Abstract
Background
Endothelial dysfunction, characterized by diminished endothelial progenitor cell (EPC) function and flow-mediated vasodilation (FMD), is a clinically significant feature of heart failure (HF). Mesenchymal stem cells (MSCs), which have pro-angiogenic properties, have the potential to restore endothelial function. Accordingly, we tested the hypothesis that MSCs increase EPC function and restore flow-mediated vasodilation (FMD).
Methods
Idiopathic dilated and ischemic cardiomyopathy patients were randomly assigned to receive autologous (n = 7) or allogeneic (n = 15) MSCs. We assessed EPC-colony forming units (EPC-CFUs), FMD, and circulating levels of vascular endothelial growth factor (VEGF) in patients before and three months after MSC transendocardial injection (n = 22) and in healthy controls (n = 10).
Findings
EPC-colony forming units (CFUs) were markedly reduced in HF compared to healthy controls (4 ± 3 vs. 25 ± 16 CFUs, P < 0.0001). Similarly, FMD% was impaired in HF (5.6 ± 3.2% vs. 9.0 ± 3.3%, P = 0.01). Allogeneic, but not autologous, MSCs improved endothelial function three months after treatment (Δ10 ± 5 vs. Δ1 ± 3 CFUs, P = 0.0067; Δ3.7 ± 3% vs. Δ-0.46 ± 3% FMD, P = 0.005). Patients who received allogeneic MSCs had a reduction in serum VEGF levels three months after treatment, while patients who received autologous MSCs had an increase (P = 0.0012), and these changes correlated with the change in EPC-CFUs (P < 0.0001). Lastly, human umbilical vein endothelial cells (HUVECs) with impaired vasculogenesis due to pharmacologic nitric oxide synthase inhibition, were rescued by allogeneic MSC conditioned medium (P = 0.006).
Interpretation
These findings reveal a novel mechanism whereby allogeneic, but not autologous, MSC administration results in the proliferation of functional EPCs and improvement in vascular reactivity, which in turn restores endothelial function towards normal in patients with HF. These findings have significant clinical and biological implications for the use of MSCs in HF and other disorders associated with endothelial dysfunction.
Keywords: Regenerative medicine, Nitric oxide, Vascular endothelium-dependent relaxation, Vasculogenesis, Autografts
Highlights
-
•
Patients with ischemic and non-ischemic cardiomyopathy have impaired endothelial function
-
•
Allogeneic, but not autologous, mesenchymal stem cells increase endothelial progenitor cell colonies and restore flow-mediated vasodilation
-
•
Allogeneic, but not autologous, mesenchymal stem cells restore VEGF levels towards normal
1. Introduction
Heart failure (HF) remains a leading cause of morbidity and mortality in the United States and worldwide (Go et al., 2014, Bui et al., 2011). The failing circulation is characterized not only by depressed cardiac function, but also by endothelial dysfunction (Blum, 2009). Endothelial dysfunction—defined by impaired flow-mediated vasodilation (FMD) and endothelial progenitor cell (EPC) function—produces increased systemic vascular resistance, which augments stress on the failing heart, and contributes to HF symptomology (Marti et al., 2012). Endothelial dysfunction is also a crucial component of the pathophysiology of numerous cardiovascular (CV) disorders and manifests in patients with CV risk factors such as atherosclerosis, hypertension, and diabetes mellitus (Schulman et al., 2006). Moreover, EPCs regulate the health of the vasculature by incorporating into the endothelium, replacing injured endothelial cells, and secreting angiogenic factors that activate mature endothelial cells (Zampetaki et al., 2008). Notably, HF patients have decreased circulating EPC levels and bioactivity (Schmidt-Lucke et al., 2005).
Mesenchymal stem cells (MSCs), under evaluation as a regenerative therapeutic approach for HF (Hare et al., 2012, Heldman et al., 2014, Karantalis and Hare, in press), have the potential for clinical benefit in CV disease by virtue of their antifibrotic, anti-inflammatory, and pro-angiogenic properties (Williams and Hare, 2011, Cao et al., 2015), and their ability to stimulate endogenous progenitor cells (Hatzistergos et al., 2010, Chen et al., 2008). Given this capacity of MSCs and the role of impaired EPCs in human HF (Werner et al., 2005, Shantsila et al., 2007a), we hypothesized that MSCs would stimulate the bioactivity of circulating EPCs and improve endothelial function in the failing circulation. Accordingly, we tested the hypothesis that MSCs stimulate EPC function and augment vascular relaxation in patients with HF due to either idiopathic dilated cardiomyopathy (DCM) or ischemic cardiomyopathy (ICM). Our results show that allogeneic, but not autologous, MSCs improve EPC bioactivity and endothelial function in HF patients, regardless of the etiology. These findings demonstrate a novel clinical beneficial effect of allogeneic MSCs transplantation in patients with HF and have implications for all disorders associated with endothelial dysfunction.
2. Materials and Methods
2.1. Experimental Design
The objective of this study was to analyze the change in endothelial function measured by EPC-CFUs and FMD% after either allogeneic or autologous MSC administration. Patients enrolled in ongoing clinical trials with both ischemic and non-ischemic cardiomyopathy were recruited to this endothelial trial. Power calculations were performed for both our primary endpoint, FMD%, and our secondary endpoint, EPC-CFUs. In order to study the response from autologous versus allogeneic patients for both endpoints, we needed five autologous and five allogeneic subjects to be able to reject the null hypothesis. Data was collected at baseline and 3 months post-treatment, and treatment group was blinded.
2.2. Study Population
This study entitled “Studying Endothelial Function and Endothelial Progenitor Stem Cells' Colonies Before and After Heart Mesenchymal Stem Cell Transplantation” is a University of Miami Institutional Review Board approved endothelial trial (#20110543). The HF patients recruited for this study are enrolled in POSEIDON-DCM (NCT01392625), “A Phase I/II, Randomized Pilot Study of the Comparative Safety and Efficacy of Transendocardial Injection of Autologous Mesenchymal Stem Cells Versus Allogeneic Mesenchymal Stem Cells in Patients with Nonischemic Dilated Cardiomyopathy” (Mushtaq et al., 2014) and in TRIDENT (NCT02013674), “The Transendocardial Stem Cell Injection Delivery Effects on Neomyogenesis Study”. In PODEIDON-DCM, patients were randomized to receive by transendocardial delivery either 100 million autologous or allogeneic MSCs. Autologous MSCs were derived from the patient's bone marrow (iliac crest aspiration) 4–6 weeks before cardiac catheterization. In the TRIDENT study, ICM patients were randomized to receive either 20 or 100 million allogeneic MSCs transendocardially. Allogeneic MSCs were manufactured by the University of Miami Cell Manufacturing Program (Da Silva and Hare, 2013). Healthy subjects (n = 10) were enrolled ranging in ages from 22–58 years and both genders. All subjects provided written informed consent.
2.3. Cell Characterization
Both autologous and allogeneic MSCs were manufactured by the Foundation for Accreditation of Cellular Therapy (FACT)-accredited Good Manufacturing Practice (GMP) Cell Production Facility at the Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, as previously described (Hare et al., 2012, Da Silva and Hare, 2013). Cells were released for patient administration after meeting the following criteria: negative for mycoplasma via polymerase chain reaction, ≥ 70% cell viability, growth assay via colony forming unit-fibroblasts assay, positive for CD105 (> 80%) and negative for CD45 by flow cytometry, and no growth of bacteria. On average, autologous MSCs were 92.9 ± 3.2% CD105 +/CD45 −, and the allogeneic MSCs were 96.4 ± 0.04% CD105 +/CD45 − (Supplemental Fig. 1).
2.4. Endothelial Colony Forming Units (EPC-CFUs)
Peripheral blood samples were obtained from patients before and three months after MSC injection. EPCs were isolated from samples using Ficoll-Paque and five million cells were seeded on 6-well fibronectin-coated dishes (BD biosciences) in CFU-Hill medium (stem cell technologies, cat#05900) (Hill et al., 2003, Solomon et al., 2012). The non-adherent cells were collected 48 h later and one million cells were seeded on 24-well fibronectin-coated dishes. On day five, EPC-CFUs were counted in five wells and the average was obtained.
2.5. Immunofluorescence
Colonies were directly fixed on fibronectin-coated dishes using 4% PFA. Cells were blocked in 10% normal donkey serum/0.3% Triton X-100/PBS for 1 h and then incubated in anti-CD31 and anti-VEGFR overnight at 4* (DAKO #235218, Cell Signaling #55B1R). Next, cells were incubated in Alexa Flour 564 anti-mouse and Alexa Flour 488 anti-rabbit for 45 min at room temperature. Lastly, wells were cover slipped with Vectashield plus DAPI. Images were obtained using immunofluorescent microscopy.
2.6. Flow-Mediated Vasodilation (FMD)
Brachial artery diameter measurements and FMD% were performed in the morning, after an overnight fast. The subjects' right arm was immobilized in an extended position, and the brachial artery was scanned via ultrasound 5–10 cm above the antecubital fossa (Hill et al., 2003, Corretti et al., 2002). A brachial cuff was then inflated to a supra-systolic pressure (40 to 50 mm Hg above systolic pressure) for 5 min. Subsequently, the cuff was deflated and the brachial artery diameter was recorded for 3 min.
2.7. VEGF ELISA
Serum vascular endothelium growth factor (VEGF) levels (Invitrogen #KHG0111) were measured in DCM patients at baseline and 6 months after allogeneic (n = 6) or autologous (n = 5) MSC treatment. In ICM patients (n = 4), VEGF was measured at baseline and three months after allogeneic MSC treatment. DCM and ICM patients who received allogeneic MSCs were combined and compared to patients who received autologous. Lastly, VEGF was measured in healthy controls (n = 9), which provided written informed consent.
2.8. Matrigel Assays
Human umbilical vein endothelial cells (HUVECs) were grown to passage seven in EGM-2 medium (LONZA). Autologous (n = 7) and allogeneic (n = 5) donor MSCs were grown to 70% confluence and the conditioned medium was obtained. Briefly, MSCs were starved in MEM alpha for 24 h at 5%, the supernatant was collected, centrifuged at 2000 g for 10 min, and stored at − 20° until use. 50,000 HUVECs were plated on Matrigel (BD Biosciences) in 24-well plates and pre-treated with 15 μM L-NAME (Cayman Chemical #80210) dissolved in alpha-MEM (GIBCO) for 45 min. 80% of either MSC conditioned medium (MSC-CM) or plain MEM alpha was added to respective treatment wells, and L-NAME was kept in the medium. After 6 h, six pictures per well were taken and Image J was used to analyze vascular index (tube length × tube number).
2.9. Statistical Analysis
To assess the difference between autologous and allogeneic groups, an unpaired, two-tailed t-test was used. To measure the difference before and after treatment in each group, both a paired, two-tailed t-test and a one-way ANOVA was utilized. Correlations were measured using Pearson correlation, assuming a Gaussian distribution. Data are presented as mean and standard deviation of the mean. Both D'Agostino-Pearson omnibus normality test and Shapiro–Wilk normality tests were run to measure within-group variability on all data (only significant differences were reported as D'Agostino-Pearson). Lastly, differences between groups regarding gender, race/ethnicity, history of smoking, and medications were analyzed using a Fisher exact test.
3. Results
3.1. Baseline Characteristics
A total of 22 patients were analyzed for this study. Allogeneic (n = 15) and autologous (n = 7) MSCs were administered transendocardially. Baseline characteristics of the study subjects are summarized in Table 1. Patients with DCM were evenly distributed for both age and sex (P = NS, ANOVA). Additionally, there was no difference in age between ICM and DCM patients receiving allogeneic MSCs (P = NS, ANOVA); however patients with ICM were older than patients with DCM receiving autologous MSCs (P < 0.01, ANOVA). There were more White/Hispanic patients with DCM compared to all other treatment groups (P = 0.022, Fisher exact test). Additionally, patients with DCM who received allogeneic MSCs had higher cholesterol than patients with ICM who received allogeneic MSCs (P < 0.05, ANOVA). As expected, there was a significant difference between groups regarding coronary artery disease (CAD); specifically, all patients with ICM had CAD (P = 0.0058).
Table 1.
Baseline characteristics of patients (n = 22) and healthy controls (n = 10). Patient data are broken down by etiology and cell treatment: Dilated cardiomyopathy (DCM) patients receiving allogeneic mesenchymal stem cells (MSCs) (n = 9), DCM patients receiving autologous MSCs (n = 7) and ischemic cardiomyopathy (ICM) patients receiving allogeneic MSCs (n = 6). *Asterisks next to P values indicate significant differences between groups. *Asterisks next to values indicate significant difference between noted groups (ANOVA). † indicates that healthy controls were left out of Fisher Exact test.
| Characteristic | DCM allogeneic (n = 9, 27%) | DCM autologous (n = 7, 21%) | ICM allogeneic (n = 6, 18%) | Healthy controls (n = 10, 33%) | P-values |
|---|---|---|---|---|---|
| Age — yr. | |||||
| Median | 60 | 55* | 71* | 44 | *< 0.01 |
| Range | 45–70 | 48–73 | 65–75 | 22–58 | |
| Male sex — no. | 8 (89%) | 6 (86%) | 6 (100%) | 7 (70%) | 0.419 |
| Race/ethnicity | |||||
| White | 8 (89%) | 3 (43%) | 6 (100%) | 7 (70%) | 0.114 |
| White/Hispanic | 0 (0%) | 4 (57%) | 0 (0%) | 3 (30%) | *0.022 |
| Black | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.451 |
| History of CVD | |||||
| CAD | 1 (11%) | 0 (0%) | 6 (100%) | 0 (0%) | **0.0058 |
| History of smoking † | 4 (44%) | 4 (57%) | 4 (67%) | 0 (0%) | 0.689 |
| Blood pressure-mm Hg (systolic/diastolic) | |||||
| Median | 126/77 | 108/65 | 113/70 | 118/67 | 0.979 |
| Range | 108–141/54–91 | 99–142/59–84 | 85–134/53–76 | 111–130/59–90 | |
| Cholesterol (mg/dL) | |||||
| Median | 202* | 175 | 147* | N/A | * < 0.05 |
| Range | 129–282 | 112–207 | 129–178 | N/A | |
| C-reactive protein (mg/mL) | |||||
| Median | 0.3 | 0.3 | |||
| 3 | 0.15 | N/A | 0.923 | ||
| Range | 0.2–0.4 | 0.2–0.5 | 0.1–0.6 | N/A | |
| Medications | |||||
| ASA/NSAIDS † | 5 (56%) | 4 (57%) | 5 (83%) | 0 (0%) | 0.5 |
| Beta-blockers † | 7 (78%) | 7 (100%) | 6 (100%) | 0 (0%) | 0.204 |
| ACE † inhibitors/ARBs | 6 (67%) | 7 (100%) | 6 (100%) | 1 (10%) | 0.166 |
| Diuretics † | 8 (89%) | 6 (86%) | 4 (67%) | 0 (0%) | 0.0814 |
| Statins † | 3 (33%) | 3 (43%) | 6 (100%) | 1 (10%) | *0.03 |
| Antiplatelet † | 4 (44%) | 2 (29%) | 3 (50%) | 0 (0%) | 0.707 |
| Other † | 9 (100%) | 7 (100%) | 6 (100%) | 0 (0%) | NS |
3.2. EPC-Colony Forming Units (CFUs) and Flow-Mediated Vasodilation (FMD) in Heart Failure Patients and Healthy Subjects
Patients with ischemic (n = 6) as well as non-ischemic (n = 16) cardiomyopathy had endothelial dysfunction at baseline, characterized by a reduced ability to form EPC-CFUs and an impaired FMD response. Specifically, patients had decreased EPC-CFU counts compared to healthy controls (4 ± 3 vs. 25 ± 16, respectively, P < 0.0001, t-test; Fig. 1A) as well as diminished FMD% (5.6 ± 3.2 vs. 9.0 ± 3.3, respectively, P = 0.01, t-test; Fig. 1B).
Fig. 1.
Endothelial function in patients with heart failure, including dilated and ischemic cardiomyopathy. (A) Patients (n = 22) have impaired endothelial progenitor cell-colony forming units (EPC-CFUs) compared to healthy controls (n = 10, *P < 0.0001, t test). (B) Patients (n = 22) have reduced flow-mediated vasodilation (FMD%) compared to healthy controls (n = 10, †P = 0. 01, t-test).
Patients were further analyzed by disease etiology. There was no difference in EPC-CFUs or FMD% comparing patients with DCM (n = 16) versus ICM (n = 6) at baseline (3 ± 3 vs. 6 ± 1 CFUs, respectively; 5.9 ± 3.6 vs. 5.1 ± 2.0%, respectively; Figs. S2A and S3A). Additionally, there was no difference at baseline in EPC-CFUs comparing DCM patients who received allogeneic MSCs (n = 9) to DCM patients who received autologous MSCs (n = 7) (3 ± 2 vs. 5 ± 4, respectively; Fig. S2B). However, DCM patients receiving autologous MSCs had a higher FMD% at baseline compared to DCM patients receiving allogeneic MSCs (8.3 ± 3.5 vs. 4 ± 2.4, P = 0.011, t-test; Fig. S3B).
Moreover, we did a sub-analysis of the patients that received allogeneic MSCs. At baseline, patients with ICM (n = 6) had more EPC-CFUs than patients with DCM (n = 9) (6 ± 1 vs. 3 ± 2, P = 0.0011, t-test), and there was no difference in FMD% (5.1 ± 2 vs. 2.4 ± 5.7, P = 0.3742, t-test). Despite ICM patients having more EPC-CFUs at baseline, the improvement from baseline to 3 months post-injection was the same (11 ± 8 vs. 10 ± 4 DCM, P = 0.6195, t-test). Comparably, there was no difference in the change in FMD % from baseline to 3 months post-injection comparing DCM vs. ICM patients that received allogeneic MSCs (3.4 ± 2.86 vs. 4.18 vs. 3.16, P = 0.6280, t-test).
3.3. EPC-CFUs in Heart Failure Patients Treated With MSCs
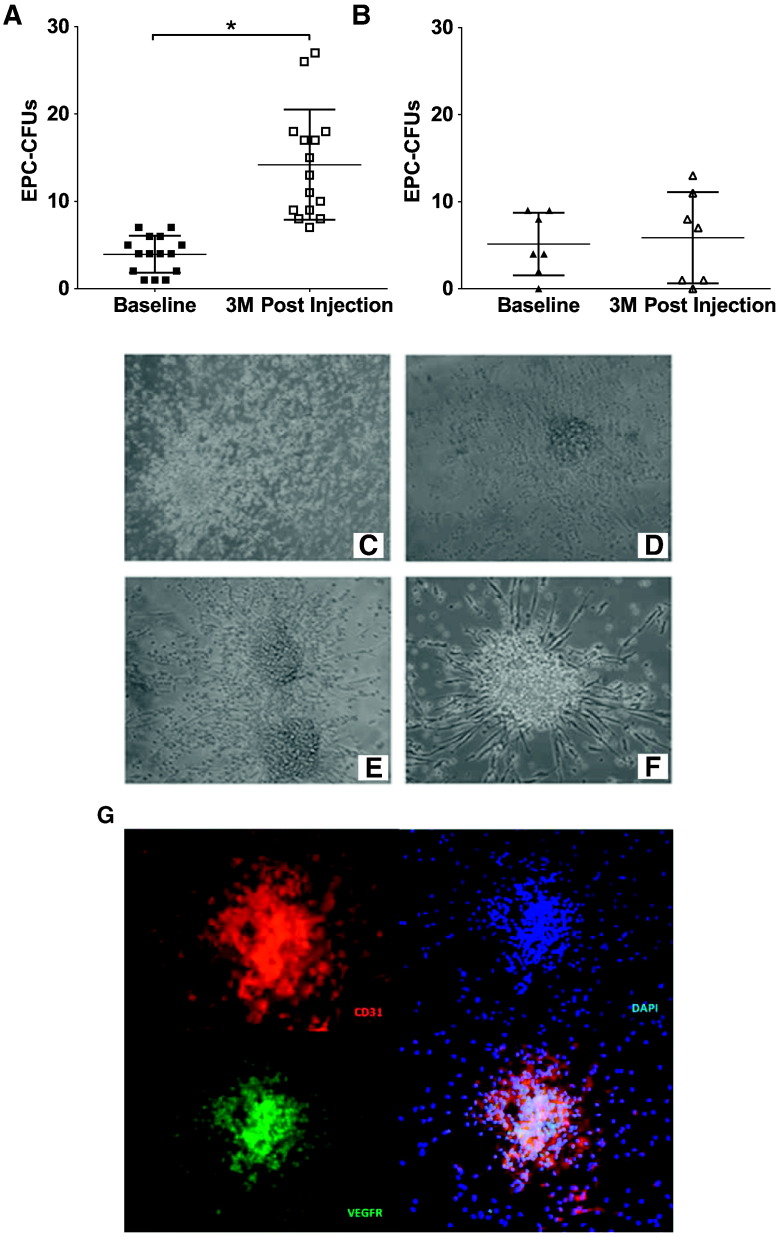
Patients were assessed for EPC-CFUs before and 3 months after MSC treatment. Patients who received allogeneic MSCs had a significant improvement in the number of EPC-CFUs post-treatment (Δ10 ± 5, P < 0.0001, t-test; Fig. 2A). On the other hand, patients who received autologous MSCs showed no improvement (Δ1 ± 3, P = NS, t-test; Fig. 2B). Moreover, we compared allogeneic versus autologous MSC treatment, and allogeneic MSCs were superior in stimulating EPC colony formation (P = 0.0067, t-test).
Fig. 2.
Endothelial colony forming units in heart failure patients treated with either allogeneic or autologous mesenchymal stem cells (MSCs). (A) Patients treated with allogeneic MSCs had a significant improvement in endothelial progenitor cell-colony forming units (EPC-CFUs) 3 months post-treatment (n = 15, *P < 0. 0001, t-test). (B) Patients treated with autologous MSCs had no change in EPC-CFUs post-treatment (n = 7, P = NS, t-test). (C–F) Representative EPC-CFUs plated on fibronectin for 5 days before (C, D) and after (E, F) MSC administration (magnification 20 ×). (G) Colonies are positive for the endothelial cell markers CD31 (red) and VEGFR (green) (magnification 20 ×).
We also compared allogeneic treatment versus autologous treatment in DCM patients. 3 months post-injection, DCM patients who received allogeneic MSCs had significantly higher EPC-CFUs compared to patients who received autologous MSCs (12 ± 4 vs. 6 ± 5, P = 0.015, t-test; Fig. S2C). Accordingly, DCM patients who received allogeneic MSCs had a significantly greater positive change in EPC-CFUs from baseline to 3 months post-injection compared to DCM patients who received autologous MSCs (10 ± 4 vs. 1 ± 3, respectively, P < 0.0001, t-test; Fig. S2D).
EPC-CFUs were examined for morphology. EPCs from HF patients had disorganized and incomplete colony formation (Fig. 2C and D), resulting in clusters that failed to form functional colonies. Three months after allogeneic MSC treatment, patient colonies were organized and healthy in appearance (Fig. 2E and F). Importantly, we found that these colonies were positive for the endothelial cell markers CD31/PECAM and VEGFR (Fig. 2G). Together, these findings suggest that transendocardial MSC therapy stimulates EPC bioactivity in patients with HF of both ischemic and non-ischemic etiology.
3.4. FMD in Heart Failure Patients Treated With MSCs
All patients were evaluated using brachial artery FMD before and 3 months after MSC injection. Patients who received allogeneic MSCs had a dramatic improvement in FMD% (Δ3.7 ± 3%, P = 0.0002, t-test; Fig. 3A). In contrast, patients who received autologous MSCs had no improvement and the majority of patients worsened 3 months post-treatment (Δ-0.46 ± 3%, Fig. 3B).
Fig. 3.
Flow-mediated vasodilation (FMD) measurements before and after mesenchymal stem cell (MSC) treatment. (A) Patients treated with allogeneic MSCs had an increase in FMD% 3 months post-injection (n = 15, *P = 0.0002, t-test). (B) Patients treated with autologous MSCs had no significant difference in FMD% 3 months post-injection (n = 7, P = NS, t-test). (C) There is a strong correlation between the absolute change in FMD% and the absolute change in endothelial progenitor cell-colony forming units (EPC-CFUs) from baseline to 3 months post-MSC injection in all patients (*P = 0.0004, R = 0.68, Pearson correlation).
We next analyzed the difference between treatment with autologous MSCs and allogeneic MSCs. There was a striking difference between the two cell types (P = 0.005, t-test), suggesting that autologous MSCs do not restore endothelial function in this patient population. We also assessed the correlation between EPC-CFUs and FMD% in all patients and found a highly significant correlation between ΔFMD% and ΔEPC-CFUs (P = 0.0004, R = 0.68, Pearson correlation; Fig. 3C).
Lastly, we analyzed DCM patients only, comparing autologous vs. allogeneic treatment. While patients who received allogeneic MSCs had similar FMD% compared to patients who received autologous MSCs 3 months after treatment (7.4 ± 4.7 vs. 7.9±3.1; Fig. S2C), patients who received allogeneic MSCs had a greater change from baseline to 3 months post-injection compared to patients who received autologous MSCs (3.8 ± 2.6 vs. 0.7 ± 2.9, P = 0.042, t-test; Fig. S3D). Notably, the majority of patients receiving autologous MSCs had no improvement or worsened, while all allogeneic patients improved (P = NS vs. P < 0.0001, t-test).
3.5. Vascular Endothelial Growth Factor (VEGF) in Patients Receiving Autologous and Allogeneic MSCs
We measured circulating VEGF levels in patients and healthy control serum. At baseline, patients had profoundly elevated circulating VEGF compared to controls (1130.3 ± 803.3 vs. 2.0 ± 5.9 pg/mL, P = 0.0009, t-test; within-group, P = 0.21, P < 0.001 respectively, D'Agostino-Pearson omnibus normality test; Fig. 4A). Allogeneic MSCs reduced VEGF levels (− 547.5 ± 350.8 pg/mL, P = 0.0015, t-test; within-group P = 0.96, D'Agostino-Pearson omnibus normality test), while patients who received autologous MSCs had an increase in VEGF levels (814.1 ± 875.8 pg/mL, Fig. 4B). Furthermore, there was a significant difference between patients who received allogeneic MSCs versus patients who received autologous MSCs (P = 0.0012, t-test; Fig. 4B).
Fig. 4.
Serum vascular endothelium growth factor (VEGF) concentration in patients and controls. (A) Patients (n = 14) have a higher level of circulating VEGF compared to controls (n = 9) at baseline (*P = 0.0009, t-test; within-group, P = 0.21, P < 0.001, respectively, D'Agostino-Pearson omnibus normality test). (B) Patients who received allogeneic MSCs (n = 9) had a decrease in VEGF serum levels post-injection (Δ-547.5 ± 350.8 pg/mL, †P = 0.0015, t-test; within-group P = 0.96, D'Agostino-Pearson omnibus normality test), while patients who received autologous MSCs (n = 5) had an increase in serum VEGF post-injection (Δ814.1 ± 875.8), and there was a difference between the groups (†P = 0.0012, t-test). (C) There is a correlation between endothelial progenitor cell-colony forming units (EPC-CFUs) and serum VEGF in patients at both baseline and 3 months post-MSC treatment (R = − 0.421, ‡P = 0.026, Pearson correlation). (D) The change in EPC-CFUs from baseline to 3 months post-treatment strongly correlated with the change in VEGF (R = − 0.863,*P < 0.0001, Pearson correlation).
VEGF levels correlated with EPC-CFUs (P = 0.026, R = − 0.421, Pearson correlation; Fig. 4C). Even more striking, there was a significant correlation between the change in VEGF and the change in EPC-CFUs from baseline to 3 months post-treatment (R = 0.863, P < 0.0001, Pearson correlation; Fig. 4D). Notably, high levels of VEGF correlated with low levels of EPCs, evidenced by the autologous group. Conversely, lower levels of VEGF correlated with high levels of EPC-CFUs, illustrated by the allogeneic group. Taken together, these data demonstrate that allogeneic MSCs stimulate EPC mobilization and suppress compensatory elevations in circulating VEGF concentrations.
3.6. Autologous and Allogeneic MSC Paracrine Effect on Endothelial Cells
Human umbilical vein endothelial cells (HUVECs), pre-treated with L-NAME to block endogenous nitric oxide (NO) synthesis, were subsequently treated with autologous or allogeneic MSC-conditioned media (CM), and vasculogenesis was examined in Matrigel assays (Fig. 5A–D). HUVECs treated with L-NAME exhibited severely impaired vasculogenesis (Fig. 5B), evident by their depressed vascular index (305.2 ± 196.8 vs. 1170.9 ± 352.6, P = 0.02, t-test; Fig. 5E). Only the addition of allogeneic MSC-CM prevented the L-NAME-induced impairment in vasculogenesis (vascular index 305.2 ± 196.8 L-NAME alone vs. 1113.4 ± 296.2 L-NAME + Allogeneic MSC-CM, P < 0.05, ANOVA; Fig. 5E). Overall, these results suggest that allogeneic MSCs are able to restore the vascular potential of endothelial cells.
Fig. 5.
Effect of autologous and allogeneic mesenchymal stem cell (MSC) treatment on vasculogenesis. (A–D) Representative pictures of human umbilical vein endothelial cells (HUVECs) alone (n = 3), HUVECs with the nitric oxide synthase inhibitor, L-NG-Nitroarginine methyl ester (L-NAME) (n = 3), HUVECs with L-NAME and allogeneic MSC-conditioned media (CM) (n = 5), and HUVECs with L-NAME and autologous MSC-CM (n = 7) after 6 h on Matrigel (magnification 10 ×). (E) L-NAME greatly reduced vascular index (*P < 0.05, ANOVA), and only allogeneic-CM restored it (*P < 0.05, ANOVA).
4. Discussion
Patients with cardiomyopathy of ischemic or non-ischemic etiology manifest endothelial dysfunction, characterized by reduced EPC colony formation, impaired FMD, and elevated VEGF levels. The major new finding of this study is that MSC administration to these patients stimulates EPC bioactivity and restores flow-mediated vasodilation towards normal. Importantly, the ability of allogeneic MSCs greatly exceeded that of autologous MSCs in restoring endothelial function, enhancing EPC colony formation, and suppressing VEGF levels. Together these findings offer major new clinical insights into the bioactivity of MSCs, suggest a novel therapeutic principle for disorders characterized by endothelial dysfunction, and have implications for the choice of allogeneic vs. autologous MSC cell therapy.
There is an increasing awareness of the central role endothelial dysfunction plays in CV disorders. Low numbers of EPC-CFUs strongly correlate with endothelial dysfunction (Werner et al., 2007) and are associated with a high Framingham risk score for adverse CV health outcomes (Hill et al., 2003). Furthermore, circulating EPC levels predict CV events—specifically in patients with coronary artery disease, heart failure, and angina (Schmidt-Lucke et al., 2005, Hill et al., 2003, Shantsila et al., 2007b). In addition, elevated levels of circulating VEGF are linked to endothelial dysfunction and HF (Chin et al., 2002, Tsai et al., 2005). In this regard, Eleuteri et al. demonstrated that elevated levels of VEGF correlated with HF disease progression (Eleuteri et al., 2011). Moreover, Wei et al. investigated circulating EPCs and VEGF levels in patients with cerebral aneurysm and found that decreased levels of circulating EPCs and increased levels of plasma VEGF were associated with chronic inflammation in the vascular walls of cerebral arteries and the development of cerebrovascular abnormalities leading to aneurysm formation and rupture (Wei et al., 2011). Thus, endothelial dysfunction is a central feature of CV disease, and may represent a powerful surrogate marker in the development of new treatments for CV disease.
MSCs are adult stem cells that are prototypically found in bone marrow and have the capacity to differentiate into multiple cell types (Williams and Hare, 2011). Importantly, they stimulate the proliferation and differentiation of endogenous precursor cells and play a crucial role in maintaining stem cell niches (Williams and Hare, 2011). In addition, MSCs secrete paracrine factors that participate in angiogenesis, cardiomyogenesis, neovascularization, stimulation of other endogenous stem cells, and regulation of the immune system (Gomes et al., 2013, Gnecchi et al., 2008). While MSCs are known to stimulate cardiac precursor cells and cell cycle activity in the heart (Hatzistergos et al., 2010), their role in stimulating other endogenous precursor populations has heretofore been unknown. Here we report that MSCs stimulated endogenous EPC activation, increasing the number and quality of functional EPCs. These findings suggest that augmentation of EPCs may represent a novel mechanism of action by which MSCs exert favorable biological effects.
Over the last decade, there has been an emerging interest in the use of MSCs in CV disorders (Karantalis and Hare, in press, Telukuntla et al., 2013). Clinical trials have demonstrated a major safety profile for MSC administration, and suggested efficacy in patients with HF (Hare et al., 2012, Telukuntla et al., 2013); however, underlying mechanism(s) of action continue to be vigorously debated. Our finding that allogeneic MSC injections in patients with both ischemic and non-ischemic HF results in an improvement in endothelial function, specifically by restoring EPC function and FMD and reducing VEGF levels towards normal, offers a major new insight into the mechanisms of action of MSCs. In the study population, increased serum VEGF correlated with diminished EPC-CFUs, consistent with the idea that VEGF plays a compensatory role, a finding also reported in patients with cerebral aneurysm (Wei et al., 2011). This is also supported by the study of Vasa et al. which showed a diminished response of EPCs to VEGF in patients with CAD (Vasa et al., 2001). Moreover, Alber et al. found that a key beneficial effect of atorvastatin therapy is reducing the levels of plasma VEGF in patients with CAD (Franz Alber et al., 2002). This coincides with our study using MSCs, rather than a pharmacological intervention, to decrease pathologic VEGF and increase endothelial function. Thus our findings establish a previously unappreciated therapeutic principle whereby allogeneic MSCs can be employed to stimulate EPC bioactivity, improve arterial physiologic vasodilatory responses, and decrease unfavorable cytokine mobilization in patients with CV disease and other disorders associated with endothelial dysfunction.
We found that allogeneic MSCs restored endothelial function in patients to a degree greatly exceeding that of autologous MSCs. One possible explanation for this may be the age of the cells. Recent studies highlight that MSC's therapeutic function declines as a result of aging (Efimenko et al., 2013, Asumda, 2013). Efimenko et al. showed that adipose-derived MSCs from aged patients with coronary artery disease have impaired angiogenic potential (Efimenko et al., 2013). Similarly, Kasper et al. demonstrated that MSC function is altered and diminished with age, specifically showing lower actin turnover and therefore decreased motility, decreased antioxidant power, decreased responsiveness to chemical and mechanical signaling, and increased senescence (Kasper et al., 2009). Stolzing et al. also reported a decline in “fitness” as a result of aging, as evidenced by a decline in colony-forming unit-fibroblasts and increase in reactive oxygen species levels and oxidative stress (Stolzing et al., 2008). In our study, all allogeneic stem cell donors were healthy, young donors between the ages of 20 and 35. Patients receiving their own stem cells not only had underlying chronic diseases, but also were older (between the ages of 45 and 75). MSC aging may impair the survival, differentiation, and ability to recruit EPCs to areas of damage, ultimately reducing their therapeutic efficacy (Asumda, 2013). Additionally, due to underlying patient comorbidities, the autologous MSC microenvironment may be negatively altered due to systematic inflammation. Consistent with this notion, Teraa et al. showed that systemic inflammation affects the bone marrow microenvironment, disturbing EPC function (Teraa et al., 2013). Although more studies are necessary to validate that the advantage evident here is due to the health and age of MSCs, this study supports the encouraging idea of using “off the shelf” allogeneic MSCs over autologous MSCs.
In this study, we report positive systemic effects from local, cardiac transendocardial MSC injections. We have previously shown that MSC engraftment after intramyocardial injection is approximately 10 to 20%, suggesting that these cells migrate and circulate systemically (Quevedo et al., 2009). MSCs are known to secrete anti-inflammatory factors and cytokines (such as IL-2, TGF-β1, hepatocyte growth factor, NO, prostaglandin 2, and stromal cell derived factor-1), which can modulate the mobilization of EPCs from bone marrow (Williams and Hare, 2011, Iyer and Rojas, 2008). Additionally, MSCs have been shown to secrete paracrine factors that stimulate resident cells (Williams and Hare, 2011). Thus, we propose a potential mechanism whereby allogeneic MSCs injected into cardiac tissue respond to local microenvironment cues, thereby secreting anti-inflammatory and EPC mobilizing factors that ultimately improve endothelial function alleviating cardiac stress.
There are several limitations of our study. All ICM patients received allogeneic MSCs, therefore we were unable to study the effect of autologous MSCs in this specific HF population. Additionally, patients who received autologous MSCs had higher FMD% at baseline compared to patients who received allogeneic MSCs. Notably, however, all patients receiving autologous MSCs had lower FMD% post-treatment, highlighting the allogeneic advantage. There was also a significantly higher White/Hispanic population in the DCM autologous group compared to other treatment groups. Despite this, there was no difference in baseline or treatment response comparing White/Hispanic patients to White patients (Supplementary Table 1). Furthermore, patients with ICM received different total number of cells (either 20 or 100 million cells). Regardless, all patients had an improvement in endothelial function and there was no intergroup variability. Lastly, there was variability within our control group for circulating VEGF levels. The majority of our controls had too low levels of circulating VEGF to detect, ultimately highlighting the elevated levels of VEGF evident in HF patients. Despite these limitations, we are confident our results provide novel insights into the positive endothelial function effect of allogeneic MSCs in patients with HF.
In conclusion, this study demonstrates a potent and clinically relevant efficacy outcome of transendocardial therapy with MSCs in patients with advanced HF. Allogeneic MSCs restore flow mediated brachial artery dilatation, EPC bioactivity, and VEGF levels towards normal. As abnormalities in the vascular function of patients with CV disease is shown to be highly predictive of adverse outcomes and disease progression, targeting endothelial function is a significant therapeutic strategy. Together, these findings offer a new mechanism of action underlying potentially clinically relevant responses to the use of allogeneic MSCs in CV disease.
Author Contributions
CP: study concept and design, EPC-CFUs, VEGF, and Matrigel experiments, data analysis, drafted manuscript; AB: study concept and design, drafted manuscript; MB: CFU assays, data analysis; IHS: study design, data analysis, manuscript editing; BH: FMD studies, data analysis; MP: FMD studies, data analysis; CD: Matrigel assay, VEGF assay, data analysis; WB: study design; DD: study design; AK: clinical cell manufacturing and characterization; JMH: study design and concept, data analysis, manuscript drafting and editing.
Funding
Dr. Hare is supported by the National Institutes of Health grants RO1 HL084275, RO1 HL107110, RO1 HL110737, and 5UM HL113460, and the Starr Foundation and the Soffer Family Foundation.
Role of Funding Sources
No funders played a role in study design, data collection, data analysis, interpretation, or writing of the report.
Conflict of Interest
Dr. Hare has a patent for cardiac cell-based therapy and reports equity interest and board membership in Vestion Inc.
Acknowledgments
The authors wish to thank Julio Sierra, Cindy Delgado, and Phillip Gonzalez for enrolling and consenting patients, collecting samples, and coordinating patient data. Additionally, the authors wish to thank Vasileios Karantalis for his statistical review. Lastly, the authors would like to thank Irene Margitich for her laboratory help and technical support.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.03.020.
Appendix A. Supplementary data
Supplementary material.
References
- Asumda F.Z. Age-associated changes in the ecological niche: implications for mesenchymal stem cell aging. Stem Cell Res. Ther. 2013;4(3):47. doi: 10.1186/scrt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. Heart failure—new insights. Isr. Med. Assoc. J. 2009;11(2):105–111. [PubMed] [Google Scholar]
- Bui A.L., Horwich T.B., Fonarow G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011;8(1):30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Gomes S., Rangel E.B. S-nitrosoglutathione reductase-dependent PPARγ denitrosylation participates in adipogenesis and osteogenesis. J. Clin. Investig. 2015;125(4) doi: 10.1172/JCI73780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tredget E.E., Wu P.Y., Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin B.S.P., Chung N.A.Y., Gibbs C.R., Blann A.D., Lip G.Y.H. Vascular endothelial growth factor and soluble P-selectin in acute and chronic congestive heart failure. Am. J. Cardiol. 2002;90(11):1258–1260. doi: 10.1016/s0002-9149(02)02848-5. [DOI] [PubMed] [Google Scholar]
- Corretti M.C., Anderson T.J., Benjamin E.J. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Da Silva J.S., Hare J.M. Cell-based therapies for myocardial repair: emerging role for bone marrow-derived mesenchymal stem cells (MSCs) in the treatment of the chronically injured heart. Methods Mol. Biol. 2013;1037:145–163. doi: 10.1007/978-1-62703-505-7_8. [DOI] [PubMed] [Google Scholar]
- Efimenko A., Dzhoyashvili N., Kalinina N. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl. Med. 2014;3(1):32–41. doi: 10.5966/sctm.2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleuteri E., Di Stefano A., Tarro Genta F. Stepwise increase of angiopoietin-2 serum levels is related to haemodynamic and functional impairment in stable chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2011;18(4):607–614. doi: 10.1177/1741826710389410. [DOI] [PubMed] [Google Scholar]
- Franz Alber H., Dulak J., Frick M. Atorvastatin decreases vascular endothelial growth factor in patients with coronary artery disease. J. Am. Coll. Cardiol. 2002;39(12):1951–1955. doi: 10.1016/s0735-1097(02)01884-3. [DOI] [PubMed] [Google Scholar]
- Gnecchi M., Zhang Z., Ni A., Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go A.S., Mozaffarian D., Roger V.L. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S.A., Rangel E.B., Premer C. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110(8):2834–2839. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J.M., Fishman J.E., Gerstenblith G. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzistergos K.E., Quevedo H., Oskouei B.N. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman A.W., DiFede D.L., Fishman J.E. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J.M., Zalos G., Halcox J.P.J. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Iyer S.S., Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert. Opin. Biol. Ther. 2008;8(5):569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- Karantalis V., Hare J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ. Res. 2015 doi: 10.1161/CIRCRESAHA.116.303614. (in Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper G., Mao L., Geissler S. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27(6):1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- Marti C.N., Gheorghiade M., Kalogeropoulos A.P., Georgiopoulou V.V., Quyyumi A.A., Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 2012;60(16):1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- Mushtaq M., DiFede D., Golpanian S. Rationale and design of the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (The POSEIDON-DCM Study) J. Cardiovasc. Transl. Res. 2014;1–12 doi: 10.1007/s12265-014-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo H.C., Hatzistergos K.E., Oskouei B.N. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Lucke C., Rössig L., Fichtlscherer S. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- Schulman I.H., Zhou M.S., Raij L. Interaction between nitric oxide and angiotensin II in the endothelium: role in atherosclerosis and hypertension. J. Hypertens. Suppl. 2006;24(1):S45–S50. doi: 10.1097/01.hjh.0000220406.46246.f2. [DOI] [PubMed] [Google Scholar]
- Shantsila E., Watson T., Lip G.Y. Endothelial progenitor cells in cardiovascular disorders. J. Am. Coll. Cardiol. 2007;49(7):741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- Shantsila E., Watson T., Lip G.Y.H. Endothelial progenitor cells in cardiovascular disorders. J. Am. Coll. Cardiol. 2007;49(7):741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- Solomon A., Blum A., Peleg A., Lev E.I., Leshem-Lev D., Hasin Y. Endothelial progenitor cells are suppressed in anemic patients with acute coronary syndrome. Am. J. Med. 2012;125(6):604–611. doi: 10.1016/j.amjmed.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Stolzing A., Jones E., McGonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech. Ageing Dev. 2008;129(3):163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Telukuntla K.S., Suncion V.Y., Schulman I.H., Hare J.M. The advancing field of cell‐based therapy: insights and lessons from clinical trials. J. Am. Heart Assoc. 2013;2(5) doi: 10.1161/JAHA.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraa M., Sprengers R.W., Westerweel P.E. Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLoS ONE. 2013;8(1):e55592. doi: 10.1371/journal.pone.0055592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W.C., Li Y.H., Huang Y.Y., Lin C.C., Chao T.H., Chen J.H. Plasma vascular endothelial growth factor as a marker for early vascular damage in hypertension. Clin. Sci. 2005;109(1):39–43. doi: 10.1042/CS20040307. [DOI] [PubMed] [Google Scholar]
- Vasa M., Fichtlscherer S., Aicher A. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001;89(1):e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Wei H., Mao Q., Liu L. Changes and function of circulating endothelial progenitor cells in patients with cerebral aneurysm. J. Neurosci. Res. 2011;89(11):1822–1828. doi: 10.1002/jnr.22696. [DOI] [PubMed] [Google Scholar]
- Werner N., Kosiol S., Schiegl T. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- Werner N., Wassmann S., Ahlers P. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res. Cardiol. 2007;102(6):565–571. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- Williams A.R., Hare J.M. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011;109(8):923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A., Kirton J.P., Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc. Res. 2008;78(3):413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.