by TCR-activated γδ T cells. We tested this hypothesis using anti-IL-18 blocking Ab (Fig. 4C). By using various Vδ2neg γδ T-cell clones and polyclonal cell lines as indicated, we observed that the anti-IL-18 blocking Ab, but not a control anti-IL-10 Ab, significantly decreased the effect of U373MG CS on γδ T-cell IFNγ production by almost 50% at high anti-CD3 stimulation. In the same manner than for cancer cell-derived CS, CS isolated from HCMV-infected HUVEC cells was able to enhance IFNγ secretion in the presence of anti-CD3 stimulation, in contrast to CS obtained from non-infected endothelial cells. This response was also decreased by almost 50% after using the anti-IL-18 blocking Ab (irrespective to anti-IL10).
To confirm these results in a more physiological setting of TCR stimulation, which involves the γδ TCR-dependent recognition of stress antigens expressed by target cells, we used our model of γδ T-cell activation by cancer cells or HCMV-infected cells.35 We tested whether target cell-release of IL-18 was also involved in γδ T-cell activation. U373MG and γδ T-cell co-culture induced a robust IFNγ secretion that was further inhibited by increasing concentrations of neutralizing anti-IL-18 monoclonal antibody (mAb; in contrast to control anti-IL-10) (Fig. 5A). Moreover, anti-IL-18 mAb also inhibited cytotoxic functions of the γδ T-cell clone as measured by expression of CD107a using flow cytometry (Fig. S3). In addition, HUVEC and γδ T-cell co-culture also induced IFNγ secretion but only when endothelial cells were infected by HCMV. Moreover, co-culture in the presence of anti-IL-18 partially but specifically blocked IFNγ secretion, as shown with anti-IL-10 negative control. Therefore, CS and co-culture experiments confirmed that tumor and infected–cell-derived IL-18 enhances γδ T-cell IFNγ production and cytotoxic function.
Figure 5.
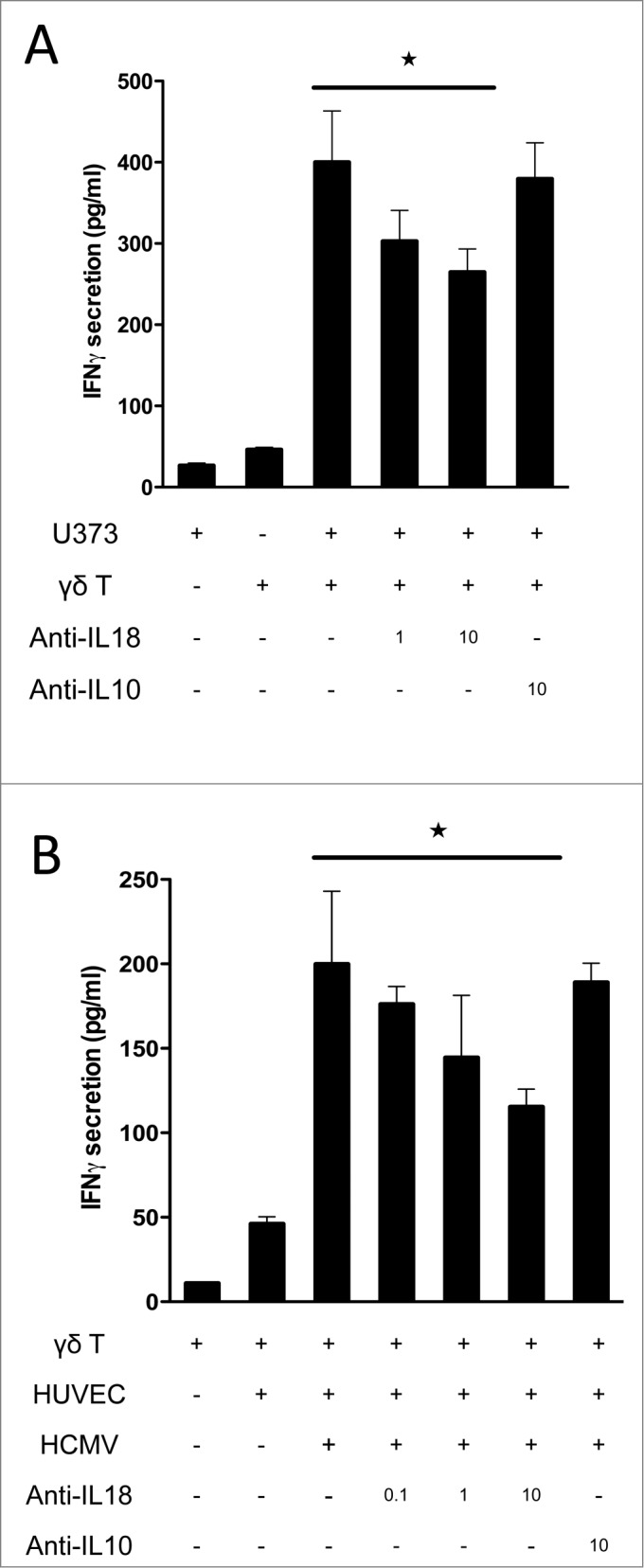
IL-18 produced by cancer cells and HCMV-infected cells contributes to IFNγ production by human Vδ2neg γδ T cells in co-culture. (A) U373MG cancer cells or (B) HUVECs uninfected or infected with HCMV (MOI 10) were cultured for 48 h at 37°C, and then co-cultured with a human Vγ4Vδ5 T-cell clone in the presence or absence of increased concentrations of anti-IL-18 or control anti-IL-10 antibodies. After 24 h at 37 °C, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). ★, P < 0.05.
IL-18 secretion from tumor and infected target cells depends on caspase-1 activation
The inactive pro-IL-18 precursor must be cleaved through caspase–1-dependent processing to generate an active cytokine that is secreted to prime the adaptive immune response. Prior to this, procaspase-1 itself needs to be activated by proteolytic cleavage upon assembly of NLR inflammasomes. However, in some circumstances including Fas signaling, IL-18 and IL-1β can be processed by a non-canonical caspase–8-dependent process. To gain insights into the mechanism of IL-18 maturation in cancer cells and HCMV-infected cells, we evaluated caspase-1 cleavage in these cells by Western blotting. THP-1 monocytic cells activated by LPS/ATP (described to induce caspase-1 processing) were used as positive controls to detect protein sizes of procaspase-1 and active cleaved forms of caspase-1 (p45 and p10, respectively). We detected a basal expression of p45 in human cancer cell lines including glioblastoma U373MG, U251, U343MG, and lung SKMES-1 (Fig. 6A). Interestingly, all cancer cell lines tested expressed the ultimate p10 active cleaved fragment, in contrast to the non-transformed MRC5 lung cell line used as a negative control. Therefore, processed and active caspase-1 is expressed to various extents in all cancer cell lines. In Figure 6B, we observed that human endothelial cells also express the inactive form p45 that is further cleaved in HCMV-infected cells into the detectable p35 cleaved form. Cell lysates were incubated with a Biotinyl-conjugated peptide that binds specifically to the active catalytic site of caspase-1 (Biotin-YVAD-fmk), and the active p10 fragment could only be precipitated from HCMV-infected HUVECs lysates. Therefore, caspase-1 is expressed and cleaved in both types of tissue-derived target cells of Vδ2neg γδ T cells (cancer cells and HCMV-infected cells).
Figure 6.
For figure legend, see next page. Figure 6 (see previous page). Active caspase-1 in cancer cells and HCMV-infected cells regulates the release of soluble molecules that enhance IFNγ production by human Vδ2neg γδ T cells. (A) Various cancer cell lines or non-transformed lung-derived MRC5 were cultured for 48 h, and lysed in denaturating buffer. Proteins were quantified to load the same amount of proteins per lane and then analyzed by SDS-PAGE immunoblotting (Western blotting) using anti-human caspase-1 p10 Ab. The p45 pro-caspase-1, p35, and p10 small catalytic subunit are indicated by dark arrowheads. LPS/ATP-treated THP-1 cells were used as the positive control for the presence of procaspase-1 p45 and p10. (B) HUVECs uninfected or infected with HCMV (MOI 10 for 48 h) were lysed in immunoprecipitation buffer (IP), and proteins were quantified to load the same amount of proteins per lane. Input indicates loading of cell lysates. THP-1 cells that were untreated or treated with LPS/ATP were used as positive control for the presence of procaspase-1 p45 and p10 using the anti-human caspase-1 p10 Ab. In parallel, solubilized caspase-1 p10 fragments contained in cleared supernatants after HUVEC cell lysis were precipitated by the addition of biotinyl-VAD-fmk; thereafter, biotinylated complexes were recovered by adding streptavidine-Sepharose beads, and the Sepharose-bound complexes were analyzed by Western blotting (WB) using anti-human caspase-1 p10 Ab. (C) U373MG cancer cells or (D) HUVECs that were uninfected or infected with HCMV (MOI 10) were cultured for 48 h at 37°C with or without various concentrations of Ac-YVAD-fmk (40 μmol/L for HUVECs). Conditioned culture supernatants (to the left) or fresh media (to the right) were then cleared by centrifugation and incubated with a Vδ1 γδ T polyclonal cell line for 24 h at 37°C, in the presence of anti-CD3. IFNγ secretion then was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (E) U373MG cancer cells were cultured for 48 h at 37°C with or without various concentrations of Ac-YVAD-fmk, and then co-cultured with a human Vγ4Vδ5 T-cell clone in the presence or absence of anti-IL-18, control anti-IL-10, or anti-CD3 antibodies. After 24 h at 37°C, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). ★, P < 0.05 and ★, P < 0.01.
Because 1 secreted IL-18 from target cells induced IFNγ production by Vδ2neg γδ T cells and 2 caspase-1 was expressed and cleaved in target cells, we tested whether inhibition of active caspase-1 might impair IL-18 secretion and, therefore, Vδ2neg γδ T-cell responsiveness. In Fig. 6C (left), we prepared conditioned cell CS of U373MG cancer cells cultured in the absence or presence of a specific caspase-1 pharmacological inhibitor (Ac-YVAD-fmk), and subsequently tested the IFNγ response of a polyclonal Vδ1 γδ T-cell line to YVAD-treated CS. The addition of Ac-YVAD-fmk to U373MG cancer cells strongly decreased the ability of YVAD-treated CS to induce IFNγ production (>50 %), even at the lowest concentration tested (11 μmol/L). Both 11 and 33 μmol/L Ac-YVAD-fmk concentrations did not induce cytotoxicity of U373MG cancer cells (data not shown). Moreover, treatment of Vδ1 cells with Ac-YVAD-fmk in fresh media did not impair IFNγ secretion (Fig. 6C, right). Similar results were obtained when using HCMV-infected cells instead of cancer cells (Fig. 6D). We then tested the effect of caspase-1 inhibition by Ac-YVAD-fmk on U373MG cancer cells directly co-cultured with Vδ5 γδ T cells (Fig. 6E). Consistent with results obtained using CS, the use of Ac-YVAD-fmk inhibited IFNγ secretion at least as efficiently as neutralizing anti-IL-18 mAb. As a control, Ac-YVAD-fmk did not inhibit the anti-CD3-induced IFNγ production by Vδ5 γδ T cells and, thus, had no direct effect on γδ T-cell activation. Therefore, caspase-1 activity in both target cells (cancer and CMV-infected cells) regulates the secretion of soluble molecules that trigger IFNγ production by γδ T cells, following TCR signaling. Caspase-1 inhibition as well as IL-18 blocking experiments resulted in a reproducible ˜50% reduction of IFNγ production, which suggest that other caspase–1-independent soluble molecules besides IL-18 may be active on γδ T cells in these conditions.
Discussion
Human Vδ2neg γδ T cells combine innate and adaptive features, and although we and others have show that they display a dual reactivity against tumor and CMV-infected cells,18,38 the mechanisms by which they participate in lymphoid stress surveillance are poorly defined. Further knowledge of the mechanisms underpinning their stress surveillance function has clear relevance for the control of malignancy and virus infection in humans. Here, we report a molecular link that orchestrates the adaptive and the innate arm of γδ T cells through the γδ TCR-dependent sensing of soluble inflammatory molecules. Specifically, γδ TCR stimulation modulates the sensitivity to the inflammatory cytokine IL-18, whose production is controlled by activation of caspase-1 inflammasomes in stressed non-myeloid human cells (malignant cells and HCMV-infected cells). γδ T-cell sensitivity to IL-18 is controlled by the expression level of the innate membrane receptor IL-18Rβ. The combination of γδ TCR and IL-18R signaling ultimately leads to enhanced IFNγ production and cytotoxicity. Importantly, tissue-derived and soluble IL-18 seems sufficient to enhance γδ T-cell effector functions as no other co-factor expressed by APCs, such as IL-12, are required.
Altogether, our data suggest that, in addition to the γδ TCR recognition of specific stress antigens expressed by tumor and infected cells,18 epithelial Vδ2neg γδ T cells fine tune their effector functions by directly sensing cues in the form of soluble molecules including innate cytokines. This observation illustrates an intricate array of molecular events involved in the lymphoid stress surveillance mediated by the immune system, which may explain the complex stress signature recognized by some Vδ2neg γδ T-cell clones.18 This array of events comprises both innate-like components (innate cytokine receptor-mediated and perhaps others) and adaptive components (TCR-specific).
The low diversity of γδ TCRs is hypothesized to allow responses to a wide range of stress antigens. The TCR-dependent innate sensing mechanism we report herein would add an extra level of safety control whether to engage in potent cytotoxicity and, therefore, potentially irreversible destruction of epithelial structures, only when disturbed epithelial cells display a complex membrane stress signature. This could include stress antigens, inflammatory signals, and co-stimulating/inhibitory receptors and adhesion molecules (NKG2D, KIRs, CD100, JAML, etc.) that are all necessary to fine tune the γδ T–cell-mediated immune outcome.2,39 In the context of tissue homeostasis, such mechanisms may restrain autoinflammation, as disturbed epithelial cells releasing all signals including inflammatory cytokines would be rapidly eliminated, thereby avoiding harmful activation of myeloid cells.
We show here that secreted inflammatory soluble molecules can originate directly from stressed epithelia or endothelium, and not only from APCs. Engagement of the γδ TCR orchestrates the expression level of innate immune sensors deployed to fine tune a stress-level appropriate response. Therefore, Vδ2neg γδ T cells may act as autonomous sentinels of epithelial structures by answering back promptly to tissue threats without needing other immune cells including APCs. Activation of caspase-1 inflammasomes in target cells becomes pivotal for the secretion of innate soluble molecules. Active caspase-1 holds several functions beyond its seminal cytokine regulation. Therefore, we can also consider a putative role in regulating the expression of γδ TCR stress ligands (for e.g., through post-translational modifications).
Peripheral Vγ9Vδ2 γδ T cells sense inflammatory cytokines (IL-1β) produced by caspase-1 inflammasomes in APCs, and trigger a Th17 response in the presence of IL-23.40 However, sensing of innate cytokines has not yet been demonstrated for epithelial Vδ2neg γδ T cells, nor has the relationship between cytokine sensing and γδ TCR signaling in the context of epithelial immunosurveillance. We found that IL-18 is secreted by various cancer cell lines (unlike IL-1β) and by HCMV-infected endothelial HUVEC cells. It has been shown previously that murine CMV infection triggers the AIM2 inflammasome in mouse macrophages followed by caspase-1 activation and the subsequent maturation and secretion of IL-18. In turn, IL-18 mediates IFNγ production by NK cells in vivo that confers protection to infected mice. 41 Similarly, we show here that HCMV triggers caspase-1 activation in non-immune human endothelial cells to secrete mature IL-18, which in turn enhances IFNγ production by Vδ2neg γδ T cells. Such observations provide new perspectives related to the intrinsic response of human cells to HCMV infection, and raise the question of which inflammasome(s) is responsible for IL-18 production by infected cells.
Interleukin-18 has a contrasting role in cancer in vivo, being either protective at early stage or pro-tumoral at later stages of disease progression. Interestingly, various epithelial cells express NLRs that assemble into inflammasome complexes to activate caspase-1.33,42 In turn, active caspase-1 in colon epithelial cells protects mice against colitis-associated colorectal cancer (CAC) in vivo through IL-18 secretion, which participates in intestinal tissue homeostasis.27,28 Moreover, these cytokines (IL-1β/IL-18) enhance the efficacy of chemotherapy-induced anticancer immunity in mice.5,43 At late stages of cancer progression in humans, IL-18 is detected in the serum of patients along with others inflammatory cytokines (TNFα, IL-6).31 In mice, an immunosuppressive role by ablating NK cells has been reported for IL-18.32 However, clinical administration of IL-18 appears to be relatively safe and beneficial against various solid tumors.31 This correlates with our in vitro observations that IL-18 improves human γδ T-cell antitumor function or may wipe out inflammation due to inflammatory cytokines. Surprisingly, cancer cell lines tested here constitutively express large amounts of caspase-1 that is cleaved into active fragments. Consistent with this, these cells also secrete mature IL-18 that triggers IFNγ secretion by γδ T cells.
Major differences exist between epithelial immune cells in their regulation of IFNγ production by the innate cytokines IL-18 and IL-12/IL-15. NK cells respond to IL-18 and IL-12, both independently and together, to produce IFNγ and develop cytotoxicity to cancer cells.44,45 The combination of both cytokines slightly increases IFNγ production and cytotoxicity due to the fact that NK cells constitutively express high amounts of cytokine membrane receptors (IL-18R and IL-12β2).45 In contrast, αβ T cells have been reported to require a combination of both cytokines IL-18 and IL-12 to synergistically induce IFNγ production in the presence or absence of TCR engagement. This synergism is mediated by the induction of IL-18R on naive T cells following IL-12 signaling, and by subsequent regulation of IL-12Rβ2 by IL-18.46,47 Importantly, naive T cells from IL-12-/- mice fail to produce IFNγ when stimulated with anti-CD3 and IL-18,47 although we cannot exclude a IL-18 response in the absence of IL-12 in other settings with differently activated αβ T cells. Therefore, our results suggest that γδ T cells may display an alternative scenario regarding the regulation of IFNγ production by innate cytokines: like NK cells, γδ T cells do not need APC products (IL-12, IL-15) in the presence of IL-18, although they do require an additional signal for being sensitized that is provided by a γδ TCR-dependent upregulation of the IL-18 receptor. Together, γδ T cells join NK cells for their capacity to sense tissue-derived molecules alone, although their sensitivity would be more finely tuned by γδ TCR engagement. Because IL-12Rβ2 signals through JAK2/TYK2-dependent activation of STAT4 to upregulate gene expression of IL-18Rβ in αβ T cells,48 it is tempting to speculate that the γδ TCR has evolved to connect to the same JAK2/TYK2 signaling pathway, thereby sensitizing γδ T cells to tissue-derived soluble triggers. This putative difference between γδ and αβ T cells regarding the IL-18 response mechanism is intriguing and deserves further investigation.
Of note, the γδ TCR sensitizes not only to soluble IL-18 but also to other unknown soluble molecules that originate from disturbed tissue-derived cells. Additional soluble triggers of γδ T cells might be metabolites that originate from dysregulated metabolic pathways, as disturbed target cells undergo major and similar metabolic changes during carcinogenesis and CMV infection that are consistent with inflammasome activation.24 Such small molecules may constitute new DAMPS, and their identification as well as their innate sensor machineries (presumably pattern recognition receptors) would constitute attractive tools to promote first-line γδ T–cell-mediated antitumor or anti-infectious immune responses. Such knowledge may, therefore, offer new clinical strategies of anticancer-or anti-infectious immunotherapy.
Materials and Methods
Human γδ T cell clone and line generation and functional assays
Peripheral blood mononuclear cells were generated from blood samples collected for medical care with the approval of the local medical ethics committee. Primary γδ T-cell lines (Vδ1, Vδ1+3, Vδ5) and Vδ2neg γδ T-cell clones (Vγ8Vδ3, Vγ2Vδ3) were generated and cultured following the same methods as described previously.14,35 For co-culture functional assays and IFNγ secretion, primary T-cell lines and clones (5 × 104 cells per well) were incubated with monolayers of tumor cell lines or HUVECs that were uninfected or infected with the human CMV clinical strain TB40-E as described.35 After 24 h at 37°C, supernatants were isolated by centrifugation, and cytokine secretion (IFNγ and TNFα) was measured by ELISA. Alternatively, primary γδ T-cell lines and clones (5 × 104 cells per well) were left untreated or treated with recombinant cytokines IL-18/IL-1β/IL-15 (50 ng/mL) or IL-12 (20 ng/mL). From co-culture experiments, the degranulation potential of γδ T cells was analyzed by measuring the expression of CD107a. Cells were co-cultured for 6 h in the presence of PE-conjugated mAb to CD107a (H4A3; BD Biosciences) and brefeldin A (10 μg/mL) and then were collected and stained with phycoerythrin–indodicarbocyanine-conjugated mAb to γδ TCR (IMMU510; Beckman Coulter); then, the expression of CD107a on γδ TCR+ cells was analyzed by flow cytometry. In some experiments, anti-IL-18 or anti-IL-10 Abs were added to culture media at various concentrations. Alternatively, conditioned culture supernatants from tumor cell lines and HUVECs uninfected or infected with HCMV obtained after 48 h were isolated by centrifugation and kept frozen at −80°C. Then, primary γδ T-cell lines and clones (5 × 104 cells per well) were incubated with immobilized anti-CD3 (OKT3 clone) in a 96-well plate. The plate was then centrifuged (2,500 rpm, 1 min) and media was replaced by conditioned culture supernatants for 24 h at 37°C. γδ T cells in the 96-well plate were then pelleted by centrifugation (2,500 rpm, 1 min) and the supernatant was used to measure cytokine secretion by ELISA.
Immunoblotting and recovery of active caspase-1
Immunoblotting was carried out as described previously.49 For recovery of active caspase-1, pellets of HUVECs uninfected or infected with HCMV were suspended in IP buffer (50 mmol/L Tris–HCl, pH 7.4, 150 mmol/L NaCl, 50 mmol/L NaF, 0.3% NP-40, 0.1 mmol/L Na3VO4), 20 μg/mL leupeptin, 20 μg/mL aprotinin, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF]). After centrifugation at 16,500 rpm/20 min at 4°C, cleared lysates were incubated with biotinyl-VAD-fmk (30 μmol/L) for 30 min at 37°C to precipitate the active cleaved fragment p10 of caspase-1. The biotinyl-VAD-fmk/caspase-1 p10 complex was recovered by using streptavidin-Sepharose beads (SIGMA), adding 30 μL of the 1:1 streptavidin-Sepharose suspension per 250 mL of IP buffer for 3 h at 4°C.50 Beads were pelleted by centrifugation (3,000 rpm/10 min at 4°C) and washed 3 times in cold IP buffer before adding an SDS loading buffer on top of the beads. Beads were then heated for 5 min, pelleted, and the supernatant was analyzed by SDS-PAGE/immunoblotting using an Ab that detects the p10 small subunit of processed human caspase-1 (sc515; Santa Cruz Biotechnology). For positive control of caspase-1 cleavage, THP-1 cell lines in suspension were treated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) for 12 h at 37°C. Adherent cells were cultured with fresh media (RPMI) and then treated with 1 μg/mL LPS for 6 h, followed by 5 mmol/L ATP (for 30 min).
Reagents and antibodies
Anti-human CD3 Ab was purchased from Biolegends (clone OKT3, 317302); anti-human Caspase-1 p10 Ab (C-20) from Santa Cruz Biotechnology (sc515); anti-human IL-18 blocking Ab from MBL (D044–3, clone 125–2H); anti-human IL-10 blocking from Mabtech (9D7); anti-human IL-18Rβ-PE conjugated from R&D Systems (FAB118P). Fluorescent dye-conjugated secondary Abs were purchased from LI-COR Biosciences. ELISA kits to measure IFNγ and TNFα secretion were purchased from Mabtech (3420–1H-20 and 3510–1H-20; respectively). Mature human recombinant IL-1β and IL-15 were obtained from Peprotech (200–01B and 200–15; respectively); human recombinant IL-12 and mature human IL-18 from MBL (JM-4161–10 and B003–5; respectively). Caspase-1 inhibitor Ac-YVAD-fmk was purchased from Calbiochem (caspase-1 inhibitor VI; 218746), and biotinyl-VAD-fmk from Enzo Life Sciences (ALX-260–098). LPS from Escherichia coli serotype 055:B5 (L6529) and ATP were purchased from SIGMA.
Statistical analysis
Most data were presented as the mean ± SD from at least 3 independent experiments. Statistical comparisons between different treatments were done by unpaired t-test, where P < 0.05 was considered statistically significant.
Acknowledgments
The authors thank the SFR TransBioMed flow cytometry facility.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the Agence Nationale de la Recherche, Fondation pour la Recherche Médicale (DEQ20110421287), INCa-Cancéropôle GSO, Ligue contre le Cancer de la Dordogne, and the Conseil Régional d'Aquitaine.
References
- 1.Chien Y, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol 2014; 32:121–55; PMID:; http://dx.doi.org/ 10.1146/annurev-immunol-032713-120216 [DOI] [PubMed] [Google Scholar]
- 2.Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 2013; 13:88–100; PMID:; http://dx.doi.org/ 10.1038/nri3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001; 294:605–9; PMID:; http://dx.doi.org/ 10.1126/science.1063916 [DOI] [PubMed] [Google Scholar]
- 4.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of gamma delta T cells. Adv Immunol 1999; 71:77–144; PMID:; http://dx.doi.org/ 10.1016/S0065-2776(08)60400-9 [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, et al.. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208:491–503; PMID:; http://dx.doi.org/ 10.1084/jem.20100269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 2003; 102:200–6; PMID:; http://dx.doi.org/ 10.1182/blood-2002-12-3665 [DOI] [PubMed] [Google Scholar]
- 7.Lafarge X, Merville P, Cazin MC, Bergé F, Potaux L, Moreau JF, Déchanet-Merville J. Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis 2001; 184:533–41; PMID:; http://dx.doi.org/ 10.1086/322843 [DOI] [PubMed] [Google Scholar]
- 8.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007; 27:334–48; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2007.05.020 [DOI] [PubMed] [Google Scholar]
- 9.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T cells. Science 2005; 309:264–8; PMID:; http://dx.doi.org/ 10.1126/science.1110267 [DOI] [PubMed] [Google Scholar]
- 10.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med 2009; 206:743–50; PMID:; http://dx.doi.org/ 10.1084/jem.20081787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology 2014; 3:e27572; PMID:; http://dx.doi.org/ 10.4161/onci.27572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegers GM, Lamb LS. Cytotoxic and regulatory properties of circulating Vδ1+ γδ T cells: a new player on the cell therapy field? Mol Ther J Am Soc Gene Ther 2014; 22:1416–22; http://dx.doi.org/ 10.1038/mt.2014.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, Michelson S, Méric C, Hallet MM, Kourilsky P, et al.. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest 1999; 103:1437–49; http://dx.doi.org/ 10.1172/JCI5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, Merville P, Moreau JF, Déchanet-Merville J. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 2008; 112:1317–24; PMID:; http://dx.doi.org/ 10.1182/blood-2008-01-136713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, Kühr J, Mascart F, Schmitt-Graeff A, Niemeyer C, Fisch P. A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest 2005; 115:3140–8; PMID:; http://dx.doi.org/ 10.1172/JCI25221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, Travers PJ, Lowdell MW. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 2010; 116:2164–72; PMID:; http://dx.doi.org/ 10.1182/blood-2010-01-255166 [DOI] [PubMed] [Google Scholar]
- 17.Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, Van Rysselberge M, Twité N, Goldman M, Marchant A, Willems F. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med 2010; 207:807–21; PMID:; http://dx.doi.org/ 10.1084/jem.20090348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, Moreau JF, Hayday AC, Willcox BE, Déchanet-Merville J. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol 2012; 13:872–9; PMID:; http://dx.doi.org/ 10.1038/ni.2394 [DOI] [PubMed] [Google Scholar]
- 19.Couzi L, Levaillant Y, Jamai A, Pitard V, Lassalle R, Martin K, Garrigue I, Hawchar O, Siberchicot F, Moore N, et al.. Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J Am Soc Nephrol 2010; 21:181–8; PMID:; http://dx.doi.org/ 10.1681/ASN.2008101072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 2014; 157:1013–22; PMID:; http://dx.doi.org/ 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 21.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 2002; 10:417–26; PMID:; http://dx.doi.org/ 10.1016/S1097-2765(02)00599-3 [DOI] [PubMed] [Google Scholar]
- 22.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol 2011; 32:373–9; PMID:; http://dx.doi.org/ 10.1016/j.it.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henao-Mejia J, Elinav E, Thaiss CA, Flavell RA. Inflammasomes and metabolic disease. Annu Rev Physiol 2014; 76:57–78; PMID:; http://dx.doi.org/ 10.1146/annurev-physiol-021113-170324 [DOI] [PubMed] [Google Scholar]
- 24.Lartigue L, Faustin B. Mitochondria: metabolic regulators of innate immune responses to pathogens and cell stress. Int J Biochem Cell Biol 2013; 45:2052–6; PMID:; http://dx.doi.org/ 10.1016/j.biocel.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 25.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012; 491:364–73; PMID:; http://dx.doi.org/ 10.1038/nature11706 [DOI] [PubMed] [Google Scholar]
- 26.Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol 2008; 26:1179–86; PMID:; http://dx.doi.org/ 10.1038/nbt.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KSB, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, et al.. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 2010; 32:367–78; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 28.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al.. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011; 145:745–57; PMID:; http://dx.doi.org/ 10.1016/j.cell.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang A-S, Humphries MM, Lavelle EC, et al.. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med 2012; 18:791–8; PMID:; http://dx.doi.org/ 10.1038/nm.2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebel-Binay S, Thiounn N, De Pinieux G, Vieillefond A, Debré B, Bonnefoy J-Y, Fridman W-H, Pagès F. IL-18 is produced by prostate cancer cells and secreted in response to interferons. Int J Cancer 2003; 106:827–35; PMID:; http://dx.doi.org/ 10.1002/ijc.11285 [DOI] [PubMed] [Google Scholar]
- 31.Vidal-Vanaclocha F, Mendoza L, Telleria N, Salado C, Valcárcel M, Gallot N, Carrascal T, Egilegor E, Beaskoetxea J, Dinarello CA. Clinical and experimental approaches to the pathophysiology of interleukin-18 in cancer progression. Cancer Metastasis Rev 2006; 25:417–34; PMID:; http://dx.doi.org/ 10.1007/s10555-006-9013-3 [DOI] [PubMed] [Google Scholar]
- 32.Terme M, Ullrich E, Aymeric L, Meinhardt K, Coudert JD, Desbois M, Ghiringhelli F, Viaud S, Ryffel B, Yagita H, et al.. Cancer-induced immunosuppression: IL-18-elicited immunoablative NK cells. Cancer Res 2012; 72:2757–67; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3379 [DOI] [PubMed] [Google Scholar]
- 33.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 2007; 55:443–52; PMID:; http://dx.doi.org/ 10.1369/jhc.6A7101.2006 [DOI] [PubMed] [Google Scholar]
- 34.Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, et al.. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 2014; 156:1045–59; PMID:; http://dx.doi.org/ 10.1016/j.cell.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, Dromer C, Emilie D, Moreau JF, Déchanet-Merville J. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 2005; 201:1567–78; PMID:; http://dx.doi.org/ 10.1084/jem.20041851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JH, Zhu HTL, Murphy TLW, Ouyang W, Murphy KM. IL-18-stimulated GADD45 β required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nat Immunol 2001; 2:157–64; PMID:; http://dx.doi.org/ 10.1038/84264 [DOI] [PubMed] [Google Scholar]
- 37.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front. Immunol 2013; 4:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, Heijhuurs S, Sebestyen Z, Gründer C, Marcu-Malina V, et al.. γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 2013; 27:1328–38; PMID:; http://dx.doi.org/ 10.1038/leu.2012.374 [DOI] [PubMed] [Google Scholar]
- 39.Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, Fischer WH, Wilson IA, Havran WL. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science 2010; 329:1205–10; PMID:; http://dx.doi.org/ 10.1126/science.1192698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009; 31:331–41; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 41.Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et al.. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 2010; 11:395–402; PMID:; http://dx.doi.org/ 10.1038/ni.1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012; 481:278–86; PMID:; http://dx.doi.org/ 10.1038/nature10759 [DOI] [PubMed] [Google Scholar]
- 43.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al.. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170–8; PMID:; http://dx.doi.org/ 10.1038/nm.2028 [DOI] [PubMed] [Google Scholar]
- 44.Tsutsui H, Matsui K, Kawada N, Hyodo Y, Hayashi N, Okamura H, Higashino K, Nakanishi K. IL-18 accounts for both TNF-α-and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J. Immunol 1997; 159:3961–7. [PubMed] [Google Scholar]
- 45.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, et al.. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol 1999; 162:1662–8; PMID: [PubMed] [Google Scholar]
- 46.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol 1998; 161:3400–7; PMID: [PubMed] [Google Scholar]
- 47.Chang JT, Segal BM, Nakanishi K, Okamura H, Shevach EM. The costimulatory effect of IL-18 on the induction of antigen-specific IFN-gamma production by resting T cells is IL-12 dependent and is mediated by up-regulation of the IL-12 receptor beta2 subunit. Eur J Immunol 2000; 30:1113–9; PMID:; http://dx.doi.org/ 10.1002/(SICI)1521-4141(200004)30:4%3c1113::AID-IMMU1113%3e3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- 48.Nakahira M, Tomura M, Iwasaki M, Ahn HJ, Bian Y, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. An absolute requirement for STAT4 and a role for IFN-gamma as an amplifying factor in IL-12 induction of the functional IL-18 receptor complex. J Immunol 2001; 167:1306–12; PMID:; http://dx.doi.org/ 10.4049/jimmunol.167.3.1306 [DOI] [PubMed] [Google Scholar]
- 49.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC . Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 2003; 423:456–461; PMID:; http://dx.doi.org/ 10.1038/nature01627 [DOI] [PubMed] [Google Scholar]
- 50.Faustin B, Lartigue L, Bruey J-M, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 2007; 25:713–24; PMID:; http://dx.doi.org/ 10.1016/j.molcel.2007.01.032 [DOI] [PubMed] [Google Scholar]