Abstract
Very long chain fatty acids (VLCFAs) with chain lengths of 20 carbons and longer provide feedstocks for various applications; therefore, improvement of VLCFA contents in seeds has become an important goal for oilseed enhancement. VLCFA biosynthesis is controlled by a multi-enzyme protein complex referred to as fatty acid elongase, which is composed of β-ketoacyl-CoA synthase (KCS), β-ketoacyl-CoA reductase (KCR), β-hydroxyacyl-CoA dehydratase (HCD) and enoyl reductase (ECR). KCS has been identified as the rate-limiting enzyme, but little is known about the involvement of other three enzymes in VLCFA production. Here, the combinatorial effects of fatty acid elongase enzymes on VLCFA production were assessed by evaluating the changes in nervonic acid content. A KCS gene from Lunaria annua (LaKCS) and the other three elongase genes from Arabidopsis thaliana were used for the assessment. Five seed-specific expressing constructs, including LaKCS alone, LaKCS with AtKCR, LaKCS with AtHCD, LaKCS with AtECR, and LaKCS with AtKCR and AtHCD, were transformed into Camelina sativa. The nervonic acid content in seed oil increased from null in wild type camelina to 6-12% in LaKCS-expressing lines. However, compared with that from the LaKCS-expressing lines, nervonic acid content in mature seeds from the co-expressing lines with one or two extra elongase genes did not show further increases. Nervonic acid content from LaKCS, AtKCR and AtHCD co-expressing line was significantly higher than that in LaKCS-expressing line during early seed development stage, while the ultimate nervonic acid content was not significantly altered. The results from this study thus provide useful information for future engineering of oilseed crops for higher VLCFA production.
Introduction
Very long chain fatty acids (VLCFAs) with chain lengths of 20 or more carbons are often modified, derivatized, or linked with other compounds to produce biologically active products that are involved in the biosynthesis of cuticular waxes, seed triacylglycerols and sphingolipids in plants [1]. VLCFAs, such as erucic acid (C22:1Δ13) and nervonic acid (C24:1Δ15), are an important renewable feedstock in plastic, cosmetic, nylon and lubricant industries [2–5]. Nervonic acid is also applied to the treatment of neurological diseases associated with multiple sclerosis, adrenoleukodystrophy and Zellweger syndrome [6–8]. As a natural component of human breast milk, nervonic acid is currently being used for infant formula supplementation [9–11]. As raw materials for industrial, pharmaceutical and nutraceutical applications, effective production of VLCFAs in oilseed, especially nervonic acid, has become an important goal of oilseed crop breeding [12–15].
Nervonic acid has been found only in the seed oils of a few known plants, primarily species of the Brassicaceae and several other family, including Lunaria annua (honesty), Borago officinalis (borage), Cannabis sativa (hemp), Acer truncatum (Purpleblow Maple), Tropaeolum speciosum (flame flower), Cardamine graeca (bittercress) and Malania oleifera [12–14, 16, 17]. Among these species, only Lunaria annua has been considered as a niche crop for future development. However, this plant is a biennial with highly variable seed yields (800–2,000 kg/ha) and the seed shattering problem. Hence, it is uneconomical to use L. annua for nervonic acid production yet [5], and it is highly demanded to improve the production of nervonic acid in other oilseed crops via metabolic engineering technology.
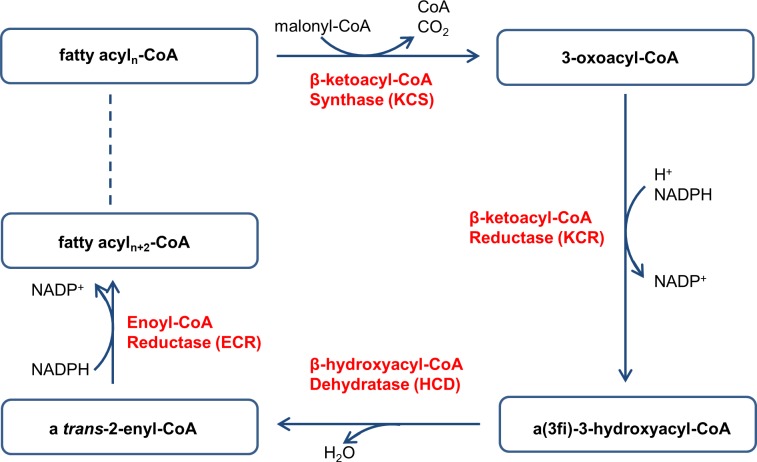
VLCFAs are synthesized in the endoplasmic reticulum (ER) by a membrane-bound fatty acid elongation complex that elongates C16 and C18 fatty acids, which are formed by the cytosolic fatty acid synthase complex. The elongase complex catalyzes the cyclic addition of two carbon units to the acyl chain, which involves four enzymatic reactions: condensation of long-chain acyl-CoA with malonyl-CoA by β-ketoacyl-CoA synthase (KCS) that results in a 3-ketoacyl-CoA, reduction of 3-ketoacyl-CoA to a 3-hydroxyacyl-CoA by β-ketoacyl-CoA reductase (KCR), dehydration of 3-hydroxyacyl-CoA to a 2-enoyl-CoA by β-hydroxyacyl-CoA dehydratase (HCD), and reduction of 2-enoyl-CoA by enoyl reductase (ECR) that generates an elongated acyl-CoA (Fig 1) [18–20]. KCS is considered to be the rate-limiting enzyme in fatty acid elongation, because it determines the substrate and tissue specificities of fatty acid elongation. Furthermore, regulating the expression of KCS affects the ultimate contents of VLCFAs [21–28]. In contrast, the other three enzymes are found to have broad substrate specificity and to be shared by all tissues exhibiting VLCFA biosynthesis [29–32]. KCR, HCD and ECR genes were successively identified and functionally characterized from Arabidopsis thaliana [30–32]. KCR1 (AtKCR) is the only β-ketoacyl-CoA reductase in Arabidopsis. Loss of KCR1 function leads to embryo lethality, while a partial loss of its function is associated with a general reduction of VLCFA contents in seed triacylglycerols, sphingolipids, cuticular waxes and root glycerolipids [30]. HCD, an essential protein for plants, is encoded by the PASTICCINO2 (AtHCD) gene in Arabidopsis. Loss-of-transcript alleles of pas2 are embryo lethal, and the knock-down mutants displayed a strong reduction but not complete absence of VLCFAs [31]. ECERIFERUM10 (AtECR) is identified as a gene coding ECR in Arabidopsis. The absence of ECR activity results in a reduction of cuticular wax load and the VLCFA content of seed triacylglycerols and sphingolipids [32]. However, the effects of the other three enzymes in enhancing VLCFA content have not yet been investigated.
Fig 1. Elongation of Fatty Acids.
Camelina (Camelina sativa) is an emerging Brassicaceae oilseed crop with many favorable agronomic attributes, such as broad distribution, cold and drought tolerance, relatively low fertilizer requirement, short life span (100–120 days) and easy transformation using Agrobacterium-mediated floral dip method [33–36]. In light of these properties, camelina is gaining popularity as an industrial platform for engineering fatty acid oil production [37–42].
The present study was aimed to systematically assess the roles of the other three enzymes of ER fatty acid elongation complex in raising the content of VLCFAs in oilseeds. As it is absent in conventional camelina seed oil, nervonic acid was chosen for the evaluation of the combinatorial effects of fatty acid elongase enzymes. A LaKCS gene from L. annua, which has been identified to be responsible for nervonic acid synthesis [12], and other three genes (AtKCR, AtHCD and AtECR) from Arabidopsis were selected as candidate genes. A previously modified extensive metabolic engineering tool box of seed-specific promoters and selection markers was used to facilitate this study [37, 43]. Nervonic acid content in seeds was analyzed to evaluate the effects of five different combinations on VLCFA production: LaKCS alone, LaKCS with AtKCR, LaKCS with AtHCD, LaKCS with AtECR, LaKCS with AtKCR and AtHCD. This study also attempted to develop camelina lines with a high nervonic acid content for potential pharmaceutical and nutraceutical applications.
Materials and Methods
Plant materials and growth conditions
Camelina (Camelina sativa cv. Sunesson) plants were grown under greenhouse conditions with 14 h day length (24–26°C) and 8 h dark (18–20°C) with natural and supplemental lights at 400–500 μmoles/m2/s as previously described [37].
Vector construction
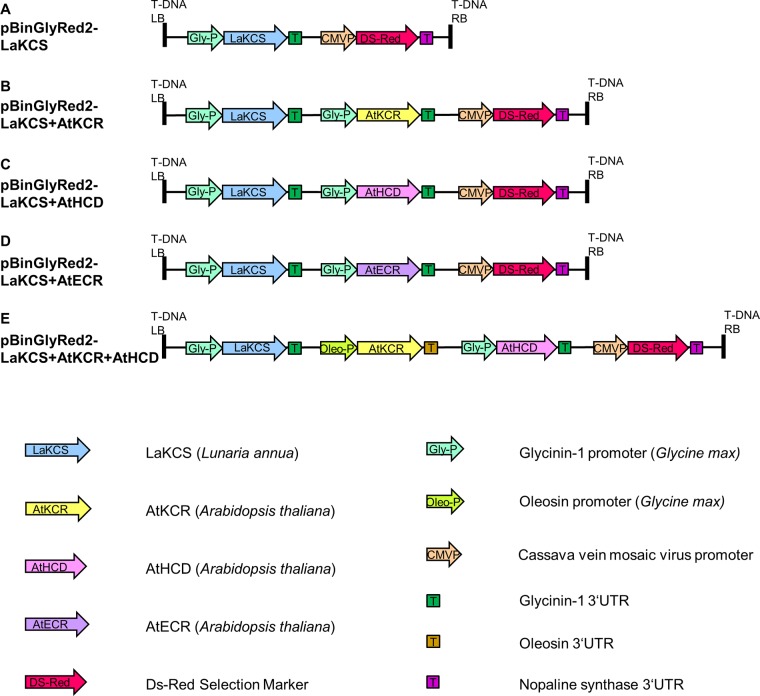
Primers for KCS were designed based on L. annua KCS (EU871787). The LaKCS gene was amplified by PCR from L.annua developing seed cDNA using the following primers with added NotI restriction sites: 5’-CATGGCGGCCGCATGACGTCCATTAACGTAAAG-3’ and 5’-CATGGCGGCCGCTTAGGACCGACCGTTTTGGGC-3’ (the added restriction sites are underlined). The NotI digested fragment was ligated into the vector pKMS3 [38] to place LaKCS under the control of seed-specific soybean glycinin-1 promoter and 3’UTR to create pKMS3-LaKCS. A cassette comprising the glycine-1 promoter and 3’UTR flanking LaKCS gene was excised using AscI and cloned into the binary vector pBinGlyRed2 containing a DsRed marker gene [38] to generate pBinGlyRed2-LaKCS (Fig 2A).
Fig 2. Transgenes used for generating nervonic acids in camelina seeds.
(A). pBinGlyRed2-LaKCS contains seed-specific cassettes for the expression of LaKCS gene. (B). pBinGlyRed2-LaKCS+AtKCR contains seed-specific cassettes for the expression of LaKCS and AtKCR genes. (C). pBinGlyRed2-LaKCS+AtHCD contains seed-specific cassettes for the expression of LaKCS and AtHCD genes. (D). pBinGlyRed2-LaKCS+AtECR contains seed-specific cassettes for the expression of LaKCS and AtECR genes. (E). pBinGlyRed2-LaKCS+AtKCR+AtHCD contains seed-specific cassettes for the expression of LaKCS, AtKCR and AtHCD genes. Selection was accomplished with a DsRed florescence marker. Constitutive and seed-specific promoters, 3’UTR sequences and arrangements of cassettes are also shown.
The Arabidopsis genes, AtKCR (At1g66730), AtHCD (At5g10480) and AtECR (At3g55360), were amplified from a cDNA library prepared from developing seeds using the following primers: 5’-CATGGAATTCATGGAGATCTGCACTTACTTC-3’ (EcoRI) with 5’-CATGCTCGAGTCATTCTTTCTTCATGGAGTC-3’ (XhoI) for AtKCR gene cloning; 5’-CATGGAATTCATGGCGGGCTTTCTCTCCGTT-3’ (EcoRI) with 5’-CATGGTCGACTTATTCCCTCTTGGATTTGGA-3’ (SalI) for AtHCD gene cloning; and 5’-CATGGAATTCATGAAGGTCACCGTCGTCTCC-3’ (EcoRI) with 5’- CATGCTCGAGCTAAAGGAATGGAGGAAGTAT-3’ (XhoI) for AtECR gene cloning. The PCR products of AtKCR and AtECR were digested with EcoRI and XhoI, while the AtHCD PCR product was digested with EcoRI and SalI. The digested fragment of each gene was linked into the corresponding site of pBinGlyRed2 vector, respectively. As a result, each gene was controlled by the seed-specific soybean glycinin-1 promoter and 3’UTR. Based on the inserted gene, the resulting plasmids were designated as pBinGlyRed2-AtKCR, pBinGlyRed2-AtHCD and pBinGlyRed2-AtECR. Then the previously described AscI fragment containing the complete expression cassette of LaKCS was cloned into the corresponding sites of pBinGlyRed2-AtKCR, pBinGlyRed2-AtHCD and pBinGlyRed2-AtECR, respectively. The final vectors were designated as pBinGlyRed2-LaKCS+AtKCR (Fig 2B), pBinGlyRed2-LaKCS+AtHCD (Fig 2C) and pBinGlyRed2-LaKCS+AtECR (Fig 2D).
To generate the vector simultaneously expressing KCS, KCR and HCD, the AtKCR gene was amplified by PCR using the primers with added NotI restriction sites: 5’-CATGGCGGCCGCATGGAGATCTGCACTTACTTC-3’ and 5’-CATGGCGGCCGCTCATTCTTTCTTCATGGAGTC-3’. The NotI digested fragment was ligated into the vector pKMS2 with a strong seed-specific soybean oleosin promoter and 3’UTR and was named as pKMS2-AtKCR. A cassette harboring the oleosin promoter, AtKCR open reading frame and the oleosin 3’UTR was restricted by AscI and cloned into MluI restriction sites of pBinGlyRed2-LaKCS+AtHCD to obtain the final vector pBinGlyRed2-LaKCS+AtKCR+AtHCD (Fig 2E).
Camelina transformation and selection of transformants
Vectors of pBinGlyRed2-LaKCS, pBinGlyRed2-LaKCS+AtKCR, pBinGlyRed2-LaKCS+AtHCD, pBinGlyRed2-LaKCS+AtECR and pBinGlyRed2-LaKCS+AtKCR+AtHCD were transformed into Camelina sativa by the Agrobacterium-mediated floral vacuum infiltration method individually [35]. DsRed-positive seeds were identified using a green LED flashlight with a red camera filter lens [35].
Gas chromatographic analysis of fatty acid compositions
Fatty acid methyl esters (FAMEs) were prepared from 25 mg DsRed positive mature camelina seeds with 2.5% (v/v) sulfuric acid/methanol as previously described [44]. For developing seeds, 20 seeds from an individual plant were collected to be analyzed as a sample, and 3–4 samples were prepared for each transgenic T3 line. FAMEs were analyzed using an Agilent 6890 gas chromatograph with flame ionization detection as previously described [44]. Fatty acids, including nervonic acid (C24:1), were identified by retention time according to previously studies [12, 13].
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from immature seeds at 25 days after flowering (DAF) using a method described by Suzuki et al. [45]. First-strand cDNA was synthesized from 2 μg of total RNA using the Invitrogen ThermoScript RT-PCR system. Primers (S1 Table) were designed using the IDT DNA Real Time PCR primer design tool (http://www.idtdna.com/scitools/Applications/RealTimePCR). Real-time PCRs were performed using paired samples with three technical replicates on a Bio-Rad CFX96 Real-Time system (Bio-Rad, http://www.bio-rad.com) and DBI Bioscience Bestar-Real Time PCR Master Mix kit following the manufacturer’s instructions. The data were analyzed with LINREG as previously described [46]. The experiment was repeated using at least three independent biological replicates, with three technical replicates for each biological sample.
Acyl-CoA profiling
Acyl-CoAs were extracted from pooled and lyophilized dry residues of the developing seeds 25 DAF from transgenic plants and wild-type plants. The samples were prepared and analyzed by electrospray ionization-mass spectrometry as described by Kim et al. [47].
Statistical analysis
For pare-wise comparison, we used t-test. For the comparison of multiple means, the test for statistical significance was performed with ANOVA and Fisher’s least significant difference (LSD) multiple-comparison test using IBM SPSS Statistics software. In all the analyses, only P < 0.05 was considered statistically significant and different probability levels were calculated.
Results
Characterization of transgenic plants
To examine the effects of different combinations of fatty acid elongase enzymes, five constructs respectively harboring (i) only LaKCS, (ii) LaKCS and AtKCR, (iii) LaKCS and AtHCD, (iv) LaKCS and AtECR, and (v) LaKCS, AtKCR and AtHCD were used for transforming camelina plants. At least seven transgenic lines were obtained for each construct. All transgenic camelina plants were first selected by visible DsRed marker and verified by PCR for the respective transgenes. None of the transgenic lines exhibited any growth anomalies, and no apparent penalty on plant vigor or seed yield was observed under greenhouse and growth chamber conditions.
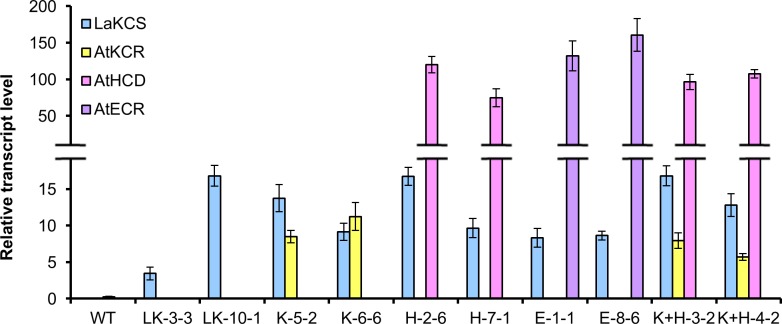
qRT-PCR analyses on the developing seeds of the highest nervonic acid-accumulating transgenic T3 lines were performed to determine the transcripts of the four target transgenes. The expression levels of the four transgenes in the non-transformed control were extremely low and not detectable (Fig 3). All the four target genes were expressed as expected in the transgenic lines (Fig 3).
Fig 3. Relative transcript levels of transgenes in immature seeds from T3 engineered camelina lines and the non-transgenic control (Wt).
LK-3-3 and LK-10-1 are the homozygous T3 lines transformed with only LaKCS. K-5-2 and K-6-6 are the homozygous T3 lines transformed with LaKCS and AtKCR. H-2-6 and H-7-1 are the homozygous T3 lines transformed with LaKCS and AtHCD. E-1-1 and E-8-6 are the homozygous T3 lines transformed with LaKCS and AtECR. K+H-3-2 and K+H-4-2 are the homozygous T3 lines transformed with LaKCS, AtKCR and AtHCD. The relative expression values of genes were measured on developing seeds harvested at 25 days after flowering. CsACTIN7 gene expression level was used as a constitutive control. Values are the means and SE of three independent biological replicates.
Expression of LaKCS significantly increases nervonic acid content in camelina seeds
Eighteen LaKCS-expressing T1 lines were obtained and the fatty acid composition of the DsRed positive seeds from each line was determined. We observed significant differences in fatty acid composition in comparison with the non-transgenic control (S2 Table). Nervonic acid was not detected in the seeds from Wt, while significant accumulation of nervonic acid was found in seeds from LaKCS-expressing lines. Two LaKCS-expressing T1 lines (LK-3 and LK-10) with a higher nervonic acid content were selected for further evaluation (S2 Table). A stable enhancement of nervonic acid and erucic acid content, accompanied with a significant reduction in eicosenoic acid (C20:1Δ11), was observed from T1 to T3 generations (Table 1, S2 and S3 Tables). The maximum nervonic acid content in the seeds from LK-10-1 amounted up to 12.8% in T3 generation, with an average level of 12.0% (Table 1 and S4 Table). A 2- to 3-fold increase in a small proportion of the saturated VLCFAs (C22:0 and C24:0) at the expense of C20:0 was also observed (Table 1).
Table 1. Total fatty acid composition (wt% of total fatty acids) in the seeds of T3 camelina transgenic lines.
| Line | Transformed genes | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | C22:0 | C22:1 | C24:0 | C24:1 | VLCFA* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | None | 7.2±0.6cd | 3.2±0.7a | 11.6±1.2a | 21.2±2.3bc | 34.9±3.2a | 1.9±0.5a | 13.1±05a | 0.4±0.1d | 3.4±0.3c | ND | ND | 18.8±0.7d |
| LK-3-3 | LaKCS | 10.8±0.6a | 3.0±0.4a | 6.5±0.5cd | 29.2±2.2a | 25.2±2.2c | 1.0±0.2b | 3.4±0.2b | 1.5±0.1ab | 10.5±1.3b | 2.0±0.1f | 5.9±0.6d | 24.2±1.5c |
| LK-10-1 | LaKCS | 8.5±0.3b | 1.6±0.2b | 5.3±0.3d | 19.2±1.7bcd | 34.4±1.8a | 0.7±0.1b | 2.2±0.1def | 0.9±0.1c | 11.6±0.3a | 2.8±0.1bc | 12.0±0.7ab | 30.2±0.8ab |
| K-5-2 | LaKCS and AtKCR | 6.6±0.3de | 1.6±0.2b | 8.6±0.8b | 17.9±2.0bcd | 35.3±1.9a | 0.6±0.2b | 2.1±0.1ef | 1.1±0.3bc | 11.2±0.2a | 2.6±0.4bcd | 11.8±0.4ab | 29.4±0.3ab |
| K-6-6 | LaKCS and AtKCR | 6.4±0.3e | 1.6±0.2b | 7.7±0.2bc | 18.9±1.0bcd | 35.5±1.2a | 0.6±0.1b | 2.4±0.5cdef | 1.2±0.2bc | 11.5±0.5a | 2.1±0.1ef | 11.3±1.3bc | 29.0±0.8b |
| H-2-6 | LaKCS and AtHCD | 8.3±0.2b | 2.3±0.2ab | 8.2±0.7bc | 20.3±0.7bcd | 28.9±1.8bc | 0.5±0.1b | 1.8±0.3f | 1.4±0.2abc | 11.5±0.4a | 3.3±0.3a | 12.9±0.7a | 31.5±1.3a |
| H-7-1 | LaKCS and AtHCD | 8.8±0.3b | 2.7±0.4a | 8.8±0.8b | 21.6±0.9b | 28.8±1.0bc | 0.6±0.2b | 2.2±0.3ef | 1.1±0.4bc | 11.2±0.8a | 2.6±0.2bcde | 10.7±0.7bc | 28.3±1.0b |
| E-1-1 | LaKCS and AtECR | 7.4±0.3cd | 2.9±0.6a | 8.6±0.7b | 19.0±1.5bcd | 31.5±2.0ab | 0.7±0.1b | 3.0±0.4bc | 1.1±0.2bc | 11.9±0.6a | 2.3±0.1cdef | 10.0±0.8c | 29.1±0.2b |
| E-8-6 | LaKCS and AtECR | 7.5±0.2c | 2.7±0.3a | 8.5±0.7b | 17.1±2.5cd | 33.8±3.5ab | 0.6±0.1b | 2.9±0.1bcd | 1.3±0.1abc | 12.5±0.2a | 2.1±0.1def | 9.9±0.1c | 29.4±0.3ab |
| H+K-3-2 | LaKCS, AtKCR and AtHCD | 8.5±0.3b | 1.7±0.5b | 7.3±0.6bc | 19.0±0.7bcd | 33.2±1.0ab | 0.7±0.1b | 2.1±0.1ef | 1.7±0.1a | 12.1±0.3a | 3.0±0.4ab | 10.0±0.3c | 29.6±0.8ab |
| H+K-4-2 | LaKCS, AtKCR and AtHCD | 7.4±0.1cd | 2.5±0.1ab | 8.6±0.3b | 16.7±1.4d | 34.9±1.3a | 0.6±0.1b | 2.6±0.3cde | 1.5±0.1ab | 11.4±0.4a | 2.3±0.2cdef | 10.8±0.3bc | 29.2±0.6ab |
ND, not detected.
*VLCFA = C20:0+C20:1+C22:0+C22:1+C24:0+C24:1.
The values shown are analyses of 30 seeds from 3 to 4 independent measurements.
Means±SD followed by different letters in each column are statistically different at P<0.05 based on ANOVA and Fisher’s least significant difference (LSD) multiple-comparison.
The content of C24:1 in seeds of LK-10-1 was set as the standard for evaluation of other gene combinations, even though we only got one line with nervonic acid content was over 10%. Because the transcription level of LaKCS in LK-10-1 was close to that of other transgenic lines, but the transcription level of LaKCS in LK-3-3 was much lower compared with that of other transgenic lines (Fig 3). In addition, if more transformed lines gained, more lines with nervonic acid content over 10% would be obtained.
Co-expression of LaKCS with one of the other three elongase is unable to further increase the nervonic acid content in camelina seeds
To examine the combinatorial effects of LaKCS with the other three enzymes separately, one of the three elongases, i.e. AtKCR, AtHCD and AtECR, were seed-specifically co-expressed with LaKCS gene in camelina, respectively. The fatty acid composition of the DsRed positive seeds from each line was determined.
Nine T1 lines co-expressing LaKCS and AtKCR were acquired. Subsequently, two lines (K-5 and K-6) with higher levels of nervonic acid were analyzed (S2 Table). Stable increases of nervonic acid, erucic acid and VLCFA contents were observed in three successive generations (Table 1, S2 and S3 Tables). K-5-2, a T3 line, showed the highest content of nervonic acid of 12.2% (with an average level of 11.8%), which was not higher than that in LK-10-1 (Table 1 and S4 Table). The same phenomenon was observed in K-6-6 (Table 1). The contents of other VLCFAs in T3 lines (K-5-2 and K-6-6), such as C22:1, C22:0 and C24:0, also did not show further increase compared with LK-10-1 (Table 1). All these results suggest that AtKCR expression in combination with LaKCS does not further enhance the production of nervonic acid and other VLCFAs.
Seven individual transgenic lines harboring LaKCS and AtHCD genes were identified with DsRed seeds. Similarly, two T1 transgenic lines H-2 and H-7 with higher nervonic acid content were chosen for further study (S2 Table). Increases in nervonic acid, erucic acid and VLCFAs in the subsequent generations were observed (Table 1, S2 and S3 Tables). For example, the average nervonic acid content was 12.9% in T3 generation line H-2-6 with a maximum level up to 13.8% (Table 1 and S4 Table). Compared with that of LK-10-1, the nervonic acid contents of H-2-6 and H-7-1 were not significantly higher (Table 1). Content of other VLCFAs including erucic acid and saturated VLCFAs in H-2-6 and H-7-1 were increased to the level of LK-10-1(Table 1). No significant difference in the content of nervonic acid and other VLCFAs was detected among H-2-6, H-7-1 and LK-10-1, suggesting that AtHCD expression also does not promote the production of nervonic acid and other VLCFAs above levels achieved with LaKCS expression alone.
Eight T1 transgenic lines harboring LaKCS and AtECR genes were collected. Two lines (E-1 and E-8) with higher nervonic acid content were selected for further analysis (S2 Table). Increases of nervonic acid, erucic acid and VLCFAs in the succeeding generations were observed (Table 1, S2 and S3 Tables). In T3 generation, the maximum nervonic acid content in the seed oil from line E-1-1 was 10.8% (with an average level of 10.0%). The maximum and average level of nervonic acid content in E-1-1 and E-8-6 were relatively lower than those in LK-10-1 (Table 1 and S4 Table). The contents of other VLCFAs in E-1-1 and E-8-6 were also approximately equal to those in LK-10-1 (Table 1). No significant difference in the contents of nervonic acid and other VLCFAs was observed among E-1-1, E-8-6 and LK-10-1, indicating that AtECR expression has no effect in promoting the production of nervonic acid and other VLCFAs above levels achieved with LaKCS expression alone.
Based on the above results, it can be concluded that two-gene combinations (LaKCS with anyone of the other three genes) cannot enhance the nervonic acid synthesis, as none of the co-expressing lines of two genes showed elevated nervonic acid content in seed oil compared with the elite LaKCS-expressing line LK-10-1.
Co-expression of LaKCS, AtKCR and AtHCD is still unable to further increase the nervonic acid content in camelina seeds
Subsequently, we investigated the effect of a three-gene combination on nervonic acid synthesis. Based on the previous studies on two-gene combinatorial effect, AtKCR and AtHCD were selected for this investigation in addition to LaKCS, as the combination of LaKCS with AtKCR or with AtHCD resulted in a relatively higher content of nervonic acid than the combination of LaKCS with AtECR (Table 1). The construct harboring LaKCS, AtKCR and AtHCD genes was transformed into wild-type camelina. Eight T1 transgenic lines were gained, and two of them (K+H-3 and K+H-4) with higher nervonic acid contents were selected for further evaluation (S2 Table). The contents of nervonic acid, erucic acid and VLCFAs were steadily increased in the next two generations (Table 1, S2 and S3 Tables). In T3 generation, the maximum nervonic acid content was determined as 11.1% in the elite line H+K-4-2 (with an average level of 10.8%) (Table 1 and S4 Table). There was no significant difference in nervonic acid content among H+K-3-2, H+K-4-2 and LK-10-1 (Table 1). In addition, the contents of erucic acid and saturated VLCFAs in K+H-3-2 and K+H-4-2 were also enhanced to the level of LK-10-1 (Table 1). These results demonstrate that the combination of LaKCS with AtKCR and AtHCD together also fails to promote the nervonic acid production.
It is unnecessary to examine the effects of combination of LaKCS with AtKCR and AtECR, combination of LaKCS with AtHCD and AtECR, and combination of all the four genes, because the nervonic acid content in the co-expressing lines of LaKCS and AtECR was relatively lower compared with that of the elite transgenic lines of other two two-gene combinations, and the combination of LaKCS with AtKCR and AtHCD together still failed to further increase the nervonic acid production.
Acyl-CoAs profile of transgenic lines
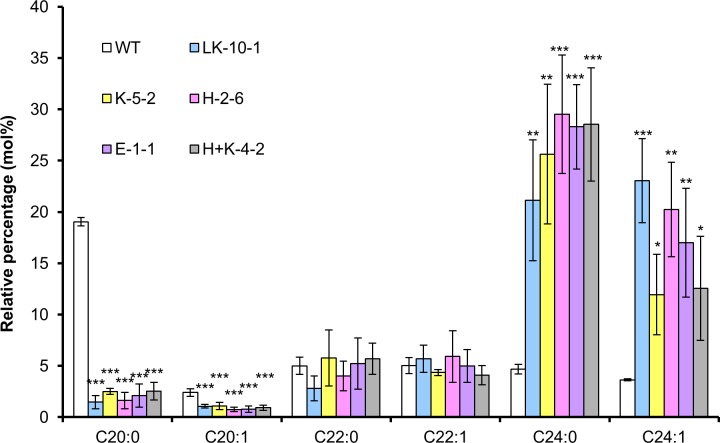
The profile of fatty acyl-CoAs was analyzed from the developing seeds of transgenic T3 lines LK-10-1, K-5-2, H-2-6, E-1-1, H+K-4-2 and non-transformed control lines, as the nervonic acid contents in these transgenic lines were the highest. The contents of C22:0- and C22:1-CoAs were decreased, while the levels of C24:0- and C24:1-CoAs were substantially increased in all the transgenic T3 lines relative to the control (Fig 4). However, the C22:0- and C22:1-CoA contents were not significantly altered in all the transgenic T3 lines compared with in the control lines (Fig 4). These results confirm that the expression of LaKCS changed the contents of VLCFA-CoAs, while the VLCFA-CoAs composition would not be further changed by the co-expression of LaKCS with any one or two of the other fatty acid elongase genes.
Fig 4. Fatty acyl-CoA profiling of non-transgenic control (Wt) and T3 engineered camelina lines.
LK-10-1 is the homozygous T3 line transformed with LaKCS. K-5-2 is the homozygous T3 line transformed with LaKCS and AtKCR. H-2-6 is the homozygous T3 line transformed with LaKCS and AtHCD. E-1-1 is the homozygous T3 line transformed with LaKCS and AtECR. K+H-4-2 is the homozygous T3 line transformed with LaKCS, AtKCR and AtHCD. Data are presented as means with standard error bars SD, which were calculated based on 3–5 independent replicates. Samples from each replicate were measured using 15 mg developing seeds harvested at 25 days after flowering (DAF). Statistical significance for the difference between Wt and each transgenic line was determined by t-test. *, ** and *** designates a significant level at P < 0.05, P < 0.01 and P < 0.001, respectively.
Fatty acid composition in developing seeds of transgenic lines
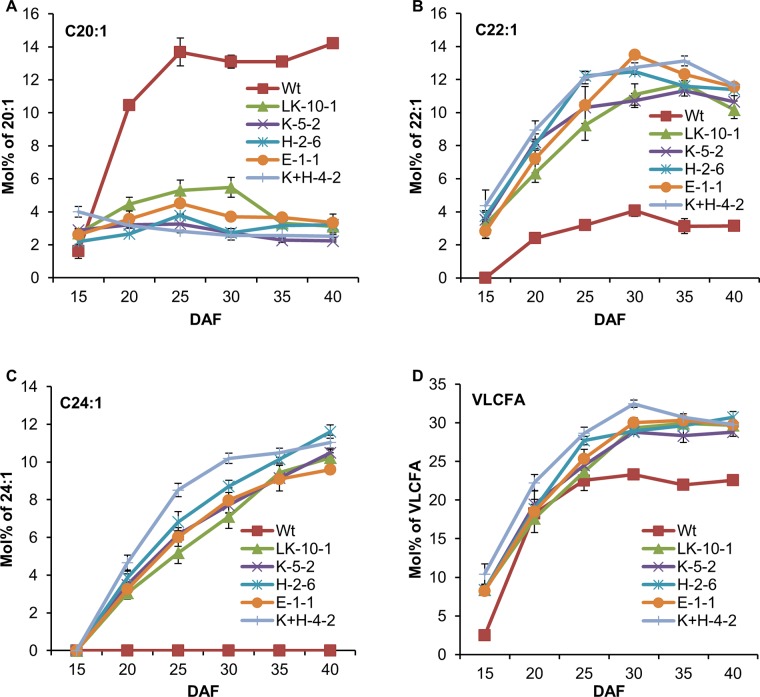
Analysis of fatty acid composition in the developing seeds of non-transformed control (Wt) and transgenic T3 lines was conducted at 15, 20, 25, 30, 35 and 40 DAF. LK-10-1, K-5-2, H-2-6, E-1-1 and H+K-4-2 were selected to represent each combination because they displayed the highest nervonic acid content. The complete analyses are presented in Supporting Information S5 Table. Some of the data are extracted and presented in graphic form in Fig 5.
Fig 5. Fatty acid composition of seeds from non-transformed control (Wt) and T3 engineered camelina lines at different days after flowering (DAF).
(A). C20:1 content; (B). C22:1 content; (C). C24:1 content; (D). VLCFA content. LK-10-1 is the homozygous T3 line transformed with only LaKCS. K-5-2 is the homozygous T3 line transformed with LaKCS and AtKCR. H-2-6 is the homozygous T3 line transformed with LaKCS and AtHCD. E-1-1 is the homozygous T3 line transformed with LaKCS and AtECR. K+H-4-2 is the homozygous T3 line transformed with LaKCS, AtKCR and AtHCD. Data are presented as means with standard error bars SD, which were calculated based on 3–4 independent replicates. Samples from each replicate were measured using 20 developing seeds harvested at 25 days after flowering (DAF). Statistical significance was evaluated by ANOVA and Fisher’s least significant difference (LSD) multiple-comparison. The complete analyses are presented in Supporting Information S5 Table.
The content of C20:1 of control lines was sharply increased during 15–25 DAF, and then was gradually decreased during 25–35 DAF followed by a slight growth in the last 5 days (Fig 5A). However, the content of C20:1 in LK-10-1, K-5-2, H-2-6 and E-1-1 moderately increased during 15–25 DAF, and then gradually decreased during the rest period except for LK-10-1, whose C20:1 content reached the peak at 30 DAF (Fig 5A). Furthermore, the content of C20:1 in H+K-4-2 showed a continuous reduction during the whole detection (Fig 5A). In addition, the contents of C20:1 in all the transgenic lines were around 2–5%, whereas that of the control was as high as 13–14% (S5 Table), indicating that the accumulation of C20:1 in transgenic lines was inhibited due to the continuous elongation of carbon chains by the expression of LaKCS.
The accumulation of C22:1 in all transgenic lines showed the same trend as in the control. During 15–30 DAF, the content of C22:1 dramatically rose to a peak (10–13% in transgenic lines and 4% in control lines), and then slightly decreased during 30–40 DAF (Fig 5B; S5 Table).
The fatty acid C24:1 was not detected during seed development in the control (Fig 5C). The content of C24:1 continuously increased to 9–11% at 40 DAF in all transgenic lines (S5 Table). The C24:1 accumulation rates in K-5-2, H-2-6 and E-1-1 were slightly higher than that in LK-10-1 at some sampling points (Fig 5C; S5 Table). The C24:1 accumulation rate in H+K-4-2 was the highest at all sampling points, particularly during the earlier stages (20–30DAF) (Fig 5C; S5 Table), suggesting that simultaneous expression of LaKCS with AtKCR and AtHCD may significantly accelerated the accumulation of C24:1 at earlier stages of seed development.
The VLCFA content in the control increased during 15–25 DAF, and then plateaued at 22–23% (Fig 5D; S5 Table). Similar patterns were observed in the VLCFA accumulations of all transgenic lines (Fig 5D). The ultimate VLCFA contents of all transgenic lines were between 28% and 30% (S5 Table).
Taken together, the above results showed that the combination of LaKCS with KCR and HCD may accelerate the accumulation of nervonic acid during the seed development, but does not alter the final levels of nervonic acid accumulation.
Discussion
VLCFA biosynthesis is controlled by a multienzyme complex known as fatty acid elongase, which is composed of KCS, KCR, HCD and ECR. Previously, only KCS was over-expressed to increase the VLCFA content, which showed that KCS is a rate-limiting enzyme for its substrate and tissue specificity [23, 28, 29, 48, 49]. However, the impact of the other three genes on VLCFA production is still not clear from these past reports. In this study, we systematically evaluated the combinatorial effects of KCR, HCD and ECR with KCS on VLCFA production.
The LaKCS gene from L. annua has been identified to be responsible for nervonic acid synthesis [12], but the other three component of the elongase complex gene, KCR, HCD and ECR in L. annua have not been identified yet. In contrast, AtKCR, AtHCD and AtECR in Arabidopsis, only have one functional copy for each enzyme and have broad substrate specificity, and different KCSs shared the other three components to form elongase [30–32]. Taken together, we chose to use AtKCR, AtHCD and AtECR with LaKCS to study the combinatorial effects of all the elongase components.
Compared with that in LaKCS-expressing lines, the nervonic acid content in two-gene co-expressing lines was not further increased (Table 1), indicating that the combinations of LaKCS with another elongase gene cannot promote the nervonic acid production. Then AtKCR and AtHCD were co-expressed with LaKCS in camelina seeds, but the nervonic acid content still was not enhanced (Table 1). The combination of LaKCS with AtKCR and AtHCD also failed to promote the nervonic acid production. We did not examine the effects of combination of LaKCS with AtKCR and AtECR, combination of LaKCS with AtHCD and AtECR, and combination of all the four genes, because the nervonic acid content in the top nervonic acid accumulating-co-expression lines of LaKCS and AtECR was relatively lower compared with that of the top nervonic acid accumulating- lines of other two two-gene combinations.
This study further confirms that KCS is the rate-limiting enzyme in the VLCFA synthesis. KCS catalyzes the first step of elongation, and the products of KCS are the precursors of KCR, HCD and ECR. As a result, the amount of ultimate product is determined by the amount of KCS, and is not affected by the amount of KCR, HCD and ECR.
Interestingly, nervonic acid accumulation in the seeds from the transgenic line of co-expressing LaKCS with AtKCR and AtHCD appeared to occur more rapidly during seed development than that in the seeds from LaKCS-expressing lines (Fig 5C). It is likely that overexpression of KCS together with KCR and HCD might result in more efficient successive catalyzes of the intermediate products. However, such a difference in mature seeds is diminished and the final content of nervonic acid in all transgenic plants did not show significant differences. Further studies are required to understand why such a developmental change could happen. In this regard, identification of all the endogenous CsKCS, CsKCR, CsHCD and CsECR genes in camelina shall be the first step for further analysis of the interaction among the transgenes and endogenous genes.
It was noticed that there was a more than ten-fold difference in the transcription levels of the AtKCR gene in K-5-2 and AtHCD in H-2-6 that are under the control of the same Glycinin-1 promoter (Fig 3). In addition, the LaKCS gene and AtHCD genes in transgenic lines of K+H-3-2 and K+H-4-2, which were transformed with the same construct, are separately driven by Glycinin-1 promoter, but the transcription level of AtHCD was much higher than that of LaKCS (Fig 3). On the other hand, the same gene under the control of different promoters may exert similar transcription level. For example, the AtKCR gene is controlled by Glycinin-1 promoter in K-5-2 and by oleosin promoter in H+K-3-2, but the transcription level of them were almost the same (Fig 3). Such differences may be attributed to the properties of the transgenes themselves. Based on the expression data in Arabidopsis e-FP browser [50], the transcription levels of AtHCD and AtECR in Arabidopsis all are approximately 20-fold higher than that of AtKCR during seed development, indicated that AtHCD and AtECR are highly expressing genes while AtKCR is not. Such a property may be related to the mRNA stability and decay rate of individual mRNA molecules, which are influenced by GC% of gene sequence, the sequence elements of DNA or the secondary structure of mRNAs [51–53]. When a highly expressing gene was overexpressed in camelina seeds, they may keep the property to show higher transcription level. In addition, the stability and decay rate of mRNAs may affect the cDNA abundance of different genes [54–55]. As a result, different genes displayed different transcription levels even driven by the same promoter. In camelina, the overall content of nervonic acid was 10–13% in the seeds from the series of transgenic lines with LaKCS (Table 1). Heterologous expression of LaKCS gene in Arabidopsis and Brassica carinata increased the nervonic acid content to 3–5% and 20–30%, respectively [12]. The nervonic acid contents are comparatively lower in seed oil from engineered camelina and Arabidopsis. The content of erucic acid, which is the major precursor of nervonic acid, is low in the seeds from camelina and Arabidopsis. In contrast, in B. carinata, erucic acid substrate is present at higher levels for elongation. The above facts suggest that substrate availability and magnitude can influence the efficiency of chain elongation and eventually the contents of VLCFAs. Similar phenomenon was also observed in other studies, in which another KCS gene from Cardamine graece was ectopically expressed in Arabidopsis, Brassica carinata and Brassica napus [13].
The desired nervonic acid oils for nutraceutical, pharmaceutical and industrial applications are the one with a high content of nervonic acid but a very low content of erucic acid. The content of erucic acid in the oil from the camelina transgenic lines in this study was 10–12%, while the ideal oil should contain no more than 5% of erucic acid and should have a higher content of nervonic acid [13]. Thus, a more suitable balance between the contents of nervonic and erucic acid for commercial applications in camelina awaits further study.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We wish to thank Tara J. Nazarenus for support of camelina transformation and Rebecca E. Cahoon for Acyl-CoAs analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Ministry of Science and Technology of China (grant 2014DFA32210) (http://www.istcp.org.cn/) to YZ, Huazhong Agricultural University Scientific & Technological Self-innovation Foundation (Program No.2015RC010)(http://kjc.hzau.edu.cn/) to EC, China Scholarship Council grant 2010676021 (http://www.csc.edu.cn/) to DH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Haslam TM, Kunst L. Extending the story of very-long-chain fatty acid elongation. Plant Science. 2013;210:93–107. 10.1016/j.plantsci.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 2. Sonntag N. Industrial utilization of long-chain fatty acids and their derivatives. Brassica Oilseeds CAB International, Oxon, UK: 1995:339–352. [Google Scholar]
- 3. Piazza GJ, Foglia TA. Rapeseed oil for oleochemical usage. European journal of lipid science and technology. 2001;103(7):450–454. [Google Scholar]
- 4. Friedrich H, Gruber R, Mees B. Salts of copolymers of ethylenically unsaturated carboxylic acids and ethylenically unsaturated fatty acid derivatives. Google Patents; 1996. [Google Scholar]
- 5. Mastebroek H, Marvin H. Breeding prospects of Lunaria annua L. Industrial Crops and Products. 2000;11(2):139–143. [Google Scholar]
- 6. Sargent J, Coupland K, Wilson R. Nervonic acid and demyelinating disease. Medical hypotheses. 1994;42(4):237–242. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka K, Shimizu T, Ohtsuka Y, Yamashiro Y, Oshida K. Early dietary treatments with Lorenzo’s oil and docosahexaenoic acid for neurological development in a case with Zellweger syndrome. Brain and development. 2007;29(9):586–589. [DOI] [PubMed] [Google Scholar]
- 8. Amminger G, Schäfer M, Klier C, Slavik J, Holzer I, Holub M, et al. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Molecular psychiatry. 2012;17(12):1150–1152. 10.1038/mp.2011.167 [DOI] [PubMed] [Google Scholar]
- 9. Sala-Vila A, Castellote AI, Campoy C, Rivero M, Rodriguez-Palmero M, López-Sabater MC. The source of long-chain PUFA in formula supplements does not affect the fatty acid composition of plasma lipids in full-term infants. The Journal of nutrition. 2004;134(4):868–873. [DOI] [PubMed] [Google Scholar]
- 10. Ntoumani E, Strandvik B, Sabel K. Nervonic acid is much lower in donor milk than in milk from mothers delivering premature infants—Of neglected importance? Prostaglandins, Leukotrienes and Essential Fatty Acids. 2013;89(4):241–244. [DOI] [PubMed] [Google Scholar]
- 11. Dingess K, Valentine C, Davidson B, Peng Y, Guerrero M, Ruiz-Palacios G, et al. Docosahexaenoic acid, nervonic acid and iso-20 (BCFA) concentrations in human milk from the Global Exploration of Human Milk Project (623.15). The FASEB Journal. 2014;28(1 Supplement):623.615. [Google Scholar]
- 12. Guo Y, Mietkiewska E, Francis T, Katavic V, Brost JM, Giblin M, et al. Increase in nervonic acid content in transformed yeast and transgenic plants by introduction of a Lunaria annua L. 3-ketoacyl-CoA synthase (KCS) gene. Plant molecular biology. 2009;69(5):565–575. 10.1007/s11103-008-9439-9 [DOI] [PubMed] [Google Scholar]
- 13. Taylor DC, Francis T, Guo Y, Brost JM, Katavic V, Mietkiewska E, et al. Molecular cloning and characterization of a KCS gene from Cardamine graeca and its heterologous expression in Brassica oilseeds to engineer high nervonic acid oils for potential medical and industrial use. Plant biotechnology journal. 2009;7(9):925–938. 10.1111/j.1467-7652.2009.00454.x [DOI] [PubMed] [Google Scholar]
- 14. Tang T-F, Liu X-M, Ling M, Lai F, Zhang L, Zhou Y-H, et al. Constituents of the essential oil and fatty acid from Malania oleifera . Industrial Crops and Products. 2013;43:1–5. [Google Scholar]
- 15. Umemoto H, Sawada K, Kurata A, Hamaguchi S, Tsukahara S, Ishiguro T, et al. Fermentative Production of Nervonic Acid by Mortierella capitata RD000969. Journal of oleo science. 2014;63(7):671–679. [DOI] [PubMed] [Google Scholar]
- 16.Lange W, Marvin H. Vegetable Oils with Specific Fatty Acids (VOSFA) Agricultural and Industrial Development of Novel Oilseed crops—Final Summary Report. Contract No AIR-CT93-1817. 2000.
- 17. Wang X-Y, WANG S-q. A new resource of nervonic acid: purpleblow maple oil. China Oils and Fats. 2005;9:021. [Google Scholar]
- 18. Fehling E, Mukherjee KD. Acyl-CoA elongase from a higher plant (Lunaria annua): metabolic intermediates of very-long-chain acyl-CoA products and substrate specificity. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism. 1991;1082(3):239–246. [DOI] [PubMed] [Google Scholar]
- 19. Kunst L, Samuels A. Biosynthesis and secretion of plant cuticular wax. Progress in lipid research. 2003;42(1):51–80. [DOI] [PubMed] [Google Scholar]
- 20. P v W-K. Elongases and epicuticular wax biosynthesis. Physiologie Vegetale. 1982;20(4):797–809. [Google Scholar]
- 21. Cahoon EB, Marillia E-F, Stecca KL, Hall SE, Taylor DC, Kinney AJ. Production of fatty acid components of meadowfoam oil in somatic soybean embryos. Plant Physiology. 2000;124(1):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han J, Lühs W, Sonntag K, Zähringer U, Borchardt DS, Wolter FP, et al. Functional characterization of β-ketoacyl-CoA synthase genes from Brassica napus L. Plant molecular biology. 2001;46(2):229–239. [DOI] [PubMed] [Google Scholar]
- 23. James DW, Lim E, Keller J, Plooy I, Ralston E, Dooner HK. Directed tagging of the Arabidopsis FATTY ACID ELONGATION1 (FAE1) gene with the maize transposon activator . The Plant Cell. 1995;7(3):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lassner MW, Lardizabal K, Metz JG. A jojoba beta-Ketoacyl-CoA synthase cDNA complements the canola fatty acid elongation mutation in transgenic plants. The Plant Cell. 1996;8(2):281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mietkiewska E, Brost JM, Giblin EM, Barton DL, Taylor DC. Cloning and functional characterization of the fatty acid elongase 1 (FAE1) gene from high erucic Crambe abyssinica cv. Prophet. Plant biotechnology journal. 2007;5(5):636–645. [DOI] [PubMed] [Google Scholar]
- 26. Mietkiewska E, Brost JM, Giblin EM, Barton DL, Taylor DC. A Teesdalia nudicaulis FAE1 complements the fae1 mutation in transgenic Arabidopsis thaliana plants and shows a preference for elongating oleic acid to eicosenoic acid. Plant science. 2007;173(2):198–205. [Google Scholar]
- 27. Mietkiewska E, Giblin EM, Wang S, Barton DL, Dirpaul J, Brost JM, et al. Seed-specific heterologous expression of a nasturtium FAE gene in Arabidopsis results in a dramatic increase in the proportion of erucic acid. Plant physiology. 2004;136(1):2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Millar AA, Kunst L. Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. The Plant Journal. 1997;12(1):121–131. [DOI] [PubMed] [Google Scholar]
- 29. Jm Joubes, Raffaele S, Bourdenx B, Garcia C, Laroche-Traineau J, Moreau P, et al. The VLCFA elongase gene family in Arabidopsis thaliana: phylogenetic analysis, 3D modelling and expression profiling. Plant molecular biology. 2008;67(5):547–566. 10.1007/s11103-008-9339-z [DOI] [PubMed] [Google Scholar]
- 30. Beaudoin F, Wu X, Li F, Haslam RP, Markham JE, Zheng H, et al. Functional characterization of the Arabidopsis β-ketoacyl-coenzyme A reductase candidates of the fatty acid elongase. Plant physiology. 2009;150(3):1174–1191. 10.1104/pp.109.137497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bach L, Michaelson LV, Haslam R, Bellec Y, Gissot L, Marion J, et al. The very-long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proceedings of the National Academy of Sciences. 2008;105(38):14727–14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng H, Rowland O, Kunst L. Disruptions of the Arabidopsis enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. The Plant Cell. 2005;17(5):1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bramm A, Dambroth M, Schulte-Körne S. Analysis of yield components of linseed, false flax and poppy. Landbauforschung Voelkenrode. 1990;40(2):107–114. [Google Scholar]
- 34. Gugel R, Falk K. Agronomic and seed quality evaluation of Camelina sativa in western Canada. Canadian journal of plant science. 2006;86(4):1047–1058. [Google Scholar]
- 35. Lu C, Kang J. Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant cell reports. 2008;27(2):273–278. [DOI] [PubMed] [Google Scholar]
- 36. Pilgeram AL, Sands DC, Boss D, Dale N, Wichman D, Lamb P, et al. Camelina sativa, a Montana omega-3 and fuel crop. Issues in new crops and new uses. 2007:129–131. [Google Scholar]
- 37. Nguyen HT, Silva JE, Podicheti R, Macrander J, Yang W, Nazarenus TJ, et al. Camelina seed transcriptome: a tool for meal and oil improvement and translational research. Plant biotechnology journal. 2013;11(6):759–769. 10.1111/pbi.12068 [DOI] [PubMed] [Google Scholar]
- 38. Nguyen HT, Park H, Koster KL, Cahoon RE, Nguyen H, Shanklin J, et al. Redirection of metabolic flux for high levels of omega-7 monounsaturated fatty acid accumulation in camelina seeds. Plant biotechnology journal. 2014. [DOI] [PubMed] [Google Scholar]
- 39. Cahoon EB, editor. Development of Camelina as Oilseed Platform for Advanced Metabolic Engineering and Synthetic Biology Annual Meeting and Exhibition 2014; 2014. July 20–24; St. Louis, MO: Simb. [Google Scholar]
- 40. Iskandarov U, Kim HJ, Cahoon EB. Camelina: an emerging oilseed platform for advanced biofuels and bio-based materials Plants and BioEnergy: Springer; 2014. pp. 131–140. [Google Scholar]
- 41. Lu C, Napier JA, Clemente TE, Cahoon EB. New frontiers in oilseed biotechnology: meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications. Current Opinion in Biotechnology. 2011;22(2):252–259. 10.1016/j.copbio.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 42. Carlsson AS, Zhu L-H, Andersson M, Hofvander P. Platform crops amenable to genetic engineering—a requirement for successful production of bio-industrial oils through genetic engineering. Biocatalysis and Agricultural Biotechnology. 2014;3(1):58–64. [Google Scholar]
- 43. Horn PJ, Silva JE, Anderson D, Fuchs J, Borisjuk L, Nazarenus TJ, et al. Imaging heterogeneity of membrane and storage lipids in transgenic Camelina sativa seeds with altered fatty acid profiles. The Plant Journal. 2013;76(1):138–150. 10.1111/tpj.12278 [DOI] [PubMed] [Google Scholar]
- 44. Cahoon EB, Dietrich CR, Meyer K, Damude HG, Dyer JM, Kinney AJ. Conjugated fatty acids accumulate to high levels in phospholipids of metabolically engineered soybean and Arabidopsis seeds. Phytochemistry. 2006;67(12):1166–1176. [DOI] [PubMed] [Google Scholar]
- 45. Suzuki Y, Kawazu T, Koyama H. RNA isolation from siliques, dry seeds, and other tissues of Arabidopsis thaliana . Biotechniques. 2004;37:542–544. [DOI] [PubMed] [Google Scholar]
- 46. Zhang YY, Li BH, Huai DX, Zhou YM, Kliebenstein DJ., The conserved transcription factors, MYB115 and MYB118, control expression of the newly evolved benzoyloxy glucosinolate pathway in Arabidopsis thaliana . Frontiers in Plant Science. 2015;6:343–362. 10.3389/fpls.2015.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim J, Jung JH, Lee SB, Go YS, Kim HJ, Cahoon R, et al. Arabidopsis 3-ketoacyl-coenzyme A synthase9 is involved in the synthesis of tetracosanoic acids as precursors of cuticular waxes, suberins, sphingolipids, and phospholipids. Plant physiology. 2013;162(2):567–580. 10.1104/pp.112.210450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cassagne C, Lessire R, Bessoule J, Moreau P, Creach A, Schneider F, et al. Biosynthesis of very long chain fatty acids in higher plants. Progress in lipid research. 1994;33(1):55–69. [DOI] [PubMed] [Google Scholar]
- 49. Katavic V, Friesen W, Barton DL, Gossen KK, Giblin EM, Luciw T, et al. Improving Erucic Acid Content in Rapeseed through Biotechnology: What Can the Arabidopsis FAE1 and the Yeast SLC1-1 Genes Contribute? Crop Science. 2001;41(3):739–747. [Google Scholar]
- 50. Winter D., Vinegar B., Nahal H., Ammar R., Wilson GV., Provart NJ. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE. 2007;2:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gutiérrez RA., MacIntosh GC., Green PJ. Current perspectives on mRNA stability in plants: multiple levels and mechanisms of control. Trends in plant science. 1999;4(11):429–438. [DOI] [PubMed] [Google Scholar]
- 52. Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-sequence determinants of gene expression in Escherichia coli . Science. 2009; 324(5924):255–258. 10.1126/science.1170160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. 10.1016/j.cell.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu Yang, Turner RJ, Switzer RL. Function of RNA secondary structures in transcriptional attenuation of the Bacillus subtilis pyr operon. PNAS. 1996;93(12):14462–14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Katz L., Burge CB. Widespread Selection for Local RNA Secondary Structure in Coding Regions of Bacterial Genes. Genome Research, 2003;13(9):2042–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.