Abstract
Objectives
Pediatric guidelines in 2008 and 2011 recommended lipid lowering therapy in children ≥ 8 with high-risk cardiovascular conditions, such as familial hypercholesterolemia (FH). Our objective was to describe the patterns and predictors of LLT initiation in commercially insured children between 2005 and 2010.
Study design
Using commercial health plan data on children ages 8–20 from 2004 to 2010, we estimated rates of LLT initiation overall and stratified by age. Using a nested case-control design, we used multivariable logistic regression to identify temporal, demographic, clinical and health utilization characteristics associated with LLT initiation.
Results
Among >13 million children, 665 initiated LLT for an incidence rate 2.6/100,000 person-years (PY). Incidence rates were highest in 2005 (4.1/100,000 PY) and 2008 (3.9/100,000 PY), with no discernable secular trend. Rates of LLT initiation were significantly greater in children ≥15 years (Odds Ratio (OR) 2.9 [95% CI 5.2 – 13.0]), males (2.1[1.7–2.4]), and those with a diagnosis of FH (165.2[129.0–211.6]), other dyslipidemia (175.5 [143.2–215.3]), diabetes type I (7.7[4.7–12.4]), diabetes type II (13.6[8.5–21.7]), hypertension (8.1[4.9–13.3]), obesity (7.8[4.7[12.7]), and ≥ 5 outpatient visits (1.5[1.2–1.7]), and children with dispensing of ≥2 non-LLT prescriptions were less likely to initiate LLT (0.2[0.2–0.3]).
Conclusions
Despite new guidelines, LLT initiation in children is low and has not increased through 2010. Although diagnosis of FH and other dyslipidemias was associated with higher probability of LLT initiation, our findings suggest LLT is underutilized in this population given the prevalence of these disorders.
Keywords: Lipid lowering therapy, children, cardiovascular disease, familial hypercholesterolemia, drug treatment
Pathologic and epidemiologic data demonstrate that atherosclerosis begins at a young age and that early treatment can reduce precursors to cardiovascular disease (CVD) later in life.1–4 Multiple observational studies and clinical trials have demonstrated the efficacy of pharmacological lipid lowering therapy in reducing markers of CVD.5–12 The majority of pediatric studies have focused on children with familial hypercholesterolemia (FH), a dominant negative genetic condition characterized by significantly elevated low-density lipoprotein cholesterol (LDL-C) levels starting at birth. It is well recognized that children with FH are at a substantially higher risk for coronary events earlier in adulthood and exhibit precursors to CVD in late adolescence and young adulthood. Although lifestyle modification alone is insufficient as a means of risk reduction, pharmacotherapy appears to be of benefit.13,14
As a result of the growing evidence to support early intervention, in 2008 the American Academy of Pediatrics (AAP) released a policy statement on “Lipid Health and Cardiovascular Screening in Childhood”, updating the 1998 policy statement “Cholesterol in Childhood”15 to include pharmacological treatment with statins of children 8 years and older - pravastatin exclusively for children 8–10, and all statins for children 10 and older - who are the highest at risk for CVD.1 The 2008 report was followed in 2011 by a report from an expert panel commissioned by the National Heart, Lung and Blood Institute (NHLBI) and endorsed by the AAP, recommending similar treatment guidelines with the exception that the minimum age recommended for initiation of lipid lowering therapy was 10.16 Both sets of guidelines were met with controversy when released. Critics argued that the guidelines promoted medicating children without sufficient evidence on the long-term safety of lipid lowering therapy use and warned of an “epidemic” of pharmacological treatment in children.17
Prior to the release of the AAP and NHLBI guidelines, estimates of the prevalence of statin use in children under the age of 18, both privately insured and Medicaid recipients, was between 0.0112%18 and 0.0168%.19 Both estimates are from studies using the CVS Caremark dispensing database, are limited to 2004 and 2007 and lack data on clinical covariates.
The primary objective of this study was to describe the patterns of, and clinical variables associated with, lipid lowering therapy initiation in a commercially insured population of children aged 8–20 between 2005 and 2010, thereby spanning the 2008 lipid management guidelines. We hypothesized that the rate of lipid lowering therapy initiation would increase over time and that older children and children with multiple comorbidities would be more likely to receive lipid lowering therapy. We further hypothesized that the presence of certain comorbidities might cause physicians to be more aggressive with screening for dyslipidemias or more likely to treat children for such dyslipidemias. Because of the particular focus on children with genetic dyslipidemias included in the 2008 AAP guidelines, a secondary objective was to investigate factors associated with initiation of lipid lowering therapy among commercially insured children with a diagnosis of FH or other dyslipidemia.
METHODS
Data on demographic characteristics, pharmacy claims, and clinical diagnoses were extracted from the Truven Health Analytics MarketScan® databases (Truven Health Analytics, Ann Arbor, MI) for calendar years 2004 through 2010. The MarketScan database includes claims data on employees and their dependents from employers and health insurers. The enrollment database was used to assess eligibility, and clinical information was collected from the inpatient and outpatient claims databases. Uses of lipid lowering therapy were collected from the outpatient pharmacy claims database and included all uses of a statin, niacin, ezetimibe, fibric acid derivative or bile acid sequestrant.
The primary outcome of the study was incident lipid lowering therapy, defined as dispensing at least one lipid lowering agent following a period of 12 consecutive months of no recorded use. We used measures of incidence instead of prevalence in order to capture changes in the decision to initiate lipid lowering therapy, as prevalence measure can reflect changes in discontinuation of lipid lowering therapy and differential right censoring. Patients aged 8–20 years between 2005 and 2010 with at least 12 months of continuous enrollment in a health plan were eligible for inclusion in the analysis. Follow-up time at-risk of lipid lowering therapy initiation began on the first day after the 12-months of continuous enrollment, therefore estimates of lipid lowering therapy initiation begin in 2005. For our analysis we assessed the presence of the age and sex as well as clinical diagnoses identified as risk factors for CVD in the consensus statement endorsed by the AAP “Cardiovascular Risk Reduction in High-Risk Pediatric Patients”21 using the following ICD-9 codes: FH (272.0) and other dyslipidemia (270.1–270.9), hypertension (401.xx-404.xx, 796.0, 796.2), diabetes mellitus types I (252.x1, 252.x3) and II (252.x0, 252.x2), obesity (278.xx), congenital heart disease (746), chronic kidney disease/end stage renal disease (585), Kawasaki disease (446.1) and heart transplant and chronic inflammatory diseases (V42.1, 996.83, 375.1, 375.3). FH was defined using the ICD-9 code 272.0, however, because this code also includes Fredrickson Type IIa hyper hyperlipoproteinemia, Hyperbetalipoproteinemia, Hyperlipidemia Group A and Low-Density-Lioid-Type (DLD) hyperlipoproteinemia, we cannot be certain that this diagnosis is meant to specifically refer to FH. As such, patients with an ICD-9 code for 272.0 are presumed, but not confirmed, to have FH. A clinical diagnosis was deemed present when identified on at least two outpatient or one inpatient claims within the 12-month look back period. As the clinical diagnoses were chronic diseases, once identified they were assumed to persist for the duration of follow-up.22 Additionally, we considered the following three binary markers of health care utilization as measured over the past 12 months: (1) the presence of five or more outpatient visits; (2) one or more inpatient visits; and (3) dispensing of two or more prescription medication other than lipid lowering therapy.23
Statistical Analyses
We plotted crude rates of incident lipid lowering therapy as well as rates stratified by age (≥15 vs <15 years) over the study period for all eligible patients. To identify the association between clinical characteristics and lipid lowering therapy initiation, we constructed a nested case-control analysis including all cases of incident lipid lowering therapy use and used incidence density sampling to select one million unmatched controls. We used a nested case-control design instead of a cohort design because of limitations in the computing power required to fit models with time varying covariates and the over 300 million person-months present in the database. Incidence density sampling, which samples controls proportional to person-time, provides direct estimates of the incidence rate ratio and maintains the distribution of covariate person time that would be found in a cohort analysis.24
We fit univariable logistic regression models, regressing the outcome of lipid lowering therapy initiation (yes/no) on demographics, clinical diagnoses and measures of health utilization. We then ran a multivariable logistic regression, modeling the relationship of year and age on initiation of lipid lowering therapy after adjusting for sex and measures of healthcare utilization. In a sub-analysis we included an interaction between age and year to test for the different trends in lipid lowering therapy initiation over time by age group. The effects of time did not differ by age group and thus the interaction term was not included in the final analysis.
We included a secondary-analysis limiting our sample to only children with a diagnosis of FH or a dyslipidemia. We compared lipid lowering therapy initiation in this subpopulation by demographics, comorbidities and health utilization. Logistic regression models were constructed along the same schema detailed in the primary analysis. All analysis was performed using SAS version 9.3 of the SAS system for Windows (SAS Institute Inc. Cary, NC). Statistical significance was assessed at p<0.05.
RESULTS
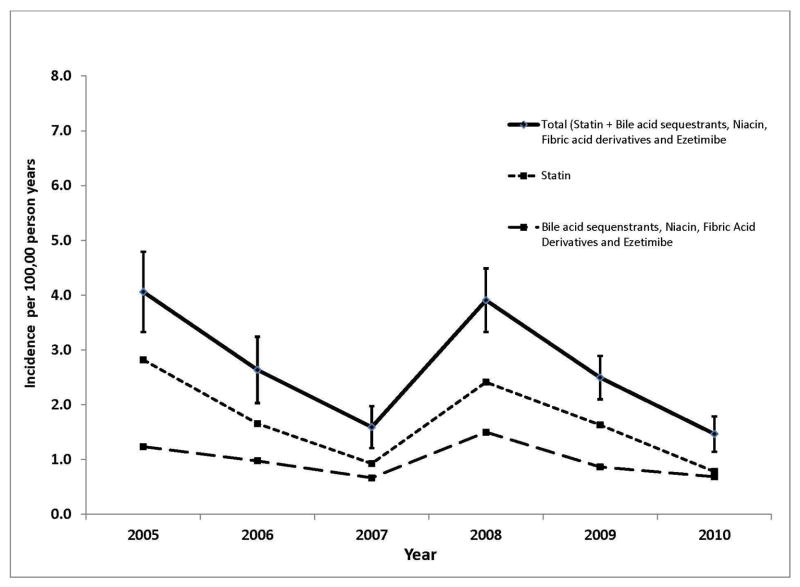
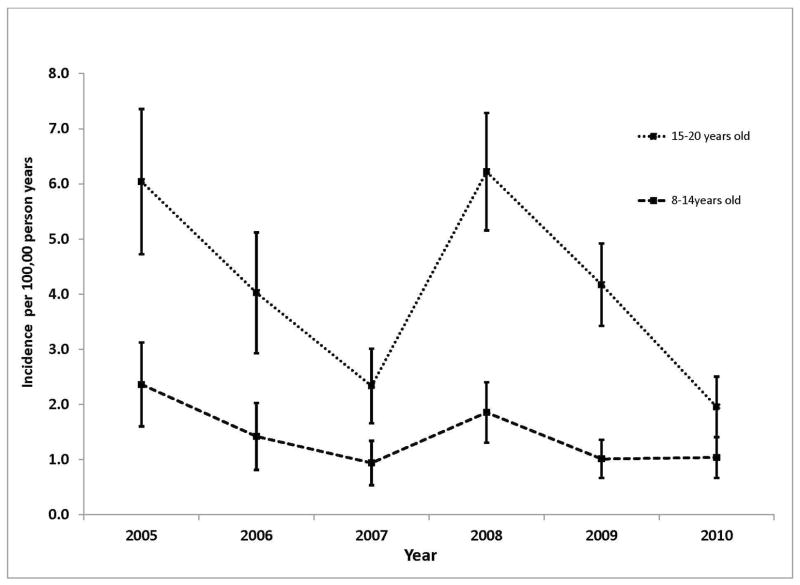
The study population consisted of 13,262,087 unique patients 8–20 years old with a mean 38 months of follow-up time. From this population we identified a total of 665 patients who initiated lipid lowering therapy between 2005 and 2010 corresponding to an overall incidence rate of 2.6 (95% CI 0.1–2.7) per 100,000 person-years (PY). The rate of lipid lowering therapy initiation was highest in 2005 with an incidence of 4.1 per 100,000 PY (95% CI 3.3–4.8) and was significantly lower in all subsequent years with the exception of 2008 in which the incidence was 3.9 per 100,000 PY (95% CI 3.3–4.5). The temporal pattern was similar whether we considered all lipid lowering therapies together, statins only, or non-statin lipid lowering therapy only, as well as when stratified by age (Figures 1 and 2; Figure 1 available at www.jpeds.com).
Figure 1.
Incidence of Lipid Lowering Therapy in Children Ages 8 to 20 between 2005 and 2010
Figure 2.
Incidence of Lipid Lowering Therapy between 2005 and 2010 by Age Group
In univariable analyses, lipid lowering therapy initiation was associated with age ≥15 years (OR 2.9, 95% CI 2.5–3.5) and male sex (OR 2.1, 95% CI 1.7–2.4). Further, with the exception of Kawasaki disease and CID, for which there were no cases of lipid lowering therapy, all clinical diagnoses in the analysis were significantly associated with a higher incidence of lipid lowering therapy initiation. Two or more prescription drug dispensings other than lipid lowering therapy in the past 12 months was the only variable studied negatively associated with initiation of lipid lowering therapy (OR 0.2, 95% CI 0.2–0.3; Table I).
Table 1.
Comparison of demographics, clinical characteristics and health utilization among among children ages 8 to 20 by use of lipid lowering therapy (n=1,000,665)
| Lipid Lowering Therapy Initiation (cases) (N=665) | No LLT Initiation (controls) (N=1,000,000) | Odds Ratio | (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| n | % | n | % | ||||
| Age ≥ 15 years | 479 | 72.0 | 532,973 | 53.3 | 2.9 | (2.5–3.5) | 0.00 |
| Male | 452 | 68.0 | 491,706 | 49.1 | 2.1 | (1.7–2.4) | 0.00 |
| CVD Risk Factors | |||||||
| Diabetes Type I | 17 | 2.6 | 3,414 | 0.3 | 7.7 | (4.7–12.4) | 0.00 |
| Diabetes Type II | 18 | 2.7 | 2,045 | 0.2 | 13.6 | (8.5–21.7) | 0.00 |
| Hypertension | 16 | 2.4 | 3,037 | 0.3 | 8.1 | (4.9–13.3) | 0.00 |
| Obesity | 16 | 2.4 | 3,171 | 0.3 | 7.8 | (4.7–12.7) | 0.00 |
| Indications for LLT | |||||||
| Congenital Heart Disease | 1 | 0.2 | 824 | 0.1 | 1.8 | (0.3–13.0) | 0.55 |
| Disorders of Lipid Metabolism | 122 | 18.3 | 1,278 | 0.1 | 175.6 | (143.2–215.3) | 0.00 |
| Familial Hypercholesterolemia | 77 | 11.6 | 792 | 0.1 | 165.2 | (129.0–211.6) | 0.00 |
| Chronic Kidney Disease | 3 | 0.5 | 232 | 0.0 | 19.5 | (6.2–61.1) | 0.0 |
| Kawasaki Disease | 0 | 0.0 | 66 | 0.0 | N/A | N/A | N/A |
| Heart transplant | 1 | 0.2 | 36 | 0.0 | 41.8 | (5.7–305.6) | 0.00 |
| Chronic Inflammatory Disease | 0 | 0.0 | 1,175 | 0.1 | N/A | N/A | N/A |
| Health Care Utilization 1 | |||||||
| Inpatient stay | 17 | 2.6 | 18,353 | 1.8 | 1.4 | (1.2–1.7) | 0.00 |
| Outpatient visit | 172 | 25.9 | 194,892 | 19.5 | 1.4 | (0.9–2.3) | 0.17 |
| Prescription Medication | 76 | 11.4 | 371,651 | 37.1 | 0.2 | (0.2–0.3) | 0.00 |
CI= confidence interval;
>1 inpatient visit in past year ≥ 5 outpatient visits ≥2 prescription drug dispensings in the last 12 months
In multivariable models adjusting for clinical diagnoses included in the univariate analysis and calendar year, age ≥15 (OR 3.1, 95% CI 2.6–3.6), male sex (OR 2.0, 95% CI 1.7–2.4) and ≥5 outpatient visits in the past 12 months (OR 2.55, 95% CI 2.1–3.1) were associated with an increase in the rate of lipid lowering therapy initiation, whereas, compared with crude estimates, there was an attenuation of the effect of >1 inpatient stay in the past 12 months (OR 1.26, 95% CI 0.8–2.1). Similar to the crude incidence rate of lipid lowering therapy, the adjusted incidence rate of lipid lowering therapy initiation was significantly greater in 2005 than in all other years with the exception of 2008 in which there was no significant difference (Table II).
Table 2.
Adjusted Odds of Lipid Lowering Therapy Use among children ages 8–20 between 2005 and 2010 (n=1,000,665)
| IRR | 95% CI | p-value | |
|---|---|---|---|
|
|
|||
| Age ≥ 15 years | 3.07 | (2.6–3.6) | 0.00 |
| Year | |||
| 2005 | 1.00 | -- | |
| 2006 | 0.65 | (0.5–0.9) | 0.00 |
| 2007 | 0.35 | (0.3–0.5) | 0.00 |
| 2008 | 0.90 | (0.7–1.1) | 0.40 |
| 2009 | 0.57 | (0.4–0.7) | 0.00 |
| 2010 | 0.35 | (0.3–0.5) | 0.00 |
| Male | 2.00 | (1.7–2.4) | 0.00 |
| Health Utilization 1 | |||
| Inpatient stay | 1.26 | (0.8–2.1) | 0.35 |
| Outpatient visit | 2.55 | (2.1–3.1) | 0.00 |
| Prescription Medication | 0.16 | (0.1–0.2) | 0.00 |
CI, confidence interval;
>1 inpatient visit in past year ≥ 5 outpatient visits ≥2 prescription drug dispensings in the last 12 months
In the sub-analysis of children with a diagnosis of FH or other dyslipidemias initiation of lipid lowering therapy was more likely among males (OR 1.9, 95% CI 1.4–2.6), and less likely in those with diabetes type II (OR 0.2, 95% CI 0.0–0.6), obesity (OR 0.4, 0.2–0.9) and dispensing of ≥2 prescription drugs, other than lipid lowering therapy, in the past 12 months (OR 0.1, 95% CI 0.0–0.1; Table III).
Table 3.
Comparison of demographics, clinical characteristics and health utilization among among children ages 8 to 20 with a diagnosis of familial hypercholesterolemia (FH) or other dyslipidemia by use of lipid lowering therapy (n=2,148)
| Lipid Lowering Therapy Initiation (Cases) (N=182) | Controls (N=1,966) | Odds Ratio | (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| n | % | n | % | ||||
| Age ≥ 15 years | 124 | 68.13 | 1,377 | 63.96 | 0.9 | (0.7–1.3) | 0.68 |
| Male | 121 | 66.48 | 1,010 | 46.91 | 1.9 | (1.4–2.6) | 0.00 |
| CVD Risk Factors | |||||||
| Diabetes Type I | 5 | 2.75 | 76 | 3.53 | 0.7 | (0.2–1.8) | 0.61 |
| Diabetes Type II | 2 | 1.10 | 124 | 5.76 | 0.2 | (0.0–0.6) | 0.00 |
| Hypertension | 9 | 4.95 | 148 | 6.87 | 0.6 | (0.3–1.3) | 0.26 |
| Obesity | 7 | 3.85 | 175 | 8.13 | 0.4 | (0.2–0.9) | 0.02 |
| Additional Indications for LLT | |||||||
| Congenital Heart Disease | 1 | 0.55 | 6 | 0.28 | 1.8 | (0.0–15.0) | 0.92 |
| Chronic Kidney Disease | 1 | 0.55 | 16 | 0.74 | 0.7 | (0.0–4.4) | 1.00 |
| Kawasaki Disease | 0 | 0.00 | 0 | 0.00 | N/A | N/A | N/A |
| Heart transplant | 0 | 0.00 | 0 | 0.00 | N/A | N/A | N/A |
| Chronic Inflammatory Disease | 0 | 0.00 | 11 | 0.51 | N/A | N/A | N/A |
| Health Utilization 1 | |||||||
| Inpatient stay | 7 | 3.85 | 142 | 6.60 | 0.7 | (0.5–1.0) | 0.04 |
| Outpatient visit | 81 | 44.51 | 1,037 | 48.17 | 0.5 | (0.2–1.1) | 0.10 |
| Prescription Medication | 15 | 8.24 | 1,027 | 47.70 | 0.1 | (0.0–0.1) | 0.00 |
CI= confidence interval;
>1 inpatient visit in past year ≥ 5 outpatient visits ≥2 prescription drug dispensings in the last 12 months
DISCUSSION
In a population of commercially insured children ages 8 to 20 between 2005 and 2010, we found extremely low rates of lipid lowering therapy initiation. Over this period there was a general decrease in incident lipid lowering therapy in this population, with the exception of an increase coincident with the publication of the 2008 AAP guidelines. The 2011 NHLBI report was released subsequent to the study period, and thus could not have contributed to our findings. This overall decreasing temporal trend was similar across different types of lipid lowering therapy (statins vs. other lipid lowering therapies) and across different age groups (<15 vs. ≥15 years old). Children with identified dyslipidemias or other measured clinical diagnoses, and those with greater healthcare utilization were more likely to initiate lipid lowering therapy, whereas children on multiple other medications were less likely.
Concern over lipid lowering therapy use in children occurs within the context of the high prevalence of childhood obesity and metabolic syndrome. Some providers fear that in pursuit of treatment for rare genetic dyslipidemias, pediatric lipid guidelines would be misinterpreted to encourage the large number of children with lifestyle related dyslipidemias to be placed on inappropriate medical therapy. Recent data from NHANES suggests that roughly 0.85% of American children ages 12–17 would be eligible for lipid lowering therapy under current AAP/NHLBI recommendations.25 Although our analysis suggests that a diagnosis of FH or other dyslipidemia increased the likelihood of lipid lowering therapy initiation, an incidence of lipid lowering therapy of 2.6 per 100,000 PY is still an order of magnitude lower than the estimated 0.2% population prevalence of FH. The unexpectedly low incidence of lipid lowering therapy initiation in this population suggests that, rather than an ‘epidemic’ of use, lipid lowering therapy is underutilized in this population.
Our finding that children with greater outpatient visits are more likely to initiate lipid lowering therapy is not surprising given that each visit provides an opportunity for initiation. Though, it may also reflect increased lipid testing or visits to a lipid specialist prior to beginning treatment or greater initiative on the part of families to seek healthcare and engage in preventive therapy which has been identified in the epidemiologic literature of administrative health claims databases as a “healthy user” bias.26 However, our finding that children with ≥2 non-lipid lowering therapy prescription dispensings in the last 12 months were significantly less likely to be initiate lipid lowering therapy was unexpected. We hypothesized that patients who initiate lipid lowering therapy would be less hesitant to use other medications and in fact, several studies have demonstrated increasing rates of polypharmacy in pediatric populations.27 However, lower rates of polypharmacy among children who initiate lipid lowering therapy in our sample may reflect provider discomfort in prescribing lipid lowering therapy to children in the first place and therefore reluctance to prescribe additional medications. In a survey of pediatric providers, 83% said that they were uncomfortable managing lipid disorders and 57% said that they were opposed to the use of lipid lowering therapy in children.28 Further, in another survey of lipid management in a pediatric population after a FH diagnosis, only 26% of physicians reported prescribing pharmacotherapy to their patients.29
When we restricted the analysis to those patients who met the definition for FH or other type of dyslipidemia (two outpatient visits in a 12 month period or 1 inpatient visit with a claim containing the ICD-9 code 272.0–272.9), only male sex and dispensing of ≥2 non-lipid lowering therapy prescription medications were positively associated with lipid lowering therapy initiation, and diabetes type II and obesity were negatively associated with lipid lowering therapy initiation. The 2008 AAP recommendation citing the presence of FH as sufficient grounds to initiate lipid lowering therapy may explain why additional risk conditions or risk factors do not incrementally add to the likelihood of pharmacotherapy in FH patients. Alternatively, it is possible that clinicians are, counter to the recommendations, electing not to treat very high risk patients with both FH and other high risk conditions like diabetes. A third possibility is clinicians miscoding more minor LDL-C elevations or other dyslipidemias as FH in children with other relevant diagnoses. Without laboratory measures, however, it is not possible to ascertain the extent of misclassification within the FH code. Though the prevalence of clinical diagnoses did not differ significantly between the cases and controls when limited to children with FH or other dyslipidemias, the prevalence of additional clinical diagnoses was distinctly greater among children with FH than in the population overall. This difference in prevalence of multiple clinical diagnoses between the full study population versus dyslipidemia-only populations may reflect a tendency for clinicians treating children with a relevant conditions, like hypertension or diabetes, to also screen and recognize dyslipidemia.
We were not able to determine the reason for underutilization of lipid lowering therapy in children relative to guideline recommendations. Statins have a favorable risk-benefit profile in adults, yet there have been no studies of the long-term risks of statins that follow participants for the 60 to 80 years expected when initiated in childhood.14,30 However, our finding that between 25% and 50% of all lipid lowering therapy initiated during the study period were non-statins is potentially concerning, as they have not been recommended for use in a pediatric population. Despite studies demonstrating the beneficial effect of lipid lowering therapy use on precursors to CVD later in life among children with FH5, 8, 10, 31 it is likely that providers remain hesitant to start a child with genetic dyslipidemia on lifelong medical treatment for their chronic condition.32
There were several limitations of our study. First, the children in our sample are commercially insured and are not representative of the general population. As such, researchers should be cautious when extrapolating the results to a non-commercially insured population. Second, because data were collected for billing purposes, we would expect a low sensitivity for conditions that do not affect reimbursement. Additionally, our estimates of lipid lowering therapy prescribing are based on reimbursement for prescriptions that were filled and thus are likely underestimates of the true rate of prescribing. Further, although we cannot identify clinical diagnoses that have not been documented, we required at least 2 outpatient visits or one inpatient admission to diagnose a clinical diagnosis. We required this definition in order to avoid misclassification of conditions due to “rule out claims”, which are sometimes included on the claim in order to justify procedures used to “rule out” a condition, rather than diagnosis it. 23, 26
Based on the number of diagnoses in Table I, and accounting for children with multiple diagnoses, we found that only 204 (25%) of children initiating lipid lowering therapy had a documented indication for lipid lowering therapy use suggesting that, although initiation rates are low, children without an indication are likely being treated with lipid lowering therapy. However, it is also possible that the relatively low prevalence of documented indications for lipid lowering therapy use may be the result of indications for lipid lowering therapy identified prior to being captured in the Marketscan database, or identified but not documented for billing purposes. In one study using health insurance claims to study the risk of cardiovascular events associated with pharmacotherapy for irritable bowel syndrome (IBS), only 32% of patients had a claim for IBS in the 6-months prior to beginning treatment.33 Similarly, in a study of Chronic Obstructive Pulmonary Disease (COPD) in patients dispensed Long Acting Beta Agonists (LABAs), less than 50% had a claim for COPD or asthma in the 6 months prior to LABA use.34 Accordingly, the lack of an indication for lipid lowering therapy use may be a result of the limitations of health insurance claims and not necessarily indicative of prescribing patterns. Thus, although misclassification may play a role in our low rates of documented indications for lipid lowering therapy use, it is unlikely to fully account for the 75% of patients without an indication for lipid lowering therapy.33, 34 Finally, the low incidence of lipid lowering therapy may have limited our power to detect an association between comorbidities and lipid lowering therapy initiation in patients with a diagnosis of FH or other dyslipidemia. However, our main findings regarding temporal trends in lipid lowering therapy initiation are robust to this type of misclassification.
Despite these limitations, our analysis covers the time period during which recommendations for lipid lowering therapy use in a pediatric population changed. The isolated increase in lipid lowering therapy initiation observed in 2008, when the new guidelines were released, followed by a return to the downward trend observed from 2005–2007, suggests that the guidelines may have had only a transient effect on prescribing practices and did not lead to overtreatment of children with lifestyle-related dyslipidemias Further, the incidence of treatment with non-statin lipid lowering therapy suggests that there is limited adherence to the 2008 AAP guidelines and the recommended treatment for high-risk children with FH is not occurring. Although we are unable to formally test the effect of the guidelines with our available data, these findings are consistent with recently published reports noting no significant increase in lipid screening rates.20
Acknowledgments
N.J. was supported by the American Heart Association- Founders Affiliate (<>); she worked as a Global Health and Economics Outcome Research fellow for Pfizer, Inc in a capacity unrelated to the report. J.Z. was supported by the National Heart, Lung, and Blood Institute (K23 award- HL111335). D.D. is a full-time employee of Optum Epidemiology; he conducted this work while a full-time employee of Brown University, during which he was a paid consultant to Optum Epidemiology. J.N. is supported by Bristol-Myer-Squibb (<>); he has consulted for Bristol-Myer-Squibb and Janssen Pharmaceutical on topics unrelated to this report.
Abbreviations
- CVD
Cardiovascular Disease
- CID
Chronic Inflammatory Disease
- CHD
Congenital Heart Disease
- CKD/ESRD
Chronic Kidney Disease/End Stage Renal Disease
- FH
Familial Hypercholesterolemia
- LDL-C
Low-Density Lipoprotein Cholesterol
- AAP
American Academy of Pediatrics
- NHLBI
National Heart, Lung and Blood Institute
- PY
Person Years
- CI
Confidence interval
Footnotes
G.W. and J.Z. declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 2.Newman WP, 3rd, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med. 1986;314:138–44. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 3.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 4.McGill HC, Jr, McMahan CA, Zieske AW, Malcom GT, Tracy RE, Strong JP. Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation. 2001;103:1546–50. doi: 10.1161/01.cir.103.11.1546. [DOI] [PubMed] [Google Scholar]
- 5.Knipscheer HC, Boelen CC, Kastelein JJ, van Diermen DE, Groenemeijer BE, van den Ende A, et al. Short-term efficacy and safety of pravastatin in 72 children with familial hypercholesterolemia. Pediatr Res. 1996;39:867–71. doi: 10.1203/00006450-199605000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Rodenburg J, Vissers MN, Wiegman A, van Trotsenburg AS, van der Graaf A, de Groot E, et al. Statin treatment in children with familial hypercholesterolemia: the younger, the better. Circulation. 2007;116:664–8. doi: 10.1161/CIRCULATIONAHA.106.671016. [DOI] [PubMed] [Google Scholar]
- 7.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Buller HR, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2004;292:331–7. doi: 10.1001/jama.292.3.331. [DOI] [PubMed] [Google Scholar]
- 8.Stein EA, Illingworth DR, Kwiterovich PO, Jr, Liacouras CA, Siimes MA, Jacobson MS, et al. Efficacy and safety of lovastatin in adolescent males with heterozygous familial hypercholesterolemia: a randomized controlled trial. JAMA: the journal of the American Medical Association. 1999;281:137–44. doi: 10.1001/jama.281.2.137. [DOI] [PubMed] [Google Scholar]
- 9.Clauss SB, Holmes KW, Hopkins P, Stein E, Cho M, Tate A, et al. Efficacy and safety of lovastatin therapy in adolescent girls with heterozygous familial hypercholesterolemia. Pediatrics. 2005;116:682–8. doi: 10.1542/peds.2004-2090. [DOI] [PubMed] [Google Scholar]
- 10.de Jongh S, Ose L, Szamosi T, Gagne C, Lambert M, Scott R, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial with simvastatin. Circulation. 2002;106:2231–7. doi: 10.1161/01.cir.0000035247.42888.82. [DOI] [PubMed] [Google Scholar]
- 11.Vuorio A, Kuoppala J, Kovanen PT, Humphries SE, Strandberg T, Tonstad S, et al. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev. 2010:CD006401. doi: 10.1002/14651858.CD006401.pub2. [DOI] [PubMed] [Google Scholar]
- 12.McCrindle BW, Ose L, Marais AD. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. The Journal of pediatrics. 2003;143:74–80. doi: 10.1016/S0022-3476(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 13.McCrindle BW. Familial hypercholesterolemia in children and adolescents. Curr Opin Lipidol. 2012;23:525–31. doi: 10.1097/MOL.0b013e3283587522. [DOI] [PubMed] [Google Scholar]
- 14.Kusters DM, Avis HJ, de Groot E, Wijburg FA, Kastelein JJ, Wiegman A, et al. Ten-year follow-up after initiation of statin therapy in children with familial hypercholesterolemia. JAMA: the journal of the American Medical Association. 2014;312:1055–7. doi: 10.1001/jama.2014.8892. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics. Committee on Nutrition. Cholesterol in childhood. Pediatrics. 1998;101:141–7. [PubMed] [Google Scholar]
- 16.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 (Suppl 5):S213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Psaty BM, Rivara FP. Universal screening and drug treatment of dyslipidemia in children and adolescents. JAMA: the journal of the American Medical Association. 2012;307:257–8. doi: 10.1001/jama.2011.1916. [DOI] [PubMed] [Google Scholar]
- 18.Lasky T. Statin use in children in the United States. Pediatrics. 2008;122:1406–8. doi: 10.1542/peds.2008-2231. author reply 8. [DOI] [PubMed] [Google Scholar]
- 19.Liberman JN, Berger JE, Lewis M. Prevalence of antihypertensive, antidiabetic, and dyslipidemic prescription medication use among children and adolescents. Archives of pediatrics & adolescent medicine. 2009;163:357–64. doi: 10.1001/archpediatrics.2009.5. [DOI] [PubMed] [Google Scholar]
- 20.Vinci SR, Rifas-Shiman SL, Cheng JK, Mannix RC, Gillman MW, de Ferranti SD. Cholesterol testing among children and adolescents during health visits. JAMA: the journal of the American Medical Association. 2014;311:1804–7. doi: 10.1001/jama.2014.2410. [DOI] [PubMed] [Google Scholar]
- 21.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22:218–53. doi: 10.1097/01.JCN.0000267827.50320.85. [DOI] [PubMed] [Google Scholar]
- 22.Brunelli SM, Gagne JJ, Huybrechts KF, Wang SV, Patrick AR, Rothman KJ, et al. Estimation using all available covariate information versus a fixed look-back window for dichotomous covariates. Pharmacoepidemiol Drug Saf. 2013;22:542–50. doi: 10.1002/pds.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dart AB, Martens PJ, Sellers EA, Brownell MD, Rigatto C, Dean HJ. Validation of a pediatric diabetes case definition using administrative health data in manitoba, Canada. Diabetes care. 2011;34:898–903. doi: 10.2337/dc10-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandenbroucke JP, Pearce N. Case-control studies: basic concepts. Int J Epidemiol. 2012;41:1480–9. doi: 10.1093/ije/dys147. [DOI] [PubMed] [Google Scholar]
- 25.McCrindle BW, Tyrrell PN, Kavey RE. Will obesity increase the proportion of children and adolescents recommended for a statin? Circulation. 2013;128:2162–5. doi: 10.1161/CIRCULATIONAHA.113.002411. [DOI] [PubMed] [Google Scholar]
- 26.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. Journal of clinical epidemiology. 2005;58:323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Feudtner C, Dai D, Hexem KR, Luan X, Metjian TA. Prevalence of polypharmacy exposure among hospitalized children in the United States. Archives of pediatrics & adolescent medicine. 2012;166:9–16. doi: 10.1001/archpediatrics.2011.161. [DOI] [PubMed] [Google Scholar]
- 28.Dixon DB, Kornblum AP, Steffen LM, Zhou X, Steinberger J. Implementation of lipid screening guidelines in children by primary pediatric providers. The Journal of pediatrics. 2014;164:572–6. doi: 10.1016/j.jpeds.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Avis HJ, Kusters DM, Vissers MN, Huijgen R, Janssen TH, Wiegman A, et al. Follow-up of children diagnosed with familial hypercholesterolemia in a national genetic screening program. The Journal of pediatrics. 2012;161:99–103. doi: 10.1016/j.jpeds.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert M, Lupien PJ, Gagne C, Levy E, Blaichman S, Langlois S, et al. Treatment of familial hypercholesterolemia in children and adolescents: effect of lovastatin. Canadian Lovastatin in Children Study Group. Pediatrics. 1996;97:619–28. [PubMed] [Google Scholar]
- 32.de Ferranti S, Ludwig DS. Storm over statins--the controversy surrounding pharmacologic treatment of children. N Engl J Med. 2008;359:1309–12. doi: 10.1056/NEJMp0805953. [DOI] [PubMed] [Google Scholar]
- 33.Loughlin J, Quinn S, Rivero E, Wong J, Huang J, Kralstein J, et al. Tegaserod and the risk of cardiovascular ischemic events: an observational cohort study. J Cardiovasc Pharmacol Ther. 2010;15:151–7. doi: 10.1177/1074248409360357. [DOI] [PubMed] [Google Scholar]
- 34.Dore DD, Ziyadeh N, Cai B, Clifford CR, Norman H, Seeger JD. A cross-sectional study of the identification of prevalent asthma and chronic obstructive pulmonary disease among initiators of long-acting beta-agonists in health insurance claims data. BMC Pulm Med. 2014;14:47. doi: 10.1186/1471-2466-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]