Abstract
Background
Ranunculus arvensis L. (R. arvensis) has long been used to treat a variety of medical conditions such as arthritis, asthma, hay fever, rheumatism, psoriasis, gut diseases and rheumatic pain. Here, we screened R. arvensis for antioxidant activity, phytochemical and high performance liquid chromatography (HPLC) analyses.
Methods
The chloroform, chloroform:methanol, methanol, methanol:acetone, acetone, methanol:water and water extracts of R. arvensis were examined for DPPH (1, 1-diphenyl-2-picrylhydrazyl) free radical scavenging assay, hydrogen peroxide scavenging assay, phosphomolybdenum assay, reducing power assay, flavonoid content, phenolic content and high performance liquid chromatography analysis.
Results
Significant antioxidant activity was displayed by methanol extract (IC50 34.71 ± 0.02) in DPPH free radical scavenging assay. Total flavonoids and phenolics ranged 0.96–6.0 mg/g of extract calculated as rutin equivalent and 0.48–1.43 mg/g of extract calculated as gallic acid equivalent respectively. Significant value of rutin and caffeic acid was observed via high performance liquid chromatography.
Conclusions
These results showed that extracts of R. arvensis exhibited significant antioxidant activities. Moreover, R. arvensis is a rich source of rutin, flavonoids and phenolics.
Keywords: Antioxidant, Ranunculus arvensis, Phenolic content, Flavonoid content, HPLC
Background
Ranunculus arvensis L., (R. arvensis) belongs to the family Ranunculaceae which is commonly known as corn buttercup. It is widely used to treat arthritis, asthma, hay fever, rheumatism, psoriasis and gut diseases [1]. It is also used as poultice around the knees and thumbs for rheumatic pain [2]. The fresh plant is toxic because it contains acrid sap that can cause blistering of skin however, its toxicity is abolished when dried [1]. From the beginning of civilization, plants have been used to treat diseases, as source of food, shelter, fodder, timber, fuel, and also in health-care [3]. Many plants are widely used in traditional medicines. They contain active chemical constituents that produce therapeutic physiological effects to treat a variety of diseases in both humans and animals [4]. Natural products from medicinal plants are considered chemically balanced, effective and least harmful with minimal side effects as compared to synthetic medicines. These medicinal plants have long been effective used in both traditional and modern medicine as nutraceuticals as well as food supplements. The World Health Organization (WHO) estimated that 60–70% of the population of developing countries use medicinal plants for the treatment of ailments [5].
Certain diseases are caused by the free radicals which can cause irreversible oxidative damage to the living system [6, 7]. The oxidation induced by reactive oxygen species results in membrane protein damage and DNA mutation, which can lead to development and propagation of many diseases, such as tissue injury, cardiovascular diseases, inflammation, mutation in genetic material, cancer and human neurological disorders [8–10]. Antioxidants can protect human from these free radicals and/or delay the development of diseases caused by these free radicals [11, 12]. Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) have been used as antioxidant agents since the beginning of this century but these are prohibited due to their in vivo carcinogenic effects. Therefore, a significant effort has been spent to find out natural antioxidants over synthetic compounds and elimination of synthetic antioxidants [13]. Polyphenols are widely distributed in plants and play an important role in medicine. Flavonoids and phenolics are a significant constituent of the human diet and many of them are natural antioxidants [14]. These phytochemicals have wide pharmacological and biological applications and can be used to treat coronary heart diseases, cancer and mutagenesis [15].
To our knowledge, there are no reports on antioxidant activity of R. arvensis. The present investigation was designed to determine antioxidant activity by DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical scavenging assay, hydrogen peroxide scavenging assay, phosphomolybdenum assay, reducing power assay, phytochemical screening (total flavonoids content and total phenolics content). Moreover, we also determined the effects of different extracts by high performance liquid chromatography (HPLC) analysis.
Methods
Preparation of plant extracts
Fresh R. arvensis (L.) was collected in May 2011 from F. R. Bannu (32°56′ 33°16′ North latitudes and 70°22′ 70°52′ longitudes), located on the East of Bannu District, Khyber Pakhtunkhwa, Pakistan. Taxonomic identification of the plant was done by taxonomist Department of Plant Sciences, Quaid-i-Azam University, Islamabad, 45320, Pakistan and Department of Botany, Government Post Graduate College, Bannu, Pakistan. The voucher specimen (AR-57) was deposited in the herbarium. The plant was rinsed with distilled water and shade dried. The extracts were prepared by soaking 30 g of ground plant powder in 300 mL of various solvents i.e. chloroform, chloroform:methanol (1:1), methanol, methanol:acetone (1:1), acetone, methanol:water (1:1) and water. They were placed in a shaking incubator (1575-2, Shel Lab., USA) at 150 rpm for 24 h at room temperature (28 ± 2°C) and sonicated for 5 min after 12 h. It was filtered with Whatmann No. 41 filter paper and concentrated in rotary evaporator (BUHI Rotavapor R-20, Switzerland) at 40°C. Fully dried extracts were packed in seal-pack containers and stored at −20°C for further experiments.
Chemicals
Aluminum chloride, ammonium molybdate, ascorbic acid (Vitamin-C), caffeic acid, catechin, dibasic sodium phosphate, 1, 1-diphenyl-2-picrylhydrazyl (DPPH), ferric chloride, Folin–Ciocalteu reagent, gallic acid, hydrogen chloride, hydrogen peroxide (H2O2), kaempferol, monobasic sodium phosphate, myrecitin, nitric acid, potassium acetate, potassium ferricyanide, quercetin, rutin sodium carbonate, sodium phosphate, trichloroacetic acid, chloroform, methanol, acetone, and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich chemical co.
DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical scavenging assay
The free radical scavenging potential of different extracts were determined according to the procedure of Kulisic with some modifications [16]. An aliquot of 50 µL of sample solution of various concentrations (25–400 μg/mL) were mixed with 950 µL of methanolic solution of DPPH (3.4 mg/100 mL). The reaction mixture was incubated at 37°C for 1 h in the dark. The free radical scavenging potential of the extracts were expressed as the disappearance of the initial purple color. The absorbance of the reaction mixture was recorded at 517 nm using UV–Visible spectrophotometer (Agilent 8453, Germany). Ascorbic acid was used as the positive control. DPPH scavenging capacity was calculated by using the following formula:
Hydrogen peroxide scavenging assay
The ability of the extract to scavenge hydrogen peroxide (H2O2) was determined according to the method of Ruch et al. [17]. Aliquot of 0.1 mL of extracts (25–400 μg/mL) was transferred into the eppendorf tubes and their volume was made up to 0.4 mL with 50 mM phosphate buffer (pH 7.4) followed by the addition of 0.6 mL of H2O2 solution (2 mM). The reaction mixture was vortexed and after 10 min of reaction time, its absorbance was measured at 230 nm. Ascorbic acid was used as the positive control. The ability of the extracts to scavenge the H2O2 was calculated using the following equation:
where: A0 = Absorbance of control, A1 = Absorbance of sample.
Phosphomolybdenum assay
For the conduction of the phosphomolybdenum assay, the method of Prieto et al. was followed [18]. An aliquot of 0.1 mL of sample solution of different concentrations (25–400 μg/mL) treated with 1 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were incubated at 95°C in a water bath for 90 min. The samples were cooled to room temperature and their absorbance was recorded at 765 nm. Ascorbic acid was used as the positive control. Antioxidant capacity was estimated by using following equation:
Reducing power assay
The reducing power was determined according to the Oyaizu et al. method with some modifications [19]. Aliquot of 0.2 mL of various concentrations of the extracts (25–400 μg/mL) were mixed separately with 0.5 mL of phosphate buffer (0.2 M, pH 6.6) and 0.5 mL of 1% potassium ferricyanide. The mixture was incubated in a water bath at 50°C for 20 min. After cooling at room temperature, 0.5 mL of 10% trichloroacetic acid was added to it followed by centrifugation at 3,000 rpm for 10 min. Supernatant (0.5 mL) was collected and mixed with 0.5 mL of distilled water. Ferric chloride (0.1 mL of 0.1%) was added to it and the mixture was left at room temperature for 10 min. The absorbance was measured at 700 nm. Ascorbic acid was used as positive control.
Determination of total flavonoid content
The total flavonoid content was determined by the aluminum chloride colorimetric method as described by Chang et al. with some modifications [20]. Aliquot of 0.5 mL of various extracts (1 mg/mL) were mixed with 1.5 mL of methanol, followed by the addition of 0.1 mL of 10% aluminum chloride, 0.1 mL of potassium acetate (1 M) and 2.8 mL of distilled water. The reaction mixture was kept at room temperature for 30 min. Absorbance of the reaction mixture was recorded at 415 nm. The calibration curve (0–8 µg/mL) was plotted using rutin as a standard. The total flavonoids were expressed as mg of rutin equivalent/gram dry weight.
Determination of total phenolic content
The amount of total phenolic content was determined according to the Velioglu method using the Folin–Ciocalteu reagent [21]. Aliquot of 0.1 mL of various extracts (4 mg/mL) was mixed with 0.75 mL of Folin–Ciocalteu reagent (10-fold diluted with dH2O). The mixture was kept at room temperature for 5 min and 0.75 mL of 6% sodium carbonate was added. After 90 min of reaction, its absorbance was recorded at 725 nm. The standard calibration (0–25 μg/mL) curve was plotted using gallic acid. The total phenolics were expressed as mg gallic acid equivalent/gram dry weight. Negative control was prepared by adding 0.1 mL of DMSO instead of extract.
High performance liquid chromatography analysis
For the analysis of flavonoids and phenolics, stock solutions of caffeic acid, catechin, kaempferol, myricetin, rutin, quercetin and gallic acid were prepared in methanol (1 mg/mL). Solutions were filtered by 0.2 µm Sartolon Polyamide membrane filter (Sartorius). The calibration curve was raised by 10, 20, 50, 100, 150 and 200 µg/mL. The crude extracts of R. arvensis were prepared at concentration of 10 mg/mL in methanol. The extracts were dissolved in methanol with the aid of sonication and were filtered through 0.2 µm Sartolon Polyamide membrane filter (Sartorius). All the samples were prepared fresh and used for analysis immediately.
The analysis was carried out by using Agilent Chem. station Rev.B.02-01-SR1(260) software and Agilent 1200 series binary gradient pump coupled with a diode array detector (DAD; Agilent technologies, Germany) having Discovery-C18 analytical column (4.6 × 250 mm, 5 µm particle size, Supelco, USA). Method followed was as described by Zu et al. with slight modification according to the system suitability [22]. Briefly, mobile phase-A was methanol:acetonitrile:water:aectic acid (10:5:85:1) and mobile phase B was methanol:acetonitrile:acetic acid (60:40:1). A gradient of time 0–20 min for 0–50% B, 20–25 min 50–100% B and then isocratic 100% B till 30 min was used. Flow rate was 1 mL/min and injection volume was 20 µL. Rutin and gallic acid were analyzed at 257 nm, catechin at 279 nm, caffeic acid at 325 nm and quercetin, myricetin, kampferol was analyzed at 368 nm. Each time the column was preconditioned for 10 min before the next analysis.
Statistical analysis
Results were expressed as mean ± standard deviation of three replicates. CoStat statistical program 6.400® (2008©, USA) was used for statistical analysis. Analysis of variance (ANOVA) was performed through Bartlett’s Test. Latin square design (LSD) was applied to testify the significance of concentrations and extracts.
Results and discussion
DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical scavenging assay
The antioxidant activity of different extracts of R. arvensis was primarily assessed by 2, 2-diphenyl-1-picrylhydrazyl (DPPH), which is based on the ability of DPPH to react with proton donors such as phenols. The other members of family Ranunculaceae were previously assessed for free radical scavenging by many groups. However, R. arvensis free radical scavenging ability remains unknown. We showed that R. arvensis exhibits significant free radical scavenging potential especially its methanol extract (IC50: 34.71 µg/mL; Table 1). The percentages of free radical scavenging are given in Figure 1. The DPPH activity demonstrated in Nigella sativa was EC50 (29.40 ± 0.35) [23], while IC50 values of chloroform extract and ethyl acetate extract were 106.56 and 121.62 μg/mL, respectively [24]. Zengin et al. reported IC50 value of crude extract of Centaurea urvillei was 137.06 μg/mL [25]. These results show that R. arvensis is a good source for DPPH free radical scavenging activity as compared to the other members of the family.
Table 1.
IC50 values of various extracts of R. arvensis
| Antioxidant assays | |||
|---|---|---|---|
| IC50 (µg/mL) | |||
| Extract | DPPH free radical scavenging assay | Hydrogen peroxide scavenging assay | Phosphomolybdenum assay |
| Chloroform extract | 330.29 ± 0.01 | 124.36 ± 0.01 | 52.58 ± 0.01 |
| Chloroform:methaol extract | 186.28 ± 0.01 | 101.6 ± 0.01 | 69.39 ± 0.03 |
| Methanol extract | 34.71 ± 0.02 | 65.73 ± 0.01 | 66.06 ± 0.01 |
| Methanol:acetone extract | 285.28 ± 0.01 | 134.68 ± 0.01 | 63.09 ± 0.01 |
| Acetone extract | 264.08 ± 0.01 | 69.55 ± 0.01 | 56.29 ± 0.01 |
| Methanol:water extract | 47.61 ± 0.02 | 43.53 ± 0.02 | 77.95 ± 0.01 |
| Water extract | 85.11 ± 0.02 | 51.27 ± 0.01 | 74.37 ± 0.01 |
| Ascorbic acida | 6.38 ± 0.01 | 39.05 ± 0.01 | 26.16 ± 0.01 |
| LSD value | 8.14 | 6.30 | 9.90 |
| CV | 12.01% | 8.49% | 12.72% |
| R2 | 0.96 | 0.97 | 0.96 |
p < 0.05.
LSD least significant difference, CV coefficient of variation, LD 50 lethal dose, 50%, IC 50 half maximal inhibitory concentration.
aPositive control values are expressed as ascorbic acid (AA) (average ± SD; n = 4).
Figure 1.
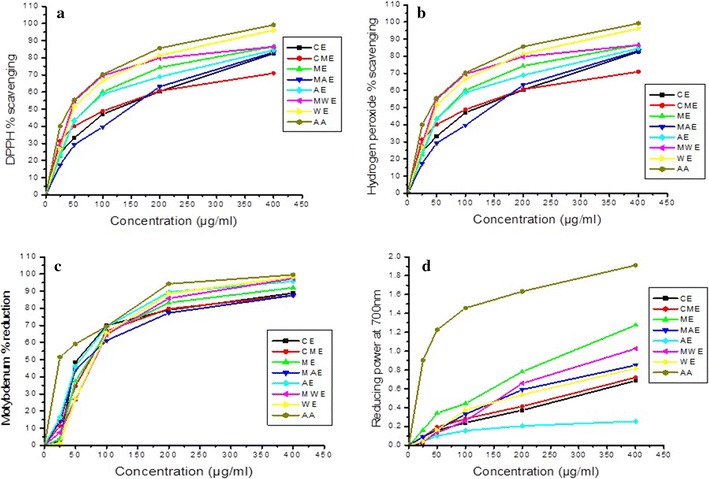
Antioxidant activities of R. arvensis extracts: a DPPH free radical scavenging activity; b hydrogen peroxide scavenging activity; c motybdenum ion percentage reduction; d reducing capacity.
Hydrogen peroxide scavenging assay
The scavenging effect of different extracts of R. arvensis on hydrogen peroxide was concentration-dependent (25–400 μg/mL) as shown in Figure 1 (P < 0.05). The methanol:water extract displayed strong H2O2 scavenging activity (IC50 43.53 µg/mL). whereas water extract exhibited IC50 51.27 µg/mL (Table 1). The significant difference in percentage inhibition of H2O2 of all extracts was compromising in Figure 1P < 0.05. Among various plants of the Ranunculaceae, Gymnema sylvestre exhibited better H2O2 scavenging activity (IC50 72.55 μg/mL) but comparatively less than R. arvensis [26]. Moreover, Spondias pinnata plant extract acquires IC50 44.74 ± 25.61 mg/mL on the scavenging of H2O2 [27]. The naturally occurring of H2O2 in the air, water, human body, plants, microorganisms and food is at low concentration levels. It is quickly decomposed into oxygen (O2) and water (H2O) and may create hydroxyl radicals (OH) that can initiate lipid peroxidation and cause DNA damage. methanol:water extract of R. arvensis capably scavenged hydrogen peroxide which may be attributed to the presence of phenolic groups that could donate electrons to hydrogen peroxide, thereby neutralizing it into H2O.
Phosphomolybdenum assay
This assay is based on the reduction of MoVI into MoV by a reductant with the formation of a green phosphate–MoV complex, which shows an absorbance maximum at 695 nm. The antioxidant activity of almost all extracts was not significantly different. Chloroform extract showed best total antioxidant capacity i.e. IC50 value was 52.58 µg/mL (Table 1). Other extracts also exhibited better IC50 value and molybdenum ions percentage reduction at P < 0.05 (Figure 1). The total antioxidant capacity of Centaurea urvillei with ascorbic acid (39.70 mg AE/g extract) and trolox equivalents (143.53 mg TE/g extract) [25]. Nigella sativa also expressed better activity in this assay (TEAC 36.38 ± 1.08) [23]. However, these findings are not comparable due to difference in solvents, measuring techniques and growth conditions.
Reducing power assay
In this assay, the presence of e−-donating compounds resulted in the reduction of Fe3+ (ferricyanide) into Fe2+ (ferrous). The results are shown in the Figure 1. The reducing potential of the extracts measured for the concentration up to 400 μg/mL showed a general increase in activity when the concentration was increased. Among the tested extracts, the methanol:water extract possessed the highest reducing capacity of free radicals scavenging (1.28 ± 0.05), with absorbance at 700 nm. The extracts had better free radical reductive ability with increasing concentrations of the extract. Hazra et al. [27] reported the same behavior in Spondias pinnata extracts. This concentration-dependent activity pattern was also followed by Consolida orientalis extracts which behaved the best at 800 μg/mL [26].
Determination of total flavonoids content
Quantitative total flavonoid determination was performed by precipitating the extracts with aluminum chloride have an intense yellow fluorescence when observed by UV spectrophotometer. Total flavonoids content were expressed as mg rutin equivalent (RE) per gram dry extract weight. Among the studied R. arvensis extracts, total flavonoid contents estimation revealed the presence of flavonoids, except in the chloroform extract. Significant amount of flavonoids were present in the methanol extract (6.00 ± 0.02 mg RE/g; Table 2), while comparative amount was present in methanol:water extract (5.72 ± 0.01 mg RE/g) and in the water extract (2.19 ± 0.01 mg RE/g). Previous study has shown that flavonoids were present in R. arvensis by the change of sample colour [28]. Hussain et al. used the titration method for identification of flavonoids in R. arvensis (1.769 mg/100 g) [29]. This difference may be due to different geographical distribution of the plant or changes in methodology.
Table 2.
Identification and quantification of flavonoids and phenolics of seven crude extracts of R. arvensis through spectrophotometry and high performance liquid chromatography
| Total flavonoids and total phenolics ± SD | HPLC profile | |||||
|---|---|---|---|---|---|---|
| Extract | mg RE/g dry extract | GAE/g dry extract | RT (min) | λ max (nm) | Compound | % of dry weight |
| Chloroform extract | – | – | – | – | – | |
| Chloroform:methaol extract | 1.95 ± 0.01 | – | – | – | – | |
| Methanol extract | 6.00 ± 0.02 | 0.48 ± 0.03 | 15.00 | 257 | Rutin | 0.44 |
| – | – | 10.79 | 325 | Caffeic acid | 0.017 | |
| Methanol:acetone extract | 1.08 ± 0.01 | – | – | – | – | |
| Acetone extract | 0.96 ± 0.01 | – | – | – | – | |
| Methanol:water extract | 5.72 ± 0.01 | 1.06 ± 0.02 | 15.02 | 257 | Rutin | 0.01 |
| Water extract | 2.19 ± 0.01 | 1.43 ± 0.01 | 10.68 | 325 | Caffeic acid | 0.008 |
SD ±standard deviation, RT retention time.
Determination of total phenolics content
The quantitative determination of total phenolic was carried out using Folin–Ciocalteu reagent in terms of gallic acid equivalent. Total phenolic content is expressed as mg gallic acid equivalent per gram dry extract weight. There is variation in total phenolics present in R. arvensis ranging from 0.48 to 1.43 mg of the total GAE/g of extract. The highest amount was shown by water extract (1.43 mg/g GAE), whereas the chloroform extract, chloroform:methanol extract, methanol:acetone extract and acetone extract remained insignificant (Table 2). Our results are more significant than the results of Hachelaf et al. which detected the presence of phenolic acid in R. arvensis by the change of sample color [28]. Hussain et al. found phenolic contents (0.848 mg/100 g) in R. arvensis using titration method [29]; the same work was performed in two other species of Ranunculus, with the highest phenolics were found in the ethyl acetate extract of R. marginatus (131.7 ± 4.2 mg/g GAE) and R. sprunerianus (140.2 ± 5.3 mg/g GAE) [30], which are comparable with our results of R. arvensis.
High performance liquid chromatography analysis
The crude extracts of R. arvensis were assessed via seven standards (caffeic acid, catechin, kaempferol, myricetin, rutin, quercetin and gallic acid) of flavonoids and phenolics to monitor their efficiency. The HPLC profile of methanol extract of R. arvensis showed the presence of rutin (0.44%) and caffeic acids (0.017%). In comparison with methanol extract, smaller amount of rutin (0.01%) in methanol:water extract and caffeic acid (0.008%) in water extract (Figure 2; Table 2). The compounds belonging to classes of flavonoid and phenolics (flavonol glycosides of quercetin, kaempferol, isorhamnetin and their aglycons) were previously identified in another species of Ranunculus, R. sardous [31]. Previous studies showed the presence of quercetin-7-O-glucoside and rutin in R. peltatus extracts [32]. Noor et al. reported many flavonoids and phenolics from R. repens [33]. The presence of rutin in high quantities can be closely related to the lowest values of IC50 obtained for methanol extract in the DPPH assay.
Figure 2.
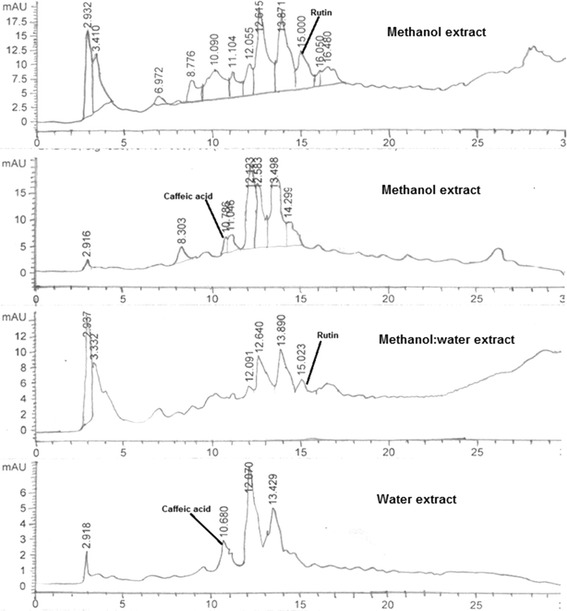
High performance liquid chromatography chromatograms of the flavonoids present in different extracts of R. arvensis.
Conclusion
To the best of our knowledge, this study provides new scientific information about R. arvensis based on the phytochemical analysis, antioxidant potential and HPLC analysis. The various extracts R. arvensis showed different potential of antioxidant activity in variety of antioxidant assays. Quantitative and qualitative analysis of various crude extracts indicated the presence of bioactive compounds as flavonoids and phenolics. Moreover, the above data indicate that, R. arvensis was also rich in rutin and caffeic acid. However, further studies are needed for the isolation of the natural products with fascinating biological and pharmacological properties.
Authors’ contributions
MZB, contributed to the study design, data collection, laboratory work, and writing of the manuscript. AA, AA, AS, participated in data analysis, interpretation and drafting and writing of the manuscript. SAM, participated in supervision and revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are thankful to Higher Education Commission (HEC) of Pakistan for logistical support to conduct this study. We would like to acknowledge Dr. Ihsan-ul-Haq, Department of Pharmacy, Quaid-i-Azam University for his technical assistance in HPLC.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Abbreviations
- CE
chloroform extract
- CME
chloroform:methaol extract
- ME
methanol extract
- MAE
methanol:acetone extract (MAE)
- AE
acetone extract (AE)
- MWE
methanol:water extract
- WE
water extract (WE)
Contributor Information
Muhammad Zeeshan Bhatti, Email: zeshan34@yahoo.com.
Amjad Ali, Email: amjad_486@yahoo.com.
Ayaz Ahmad, Email: ahdayazb5@awkum.edu.pk.
Asma Saeed, Email: asmasaeed78@gmail.com.
Salman Akbar Malik, Email: samalikqau@yahoo.com.
References
- 1.Orak M, Ustundag M, Guloglu C, Tas M, Baylan B. A skin burn associated with Ranunculusarvensis. Ind J Dermatol. 2009;54:19–20. doi: 10.4103/0019-5154.45435. [DOI] [Google Scholar]
- 2.Akbulut S, Semur H, Kose O, Ozhasenekler A, Celiktas M, Basbug M, et al. Phytocontact dermatitis due to Ranunculus arvensis mimicking burn injury: report of three casas and literature review. Intern J Emerg Med. 2011;4:1–5. doi: 10.1186/1865-1380-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabri RL, Nogueira MS, Braga FG, Coimbra ES, Scio E. Mitracarpusfrigidus aerial parts exhibited potent antimicrobial, antileishmanial, and antioxidant effects. Bioresour Technol. 2009;100:428–433. doi: 10.1016/j.biortech.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 4.Kanwal S, Ullah N, Haq IU, Afzal I, Mirza B. Antioxidant, antitumor activities and phytochemical investigation of Hedra neplalensis K. Koch an important medicinal plant from Pakistan. Pak J Bot. 2011;43:85–89. [Google Scholar]
- 5.Boligon AA, Pereira RP, Feltrin AC, Machado MM, Janovik V, Rocha JBT, et al. Antioxidant activities of flavonol derivatives from the leaves and stem bark of Scutia buxifolia Reiss. Bioresour Technol. 2009;100:6592–6598. doi: 10.1016/j.biortech.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 6.Sikder MAA, Rahman MA, Islam MR, Abul KM, Kiasar MA, Rahman MS, et al. In-vitro antioxidant, reducing power, free radical scavenging and membrane stabilizing activities of Spilanthes calva. Bangladesh Pharmaceut J. 2010;13:63–67. [Google Scholar]
- 7.Blazquez S, Olmos E, Hernandez JA, Fernandez-Garcıa N, Fernandez JA, Piqueras A. Somatic embryogenesis in saffron (Crocus sativus L.) Histological differentiation and implication of some components of the antioxidant enzymatic system. Plant Cell Tiss Organ Cult. 2009;97:49–57. doi: 10.1007/s11240-009-9497-y. [DOI] [Google Scholar]
- 8.Zur I, Dubas E, Krzewska M, Janowiak F, Hura K, Pociecha E et al (2014) Antioxidant activity and ROS tolerance in triticale (xTriticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-014-0515-3
- 9.Li L, Yi H. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol Biochem. 2012;58:46–53. doi: 10.1016/j.plaphy.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Hajaji HE, Lachkar N, Alaoui K, Cherrah Y, Farah A, Ennabili A, et al. Antioxidant activity, phytochemical screening, and total phenolic content of extracts from three genders of carob tree barks growing in Morocco. Arabian J Chem. 2011;4:321–324. doi: 10.1016/j.arabjc.2010.06.053. [DOI] [Google Scholar]
- 11.Voravuthikunchaia SP, Kanchanapoomb T, Sawangjaroena N, Towatana NH. Antioxidant, antibacterial and antigiardial activities of Walsura robusta Roxb. Nat Prod Res. 2010;24:813–824. doi: 10.1080/14786410902819152. [DOI] [PubMed] [Google Scholar]
- 12.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Miladi S, Damak M. In-vitro antioxidant activities of Aloe vera leaf skin extracts. J Soc Chim Tunisie. 2008;10:101–109. doi: 10.1186/1476-511X-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velasco P, Francisco M, Moreno DA, Ferreres F, García-Viguera C, Cartea ME. Phytochemical fingerprinting of vegetable Brassicaoleracea and Brassica napus by simultaneous identification of glucosinolates and phenolics. Phytochem Anal. 2011;22:144–152. doi: 10.1002/pca.1259. [DOI] [PubMed] [Google Scholar]
- 15.Shah SMM, Sadiq A, Shah SMH, Ullah F. Antioxidant, total phenolic contents and antinociceptive potential of Teucriumstocksianum methanolic extract in different animal models. BMC Complement Altern Med. 2014;14:181. doi: 10.1186/1472-6882-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]
- 17.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 18.Prieto P, Pineda M, Aguliar M. Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 19.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Japanese J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 20.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 21.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agri Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 22.Zu Y, Li C, Fu Y, Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharmaceut Biomed Anal. 2006;41:714–719. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 23.Kirca A, Arslan E. Antioxidant capacity and total phenolic content of selected plants from Turkey. Intern J Food Sci Technol. 2008;43:2038–2046. doi: 10.1111/j.1365-2621.2008.01818.x. [DOI] [Google Scholar]
- 24.Meziti A, Meziti H, Boudiaf K, Mustapha B, Bouriche H. Polyphenolic profile and antioxidant activities of Nigella Sativa seed extracts in vitro and in vivo. World Acad Sci Eng Technol. 2012;64:24–32. [Google Scholar]
- 25.Zengin G, Aktumsek A, Guler GO, Cakmak YS, Yildiztugay E. Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. Hayekiana Wagenitz. J Nat Prod. 2011;5:123–132. [Google Scholar]
- 26.Shah KA, Patel MD, Parmar PK, Patel RJ. In-vitro evaluation of antioxidant activity of Gymnema Sylvestre. Deccan J Nat Prod. 2010;1:1–7. [Google Scholar]
- 27.Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondiaspinnata. BMC Complement Altern Med. 2008;8:1–10. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hachelaf A, Zellagui A, Touil A, Rhouati S (2013) Chemical composition and analysis antifungal properties of Ranunculusarvensis L. Pharmacophore 4(3):89–91. ISSN 2229-5402 USA CODEN: PHARM7
- 29.Hussain I, Ullah R, Ullah R, Khurram M, Ullah N, Baseer A, et al. Phytochemical analysis of selected medicinal plants. Afr J Biotechnol. 2011;10:7487–7492. [Google Scholar]
- 30.Kaya GI, Somer NU, Konyalioglu S, Yalcin HT, Yavasoglu NUK, Sarikaya B, et al. Antioxidant and antibacterial activities of Ranunculus marginatus var. trachycarpus and R. sprunerianus. Turk. J Biol. 2010;34:139–146. [Google Scholar]
- 31.Campos MG, Webby RF, Markham KR, Mitchell KA, Dacunha AP. Age-induced diminution of free radical scavenging capacity in bee pollens and the contribution of constituent flavonoids. J Agri Food Chem. 2003;51:742–745. doi: 10.1021/jf0206466. [DOI] [PubMed] [Google Scholar]
- 32.Prieto JM, Recio MC, Giner RM, Schinella GR, Manez S, Rios JL. In-vitro and in vivo effects of Ranunculuspeltatus subsp. baudotii methanol extract on models of eicosanoid production and contact dermatitis. Phytother Res. 2008;22:297–302. doi: 10.1002/ptr.2309. [DOI] [PubMed] [Google Scholar]
- 33.Noor W, Gul R, Ali I, Choudary MI. Isolation and antibacterial activity of the compound from Ranunculus repens L. J Chem Soc Pak. 2006;28:271–274. [Google Scholar]


