Abstract
Filifactor alocis, a previously unrecognized Gram-positive anaerobic rod, is now considered a new emerging pathogen that may play a significant role in periodontal disease. F. alocis’ unique characteristics and variations at the molecular level that may be responsible for the functional changes required to mediate the pathogenic process are discussed.
Keywords: Filifactor alocis, Polymicrobial infection, Periodontitis, Community dynamics
1. Introduction
Recent technological advances that have more clearly defined the oral microbiome have yielded novel insights and a paradigm shift in the etiology of periodontal diseases. Periodontitis, characterized by chronic inflammation, alveolar bone loss and destruction of gingival and periodontal ligament attachments to the teeth, affects approximately 65 million people in the United States [1]. Its occurrence is also associated with systemic diseases such as cardiovascular disease [2], rheumatoid arthritis [3], Alzheimer’s disease [4]. With more than 650 species of bacteria identified in the human oral cavity, only a subset of these microbes which now includes previously unrecognized and yet-unculturable species [5] are associated with the disease. In addition to the important “red complex” bacteria along with other cultivable bacterial species such as Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Selenomonas noxia, and Eubacterium nodatum are associated with periodontitis, organisms such as Selenomonas, Synergistes, Desulfobulbus, TM7 (new candidate bacterial division) and Filifactor alocis have now been identified as new potential pathogens in a number of independent studies [6,7]. Moreover, with 20–60% of the phylotypes identified in the oral cavity are yet-to-be cultivated [6,7] raises questions on the relative significance of these microbes in the disease process. This review focuses on the new organism F. alocis and explores its unique characteristics, virulence potential and capacity to influence the oral microbiome in community dynamics and a role in periodontitis.
2. Incidence
Multiple studies have documented the increasing incidence and importance of F. alocis (reviewed in Ref. [8]) (Table 1). In comparison to the other traditional periodontal pathogens, the high incidence of F. alocis in the periodontal pocket compared to its absence in healthy or periodontitis resistant patients has highlighted its importance in the infectious disease process [9]. F. alocis has also been discovered in the canals of root-filled teeth with periapical lesions and is associated with signs and symptoms of endodontic infections [10]. It has also been identified as one of the prevalent phylotypes in cases of failed endodontic treatment [11]. There is documented evidence that F. alocis is associated with peri-implantitis [12]. Tamura et al. [12] have shown that the sulcus around oral implants with peri-implantitis harbors high levels of asaccharolytic anaerobic Gram-positive rods (AAGPRs) including F. alocis which is one of the most prominent in that environment [12]. Collectively, these studies implicate F. alocis as one of few organisms associated with multiple oral infections.
Table 1.
Pubmed information on F. alocis.
| Relevant information on F. alocis | Reference |
|---|---|
|
F. alocis a common taxa noted in the subgingival microbiome of smokers compared to non-smokers |
[47] |
|
F. alocis one among the four predominant bacteria in subgingival plaque samples that could produce hydrogen sulphide |
[48] |
|
F. alocis showed variation in proteome modulation during co- infection with P. gingivalis |
[17] |
|
F. alocis showed strain variation in virulence and expression of proteins. Showed major virulence factors common to other bacteria. |
[20] |
| Incidence of F. alocis in root canals and adjacent periodontal pockets in combined periodontal-endodontic lesions. |
[49] |
| F. alocis virulence in mouse subcutaneous chamber model | [19] |
|
F. alocis was more frequently identified at higher levels in the salivary bacterial profile in periodontitis patients than in control samples |
[50] |
|
F. alocis was one of the predominant bacterial species present in peri-implantitis cases. |
[12] |
|
F. alocis showed variations in dual species community interactions. Showed synergism with F. nucleatum and was antagonistic to the accumulation of S. gordonii. A. actinomycetemcomitans showed accumulation or no effect and F. alocis with P. gingivalis formed synergistic relationship and P. gingivalis was shown benefited from the relationship. |
[35] |
| Investigation of specific anaerobic species in necrotic teeth in the pulp chamber and root canal showed the frequency of incidence of F. alocis to be 73.3%. |
[51] |
| Prevalence of F. alocis in aggressive periodontitis | [52] |
| Prevalence of F. alocis in endodontitis | [53-57] |
| Prevalence of F. alocis in subgingival microbiota | [7,58] |
| F. alocis virulence attributes | [15,18,34] |
| Incidence of F. alocis | [9,10,59] |
| F. alocis classification, isolation and phylogeny | [60-62] |
3. Morphological and cultural characteristics
F. alocis is a non-spore forming, Gram-positive obligate anaerobic rod that is slow growing and generally unreactive to conventional biochemical tests, hence difficult to identify [13]. The main habitat of F. alocis is the gingival sulcus [14]. F. alocis was first isolated in 1985 from the gingival sulcus in gingivitis and periodontitis patients and originally classified as Fusobacterium alocis, [14], but later reclassified into the genus Filifactor [13]. In silico analysis of F. alocis has shown close relatedness to Clostridium and Fusobacterium [15]. Common to these genera is their asaccharolytic nature thus an ability to utilize specific amino acids including arginine. Consistent with this observation, arginine has been shown to stimulate the growth of F. alocis [16].
4. Virulence factors
F. alocis appears to have unique properties such as resistance to oxidative stress with its stimulated growth under these condition and genes coding for a well-developed amino acid metabolic pathway that can allow it to colonize and survive with other traditional periodontal pathogens in the stress environment of the periodontal pocket [15,17]. These unique properties of F. alocis in addition to its ability to interact with other microbial species forming a polymicrobial synergistic relationship can enhance its invasive capacity [15] and cause chronic inflammation [18] in prevailing adverse conditions including fluctuations in nutrient availability, temperature, pH and oxygen tension. Additionally, an impact of F. alocis on the host is its ability to induce proinflammatory cytokines triggering apoptosis of gingival epithelial cells [19]. Other interactions with the host have triggered in F. alocis the upregulation of several proteins (e.g. proteases, proteins involved in secretion systems and proteins with cell wall anchor motifs) that are considered to have virulence properties in other bacteria [20]. Collectively, these observations implicate specific F. alocis that may be of significance in the pathogenic process.
4.1. Proteases
Proteases play a significant role in virulence among the major oral pathogens. Similarities in virulence attributes among these organisms have been notably due to the presence of a battery of proteases. In Gram-positive bacteria, proteolysis plays a pivotal role in major biological processes such as post-translational regulation of gene expression, processing and maturation of proteins. Also, expression of various surface proteins involved in virulence modulation depends on proteolysis which could strongly influence the levels of activity of proteases and their cellular mobility.
The F. alocis genome possesses at least 15 different proteases [20]. Moreover, there are variations in the expression of some of these proteases among strains of F. alocis when a low passaged strain was compared to the type strain [20]. Several of these proteases may have functional similarity to those of other periodontal pathogens. A comparison of proteases between F. alocis and the red complex bacteria is given in Table 2. Membrane bound proteases of F. alocis, Caax protease (HMPREF0389_00590) could be involved in protein and/or peptide modification and secretion [21]. There was higher expression of Caax proteases during F. alocis co-culture with Porphyromonas gingivalis [20]. Other than their metalloprotease activity, the Caax amino-terminal proteases in other oral bacteria such as Streptococcus gordonii, have been shown to play an important role in transport of proteins and also protect the bacteria against bacteriocins. Additionally, the Xaa-pro-dipeptidase (HMPREF0389_01538), O-sialoendo-peptidase (HMPREF0389_01445), Nlp/P60 family protein (peptidase M23/37) (HMPREF0389_00239) and oligo endo-peptidase M3 family (HMPREF0389_00926), were shown to be present only in the membrane fraction of F. alocis. However, the protease (HMPREF0389_00122) was identified only in the extracellular fraction. Additionally, this protease is predicted to possess a collagen peptidase function. The role of this enzyme could be important in F. alocis pathogenesis since several oral pathogens are known to produce or induce host-derived collagenases that are implicated in tissue destruction in periodontal diseases [22].
Table 2.
Comparison of proteases and MSCRAMMs between F. alocis and the red complex bacteria.
| No. | Protease | F. alocis | P. gingivalis | T. denticola | T. forsythia |
|---|---|---|---|---|---|
| 1. | RIP metalloprotease | RIP metalloprotease RseP (HMPREF0389_00112) |
Membrane-associated zinc metalloprotease (PGTDC60_1498) 97 Zinc metalloprotease (PGN_1582) [RIP metalloprotease domain] Membrane-associated zinc metalloprotease (PG0383) [RIP metalloprotease domain] |
Membrane-associated zinc metalloprotease (TDE2341) [RIP metalloprotease domain] |
Putative RIP metalloprotease RseP (BFO_2027) [RIP metalloprotease domain] |
| 2. | Protease | Protease (HMPREF0389_00122) [Collagenase domain] |
Protease (PG0753) [Collagenase domain] |
Protease II (TDE2140) [Peptidase_S9; Prolyl oligopeptidase family] |
Protease 2 (BFO_3078) [Peptidase_S9; Prolyl oligopeptidase family] |
| 3. | ATP-dependent protease La |
ATP-dependent protease La (HMPREF0389_00279) |
ATP-dependent protease La (PG0620) ATP-dependent protease La (PGN_0662) Lon ATP-dependent protease La (PGTDC60_1748) |
ATP-dependent protease La (TDE0670) |
Lon endopeptidase La (BFO_3077) [LON; ATP-dependent protease La (LON) domain] |
| 4. | Zinc protease | Zinc protease (HMPREF0389_00298) |
Zinc protease (PGN_0303) Membrane-associated zinc metalloprotease (PGTDC60_1498) [S2P-M50; Site-2 protease (S2P) class of zinc metalloproteases] ATP-dependent protease ATP- binding subunit ClpX (PG0417) [zf-C4_ClpX; ClpX C4-type zinc finger domain] ATP-dependent protease ATP- binding subunit ClpX (PGTDC60_1529) [zf- C4_ClpX; ClpX C4-type zinc finger] Zinc metalloprotease (PGN_1582) [RIP metalloprotease RseP domain] Membrane-associated zinc metalloprotease (PG0383) [RIP metalloprotease RseP domain] ATP-dependent protease ATP- binding subunit ClpX (PGN_1550) [zf-C4_ClpX; ClpX C4-type zinc finger] |
Membrane-associated zinc metalloprotease (TDE2341) [PDZ_metalloprotease; PDZ domain of bacterial and plant zinc metalloproteases] ATP-dependent protease ATP- binding subunit ClpX (TDE1673) [clpX; ATP-dependent protease ATP-binding subunit ClpX; Provisional] Hypothetical protein (TDE2485) [zf-ribbon_3; zinc-ribbon domain] |
ATP-dependent Clp protease ATP- binding subunit ClpX (BFO_1501) [zf-C4_ClpX; ClpX C4-type zinc finger] Putative RIP metalloprotease RseP (BFO_2027) [zinc metalloproteases] Thermolysin metallopeptidase, catalytic domain-containing protein (BFO_0703) [LasB; Zinc metalloprotease] Hypothetical protein BFO_2238 [S2P-M50; Site-2 protease (S2P) class of zinc metalloproteases] |
| 5. | ATP-dependent zinc metalloprotease FtsH (neutral zinc metallopeptidase family protein) |
Neutral zinc metallopeptidase family protein (HMPREF0389_01001) [Zn_peptidase_2; Putative neutral zinc metallopeptidase] |
ftsH transmembrane AAA- metalloprotease FtsH (PGTDC60_0044) [FtsH_fam; ATP-dependent metalloprotease FtsH] Transmembrane AAA- metalloprotease FtsH(PGN_0043) [FtsH_fam; ATP-dependent metalloprotease FtsH] Cell division protein FtsH(PG0047) [FtsH_fam; ATP-dependent metalloprotease FtsH domain] |
FtsH cell division protein FtsH (TDE0470) [ATP-dependent metalloprotease FtsH domain] |
hflB ATP-dependent metallopeptidase HflB (BFO_2507) [FtsH_fam; ATP- dependent metalloprotease FtsH domain] Putative neutral zinc metallopeptidase (BFO_1527) [Zn_peptidase_2; Putative neutral zinc metallopeptidase domain] Thermolysin metallopeptidase, catalytic domain-containing protein (BFO_0703) Zinc [metalloprotease (elastase) [Amino acid transport and metabolism] domain] |
| 6. | Caax amino protease family protein |
CAAX amino protease family protein (HMPREF0389_00590) |
Abortive infection protein (PGN_1454) [Abi; CAAX protease self-immunity domain] Putative abortive infection protein (PGTDC60_1637) [Abi; CAAX protease self- immunity] PG0518 hypothetical protein [Abi; CAAX protease self- immunity] |
CAAX amino terminal protease (TDE0275) CAAX amino terminal protease (TDE1288) CAAX amino terminal protease (TDE0716) CAAX amino terminal protease (TDE1870) Hypothetical protein (TDE1873) [Abi; CAAX protease self-immunity] Hypothetical protein (TDE1871) [Abi; CAAX protease self- immunity] Hypothetical protein (TDE1317) [Abi; CAAX protease self- immunity] |
CAAX amino terminal protease family protein (BFO_1873) CAAX amino terminal protease family protein (BFO_0185) Pseudo (BFO_1506) [CAAX amino terminal protease family protein] |
| 7. | Caax amino protease | CAAX amino protease (HMPREF0389_00677) |
Abortive infection protein (PGN_1454) [Abi; CAAX protease self-immunity domain] Putative abortive infection protein (PGTDC60_1637) [Abi; CAAX protease self- immunity] PG0518 hypothetical protein [Abi; CAAX protease self- immunity] |
CAAX amino terminal protease (TDE0275) CAAX amino terminal protease (TDE1288) CAAX amino terminal protease (TDE07160) CAAX amino terminal protease (TDE1870) Hypothetical protein(TDE1873) [Abi; CAAX protease self-immunity] Hypothetical protein (TDE1871) [Abi; CAAX protease self-immunity] Hypothetical protein (TDE1317) [Abi; CAAX protease self- immunity] |
CAAX amino terminal protease family protein (BFO_1873) CAAX amino terminal protease family protein (BFO_0185) Pseudo (BFO_1506) [CAAX amino terminal protease family protein] |
| 8. | Metalloprotease | Metalloprotease (HMPREF0389_00692) |
Membrane-associated zinc metalloprotease (PGTDC60_1498) Transmembrane AAA- metalloprotease FtsH (PGTDC60_0044) Zinc metalloprotease (PGN_1582) Membrane-associated zinc metalloprotease (PG0383) Transmembrane AAA- metalloprotease FtsH (PGN_0043) |
Metalloprotease (TDE1242) Membrane-associated zinc metalloprotease (TDE2341) (TDE2337) aminopeptidase [Peptidase_M29; Thermophilic metalloprotease (M29)] |
Hypothetical protein (BFO_1297) [Zinc-dependent metalloprotease] Hypothetical protein (BFO_2661) [Zinc-dependent metalloprotease] |
| 9. | Glycoprotease family protein |
Glycoprotease family protein (HMPREF0389_01443) |
Putative glycoprotease (PGTDC60_1893) Glycoprotease (PGN_0802) Hypothetical protein (PG0778) [Peptidase_M22; Glycoprotease family] DNA-binding/iron metalloprotein/AP endonuclease (PGN_0393) [PRK09604; UGMP family protein] |
Glycoprotease (TDE1468) DNA-binding/iron metalloprotein/AP endonuclease (TDE2504) [COG1214; Inactive homolog of metal-dependent proteases] |
Putative glycoprotease GCP (BFO_0842) Hypothetical protein (BFO_2190) [Peptidase_M22; Glycoprotease family] |
| 10. | Xaa pro dipeptidase | Xaa-Pro dipeptidase (HMPREF0389_01538) |
PepD-1 aminoacyl-histidine dipeptidase (PGTDC60_0414) [aa-his-dipept; Xaa-His dipeptidase] PepD-2 aminoacyl-histidine dipeptidase (PGTDC60_1655) [a-his-dipept; Xaa-His dipeptidase] Peptidase M24 family (PGTDC60_1183) [PepP; Xaa-Pro aminopeptidase] M24 family peptidase (PGTDC60_0816) [PepP; Xaa-Pro aminopeptidase] Aminoacyl-histidine dipeptidase (PGN_0250) [aa- his-dipept; Xaa-His dipeptidase] Peptidase M24 family Aminoacyl-histidine (PGN_0914) [PepP; Xaa-Pro aminopeptidase] Dipeptidase (PGN_1434) [aa- his-dipept; Xaa-His dipeptidase] M24 family peptidase (PG0889) [PepP; Xaa-Pro aminopeptidase] pepD-2 aminoacyl-histidine dipeptidase(PG0537) [aa-his- dipept; Xaa-His dipeptidase] pepD-1 aminoacyl-histidine dipeptidase(PG0137) [aa-his- dipept; Xaa-His dipeptidase] M24 family peptidase (PG1210) [Xaa-Pro aminopeptidase] Hypothetical protein (PGN_1050) [Xaa-Pro aminopeptidase] |
TDE2228 aminoacyl-histidine dipeptidase [aa-his-dipept; Xaa- His dipeptidase] TDE1482 peptidase, M24 [PepP; Xaa-Pro aminopeptidase] |
pepD_1 Xaa-His dipeptidase (BFO_1038) [aa-his-dipept; Xaa- His dipeptidase] pepD_2 Xaa-His dipeptidase (BFO_2543) [aa-his-dipept; Xaa- His dipeptidase] Aminopeptidase P, N-terminal domain-containing protein (BFO_2955) [Prolidase; Prolidase. E.C. 3.4.13.9. Also known as Xaa- Pro dipeptidase] Creatinase (BFO_2658) [PepP; Xaa-Pro aminopeptidase] Putative glycoprotease GCP (BFO_0842) YeaZ hypothetical protein (BFO_2190) [Peptidase_M22; Glycoprotease family] |
| 11. | O-sialoglycoprotein endopeptidase |
O-sialoglycoprotein endopeptidase (HMPREF0389_01445) |
gcp putative DNA-binding/iron metalloprotein/AP endonuclease (PG1724) Hypothetical protein (PG0778) [Glycoprotease family] Glycoprotease (PGN_0802) [Glycoprotease family] |
– | Putative glycoprotease GCP (BFO_0842) yeaZ hypothetical protein (BFO_2190) [Peptidase_M22; Glycoprotease family] |
| 12. | Serine protease HtrA | Serine protease HtrA (HMPREF0389_01460) |
HtrA protein (PG0593) [PDZ_serine_protease] Heat shock-related protease htrA (PGTDC60_1718) [PDZ_serine_protease] Heat shock-related protease htrA protein (PGN_0637) [PDZ_serine_protease] PDZ domain-containing protein (PGTDC60_0574) [PDZ_serine_protease] Hypothetical protein (PGN_0391) [PDZ_serine_protease] |
Trypsin domain/PDZ (TDE1966) [PDZ_serine_protease domain] Trypsin domain/PDZ (TDE2300) [PDZ_serine_protease] Trypsin domain/PDZ (TDE1343) [PDZ_serine_protease] Membrane-associated zinc metalloprotease (TDE2341) [PDZ_serine_protease] |
DegP peptidase Do (BFO_0430) [PDZ_serine_protease] |
| 13. | ATP-dependent Clp protease |
ATP-dependent Clp protease ATP-binding subunit ClpX (HMPREF0389_01648) |
ATP-dependent Clp protease, proteolytic subunit (PGTDC60_1530) ATP-dependent Clp protease proteolytic subunit (PG0418) ATP-dependent Clp protease, ATP-binding subunit ClpC (PGTDC60_0010) ATP-dependent Clp protease, ATP-binding subunit ClpC (PG0010) ATP-dependent Clp protease proteolytic subunit (PGN_1549) ATP-dependent Clp protease ATP-binding subunit ClpC (PGN_0008) |
TP-dependent Clp protease, ATP- binding subunit ClpA (TDE2124) ATP-dependent Clp protease, ATP-binding subunit ClpB (TDE2327) ATP-dependent Clp protease proteolytic subunit (TDE2388) ATP-dependent Clp protease proteolytic subunit (TDE1672) Hypothetical protein (TDE2123) [ClpS; ATP-dependent Clp protease adaptor protein ClpS] |
ATP-dependent Clp protease ATP- binding subunit ClpX (BFO_1501) ATP-dependent Clp endopeptidase, proteolytic subunit ClpP (BFO_1502) Hypothetical protein (BFO_2017) [Clp_protease_like; Caseinolytic protease (ClpP) is an ATP- dependent protease] |
| 14. | Carboxy-processing protease |
Carboxy- processing protease (HMPREF0389_00522) |
Zinc carboxypeptidase (PG0232) [Peptidase_M14_like; M14 family of metallocarboxypeptidases and related proteins] Hypothetical protein (PGN_0335) [Peptidase_M14_like; M14 family of metallocarboxypeptidases and related proteins] Carboxyl-terminal protease (PGTDC60_0120) [C-terminal peptidase domain] Carboxyl-terminal protease (PGTDC60_1149) [C-terminal processing peptidase domain] Carboxyl-terminal protease (PGTDC60_0515) [C-terminal processing peptidase domain] Carboxyl-terminal protease- like protein (PGTDC60_0655) Carboxyl-terminal processing protease (PGN_1788) [C- terminal processing peptidase domain] Carboxyl-terminal protease (PG1855) [C-terminal processing peptidase domain] Carboxyl-terminal processing protease) (PGN_0340) [C- terminal processing peptidase domain] Carboxyl-terminal processing protease (PGN_0952) [C- terminal processing peptidase domain] Carboxyl-terminal processing protease (PGN_1914) [C- terminal processing peptidase domain] Carboxyl-terminal protease (PG1060) [C-terminal processing peptidase domain] Carboxyl-terminal protease (PG0235) [C-terminal processing peptidase domain] Carboxyl-terminal protease- like protein (PG1620) |
– | – |
| 15. | Oligoendopeptidase F | Oligoendopeptidase F (HMPREF0389_00926) Oligoendopeptidase F (HMPREF0389_00527) |
– | Oligoendopeptidase F (TDE2001) Oligoendopeptidase F (TDE2639) Oligoendopeptidase F (TDE2738) |
– |
| No. | MSCRAMMs (putative) |
F. alocis | P. gingivalis | T. denticola | T. forsythia |
|---|---|---|---|---|---|
| 1. | Fibronectin- binding protein |
Fibronectin-binding protein (HMPREF0389_00575) [Fibronectin binding domain] |
Hypothetical protein (PGN_1211) –[Fibronectin binding domain] |
Fibronectin/fibrinogen-binding protein, internal deletion (TDE1579) [Fibronectin/ fibrinogen binding domain] Fibronectin type III (TDE0446) [Fibronectin 3 domain] |
Fibronectin type III domain- containing protein (BFO_0565) [Fibronectin 3 domain] Fibronectin type III domain- containing protein (BFO_0035) [Fibronectin 3 domain] Hypothetical protein (BFO_2860) [Fibronectin 3 domain] Hypothetical protein (BFO_2865) [Fibronectin type 3 domain] Hypothetical protein (BFO_2862) [Fibronectin type 3 domain] Hypothetical protein (BFO_2644) [Fibronectin type 3 domain] Hypothetical protein (BFO_0575) [Fibronectin type 3 domain] Glycosyl hydrolase family 3, C- terminal domain-containing protein (BFO_0699) [Fibronectin type 3-like domain] Glycosyl hydrolase family 3, N- terminal domain-containing protein (BFO_2182) [Fibronectin type 3-like domain] |
| 2. | Heparin- binding protein |
50S ribosomal protein L29(HMPREF0389_00839) [Ribosomal_L29_HIP; Ribosomal L29 protein/HIP* heparin/heparan sulfate interacting protein (HIP)] |
RpmC 50S ribosomal protein L29 (PGTDC60_0200) [Ribosomal_L29_HIP; Ribosomal L29 protein/HIP] RpmC 50S ribosomal protein L29 (PGN_1860) [Ribosomal_L29_HIP; Ribosomal L29 protein/HIP] 50S ribosomal protein L29 (PG1930) [Ribosomal_L29_HIP; Ribosomal L29 protein/HIP.] |
50S ribosomal protein L29 (TDE0775) [Ribosomal_L29_HIP; Ribosomal L29 protein/HIP] |
RpmC 50S ribosomal protein L29 (BFO_1554) [Ribosomal_L29_HIP; Ribosomal L29 protein/HIP] |
| 3. | Fibrinogen- binding protein |
– | – | Fibronectin/fibrinogen-binding protein, internal deletion (TDE1579) |
– |
| 4. | Collagenase | Protease (HMPREF0389_00122) [Collagenase domain] Peptidase, U32 family (HMPREF0389_00504) [Collagenase domain] |
Collagenase (PG1542) Collagenase (PGN_0567) PrtC collagenase (PGTDC60_0756) PrtQ PrtQ, protease (PGTDC60_1870) PrtQ PrtQ, protease (PGN_0780) Protease (PG0753) [All have Collagenase domains] |
U32 family peptidase (TDE0071) [Collagenase domain] U32 family peptidase (TDE2262) [Collagenase domain] |
PrtC collagenase (BFO_1352) Peptidase, (U32 family BFO_0839) [Collagenase domain] |
| 5. | Collagen adhesin/ binding protein |
Collagen adhesin protein (HMPREF0389_01006) [Collagen binding domain] Gram positive anchor (HMPREF0389_01336) [Collagen binding domain] Hypothetical protein (HMPREF0389_01750) [Collagen binding domain] |
– | – | – |
| 6. | Laminin binding protein |
– | – | Fibronectin type III (TDE0446) [Fibronectin type 3 domain] Hypothetical protein (TDE1139) [Laminin_G_3; Concanavalin A-like lectin/ glucanases superfamily] |
Putative lipoprotein (BFO_1193) [Laminin_G_3; Concanavalin A-like lectin/glucanases superfamily] |
( ) Gene annotation from NCBI.
[Italics] conserved domains.
4.2. Adhesion
Attributes such as adherence and invasion of host cells, are considered important to the success of a pathogen. Genes encoding for proteins such as CaaX aminopeptidases may be crucial for masking the host ubiquitin system and facilitate invasion of host cells [23].
F. alocis was shown to adhere and invade epithelial cells [15]. These attributes were enhanced in the presence of P. gingivalis [15]. While a similar enhancement of invasion was observed between P. gingivalis, F. nucleatum and P. intermedia [24], F. nucleatum and Streptococcus cristatus [25], and F. nucleatum and Pseudomonas aeruginosa [26], the exact mechanism for F. alocis is unclear.
Co-infection of F. alocis with P. gingivalis showed filapodial projections on the surface of the host cell that were believed to mediate the organism’s internalization. Also, vesicle-mediated internalization of P. gingivalis and F. alocis was observed during invasion of epithelial cells in co-infection studies [15]. This process may protect the pathogen after invasion, facilitating its pathogenic potential. The mechanism of membrane-ruffling commonly noted among Gram-positive bacteria during invasion strategies, were not noted in F. alocis. Since vesicle-mediated endocytic internalization of Gram-positive bacteria are generally mediated by type II and III exotoxins [27], it is likely that such exotoxins may contribute to the enhancement of internalization observed during F. alocis–P. gingivalis co-infection. It is worthy to note that several exotoxins have been identified in F. alocis but their role in invasion remains unclear.
Proteomic analysis of F. alocis during co-infection of epithelial cells with P. gingivalis using tandem mass tagging technique revealed increase in several membrane adhesion proteins and Microbial Surface Components Recognizing Adhesive Matrix Molecules (MSCRAMMs). In silico analysis of the mass spectrometry data using database search and domain prediction revealed some of these adhesion proteins to be known virulence factors in other systems, however, the functions of several unique hypothetical proteins with transmembrane domains needs to be elucidated. Taken together, this suggests that community dynamics through the interaction of F. alocis and P. gingivalis may result in the upregulation of specific factor(s) that may enhance their virulence potential [15]. In our preliminary studies, F. alocis co-cultured with P. gingivalis showed adhesion to epithelial cells and alteration in their morphology leading to cell death. This was in contrast to mono-infections with either F. alocis or P. gingivalis which did not trigger the same morphological alteration, although these bacteria were still able to induce cell death over a longer time period [15].
4.2.1. MSCRAMMs
MSCRAMMs are known to play an important role in Gram-positive bacterial virulence by mediating adherence to and colonization of host tissues as an early step toward clinically manifested infection. There is also evidence to suggest that these extracellular matrix adhesion proteins can be regulated by quorum-sensing, which implies that some environmental signals can modulate their expression and hence promote adhesion and colonization. MSCARMMs are evidently important in the pathogen attachment and virulence modulation. Expression of F. alocis MSCRAMMs have been identified in the cell membrane and cell wall fractions [20]. Genome annotations of putative F. alocis MSRAMMs compared with the three red complex bacteria are given in Table 2. Among the MSCRAMMs of F. alocis, the collagenolytic MSCRAMMs and collagenase related proteins could be important to bring about initial pathogen interaction and host adherence due to proteolysis of the common extracellular matrix (ECM)-collagen. The role of collagenases in periodontal pathogenesis has been well documented in other pathogens [28-30]. Our preliminary studies based on in silico analysis of F. alocis collagenases also reveal molecular relatedness to some of the collagenolytic MSCRAMMs of other pathogenic bacteria. It has been reported over the past decade that many organisms that express cell surface adhesins can mediate microbial adhesion to the ECM of the host tissues. Hence, F. alocis can also target the ECM in a similar manner through interaction with collagenolytic MSCRAMMs.
5. Unique amino acid metabolism
Even though F. alocis showed low gingipain-type activity, it had increased non-gingipain protease activity [15]. The amino acids mostly utilized by F. alocis include arginine and lysine, followed by cysteine. The F. alocis arginine metabolic pathway predicts the enzymatic degradation of arginine by arginine deiminase, leading to the conversion of arginine to ornithine and ammonia [16]. Arginine degradation could favor increase in the pH that would counteract acidic conditions generated from carbohydrate catabolism in a mixed bacterial oral flora. In the periodontal pocket, these amino acids can also be made available from the degradation of various protein substrates by other bacteria and host-derived proteases for nutritional support, survival and virulence [31].
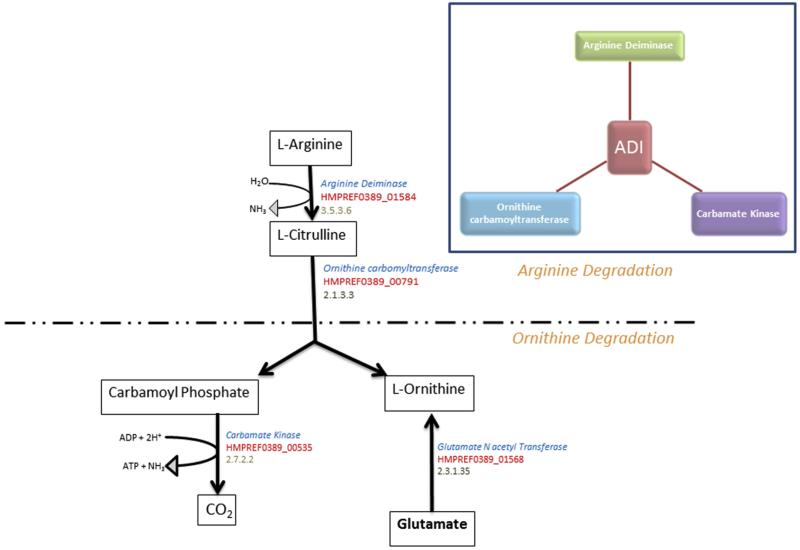
In silico analysis of the F. alocis genome predicts a well-developed amino acid metabolism pathway that should be functional to catabolize protein and amino acids. One among the well-developed pathways identified in F. alocis is the arginine deiminase pathway which is involved in arginine metabolism leading to citrulline and ornithine (Fig. 1). Earlier studies have shown that F. alocis can convert arginine to ornithine through an enzymatic process without the intermediate step of citrulline production [16]. This process may be vital for its survival as studies from our laboratory have shown that the arg operon consisting of 5 genes involved in arginine metabolism were highly upregulated during co-infection studies with P. gingivalis. While the three genes involved in arginine deiminase pathway namely the arginine deiminase (HMPREF0389_ 001584), ornithine carbamoyltransferase (HMPREF0389_ 00791) and carbamate kinase (HMPREF0389_ 00535) were annotated in the genome of F. alocis, in silico analysis of ornithine carbamoyltransferase the enzyme involved in conversion of l-citrulline to l-Ornithine show an ornithine/aspartate carbamoyltransferase domain inferring a dual role of the enzyme. F. alocis also possess glutamate N acetyl transferase (HMPREF0389_ 001568) an enzyme know to have dual role in other bacteria that converts glutamate one of the important intermediary metabolites during energy metabolism to Ornithine.
Fig. 1.
Arginine degradation in F. alocis through the arginine deiminase pathway. The boxes show the metabolites. The NCBI annotations are given in red, gene descriptions are given in blue and enzyme nomenclature (EC number) is given in gray. The inset showing the three enzymes of the arginine deiminase pathway.
Certain oral bacteria like F. nucleatum lack essential amino acid synthetic pathways and rely on the ability to import and degrade di and oligo peptides [32]. Consistent with the assacharolytic properties of F. alocis, several proteins that play an important role in amino acid metabolism (e.g. Arginine degradation pathway proteins referred above) including many that may contribute to protein degradation (such as peptidases and proteases) are encoded in its genome (http://www.ncbi.nlm.nih.gov/bioproject/46625). Even if many inherent amino acid synthesis pathways may be nonfunctional, the occurrence of a wide range of such dipeptidases, metalloproteases and o-sialoglycoproteases, could likely provide F. alocis with the appropriate substrates to compensate for its nutritional needs. Additionally, certain proteins, such as the oxy acyl carrier protein (HMPREF0389_ 01112) which is involved in fatty acid metabolism and not usually identified among the oral biofilm forming pathogens, fibronectin binding protein (HMPREF0389_00575) and dipicolinate reductase (HMPREF0389_01077) which are involved in amino acid metabolism and virulence [33], were also identified in F. alocis [20]. Taken together, it is likely that F. alocis may be well adapted to provide for its own nutritional needs. However, the role these systems play in bacterial community dynamics should be further elucidated.
6. Co-existence, polymicrobial synergy and biofilm formation
Oral bacterial composition varies during the progression of disease from a scanty biofilm forming Gram-positive bacteria to increased number of Gram-negative bacteria. Oral biofilms are primary initiating factors of periodontal disease. Biofilm formation involving F. alocis has been demonstrated both in periodontic and endodontic cases [34]. While some interspecies interaction can inhibit biofilm formation, P. gingivalis ATCC 33277 co-cultured with F. alocis showed significant increase in biofilm formation [15]. This enhanced biofilm forming capacity may be due to the ability of both species to auto aggregate and express unique components. This could indicate a commensal relationship between F. alocis and P. gingivalis. Thus, F. alocis and P. gingivalis, each with different growth rates, could form a mixed species biofilm and coexist [15]. As a result, F. alocis proteins could enable P. gingivalis to proliferate and disseminate from these biofilms thus facilitating its virulence.
A recent in vitro study, evaluating the community interactions of two strains of F. alocis with S. gordonii, F. nucleatum, P. gingivalis and A. actinomycetemcomitans, which are organisms of differing pathogenic potential in the oral cavity, suggests that F. alocis is likely to interact with a variety of oral bacteria and participate in community development [35]. Further, F. alocis colonization seemed influenced by the spatial composition of microbial microenvironments through organism preferentially accumulating at sites rich in F. nucleatum. S. gordonii was antagonistic to the accumulation of F. alocis in a dual species community. This was consistent with the observation that streptococcal rich dental plaques were resistant to colonization by F. alocis [35]. In three species communities of S. gordonii, F. nucleatum and F. alocis, the antagonistic effects of S. gordonii superseded the synergistic effects of F. nucleatum toward F. alocis [35]. The interaction between A. actinomycetemcomitans and F. alocis was strain specific. It was also noted that A. actinomycetemcomitans could either stimulate F. alocis accumulation or have no effect, depending on the strain. P. gingivalis and F. alocis formed heterotypic communities, with the abundance of P. gingivalis being enhanced in the presence of F. alocis [35]. While the mechanism of the interaction is complex with inhibitory and counterbalancing measures, It is likely that F. alocis proteins induced under those conditions may facilitate adhesion and nutrient support for P. gingivalis. Also the question of how arginine deiminase affects the community dynamics can be raised. The inhibitory effect of P. gingivalis on F. alocis was observed to be partially dependent on the minor fimbriae [35]. The arginine deiminase of S. cristatus is known to suppress fimbrial production in P. gingivalis [36]. Based on the relative abundance of F. alocis in the periodontal pocket compared to P. gingivalis, it is unclear if the F. alocis arginine deiminase is induced in that microenvironment and could have an effect on P. gingivalis fimbrial expression. However, the primary role of a well-developed arginine degradation pathway leads to the concept of reduction in pH and increased CO2 production that might have an effect on the expression of virulence factors.
7. Oxidative stress resistance
Our earlier studies have shown that F. alocis is relatively resistant to oxidative stress compared to P. gingivalis and that its growth is stimulated under those conditions [15]. These observations may indicate an important attribute for the survival and relative abundance of F. alocis compared to other organisms in the inflammatory microenvironment of the periodontal pocket. It is also likely that F. alocis may play a role as an “oxidative sink” to stabilize the microbial community in the microenvironment of the periodontal pocket. It was also noted that survival of P. gingivalis under hydrogen peroxide-induced oxidative stress is enhanced in the presence of F. alocis. A likely mechanism could be due to the presence of sialidase activity in F. alocis that not only satisfy its asaccharolytic property by breakdown of sialated glycoproteins found in saliva, but the released sialic acid can also act as a reactive oxygen species (ROS) scavenger to reduce the oxidative stress in the inflammatory environment of the periodontal pocket. F. alocis possesses a superoxide reductase (GenBank accession no. EFE28874) that could help to facilitate its growth in the presence of hydrogen peroxide (http://www.ncbi.nlm.nih.gov/bioproject/46625).
The interaction of F. alocis with other organisms can also enhance its oxidative stress resistance and hence its virulence potential. In co-culture with P. gingivalis, an upregulation of many proteins involved in oxidative stress resistance such as superoxide reductase, iron–sulfur cluster protein, iron permease, rubrerythrin, ferrous hydrogenase family protein and thioredoxin family proteins were observed in F. alocis [20]. One of the key attributes of F. alocis is the 3-methyladenine DNA glycosylase (HMPREF0389_1529), an enzyme reported to be involved in oxidative and nitrosative stress resistance in other pathogenic bacteria; although, its function under those conditions is unclear. The genome of F. alocis also includes genes that encode for a well-developed group of iron sulfur cluster proteins and a ferrous iron transport system which are unique to this organism compared to other “red complex” bacteria. Additionally, F. alocis seems to possess a well-developed protein sorting/transport system which is evident by the presence of a large number of membrane proteins [20]. It is likely that surface and secretory proteins from F. alocis may play a role in this protein transport process. With a well-developed arginine deiminase pathway leading to Ornithine production, hence similar to other bacteria, ornithine production could also favor oxidative stress resistance in F. alocis. Together, these systems could possibly facilitate the efflux of ROS.
8. CRISPR-associated genes
Regions of unusual DNA composition in the bacterial genome such as the clustered regularly interspaced short palindromic repeats (CRISPR) locus together with the CRISPR-associated genes are thought to act as adaptive immunity systems in bacteria and recently found to play a role in bacterial virulence [37]. The virulence attribute may partly be due to genome rearrangement and/or regulation of gene expression that will facilitate host environment adaptation.
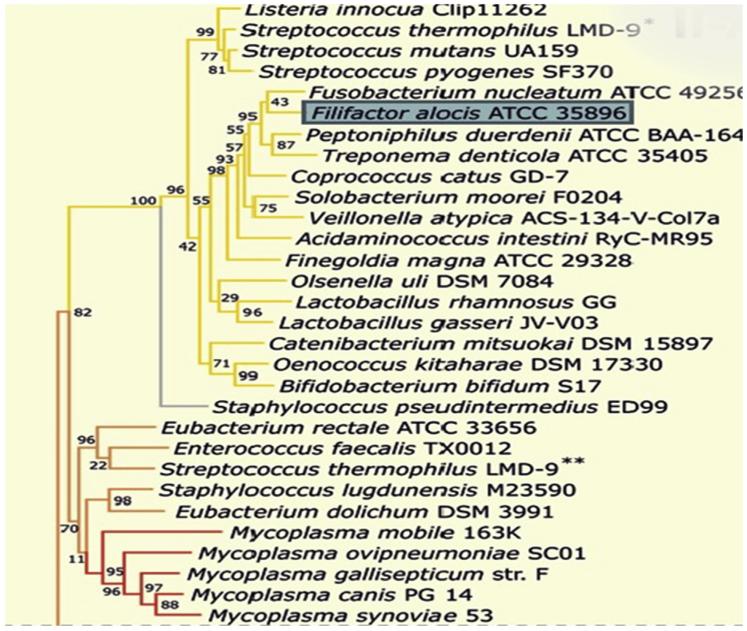
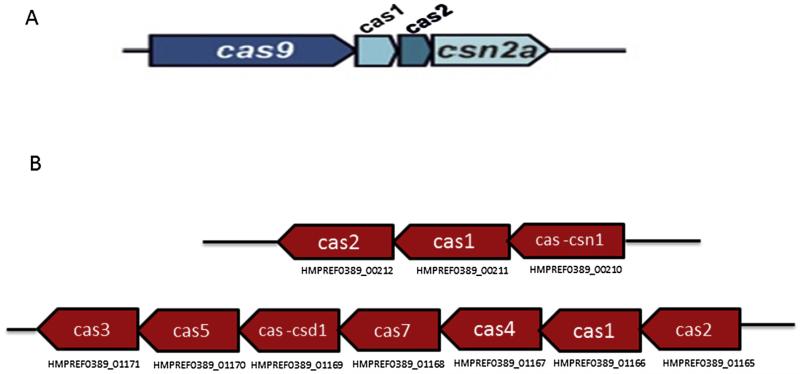
CRISPRs are widely distributed amongst bacteria and archaea [38] and show some sequence similarities [39], however their most notable characteristic is their repeating spacers and direct repeats which is the basis for their division into three groups. The three groups are further divided into subgroups depending on a specific combination of Cas genes. Genes encoding Cas 1 and 2 are present in each group. Of the three major groups, type II is the most studied. Type I CRISPR–Cas system is based on the ubiquitous presence of a signature protein, the Cas3 helicase/nuclease [40]. The Type II CRISPR/Cas locus contains Cas1, Cas2 and Cas9, as well as a predicted trans-activating crRNA (tracrRNA), and a small CRISPR/Cas-associated RNA (scaRNA) [41]. The Type III CRISPR/Cas system is typified by the presence of Cas10 [40]. Based on phylogenetic analyses of Cas9 (member of type II system) and its orthologues, F. alocis is predicted to be a member of the Type II-A CRISPR–Cas system (Fig. 2) [42]. The locus architecture of Type II CRISPR–cas system consists of cas9, cas1, cas2, csn2a in other bacteria (Fig. 3A) [42]. The orientation of CRISPR–Cas system in F. alocis is found to be localized in two clusters (Fig. 3B). The upregulation of CRISPR/Cas system components during the co-infection of epithelial cells with F. alocis and P. gingivalis (Table 3) could suggest their role in virulence and pathogen synergy. The virulence of F. alocis was enhanced in the presence of P. gingivalis [17]. The significance of the CRISPR–Cas system in this process needs further investigation.
Fig. 2.
Phylogenetic tree of representative Cas9 sequences. Bootstrap values calculated for each node are indicated. Blue box shows the location of F. alocis. Subclusters of similar Cas9 orthologs are indicated by the same branch colors.
Fig. 3.
A. Genome organization of Type II CRISPR–Cas system [42]. B. F. alocis genome organization of CRISPR–Cas system genes located in two clusters.
Table 3.
Relative abundance of CRISPR associated proteins of F. alocis strains during co-infection with P. gingivalis [17].
| Gene ID | Annotation | Fold change |
|
|---|---|---|---|
| D- 62D |
ATCC- 35896 |
||
| HMPREF 0389_00212 |
CRISPR-associated protein (Cas2) |
2.12 | 1.40 |
| HMPREF 0389_00211 |
CRISPR-associated protein (Cas1) |
1.4 | 0.70 |
| HMPREF 0389_00210 |
CRISPR-associated protein (Csn1) |
1.046 | 1.00 |
| HMPREF 0389_01169 |
CRISPR-associated protein (Csd1) |
0.960 | 0.95 |
| HMPREF 0389_01170 |
CRISPR-associated protein (Cas5) |
0.748 | 0.78 |
| HMPREF 0389_01165 |
CRISPR-associated protein (Cas2) |
1.30 | 1.20 |
| HMPREF 0389_0167 | CRISPR-associated protein (Cas4) |
1.01 | 1.00 |
9. Host cell modulation
Various studies have demonstrated the ability of F. alocis to impact host cells, enabling its persistence in the periodontal pocket. F. alocis infection demonstrated its ability to induce the secretion of the proinflammatory cytokines IL-1β, IL-6 and TNF-α from epithelial cells, while also inducing their apoptosis through pathways involving caspase-3 [19]. The in vivo pathogenicity of F. alocis tested using a mouse subcutaneous chamber model induced a local inflammatory response as observed by the influx of neutrophils in addition to an increase in the IL-1β, IL-6 and TNF-α proinflammatory cytokine levels [43]. Overall, with respect to the various mechanisms by which F. alocis could possibly impact host cells, proteome analysis of F. alocis in co-infection of epithelial cells with P. gingivalis revealed proteins that could contribute to several functions including host cell signaling, metabolic host response, cell–cell interaction, and activation of oncogenes, among others [17].
Host responses to bacterial infections may favor survival and play a role in pathogenesis through modulation of metabolic processes. High amounts of arginine in the periodontal pocket and the abundance of F. alocis proteins involved in arginine metabolism and citrulline synthesis, such as arginine deiminase (HMPREF 0389_01584), acetyl ornithine transferase (HMPREF0389_01570) [20], aminotransferases (HMPREF0389_01352 and HMPREF0389_01353), amino-transferase family protein (HMPREF 0389_00349), arginine – tRNA ligase (HMPREF0389_00390), and arginine decarboxylase (HMPREF0389_00102) (http://www.ncbi.nlm.nih.gov/bioproject/46625), indicate that the nutritional needs of the bacterium could be adequately met during infection and vital for its survival in the harsh microenvironment of the periodontal pocket. Furthermore, its interaction with other microbes may collectively enhance their survival. Ammonia production from arginine metabolism has been identified as an important mechanism by which oral bacteria are protected against acid killing. Among the three key enzymes, namely, arginine deiminase, ornithine carbamoyltransferase and carbamate kinase important in arginine metabolism [44], F. alocis genome shows genes coding for arginine deiminase, and carbamate kinase but not the ornithine carbamoyltransferase. It is noteworthy that P. gingivalis and F. alocis interspecies interaction resulted in the upregulation of arginine deiminase (HMPREF_0389_01584) and carbamate kinase (HMPREF_0389_00535) in F. alocis. While it may use a novel arginine catabolic pathway compared to other AAGPRs in the periodontal pocket [16], its relative abundance in the periodontal pocket and it’s enhanced ability to produce ammonia could promote species co-habitation and survival. Because butyrate is a metabolic end product from arginine, it could likely also have an impact on other microbial interactions including viruses in the oral cavity. Butyric acid produced by periodontopathic bacteria including P. gingivalis can lead to viral reactivation. The impact of viral infection on periodontal disease is now being recognized as the active inflamed lesion appears to be a major site for re-activation and accumulation of Herpes virus resulting in enhanced tissue breakdown.
Interrogation of the F. alocis genome also revealed templates for a well-developed citrulline synthesis mechanism using arginine [16]. Citrullination of proteins is understood to be an important posttranslational modification with systemic implications. Previous studies have shown that upregulation of peptidylarginine deiminase (PAD) expression and the associated increase in citrullinated proteins were found in patients with rheumatoid arthritis [45]. A mechanistic link between periodontal infection and rheumatoid arthritis has been established, collagen-induced arthritis was dependent on the expression of a unique P. gingivalis peptidylarginine deiminase (PPAD) [46]. The arginine deiminase from pathogens was shown to possess multiple regulatory roles similar to PAD function [47]. Bioinformatic analyses indicate that P. gingivalis PAD has major sequence and structural homology with the F. alocis arginine deiminase enzyme (unpublished data). It is likely that in F. alocis, arginine deiminase could have citrullination-induced systemic implications.
It is also important to note that the proteins ornithine transaminase (HMPREF0389_01570), acetyl glutamate kinase (HMPREF0389_01569), glutamate racemase (HMPRE F0389_00100) and aminotransferase (HMPREF0389_00478) involved in ornithine biosynthesis were identified in F. alocis. In fact, arginine deiminase (HMPREF0389_01584) involved in ornithine catabolism and urea breakdown was found both in the membrane and the extracellular fractions of F. alocis [20] suggesting a well-developed nitrogen assimilatory pathway that may play a role as an alternative mode of amino acid synthesis in F. alocis.
10. Conclusion
F. alocis is one of a few bacteria that is associated with multiple oral diseases including periodontitis, localized aggressive periodontitis, endodontitis and peri-implantitis. Its relative abundance in the periodontal pocket of patients with periodontitis may support the hypothesis to include F. alocis as a diagnostic marker organism. This organism has unique potential virulence characteristics such as resistance to oxidative stress and genes coding for a well-developed amino acid metabolic pathway that can modulate multiple changes to the oral microbial community and the host cell proteome, that collectively can lead to the disease process. Most of the potential virulence attributes of this assacharolytic bacteria is unexplored and deserves further extensive study.
Acknowledgments
This work was supported by Loma Linda University and Public Health Services Grants R-56-DE13664, DE019730, DE019730 04S1, DE022508, DE022724 from NIDCR (to H.M.F). Due to the Journal’s editorial limitations, we apologize to our colleagues/fellow scientists for not including their references in the review.
Footnotes
Conflict of interest
The authors disclose no conflict of interest.
References
- [1].Thornton-Evans G, Eke P, Wei L, Palmer A, Moeti R, Hutchins S, et al. Periodontitis among adults aged ≥30 years – United States, 2009–2010. 2013;62(Suppl. 3):129–35. [PubMed] [Google Scholar]
- [2].Genco RJ, Van Dyke TE. Prevention: reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol. 2010;7:479–80. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- [3].Bingham CO, Moni M. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol. 2013;25:345–53. doi: 10.1097/BOR.0b013e32835fb8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis. 2013;36:665–77. doi: 10.3233/JAD-121918. [DOI] [PubMed] [Google Scholar]
- [5].Belstrom D, Fiehn NE, Nielsen CH, Kirkby N, Twetman S, Klepac-Ceraj V, et al. Differences in bacterial saliva profile between periodontitis patients and a control cohort. J Clin Periodontol. 2014;41:104–12. doi: 10.1111/jcpe.12190. [DOI] [PubMed] [Google Scholar]
- [6].Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–85. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aruni W, Chioma O, Fletcher HM. Filifactor alocis: the newly discovered kid on the block with special talents. J Dent Res. 2014;93:725–32. doi: 10.1177/0022034514538283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–73. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gomes BP, Jacinto RC, Pinheiro ET, Sousa EL, Zaia AA, Ferraz CC, et al. Molecular analysis of Filifactor alocis, Tannerella forsythia, and Treponema denticola associated with primary endodontic infections and failed endodontic treatment. J Endod. 2006;32:937–40. doi: 10.1016/j.joen.2006.05.003. [DOI] [PubMed] [Google Scholar]
- [11].Zhang C, Hou BX, Zhao HY, Sun Z. Microbial diversity in failed endodontic root-filled teeth. Chin Med J Engl. 2012;125:1163–8. [PubMed] [Google Scholar]
- [12].Tamura N, Ochi M, Miyakawa H, Nakazawa F. Analysis of bacterial flora associated with peri-implantitis using obligate anaerobic culture technique and 16S rDNA gene sequence. Int J Oral Maxillofac Implants. 2013;28:1521–9. doi: 10.11607/jomi.2570. [DOI] [PubMed] [Google Scholar]
- [13].Jalava J, Eerola E. Phylogenetic analysis of Fusobacterium alocis and Fusobacterium sulci based on 16S rRNA gene sequences: proposal of Filifactor alocis (Cato, Moore and Moore) comb. nov. and Eubacterium sulci (Cato, Moore and Moore) comb. nov. Int J Syst Bacteriol. 1999;49(4):1375–9. doi: 10.1099/00207713-49-4-1375. [DOI] [PubMed] [Google Scholar]
- [14].Cato Elizabeth P, Moore LVH, Moore WEC. Fusobacterium alocis sp. nov. and Fusobacterium sulci sp. nov. from the human gingival sulcus. 1985;35:475–7. [Google Scholar]
- [15].Aruni AW, Roy F, Fletcher HM. Filifactor alocis has virulence attributes that can enhance its persistence under oxidative stress conditions and mediate invasion of epithelial cells by Porphyromonas gingivalis. Infect Immun. 2011;79:3872–86. doi: 10.1128/IAI.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Uematsu H, Sato N, Hossain MZ, Ikeda T, Hoshino E. Degradation of arginine and other amino acids by butyrate-producing asaccharolytic anaerobic Gram-positive rods in periodontal pockets. Arch Oral Biol. 2003;48:423–9. doi: 10.1016/s0003-9969(03)00031-1. [DOI] [PubMed] [Google Scholar]
- [17].Aruni AW, Zhang K, Dou Y, Fletcher H. Proteome analysis of coinfection of epithelial cells with Filifactor alocis and Porphyromonas gingivalis shows modulation of pathogen and host regulatory pathways. Infect Immun. 2014;82:3261–74. doi: 10.1128/IAI.01727-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fine DH, Markowitz K, Fairlie K, Tischio-Bereski D, Ferrendiz J, Furgang D, et al. A consortium of Aggregatibacter actinomycetemcomitans (Aa), Streptococcus parasanguinis and Filifactor alocis are present in sites prior to bone loss in a longitudinal study of localized aggressive periodontitis. J Clin Microbiol. 2013;51(9):2850–61. doi: 10.1128/JCM.00729-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moffatt CE, Whitmore SE, Griffen AL, Leys EJ, Lamont RJ. Filifactor alocis interactions with gingivalis epithelial cells. Mol Oral Microbiol. 2011;26:365–73. doi: 10.1111/j.2041-1014.2011.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aruni AW, Roy F, Sandberg L, Fletcher HM. Proteome variation among Filifactor alocis strains. Proteomics. 2012;12:3343–64. doi: 10.1002/pmic.201200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pei J, Grishin NV. Type II CAAX prenyl endopeptidases belong to a novel superfamily of putative membrane-bound metalloproteases. Trends Biochem Sci. 2001;26:275–7. doi: 10.1016/s0968-0004(01)01813-8. [DOI] [PubMed] [Google Scholar]
- [22].Kumagai Y, Yagishita H, Yajima A, Okamoto T, Konishi K. Molecular mechanism for connective tissue destruction by dipeptidyl aminopeptidase IV produced by the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2005;73:2655–64. doi: 10.1128/IAI.73.5.2655-2664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Price CT, Abu KY. Exploitation of poly ubiquitination machinery through molecular mimicry by eukaryotic-like bacterial F-box effectors. Front Microbiol. 2010;1:1–12. doi: 10.3389/fmicb.2010.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Saito A, Inagaki S, Ishihara K. Differential ability of periodontopathic bacteria to modulate invasion of human gingival epithelial cells by Porphyromonas gingivalis. Microb Pathog. 2009;47:329–33. doi: 10.1016/j.micpath.2009.09.012. [DOI] [PubMed] [Google Scholar]
- [25].Edwards AM, Grossman TJ, Rudney JD. Fusobacterium nucleatum transports non invasive Streptococcus cristatus into human epithelial cells. Infect Immun. 2006;74:654–62. doi: 10.1128/IAI.74.1.654-662.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pan Y, Teng D, Burke AC, Haase EM, Scannapieco FA. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb Pathog. 2009;46:73–9. doi: 10.1016/j.micpath.2008.10.012. [DOI] [PubMed] [Google Scholar]
- [27].Nitsche-Schmitz DP, Rohde M, Chhatwal GS. Invasion mechanisms of Gram-positive pathogenic cocci. Thromb Haemost. 2007;98:488–96. [PubMed] [Google Scholar]
- [28].Bedi GS, Williams T. Purification and characterization of a collagen-degrading protease from Porphyromonas gingivalis. J Biol Chem. 1994;269:599–606. [PubMed] [Google Scholar]
- [29].Lawson DA, Meyer TF. Biochemical characterization of Porphyromonas (Bacteroides) gingivalis collagenase. Infect Immun. 1992;60:1524–9. doi: 10.1128/iai.60.4.1524-1529.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sojar HT, Lee JY, Bedi GS, Genco RJ. Purification and characterization of a protease from Porphyromonas gingivalis capable of degrading salt-solubilized collagen. Infect Immun. 1993;61:2369–76. doi: 10.1128/iai.61.6.2369-2376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eley BM, Cox SW. Cathepsin B/L-, elastase-, tryptase-, trypsin- and dipeptidyl peptidase IV-like activities in gingival crevicular fluid: a comparison of levels before and after periodontal surgery in chronic periodontitis patients. J Periodontol. 1992;63:412–7. doi: 10.1902/jop.1992.63.5.412. [DOI] [PubMed] [Google Scholar]
- [32].Kapatral V, Anderson I, Ivanova N, Reznik G, Los T, Lykidis A, et al. Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J Bacteriol. 2002;184:2005–18. doi: 10.1128/JB.184.7.2005-2018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Berges DA, DeWolf WE, Jr, Dunn GL, Newman DJ, Schmidt SJ, Taggart JJ, et al. Studies on the active site of succinyl-CoA:tetrahydrodipicolinate N-succinyltransferase. Characterization using analogs of tetrahydrodipicolinate. J Biol Chem. 1986;261:6160–7. [PubMed] [Google Scholar]
- [34].Schlafer S, Riep B, Griffen AL, Petrich A, Hubner J, Berning M, et al. Filifactor alocis – involvement in periodontal biofilms. BMC Microbiol. 2010;10:66. doi: 10.1186/1471-2180-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Q, Wright CJ, Dingming H, Uriarte SM, Lamont RJ. Oral community interactions of Filifactor alocis in vitro. PLoS One. 2013;8:e76271. doi: 10.1371/journal.pone.0076271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xie H, Lin X, Wang BY, Wu J, Lamont RJ. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology. 2007;153:3228–34. doi: 10.1099/mic.0.2007/009050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Louwen R, Staals RH, Endtz HP, van BP, van der Oost J. The role of CRISPR–Cas systems in virulence of pathogenic bacteria. Microbiol Mol Biol Rev. 2014;78:74–8. doi: 10.1128/MMBR.00039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chylinski K, Makarova KS, Charpentier E, Koonin EV. Classification and evolution of type II CRISPR–Cas systems. Nucleic Acids Res. 2014;42:6091–105. doi: 10.1093/nar/gku241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kunin V, Sorek R, Hugenholtz P. Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol. 2007;8:R61. doi: 10.1186/gb-2007-8-4-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bhaya D, Davison M, Barrangou R. CRISPR–Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu Rev Genet. 2011;45:273–97. doi: 10.1146/annurev-genet-110410-132430. [DOI] [PubMed] [Google Scholar]
- [41].Sampson TR, Saroj SD, Llewellyn AC, Tzeng YL, Weiss DS. A CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature. 2013;497:254–7. doi: 10.1038/nature12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chylinski K, Le RA, Charpentier E. The tracrRNA and Cas9 families of type II CRISPR–Cas immunity systems. RNA Biol. 2013;10:726–37. doi: 10.4161/rna.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang Q, Jotwani R, Le J, Krauss JL, Potempa J, Coventry SC, et al. Filifactor alocis infection and inflammatory responses in the mouse subcutaneous chamber model. Infect Immun. 2014;82:1205–12. doi: 10.1128/IAI.01434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Griswold A, Chen YY, Snyder JA, Burne RA. Characterization of the arginine deiminase operon of Streptococcus rattus FA-1. Appl Environ Microbiol. 2004;70:1321–7. doi: 10.1128/AEM.70.3.1321-1327.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al BR, Mechin MC, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–53. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- [46].Maresz KJ, Hellvard A, Sroka A, Adamowicz K, Bielecka E, Koziel J, et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD) PLoS Pathog. 2013;9:1–10. doi: 10.1371/journal.ppat.1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Touz MC, Ropolo AS, Rivero MR, Vranych CV, Conrad JT, Svard SG, et al. Arginine deiminase has multiple regulatory roles in the biology of Giardia lamblia. J Cell Sci. 2008;121:2930–8. doi: 10.1242/jcs.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Basic A, Dahlen G. Hydrogen sulfide production from subgingival plaque samples. Anaerobe. 2014 doi: 10.1016/j.anaerobe.2014.09.017. http://dx.doi.org/10.1016/j.anaerobe.2014.09.017. [DOI] [PubMed] [Google Scholar]
- [49].Li H, Guan R, Sun J, Hou B. A Bacteria community study of combined periodontal-endodontic lesions using denaturing gradient gel electrophoresis and sequencing analysis. J Periodontol. 2014;85(10):1442–9. doi: 10.1902/jop.2014.130572. [DOI] [PubMed] [Google Scholar]
- [50].Belstrom D, Fiehn NE, Nielsen CH, Kirkby N, Twetman S, Klepac-Ceraj V, et al. Differences in bacterial saliva profile between periodontitis patients and a control cohort. J Clin Periodontol. 2014;41:104–12. doi: 10.1111/jcpe.12190. [DOI] [PubMed] [Google Scholar]
- [51].Gomes GB, Sarkis-Onofre R, Bonow ML, Etges A, Jacinto RC. An investigation of the presence of specific anaerobic species in necrotic primary teeth. Braz Oral Res. 2013;27:149–55. doi: 10.1590/s1806-83242013000100020. [DOI] [PubMed] [Google Scholar]
- [52].Shaddox LM, Huang H, Lin T, Hou W, Harrison PL, Aukhil I. Microbiological characterization in children with aggressive periodontitis. J Dent Res. 2012;91:927–33. doi: 10.1177/0022034512456039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang C, Hou BX, Zhao HY, Sun Z. Microbial diversity in failed endodontic root-filled teeth. Chin Med J (Engl) 2012;125:1163–8. [PubMed] [Google Scholar]
- [54].Montagner F, Jacinto RC, Signoretti FG, Sanches PF, Gomes BP. Clustering behavior in microbial communities from acute endodontic infections. J Endod. 2012;38:158–62. doi: 10.1016/j.joen.2011.09.029. [DOI] [PubMed] [Google Scholar]
- [55].Siqueira JF, Jr, Rocas IN. Detection of Filifactor alocis in endodontic infections associated with different forms of periradicular diseases. Oral Microbiol Immunol. 2003;18:263–5. doi: 10.1034/j.1399-302x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- [56].Siqueira JF, Jr, Rocas IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:85–94. doi: 10.1016/s1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- [57].Siqueira JF, Jr, Rocas IN, Alves FR, Silva MG. Bacteria in the apical root canal of teeth with primary apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:721–6. doi: 10.1016/j.tripleo.2009.01.042. [DOI] [PubMed] [Google Scholar]
- [58].Colombo AP, Bennet S, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol. 2012;83:1279–87. doi: 10.1902/jop.2012.110566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–44. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- [60].Da Silva ES, Feres M, Figueiredo LC, Shibli JA, Ramiro FS, Faveri M. Microbiological diversity of peri-implantitis biofilm by Sanger sequencing. Clin Oral Implants Res. 2014;25:1192–9. doi: 10.1111/clr.12231. [DOI] [PubMed] [Google Scholar]
- [61].Citron DM. Update on the taxonomy and clinical aspects of the genus Fusobacterium. Clin Infect Dis. 2002;35:S22–7. doi: 10.1086/341916. [DOI] [PubMed] [Google Scholar]
- [62].Downes J, Munson MA, Spratt DA, Kononen E, Tarkka E, Jousimies-Somer H, et al. Characterisation of Eubacterium-like strains isolated from oral infections. J Med Microbiol. 2001;50:947–51. doi: 10.1099/0022-1317-50-11-947. [DOI] [PubMed] [Google Scholar]