Abstract
Traumatic brain injury (TBI) affects millions of people worldwide every year. The primary impact initiates the secretion of pro- and anti-inflammatory factors, subsequent recruitment of peripheral immune cells and activation of brain-resident microglia and astrocytes. Chemokines are major mediators of peripheral blood cell recruitment to damaged tissue, including the TBI brain. Here we review the involvement of specific chemokine pathways in TBI pathology and attempts to modulate these pathways for therapeutic purposes. We focus on chemokine (C-C motif) ligand 2/chemokine (C-C motif) receptor 2 (CCL2/CCR2) and chemokine (C-X-C motif) ligand 12/chemokine (C-X-C motif) receptor 4 (CXCL12/CXCR4). Recent micro-array and multiplex expression profiling have also implicated CXCL10 and CCL5 in TBI pathology. Chemokine (C-X3-C motif) ligand 1/ chemokine (C-X3-C motif) receptor 1 (CX3CL1/CX3CR1) signaling in the context of TBI is also discussed. Current literature suggests that modulating chemokine signaling, especially CCL2/CCR2, may be beneficial in TBI treatment.
Keywords: Traumatic brain injury, inflammation, chemokines, CCR2
Traumatic brain injury
Traumatic brain injury (TBI) is defined as an open or closed head injury that disrupts brain function. Millions of people worldwide seek medical attention for TBI as a result of falls, motor vehicle accidents, sports- and war-related activities, among others. Depending on the etiology, TBIs can be closed head or penetrating, and occur in a single event or in a repetitive fashion [1]. Any of these types of injuries can result in various severities that are clinically classified as mild, moderate or severe based on a series of neurological tests [2].
Regardless of origin, TBI sufferers experience a relatively stereotyped array of symptoms associated with the injury: dizziness, confusion and sometimes loss of consciousness (especially in severe injury). Even after the initial injury is managed and resolved, ~70–80% of TBI patients develop long-lasting effects such as changes in personality and cognition, anxiety and depressive-like behaviors [3–6]. TBI also increases the risk for certain neurodegenerative conditions. For instance, repeated concussive TBI has been associated with the development of chronic traumatic encephalopathy (CTE) in athletes [7–9]. Furthermore, both repeated and single TBI show a strong association with increased Alzheimer’s disease (AD) risk or earlier AD onset [10–12]. Correlations with Parkinson’s disease and amyotrophic lateral sclerosis have also been reported, but the supporting evidence is not as strong as for CTE and AD [13].
An important determinant of brain pathology and functional recovery after TBI is whether the injury was focal or diffuse: whether it affected only the site of impact or led to the involvement of functionally and anatomically connected brain regions [14]. The minutes and hours after any TBI are characterized by acute pathology that includes – depending on the nature and extent of injury – tissue and blood-brain-barrier (BBB) disruption, release of excitotoxic compounds, axonal injury and neuronal death [15]. This primary pathology sets in motion events that perpetuate dysfunction over time, often associated with spreading of pathological changes to surrounding brain regions (diffuse injury) [14]. One of the main driving forces of this secondary pathology appears to be the inflammatory reaction after TBI [12].
TBI initiates an inflammatory reaction encompassing several inter-related components: release of intracellular components to the parenchyma from damaged cells; activation of brain-resident microglia and astrocytes; production of cytokines and chemokines; and recruitment of peripheral immune cells into the brain. Many of these processes influence each other to lead to complex interactions. For example, brain-resident cells secrete chemokines that attract peripheral cells, which in turn release signaling factors. These serve to recruit additional cells from the periphery, perpetuate activation of microglia and astrocytes, and damage neurons.
Considering the high prevalence of TBI and its association with serious neurological problems and risk for neurodegenerative diseases, there is strong impetus to develop new TBI therapies that not only promote cell survival immediately after the injury, but also address the development of secondary pathology. Because of the close association between neuropathology and inflammation in space and time, the latter has emerged as an important target for the amelioration of TBI [12, 16, 17]. Moreover, the persistent nature of inflammation suggests that modulating inflammatory pathways may provide an extended therapeutic window to prevent the development of secondary pathology and in this way to promote subsequent neurological recovery.
Inflammatory reaction following TBI
Molecular mediators
The use of animal models (Box 1), human surgical and post-mortem tissue samples, and analysis of cerebrospinal fluid (CSF) and plasma from TBI patients has elucidated the temporal profile of inflammatory events following TBI (Figure 1) [18]. Within minutes and hours of the injury, damaged cells release certain intracellular components that act as “Danger Associated Molecular Patterns” (DAMPs) and signal to other cells via pattern recognition receptors (Figure 1A). DAMPs include Heat shock proteins (HSP) 60 and 70, nucleic acids, and high mobility group protein B1 (HMGB1), which signal principally to tolllike receptors 2 and 4 (TLR2 and TLR4) [19]. In response to these and other stimuli, astrocytes, microglia and damaged neurons at the injury site start secreting cytokines and chemokines. This initial wave of inflammatory signals serves to activate microglia and astrocytes, possibly increase their migration to the site of damage and recruit peripheral immune cells. After they enter the brain, leukocytes initiate a second wave of inflammatory mediators that contributes to the tissue damage.
Box 1: Animal models of TBI.
There are several established animal models of TBI that simulate human injury to varying extents. Below are brief descriptions of models used in the studies that we reference. For more information, see Xiong et al. [86] for a nice review of the most commonly used models.
Stab injury
Stab injury is a type of penetrating injury that is delivered through a small craniotomy. Fine scissors or a thin membrane are inserted a defined distance (1–5 mm) into the brain parenchyma [87]. This type of injury was used for earlier studies of TBI in mice.
Weight drop/closed head injury (CHI)
There are several weight drop models developed by different groups that simulate CHI. Although generally applied to the closed skull, some of these injuries are delivered through a craniotomy, with the dura mater intact. Injury intensity is controlled by adjusting the weight of the dropped object and the height from which it is dropped. Depending on the exact model and injury intensity, CHI models induce concussions, brain contusion, diffuse axonal injury, hemorrhage and other features of human TBI. However, different weight drop models can have significant inter-animal variability.
Controlled cortical impact (CCI)
A piston driven by air pressure or electromagnetism impacts the head at a controlled angle, velocity and depth. CCI is most often delivered through a craniotomy, but newer variations of this model deliver an injury to the intact skull. Similar to CHI, CCI simulates aspects of concussions, brain contusion, and hemorrhage seen in human TBI. Unlike CHI, the injury is highly reproducible between animals.
Fluid percussion injury (FPI)
For FPI, rodents are connected through a craniotomy to a fluid-filled chamber with a small opening; a swinging pendulum hits one end of the chamber to generate a water pulse that impacts the exposed brain at the other end of the chamber. Depending on the location of the craniotomy, the injury can be delivered to the side of the brain (lateral FPI) or the midline (central FPI). Injury intensity is controlled by adjusting the height from which the pendulum is dropped. Like CCI, FPI delivers reproducible injury that mimics aspects of concussions, brain contusion, diffuse axonal injury and hemorrhage seen in humans.
Utility of models
CCI and lateral FPI are commonly used to generate focal injuries. Central FPI or the Marmarou weight drop models are most appropriate to simulate diffuse injuries.
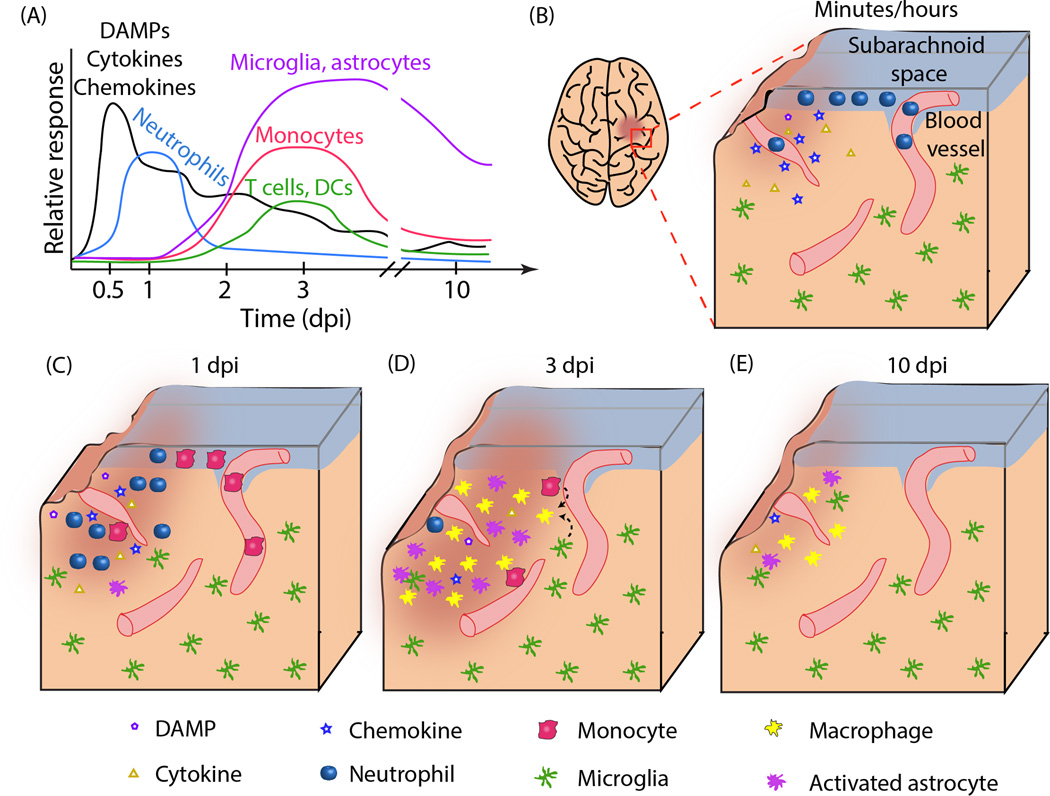
Figure 1. Inflammatory response to TBI.
A. Time course of molecular and cellular mediators after TBI. Molecular mediators such as danger-associated molecular patterns (DAMPs), cytokines and chemokines are released immediately after injury and peak within hours. They continue to be released at later time points by the tissue damage and infiltrating cells. Neutrophil infiltration in the brain parenchyma is maximal at 1 day post injury (dpi), while monocyte, T and dendritic cell accumulation peaks at about 3 dpi. The inflammatory reaction is mostly resolved by 10 dpi. The relative response for each cells type is normalized to the maximum cellular response. B-E. Histological representation of the inflammatory reaction. B. Acute (minutes/hours) inflammation at the site of tissue lesion. A focal injury is characterized by tissue damage and secretion of DAMPs, cytokines and chemokines by the damaged cells. Neutrophils start to accumulate in the subarachnoid space (SAS) and in blood vessels (parenchymal and pial) at the injured area. C. At 1 dpi, DAMPs, cytokine and chemokine levels are reduced. Neutrophils are found throughout the parenchyma. Monocytes are being recruited to the damaged area, but most of them are still inside blood vessels. D. By 3 dpi, neutrophils are almost undetectable and monocytes predominate at the site of lesion. Astrocytes and resident microglia also assume an activated morphology. Amoeboid microglia are visually indistinguishable from monocyte-derived macrophages. Some T lymphocytes and dendritic cells are also present in the parenchyma. E. By 10 dpi, the inflammatory reaction is mostly resolved. Some activated astrocytes and macrophage-like cells can still be detected, especially in deeper brain regions (not shown).
The number of inflammatory mediators recognized to participate in the response to TBI has expanded over the last few years through application of multiplex assays that measure multiple unique mRNA or protein analytes at once. These approaches enable kinetic studies of mediator production in TBI animal models [20–23]. For example, more than one study reported expression of IL-1β, TNF-α, IL-6, CCL2, CCL3, CXCL1, CXCL2, CXCL8/IL-8, CXCL10, CCR2, CCR5, CXCR4 and CX3CR1within 6 hr of TBI. Importantly, the same mediators have been detected in the early stages after injury in TBI patients, at the mRNA level by ribonuclease protection assay, or the protein level by multiplex analysis of microdialysis samples. As in animal models, the levels of many cytokines and chemokines peak within 4–12 hr after TBI [24, 25]. Moreover, most chemokines are present at higher levels in the cerebrospinal fluid than plasma, indicating local production by brain-resident cells [24, 25].
In the past few years, the central role of inflammatory signaling in TBI response has been even further supported by large-scale microarray analyses covering much or all of the genome [26–30]. In bioinformatic analysis of all studies so far, functional pathways related to inflammation, stress, (inflammatory) cell movement and cell signaling were among the top pathways differentially affected by TBI. These data were obtained by several groups using different animal models, showing that the importance of inflammation is model- and platform-independent. Examination of specific genes differentially affected by TBI in microarray experiments converged on cytokine and chemokine pathways similar to the ones identified by the narrowly focused multiplex assays [24–30]. Together, these studies confirm that early upregulation of inflammatory mediators is a robust response that likely contributes to the subsequent neuropathological sequelae of TBI.
Cellular response
The tissue damage and subsequent release of inflammatory mediators after TBI lead to changes in the function of brain-resident cells and recruitment of peripheral cells (Box 2). If one imagines a prototypical cortical impact, among the first cell types to respond to injury are neutrophils, whose accumulation starts in the subarachnoid space and vascular elements near the injury. Neutrophils subsequently enter the brain parenchyma around 1 day post injury (dpi) (Figure 1A), mediated at least in part by upregulation of adhesion molecules on the endothelium [18, 31–35]. While neutrophil recruitment to tissues is essential in responses to peripheral infections and damage, they release reactive oxygen and nitrogen species that damage the brain parenchyma. Neutrophil presence in the brain is greatly reduced by 3–5 dpi when mononuclear leukocyte accumulation predominates (Figure 1B, C) [18, 31, 36]. Identification by immunohistochemistry and flow cytometry indicates that most recruited cells are inflammatory CD45hiCCR2+Ly6C+ monocytes, with a small contribution of T lymphocytes, NK and dendritic cells (Figure 1C) [35–37]. At the same time, brain-resident microglia and astrocytes become activated. Microglia assume an amoeboid morphology, secrete inflammatory factors, perform phagocytosis, and are largely indistinguishable from infiltrating monocyte-derived macrophages (Figure 1C) [31, 38–42]. While peripheral cells are largely absent by 10–14 dpi (Figure 1D), the presence of F4/80+ macrophages and GFAP+ astrocytes at sites far from the primary injury, such as thalamic projection fields of injured neurons, for months and even years after the primary insult is indicative of diffuse injury [38, 39, 43, 44].
Box 2: Cellular response to TBI.
The cellular response to TBI involves contributions from both brain-resident and peripheral cells. Depending on the intensity of injury and whether it is penetrating or not, the injury induces instantaneous death of the cells at the site of impact. In the surrounding tissue, intracellular components spill out of damaged cells to serve as DAMPs to resident and infiltrating immune cells, and cytokines and chemokines mediate cell-cell interactions. There are also responses that are specific to each cell type.
Neurons that lie in the damaged tissue experience mechanical forces to their dendrites, cell body and axon. Damage to the axon leads to it stretching, bending or shearing off. This TBI-associated axonal injury can be seen even at sites away from the primary injury, especially in the corpus callosum. Neurons are also damaged during the secondary injury phase by excitotoxic compounds and inflammatory mediators present in the extracellular space. Neurons are often identified in tissue sections as NeuN+ cells.
Astrocytes surrounding the lesion area produce many of the inflammatory mediators (cytokines and chemokines) that damage neurons, recruit peripheral cells and activate microglia. Astrocytes are themselves activated by the presence of cell debris and inflammatory mediators. Reactive astrocytes can be identified as GFAP+ cells.
Microglia are brain-resident cells with hematopoietic origin. After injury, they try to clear tissue debris by phagocytosis. They also secrete and respond to inflammatory mediators. Microglial activation under inflammatory conditions is accompanied by a morphological transformation from a ramified to an amoeboid morphology; amoeboid microglia are morphologically indistinguishable from blood-derived macrophages. Healthy microglia express the myeloid marker CD11b and low levels of CD45 and Iba1; in flow cytometry experiments, they are CD11b+CD45lowCX3CR1+ cells. Activated microglia increase the expression of Iba1, F4/80 and other phagocytic markers. In flow cytometry, they remain CD11b+CD45lowCX3CR1+ cells.
Neutrophils are the first peripheral cell type to accumulate in the brain after injury. They attempt to clear cell debris by phagocytosis, but also contribute to the ongoing damage by releasing toxic mediators such as reactive oxygen species. They can be identified as Ly6G+ cells. Myeloperoxidase, which is sometimes used as a marker for neutrophils, is also present in other phagocytic cells such as macrophages.
Monocytes follow chemokine gradients to be recruited to the brain after TBI. Once in the brain, they differentiate into macrophages, perform phagocytosis and secrete inflammatory mediators. Morphologically, they resemble microglia-derived macrophages. In the healthy body, monocytes are classified as “inflammatory” CD11b+CD45hiCCR2+Ly6Chi or “patrolling”CD11b+CD45hiCX3CR1+ monocytes, with the CD11b+CD45hiCCR2+Ly6Chi subtype preferentially recruited after TBI. Monocyte-derived macrophages that accumulate in the brain display upregulated F4/80 and Iba1 expression and reduced CCR2 expression. Activated monocytes can be separated from activated microglia by flow cytometry as CD11b+CD45hi and CD11b+CD45lo cells, respectively.
Dendritic cells (DCs) and T cells enter the brain with approximately the same kinetics as monocytes, but at much lower numbers. The functions they perform will depend on the specific subpopulation of cells present. Dendritic cells are classified as conventional (cDCs), which stimulate T cells, and plasmacytoid (pDCs), which secrete interferon-α. Different subpopulations of T cells include T helper, T memory, T cytotoxic, Nature Killer cells, and others, each with distinct function. The exact role of DCs and T cells in TBI pathology has not been established.
Consistent with coup-countercoup injury, a focal brain insult induces inflammatory gene expression on the opposite side of the brain [21, 30]. While some genes respond concordantly on the ipsilateral and contralateral sides, the expression of other genes changes in opposite directions. These studies confirm that, despite the lack of detectable cellular reaction on the contralateral side (see above), an injury event affects the whole brain.
Neutrophils were the first cells targeted for therapeutic intervention, probably because of their prominent accumulation in the brain early after TBI and their contribution to tissue damage through oxidative bursts [32, 33, 45, 46]. Disappointingly, multiple studies showed that blocking neutrophil entry into the brain by deletion of cell adhesion molecules required for neutrophil entry was not associated with an improved neurological outcome on a variety of motor and cognitive tests [18, 45, 47–49]. Since then, the focus has shifted to anti-inflammatory therapies that inhibit macrophage/microglial activation (for example, with minocycline) or therapeutic modulation of the molecular components of TBI-induced inflammation, including cytokine, chemokine and TLR signaling. Indeed, there is extensive literature on targeting cytokine signaling after TBI [17, 50–52, Reviewed in 53]. This review will concentrate on efforts to target chemokine/chemokine receptor signaling to modulate brain-periphery communication after TBI.
Strategies aimed at chemokine signaling
Chemokine biology
Chemokines are a large subgroup in the cytokine family [54]. They were originally defined as “chemotactic cytokines” because of their ability to attract immune system cells to a site of injury or infection. Since then, chemokines have been implicated in a variety of non-inflammatory functions, including nervous, hematopoietic and urogenital system development, cancer and bone marrow homeostasis. Chemokines elicit their effects after binding to seven transmembrane domain G protein-coupled chemokine receptors. While all typical chemokine receptors couple to Gαi G proteins, ligand binding stimulates a wide variety of intracellular signaling pathways such as activation of phospholipases, mitogen kinases and small G proteins (Rac, Rho, cdc42) that ultimately lead to changes in actin polymerization and cell movement [54].
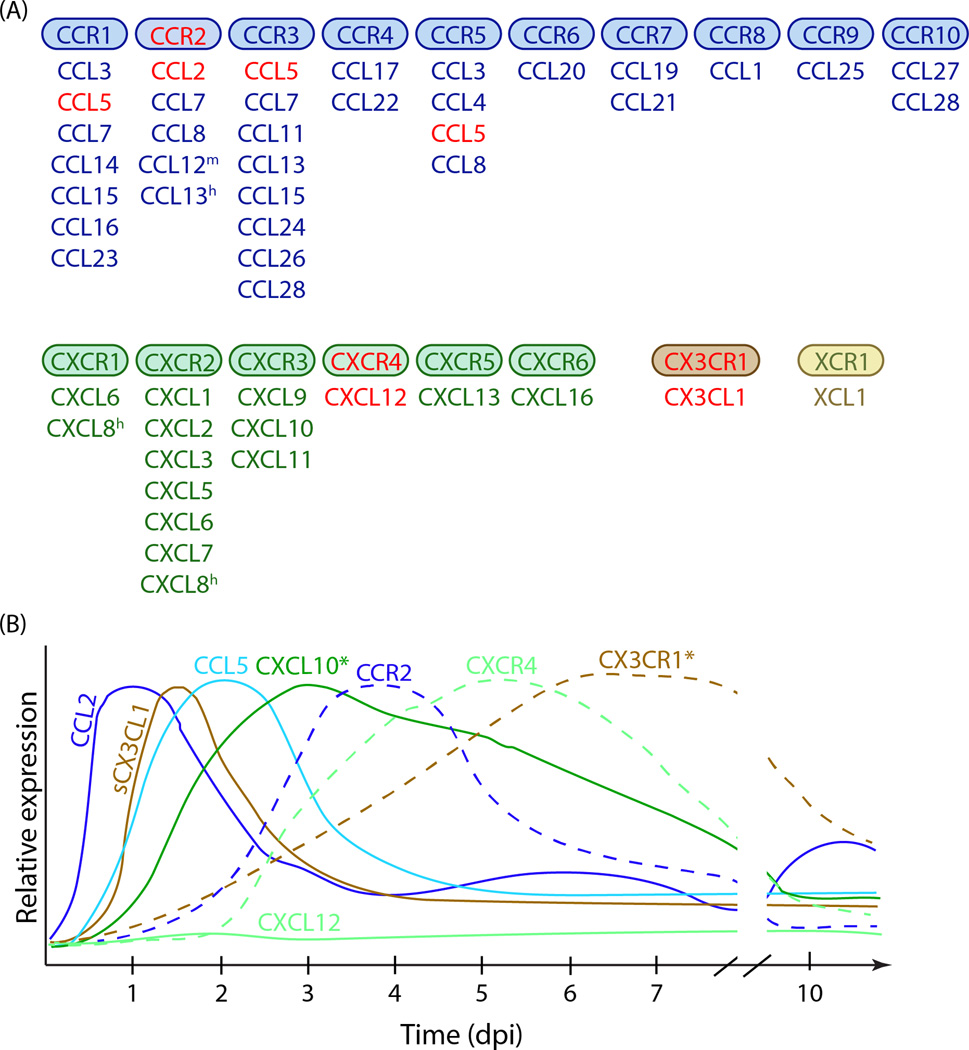
Chemokines can be divided into four groups based on the presence of positionally conserved cysteine residues in their primary structure: XC, CC, CXC, and CX3C chemokines (Figure 2A). Although there are no absolutes, chemokines within each group also tend to share similar functions and bind to related receptors (named XLRs, CCRs, CXCRs, CX3CRs). One subfamily, CXC chemokines containing Glu-Leu-Arg (ELR) motif, are involved in neutrophil attraction, whereas many CC chemokines/CCRs are involved in mononuclear leukocyte trafficking [54]. In vitro, chemokines exhibit considerable promiscuity of ligand/receptor interactions, as individual ligands can bind several receptors and certain receptors can be activated by multiple ligands (Figure 2A). CX3CL1/CX3CR1, which will be discussed below, represents one of few monogamous chemokine/receptor pairs [54–56].
Figure 2. Chemokines in the context of TBI.
A. Chemokine receptor/chemokine signaling. There are four major chemokine families (CCL, CXCL, CX3CL and XCL) that bind to four families of chemokine receptors (CCR, CXCR, CX3CR and XCR). The figure shows the chemokine receptors and the ligands that have been shown to activate them. Only typical chemokine receptors that activate G proteins upon ligand binding are included. m, present only in mice; h, present only in humans. Chemokines in red are referenced in this review. B. General time course of chemokine and chemokine receptor expression in TBI. Only chemokines and receptors discussed in this review are included. The graphs show protein expression of each analyte, except for CXCL10* and CX3CR1* (*, mRNA expression shown). The relative expression for each chemokine/receptor is normalized to its own peak expression. Chemokine expression generally precedes the expression of the receptor it signals through.
Consistent with the diverse functions of the chemokine system, chemokines and their receptors are also expressed in the central nervous system (CNS) where they play key functions in development and maintenance [55, 56]. For example, CXCL12/CXCR4 signaling is involved in maintaining the neural stem cell niche and CX3CL1/CX3CR1 signaling modulates stimulus-dependent microglial activation [56].
Several chemokines and their receptors have been examined in the context of TBI. CXCL8/CXCR2 signaling has been extensively researched in this context. Specifically, multiple studies report that CXCL8 levels in CSF and/or plasma of TBI patients show some evidence of correlation with poorer outcome [17, 52, Reviewed in 53, 57]. This literature has been reviewed, so we will not reexamine it here. We will instead describe the involvement of several other chemokine signaling pathways, focusing on CCL2/CCR2, CXCL12/CXCR4, CXCL10/CXCR3, CX3CL1/CX3CR1 and CCL5/CCR1,3,5.
CCL2/CCR2
CCR2 is mainly expressed on a subset of monocytes (inflammatory, CD45hiLy6Chi monocytes) and is important in monocyte mobilization out of the bone marrow and recruitment to tissues (including brain) in inflammatory states [54, 58]. Indeed, Ccr2−/− mice have significantly reduced numbers of circulating inflammatory monocytes with a corresponding accumulation of these cells in the bone marrow, indicating their non-physiological retention [59] (Figure 3). As expected, these mice demonstrate failure to accumulate monocytes in the CNS in neurological disease or injury models [58] (Figure 3). CCR2 is activated by CCL2 (also known as monocyte chemotactic protein-1, MCP-1), CCL7, CCL8 and CCL12 (in mice)/CCL13 (in humans), with CCL2 being the most intensely studied ligand [58].
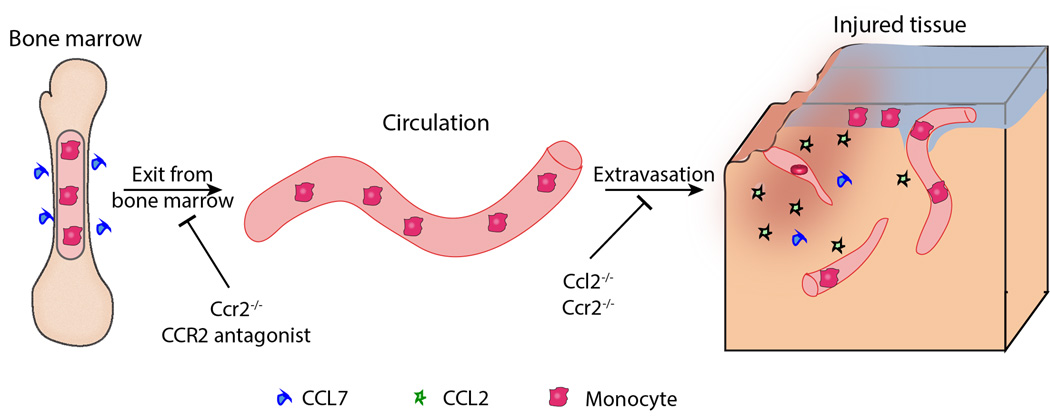
Figure 3. CCR2 signaling in the turnover of CD11b+CD45hi monocytes.
Monocytes produced in the bone marrow require CCR2 signaling – mostly activated by CCL7 – to exit the bone marrow and enter the circulation. In the presence of tissue injury, including brain injury, CD11b+CD45hiCCR2+ monocytes are recruited to the damaged area. Several chemokines, such as CCL2, CCL7, CCL8, CCL12 (mouse) and CCL13 (human) can mediate monocyte extravasation. CCR2 deletion and antagonism inhibits both monocyte egress out of the bone marrow and into tissues, while CCL2 deletion affects primarily monocyte recruitment to the site of damage.
There is substantial evidence to support the involvement of CCL2/CCR2 signaling in TBI. CCL2 was one of the initial chemokines identified as significantly upregulated within 24 hr in rodent models of TBI at both the mRNA and protein level (Figure 2B) [20–23, 41, 42, 60–64]. In situ hybridization, immunohistochemistry and immunofluorescence techniques have shown CCL2 expression in both GFAP-positive astrocytes [60, 61] and cells with macrophage morphology and markers [61, 64]. CCL2 is also detected in surgically resected samples from TBI patients and in patient CSF and plasma [42, 65–67]. Interestingly, older mice subjected to TBI experience a larger induction of CCL2 (and other chemokines) than young mice [41], which could have clinical significance because of increased incidence of TBI in the elderly.
The functional contribution of CCL2/CCR2 to TBI pathology has been examined recently. In the weight-drop model of closed head injury, CCL2 deficiency in mice only slightly affected cell loss and lesion volume in the first 7 dpi, but resulted in reduced lesion size, decreased F4/80 and GFAP immunoreactivity and improved neurological recovery by 28 dpi [42].
Additional studies utilizing CCR2 inhibition have expanded on the role of CCL2/CCR2 signaling in TBI. Liu et al. showed beneficial effects of CCR2 antagonism in the acute stages after weight drop TBI. Treatment of rats with a high dose of the CCR2 antagonist RS504393 after TBI decreased the number of apoptotic cells at 3 dpi [64]. Moreover, RS504393 significantly improved rat performance on the Morris Water Maze test of spatial memory, reducing escape latency on a probe trial at 3 dpi [64]. Similarly, Morganti et al. [68] used a different CCR2 antagonist, CCX872, in the controlled cortical impact model of TBI in mice. The authors showed that pretreatment with CCX872 led to reduced CD11b+F4/80hiCD45hi macrophage accumulation at 1 dpi, especially in the hippocampus, overall decreased inflammatory gene expression, and improved performance on the radial arm water maze test at 28 dpi [68].
These findings are also supported by data obtained in Ccr2−/− mice subjected to controlled cortical impact in two separate studies [69, 70]. Acutely, at 3 dpi, Ccr2−/− mice had decreased lesion cavity and inflammatory gene expression, decreased number of infiltrating CD45hiCCR2+ monocytes by flow cytometry and decreased number of F4/80+ phagocytic cells by immunohistochemistry [70]. At the 8 week time point, these mice displayed higher neuronal density in CA1 region of hippocampus and improved performance on Morris Water Maze test [69]. As expected, CCR2 deficiency did not affect the number of infiltrating Ly6G+ neutrophils which do not express CCR2 [69], but reduced dendritic cell numbers (both CD11c+ and PDCA-1+ subtypes, both of which can express CCR2) [70] after TBI.
The examination of CCR2 signaling in the context of TBI has provided important information about general myeloid cell biology. Both Hsieh et al. [37] and Morganti et al. [68] studied gene expression in specific cell populations after TBI by qPCR and microarray analysis, respectively. Hsieh et al. [37] isolated macrophages expressing arginase-1, a marker that has been associated with wound repair and the so-called alternatively activated (M2) macrophages. However, the authors determined that these “M2” macrophages concurrently express many markers associated with classical activation (M1). Similarly, Morganti et al. [68] showed that Ccr2+ macrophages in the brain after TBI express a range of M1 and M2 markers during the different time points examined. Each gene changed with its own characteristic time course such that not all M1 genes were expressed at the same time and not all M2 genes were expressed at the same time. Instead, there was a mixture of M1 and M2 genes at all time points. These studies highlight the plastic nature of macrophages – and also likely microglia – that cannot be described by a simple M1/M2 (or M2a, M2c, etc) designation. Instead, myeloid cells adopt a phenotype (responsive state) that is characterized by gene expression specific to each stimulus.
Overall, deficiency in either CCL2 or CCR2 signaling appears to be beneficial after TBI by improving both histological and neurological outcomes. Yet, it should be pointed out that interfering with CCL2 or CCR2 signaling will have different effects in the context of TBI. Ccr2−/− mice show a larger retention of CD11b+CD45hi monocytes in the bone marrow than Ccl2−/− mice [59], making these inflammatory monocytes unavailable to respond to TBI (Figure 3). CCR2 antagonists would act in a similar fashion, preventing monocyte exit from the bone marrow and extravasation into the brain (Figure 3). In contrast, monocytes in Ccl2−/− mice still express CCR2 receptors and could enter the brain after TBI if induced by other ligands (Figure 3).
CXCL12/CXCR4
Another chemokine signaling pathway implicated in TBI pathology is the CXCL12/CXCR4 pathway, which is also involved in adult neural stem cell population maintenance [56]. CXCR4 but not CXCL12 is upregulated in the cortex surrounding the damaged area at 3 and 7 days after TBI (Figure 2B) [71, 72]. CXCR4 is expressed by cells displaying neural stem cell markers (nestin, SOX2, neurofilaments) [71]. Intracranial injection of CXCL12 (also known as stromal derived factor-1α, SDF-1α) induced angiogenesis, improved edema, lessened BBB permeability and reduced numbers of TUNEL+ apoptotic cells and intracellular apoptosis mediators in the controlled cortical impact and weight drop models of TBI [72, 73]. When peripheral blood cells differentiated in vitro to CXCR4+ stem-like cells were injected into the lateral ventricle one day after lateral fluid percussion injury, the cells accumulated at the injury site, appeared to secrete neurotrophic factors, and expressed markers of immature and mature neurons at 1 and 3 months after TBI [74]. The expression of neuronal markers was not explained by cell fusion with neurons as the CXCR4+ cells were not polyploid by flow cytometry and had only one DAPI-labeled nucleus by confocal microscopy [74]. In contrast, CXCR4+ cells injected into sham mice that did not receive injury did not migrate out of the ventricles and into the brain parenchyma [74]. The stem cell-treated TBI rats showed improved learning in the training phase of the Morris Water Maze test on 11–15 dpi, but not in the testing phase with submerged platform [74]. The rats were not subjected to cognitive testing at later time points.
CXCL10
CXCL10 (also known as inhibitory protein-10, IP-10), a chemokine involved in Th1 immune responses [54], is consistently upregulated after TBI in gene array experiments and in samples from TBI patients (Figure 2B) [20, 21, 23–25, 27, 65]. Interestingly, Cxcl10 mRNA is expressed in clusters of Fcgr1+ cells with highest density seen close to the injury site at 3 and 7 dpi, but also detected throughout the brain [20]. The Fcgr1+ clusters are still present in Cxcl10-/- mice, indicating that the presence of the chemokine does not drive their formation. Furthermore, while Cxcl10 mRNA is reduced in Ccr2-/- mice, the clusters are not eliminated, suggesting that CCR2-positive cells do not participate in cluster formation. In situ hybridization analysis of Itgax mRNA (encoding CD11c, a dendritic cell marker) [20] or Fcrg1 (Fcγ receptor) show similar cluster formation in the same anatomical locations as the Cxcl10 clusters not only after TBI, but also in the SOD1G93A model of amyotrophic lateral sclerosis and the experimental autoimmune encephalomyelitis model of multiple sclerosis [75]. Based on appearance, the clusters may be formed around microvessels and represent cells extravasating into the brain parenchyma [20]. However, the cellular sources of CXCL10 and the cellular composition of CXCL10+ clusters in TBI have not been definitively characterized [75].
CX3CL1/CX3CR1
Despite the considerable attention they have attracted in many brain pathologies [56], the data on CX3CL1/CX3CR1 signaling in the context of TBI are very sparse. Analysis of CX3CL1 (also known as fractalkine) and CX3CR1 is limited to mRNA and free peptide by ELISA and Western blot, as tissue immunohistochemistry is not informative due to antibody non-specificity. Cx3cr1 mRNA levels gradually increase over one week after closed head injury in mice (Figure 2B), but there is no change in Cx3cl1 mRNA expression [76]. However, CSF levels of soluble CX3CL1 are significantly higher in TBI patients that in healthy controls at 1 dpi (Figure 2B) [76], suggesting that CX3CL1 function might be regulated by chemokine cleavage after TBI. Indeed, CX3CL1 may be either membrane-bound or soluble, and the two isoforms exert distinct effects [77–80]. More work must be performed to establish whether TBI regulates CX3CL1 cleavage and to define the roles of soluble or membrane-bound CX3CL1 in TBI pathology.
While its functional significance in TBI has not been characterized, data from many other studies suggest that CX3CR1 ligation might play an important role in TBI. First, CX3CR1 regulates microglial toxicity in the lipopolysaccharide model of neuroinflammation and in neurodegeneration models of AD, Parkinson’s disease and amyotrophic lateral sclerosis [80, 81], all of which have also been linked to TBI incidence. Second, activated microglia are prominent in the early stages after TBI (Figure 1). Hence, it is plausible that CX3CL1/CX3CR1 signaling will also play a role in TBI pathogenesis. Interestingly, CX3CR1+ blood-derived monocytes, rather than resident microglia, appear to contribute to axonal damage in the dorsal column crush model of spinal cord injury [82]. Therefore, the effects of CX3CR1 modulation of TBI pathology need to be carefully examined before this signaling pathway can be considered for therapeutic intervention.
CCL5
CCL5 (also known as RANTES) is a chemoattractant and activating cytokine for T cells, monocytes, eosinophils and basophils that can signal through CCR1, CCR3 or CCR5 [54]. In both animal models and TBI patients, Ccl5 mRNA is upregulated in the cortex after TBI (Figure 2B) [41, 65]. CCL5 is also elevated in plasma of TBI patients and its concentration at admission may correlate with poor outcome in TBI patients [24, 83]. However, to date there are no functional or mechanistic studies to enhance our understanding of this association. However, there are clues about how CCL5 may modulate pathology in other experimental conditions. In spinal cord injury, CCL5 (and CXCL10) promotes T cell recruitment [84]. Although T cells represent only a small proportion of the infiltrating leukocytes in TBI, they have the potential to secrete monocyte-attracting chemokines such as CCL2 [84]. Furthermore, CCL5 signals through several receptors (CCR1, CCR3, CCR5), expressed both on T cells and monocytes [54], resulting in a large number of possible functional outcomes. This highlights the complexity of inflammatory cell interactions via chemokines, necessitating the careful elucidation of chemokine signaling in TBI.
Concluding remarks
With its high incidence, neuropsychological morbidity and link to neurodegenerative diseases, TBI represents a significant public health and financial burden. Because injuries cannot be entirely prevented, one attractive treatment strategy is to interdict the development of secondary brain pathology. Inflammatory pathways become active immediately after injury and therefore represent an appealing target to influence the ongoing pathology.
The inflammatory reaction following TBI comprises a complex interaction of cellular and molecular components that cooperate in a restricted spatial and temporal pattern: specific cell populations are recruited to the injury site at specific times and express a limited array of mediators. For this reason, a potential therapeutic approach could be directed at only one TBI-induced target, giving specificity to the approach, but influencing multiple downstream mediators. Unfortunately, initial efforts to reduce neutrophil infiltration in the brain, the first peripheral population to be recruited to the brain after TBI, have not improved motor or neurological recovery [18, 45, 47–49].
Certain chemokines and their receptors are specifically altered in TBI, and thus the chemokine system has arisen as a potential target for TBI treatment. Early studies examining the effects of reduced CCR2 signaling show promising results, improving cognitive and neurological function in addition to neuropathological features of tissue damage [68–70]. It should be noted that CCR2 is expressed in multiple cell types recruited to the brain, including monocytes, and to a lesser extent, dendritic and T cells [58]. As CCR2 inhibition emerges as a therapeutic approach, the exact contribution of CCR2 signaling in each of these cell types should be examined to determine the possibility of unwanted side effects of CCR2 reduction. Moreover, with future advancements in stem cell therapy, transplantation of CXCR4+ neural stem cells could become a viable therapeutic option. Alternatively, approaches to activate resident CXCR4+ cells in situ need to be examined. Finally, there are additional chemokines that have been consistently associated with TBI (CCL5 and CXCL10) and the functional significance of their upregulation in TBI should be elucidated.
An advantage of targeting chemokines and their receptors is that chemokine receptors are GPCRs that lend themselves to drug development. However, despite the involvement of chemokine signaling in a variety of disease states, only two drugs targeting chemokine receptors have reached the market [54]. A widely cited difficulty in targeting the chemokine system is that many ligands bind to multiple receptors and vice versa (Figure 2A). This apparent promiscuity largely vanishes when one considers that chemokine-receptor signaling during tissue pathology is typically limited to one of a potential array of ligands. Focusing on monogamous ligand-receptor pairs, such as the CX3CL1/CX3CR1, avoids even this potential concern. Alternatively, in certain cases, it may be advantageous to use a broad-spectrum chemokine inhibitor. For example, one such inhibitor (NR58-3.14.3) has been shown to be protective in a mouse model of ischemia-reperfusion injury, reducing leukocyte infiltration and lesion volume up for 72 hr after reperfusion and improving functional performance [85].
In summary, certain features of the chemokine system, such as temporal and spatial regulation of chemokines and their receptors in TBI, provide targets for drug development. Modulating chemokine signaling in TBI has the potential to result in therapeutic benefit by preventing aspects of the secondary pathology initiated after the initial insult. Because of the link between TBI and neurodegenerative disease risk, successful treatment of TBI can potentially decrease the incidence of tragically debilitating and costly neurodegenerative conditions.
Highlights.
The inflammatory reaction after TBI follows a stereotyped time course
Multiple cellular and molecular mediators may contribute to secondary injury
Chemokines mediate peripheral immune cell recruitment after TBI
Chemokine pathways are altered after TBI and may represent therapeutic targets
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faul M, et al. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention. National Centers for Injury Prevention and Control. 2010 [Google Scholar]
- 2.Teasdale G, Jennett B. Assessment of coma and Impaired consciousness: A practical scale. Lancet. 1974;304(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 3.Whitnall L, et al. Disability in young people and adults after head injury: 5–7 year followup of a prospective cohort study. J Neurol Neurosurg Psychiatry. 2006;77(640–645):640. doi: 10.1136/jnnp.2005.078246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiraly MA, Kiraly SJ. Traumatic Brain Injury and Delayed Sequelae: A Review -Traumatic Brain Injury and Mild Traumatic Brain Injury (Concussion) are Precursors to Later-Onset Brain Disorders, Including Early-Onset Dementia. The Scientific World Journal. 2007;7:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Draper K, Ponsford J. Cognitive Functioning Ten Years Following Traumatic Brain Injury and Rehabilitation. Neurophyschology. 2008;22(5):618–625. doi: 10.1037/0894-4105.22.5.618. [DOI] [PubMed] [Google Scholar]
- 6.Ponsford J, Draper K, Schönberger M. Functional outcome 10 years after traumatic brain injury: Its relationship with demographic, injury severity, and cognitive and emotional status. Journal of the International Neuropsychological Society. 2008;14:233–242. doi: 10.1017/S1355617708080272. [DOI] [PubMed] [Google Scholar]
- 7.Stern RA, et al. Long-term Consequences of Repetitive Brain Trauma: Chronic Traumatic Enchephalopathy. PM&R. 2011;3(10S2):S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Yi J, et al. Chronic Traumatic Encephalopathy. Current Sports Medicine Reports. 2013;12(1):28–32. doi: 10.1249/JSR.0b013e31827ec9e3. [DOI] [PubMed] [Google Scholar]
- 9.McKee AC, et al. Chronic Traumatic Enchephalopathy in Athletes: Progressive Taupathy After Repetitive Head Injury. Journal of Neuropathology and Experimental Neurology. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemetz PN, et al. Traumatic Brain Injry and Time to Onset of Alzheimer's Disease: A Population-based Study. American Journal of Epidemiology. 1999;149(1):32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- 11.Guo Z, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 12.Das M, Mohapatra S, Mohapatra SS. New Perspectives on central and peripheral immune responses to acute traumatic brain injury. Journal of Neuroinflammation. 2012;9:236. doi: 10.1186/1742-2094-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, et al. Head Injury and Amyotropic Lateral Sclerosis. American Journal of Epidemiology. 2007;166(7):810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Povlishock JT, Katz DI. Update of the Neuropathological Recovery After Traumatic Brain Injury. J Head Trauma Rehabil. 2005;20(1):76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hellmich HL, et al. Dose-dependent neuronal injury after traumatic brain injury. Brain Research. 2005;1044:144–154. doi: 10.1016/j.brainres.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 16.Giunta B, et al. The immunology of traumatic brain injury: a prime target for Alzheimer's disease prevention. Journal of Neuroinflammation. 2012;9:185. doi: 10.1186/1742-2094-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Frontiers in Neurology. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes J. Peripheral immune cells in the pathology of traumatic brain injury? Current Opinion in Critical Care. 2011;17:122–130. doi: 10.1097/MCC.0b013e3283447948. [DOI] [PubMed] [Google Scholar]
- 19.Kigerl KA, et al. Pattern recognition receptors and central nervous system repair. Experimental Neurology. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Israelsson C, et al. Distinct Cellular Patterns of Upregulated Chemokine Expression Supporting a Prominent Inflammatory Role in Traumatic Brain Injury. Journal of Neurotrauma. 2008;25:959–974. doi: 10.1089/neu.2008.0562. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee S, et al. Early TBI-induced cytokine alterations are similarly detected by two distinct methods of multiplex assay. Frontiers in Molecular Neuroscience. 2011;4:21. doi: 10.3389/fnmol.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalgard CL, et al. The cytokine temporal profile in the rat cortex after controlled cortical impact. Frontiers in Molecular Neuroscience. 2012;5:6. doi: 10.3389/fnmol.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shein SL, et al. Hemorrhagic Shock Shifts the Serum Cytokine Profile from Pro- to Anti-Inflammatory after Experimental Traumatic Brain Injury in Mice. Journal of Neurotrauma. 2014;31:1386–1395. doi: 10.1089/neu.2013.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helmy A, et al. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. Journal of Cerebral Blood Flow & Metabolism. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmy A, et al. Principal Component Analysis of the Cytokine and Chemokine Response to Human Traumatic Brain Injury. PLoS One. 2012;7(6):e39677. doi: 10.1371/journal.pone.0039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crack PJ, et al. The genomic profile of the cerebral cortex after closed head injury in mice: effects of minocycline. Journal of Neural Transmission. 2009;116:1–12. doi: 10.1007/s00702-008-0145-1. [DOI] [PubMed] [Google Scholar]
- 27.Israelsson C, et al. Closed Head Injury in a Mouse Model Results in Molecular Changes Indicating Inflammatory Responses. Journal of Neurotrauma. 2009;26:1307–1314. doi: 10.1089/neu.2008.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shojo H, et al. Genetic and histologic evidence implicates role of inflammation in traumatic brain injury-induced apoptosis in the rat cerebral cortex following moderate fluid percussion injury. Neuroscience. 2010;171:1273–1282. doi: 10.1016/j.neuroscience.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Redell JB, et al. Analysis of Functional Pathways Altered after Mild Traumatic Brain Injury. Journal of Neurotrauma. 2013;30:752–764. doi: 10.1089/neu.2012.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White TE, et al. Gene expression patterns following unilateral traumatic brain injury reveals a local pro-inflammatory and remote anti-inflammatory response. BMC Genomics. 2013;14:282. doi: 10.1186/1471-2164-14-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soares HD, et al. Inflammatory Leukocytic Recruitment and Diffuse Neuronal Degeneration Are Separate Pathological Processes Resulting from Traumatic Brain Injury. Journal of Neuroscience. 1995;15(12):8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlos T, et al. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. Journal of Leukocyte Biology. 1997;61(3):279–285. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- 33.Biagas KV, et al. Assessment of Posttraumatic Polymorphonuclear Leukocyte Accumulation in Rat Brain Using Tissue Myeloperoxidase Assay and Vinblastine Treatment. Journal of Neurotrauma. 1992;9(4):363–371. doi: 10.1089/neu.1992.9.363. [DOI] [PubMed] [Google Scholar]
- 34.Clark RS, et al. Neutrophil Accumulation After Traumatic Brain Injury in Rats: Comparison ofWeight Drop and Controlled Cortical Impact Models. Journal of Neurotrauma. 1994;11(5):499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- 35.Hausmann R, et al. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. International Journal of Legal Medicine. 1999;112:227–232. doi: 10.1007/s004140050241. [DOI] [PubMed] [Google Scholar]
- 36.Holmin S, et al. Intracerebral Inflammatory Response to Experimental Brain Contusion. Acta Neurochirurgica (Wien) 1995;132:110–119. doi: 10.1007/BF01404857. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh CL, et al. Traumatic brain injury induces macrophage subsets in the brain. European Journal of Immunology. 2013;43:2010–2022. doi: 10.1002/eji.201243084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory Responses After Experimental Diffuse Traumatic Brain Injury. Journal of Neuropathology and Experimental Neurology. 2007;66(11):989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- 39.Cao T, et al. Morphological and Genetic Activation of Microglia after Diffuse Traumatic Brain Injury in the Rat. Neuroscience. 2012;225:65–75. doi: 10.1016/j.neuroscience.2012.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muessel MJ, et al. Ablation of the chemokine monocyte chemoattractant protein-1 delays retrograde neuronal degeneration, attenuates microglial activation, and alters expression of cell death molecules. Molecular Brain Research. 2002;103:12–27. doi: 10.1016/s0169-328x(02)00158-4. [DOI] [PubMed] [Google Scholar]
- 41.Sandhir R, et al. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neuroscience Letters. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 42.Semple BD, et al. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. Journal of Cerebral Blood Flow & Metabolism. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernandez-Ontiveros DG, et al. Microglia activation as a biomarker for traumatic brain injury. Frontiers in Neurology. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loane DJ, et al. Progressive Neurodegeneration After Experimental Brain Trauma: Association With Chronic Microglial Activation. Journal of Neuropathology and Experimental Neurology. 2014;73(1):14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weaver KD, et al. Effect of Leukocyte-Endothelial Adhesion Antagonism on Neutrophil Migration and Neurologic Outcome after Cortical Trauma. Journal of Trauma. 2000;48(6):1081–1090. doi: 10.1097/00005373-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Knoblach S, Faden AI. Administration of Either Anti-Intercellular Adhesion Molecule-1 or a Nonspecific Control Antibody Improve Recovery after Traumatic Brain Injury in the Rat. Journal of Neurotrauma. 2002;19(9):1039–1050. doi: 10.1089/089771502760341956. [DOI] [PubMed] [Google Scholar]
- 47.Whalen MJ, et al. Reduced brain edema after traumatic brain injury in mice deficient in P-selectin and intercellular adhesion molecule-1. Journal of Leukocyte Biology. 2000;67(2):160–168. doi: 10.1002/jlb.67.2.160. [DOI] [PubMed] [Google Scholar]
- 48.Isaksson J, Hillered L, Olsson Y. Cognitive and histopathological outcome after weight-drop brain injury in the rat: influence of systemic administration of monoclonal antibodies to ICAM-1. Acta Neuropathologica. 2001;102(3):246–256. doi: 10.1007/s004010100361. [DOI] [PubMed] [Google Scholar]
- 49.Semple BD, et al. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiology of Disease. 2010;40:394–403. doi: 10.1016/j.nbd.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Morganti-Kossmann MC, et al. Role of cerebral inflammation after traumatic brain injury: A revisited concept. Shock. 2001;16(3):165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- 51.Kadhim HJ, Duchateau J, Sebire G. Cytokines and Brain Injury: Invited Review. Journal of Intensive Care Medicine. 2008;23(4):236–249. doi: 10.1177/0885066608318458. [DOI] [PubMed] [Google Scholar]
- 52.Ziebell JM, Morganti-Kossmann MC. Involvement of Pro- and Anti-Inflammatory Cytokines and Chemokines in the Pathophysiology of Traumatic Brain Injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helmy A, et al. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Progress in Neurobiology. 2011;95:352–372. doi: 10.1016/j.pneurobio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Bachelerie F, et al. International Union of Basic Clinical Pharmacology. LXXXIX. Update on the Extended Family of Chemokine Receptors and Introducing a New Nomenclature for Atypical Chemokine Receptors. Pharmacological Reviews. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajetto A, et al. Chemokines and Their Receptors in the Central Nervous System. Frontiers in Neuroendocrinology. 2001;22:147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- 56.Ransohoff RM. Chemokines and Chemokine Receptors: Standing at the Crossroads of Immunobiology and Neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veenstra M, Ransohoff RM. Chemokine receptor CXCR2: physiology regulator and neuroinflammation controller? Journal of Neuroimmunology. 2012;246(1–2):1–9. doi: 10.1016/j.jneuroim.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu HX, et al. Role of CCR2 in inflammatory conditions of the central nervous system. Journal of Cerebral Blood Flow & Metabolism. 2014;34:1425–1429. doi: 10.1038/jcbfm.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsou C-L, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. Journal of Clinical Investigation. 2007;117(4):902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glabinski AR, et al. Chemokine Monocyte Chemoattractant Protein-1 Is Expressed by Astrocytes After Mechanical Injury to the Brain. Journal of Immunology. 1996;156(11):4363–4368. [PubMed] [Google Scholar]
- 61.Berman JW, et al. Localization of Monocyte Chemoattractant Peptide-1 Expression in the Central Nervous System in Experimental Autoimmune Encephalomyelitis and Trauma in the Rat. Journal of Immunology. 1996;156(8):3017–3023. [PubMed] [Google Scholar]
- 62.Rancan M, et al. Upregulation of ICAM-1 and MCP-1 but not of MIP-2 and Sensorimotor Deficit in Response to Traumatic Axonal Injury in Rats. Journal of Neuroscience Research. 2001;63:438–446. doi: 10.1002/1097-4547(20010301)63:5<438::AID-JNR1039>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 63.Rhodes JK, Sharkey J, Andrews PJ. The Temporal Expression, Cellular Localization, and Inhibition of the Chemokines MIP-2 and MCP-1 after Traumatic Brain Injury in the Rat. Journal of Neurotrauma. 2009;26:507–525. doi: 10.1089/neu.2008.0686. [DOI] [PubMed] [Google Scholar]
- 64.Liu S, et al. Chemokine CCL2 Induces Apoptosis in Cortex Following Traumatic Brain Injury. Journal of Molecular Neuroscience. 2013;51:1021–1029. doi: 10.1007/s12031-013-0091-8. [DOI] [PubMed] [Google Scholar]
- 65.Stefini R, et al. Chemokine detection in the cerebal tissue of patients with posttraumatic brain contusions. Journal of Neurosurgery. 2008;108:958–962. doi: 10.3171/JNS/2008/108/5/0958. [DOI] [PubMed] [Google Scholar]
- 66.Rhodes J, Sharkey J, Andrews P. Serum IL-8 and MCP-1 Concentration Do Not Identify Pateitns With Enlarging Contusions After Traumatic Brain Injury. Journal of Trauma. 2009;66:1591–1598. doi: 10.1097/TA.0b013e31819a0344. [DOI] [PubMed] [Google Scholar]
- 67.Ho L, et al. Elevated Plasma MCP-1 Concentration Following Traumatic Brain Injury as a Potential “Predisposition” Factor Associated with an Increased Risk for Subsequent Development of Alzheimer’s Disease. Journal of Alzheimers Disease. 2012;31(2):301–313. doi: 10.3233/JAD-2012-120598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morganti JM, et al. CCR2 Antagonism Alters Brain Macrophage Polarization and Ameliorates Cognitive Dysfunction Induced by Traumatic Brain Injury. Journal of Neuroscience. 2015;35(2):748–760. doi: 10.1523/JNEUROSCI.2405-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsieh CL, et al. CCR2 Deficiency Impairs Macrophage Infiltration and Improves Cognitive Function after Traumatic Brain Injury. Journal of Neurotrauma. 2014;31:1677–1688. doi: 10.1089/neu.2013.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Israelsson C, et al. Interacting Chemokine Signals Regulate Dendritic Cells in Acute Brain Injury. PLoS One. 2014;9(8):e104754. doi: 10.1371/journal.pone.0104754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Itoh T, et al. The relationship between SDF-1α/CXCR4 and neural stem cells appearaing in damaged area after traumatic brain injury in rats. Neurology Research. 2009;31:90–102. doi: 10.1179/174313208X332995. [DOI] [PubMed] [Google Scholar]
- 72.Sun W, et al. Intracranial injection of recombinant stromal-derived factor-1 alpha (SDF-1α) attenuates traumatic brain injury in rats. Inflammation Research. 2014;63:287–297. doi: 10.1007/s00011-013-0699-8. [DOI] [PubMed] [Google Scholar]
- 73.Li S, et al. SDF-1α induces angiogenesis after traumatic brain injury. Brain Research. 2012;1444:76–86. doi: 10.1016/j.brainres.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 74.Nichols JE, et al. Neurogenic and nero-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+CXCR4+ mesencgymal stem cells: development of autologous cell-based therapeutics for traumatic brain injury. Stem Cell Research & Therapy. 2013;4:3. doi: 10.1186/scrt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Israelsson C, et al. Appearance of Cxcl10-expressing cell clusters is common for traumatic brain injury and neurodegenerative disorders. European Journal of Neuroscience. 2010;31:852–863. doi: 10.1111/j.1460-9568.2010.07105.x. [DOI] [PubMed] [Google Scholar]
- 76.Rancan M, et al. The Chemokine Fractalkine in Patients With Severe Traumatic Brain Injury and a Mouse Model of Closed Head Injury. Journal of Cerebral Blood Flow & Metabolism. 2004;24:1110–1118. doi: 10.1097/01.WCB.0000133470.91843.72. [DOI] [PubMed] [Google Scholar]
- 77.Bazan JF, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 78.Haskell CA, Cleary MD, Charo IF. Molecular Uncoupling of Fractalkine-mediated Cell Adhesion and Signal Transduction. Journal of Biological Chemistry. 1999;274(15):10053–10058. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]
- 79.Kim K-W, et al. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118(22):e156–e167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee S, et al. Opposing Effects of Membrane-Anchored CX3CL1 on Amyloid and Tau Pathologies via the p38 MAPK Pathway. Journal of Neuroscience. 2014;34(37):12538–12546. doi: 10.1523/JNEUROSCI.0853-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cardona AE, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nature Neuroscience. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 82.Evans TA, et al. High-resolution intravital imaging revelas that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Experimental Neurology. 2014;254:109–120. doi: 10.1016/j.expneurol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lumpkins K, et al. Plasma Levels of the Beta Chemokine Regulated Upon Activation, Normal T Cell Expressed, and Secreted (RANTES) Correlate With Severe Brain Injury. Journal of Trauma. 2008;64:358–361. doi: 10.1097/TA.0b013e318160df9b. [DOI] [PubMed] [Google Scholar]
- 84.Jones TB, Hart RP, Popovich PG. Molecular Control of Physiological and Pathological T-Cell Recruitment after Mouse Spinal Cord Inury. Journal of Neuroscience. 2005;25(28):6576–6583. doi: 10.1523/JNEUROSCI.0305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beech JS, et al. Neuroprotection in Ischemia-Reperfusion Injury: An Antiinflammatory Approach Using a Novel Broad-Spectrum Chemokine Inhibitor. Journal of Cerebral Blood Flow & Metabolism. 2001;21:683–689. doi: 10.1097/00004647-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nature Reviews Neuroscience. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balasingam V, et al. Reactive Astrogliosis in the Neonatal Mouse Brain and Its Modulation by Cytokines. Journal of Neuroscience. 1994;14(2):846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]