Abstract
Significant advances have been made in the understanding of disease progression in cystic fibrosis (CF), revealing a complex interplay between host and pathogenic organisms. The diverse CF microbiota within the airway activates an aberrant immune response that is ineffective in clearing infection. An appreciation of how the CF host immune system interacts with these organisms is crucial to understanding the pathogenesis of CF pulmonary disease. Here we discuss the microbial complexity present in the lungs of individuals with CF, review emerging concepts of innate and adaptive immune responses to pathogens that chronically inhabit the CF lung, and discuss therapies that target the aberrant inflammatory response that characterizes CF. A greater understanding of the underlying mechanisms will shed light on pathogenesis and guide more targeted therapies in the future that serve to reduce infection, minimize lung pathology, and improve the quality of life for patients with CF.
Keywords: Cystic fibrosis, host-pathogen interaction, microbiota, innate and adaptive immunity, lung disease
Overview of infection and disease
Respiratory failure resulting from chronic infection and inflammation of the airways still represents the primary cause of death for most individuals with cystic fibrosis (CF) [1]. As such, a better understanding of infection and immunity related pathology of the CF airways is needed. Pseudomonas aeruginosa, Staphylococcus aureus including the methicillin-resistant (MRSA), Haemophilus influenzae, and Burkholderia cepacia complex, remain the primary pathogens associated with airway inflammation, although additional important opportunistic pathogens including Achromobacter xylosoxidans, Stenotrophomonas maltophilia and non-tuberculous mycobacterium are emerging [2]. Newly developed culture and molecular approaches have allowed for greater appreciation of new and/or emerging pathogens, and complex bacterial communities, or microbiota, in the CF airways. Respiratory tract infection contributes towards a dysregulated host immune response in CF, impacting both innate and adaptive immunity and perpetuating a cycle of inflammation and disordered microbiota. Recent research has unveiled the complexity of the relationship between the traditional pathogens, overlooked lung microbiota and host immune response (Fig 1), although our understanding is still incomplete. In this article, we review new insights into CF pathogenesis and discuss their potential importance in prevention and treatment of pulmonary disease. The key points presented herein are summarized in the highlights.
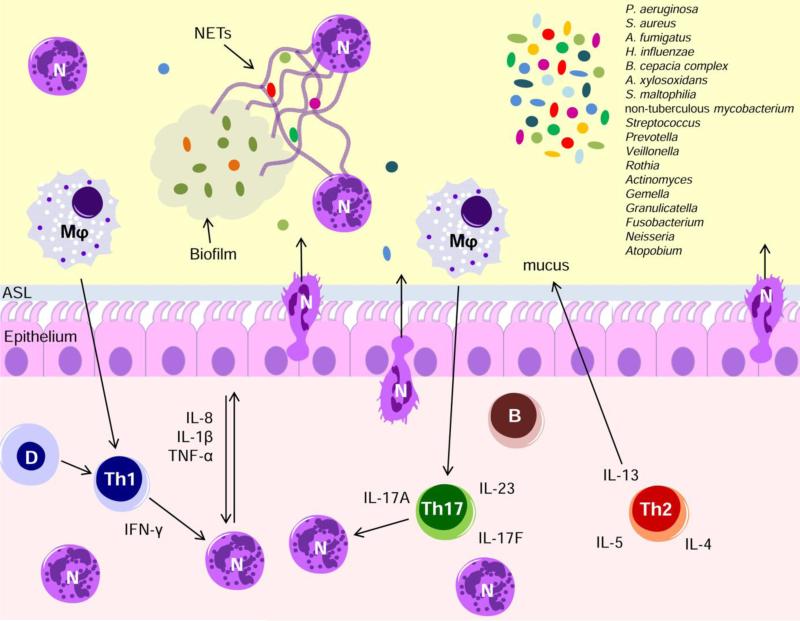
Figure 1. Innate and adaptive immune response to infections in CF.
Infection by several different pathogens triggers perpetual bouts of neutrophil recruitment and breach of the airway epithelium. In the airspace, neutrophils encounter persistent microbial biofilms and release neutrophil extracellular traps (NETs) that are ineffective and ultimately repurposed for the benefit of the microbial biofilms. Following continuous cycles of acute infection or once chronic infection is established, T lymphocytes are recruited and polarized to Th1, Th2 and/or Th17 cells. IL-17A and related cytokines, as well as IFN-γ play an important role in the excessive recruitment of neutrophils. The effector phase of ABPA, associated to A. fumigatus infections, is determined by Th2-mediated allergic responses, including eosinophilia, mucus production, and airway hyperresponsiveness. The relevance of other emerging pathogens in CF lung disease progression still remains to be fully elucidated. N, Neutrophils; Mφ, macrophages; D, dendritic cells; B, B cells.
Role of lung microbiota and pathogenesis
The CF airway represents a permissive environment for microbial colonization [3]. The natural history of CF is characterized by early colonization of the lung by S. aureus, followed occasionally by H. influenzae [4]. Although the consequences of H. influenza remain unclear, evidence suggests that S. aureus worsens pulmonary disease [5]. Predisposition and factors contributing to colonization with these early pathogens are still unclear. Although the lungs were classically believed to be sterile, recently published investigations have identified microbial communities in the airway of healthy humans [6]. Similarly, microflora in infants with CF have been described [7]. In addition, discrepancies in lung microbiota composition between clinically stable children with CF and children without CF suggest a modification of the airway microflora occurring early in life in CF [19]. Whether a specific microbiome may predispose CF patients to early colonization is still to be established. In this context, it is hypothesized that early pathogens cause inflammation and damage to the airway and act as gateway organisms paving the way for colonization with P. aeruginosa, which remains the most significant pathogen in CF. The source of the initial acquisition of P. aeruginosa seems to be environmental reservoirs, although cross-infection outbreaks have been increasingly reported [8]. Early P. aeruginosa colonization has a significant impact on the prognosis of patients with CF, especially if associated with initial S. aureus colonization, likely related to its extensive set of virulence factors and ability to evolve strategies to escape antibiotic treatments and immune response [9].
Other organisms such as Burkholderia sp., Stenotrophomonas maltophilia, Achromobacter xylosoxidans, fungi including Aspergillus, and nontuberculous mycobacteria (NTM) have emerged. Several organisms, including S. maltophilia, were initially considered to be harmless colonizers of the lung, however, newer data suggest they may participate in pulmonary deterioration in CF lung disease [10]. The perception of NTM disease has undergone a notable shift as well [11]. NTM, such as Mycobacterium abscessus Complex and Mycobacterium avium Complex, are now recognized as insidious opportunists, rather than colonizers, that can lead to increased morbidity and mortality in CF, and identification and speciation are important for tailoring therapy. Fungi, including Aspergillus fumigatus, are also increasingly recognized as disease causing in CF and may be associated with a more rapid decline in pulmonary function [12].
Clinical culture-based laboratory testing for specific pathogenic species has led to a reductionist view of the microbiology in the CF niche. The list of new and/or emerging pathogens associated with CF patients has been increasing with the introduction of culture-independent, DNA-based methods and the advent of next-generation sequencing methods [2]. It is now recognized that numerous bacterial species, with a high prevalence of anaerobic bacteria, permanently reside in CF airways. The top ten most common genera recovered from the CF microbiome independent of the conventional pathogens included Streptococcus, Prevotella, Veillonella, Rothia, Actinomyces, Gemella, Granulicatella, Fusobacterium, Neisseria, and Atopobium [13]. Airway microbiota changes in response to a range of factors, most notably host immune response and treatment [14]. Furthermore, diverse and spatially heterogeneous microbiotas, are present in the CF lung disease [15]. However, several studies have shown that the combination of species colonizing the lungs in CF differs between individuals, with a loss of bacterial diversity associated with increasing age, reduced lung function, and disease progression [16] [17]. The role of these emerging bacteria in the pathophysiology of infection and inflammation in chronic lung disease is still unclear and needs further investigation.
Role of immunity in CF disease pathogenesis
A major hallmark of CF lung disease is a chronic, aberrant state of inflammation, which is ineffective in clearing infection. There is much debate over the underlying cause for this state; with evidence to suggest CF immune responses are defective in their ability to respond to chronic infection, and also with evidence for the establishment of a permissive environment for chronic infection in the CF airway encouraging unresolved inflammation and immune activity [18]. Microbes found in CF airways have been shown to evade immune response or harness host responses to their benefit, further complicating matters. In the sections below we report new insights on the contribution of host-pathogen interplay to CF pathogenesis and discuss its potential importance for therapeutic treatments.
i) Innate immunity
Recognition of infection and recruitment of neutrophils
Innate immunity operating in the lung pairs physical barriers to infection with the ability of resident cells to sense and respond to infection eliciting the mucosal recruitment of effector cells that facilitate rapid clearance. Mucous barriers and mucociliary transport block infection from reaching the airway epithelium in a gel-on-brush manner [19] and transport mucus and debris out of the airways to the oropharynx where it can be coughed out or swallowed. Cystic Fibrosis Transmembrane conductance Regulator (CFTR) dysfunction is associated with impaired mucociliary clearance due to reduced height of the periciliary layer and increased mucus viscosity in the airway [20]. Defects in the airway surface layer and mucociliary clearance are further outlined in the review by Cantin et al. Airway epithelial cells, macrophages, and dendritic cells recognize pathogen-associated molecular patterns (PAMPs) that evade mucociliary clearance. Pattern recognition receptors (PRRs) including Toll Like Receptors (TLRs) and intracellular Nod Like Receptors (NLRs) recognize PAMPs such as cell wall lipoproteins, LPS, and flagellin, facilitating pathogen identification. These interactions result in immune signaling through the adapter proteins myeloid differentiation factor 88 (MyD88) or apoptosis-associate speck-like protein containing a caspase recruitment domain (ASC) triggering further cascades and production of pro-inflammatory mediators, such as TNFα, IL-8, and IL-1β [21]. The sensing of pathogen and release of inflammatory mediators are thought to be exquisitely orchestrated to promote neutrophil recruitment and rapid pathogen clearance followed by swift resolution. In CF, many aspects to this process appear to be dysregulated. For example, CF bronchial epithelial cell lines display aberrant PRR signaling and constitutively elevated NF-κB activity [22-24]. Sputum and bronchoalveolar lavage (BAL) reveal increased levels of pro-inflammatory cytokines, such as TNFα, IL-6, IL-8 and IL-1β, and decreased levels or anti-inflammatory cytokines, such as IL-10 [25, 26].
Dysregulation of CF innate immune signaling likely impacts neutrophil recruitment to the airway as well as neutrophil function at the mucosal surface. In CF, neutrophil recruitment to the airspace is excessive and perpetual, yet fails to eradicate airway infection. Circulating neutrophils respond rapidly to signals triggered by infections at mucosal surfaces and exit the bloodstream. The process of neutrophil migration in response to infection relies on carefully orchestrated cytokine deployment, signaling pathways, adhesion interactions, multiple chemokines such as IL-8 and additional chemo-attractants that cooperate to direct neutrophils to the infected airway [27]. Upon exiting circulation and entering the extracellular space, pericytes and fibroblasts contribute to the guidance of neutrophils towards the infected mucosal surface [27]. A recently identified collagen breakdown product, proline-glycine-proline (PGP), has been established as a neutrophil chemoattractant. PGP interacts with CXCR2 to stimulate migration [28] and is then degraded by a leukotriene hydrolase (LTA4H), facilitating the resolution of inflammation [29]. Additional intercellular signaling among neutrophils occurs via leukotriene B4 (LTB4), an arachidonic acid metabolite generated by 5-lipoxygenase that serves as a potent neutrophil chemo-attractant capable of amplifying recruitment [30, 31]. CF patients display abnormalities in levels of PGP peptides [28] and LTB4 [32], although the effect of these alterations are not fully appreciated.
Upon reaching the airway mucosal surface, neutrophilic transepithelial migration occurs. Bacterial infection of the epithelium results in generation of a variety of inflammatory mediators, including another arachidonic acid metabolite and neutrophil chemo-attractant known as hepoxilin A3 (HXA3) that is generated by12-lipoxygenase. Both in vitro and in vivo models have implicated a discrete role for HXA3 as a mucosal neutrophil chemoattractant secreted into the airways to drive neutrophils across the mucosal barrier [33]. Further, it appears that epithelial derived HXA3 operates in tandem with neutrophil derived LTB4 for fully maximize neutrophil trans-epithelial migration [31]. Along with chemotactic signals, neutrophil migration across the airway epithelial barrier also requires multiple adhesion interactions [34]. A recent study suggests a role for neutrophil triggering receptor expressed on myeloid cells 1 (TREM-1) in the process of trans-epithelial migration in the airway [35]. At present, little is known about mechanisms of neutrophil transepithelial migration in the context of the CF, however, once the neutrophil has reached the airway, the neutrophil undergoes activation, releasing elevated levels of proteases and cationic peptides, including neutrophil elastase [36] resulting in altered airway structure and permeability [37]. A better understanding of neutrophil recruitment mechanisms will inform our understanding of the key drivers of dys-regulated neutrophil recruitment in CF.
Neutrophil in the airspace; interaction with biofilms and formation of neutrophil extracellular traps
Upon entering the airspace of the CF lung, neutrophils seek to eradicate the inciting infection, but are faced with persistent aggregates of microbes called biofilms that frustrate neutrophil killing programs. Biofilms are organized by microorganisms and contain a matrix of extracellular DNA, proteins, and polysaccharides. P. aeruginosa is particularly adept at creating biofilms [38], although other CF pathogens including S. aureus, NTM, Burkholderia sp., A. fumigatus and mixed species are also capable of biofilms formation [39]. The benefit to microbes in producing a biofilm is speculated to be that the organized community of microbes within the biofilm have better access to nutrients, while also being shielded from both the immune response as well as antibiotics [40, 41]. Biofilms are problematic for the host for several reasons including contributing to antibiotic/antifungal resistance [42] and perpetuating the inflammatory state. Biofilms factors, such as 3OC12-HSL N-(3-oxododecanoyl)-L-homoserine lactone [43], and extracellular DNA from microorganisms induce neutrophil migration and further activate neutrophils [44]. Recruited neutrophils associated with biofilms are less effective at eradicating bacteria. Biofilm-entrapped neutrophils have decreased mobility, decreased enzyme production and ineffective oxidative burst [45]. Additionally, P. aeruginosa engaged in quorum-sensing within the growing biofilm produce rhamnolipids, a bacterial product that can induce rapid neutrophil necrosis [46], shielding biofilm bacteria from incoming neutrophils [47]. P. aeruginosa then repurpose the contents of the necrotic neutrophil to enhance biofilm development, incorporating neutrophil derived actin and DNA into the biofilm further promoting attachment of additional bacteria [48]. Therefore, despite the pronounced ability to recruit neutrophils into the CF airway, neutrophils are ineffective in clearing the inciting infection and ultimately serve to the detriment of the lung by contributing to biofilm formation and host tissue destruction.
An important component of innate host-microbe interactions includes the ability of neutrophils to form antimicrobial neutrophil extracellular traps (NETs). NETs are made up of neutrophil DNA, histones and proteolytic enzymes, actively expelled by the neutrophil as a means to ensnare nearby microbes including those that are predominant in the CF airspace, such as P. aeruginosa, H. influenzae and S. aureus, as well as fungal pathogens A. fumigatus and C. albicans [49]. Each of these pathogens, as well as various endogenous signals, is in turn capable of stimulating NETs formation. NETs signaling predominantly occurs through the Raf-MEK-ERK pathway [50] and requires NADPH activation with ROS production in many cases [51]. Resolution of the inflammatory debris created by NET formation is mediated by endogenous DNAse and neutrophil derived SerpinB1 [52], which is an inhibitor of neutrophil serine proteases, elastase, cathepsin G, and proteinase-3 [53]. NETs are an important part of the immune response, however, in CF, NETs are detrimental and contribute to the thick tenacious airway secretions that lead to progressive lung disease, entangling, rather than clearing airway infection [54]. One of the mechanisms involved in Pseudomonas-derived NETs formation requires pyocyanin, which promotes NETs formation in a time- and dose-dependent manner [55]. Despite the drive to create NETs, clinical isolates of P. aeruginosa obtained from CF patients demonstrated an acquired capacity to withstand NETs-mediated killing, which may be related to the mucoid phenotype of the microbe but not necessarily a direct result of excess alginate production [56]. Furthermore, membrane sialoglycoproteins of P. aeruginosa interact with neutrophils to reduce ROS production, elastase release and NETs formation while prolonging Pseudomonas survival [57]. S. aureus drive NETs formation, however, they also secrete enzymes capable of converting NETs to deoxyadenosine, diminishing their antimicrobial capacity while simultaneously providing a trigger for host immune cell death [58]. Thus, in CF, NETs formation most likely fails to destroy the plethora of pathogen encountered in the airspace and may only contribute to neutrophil mediated damage of lung tissue. Furthermore, the formation of NETs likely reinforces microbial biofilms in CF, facilitating microbial persistence and adding to the dysregulation of the innate immune system, as is the case with H. Influenzae whereby recruited neutrophils produce ineffective NETs that merely serve the construction of the biofilm [59]. The extracellular DNA critical for P. aeruginosa biofilms previously ascribed mostly to necrotic neutrophils is now thought to also originate to a large extent from NETs release [60]. A better understanding of potential merits and drawbacks of NETs formation in the CF airway with regard to pathogen clearance, resolution of inflammation, and lung function will be critical when considering therapeutic strategies capable of modulating NETs.
Macrophage-mediated inflammation
Macrophages likely play a significant yet underappreciated role in CF, via aberrant scavenger signaling cascades, resulting in chronic inflammation yet ineffective clearance of infection in CF. CFTR mutations result in impaired bacterial killing in murine and human macrophages with dysregulated cytokine production [61] [62], including high concentrations of pro-inflammatory cytokines released by macrophages, such as IL-1α, IL-6, G-CSF and IL-8. Elevated production of these cytokines was also confirmed in bone marrow and alveolar macrophages from cftr-deficient mice after in vitro stimulation with LPS from P. aeruginosa [63]. There is debate over whether CFTR-deficient macrophages fail to acidify lysosomes and phagolysosomal compartments resulting in altered bactericidal activity [64, 65]. CFTR mutations in human and murine macrophages have also been associated with abnormal signaling and trafficking of TLR-4[66]. Finally, there is also evidence that virulence factors as pyocyanin released from P. aeruginosa compromises macrophage efferocytosis of apoptotic neutrophils indicating that the microbial environment could further suppress phagocytic functions [67]. These data suggest that CFTR directly contributes to microbicidal dysfunction of macrophages.
ii) Adaptive immunity
Cellular adaptive immune response to infection in CF
While neutrophils are the dominant cell population in the broncho-alveolar compartment of CF patients, recent studies indicate that T lymphocytes accumulate within the subepithelial bronchial tissue, although the bronchial space is almost devoid of T cells. CFTR present on circulating T lymphocytes participates in immune cell signaling, and this CFTR mediated signaling is absent in CF patients [68] further suggesting a role for T cells in the pathogenesis of CF disease. The adaptive immune response to infections and, in particular, the specific subsets of Th cells recruited in CF are still debated and may be pathogen specific. Historically, the predilection to mount a Th2 response appeared to be central to the CF T-cell phenotype, although a Th2 response is pro-allergic and is geared for fighting parasites, which are rare in CF, and less effective at fighting pathogens such as P. aeruginosa. In CF, approximately 4-15% of patients develop allergic bronchopulmonary aspergillosis (ABPA) upon exposure to A. fumigatus [69]. Although the mechanisms for developing ABPA are not fully understood, they are characterized by an exaggerated IgE response toward A. fumigatus, a shift to a predominantly Th2 –related cytokine profile (IL-4 and IL-13) and increased sensitivity to IL-4 [70, 71]. Th2 cells are considered to be deleterious during fungal infections in part because they dampen the protective Th1 cell response. In this context, Th cells from patients with CF produce lower levels of Th1 –related cytokine IFN-γ, and higher levels of Th2 cytokine IL-10 [72]. The observation that IL-4-deficient mice are resistant to invasive pulmonary aspergillosis owing to enhanced IFN-γ production, while IFN-γ-deficient mice are highly susceptible [73], further underscores the importance of the Th2/Th1 balance. The role of Th17 cells in CF host response to A. fumigatus is still unclear. A robust antigen-specific Th17 response has been seen in the lungs of CF patients when stimulated with antigens from Aspergillus spp.. However, intranasal infection of mice with A. fumigatus induced detrimental Th17 responses because of the negative regulation of protective Th1 responses. Neutralization of Th17-associated cytokines greatly increased resistance to Aspergillus infections [74].
A Th1 response has been associated with S. aureus infection in CF. Exposure of CF epithelial cells to S. aureus increased MIG and MIP-3β secretions, a chemoattractant for Th1 lymphocytes [75], suggesting that S. aureus bias the immune response toward a Th1 phenotype. Recently it has been shown that the presence of S. aureus in the sputum from CF patients did not affect the IL-17 or IL-23 levels [76], suggesting that Th17 cells are not involved in the response to this pathogen.
A Th2-dominated immune response in CF patients with chronic P. aeruginosa lung infection as compared with CF patients without chronic infection was observed, whereas Th1 responses were accompanied by a better pulmonary outcome [77] [78]. Another study reported significantly higher Th1 – (IFN-γ) cytokine IFN-γ expression in bronchial biopsies from chronic stable compared with CF patients with acute exacerbations [79]. Evidence in murine model further supports this view. When cftr-deficient mice were infected with P. aeruginosa, recovery from infection was accompanied by a shift to a Th1 response while deficiency to produce the IFN-γ increased risk to P. aeruginosa infections [80]. Although there is growing evidence that a Th1-dominated immune response might improve the prognosis of CF patients with chronic P. aeruginosa lung infection, the host would presumably benefit also of a Th17 response. Recently, the potential key role of Th17 signaling in the host response to this extracellular pathogen is increasingly emerging. Higher levels of IL-17 and IL-23 cytokines were described in patients chronically infected with P. aeruginosa as compared to those who were not chronically infected with P. aeruginosa [81]. Furthermore, increased levels of IL-17 in bronchoalveolar lavage fluid during exacerbations was found associated with positive Pseudomonas species sputum cultures [82]. In addition, a crucial role has been proposed for IL-17–producing cells in inflammation in the lung of patients with CF promoting inflammation-related destruction of lung tissue, supporting neutrophil recruitment, and defined IL-17A to be a marker preceding infection with P. aeruginosa [76]. How different Th cell sub-types respond to different and emerging pathogens is still unknown.
Novel insight in new T-cell subsets (Th17-Th2, Th17-Th1) and the capacity of Th17 cells to produce Th2-type cytokines strengthens the complexity and plasticity of the adaptive immune system [76]. Moreover, the presence of a complex microbiota and multiple pathogens which vary among CF patients combined with a lack of comprehensive clinical studies on adaptive immune responses align to complicate characterization of the adaptive immune response in CF. Cross-sectional data collection, inspiring the majority of the past studies, does not inform whether any causal relationship exists among original lung microbiota composition, infection, lymphocytes subsets and clinical parameters. Future longitudinal studies dissecting the kinetics and dynamics of the adaptive immune response to infection will be essential to identify specific lymphocytes subtypes involved in CF lung disease, and to define the sequence of causal events occurring from birth.
Humoral adaptive immune response to infection in CF
B cells are critical for pulmonary protection against encapsulated bacteria. High numbers of B cells were seen in CF subjects infected with P. aeruginosa and low levels in subjects under treatment for an acute pulmonary exacerbation suggesting a role in CF pathogenesis. Patients with persistent P. aeruginosa infection present also very high titers of immunoglobulins (Ig) against P. aeruginosa in the airway surface liquid, in particular secretory IgA. However, this response appears to be insufficient to remove P. aeruginosa from the lung, or to prevent the development of a persistent infection, or the acquisition of new strains of P. aeruginosa. It is likely that this vigorous antibody response is mainly responsible for preventing bacterial systemic spread of P. aeruginosa as patients with persistent infection rarely develop pseudomonal bacteraemia or sepsis. Indeed, mice with a defective poly-Ig receptor, involved in the transport of secretory IgA into the lumen of the lung, are unable to produce secretory IgA in lung secretions and show a threefold increase in mortality from intratracheal infection with Pseudomonas [83]. The role of Ig in defense against P. aeruginosa has also been addressed by vaccination. The pseudomonal flagellum is a potent immunogen that has been developed into a vaccine candidate and tested in phase III trials [84]. However, the results of trials published up to now warned from relying on vaccines against P. aeruginosa to prevent initial infection in CF [85].
Therapeutic options to target host and pathogen response
Multiple therapies are currently available that serve to modulate the immune response in CF patients and are discussed below. For further details see the review by Cantin et al. Recombinant human DNAse (dornase alpha) acts to degrade the extracellular DNA that is present in the sputum as a result of NET formation serving to dampen inflammation mediated by IL-8 and Il-1β [86]. High dose ibuprofen decreases neutrophil migration as measured through oral mucosal washes in clinical trials [87] and slows the progression of CF lung disease, particularly in children [88]. The mechanism involved in ibuprofen-affected neutrophil migration has not been clearly delineated but may be due to reduced CD11 upregulation and polymerization of actin in response to IL-8 and C5a [89]. Azithromycin has been shown to reduce pulmonary exacerbations and improve CF lung function [90] by different mechanisms, which include suppression of cytokine production, increased tight junctions and protection of respiratory epithelium from damage. N-acetylcysteine (NAC) exhibits anti-inflammatory properties, breaks disulfide bonds to inhibit biofilm formation [91], depolymerizes mucous [92], and inhibits NETs formation [93], however, the clinical effectiveness of this approach has yet to be realized [94]. Corticosteroids are potent anti-inflammatory agents and have been shown to reduce neutrophil adhesion and tissue infiltration as well as decrease both endothelial and epithelial transmigration [95] and reduce the rate of decline of pulmonary function [96]. However, the adverse effects related to steroids, limit their use in CF patients, while inhaled steroids appear ineffective unless treating comorbid asthma or ABPA [97]. Leukotrienes are over-produced in CF airways, however the effect of leukotriene inhibitors on lung function is unclear [97]. Generally, leukotriene inhibitors, common in asthma treatment, are not implemented in the treatment of lung disease related to CF, perhaps due to their non-specific effect on leukotrienes rather than specifically targeting subtypes of leukotriene involved in neutrophilic inflammation. A clinical study to investigate the LTB4-receptor antagonist BIIL 284 administered orally in CF patients was prematurely terminated due to a significantly increased risk of adverse pulmonary events [98]. Subsequently animal models revealed that BIIL 284 decreased pulmonary neutrophils but increased P. aeruginosa burden leading to high bacteremia rates [99]. These data suggest that caution should be taken when administering anti-inflammatory compounds to patients with bacterial infections. The challenge ahead is the development of more targeted and effective approaches that can diminish excessive inflammation while maintaining pathogen control.
Several targets for the treatment of CF pulmonary inflammation are under investigation as reported in the CF Foundation Drug Development Pipeline [100]. Neutrophils release large amounts of elastase following activation, which is normally neutralized by alpha 1 anti-trypsin (AAT). AAT has been shown to significantly reduce airway disease in mice infected with P. aeruginosa pneumonia [101]. Human recombinant AAT (rAAT) is currently under investigation in patients with CF [102]. KB001-A, a humanized monoclonal antibody targets the type III secretion system in P. aeruginosa. Phase 2 trials in non-CF Pseudomonas-colonized ventilated patients displayed safety and tolerability and reduced colonization in patients receiving treatment as compared to placebo [103]. Preliminary studies in CF showed a trend towards decreased sputum MPO, IL-1, IL-8, elastase, and neutrophil counts in CF patients receiving a single dose of KB001-A. However, there were no differences in P. aeruginosa density, clinical symptoms, or lung function [104]. Phase 2 trials in CF patients are underway. Several opportunities exist going forward to design additional therapies when considering the wealth of newly described aspects of the host-pathogen interplay reviewed above. Novel anti-inflammatory approaches when combined with currently effective treatments such as CFTR modulation and correctors, hypertonic saline, mannitol, chest physiotherapy, and antibiotics may work more effectively to both combat infection and reduce destructive inflammation.
Conclusions
CF is an extremely complex disease resulting from the inheritance of a single mutated gene. Clearly compromised expression of CFTR has wide ranging consequences with regard to innate and adaptive immunity permitting the growth of an evolving polymicrobial community in the airspace that cannot be cleared or accommodated. Recent significant advances in our understanding of host-pathogen interactions in this disease continue to provide novel strategies for therapeutic development (see also the review by Cantin et al). However, several challenges lie ahead in the quest to improve upon the current list of available therapies. The true nature of the evolving polymicrobial community that thrives in the CF airway as well as the architecture of the biofilms that these varied species generate will need further exploration. It is critical to gain a clearer picture of the mechanism behind perpetual neutrophil recruitment to the airspace both in the context of CFTR deficiency and in the face of continued exposure to microbial triggers of the evolving polymicrobial community. Furthermore, many questions remain regarding the adaptive immune response to individual pathogens as well to the collective microbial community in the CF airway. Improving our knowledge of T cell population dynamics in CF and how the balance between inflammatory and regulatory T cells impact microbial persistence, inflammatory mediated damage, and lung function will guide therapeutic options and define potential “windows of opportunity” to target adaptive immune processes. Clever strategies are needed to dampen aggressive persistent inflammation, while preserving the ability of the host to compartmentalize chronic infection. A better understanding of these issues has great potential to usher the development of more targeted therapies to reduce infection, minimize lung pathology, and improve the quality of life for patients with CF.
Highlights.
CF pulmonary microbiota differs spatially and temporally within and between individuals
Innate and adaptive immune responses, including emerging cell-subset and pathways, are sustained, but ineffective at eradicating pathogens
Longitudinal studies and appropriate animal models are required to dissect the sequence of immune and infectious events occurring in CF
Careful understanding of immune system dysfunctions in CF is critical to identifying opportunities to dampen inflammation without compromising pathogen clearance.
Acknowledgments
Supported by Ministero della Salute (project GR/2009/1579812) to AB, Italian Cystic Fibrosis Foundation (FFC#9/2014) to AB and (FFC#14/2013) to CC. Support for BPH and LMY include NIH/NIAID R01 AI095338 and the Department of Pediatrics at MGHfC respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gibson RL, B.J., Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Lipuma J. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev. 2010;23(2):299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevivino A, Bragonzi A. The evolving polymicrobial composition in the airways of patients with Cystic Fibrosis: implications for disease progression and clinical management. CML – Cystic Fibrosis. 2013;3(4):93–104. [Google Scholar]
- 4.Armstrong D, Grimwood K, Carlin JB, Carzino R, Gutierrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156:1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 5.Kahl B. Impact of Staphylococcus aureus on the pathogenesis of chronic cystic fibrosis lung disease. Int J Med Microbiol. 2010;300(8):514–9. doi: 10.1016/j.ijmm.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Beck JM, Young VB, Huffnagle GB. The microbiome of the lung. Transl Res. 2012;160(4):258–66. doi: 10.1016/j.trsl.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madan J, Koestler DC, Stanton BA, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012;21(3):4. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiman L. Infection prevention and control in cystic fibrosis. Current Opinion in Infectious Diseases. 2011;24:390–395. doi: 10.1097/QCO.0b013e32834748ff. [DOI] [PubMed] [Google Scholar]
- 9.Cigana C, Lorè NI, Bernardini ML, Bragonzi A. Dampening host sensing and avoiding recognition in Pseudomonas aeruginosa pneumonia. J Biomed Biotechnol. 2011:852513. doi: 10.1155/2011/852513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters V, et al. Stenotrophomonas maltophilia in cystic fibrosis: serologic response and effect on lung disease. Am J Respir Crit Care Med. 2011;183(5):635–40. doi: 10.1164/rccm.201009-1392OC. [DOI] [PubMed] [Google Scholar]
- 11.Tavs Q. Tania Pressler, Niels Høiby, Terese L Katzenstein, Shifting paradigms of nontuberculous mycobacteria in cystic fibrosis. Respir Res. 2014;15(1):41. doi: 10.1186/1465-9921-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin R, Dupuis A, Aaron SD, Ratjen F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest. 2010;137(1):171–6. doi: 10.1378/chest.09-1103. [DOI] [PubMed] [Google Scholar]
- 13.Surette M. The cystic fibrosis lung microbiome. Ann Am Thorac Soc. Suppl. 2014;1:S61–5. doi: 10.1513/AnnalsATS.201306-159MG. [DOI] [PubMed] [Google Scholar]
- 14.Conrad D, Haynes M, Salamon P, Rainey PB, Youle M, Rohwer F. Cystic fibrosis therapy: a community ecology perspective. Am J Respir Cell Mol Biol. 2013;48(2):150–6. doi: 10.1165/rcmb.2012-0059PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown P, Pope CE, Marsh RL, Qin X, McNamara S, Gibson R, Burns JL, Deutsch G, Hoffman LR. Directly sampling the lung of a young child with cystic fibrosis reveals diverse microbiota. Ann Am Thorac Soc. 2014;11(7):1049–55. doi: 10.1513/AnnalsATS.201311-383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox M, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One. 2010;5:e11044. doi: 10.1371/journal.pone.0011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Schloss PD, Kalikin LM, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieber N, et al. Current concepts of immune dysregulation in cystic fibrosis. Int J Biochem Cell Biol. 2014;52:108–12. doi: 10.1016/j.biocel.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Button B, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–41. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13(6):231–40. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Skerrett SJ, et al. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L312–22. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 23.John G, et al. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol. 2010;42(4):424–31. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkatakrishnan A, et al. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;23(3):396–403. doi: 10.1165/ajrcmb.23.3.3949. [DOI] [PubMed] [Google Scholar]
- 25.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133(2):489–95. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 26.Sagel SD, et al. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1425–31. doi: 10.1164/ajrccm.164.8.2104075. [DOI] [PubMed] [Google Scholar]
- 27.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83(2):309–36. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 28.Gaggar A, et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180(8):5662–9. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snelgrove RJ, et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330(6000):90–4. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lammermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498(7454):371–5. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pazos MA, et al. Distinct cellular sources of hepoxilin a3 and leukotriene b4 are used to coordinate bacterial-induced neutrophil transepithelial migration. J Immunol. 2015;194(3):1304–15. doi: 10.4049/jimmunol.1402489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodini A, et al. Biomarkers of neutrophilic inflammation in exhaled air of cystic fibrosis children with bacterial airway infections. Pediatr Pulmonol. 2005;40(6):494–9. doi: 10.1002/ppul.20336. [DOI] [PubMed] [Google Scholar]
- 33.Tamang DL, et al. Hepoxilin A(3) facilitates neutrophilic breach of lipoxygenase-expressing airway epithelial barriers. J Immunol. 2012;189(10):4960–9. doi: 10.4049/jimmunol.1201922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurley BP, Sin A, McCormick BA. Adhesion molecules involved in hepoxilin A3-mediated neutrophil transepithelial migration. Clin Exp Immunol. 2008;151(2):297–305. doi: 10.1111/j.1365-2249.2007.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klesney-Tait J, et al. Transepithelial migration of neutrophils into the lung requires TREM-1. J Clin Invest. 2013;123(1):138–49. doi: 10.1172/JCI64181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sly PD, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963–70. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 37.Gifford AM, Chalmers JD. The role of neutrophils in cystic fibrosis. Curr Opin Hematol. 2014;21(1):16–22. doi: 10.1097/MOH.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 38.Parsek MR, Tolker-Nielsen T. Pattern formation in Pseudomonas aeruginosa biofilms. Curr Opin Microbiol. 2008;11(6):560–6. doi: 10.1016/j.mib.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Elias S, Banin E. Multi-species biofilms: living with friendly neighbors. FEMS Microbiol Rev. 2012;36(5):990–1004. doi: 10.1111/j.1574-6976.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- 40.Bjarnsholt T, et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005;151(Pt 2):373–83. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 41.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang WC, et al. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2013;57(5):2352–61. doi: 10.1128/AAC.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmermann S, et al. Induction of neutrophil chemotaxis by the quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone. Infect Immun. 2006;74(10):5687–92. doi: 10.1128/IAI.01940-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trevani AS, et al. Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol. 2003;33(11):3164–74. doi: 10.1002/eji.200324334. [DOI] [PubMed] [Google Scholar]
- 45.Jesaitis AJ, et al. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol. 2003;171(8):4329–39. doi: 10.4049/jimmunol.171.8.4329. [DOI] [PubMed] [Google Scholar]
- 46.Jensen PO, et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153(Pt 5):1329–38. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 47.Alhede M, et al. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology. 2009;155(Pt 11):3500–8. doi: 10.1099/mic.0.031443-0. [DOI] [PubMed] [Google Scholar]
- 48.Walker TS, et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun. 2005;73(6):3693–701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 50.Hakkim A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7(2):75–7. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farley K, et al. A serpinB1 regulatory mechanism is essential for restricting neutrophil extracellular trap generation. J Immunol. 2012;189(9):4574–81. doi: 10.4049/jimmunol.1201167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benarafa C, Priebe GP, Remold-O'Donnell E. The neutrophil serine protease inhibitor serpinb1 preserves lung defense functions in Pseudomonas aeruginosa infection. J Exp Med. 2007;204(8):1901–9. doi: 10.1084/jem.20070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo DG, et al. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes. Immunol Lett. 2014;160(2):186–94. doi: 10.1016/j.imlet.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Rada B, et al. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLoS One. 2013;8(1):e54205. doi: 10.1371/journal.pone.0054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young RL, et al. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6(9):e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khatua B, Bhattacharya K, Mandal C. Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J Leukoc Biol. 2012;91(4):641–55. doi: 10.1189/jlb.0511260. [DOI] [PubMed] [Google Scholar]
- 58.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342(6160):863–6. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langereis JD, Hermans PW. Novel concepts in nontypeable Haemophilus influenzae biofilm formation. FEMS Microbiol Lett. 2013;346(2):81–9. doi: 10.1111/1574-6968.12203. [DOI] [PubMed] [Google Scholar]
- 60.Rahman S, Gadjeva M. Does NETosis Contribute to the Bacterial Pathoadaptation in Cystic Fibrosis? Front Immunol. 2014:378. doi: 10.3389/fimmu.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartl D, et al. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros. 2012;11(5):363–82. doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggiò MA, Spadaro F, Guglietta S, Anile M, Venuta F, Quattrucci S, Ascenzioni F. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One. 2011;6(5):e19970. doi: 10.1371/journal.pone.0019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruscia E, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator-/- mice. Am J Respir Cell Mol Biol. 2009;40(3):295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di A, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8(9):933–44. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 65.Haggie PM, Verkman AS. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J Biol Chem. 2007;282(43):31422–8. doi: 10.1074/jbc.M705296200. [DOI] [PubMed] [Google Scholar]
- 66.Bruscia E, Zhang PX, Satoh A, Caputo C, Medzhitov R, et al. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol. 2011;186:6990–6998. doi: 10.4049/jimmunol.1100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bianchi SM, et al. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2008;177(1):35–43. doi: 10.1164/rccm.200612-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong YJ, et al. Activation of CFTR chloride current by nitric oxide in human T lymphocytes. EMBO J. 1995;14(12):2700–7. doi: 10.1002/j.1460-2075.1995.tb07270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal R, et al. Link between CFTR mutations and ABPA: a systematic review and meta-analysis. Mycoses. 2012;55(4):357–65. doi: 10.1111/j.1439-0507.2011.02130.x. [DOI] [PubMed] [Google Scholar]
- 70.Mueller C, et al. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. 2011;44(6):922–9. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knutsen AP, et al. Increased sensitivity to IL-4 in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy. 2004;59(1):81–7. doi: 10.1046/j.1398-9995.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 72.Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;120(3):518–25. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cenci E, et al. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis. 1999;180(6):1957–68. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 74.Zelante T, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37(10):2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 75.Al Alam D, et al. Impaired interleukin-8 chemokine secretion by staphylococcus aureus-activated epithelium and T-cell chemotaxis in cystic fibrosis. Am J Respir Cell Mol Biol. 2010;42(6):644–50. doi: 10.1165/rcmb.2008-0021OC. [DOI] [PubMed] [Google Scholar]
- 76.Tiringer K, et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2013;187(6):621–9. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- 77.Hartl D, et al. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. 2006;117(1):204–11. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 78.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Høiby N. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS. 2000;108(5):329–35. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 79.Wojnarowski C, Frischer T, Hofbauer E, Grabner C, Mosgoeller W, Eichler I, Ziesche R. Cytokine expression in bronchial biopsies of cystic fibrosis patients with and without acute exacerbation. 1999;145:1136–44. doi: 10.1183/09031936.99.14511369. [DOI] [PubMed] [Google Scholar]
- 80.Moser C, et al. Improved outcome of chronic Pseudomonas aeruginosa lung infection is associated with induction of a Th1-dominated cytokine response. Clin Exp Immunol. 2002;127(2):206–13. doi: 10.1046/j.1365-2249.2002.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Decraene A, et al. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable Cystic Fibrosis patients. Respir Res. 2010;11:177. doi: 10.1186/1465-9921-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan Y, Chen K, Duncan SR, Lathrop KL, Latoche JD, et al. Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes. The Journal of allergy and clinical immunology. 2013;131:1117–1129. doi: 10.1016/j.jaci.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amin PB, Diebel LN, Liberati DM. Secretory immunoglobulin A abrogates inflammatory responses and improves mortality after pseudomonas pneumonia. J Trauma. 2010;68(4):827–33. doi: 10.1097/TA.0b013e3181d486fe. [DOI] [PubMed] [Google Scholar]
- 84.Doring G, Meisner C, Stern M. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc Natl Acad Sci U S A. 2007;104(26):11020–5. doi: 10.1073/pnas.0702403104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johansen HK, Gotzsche PC. Vaccines for preventing infection with Pseudomonas aeruginosa in cystic fibrosis. Cochrane Database Syst Rev. 2013;6:CD001399. doi: 10.1002/14651858.CD001399.pub3. [DOI] [PubMed] [Google Scholar]
- 86.Fuxman Bass JI, et al. Extracellular DNA: a major proinflammatory component of Pseudomonas aeruginosa biofilms. J Immunol. 2010;184(11):6386–95. doi: 10.4049/jimmunol.0901640. [DOI] [PubMed] [Google Scholar]
- 87.Konstan MW, et al. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. J Pharmacol Exp Ther. 2003;306(3):1086–91. doi: 10.1124/jpet.103.052449. [DOI] [PubMed] [Google Scholar]
- 88.Lands LC, Stanojevic S. Oral non-steroidal anti-inflammatory drug therapy for cystic fibrosis. Cochrane Database Syst Rev. 2007;(4):CD001505. doi: 10.1002/14651858.CD001505.pub2. [DOI] [PubMed] [Google Scholar]
- 89.Bertolotto M, et al. Neutrophil migration towards C5a and CXCL8 is prevented by non-steroidal anti-inflammatory drugs via inhibition of different pathways. Br J Pharmacol. 2014;171(14):3376–93. doi: 10.1111/bph.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saiman L, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 91.Drago L, et al. Activity of N-acetyl-L-cysteine against biofilm of Staphylococcus aureus and Pseudomonas aeruginosa on orthopedic prosthetic materials. Int J Artif Organs. 2013;36(1):39–46. doi: 10.5301/ijao.5000135. [DOI] [PubMed] [Google Scholar]
- 92.Sprenger L, et al. Dexamethasone and N-acetyl-cysteine attenuate Pseudomonas aeruginosa-induced mucus expression in human airways. Pulm Pharmacol Ther. 2011;24(2):232–9. doi: 10.1016/j.pupt.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 93.Kirchner T, et al. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediators Inflamm. 2013;2013:710239. doi: 10.1155/2013/710239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Conrad C, et al. Long-term treatment with oral N-acetylcysteine: Affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J Cyst Fibros. 2014 doi: 10.1016/j.jcf.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 95.van Overveld FJ, et al. Inhibitory capacity of different steroids on neutrophil migration across a bilayer of endothelial and bronchial epithelial cells. Eur J Pharmacol. 2003;477(3):261–7. doi: 10.1016/s0014-2999(03)02153-8. [DOI] [PubMed] [Google Scholar]
- 96.Eigen H, et al. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. Cystic Fibrosis Foundation Prednisone Trial Group. J Pediatr. 1995;126(4):515–23. doi: 10.1016/s0022-3476(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 97.Flume PA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957–69. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 98.Konstan MW, et al. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J Cyst Fibros. 2014;13(2):148–55. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doring G, et al. BIIL 284 reduces neutrophil numbers but increases P. aeruginosa bacteremia and inflammation in mouse lungs. J Cyst Fibros. 2014;13(2):156–63. doi: 10.1016/j.jcf.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. http://www.cff.org/research/DrugDevelopmentPipeline/
- 101.Pott GB, et al. Alpha-1 antitrypsin reduces severity of pseudomonas pneumonia in mice and inhibits epithelial barrier disruption and pseudomonas invasion of respiratory epithelial cells. Front Public Health. 2013;1:19. doi: 10.3389/fpubh.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martin SL, et al. Safety and efficacy of recombinant alpha(1)-antitrypsin therapy in cystic fibrosis. Pediatr Pulmonol. 2006;41(2):177–83. doi: 10.1002/ppul.20345. [DOI] [PubMed] [Google Scholar]
- 103.Francois B, et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized,double-blind, placebo-controlled trial. Crit Care Med. 2012;40(8):2320–6. doi: 10.1097/CCM.0b013e31825334f6. [DOI] [PubMed] [Google Scholar]
- 104.Milla CE, et al. Anti-PcrV antibody in cystic fibrosis: a novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr Pulmonol. 2014;49(7):650–8. doi: 10.1002/ppul.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]