Abstract
In a recent issue of Chemistry & Biology, Bone et al. (2009) employed a chemical biology approach to probe the role of glycogen synthase kinase-3 (GSK-3), a key regulator of pluripotentiality, providing new insights and tools for modulating this process.
The multifunctional serine-threonine kinase, glycogen synthase kinase-3 (GSK-3) has recently been identified as a critical modulator of embryonic stem cell (ESC) properties, largely based on experiments using selective GSK-3 inhibitors. Due to the use of various cell lines (from mouse and human), inconsistency in cell culture conditions and experimental assays, as well as different small molecule inhibitors of GSK-3, the precise role that GSK-3 plays in ESCs has been somewhat ambiguous. Bone et al. used a macrocyclic bisindolylmaleimide compound 1i, previously identified as a selective small-molecule ATP-competitive inhibitor of GSK-3, and contrasted its effects with two structurally distinct GSK-3 inhibitors that have previously been reported; 6-bromoindirubin-3-oxime (BIO) and TWS119. BIO was initially demonstrated to be capable of maintaining the pluripotency of both mouse and human ESCs through a mechanism dependent upon activation of Wnt/β-catenin signaling (Sato et al., 2004). Subsequently, it was reported that sustained GSK-3 inhibition or canonical Wnt activation in both mouse and human ESCs promotes their differentiation into multi-potent mesendodermal progenitors or their differentiated progeny (Bakre et al., 2007; Dravid et al., 2005). While most studies with GSK-3 inhibitors and ESCs support a role for GSK-3 in the maintenance of undifferentiated pluripotent cells, the GSK-3 inhibitor TWS119 was reported to induce neuronal differentiation of ESCs (Ding et al., 2003).
The standard method used to propagate mouse ESCs (mESCs) is in rich medium, containing high levels of fetal bovine serum, supplemented with the cytokine leukemia inhibitory factor (LIF), although serum-free medium containing only LIF and bone morphogenetic protein-4 (BMP4) is sufficient to maintain these cells in a self-renewing, undifferentiated state. The proposed mode of action of these extrinsic factors is that LIF activation of Stat3 enhances ESC self-renewal, while BMP4, acting through Smad-mediated induction of inhibitor of differentiation (Id) proteins, blocks neuronal differentiation and supports self-renewal (Ying et al., 2003). In vitro GSK-3 activity assays indicated that 1i inhibits GSK-3 present in mESC lysates with an IC50 of 250 nM, some 5-fold higher than that obtained with BIO. Bone et al. (2009) then sought to determine if 1i was capable of enhancing the propagation of undifferentiated mESCs as previously reported for BIO. Undifferentiated ESCs express high levels of alkaline phosphatase (AP), which is easily detected through histochemical staining for enzymatic activity. The authors used AP staining as a surrogate marker for undifferentiated mESC colonies in a clonal assay in which limiting dilutions of ESCs were plated out in serum and LIF for 5 days. Colonies present after this 5 day period were scored based on their AP levels. The inhibitors TWS119 (2 μM), BIO (0.5 μM) or 1i (5 μM) each elevated the number of undifferentiated ESC clones scored in this manner, and thus were interpreted to enhance ESC self-renewal.
One of the best-characterized GSK-3 substrates is the transcriptional co-activator β-catenin, a mediator of canonical Wnt signaling. GSK-3-mediated phosphorylation of β-catenin promotes its proteasomal degradation; inhibition of GSK-3 therefore results in β-catenin accumulation in the cytoplasm and its subsequent translocation to the nucleus where it activates T-cell factor/lymphoid enhancer-binding factor (TCF/LEF)-mediated transcription of target genes. To assess the relative extent of GSK-3 inhibition obtained by 1i, BIO or TWS119 in mESCs, Bone et al., monitored the stabilization of β-catenin and the subsequent activation of the β-catenin/TCF reporter constructs as indicators of GSK-3 inhibition in intact cells. The minimal concentrations of BIO and 1i that resulted in detectable β-catenin dephosphorylation on GSK-3 target residues after a 30-minute incubation also enhanced TCF-reporter activity to a similar extent and correlated with the concentrations of each inhibitor that enhanced maintenance of AP-positive mESC colonies. In contrast, the inhibitor TWS119, increased the number of AP-positive mESC colonies scored at 5 days at 2 μM, yet only modestly elevated TCF-reporter activity at this dose, suggesting that it might have other targets in mESCs that may be responsible for its distinct effects, such as neuronal differentiation.
Bone et al. went on to synthesize 48 derivatives of 1i with the goal of enhancing the potency of the lead compound. The new compounds were screened based on the clonal mESC self-renewal assay described above and twelve were found to enhance maintenance of undifferentiated mESC with potency equal to or better than 1i. A more detailed characterization of the twelve most effective compounds revealed a direct correlation between a compound’s potency for GSK-3 inhibition and ability to enhance the retention of undifferentiated mESC colonies. The three most potent derivatives (1l, 1m and 1o) were approximately 10X more potent than the lead compound, 1i. The extent to which GSK-3 inhibitors 1i, 1l, 1m, 1o, BIO and TWS119 exerted off-target effects on three pathways regulating mESC self-renewal: the Stat3, PI3K/Akt, and MAPK pathways, was tested. While 1i, 1l, 1m and 1o had no apparent affect on these signaling pathways, both BIO and TWS119 caused significant suppression of Erk1/2 signaling and Stat3 phosphorylation. All of the GSK-3 inhibitors tested by Bone et al. enhanced maintenance of undifferentiated mESCs only in the presence of LIF and serum.
While the Bone et al. analysis strengthens the notion that small molecule inhibitors of GSK-3 potentiate self-renewal of pluripotent mESCs, the mechanism through which GSK-3 inhibition imparts this effect is still unclear. That GSK-3 inhibition in and of itself enhances self-renewal of mESCS is not surprising given that GSK-3 nullizygous ESCs display this phenotype (Doble et al., 2007). Still, the newly synthesized, highly specific and potent 1i–based GSK-3 inhibitor series should prove very useful for further dissection of GSK-3’s role in stem cell biology. GSK-3 has the unique property of regulating multiple signaling pathways including Wnt/β-catenin, Hedgehog, Notch and PI3K pathways (Figure 1), and also directly regulates numerous transcription factors including c-Myc, which has been directly linked to mESC self-renewal and induced pluripotent stem cell (iPSC) generation. There is accumulating evidence that the PI3K pathway plays a role in the regulation of pluripotency in mESCs and human ESCs (Welham et al., 2007). The precise mechanism(s) through which PI3K signaling regulates ESC self-renewal and pluripotency is still unclear, but regulation of Nanog transcript levels plays at least a partial role. GSK-3 has been implicated in the cascade involving PI3K and Nanog, although the relevant, direct GSK-3 substrates remain unknown. Further studies remain to be completed before GSK-3 inhibition-dependent β-catenin stabilization can be excluded from playing a role in ESC self-renewal, especially given recent data suggesting that TCF3 is a component of the core transcription factors regulating ESC pluripotency (Cole et al., 2008). Inhibition of GSK-3, combined with inhibition of FGF-mediated MAPK signaling, has been reported to be sufficient for maintenance of mESCs in serum-free culture conditions (Ying et al., 2008). Under these conditions, inhibition of GSK-3 has been proposed to act primarily via promotion of cell viability. The study by Bone et al., in which GSK-3 inhibition retains an effect in the presence of serum and LIF, suggests that GSK-3 inhibition exerts direct effects on ESC self-renewal machinery, in addition to any function as a survival factor. The development of defined chemical cocktails that maintain stem cell pluripotency would simplify expansion of ESC and iPSCs by overcoming the current need for animal sera and protein factors. A chemical genetics approach, such as that used by Bone et al., uses the phenotype of a classical genetic modification (e.g. GSK-3 DKO) as the “gold standard” that is to be mimicked through chemical means. This powerful strategy allows for the development of “clean” chemical tools with minimal undesired off-target effects, which will be invaluable for future stem cell applications.
Figure 1. GSK signaling cascades in ESCs.
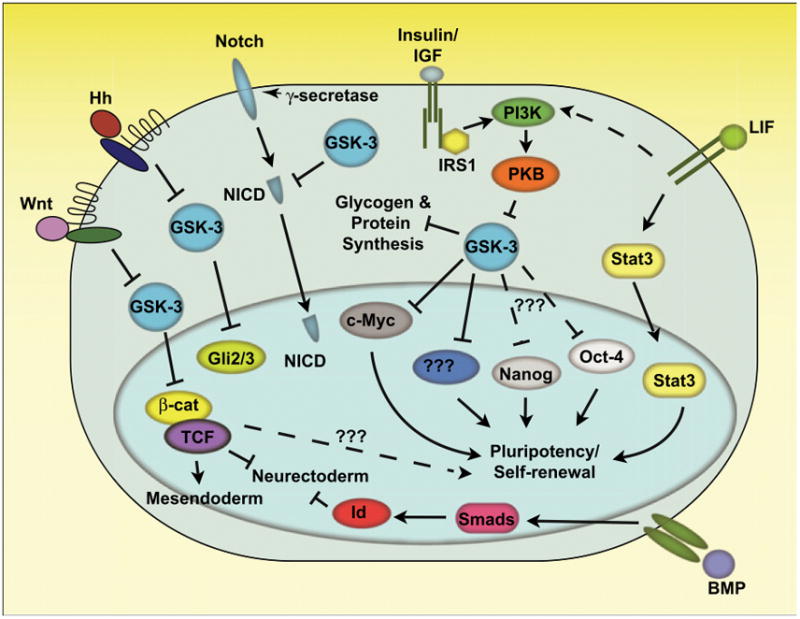
GSK-3 plays a central role in the Wnt, Hedgehog, Notch, and PI3K signaling pathways, serving to negatively regulate the transcription factors β-catenin/TCF, Gli2/3, NICD as well as metabolic enzymes involved in glycogen and protein synthesis. Inhibition of GSK-3 in ESCs, in the presence of the cytokine LIF, enhances their self-renewal and prevents downregulation of core pluripotency factors, Oct-4 and Nanog through an unknown mechanism. There are likely GSK-3 target molecules, yet to be identified, that are involved in its regulation of stem cell properties, which may be cytosolic or nuclear (as depicted). The transcription factor c-Myc, which has been linked to ESC pluripotency as well as iPSC generation, is negatively regulated by GSK-3. Prolonged activation of Wnt/β-catenin signaling has been linked to mesendoderm differentiation. GSK-3 nullizygous mouse ES cells are refractory to neurectodermal differentiation, potentially due to hyperactivated Wnt signaling. Although controversial, a role for β-catenin, perhaps independent of TCF, has been suggested to enhance ESC pluripotency under some conditions.
Abbreviations
- β-cat
β-catenin
- BMP
Bone morphogenetic protein
- Hh
Hedgehog
- GSK-3
Glycogen synthase kinase-3
- Id
Inhibitor of differentiation
- IGF
Insulin-like growth factor
- LIF
Leukemia inhibitory factor
- NICD
Notch intracellular domain
- PI3K
Phosphoinositide 3-kinase
- PKB
Protein kinase B (also known as Akt)
References
- Bakre MM, Hoi A, Mong JC, Koh YY, Wong KY, Stanton LW. J Biol Chem. 2007;282:31703–31712. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wu TY, Brinker A, Peters EC, Hur W, Gray NS, Schultz PG. Proc Natl Acad Sci U S A. 2003;100:7632–7637. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Welham MJ, Storm MP, Kingham E, Bone HK. Biochem Soc Trans. 2007;35:225–228. doi: 10.1042/BST0350225. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]


