Abstract
Pharmacologic inhibition of the mutant BRAFV600E protein in advanced BRAFV600E melanoma results in a high proportion of patients that respond, but few with durable responses. We have recently revealed that Natural Killer (NK) cells play an essential role in the BRAFV600E inhibitor control of melanoma metastases in mice that may be therapeutically exploited to help overcome drug resistance.
Keywords: BRAF, cancer immunotherapy, metastases, NK cells, resistance
The discovery of the gain-of-function BRAFV600E mutation in half of all human melanoma diagnosed was followed by the development of selective inhibitors for the mutant BRAF. Vemurafenib (PLX4032) and its research analog PLX4720 are BRAFV600E inhibitors capable of preventing tumor cell proliferation by inhibiting the mitogen activated protein kinase (MAPK) pathway. In phase III clinical trials, the treatment of melanoma patients with Vemurafenib substantially increased overall survival; however, durable responses or complete remissions were rarely observed.1 This is due to the drug resistance, and thus strategies to either overcome resistance with additional drugs or by boosting the patient's antitumor immune response have become fashionable. 2
Vemurafenib and PLX4720 were developed in vitro using human melanoma cell lines or in vivo using human melanomas inoculated in the severe combined immune-deficient (SCID) mice. 3,4 SCID mice do not allow assessment of the potential role of the immune system in the therapeutic response, nor the effect of T regulatory (Treg) cells in promoting tumor growth.3-5 More recently, several immune competent mouse models of BRAFV600E-mutated melanoma have been developed and the role of the host immune system and potential for combination cancer immunotherapy explored.6 We have previously used a transplantable BRAFV600E mutant melanoma cell line, SM1WT1, to show that PLX4720 anti-melanoma effect is dependent on CD8+ T cells, consistent with the changes of intratumoral CD8+ T cells, NK cells, and Treg cells.6 The primary SM1WT1 tumor did not overtly metastasize. However, we recently developed a metastatic SM1WT1 variant through in vivo passage in the lungs of C57BL/6 mice.7 This technique was firstly used many years ago for the generation of the B16F10 melanoma, a mouse melanoma cell line sensitive to NK cell recognition and therefore suitable for studies focused on NK cell anti-metastatic function.8
The variant of SM1WT1 we created, named LWT1, produces consistent metastases in the lungs of C57BL/6 mice and these are naturally controlled by host NK cells through DNAM-1 receptor, and interferon (IFN)γ and perforin effector pathways. Given the importance of melanoma metastasis in the death of patients, these features of LWT1 allowed us to investigate the role of NK cells in the therapeutic control of BRAFV600E mutant melanoma metastases by PLX4720. Although PLX4720 controlled the lung metastasis of LWT1, by contrast, when NK cells were depleted, the BRAF inhibitor was ineffective.7 This revealed that NK cells were critical for PLX4720 to have therapeutic effects in vivo against a mouse BRAF mutant melanoma cell line.
The mechanism by which the BRAFV600E inhibitor activates BRAFwild type NK cells is not entirely clear. We showed that PLX4720 increases the phosphorylation of ERK1/2, CD69 expression, and proliferation of mouse NK cells in vitro. NK cell frequencies were significantly enhanced by PLX4720 specifically in the lungs of mice with BRAFV600E lung metastases. Furthermore, PLX4720 also increased human NK cell pERK1/2, CD69 expression, and IFNγ release in the context of anti-NKp30 and Interleukin-2 (IL-2) stimulation. However, translation of these findings into humans is cautioned, and proper analysis of NK cells in patients undergoing BRAF inhibitor therapy is a must. Now that MEK inhibitors are used clinically in combination with BRAF inhibitors, any stimulatory effect of BRAF inhibitors on NK cells may be lost and thus patients receiving BRAF inhibitor alone or BRAF inhibitor plus MEK inhibitor might be compared.
Much evidence shows the immune system plays a critical role in cancer therapy.9 It is not known why only a small proportion of advanced melanoma patients on BRAF inhibitors have survived long-term thus far, but one simple hypothesis is that these patients have an effective and active immune surveillance of their disease. Analysis of the innate NK cell activity of these patients pre- and post-BRAF inhibitor treatment may be an interesting comparator with the majority that ultimately fails therapy. Despite the promise of combination of BRAF inhibitors with the immunotherapies that block the T-cell checkpoint inhibitors, such as CTLA-4, these initial combinations tested in humans produced severe toxicity to melanoma patients and further studies were discouraged. 10 Safer immune checkpoint inhibitors, such as anti-PD-L1, may well be worthy of examination in combination with BRAF inhibitors. Now, with the discovery that NK cells are also essential for the therapeutic activity of PLX4720, future strategies to overcome drug resistance might be designed based on a combination therapy of the BRAF inhibitor with NK cell activating immunotherapies.
Indeed, we showed that IL-2 activation of NK cells combined with PLX4720 to more powerfully suppress LWT1 melanoma metastases in mice (Fig. 1).7 The scheduling of NK cell based immunotherapy with BRAF inhibitors needs to be examined pre-clinically in more detail since thus far only concurrent therapy has been evaluated. These results should stimulate further pre-clinical and clinical studies with different NK cell activators (e.g. type I IFN, IL-15, TLR agonists, anti-CD137, anti-KIR antibodies) in combination with BRAFV600E inhibition. The combination of BRAF inhibitor and anti-CD137 was already demonstrated as very effective in pre-clinical models of primary BRAF mutant melanomas, but metastases have not yet been evaluated.6 In particular early phase trials with immune checkpoint blocking anti-KIR antibodies (Lirilumab), which activate human NK cells, have been promising and safe in hematological malignancies. The examination of anti-KIR with anti-PD-1 in advanced solid cancers is also underway in clinical trials. Our work indicates that BRAF inhibitor and anti-KIR therapy may be an interesting combination to test in earlier stage BRAF mutant melanoma where metastases may be preventable.
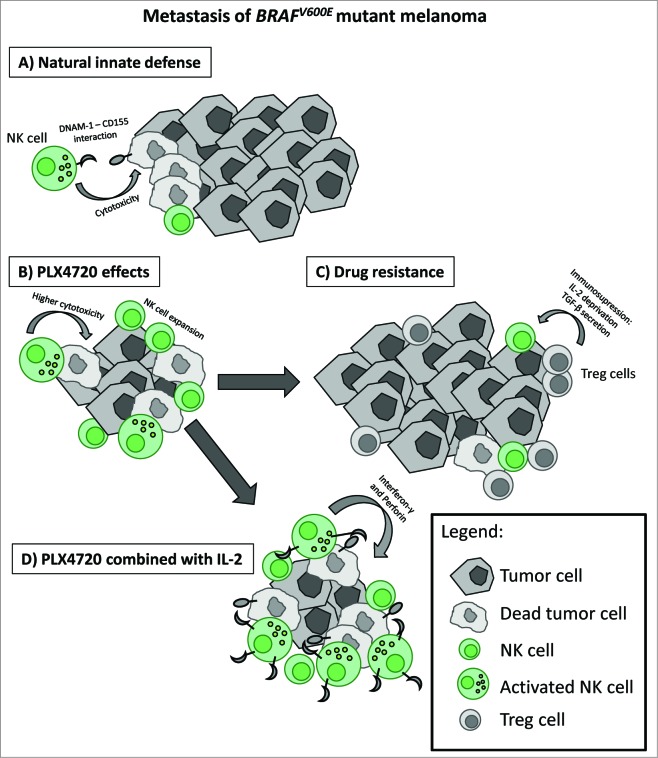
Figure 1.
The combination of PLX4720 with a NK cell activator (IL-2) results in better pre-clinical outcome for melanoma metastases suppression. (A) BRAFV600E mutant melanoma tumors are naturally controlled by NK cells through DNAM-1 pathway. (B) PLX4720 reduces tumor growth through inhibition of MAPK pathway and promotes NK cell cytotoxic function in the tumors. (C) But tumors became PLX4720 resistant and develop an immunosuppressive environment through recruitment of Treg cells. (D) Synergistic effects by the combination of PLX4720 with IL-2 promotes both effective control of tumor metastases by the BRAF inhibitor and clearance of any remaining or potentially drug resistant tumor cells by NK cells7.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with Vemurafenib in melanoma with BRAF V600E mutation. The New England Journal of Medicine 2011; 364:2507-16; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014; 343:84-7; PMID:; http://dx.doi.org/; http://dx.doi.org/ 10.1126/science.1247005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A 2008; 105:3041-6; PMID:; http://dx.doi.org/ 10.1073/pnas.0711741105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, Halilovic E, Persaud Y, Xing F, Viale A, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A 2010; 107:14903-8; PMID:; http://dx.doi.org/ 10.1073/pnas.1008990107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dorshkind K, Pollack SB, Bosma MJ, Phillips RA. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (SCID). J Immunol 1985; 134:3; PMID: [PubMed] [Google Scholar]
- 6. Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, Haynes NM, Kinross K, Yagita H, Koya RC, et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest 2013; 123:1371-81; PMID:; http://dx.doi.org/ 10.1172/JCI66236 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7. Ferrari de Andrade L, Ngiow SF, Stannard K, Rusakiewicz S, Kalimutho M, Khanna KK, et al. Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E mutant metastatic melanoma. Cancer Res 2014; 74:7298-308; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1339 [DOI] [PubMed] [Google Scholar]
- 8. Fidler IF. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res 1975; 35:6; PMID: [PubMed] [Google Scholar]
- 9. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 10. Johnson DB, Wallender EK, Cohen DN, Likhari SS, Zwerner JP, Powers JG, Shinn L, Kelley MC, Joseph RW, Sosman JA. Severe cutaneous and neurologic toxicity in melanoma patients during vemurafenib administration following anti-PD-1 therapy. Cancer Immunol Res 2013; 1:373-7; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]