Abstract
Androgen-deprivation therapy (ADT) induces prostate cancer immunogenic modulation (IM) by reducing human tumor cell expression of anti-apoptotic genes thus facilitating increased sensitivity to immune-mediated lysis. Through its stimulation of IM, ADT has been shown to synergize with active immunotherapy thereby significantly improving overall survival in a mouse model of prostate cancer.
Keywords: Androgen-deprivation therapy, cancer immunotherapy, enzalutamide, immunogenic modulation, therapeutic cancer vaccine
Introduction
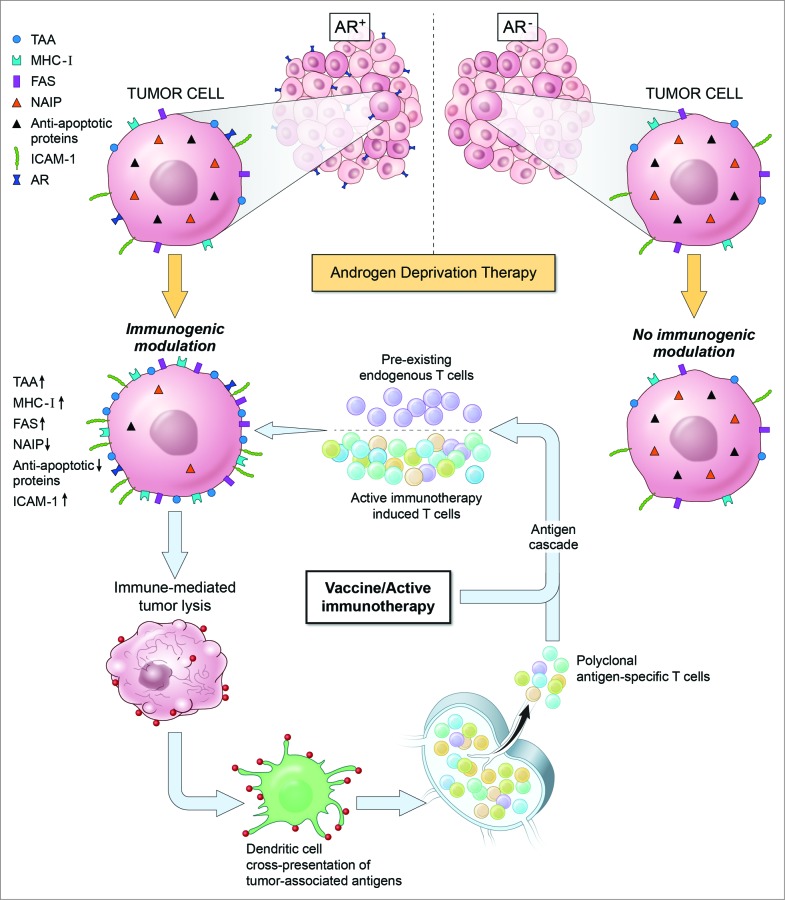
Certain conventional and emerging anticancer therapies possess immunostimulatory properties capable of enhancing the therapeutic effectiveness of immunotherapy. Such therapies achieve this outcome through the process of immunogenic modulation (IM). IM encompasses a spectrum of molecular alterations in the biology of cancer cells surviving therapy that independently or collectively render the tumor more amenable to immune attack (Fig. 1). Standard-of-care therapies, including chemotherapy, radiotherapy and small molecule inhibitor therapy, have demonstrated the ability to induce IM by altering the expression of proteins implicated in immune recognition and/or antigen processing.1,2
Figure 1.
Androgen-deprivation therapy induces immunogenic modulation in an androgen receptor-dependent manner. Immunogenic modulation promotes synergy between androgen-deprivation therapy and active immunotherapy.
Prostate cancer (PC) is the second leading cause of death from cancer among men in the United States. Localized PC is treated with surgery and radiotherapy, while recurrent disease is further treated with androgen-deprivation therapy (ADT).3 However, most patients eventually progress to castration-resistant prostate cancer (CRPC). The concept that CRPC is resistant to ADT has been challenged by emerging evidence indicating that CRPC remains dependent on androgen-receptor signaling for growth and is sensitive to further manipulation of androgen signaling.4 Here, we examine published studies examining how ADT induces IM and how this phenomenon can be exploited to improve clinical benefit when ADT is combined with immunotherapy in the treatment of PC patients.
Immunogenic Modulation Induced by Androgen-Deprivation Therapy
The IM potential of ADT was first described using the murine TRAMP (transgenic adenocarcinoma of the mouse prostate) model of prostate carcinoma.5 Exposure of TRAMP-C2 prostate tumor cells to enzalutamide, an androgen-receptor antagonist, significantly enhanced cell-surface expression of the death receptor Fas and MHC Class I resulting in improved sensitivity to immune-mediated lysis in vitro (Fig. 1). enzalutamide treatment in vivo significantly reduced genitourinary tissue weight, enlarged the thymus and increased the presence of T-cell receptor excision circles, suggesting augmented thymic function. These immunomodulatory properties of enzalutamide were exploited in combination with a therapeutic cancer vaccine targeting a transcription factor associated with the metastatic process. Combination treatment significantly increased antigen specific CD4+ T-cell proliferation as compared to the levels observed in mice receiving either no treatment or enzalutamide alone. Mice receiving combination treatment also exhibited significantly increased overall survival (OS) as compared to mice receiving no treatment or either monotherapy alone. Interestingly, mice harboring more advanced disease received the greatest survival benefit, possibly due to greater target antigen expression. Tumor-bearing mice receiving combination therapy displayed increased antigen specific CD8+ T-cell cytokine production as well as increased CD8+ T-cell responses against antigens not present in the vaccine, indicative of antigen cascade.
A follow-up study examined whether ADT could induce IM phenotypes in human PC cell lines.6 In this case, the effects of enzalutamide or abiraterone, an inhibitor of androgen biogenesis, were examined. Both agents were able to render androgen receptor positive (AR+) LNCaP human prostate tumor cells more sensitive to T cell-mediated lysis but not tumor cells lacking the androgen receptor, AR− PC-3 cells, or LNCaP cells engineered to express reduced levels of AR. Together, these data supported the conclusion that IM mediated by ADT was strictly dependent on the expression of AR. Treatment of LNCaP cells with enzalutamide did not induce significant changes in cell-surface expression of immunostimulatory molecules or antigen processing machinery; however, such treatment did alter the expression of several apoptotic genes including neuronal apoptosis inhibitory protein 1 (NAIP) (Fig. 1). NAIP reportedly prevents cell death by inhibiting activated caspases and increased expression has been linked to the development of CRPC.7 NAIP expression was significantly downregulated in AR+ LNCaP cells treated with enzalutamide in vitro or in vivo; however, alteration of NAIP expression was not observed in AR− PC-3 cells (Fig. 1). Knockdown of NAIP improved the sensitivity of PC cell lines to T cell-mediated killing regardless of AR expression, supporting downregulation of NAIP as a mechanism by which ADT induced IM. LNCaP cells harboring AR amplification, a major mechanism of ADT resistance, were also rendered more sensitive to immune-mediated lysis in response to enzalutamide treatment, highlighting the potential efficacy of ADT-induced IM even in ADT-resistant patients.8 Another intriguing observation from this study was that enzalutamide was found to significantly improve the sensitivity of LNCaP cells to prostate specific antigen (PSA)-targeted T-cell killing despite the enzalutamide-mediated reduction in PSA levels.
Clinical Implications
Findings from these studies provide a rationale for the use of ADT in combination with active immunotherapy, such as PROSTVAC-VF, for the treatment of CRPC patients, including those with minimal response to enzalutamide or who have developed resistance to ADT. PROSTVAC-VF, a cancer vaccine composed of a series of poxviral vectors engineered to express PSA and a TRiad of human T-cell COstimulatory Molecules (TRICOM), has been observed to be well tolerated and associated with a 44% reduction in death rate and an 8.5-month improvement in median OS compared to placebo in a randomized placebo controlled Phase 2 trial.9 Most patients receiving PROSTVAC have been shown to exhibit a ≥2-fold increase in the occurrence of PSA-specific T cells. Furthermore, patients with the greatest increases in PSA-specific T cells displayed the most improved OS.10 In light of this, the observation that enzalutamide significantly improved the sensitivity of PC cells to PSA-specific T-cell killing despite reducing their PSA levels is of particular interest. These data suggest that enzalutamide would not reduce the sensitivity of patient tumor cells to PSA-specific immunity stimulated by this vaccine. Based on these observations, clinical trials evaluating the efficacy of enzalutamide in combination with PROSTAC-VF in patients with CRPC (NCT01867333) and non-metastatic castration-sensitive prostate cancer (NCT01875250) are currently underway (www.clinicaltrials.gov). These findings also suggest improved clinical benefit could be attained by combining ADT with other immunotherapies, including Provenge® (sipuleucel-T) and checkpoint inhibitors (e.g., anti-PD-1/PD-L1 and anti-CTLA-4 blocking antibodies). In addition, one can envision that other hormone-deprivation therapies, such as estrogen depletion in the treatment of breast cancer might also induce IM, offering additional therapeutic options for breast cancer patients including those with hormone-deprivation resistant triple-negative breast carcinoma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1.Hodge JW, et al.. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol 2012; 39(3): 323–39; PMID:; http://dx.doi.org/ 10.1053/j.seminoncol.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwilas AR, et al.. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med 2014; 12(1): 294; PMID:; http://dx.doi.org/ 10.1186/s12967-014-0294-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich A, et al.. EAU guidelines on prostate cancer. Eur Urol 2008; 53(1): 68–80; PMID:; http://dx.doi.org/ 10.1016/j.eururo.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol 2011; 29(27): 3651–8; PMID:; http://dx.doi.org/ 10.1200/JCO.2011.35.2005 [DOI] [PubMed] [Google Scholar]
- 5.Ardiani A, et al.. Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res 2013; 19(22): 6205–18; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardiani A, et al.. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen receptor dependent modulation of the apoptotic pathway. Oncotarget 2014; 5(19): 9335–48; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu HH, et al.. Induction of neuronal apoptosis inhibitory protein expression in response to androgen deprivation in prostate cancer. Cancer Lett 2010; 292(2): 176–85; PMID:; http://dx.doi.org/ 10.1016/j.canlet.2009.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013; 32(49): 5501–11; PMID:; http://dx.doi.org/ 10.1038/onc.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantoff PW, et al.. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28(7): 1099–105; PMID:; http://dx.doi.org/ 10.1200/JCO.2009.25.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulley JL, et al.. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010; 59(5): 663–74; PMID:; http://dx.doi.org/ 10.1007/s00262-009-0782-8 [DOI] [PMC free article] [PubMed] [Google Scholar]