Abstract
Background
Silencing of P16 through methylation and locus deletion is the most frequent early events in carcinogenesis. The aim of this study is to prospectively determine if early P16 methylation is a predictor for oral cancer development.
Methods
Patients (n = 181) with mild or moderate oral epithelial dysplasia (OED) were recruited into the double blind multicentre cohort. P16 methylation was analyzed using the MethyLight assay. Progression of OEDs was monitored for a minimum 3 year follow-up period.
Findings
P16 methylation-informative cases (n = 152) were enrolled in the prospective multicenter cohorts with an ultimate compliance of 96.7%. OED-derived squamous cell carcinomas were observed in 21 patients (14.3%) during the follow-up (median, 41.0 months). The cancer progression rate from the P16 methylation-positive patients was significantly increased when compared to P16 methylation-negative patients [27.1% vs 8.1%; adjusted odds ratio = 4.6; P = 0.006]. When the P16 methylation-positive criteria were used as a biomarker for early prediction of cancer development from OEDs, sensitivity and specificity of 62% and 76% were obtained, respectively.
Interpretation
P16 methylation is unequivocally a marker for determining the malignant potential of OED and there is no need for further research regarding this aspect.
Funding
National Basic Research Programs of China (2011CB504201 and 2015CB553902), Beijing Science and Technology Commission (Z090507017709016), and Beijing Municipal Administration of Hospital (XM201303) to Dajun Deng. The funding agencies have no role in the actual experimental design, patient recruitment, data collection, analysis, interpretation, or writing of this manuscript.
Keywords: P16, Methylation, Oral dysplasia, Transformation, Prospective cohort
Highlight
-
•
P16 is most frequently inactivated through methylation and deletion in many cancers.
-
•
A multicentral prospective study was carried out to test association between P16 methylation and oral cancer development.
-
•
P16 methylation significantly increased risk of malignant transformation of oral epithelial dysplasia in Chinese patients.
1. Introduction
P16 (CDKN2A) is the most frequently deleted locus in cancer genomes and has been studied extensively (Beroukhim et al., 2010, Liu et al., 1995, Hussussian et al., 1994, Kannengiesser et al., 2009). While genetic alterations in P16 do occur, gene methylation is far more prevalent in human cancers such as oral squamous cell carcinoma (OSCC) (Merlo et al., 1995, Serrano et al., 2000, Herman et al., 1995, Gonzalez-Zulueta et al., 1995, Kresty et al., 2002). Studies have also shown that increased P16 methylation correlates with decreased levels of expression and has been linked to the development and prognosis in many cancers (Sun et al., 2004, Belinsky et al., 2006, Luo et al., 2006, Hall et al., 2008, Cao et al., 2009). P16 methylation may also play important roles in carcinogenesis (Yu et al., 2014).
Oral leukoplakia is the most common precancerous condition (Kramer et al., 1978). Studies have shown that 17–25% of oral leukoplakia lesions contain oral epithelial dysplasia (OED), and about 8% will progress to OSCC (Bouquot and Gorlin, 1986, Bouquot and Whitaker, 1994). The P16 inactivation rate in end-stage head and neck carcinomas has been reported to be as high as 70–85% (Cairns et al., 1995, Reed et al., 1996, Riese et al., 1999, Danahey et al., 1999). One study found that the P16 methylation rate (58%) was much higher than the P16 mutation rate (15%) in 28 severe OED lesions (Kresty et al., 2002). P16 methylation is also frequently detected in mild/moderate OED lesions (25–40%) and may be associated with an increased risk of progression (Hall et al., 2008, Cao et al., 2009). However, the feasibility of using P16 methylation to predict malignant progression of OED has not yet been validated in a multicenter study. Therefore, such a study among Chinese patients with OED was carried out using P16 methylation as a clinical classifier. This study provides evidence strongly supporting that P16 methylation could be used as a biomarker for predicting the malignant transformation of OED early in the course of the disease.
2. Methods
2.1. Study Design
181 patients with mild or moderate OED were selected from cases of oral leukoplakia, lichen planus, or chronic discoid erythematosus at Peking University School of Stomatology (Central-A, n = 82), Capital Medical University School of Stomatology (Central-B, n = 68), and Fourth Military Medical University Hospital of Stomatology (Central-C, n = 31) between 2009 and 2011. The biopsy specimens were fixed in neutral buffered formalin, embedded in paraffin, and stained with H&E. The baseline OED lesions were classified as mild, moderate, or severe by at least two senior pathologists using the same criteria from the 2005 WHO Classification System (Gale et al., 2005). The sample size was calculated according to results of our previous single center study (Cao et al., 2009).
All cases involved primary lesions with no prior surgical, LASER, radiation therapy, or chemotherapy treatments. Information on clinical variables, lesion site, cigarette smoking, alcohol use, and past medical history was also collected. Follow-up examination was regularly performed every three months in a double-blind pattern for at least three years as described previously (Cao et al., 2009). If malignant development was observed, an additional examination and re-biopsy were carried out. Patients who did not require a re-biopsy during the follow-up examination period due to an obvious disappearance/regression of the baseline lesion continued to undergo extensive examinations (Fig. 1). The study was approved by the Institutional Review Boards of the Peking University Cancer Hospital, and all patients gave written informed consent. This trial is registered in the U.S. National Institutes of Health Clinical Trials Protocol Registration System in accordance with the criteria outlined by the International Committee of Medical Journal Editors (trial number NCT01695018, available at http://ClinicalTrials.gov).
Fig. 1.

Participant flow diagram.
2.2. Quantification of P16 Methylation Using the 115-bp MethyLight
Genomic DNA from the OED biopsy samples was extracted from the frozen tissues or paraffin blocks (Cao et al., 2009). Genomic DNA samples (2 μg) were treated with 5 M sodium bisulfite for 16 h at 50 °C without desulfonation as described. The proportion of 115-bp methylated fragments from the sense-strand of P16 exon-1 copies was analyzed using the 115-bp MethyLight assay previously established to quantify the P16 methylation level for clinical diagnosis (Zhou et al., 2011), however, uracil DNA glycosylase and dUTP were not added into the reaction mixture. The 115-bp sense-fragment is completely included within P16 exon-1, the most prevalent sequence investigated for P16 methylation. Methylation in this region is not only correlated with inactivation of this gene (Herman et al., 1995), but also associated with prognosis of epithelial dysplasia (Sun et al., 2004, Cao et al., 2009, Zhou et al., 2011). Briefly, the 115-bp methylated fragment in the sense-strand of P16 exon-1 was amplified using forward primer (5′-CgCggtCgtggttagttagt-3′), reverse primer (5′-tacGctcGacGactaCgaaa-3′), and P16-specific probe (6FAM-gttgtttttCgtCgtCggtt-TAMRA). The 25 μl MethyLight reaction mixture contained 12.5 μl Maxima Probe/ROX qPCR Master Mix (2 ×) (#K0233, Thermo Scientific Fermentas Mix), 0.75 μl of 10 μmol/L of each primer (TaKaRa, Beijing), 0.75 μl of 10 μmol/L probe (TaKaRa), and 8 ng of template. An ABI7500 fast thermal cycler was used to perform the PCR reactions using the following thermal conditions: 37 °C for 10 min → 95 °C for 30 min → (95 °C for 15 s → 62 °C for 1 min) × 45 cycles. The fluorescence signal was detected at 62 °C. Samples were run in duplicate, and the average Ct value was calculated. The COL2A1 gene, a CpG island-free gene whose copy number was not effected by methylation status in the MethyLight assay, was used as input reference and amplified with forward primer (5′-tctaacaattataaactccaaccaccaa-3′), reverse primer (5′-gggaagatgggatagaagggaatat-3′), and COL2A1-specific probe (6FAM-ccttcattctaacccaatacctatcccacctctaaa-BHQ1) (Widschwendter et al., 2004). Relative copy number (RCN) of methylated-P16 was calculated according to the formula [2− ΔCt, (ΔCt = Ctmethylated-P16 − CtCOL2A1)]. Genomic DNA samples from the human colon cancer cell line RKO containing completely methylated P16 alleles and P16 unmethylated MGC803 cell xenografts were used as P16 methylation-positive and -negative controls for each experiment, respectively (Cao et al., 2009). These cell lines were tested and authenticated by Beijing Jianlian Genes Technology Co., Ltd before being used in this study as we described previously (Liu et al., 2014).
2.3. Determining P16 Methylation State Definition
In order to steadily detect fluorescence signal for methylated-P16 at the proportion of 1/64 (1.56%), each MethyLight reaction (25 μl) should contain at least 8 ng bisulfite-modified template DNA that would result in the Ct value for the input reference COL2A1 ≤ 29.3. Using less template DNA would lead to false methylation-negative results. Therefore, when the cutoff value for P16 methylation-positive was set at 1/64, only DNA samples with an average COL2A1-Ct value ≤ 29.3 were considered as P16 methylation-informative. All of the frozen samples and 80% of the paraffin embedded samples met the P16 methylation-informative cut-off. When an amplification signal for the methylated-P16 is detected in the informative samples, it is defined as P16 methylation-positive; otherwise, it is considered P16 methylation-negative.
2.4. P16 Immunohistochemical Staining (IHC)
Mouse monoclonal antibody against human P16 protein (ZM-0205, ZYMED, USA) and the 2-step plus poly-HRP anti-mouse IgG detection system (PV-9000, GBI, USA) were used to stain oral mucosal biopsy slides according to the manufactures' protocol. Slides were then counterstained with hematoxylin.
2.5. Statistical Analysis
Results were displayed by constituent ratios of enumeration or ranked data. SPSS 13.0 software was used to perform univariate and multivariate analyses with the Chi Square test and binary logistic regression analysis. Student's t-test was used to analyze age data. All P-values were two-sided, and P < 0.05 was considered statistically significant. Each biopsy at baseline and follow-up analysis was assigned a severity score according to its histopathologic diagnosis: 1 for carcinoma, 2 for severe grade OED, 3 for moderate grade OED, 4 for mild OED, 5 for hyperplasia, and 6 for normal.
3. Results
3.1. Demographics and Clinical Characteristics of the Patients
Of the baseline mild and moderate OED cases, 152 of 181 cases met the criteria to appropriately classify the P16 methylation state using the 115-bp MethyLight analysis (COL2A1-Ct value ≤ 29.3) and 29 cases were classified as non-methylation-informative (Fig. 1). 5 methylation-negative cases were lost during the follow-up period because of changes of contact information. Thus, 147 cases with follow-up information were ultimately enrolled into the final cohort analysis giving an overall compliance of 96.7%. 48 samples were ultimately classified as P16 methylation-positive. The average age of patients with P16 methylation-positive OED was significantly higher than that of the P16 methylation-negative patients (59.3 years vs 54.7 years, Student's t-test, P = 0.011; Table 1). Most patients with a positive smoking (40/43) or drinking history (23/26) were male.
Table 1.
Demographic and clinical characteristics of the patients with oral epithelial dysplasia enrolled into the final follow-up analysis.
| Status of p16 methylation | n | Age (years) |
Sex |
||
|---|---|---|---|---|---|
| Range | Mean ± SD | Male (%) | Female (%) | ||
| Methylation-positive | 48 | 25–78 | 59.3 ± 10.4⁎ | 20 (41.7) | 28 (58.3) |
| Methylation-negative | 99 | 33–77 | 54.7 ± 11.2 | 46 (46.5) | 53 (53.5) |
| (Total) | 147 | 25–78 | 56.2 ± 11.1 | 66 (44.9) | 81 (55.1) |
Methylated group vs. unmethylated group, Student's t-test, P = 0.011.
Malignant transformation of OED to OSCC was observed in 21 of 147 (14.3%) patients during the follow-up (range, 3 to 129 months; median, 41 months) (Supplementary figure). The average baseline age of the patients that underwent malignant progression was similar to that of patients that remained stable (56.5 years vs 56.1 year). The overall cancer rate was significantly higher in females than males (21.0% vs 6.1%, Chi-square test, P < 0.020; Table 2). Similarly, OED lesions of the tongue showed significantly higher rates of cancer progression than those at other sites (23.9% vs 5.3%; P < 0.003). Interestingly, the risk of OSCC development was slightly increased in these patients without smoking and alcohol use history.
Supplementary figure.
Photos of the baseline oral epithelial dysplasia (OED) and oral squamous cell carcinoma (OSCC) from two representative samples. H&E-stained; white bar: 50 μm.
Table 2.
Comparison of P16 methylation-positive rate and malignant transformation of oral epithelial dysplasia between different subgroups with and without P16 methylation (P16 M) in univariate and multivariate analyses.
| Item | All cases |
P16 M-positive cases |
P16 M-negative cases |
Odd ratio in univariate analysis (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | P16 M-positive rate | Cancer rate | n | Cancer cases (%) | n | Cancer cases (%) | |||
| Sex | Male | 66 | 30.3% | 6.1% | 20 | 3 (15.0) | 46 | 1 (2.2) | |
| Female | 81 | 34.6% | 21.0%a,d | 28 | 10 (35.7) | 53 | 7 (13.2) | 3.65 (1.06–12.82) | |
| Age (y) | < 60 | 93 | 29.0% | 14.0% | 27 | 8 (29.6) | 66 | 5 (7.6) | 5.14 (1.31–20.97) |
| ≥ 60 | 54 | 38.9% | 14.8% | 21 | 5 (23.8) | 33 | 3 (9.1) | ||
| Cigarette smoking | Yes | 43 | 30.2% | 7.0% | 13 | 2 (15.4) | 30 | 1 (3.3) | |
| No | 104 | 33.7% | 17.3% | 35 | 11 (31.4) | 69 | 7 (10.1) | 4.06 (1.26–13.36) | |
| Alcohol use | Yes | 26 | 26.9% | 3.8% | 7 | 1 (14.3) | 19 | 0 | |
| No | 121 | 33.9% | 16.5% | 41 | 12 (29.3) | 80 | 8 (10.0) | 3.72 (1.25–11.29) | |
| Baseline grade | Mild | 97 | 32.0% | 16.5% | 31 | 11 (35.5) | 66 | 5 (7.6) | 6.71 (1.85–25.65) |
| Moderate | 50 | 34.0% | 10.0% | 17 | 2 (11.8) | 33 | 3 (9.1) | ||
| Lesion sites | Tongue | 71 | 40.8% | 23.9%b,e | 29 | 11 (37.9) | 42 | 6 (14.3) | 3.67 (1.03–13.52) |
| Others | 76 | 25.5% | 5.3% | 19 | 2 (10.5) | 57 | 2 (3.5) | ||
| Central | A | 69 | 36.2% | 10.1% | 25 | 6 (24.0) | 44 | 1 (2.3) | 13.58 (1.43–320.9) |
| B | 62 | 29.0% | 19.4% | 18 | 6 (33.3) | 44 | 6 (13.6) | ||
| C | 16 | 31.3% | 12.5% | 5 | 1 (20.0) | 11 | 1 (9.1) | ||
| Sample storages | Frozen | 41 | 31.7% | 9.8% | 13 | 4 (30.8) | 28 | 0 | Undefined |
| Paraffin | 106 | 35.7% | 16.0% | 35 | 9 (25.7) | 71 | 8 (11.3) | ||
| (Total) | 147 | 32.7% | 14.3% | 48 | 13 (27.1) | 99 | 8 (8.1) | 4.22 (1.47–12.35)c,f | |
a,b,cDifferences of cancer rates between two subgroups are statistically significant in univariate analysis (Fisher's exact test, P = 0.020, 0.003, 0.002, two sides, respective. d,e,fAdjusted odds ratio: 3.41 (95% CI: 0.66–17.72), 4.75 (1.31–17.16), 3.64 (1.29–10.27), respectively, after sex, age, smoking, alcohol use, lesion site, and OED grade were adjusted in multivariate analysis. The values are presented in the bold letters when difference between two subgroups is statistically significant.
Next, the expression status of P16 in representative OED and OSCC samples was analyzed using the IHC assay (Fig. 2). Results of IHC analysis showed that P16 protein was located in both the cytoplasm and nucleus of epithelial cells in OED lesions, but only in the cytoplasm of OSCC cells. Additionally, a greater quantity of P16-positive staining epithelial cells was observed in the P16 methylation-negative samples than in the P16 methylation-positive samples.
Fig. 2.
P16 immunohistochemical staining images. Photos of the baseline oral epithelial dysplasia (OED) and oral squamous cell carcinoma (OSCC). A, B, and C: OED lesions with strong, weak, and negative P16 staining in the cytoplasm and nucleus of squamous epithelial cells, respectively; D and E: OSCC with extensively and focally moderate staining in the cytoplasm of cancer cells, respectively; A, B, D: P16 methylation-negative; C and E: P16 methylation-positive; F: P16-negative control, an xenograft originated from human colon cancer cell line RKO containing only silenced P16 alleles by methylation; white black bar: 60 μm.
3.2. P16 Methylation Is an Early Predictor for Malignant Progression of OED
The rate of progression of OED to OSCC in the 48 P16 methylation-positive patients was consistently higher than that of 99 P16 methylation-negative patients when analyzing different subgroups including: sex, age, baseline grade, lesion site, center, and specimen storage medium. Multivariate analysis showed that the risk of malignant transformation for P16 methylation-positive OEDs was significantly higher than the negative OEDs after adjustment for age, sex, smoking, alcohol use, lesion site, and OED grade (adjusted odds ratio = 4.28, 95% confidence interval [CI]: 1.42–12.87; P = 0.002) (Table 2). The malignant transformation Kaplan–Meier curve for patients with P16 methylation-positive OED was also significantly different from those with the P16 methylation-negative OED (log-rank test, P = 0.002; Fig. 3).
Fig. 3.
Malignant transformation Kaplan–Meier curves for patients with P16 methylation-positive and negative oral epithelial dysplasia. The log-rank test, P = 0.002.
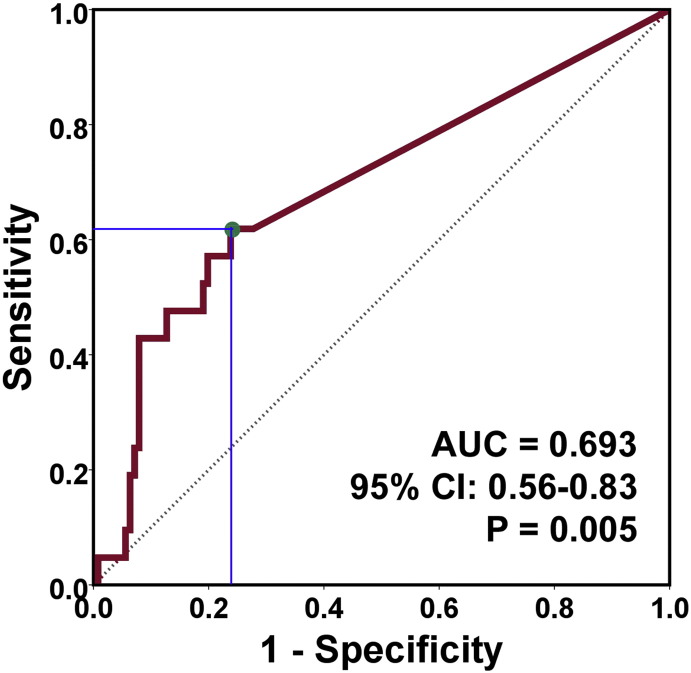
The area under the receiver operating characteristic curve (ROC) is 0.693 (95% CI: 0.56–0.83; P = 0.005; Fig. 4). Using a relative copy number cutoff point of 8.21 × 10− 5, the sensitivity and specificity for early prediction of OED malignant transformation using the P16 methylation-positive classification as a biomarker were 62% (13/21) and 76% (96/126), respectively.
Fig. 4.
ROC curve of prediction of malignant progression of oral epithelial dysplasia by different P16 methylation levels. The area under the curve is 0.693 (95% CI: 0.56-0.83, P = 0.005). Green dot, cutoff point of relative copy number of methylated-P16, 8.21 × 10− 5.
4. Discussion
Early prediction of the malignant potential of OED is crucial for clinical management of patients with the disease. Since methylation changes occurring in only a small number of cells can now be sensitively detected in tissues containing multiple cell types, alterations in promoter methylation may ultimately offer optimal biomarkers for predicting the malignant potential of precancerous lesions (Deng et al., 2010). In this multicentre prospective study, we observed that P16 methylation was a significant, independent predictor of malignant progression of OED with a sensitivity and specificity of 62% and 76%, respectively. This is consistent with the results of two small single-center studies in China and Britain (Hall et al., 2008, Cao et al., 2009). Collectively, these studies indicate that P16 methylation significantly correlates with malignant progression of OED and could be used as a useful biomarker for personalizing the management of patients with this disease.
In regular methylation analysis using MSP and other PCR-based methylation assays, a tested sample is generally considered methylation-informative after a PCR product for the methylated- or unmethylated-fragment of CpG islands is obtained. However, we found that the quantity of input DNA was a crucial factor in accurately determining the level of P16 methylation in paraffin embedded samples when using the MethyLight assay. We found that at least 8 ng of bisulfite-modified, single-stranded DNA in the 25 μl MethyLight reaction mixture was required to generate Ct values for the input reference COL2A1 < 29.3, which allowed the proportion of 1/64 of methylated-P16 to be consistently detected. Therefore, in order to ensure accurate results, only samples with the reference Ct value < 29.3 were considered as P16 methylation-informative. The significance of this finding is that sufficient amounts of input DNA are required for all accurate quantitative methylation analyses.
The proportion of 1/64 (or higher) of methylated-P16 indicates that at least 1.56% of input cells may be malignantly transformed. However, increasing the amount of input DNA could increase the number of samples detected as methylation-positive, especially for paraffin samples containing < 1.56% P16-methylated cells. Therefore, because it is unknown if OEDs containing < 1.56% methylated-P16 have a higher risk of malignant progression when compared to OEDs with no methylated-P16, we could not exclude that a P16 methylation-positive classification for this part of samples could be clinically false methylation-positive.
Although the average age of the P16 methylation-positive patients was higher than that of P16 methylation-negative patients, multivariate analysis revealed that P16 methylation is still significantly associated with malignant transformation of OEDs after adjusting for age. This indicates that P16 methylation is an independent predictor of this disease prognosis.
It is well known that 5-methylcytosine in DNA may further be oxidized into hydroxymethylation, which cannot be distinguished from true methylation using regular methylation assays. We have recently reported hydroxymethylation of P16 CpG islands in cancer cells Qin et al., 2014 and observed that P16-specific hydroxymethylation can reactivate transcription of methylated-P16 alleles (unpublished data; Deng D et al.). It is necessary to further clarify if combined analysis of both true P16 methylation and hydroxymethylation (or other derivatives) might improve clinical performance of the methylation marker. The causality of P16 methylation with OSCC development also requires further study.
An Italian group has reported that there is no eminent benefit to surgical intervention of OED in preventing recurrences and malignant development (Arduino et al., 2009). In our previous study, we described that the risk of cancer progression in OED patients with surgical excision was even slightly higher than those without surgical excision, especially in patients with P16 methylation-positive OED (Cao et al., 2009). Therefore, surgical intervention might need to be avoided in the management for mild and moderate OED patients. Only OED patients without surgical excision were included in the present multicentre prospective study.
Ho M et al. have reported that non-smoking status is a significant predictor for malignant transformation of OED patients in the UK (Ho et al., 2012). In the present study, OED patients with a negative smoking or alcohol use history showed a higher OSCC progression rate than patients with a positive history, although the difference in cancer risk was not significant.
In conclusion, P16 methylation could be used as an additional tool in helping to predict the malignant potential of OED. Combined with other diagnostic and prognostic factors, P16 methylation could help to shape an individualized treatment plan for OED patients. While it is still unknown if these results can be generalized across all populations, detection of P16 methylation should be carried out at least for Chinese OED patients.
The following is the supplementary data related to this article.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.03.015.
Authors' Contribution
HL, XWL, GD, YL, and ZS recruited the patients, collected tissue samples, and did follow-up examinations. YG and XYL made pathological diagnosis. JZ and LG extracted DNA and carried out methylation analysis. DD, HL, and ZS designed the study, got financial support, analyzed the results, and wrote the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mr. James Wilson at Georgia Regents University for English language editing. We also thank Dr. Zhaojun Liu at Peking University Cancer Hospital for her help on statistical analyses.
Contributor Information
Zheng Sun, Email: sunzheng12@vip.sohu.com.
Dajun Deng, Email: dengdajun@bjmu.edu.cn.
References
- Arduino P.G., Surace A., Carbone M. Outcome of oral dysplasia: a retrospective hospital-based study of 207 patients with a long follow-up. J. Oral Path. Med. 2009;38(6):540–544. doi: 10.1111/j.1600-0714.2009.00782.x. [DOI] [PubMed] [Google Scholar]
- Belinsky S.A., Liechty K.C., Gentry F.D. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66(6):3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- Beroukhim R., Mermel C.H., Porter D. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquot J.E., Gorlin R.J. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg. Oral Med. Oral Pathol. 1986;61(4):373–381. doi: 10.1016/0030-4220(86)90422-6. [DOI] [PubMed] [Google Scholar]
- Bouquot J.E., Whitaker S.B. Oral leukoplakia–rationale for diagnosis and prognosis of its clinical subtypes or “phases”. Quintessence Int. 1994;25(2):133–140. [PubMed] [Google Scholar]
- Cairns P., Polascik T.J., Eby Y. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat. Genet. 1995;11(2):210–212. doi: 10.1038/ng1095-210. [DOI] [PubMed] [Google Scholar]
- Cao J., Zhou J., Gao Y. Methylation of p16 CpG island associated with malignant progression of oral epithelial dysplasia: a prospective cohort study. Clin. Cancer Res. 2009;15(16):5178–5183. doi: 10.1158/1078-0432.CCR-09-0580. [DOI] [PubMed] [Google Scholar]
- Danahey D.G., Tobin E.J., Schuller D.E., Bier-Laning C.M., Weghorst C.M., Lang J.C. p16 mutation frequency and clinical correlation in head and neck cancer. Acta Otolaryngol. 1999;119(2):285–288. doi: 10.1080/00016489950181837. [DOI] [PubMed] [Google Scholar]
- Deng D.J., Liu Z.J., Du Y.T. Epigenetic alterations as cancer diagnostic, prognostic, and predictive biomarkers. Epigenet. Cancer Pt B. 2010;71:125–176. doi: 10.1016/B978-0-12-380864-6.00005-5. [DOI] [PubMed] [Google Scholar]
- Gale N., Westra W., Pilchy B.Z. Epithelial precursors lesions. In: Barnes L., Eveson J.W., Reichart P., editors. World Health Organization Classification of Tumors: Pathology and Genetics of Head and Neck Tumors. IARC Press; Lyon: 2005. pp. 177–179. [Google Scholar]
- Gonzalez-Zulueta M., Bender C.M., Yang A.S. Methylation of the 5′ CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995;55(20):4531–4535. [PubMed] [Google Scholar]
- Hall G., Shaw R., Field E. p16 promoter methylation is a potential predictor of malignant transformation in oral epithelial dysplasia. Cancer Epidemiol. Biomarkers Prev. 2008;17(8):2174–2179. doi: 10.1158/1055-9965.EPI-07-2867. [DOI] [PubMed] [Google Scholar]
- Herman J.G., Merlo A., Mao L. Inactivation of the CDKN2/P16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55(20):4525–4530. [PubMed] [Google Scholar]
- Ho M.W., Risk J.M., Woolgar J.A. The clinical determinants of malignant transformation in oral epithelial dysplasia. Oral Oncol. 2012;48(10):969–976. doi: 10.1016/j.oraloncology.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Hussussian C.J., Struewing J.P., Goldstein A.M. Germline p16 mutations in familial melanoma. Nat. Genet. 1994;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Kannengiesser C., Brookes S., del Arroyo A.G. Functional, structural, and genetic evaluation of 20 CDKN2A germ line mutations identified in melanoma-prone families or patients. Hum. Mutat. 2009;30(4):564–574. doi: 10.1002/humu.20845. [DOI] [PubMed] [Google Scholar]
- Kramer I.R., Lucas R.B., Pindborg J.J., Sobin L.H. Definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg. Oral Med. Oral Pathol. 1978;46(4):518–539. [PubMed] [Google Scholar]
- Kresty L.A., Mallery S.R., Knobloch T.J. Alterations of p16(INK4a) and p14(ARF) in patients with severe oral epithelial dysplasia. Cancer Res. 2002;62(18):5295–5300. [PubMed] [Google Scholar]
- Liu L., Lassam N.J., Slingerland J.M. Germline p16INK4A mutation and protein dysfunction in a family with inherited melanoma. Oncogene. 1995;11(2):405–412. [PubMed] [Google Scholar]
- Liu Z.J., Zhang J., Gao Y.H. Large-scale characterization of DNA methylation changes in human gastric carcinomas with and without metastasis. Clin. Cancer Res. 2014;20(17):4598–4612. doi: 10.1158/1078-0432.CCR-13-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D.Y., Zhang B.Z., Lv L.B. Methylation of CpG islands of p16 associated with progression of primary gastric carcinomas. Lab. Invest. 2006;86(6):591–598. doi: 10.1038/labinvest.3700415. [DOI] [PubMed] [Google Scholar]
- Merlo A., Herman J.G., Mao L. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat. Med. 1995;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Qin S., Li Q., Zhou J. Homeostatic maintenance of allele-specific p16 methylation in cancer cells accompanied by dynamic focal methylation and hydroxymethylation. PLoS One. 2014;9(5):e97785. doi: 10.1371/journal.pone.0097785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A.L., Califano J., Cairns P. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56(16):3630–3633. [PubMed] [Google Scholar]
- Riese U., Dahse R., Fiedler W. Tumor suppressor gene p16 (CDKN2A) mutation status and promoter inactivation in head and neck cancer. Int. J. Mol. Med. 1999;4(1):61–65. doi: 10.3892/ijmm.4.1.61. [DOI] [PubMed] [Google Scholar]
- Serrano J., Goebel S.U., Peghini P.L., Lubensky I.A., Gibril F., Jensen R.T. Alterations in the p16INK4a/CDKN2A tumor suppressor gene in gastrinomas. J. Clin. Endocrinol. Metab. 2000;85(11):4146–4156. doi: 10.1210/jcem.85.11.6970. [DOI] [PubMed] [Google Scholar]
- Sun Y., Deng D.J., You W.C. Methylation of p16 CpG islands associated with malignant transformation of gastric dysplasia in a population-based study. Clin. Cancer Res. 2004;10(15):5087–5093. doi: 10.1158/1078-0432.CCR-03-0622. [DOI] [PubMed] [Google Scholar]
- Widschwendter M., Siegmund K.D., Müller H.M. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64(11):3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- Yu D.H., Waterland R.A., Zhang P. Targeted p16(Ink4a) epimutation causes tumorigenesis and reduces survival in mice. J. Clin. Invest. 2014;124(9):3708–3712. doi: 10.1172/JCI76507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Cao J., Lu Z., Liu H., Deng D. A 115-bp MethyLight assay for detection of p16 (CDKN2A) methylation as a diagnostic biomarker in human tissues. BMC Med. Genet. 2011;12:67. doi: 10.1186/1471-2350-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]