Abstract
Maternal immunization is successfully applied against some life-threatening infectious diseases as it can protect the mother and her offspring through the passive transfer of maternal antibodies. Here, we sought to evaluate whether the concept of maternal immunization could also be applied to cancer immune-prevention. We have previously shown that antibodies induced by DNA vaccination against rat Her2 (neu) protect heterozygous neu-transgenic female (BALB-neuT) mice from autochthonous mammary tumor development. We, herein, seek to evaluate whether a similar maternal immunization can confer antitumor protection to BALB-neuT offspring. Significantly extended tumor-free survival was observed in BALB-neuT offspring born and fed by mothers vaccinated against neu, as compared to controls. Maternally derived anti-neu immunoglobulin G (IgG) was successfully transferred from mothers to newborns and was responsible for the protective effect. Vaccinated mothers and offspring also developed active immunity against neu as revealed by the presence of T–cell-mediated cytotoxicity against the neu immunodominant peptide. This active response was due to the milk transfer of immune complexes that were formed between the neu extracellular domain, shed from vaccine-transfected muscle cells, and the anti-neu IgG induced by the vaccine. These findings show that maternal immunization has the potential to hamper mammary cancer in genetically predestinated offspring and to develop into applications against lethal neonatal cancer diseases for which therapeutic options are currently unavailable.
Keywords: antitumor vaccine, cancer immune-prevention, DNA vaccination, Her2/neu, mammary cancer, maternal immunization
Abbreviations: Amot, Angiomotin p80; BALB-neuT mice, BALB/c mice heterozygous for the transforming form of the neu transgene; BKO mice, BALB/c female mice KO for the μIg chain; ECTM, extracellular and transmembrane; FcγKO mice, BALB/c mice KO for the Fc-gamma I/III receptors; FcγRI/III, Fc-gamma I/III receptors; IFNγ, interferon gamma; KO, knockout; LNs, lymph nodes; RSI, rate of stimulation index; SFU, spot-forming unit; SPC, splenocytes; Treg, T-regulatory cell
Introduction
Vaccination is the most powerful and versatile tool in preventive medicine and has recently extended its reach beyond infectious diseases to other life-threatening illnesses, such as cancer. Examples can be found in vaccines that have been designed to prevent infection-associated tumors1 and in those targeting non–infection-related cancer diseases now in preclinical phases or in clinical trials.2
Maternal immunization against life-threatening disease-inducing pathogens has been shown to be a viable approach against many childhood pathologies. The induction of high antibody levels following vaccination is fundamental to maternal immunization success. It has long been known that maternally derived IgG is the only antibody class that causes short-term passive immunity to be transferred from mother to fetus across the placenta and via the proximal small intestine during breastfeeding.3,4 This passive protection slowly declines over the first year of life while the infant's immune system become more mature.5
The vaccination of pregnant women against tetanus and the influenza virus has recently been proven to be safe and highly effective in providing newborn children with maternally transferred antibodies and, thus, protection from pathogens.6-8 Several antenatal vaccines are now available and recommended for pregnant women whereas others are in development.9,10 Maternal immunization against tumor-associated antigens, instead, is not a currently rated field and very few preclinical attempts have been made to prevent neonatal congenital tumors by maternal vaccination.11,12
Most of our recent studies have focused on DNA vaccination against what we have defined as oncoantigens: tumor-associated antigens that have a causal role in the promotion of tumor progression.13,14 Membrane tyrosine kinase Her2, of the epidermal growth factor receptor family, is expressed by many human carcinomas in association with poor prognosis15 and fulfills the definition of an oncoantigen. We have demonstrated that vaccination with a plasmid that codes for the extracellular and transmembrane (ECTM) domains of rat Her2 (neu) effectively inhibits mammary carcinogenesis16-18 in female BALB/c mice heterozygous for the transforming form of the neu transgene under the transcriptional control of the mouse mammary tumor virus promoter (BALB-neuT mice).19,20 Vaccine-elicited tumor inhibition in these mice is driven by anti-neu antibody generation,16,17 whereas the T-cell cytotoxic response is marginal as T cells that react against neu with high affinity are wiped out by central tolerance.21,22
The induction of high levels of antibodies following vaccination is also the mainstay of the success of maternal immunization strategy against infectious diseases. In the present study, we seek to evaluate whether maternal immunization can also induce an anti-neu immune response capable of hampering spontaneous tumor progression in BALB-neuT offspring.
Results
Vaccine-induced antitumor antibodies are transferred from mothers to their offspring and delay tumor development
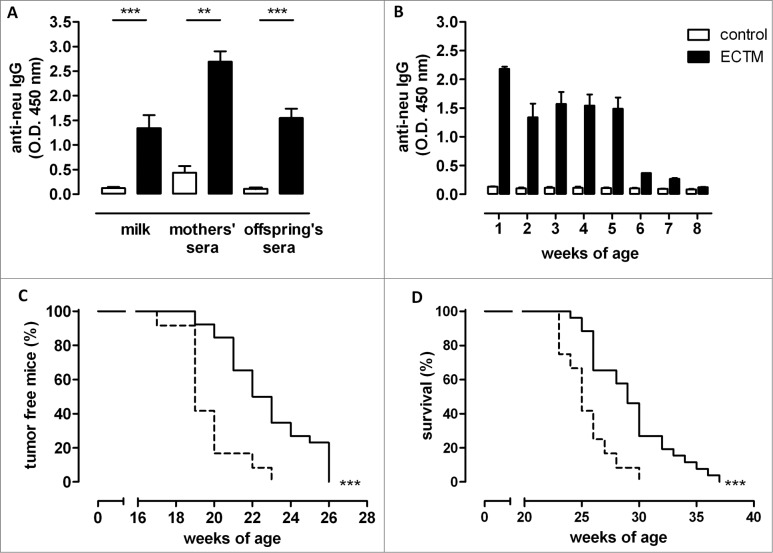
Virgin BALB/c female mice were twice vaccinated via electroporation of ECTM plasmid (ECTM mothers) or its empty control vector (control mothers) and mated with a BALB-neuT male soon after their last immunization. No fertility impairment, reduction in litter number, newborn size, or in the percentage of BALB-neuT mice was evident in the comparison between offspring of ECTM mothers, those of control mothers, and those of untreated mothers (data not shown). The presence of anti-neu antibodies in sera and milk of ECTM mothers was confirmed 2 weeks after the last immunization and 3 weeks after delivery, respectively (Fig. 1A). As expected, passively transferred anti-neu antibodies were found in the sera of offspring born from and fed by ECTM mothers (ECTM offspring), but not in the sera of offspring born from and fed by control mothers (control offspring) (Fig. 1A and B). The highest anti-neu antibody amount was found at 1 week of age, probably due to colostrum ingestion, and remained high until the fifth weeks. The anti-neu antibody titer dropped from week 6, probably because of offspring weaning at 4 weeks (Fig. 1B).
Figure 1.
DNA vaccine-induced anti-neu antibodies are successfully transferred from mothers to their pups and induce delayed mammary carcinoma onset in neu+ offspring. (A) Detection of vaccination-induced anti-neu antibodies in the milk and sera of control (white bars) and ECTM-(black bars) vaccinated mothers and in the sera of their 4-week-old offspring. **, p = 0.004; ***, P ≤ 0.0003, Student's t-test. Data are representative of 2 independent experiments and represented as mean ± SEM. (B) Detection of anti-neu IgG in control (white bars) and ECTM (black bars) offspring's sera collected from the first to the eighth week after birth. (C) Appearance of the first palpable mammary tumor in control (dotted black line, n = 12) and ECTM (continuous black line, n =26) neu+ offspring. Data are representative of 4 independent experiments. ***, P < 0.0001, Mantel–Haenszel Log-rank test. (D) ECTM offspring displayed a significant extension in overall survival as compared to control offspring. ***, p < 0.0003, Mantel-Haenszel Log-rank test.
We have previously shown that the anti-neu antibodies induced by ECTM vaccination of BALB-neuT females halt autochthonous mammary carcinogenesis.16,17,23 Having found specific anti-neu antibodies in ECTM offspring, we investigated whether these antibodies were able to inhibit mammary carcinogenesis in female BALB-neuT pups (neu+ offspring). Indeed, neu+ ECTM offspring showed significantly extended tumor-free (Fig. 1C) and overall (Fig. 1D) survival over neu+ control offspring. At week 23, approximately 35% of neu+ ECTM offspring were free from palpable lesions, whereas all neu+ control offspring displayed at least one palpable tumor. At week 30, 27% of ECTM offspring were still alive when all control offspring were dead.
The passage of antitumor immunity from mother to offspring was further confirmed by the ability of non-transgenic pups (neu- offspring) from ECTM mothers to hamper the growth of a transplantable tumor induced by a neu+ cancer cell line challenge (TUBO cells).24 Although 100% neu- control offspring developed TUBO tumors, 2 of the 22 neu- ECTM offspring did not develop a palpable tumor (Table 1). Moreover, the time required for the TUBO cells to give rise to 2, 4, 6, or 8-mm mean diameter tumors was significantly longer in neu- ECTM offspring than in neu- control offspring. The 10-mm mean diameter threshold (survival time) was reached in an average time of 34.8 ± 1.4 d in neu- ECTM offspring and in 26.8 ± 1.3 d in neu- control offspring (Table 1).
Table 1.
Maternal immunization against neu hampers the growth of a transplantable mammary tumor
| Latency time (days)a | Survival time (days)a | |||||
|---|---|---|---|---|---|---|
| Tumor takes/challenged mice | 2 mmb | 4 mm | 6 mm | 8 mm | 10 mm | |
| Control offspring | 22/22 (100%)c | 12.1 ± 0.6 | 15.5 ± 0.6 | 18.9 ± 1 | 22.5 ± 1.2 | 26.8 ± 1.3 |
| ECTM offspring | 20/22 (91%) | 14.5 ± 0.8* | 19.8 ± 0.7*** | 24 ± 0.6*** | 28.6 ± 1.1*** | 34.8 ± 1.4*** |
The time taken by TUBO cells to give rise to tumors with a mean diameter of 2, 4, 6, and 8 mm (latency times) or 10 mm (survival time). Data are representative of 3 independent experiments and are expressed as mean ± SEM
mean tumor diameter
percentage of survival in parentheses
,p = 0.02; ***,P ≤ 0.0006 as compared to control offspring, Student's t-test
,P ≤ 0.0006 as compared to control offspring, Student's t-test
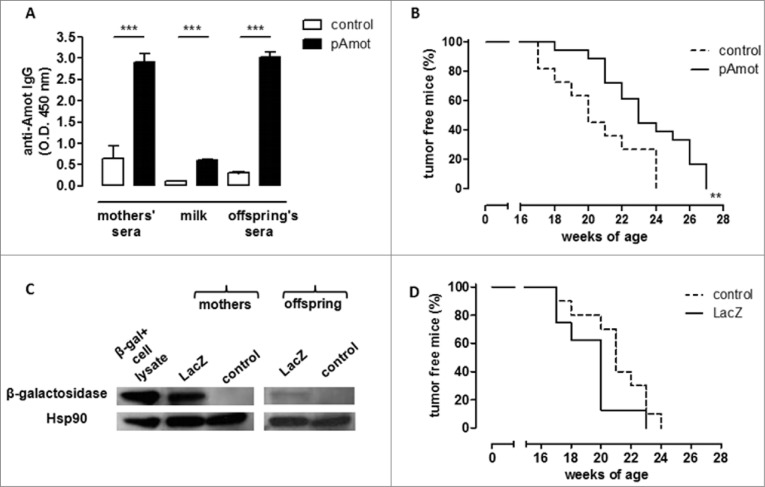
To determine whether these results obtained with antenatal vaccination can be generalized, BALB/c females were vaccinated against Angiomotin p80 (Amot), an oncoantigen expressed on tumor vasculature,25 using a plasmid coding for this protein (pAmot). Vaccinated females were then mated with a BALB-neuT male and neu+ offspring was evaluated for mammary tumor development. We have previously shown that vaccination-induced anti-Amot antibodies impair tumor vascularization26 and significantly delay autochthonous tumor progression27 in female BALB-neuT mice. Post-vaccination anti-Amot antibody induction was confirmed in pAmot-vaccinated mother sera and milk as well as in the sera of their offspring (pAmot offspring) (Fig. 2A). Mammary carcinoma onset in neu+ female offspring born from and fed by pAmot-vaccinated mothers was significantly delayed compared to female offspring born from and fed by control vaccinated mothers (Fig. 2B).
Figure 2.
Maternal immunization against an oncoantigen, but not an unrelated antigen, delayed mammary carcinoma onset in neu+ offspring. (A) Detection of vaccination-induced anti-Amot antibodies in sera and milk of control (white) and pAmot (black) mothers and in the sera of their 4-week-old offspring. ***, P ≤ 0.0003, Student's t-test. (B) Tumor incidence in control (dotted black line, n = 11) and pAmot (continuous black line, n = 18) neu+ offspring. Data are representative of 3 independent experiments. **, p = 0.001, Mantel–Haenszel Log-rank test. (C) Western blotting analysis of β-galactosidase protein. Sera from control and LacZ mothers and their offspring were used as primary antibodies, recombinant β-galactosidase as positive control, and HSP90 protein as loading control. (D) Tumor incidence in control (dotted black line, n = 10) and LacZ (continuous black line, n = 8) neu+ offspring.
Finally, to demonstrate that the observed antitumor protection was due to the choice of an oncoantigen as the DNA vaccination target, we vaccinated BALB/c females with a plasmid coding for Escherichia coli β-galactosidase (LacZ plasmid), a tumor-unrelated protein. Vaccinated females were soon mated with a BALB-neuT male and carcinogenesis progression was evaluated in neu+ female offspring. Although anti-β-galactosidase antibodies were found in the sera of both LacZ-vaccinated mothers and their offspring, no statistically significant difference in tumor incidence was observed between neu+ female offspring born from and fed by LacZ-vaccinated mothers and those born from and fed by control mothers (Fig. 2C and D).
Presence of antibodies and functional FcγRI/III is required to delay mammary carcinogenesis in ECTM offspring
To confirm the role of antibodies in mammary carcinogenesis delay, BALB/c female mice knockout (KO) for the μIg chain (BKO mice),28 that are thus unable to produce antibodies, were electroporated with ECTM or the empty control vector. Females were mated with a BALB-neuT/BKO male a few days after the last immunization. No statistically significant difference in tumor incidence was observed when autochthonous mammary tumor growth was evaluated in BKO neu+ ECTM and control offspring (Fig. 3A), thus proving that maternally derived antibodies are necessary for effective antitumor protection.
Figure 3.
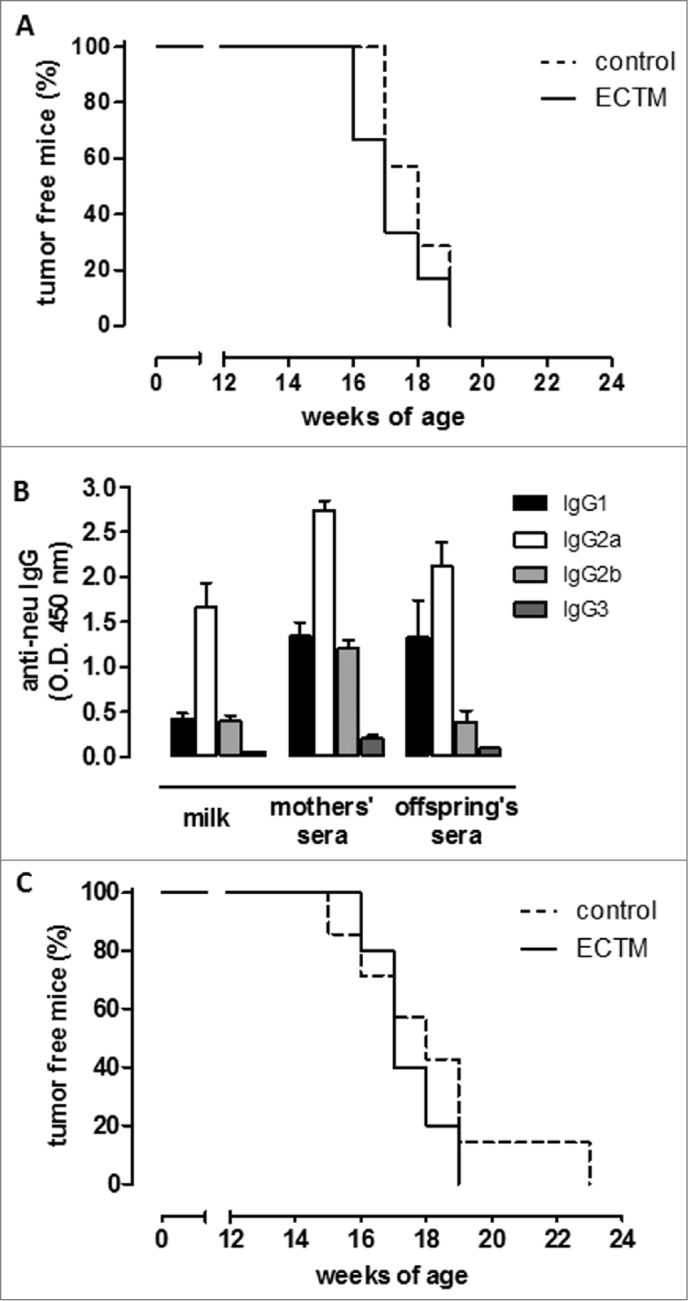
The presence of anti-neu antibodies and functional FcγRI/III are required to delay mammary carcinogenesis in neu+ offspring. (A) Tumor incidence of mammary carcinomas in neu+ offspring of BKO control (dotted black line; n = 7) and ECTM (continuous black line; n = 6) mothers. Data are representative of 2 independent experiments. (B) Characterization of IgG subclasses of anti-neu antibodies in the sera and in the milk of ECTM mothers and in the sera of their 3- to 4-week-old offspring. (C) Tumor incidence of mammary carcinomas in neu+ offspring of FcγKO control (dotted black line; n = 7) and ECTM (continuous black line; n = 5) mothers. Data are representative of 2 independent experiments.
Immunoglobulin G2a (IgG2a) was the most abundant IgG subclass in the milk and sera of ECTM mothers and in the sera of their pups, whereas IgG3 was the least common (Fig. 3B). This is in line with our previous findings, which demonstrate that ECTM vaccination elicits the activation of T helper cells producing interferon gamma (IFNγ), the primary switch factor for IgG2a.17 IgG2a activate the complement and interact very efficiently with the Fcγ receptors on various effector cells.29
To further elucidate how these passively transferred antibodies induce tumor delay, BALB/c mice KO for the Fc-gamma I/III receptors (FcγRI/III) (FcγKO mice)30 were immunized with ECTM or its empty control vector and mated with a BALB-neuT/FcγKO male. FcγKO neu+ ECTM female offspring did not display any significant tumor-onset delay over FcγKO neu+ control offspring (Fig. 3C), suggesting that antibody-dependent, cell-mediated cytotoxicity is one of the mechanisms behind vaccine-induced IgG triggering of antitumor protection.
neu extracellular domain–IgG immune complexes are present in ECTM mother's milk and induce an active immune response in offspring
Transfer of antigen–IgG immune complexes with breastfeeding is an important mechanism of active immunization in the offspring. We thus set up an ELISA assay to detect extracellular domain (EC)–IgG immune complexes, showing their presence in ECTM mothers' sera and milk (Fig. 4A). We then evaluated the presence of anti-neu IgM in the offspring sera. As shown in Figure 4B, sera from ECTM offspring had a significant higher level of IgM over that of controls. Moreover, exploiting a B-cell ELISPOT analysis, a significantly higher amount of neu-specific IgM+ memory B cells was found in the spleen of 5-week-old ECTM offspring as compared to age-matched control offspring (Fig. 4C).
Figure 4.
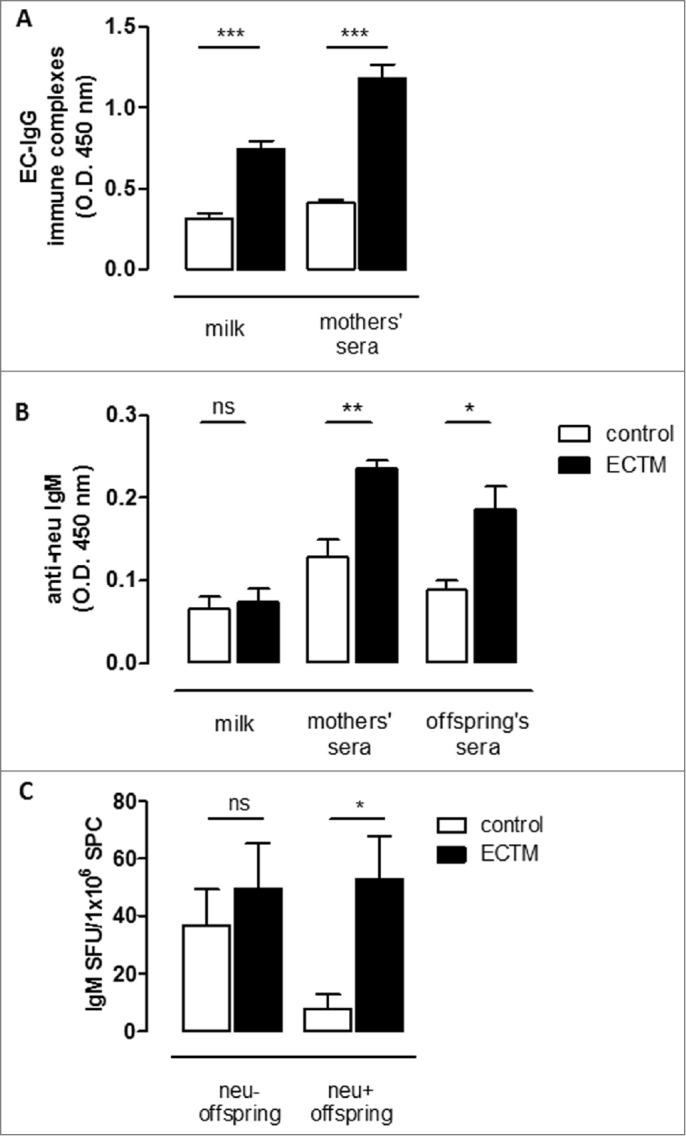
The passive transfer of maternal immunity induces an active humoral immune response in offspring. (A) Detection of EC–IgG immune complexes in mothers' milk and sera. (B) Detection of anti-neu IgM in mothers' milk and sera and in the sera of their 3- to 4-week-old offspring. (C) Presence of neu-specific IgM+ memory B cells in 5-week-old neu- and neu+ offspring. neu-specific IgM-secreting cells are expressed as SFU/1 ×106 B cells. In all panels, white bars refer to control vaccinated mice, black bars to ECTM vaccinated mice. Data show the mean ± SEM of values obtained from 2 to 3 independent experiments. *, p = 0.01; **, p = 0.004; ***, P ≤ 0.0007, Student's t-test.
A further confirmation of an active immune response against neu in ECTM offspring came from the evaluation of the T-cell response. We have previously shown that the ECTM vaccination of wild-type (neu-) BALB/c mice induces the expansion of a public CD8+ T-cell clone, which reacts at high avidity against the immunodominant neu nonamer peptide (p63–71). On the other hand, this high-avidity CD8+ T-cell clone is deleted in BALB-neuT mice because of neu expression in the thymus and its early overexpression in the mammary gland.21 Indeed, our ECTM mothers displayed increased in vivo cytotoxic activity against splenic cells pulsed with p63–71 over control mothers (Fig. 5A). In a similar assay, no specific cytotoxic response was found against p63–71 in control offspring, whereas it was evident in the ECTM offspring (Fig. 5A). Surprisingly, no significant differences in lysis percentage were found in neu- and neu+ ECTM offspring, while a lower, if any, cytotoxic response was expected in neu+ ECTM offspring. We then checked the T-cell receptor (TCR) repertoire used to react against p63–71 in these mice in order to shed light onto the origin of the cytotoxic activity found in the pups. Popliteal, inguinal, and mesenteric lymph nodes (LNs) were collected from ECTM mothers 5 weeks post delivery as well as from their 5-week-old offspring and the expansion of specific p63–71 TCR repertoires of CD8+ T cells was evaluated. We have previously identified a public TCR rearrangement, the Vβ9-Jβ1.2 recombination, elicited by ECTM vaccination in BALB/c mice that recognizes the p63–71 peptide with high avidity and is wiped out by central tolerance in BALB-neuT mice.21 The expansion of this repertoire was found in all ECTM mothers as well as in 3 out of 5 neu- ECTM offspring whereas, as expected, none of the neu+ ECTM offspring presented this TCR rearrangement (Fig. 5B). We then evaluated the expansion of the Vβ6-Jβ2.7 rearrangement. This is a low-avidity CD8+ T-cell clone that is specific for p63–71 and is induced by ECTM vaccination, but normally controlled by peripheral tolerance mechanisms such as T-regulatory cell (Treg) expansion in adult BALB-neuT mice.22 The Vβ6-Jβ2.7 rearrangement expansion was found in 5 out of 7 neu+ ECTM offspring and in none of the neu+ control offspring (Fig. 5B). These data rule out the possibility that the cytotoxic response found in the ECTM offspring is due to maternal CD8+ T cells being passively transferred via the milk and confirm the induction of an active immune response in the pups.
Figure 5.
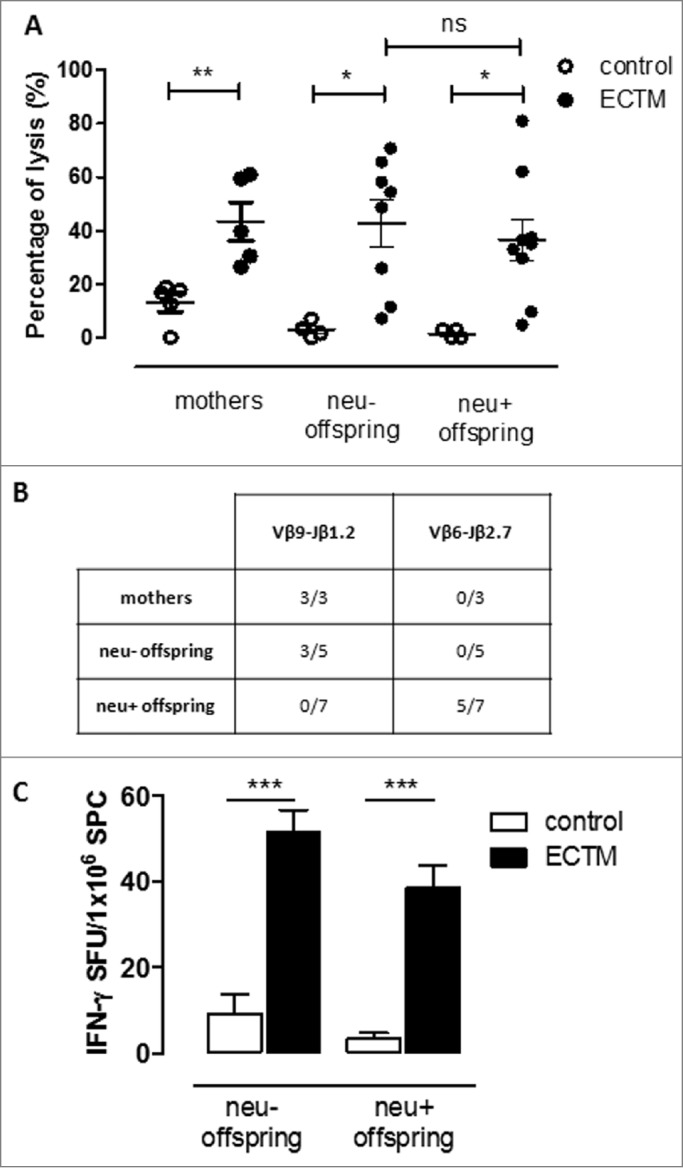
The passive transfer of maternal immunity induces an active cytotoxic immune response and the expansion of a distinct TCR repertoire in the offspring. (A) In vivo cytotoxic response against p63–71 peptide in control (white dots) or ECTM (black dots) mothers and in their neu- and neu+ 5-week-old offspring. (B) TCR repertoires in ECTM vaccinated mothers and in their neu- and neu+ offspring. Immunoscope analysis was conducted on cDNA pools obtained from LNs of ECTM mothers (n = 3) and their neu+ (n = 7) and neu- (n = 5) 5-week-old offspring. LNs cells were re-stimulated in vitro with the p63–71 peptide. Vβ9-Jβ1.2 and Vβ6-Jβ2.7 rearrangement frequencies are shown. Data are representative of 2 independent experiments. (C) T-cell response against p63–71 peptide quantified in vitro with an IFNγ-based ELISPOT assay. IFNγ-producing cells from control (white bars) and ECTM (black bars) neu- and neu+ offspring are expressed as SFU/1 ×106 SPC. *, p = 0.01; **, p = 0.005; ***, p = 0.0008, Student's t-test. Graphs display mean ± SEM and are representative of 2 independent experiments.
CD8+ T-cell activation in ECTM offspring was also confirmed in vitro with an IFNγ-based ELISPOT assay after splenic cell stimulation using the p63–71 peptide (Fig. 5C). Again, no statistically significant difference between neu- and neu+ ECTM offspring was found.
Discussion
The data reported in this article show that maternal DNA immunization against neu impairs the onset of mammary tumors in cancer-prone neu+ offspring. After DNA vaccination, high levels of IgG against the target oncoantigen can be detected in both, sera and milk of vaccinated mothers, although at lower levels in the milk. We observed that vaccine-induced IgG were successfully transferred to the offspring. Similar results were obtained when maternal DNA immunization was carried out against another oncoantigen, Amot, but not against the unrelated antigen β-galactosidase.
The passive transfer of maternal antibodies, in humans and other mammals, occurs via placental and milk transfer of IgG through the neonatal Fc receptor (FcRn) and via polymeric IgA milk transfer. In humans, FcRn is expressed by the syncytiotrophoblast where it antenatally transports IgG from maternal circulation to the fetal capillaries of the placental villi.4 By contrast, in rodents the FcRn functions most efficiently in the neonatal period when it transports maternally derived IgG in ingested milk across the epithelial-cell layer of the proximal intestine.4 Besides being the main source of passive immunity in very early life, breastfeeding is also an important route for active immunization thanks to the efficient transfer of antigen–IgG immune complexes, contained in the milk, to the breastfed pups via the FcRn and across the proximal intestine.31 We thus hypothesized that vaccine-transfected muscle cells might be the source of the neu protein, otherwise not present in a wild-type mouse. As happens normally, EC may be shed from transfected muscle cell membranes,32 form complexes with vaccine-induced anti-neu antibodies, and accumulate in the milk and be passed to the pups, triggering an active immune response. To confirm this hypothesis, an ELISA assay was set up to detect EC–IgG immune complexes that were found in the sera and milk of ECTM mothers.
We then sought to evaluate whether these EC–IgG immune complexes were able to induce an active immune response in the pups. An initial indication of this came from a significantly higher level of IgM against neu and of neu-specific IgM+ memory B cells in ECTM offspring over control. However, the proof of active immunization in breastfed pups came from the observation of an in vivo cytotoxic response against the neu p63–71 peptide in ECTM offspring. It is known that live-activated leukocytes, including CD8+ T cells, are present in mother's milk, and that they can be transferred during breastfeeding and that they can enter pups' intestinal tract tissue and mesenteric LNs in some species.31 It can be hypothesized, in the case of syngeneic strains, that these passively transferred cells may survive in the pups and be found in mesenteric LNs 5 weeks after birth, when the in vivo cytotoxicity assay was conducted. Nevertheless, this possibility was ruled out by the results of the Vβ-Jβ spectratype analysis. We observed the expansion of CD8+ T cells bearing the Vβ9-Jβ1.2 rearrangement in all ECTM mothers but in none of their neu+ offspring; this Vβ-Jβ rearrangement is normally used as public in ECTM-vaccinated BALB/c mice.21 On the other hand, in 71% of neu+ ECTM offspring, there was expansion of low-avidity CD8+ T cells bearing the Vβ6-Jβ2.7 TCR rearrangement that is typical of ECTM-vaccinated BALB-neuT, but not BALB/c, mice.22 Indeed, we have recently shown how a temporary Treg depletion and ECTM vaccination in BALB-neuT mice was able to induce the expansion of latent pools of low-avidity CD8+ T cells bearing TCR repertoires that react with p63–71.22 The Vβ6-Jβ2.7 rearrangement, the same that expanded in neu+ ECTM offspring in the present study, was among them. Preliminary data show a decrease in spleen-derived Treg percentage in neu+ ECTM offspring as compared to neu+ control offspring at the fifth week of age (data not shown). This may explain the presence of reactive, although low-avidity, CD8+ T-cell clones in neu+ ECTM offspring.
In conclusion, taken together these findings are proof of concept of the efficacy of maternal immunization against an oncoantigen. They are of particular relevance because, in BALB-neuT mice, neu is already expressed in nascent tumors and is the driving force of mammary transformation.20 We herein suggest that the concept of maternal immunization, being a potent weapon against pathogen-induced diseases in newborns, can be extended and used to delay cancer development in genetically predestinated offspring. The potential applications of this groundbreaking approach to neonatal cancer diseases such as neuroblastoma, rhabdomyosarcoma, Wilms' tumor, and retinoblastoma may have a substantial impact on clinical practice.
Materials and Methods
Mice
BKO28 and FcγKO mice30 were crossed with BALB-neuT mice to generate BALB-neuT/BKO and BALB-neuT/FcγKO mice, respectively. All mice were bred under specific pathogen-free conditions at the Molecular Biotechnology Center (Torino, Italy) and treated in conformity with European Guidelines and policies, as approved by the Ethical Committee of the University of Torino.
Cells
TUBO cells, an in vitro-established neu+ cell line derived from a lobular carcinoma arising in a BALB-neuT female mouse,24 were cultured in Dulbecco's modified Eagle's Medium (DMEM) supplemented with GlutaMAX™, D-glucose, HEPES buffer (Gibco), and 20% fetal bovine serum (FBS; Sigma-Aldrich).
Immunization and tumor growth
The pCMV3.1 control and the ECTM,16 the pcDNA3 and pAmot26 plasmids were generated as previously described. The pAAV-MCS (control plasmid) and the pAAV-MCS plasmid coding for Escherichia coli β-galactosidase (LacZ plasmid), were from the AAV Helper-Free System (Agilent Technologies Inc.). Fifty micrograms of plasmids diluted in saline were injected into the quadriceps muscle of anesthetized mice. Immediately after injection, 2 25-ms transcutaneous low-voltage electric pulses (amplitude 150 V; interval 300 ms) were administered at the injection site via a multiple-needle electrode connected to a Cliniporator™ (IGEA Srl). Female mice were immunized at 10 and 12 weeks of age and mated at week 13. All pups were fed by their own mothers and weaned at 4 weeks of age. The mammary glands of BALB-neuT female offspring were inspected weekly for tumor appearance from the twelfth week of age. The 5-week-old neu- offspring were challenged subcutaneously in the inguinal region with 1 ×105 TUBO cells. Tumor masses were measured as previously described.35
Assessment of anti-neu and anti-Amot antibodies
Sera from mothers were collected 2 weeks after the last vaccination. Pups' sera were collected from 1 to 8 weeks after birth. Mothers were separated from their litters 3 weeks after delivery for milk collection and fed with hydrated food for 24 h. Then, 2 IU of oxytocin (PitocinaIniet; Farmaceutici Gellini Srl) were injected twice intraperitoneally at an interval of 5 min between each administration. Milk was manually expressed from anesthetized mice, collected, and mixed with a protease inhibitor cocktail (Sigma-Aldrich). Defatted milk was obtained after an initial room temperature centrifugation for 10 min, at 2,000 rpm and 2 subsequent 4°C centrifugations at 12,000 rpm for 90 min. Sera and milk samples were tested by ELISA. Then, 96-well plates (Costar®, Sigma-Aldrich) were coated with 100 ng/well of recombinant EC neu (Genway) or recombinant human-Amot (Origene) protein, overnight at 4°C. Coated plates were then blocked with 10% Newborn Calf Serum (NCBS; Sigma-Aldrich) in phosphate-buffered saline (PBS; Invitrogen)-Tween (Sigma-Aldrich) 0.05% buffer for 2 h at 37°C. Plates were incubated with samples diluted 1:100 in 1% blocking buffer for 1 h at 37°C. Plates were washed 3 times with a PBS-Tween buffer. The horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody (Sigma-Aldrich; 1:2,000 dilution in blocking buffer) was incubated for 1 hour at 37°C. Plates were washed 6 times and chromogenic 3,3′,5,5′-tetramethylbenzidine substrate was added (TMB; Sigma-Aldrich). The reaction was stopped by the addition of 2N hydrochloric acid and optical density was measured at 450 nm using a microplate reader (680XR, BioRad). IgG isotype titration was carried out using rat biotin-conjugated anti-mouse IgG1, IgG2a, IgG2b, IgG3, and IgM (BD PharMingen) as secondary antibodies. Plates were then incubated for 30 min at room temperature with streptavidin-HRP (R&D Systems) diluted 1:200 in a PBS-Tween buffer and reactions were carried forward as described earlier.
Memory B-cell ELISPOT
Splenocytes (SPC) from 5-week-old control and ECTM offspring were collected and stimulated with a mixture of R848 (1 μg/mL, Mabtech) and rmIL-2 (10 ng/mL, Mabtech). Then, 72 h later, B cells were isolated by positive immune selection after SPC incubation with a biotin-antibody cocktail against CD43 (BD PharMingen) and CD11c (eBioscience) for 20 min at 4°C, followed by an incubation for 10 min at 4°C with Anti-Biotin MicroBeads (Miltenyi Biotec). Thereafter, 1 ×105B cells were plated in triplicate on polyvinylidene fluoride (PVDF) ELISPOT plates (Mabtech) pre-coated with 20 μg/mL of recombinant EC neu protein. Plates were incubated for 24 h at 37°C and developed according to manufacturer's instructions (ELISpotPLUS, Mabtech). Specific spots were enumerated using the Transtec 1300 ELISPOT Reader (AMI Bioline). The number of specific spots was calculated by subtracting the number of spontaneously produced spots and expressed as spot-forming unit (SFU)/106 cells.
Immunoblotting
A total cell lysate from mouse cardiomyocytes that express β-galactosidase protein after recombinant Adeno-associated virus 2 viral particles (kindly provided by Prof. Emilia Turco, Molecular Biotechnology Center, University of Torino) infection was separated by SDS-PAGE. Sera from vaccinated mothers and from their 3-week-old offspring were collected, pooled, diluted 1:50 in Tris-buffered saline and Tween 20 (TTBS) and incubated overnight at 4°C on the membrane, as the primary antibody. Goat anti-mouse HRP secondary antibody (Sigma-Aldrich) was used for detection. Polyclonal rabbit anti-β-galactosidase (Thermo Scientific) and anti-HSP90 antibody (Santa Cruz Biotechnology) were used as positive and loading control, respectively, and detected by goat anti-rabbit HRP (Sigma-Aldrich). Proteins were detected by enhanced chemiluminescence (ECL®, Amersham Biosciences).
In Vivo cytotoxicity assay
For this assay, 107 naive SPC/mL were labeled with two different concentrations (0.5 or 5.0 μmol/L) of the fluorescent dye CFSE (Molecular Probes); 5 μmol/L labeled-SPC were also pulsed with p63–71 (TYVPANASL; InBios Srl) for 90 min at 37°C. The two SPC populations were mixed in equal amounts and injected into the tail vein of control or ECTM mothers 2 weeks after the last vaccination and into 5-week-old control or ECTM offspring. Forty-eight hours later, single-cell suspensions from the spleen of each mouse were processed to evaluate the presence of CFSEhigh- and CFSElow-labeled SPC on a CyAn ADP Flow Cytometer (DakoCytomation). The low peak percentage was normalized to control untreated low peaks and the specific cytolytic activity was calculated as: 100 – [(CFSElow untreated cells / CFSElow experimental cells) × CFSEhigh experimental cells] × 100 / CFSEhigh untreated cells.
TCR repertoire analysis
Popliteal, inguinal, and mesenteric LNs were collected from 5-week-old control and ECTM offspring and cultured for 3 d with or without 15 μmol/L of p63–71. Total RNA was isolated from the recovered LNs cells using the RNeasy Mini Kit (Qiagen) according to manufacturer's instructions. cDNA was synthesized using an oligo-dT primer (dT15; Invitrogen). cDNA was subjected to PCR amplification using a common Constant (C) β primer (CACTGATGTTCTGTGTGACA) in combination with the following Vβ primers: 6, CTCTCACTGTGACATCTGCCC; 9, TCTCTCTACATTGGCTCTGCAGGC. Using 2 μL of PCR product as a template, run-off reactions were carried out with the following internal fluorescent Jβ: 1.2, AAAGCCTGGTCCCTGAGCCGAAG; 2.7, CTAAAACCGTGAGCCTGGTGC. Run-off products were denatured in formamide and analyzed on an Applied Biosystem 3100 Prism using Gene-scan 2.0 software (Applied Biosystem). Data were reported as the rate of stimulation index (RSI): normalized peak area from stimulated cells/normalized peak area of non-stimulated cells. T cells carrying a TCR rearrangement were considered to be expanded in a vaccination-driven manner when RSI was >2.21
EC-IgG immune complex detection
The 96-well/plates (Costar®, Sigma-Aldrich) were coated with 200 ng/well of the anti-CD340 monoclonal antibody (Sino Biological Inc.), saturated with 5% NCBS in PBS-Tween 0.05 % for 1 h at 37°C and, after several washes, the plates were incubated for 2 h at 37°C with a 1:50 dilution of both milk and serum followed by HRP-conjugated anti-mouse IgG antibody. The following reactions were carried forward as described for the detection of anti-neu antibodies.
IFNγ ELISPOT assay
For this assay, 1 ×106 SPC from 5-week-old control and ECTM offspring were plated in triplicate into nitrocellulose 96-well HTS IP plates (Millipore, Bedford, MA) that had been pre-coated with 5 μg/mL of rat anti-mouse IFNγ antibody (clone R4–6A2, BD Biosciences). SPCs were stimulated for 48 h at 37°C with 15 μg/mL of p63–71. Plates were developed according to manufacturer's instructions (BDTM ELISPOT Set, BD Biosciences). Spots were enumerated as described earlier.
Statistical analysis
Statistical differences were evaluated using the GraphPad software 5.0 (GraphPad Inc.). The Mantel–Haenszel Log-rank test was used to analyze differences in the incidence of tumors, while the Student's t-test was used for the evaluation of all other statistical differences.
Acknowledgments
The authors thank Dr. Dale Lawson for revising and editing this manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Italian Association for Cancer Research (IG 5377 and IG 11675) and the Compagnia di San Paolo (Progetti di Ricerca Ateneo/CSP, TO_call02_2012_0026) to F. Cavallo.
References
- 1. De Flora S, Bonanni P. The prevention of infection-associated cancers. Carcinogenesis 2011; 32:787-95; PMID:; http://dx.doi.org/ 10.1093/carcin/bgr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smit MA, Jaffee EM, Lutz ER. Cancer immunoprevention-the next frontier. Cancer Prev Res (Phila) 2014; 7:1072-80; PMID:; http://dx.doi.org/ 10.1158/1940-6207.CAPR-14-0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morphis LG, Gitlin D. Maturation of the maternofoetal transport system for human gamma-globulin in the mouse. Nature 1970; 228:573; PMID:; http://dx.doi.org/ 10.1038/228573a0 [DOI] [PubMed] [Google Scholar]
- 4. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715-25; PMID:; http://dx.doi.org/ 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- 5. Hanson LA, Korotkova M, Lundin S, Haversen L, Silfverdal SA, Mattsby-Baltzer I, Strandvik B, Telemo E. The transfer of immunity from mother to child. Ann New York Acad Sci 2003; 987:199-206; PMID: ; http://dx.doi.org/ 10.1111/j.1749-6632.2003.tb06049.x [DOI] [PubMed] [Google Scholar]
- 6. Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet 2007; 370:1947-59; PMID:; http://dx.doi.org/ 10.1016/S0140-6736(07)61261-6 [DOI] [PubMed] [Google Scholar]
- 7. Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555-64; PMID:; http://dx.doi.org/ 10.1056/NEJMoa0708630 [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged. MMWR 2011; 60(41):1424-6. [PubMed] [Google Scholar]
- 9. Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol 2011; 11:865-72; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rasmussen SA, Watson AK, Kennedy ED, Broder KR, Jamieson DJ. Vaccines and pregnancy: Past, present, and future. Sem Fet Neonat Med 2014; 19(3):161-9; PMID: 24355683. [DOI] [PubMed] [Google Scholar]
- 11. Botelho F, Clark DA. How might pregnancy immunize against breast cancer? Am J Reprod Immunol 1998; 39:279-83; PMID:; http://dx.doi.org/ 10.1111/j.1600-0897.1998.tb00365.x [DOI] [PubMed] [Google Scholar]
- 12. Sandler B, Smirnoff P, Gurevich P, Zusman I. Transplacental tumor-preventive effects of polyclonal antibodies generated against the soluble 53 kDa antigen on mammary tumorigenesis in offspring. Oncol Rep 1999; 6:897-900; PMID: [DOI] [PubMed] [Google Scholar]
- 13. Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer 2006; 6:204-16; PMID:; http://dx.doi.org/ 10.1038/nrc1815 [DOI] [PubMed] [Google Scholar]
- 14. Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Can Immunol Immunother. CII 2011; 60:319-26 ; http://dx.doi.org/ 10.1007/s00262-010-0968-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001; 2:127-37; PMID: [DOI] [PubMed] [Google Scholar]
- 16. Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C, et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res 2004; 64:2858-64; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-2962 [DOI] [PubMed] [Google Scholar]
- 17. Quaglino E, Rolla S, Iezzi M, Spadaro M, Musiani P, De Giovanni C, Lollini PL, Lanzardo S, Forni G, Sanges R, et al. Concordant morphologic and gene expression data show that a vaccine halts HER-2/neu preneoplastic lesions. J Clin Invest 2004; 113:709-17; PMID:; http://dx.doi.org/ 10.1172/JCI19850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quaglino E, Mastini C, Iezzi M, Forni G, Musiani P, Klapper LN, Hardy B, Cavallo F. The adjuvant activity of BAT antibody enables DNA vaccination to inhibit the progression of established autochthonous Her-2/neu carcinomas in BALB/c mice. Vaccine 2005; 23:3280-7; PMID:; http://dx.doi.org/ 10.1016/j.vaccine.2005.01.086 [DOI] [PubMed] [Google Scholar]
- 19. Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De Giovanni C, et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med 1998; 188:589-96; PMID:; http://dx.doi.org/ 10.1084/jem.188.3.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quaglino E, Mastini C, Forni G, Cavallo F. ErbB2 transgenic mice: a tool for investigation of the immune prevention and treatment of mammary carcinomas. Curr Protoc Immunol. 2008; 20 (20):91-9.10 [DOI] [PubMed] [Google Scholar]
- 21. Rolla S, Nicolo C, Malinarich S, Orsini M, Forni G, Cavallo F, Ria F. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J Immunol 2006; 177:7626-33; PMID:; http://dx.doi.org/ 10.4049/jimmunol.177.11.7626 [DOI] [PubMed] [Google Scholar]
- 22. Rolla S, Ria F, Occhipinti S, Di Sante G, Iezzi M, Spadaro M, Nicolò C, Ambrosino E, Merighi IF, Musiani P, et al. Erbb2 DNA vaccine combined with regulatory T cell deletion enhances antibody response and reveals latent low-avidity T cells: potential and limits of its therapeutic efficacy. J Immunol 2010; 184:6124-32; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0901215 [DOI] [PubMed] [Google Scholar]
- 23. Cavallo F, Offringa R, van der Burg SH, Forni G, Melief CJ. Vaccination for treatment and prevention of cancer in animal models. Adv Immunol 2006; 90:175-213; PMID:; http://dx.doi.org/ 10.1016/S0065-2776(06)90005-4 [DOI] [PubMed] [Google Scholar]
- 24. Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, Porcedda P, Boggio K, Smorlesi A, Lollini PL, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol 2000; 165:5133-42; PMID:; http://dx.doi.org/ 10.4049/jimmunol.165.9.5133 [DOI] [PubMed] [Google Scholar]
- 25. Iezzi M, Quaglino E, Amici A, Lollini PL, Forni G, Cavallo F. DNA vaccination against oncoantigens: a promise. Oncoimmunology 2012; 1:316-25; PMID:; http://dx.doi.org/ 10.4161/onci.19127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holmgren L, Ambrosino E, Birot O, Tullus C, Veitonmaki N, Levchenko T, Carlson LM, Musiani P, Iezzi M, Curcio C, et al. A DNA vaccine targeting angiomotin inhibits angiogenesis and suppresses tumor growth. Proc Natl Acad Sci U S A 2006; 103:9208-13; PMID:; http://dx.doi.org/ 10.1073/pnas.0603110103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arigoni M, Barutello G, Lanzardo S, Longo D, Aime S, Curcio C, Iezzi M, Zheng Y, Barkefors I, Holmgren L, et al. A vaccine targeting angiomotin induces an antibody response which alters tumor vessel permeability and hampers the growth of established tumors. Angiogenesis 2012; 15:305-16; PMID:; http://dx.doi.org/ 10.1007/s10456-012-9263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med 1998; 4:627-30; PMID:; http://dx.doi.org/ 10.1038/nm0598-627 [DOI] [PubMed] [Google Scholar]
- 29. Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119:5640-9; PMID:; http://dx.doi.org/ 10.1182/blood-2012-01-380121 [DOI] [PubMed] [Google Scholar]
- 30. Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci U S A 1998; 95:652-6; PMID:; http://dx.doi.org/ 10.1073/pnas.95.2.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mosconi E, Rekima A, Seitz-Polski B, Kanda A, Fleury S, Tissandie E, Monteiro R, Dombrowicz DD, Julia V, Glaichenhaus N, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol 2010; 3:461-74; PMID:; http://dx.doi.org/ 10.1038/mi.2010.23 [DOI] [PubMed] [Google Scholar]
- 32. Tse C, Gauchez AS, Jacot W, Lamy PJ. HER2 shedding and serum HER2 extracellular domain: biology and clinical utility in breast cancer. Cancer Treat Rev 2012; 38:133-42; PMID:; http://dx.doi.org/ 10.1016/j.ctrv.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 33. Grange C, Lanzardo S, Cavallo F, Camussi G, Bussolati B. Sca-1 identifies the tumor-initiating cells in mammary tumors of BALB-neuT transgenic mice. Neoplasia 2008; 10:1433-43; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conti L, Lanzardo S, Arigoni M, Antonazzo R, Radaelli E, Cantarella D, Calogero RA, Cavallo F. The noninflammatory role of high mobility group box 1/toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J 2013. 27(12):4731-44 [DOI] [PubMed] [Google Scholar]
- 35. Quaglino E, Mastini C, Amici A, Marchini C, Iezzi M, Lanzardo S, De Giovanni C, Montani M, Lollini PL, Masucci G, et al. A better immune reaction to Erbb-2 tumors is elicited in mice by DNA vaccines encoding rat/human chimeric proteins. Cancer Res 2010; 70:2604-12; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-2548 [DOI] [PubMed] [Google Scholar]