Abstract
Cardiovascular disease (CVD) remains the largest cause of mortality in most developed countries. Although recent failed clinical trials and Mendelian randomization studies have called into question the high density lipoprotein (HDL) hypothesis, it remains well accepted that stimulating the process of reverse cholesterol transport (RCT) can prevent or even regress atherosclerosis. The prevailing model for RCT is that cholesterol from the artery wall must be delivered to the liver where it is secreted into bile before leaving the body through fecal excretion. However, many studies have demonstrated that RCT can proceed through a non-biliary pathway known as transintestinal cholesterol excretion (TICE). The goal of this review is to discuss the current state of knowledge of the TICE pathway, with emphasis on points of therapeutic intervention.
Keywords: cholesterol, lipoprotein, bile, reverse cholesterol transport, transintestinal cholesterol excretion
THE EVOLVING LANDSCAPE OF RCT
Atherosclerosis and associated cardiovascular disease (CVD) remains the largest cause of mortality in developed countries [1]. Despite widespread use of statin drugs to reduce levels of low density lipoprotein (LDL), CVD-associated mortality and morbidity has been reduced by only ~30% [1], demonstrating a clear need for better therapeutic strategies. Elevation of high density lipoprotein (HDL) function is thought to be an attractive therapeutic strategy [2]. However, recent clinical trials [3,4] and Mendelian randomization studies [5] have failed to show clinical benefits of HDL cholesterol elevation, calling into question the importance of HDL cholesterol as a surrogate marker of protection from atherosclerosis [2]. Both proponents and critics alike of the “HDL hypothesis” agree on one thing – further studies are needed to understand the mechanism regulating the fundamental process of HDL-driven reverse cholesterol transport (RCT). The prevailing model for RCT is that cholesterol from the artery wall is delivered to the liver via HDL, where it is then secreted into bile before leaving the body through fecal excretion [6–8]. However, we and others have recently demonstrated that RCT can also proceed through a non-biliary pathway known as transintestinal cholesterol excretion (TICE), which involves the direct secretion of plasma lipoprotein-derived cholesterol by the small intestine [9–18]. Although mechanisms regulating the classic biliary pathway of RCT have been well defined [19–23], almost no mechanistic information exists for the non-biliary TICE pathway [10]. Therefore, the purpose of this review is to discuss the most up to date understanding of the non-biliary TICE pathway, with particular emphasis on strategies to stimulate intestinal cholesterol excretion for the prevention or treatment of atherosclerotic CVD.
RCT is a multi-organ pathway that facilitates the removal of excess cholesterol from the body, and this homeostatic pathway is conserved across a wide range of organisms [6–8,24–26]. Importantly, elevated flux through the RCT pathway is thought to protect against CVD primarily by facilitating cholesterol removal from macrophage foam cells within the atherosclerotic plaque [6–8]. The vast majority of literature assumes that RCT involves the sequential movement of macrophage-derived cholesterol from peripheral tissues to the liver for excretion into bile and subsequent loss through the feces [6–8]. In this well accepted model, biliary secretion of cholesterol is requisite for the final step of RCT, namely fecal cholesterol and bile acid excretion [6–8]. Relevant to this review, emerging evidence supports an unexpected role for the small intestine in actively secreting plasma-derived cholesterol through a process that does not rely on hepatobiliary cholesterol secretion [9–18]. The identification of this non-biliary pathway largely stems from multiple observations where biliary cholesterol secretion does not predict the amount of cholesterol in the feces [10,27–32]. Given that several recent reviews have been written surrounding the mounting evidence for the non-biliary TICE pathway [10,27–32], we refer the reader to these excellent resources for a historical perspective. Briefly, previous studies in humans [18,33–38], dogs [39,40], rats [41–43], and mice [9–18] have unequivocally demonstrated the existence of a non-biliary pathway for RCT, especially under conditions where biliary cholesterol loss is surgically or genetically interrupted. However, there is still controversy in the field regarding the relative importance of the classic biliary and non-biliary pathways for RCT. Furthermore, there is incomplete understanding regarding the therapeutic utility of targeting one or the other pathway. Here we wish to highlight the consensus and controversies surrounding both biliary and non-biliary RCT pathways, and critically discuss the realistic potential for targeting the non-biliary TICE pathway for prevention or treatment of CVD.
Although most in the field agree that a non-biliary RCT pathway exists, there has been some disagreement and confusion as to the relative contributions of biliary and non-biliary pathways. When considering the relative contribution of biliary versus non-biliary routes to RCT flux, it is important to discuss this in the context of normal physiology versus pathophysiologic conditions where biliary cholesterol movement to the intestine is interrupted. First, under normal physiological conditions, the biliary route predominates while the non-biliary pathway typically makes up less than ~30% of the total cholesterol found in the feces [10,27–32]. For instance, in chow-fed mice the non-biliary TICE pathway accounts for approximately 20–33% of total fecal neutral sterol loss [11,14]. Although validation studies are needed, early intestinal perfusion studies estimated as much as 44% of fecal cholesterol loss originated from non-biliary origins in humans [34]. Although these estimations confirm the assumption that the biliary route is the predominant RCT pathway under normal physiological conditions, the non-biliary TICE pathway is highly dynamic and can be stimulated by both pathophysiologic and pharmacologic stimuli. For example, in genetically modified mouse models that lack the ability to normally secreted cholesterol into bile (ABCG5/G8−/−, Mdr2−/−, and NPC1L1-LiverTg), or under conditions where the common bile duct is surgically excluded from the small intestine (acute or chronic biliary diversion), fecal cholesterol loss remains either unchanged or in some cases increased [9,19,22,33,34,39,40,44,45]. These studies strongly suggest that under the pathophysiologic condition of biliary cholesterol insufficiency, the non-biliary TICE pathway can fully compensate to maintain normal levels of fecal cholesterol loss. Another important implication from these studies is that when biliary cholesterol movement to the intestine is blocked, there must be a signaling mechanism to instruct the liver to stimulate TICE to maintain cholesterol homeostasis. In addition to dynamic regulation under pathophysiologic conditions, the non-biliary TICE pathway can also be stimulated pharmacologically [10,27–32], which will be discussed under points of therapeutic intervention later in this review. Collectively, fecal cholesterol disposal (i.e. the end result of RCT) relies on a dynamic interplay between both biliary and non-biliary pathways, and the contribution of each can be quite different under physiological, pathophysiological, and pharmacological conditions. As cholesterol lowering drug discovery efforts move forward, it will be extremely important to consider the shared and distinct mechanisms regulating both biliary and non-biliary branches of the RCT pathway.
THE NEW INTEGRATED MODEL OF RCT
Although the non-biliary TICE pathway is highlighted here, it is not the only concept challenging the widely accepted hepatobiliary model for RCT [6–8]. In fact, several lessons learned during the past decade have suggested that substantial revisions are needed to the original model of RCT proposed by John Glomset and colleagues [46,47]. Unfortunately, many of these paradigm-shifting discoveries are largely ignored, perpetuating a theoretical RCT model that is more based on dogma than experimental evidence. For instance, it has long been assumed that HDL are rate limiting for RCT flux of cholesterol out of the body, because it is predicted that they deliver peripheral cholesterol to the liver for biliary secretion and ultimately fecal excretion [7,8]. However, recent discoveries highlight that this assumption is only partially correct. Without dispute, lipid poor apolipoprotein AI (apo-AI) or spherical HDL can initiate RCT by promoting the efflux of cholesterol from many cells including macrophage foam cells in the artery wall [8,48,49]. Likewise, there is strong evidence that HDL cholesterol can be efficiently delivered to the liver, primarily via the action of scavenger receptor class B type I (SR-BI) [50–52]. However, there is now evidence from several well-respected laboratories that HDL-mediated cholesterol efflux and HDL-driven centripetal movement of cholesterol back to the liver does not correlate with how much cholesterol is lost in bile or the feces [53–56]. In fact, mice genetically lacking apoA-I or ATP-binding cassette transporter AI (ABCA1) (i.e. mice that have extremely low circulating levels of HDL) have normal biliary and fecal cholesterol loss [53–56]. These data provocatively suggest that circulating HDL cholesterol levels have little to do with the amount of cholesterol actually leaving the body through the bile or feces, prompting revision of the classic RCT model. Although typically excluded in most RCT models, it is likely that apoB-containing lipoproteins play quite a substantial role in RCT [58–61]. It is important to note that the activity of cholesteryl ester transfer protein (CETP) plays a major role in RCT, given its ability to transfer HDL-derived cholesteryl esters into triglyceride-rich apolipoprotein B (apoB)-containing lipoproteins [58–61]. Therefore, the relative contributions of HDL and apoB-containing lipoproteins to biliary and fecal cholesterol loss may be markedly different in CETP-containing species like humans [58–61]. Collectively, progress made within the last decade clearly demonstrates that the dogmatic model of RCT requires revision.
With such progress in mind, here we propose a new integrated model for RCT considering the shared and distinct branch points for biliary and non-biliary RCT (Figure 1). Much like the original model proposed by Glomset and Norum [46,47], this updated model begins with the de novo synthesis of HDL. There is now overwhelming evidence that the process of RCT is initiated by the ABCA1-dependent lipidation of apoA-I to form nascent pre-beta HDL particles primarily in hepatocytes and enterocytes [62,63]. These liver and intestine-derived pre-beta HDL particles can then accept free cholesterol from peripheral tissues, and be further matured by the enzymatic actions of lecithin:cholesterol acyltransferase (LCAT), phospholipid transfer protein (PLTP), and other enzymes into spherical HDL particles [64–66]. This stepwise HDL maturation process collectively facilitates the centripetal flux of cholesterol to the liver, while avoiding apoA-I/HDL catabolism by the kidney [62–69]. Once matured with both free and esterified cholesterol cargo, HDL is cleared by the liver either via SR-BI-dependent selective uptake [51,52] or by holoparticle uptake mechanisms involving proteins such as F1F0-ATPase [70] and the nucleotide purinergic receptor P2Y G protein receptor 13 (P2Y13) [71–73]. Importantly, in CETP-containing species, a large portion of HDL’s cholesteryl ester cargo can be transferred to apoB-containing lipoproteins by CETP, which are taken up by the liver by hepatic low density lipoprotein receptors (LDLr) contributing to overall RCT flux [60,61]. All steps up to this point represent well understood pathways in RCT, and this shared centripetal flux collectively delivers cholesterol to the liver where this cargo can then branch into either biliary or less well understood non-biliary pathways. As classically understood, once delivered to the liver, a large portion of the RCT-derived cholesterol pool can be secreted into bile via the actions of proteins such as ATP-binding cassette transporters G5 and G8 [19–20] and ATP8B1 [23]. An additional key determinate of biliary cholesterol flux is hepatic SR-BI, given that SR-BI can facilitate directional (basolateral to apical) trafficking of cholesterol in polarized cells [74–77] and can facilitate biliary cholesterol secretion in vivo [78]. Once cholesterol is secreted into bile a large portion of this pool is physically delivered to the lumen of the small intestine via the common bile duct, where it can ultimately provide substrate for fecal cholesterol loss.
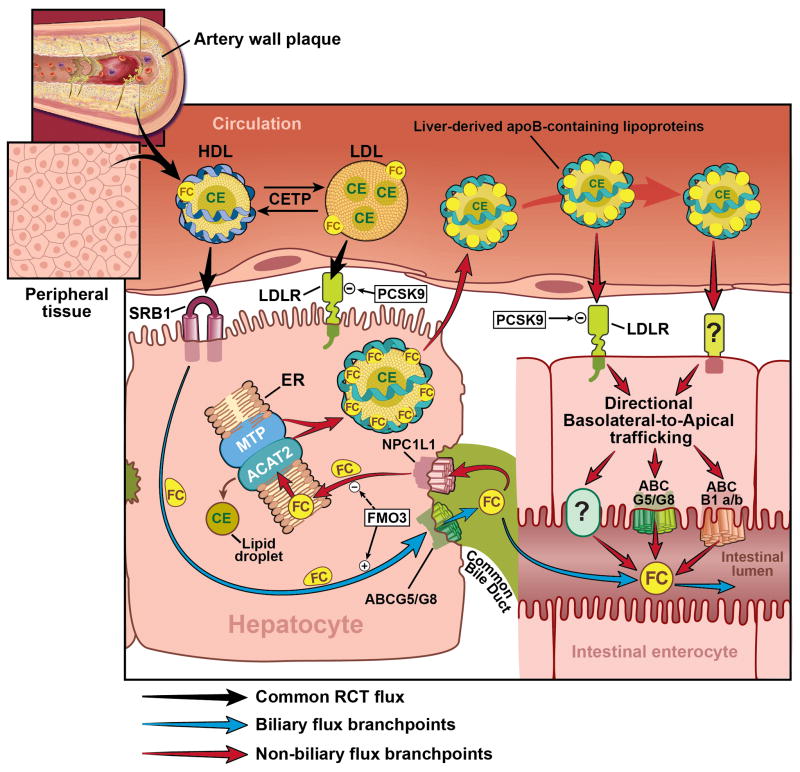
Figure 1. Model for Integrated Biliary and Non-Biliary Reverse Cholesterol Transport.
Nascent HDL particles circulate to remove cholesterol from peripheral tissues and macrophage foam cells in the artery wall plaque. Once matured with both free and esterified cholesterol cargo, HDL is cleared by the liver primarily via SR-BI-dependent selective uptake. Importantly, in CETP-containing species, a large portion of HDL’s cholesteryl ester cargo can be transferred to apoB-containing lipoproteins by CETP, which are taken up by the liver by hepatic low density lipoprotein receptors (LDLR) contributing to overall RCT flux. As shown by black arrows, all steps up to this point represent well understood pathways in RCT, and this shared centripetal flux collectively delivers cholesterol to the liver where this cargo then can branch into either biliary or less understood non-biliary pathways. As denoted with blue arrows, a large portion of the HDL-derived cholesterol pool can be secreted into bile via the actions of proteins such as ATP-binding cassette transporters G5 and G8 and other minor mechanisms. Once cholesterol is secreted into bile a large portion of this pool is physically delivered to the lumen of the small intestine via the common bile duct, where it can ultimately provide substrate for fecal cholesterol loss. Alternatively, highlighted by red arrows, the liver can initiate the non-biliary TICE pathway to eliminate excess cholesterol. Current evidence suggests that the non-biliary branch of RCT can be initiated by either re-uptake of biliary cholesterol via the cannalicular sterol transporter NPC1L1 among other mechanisms. Following NPC1L1-dependent recovery of biliary cholesterol, the excess free cholesterol is moved to the ER where it is repackaged onto nascent apoB-containing lipoproteins, which are ultimately secreted from the liver into the bloodstream. The liver-derived apoB-containing lipoproteins are then recognized by the proximal small intestine through lipoprotein receptors such as LDLr, and likely other mechanisms. Once cleared by the proximal small intestine, TICE-derived cholesterol is directionally trafficked across the enterocyte in a basolateral to apical fashion, and this cholesterol can be effluxed across the apical membrane via the actions of ATP binding cassette transporters ABCG5/ABCG8, ABCB1a/b, and likely other mechanisms. Collectively this TICE flux through the intestine, coupled with biliary cholesterol secretion, and dietary cholesterol make up the sum total of cholesterol available for excretion into the feces. New evidence suggests that the hepatic enzyme flavin containing monooxygenase 3 add another level of control, functioning to balance RCT flux by enhancing the biliary pathway and suppressing the TICE pathway. Abbreviations used: ABCB1a/b = ATP-binding cassette transporter 1a/b; ABCG5/G8 = ATP-binding cassette transporters G5 and G8; ACAT2 = acyl-CoA:cholesterol acyltransferase 2; apoB = apolipoprotein B; CE = cholesteryl ester; ER = endoplasmic reticulum; FC = free cholesterol; FMO3 = flavin containing monooxygenase 3; HDL = high density lipoprotein; LDL = low density lipoprotein; LDLR = low density lipoprotein receptor; MTP = microsomal triglyceride transfer protein; NPC1L1 = Niemann-Pick C1-like 1; PCSK9 = proprotein convertase subtilisin/kexin type 9; SR-BI = scavenger receptor class B class I; TICE = transintestinal cholesterol excretion; ? = unknown proteins.
Alternatively, the liver can initiate the non-biliary TICE pathway to eliminate excess cholesterol, especially under conditions where biliary cholesterol secretion is limited [9–18]. Current evidence suggests that the non-biliary branch of RCT can be initiated by either re-uptake of biliary cholesterol via the cannalicular sterol transporter Niemann-Pick C1-like 1 (NPC1L1) [9,22], or by blocking cholesterol acyl-CoA:cholesterol acyltransferase 2 (ACAT2)-driven cholesterol esterification [13,79]. Both of these conditions are expected to cause an accumulation of hepatic free cholesterol, yet under these conditions the excess free cholesterol is repackaged onto nascent apoB-containing lipoproteins, which are ultimately secreted from the liver into the bloodstream [9,13,22,79]. These liver-derived apoB-containing lipoproteins are then recognized by the proximal small intestine through lipoprotein receptors such as LDLr [18], and likely other mechanisms given that TICE can still occur in LDLr−/− mice [13]. Importantly, there is no evidence that the HDL receptor SR-BI is involved in non-biliary TICE [80]. Once cleared by the proximal small intestine, the trafficking itinerary of TICE-derived cholesterol within the intestinal enterocyte is not well understood. However, given that apoB-containing lipoproteins are the TICE donor particles, it is tempting to speculate that the trafficking itinerary would involve endosomal/lysomal compartments. Once the free cholesterol is liberated from TICE lipoproteins in the intestine, this cholesterol can be effluxed across the apical membrane via the actions of ATP binding cassette trasporters ABCG5/ABCG8 [17,45,81] and ABCB1a/b [18]. Collectively this TICE flux through the intestine, coupled with biliary cholesterol secretion, and dietary cholesterol make up the sum total of cholesterol available for excretion into the feces (Figure 2). As will be discussed later, once within the lumen of the intestine, RCT-derived cholesterol can be efficiently re-absorbed via the actions of intestinal NPC1L1 [82]. The process of intestinal NPC1L1-driven cholesterol absorption directly opposes biliary and non-biliary RCT flux [83] (Figure 2). Within this new integrated model of RCT (Figure 1), there are several points of therapeutic intervention that warrant discussion.
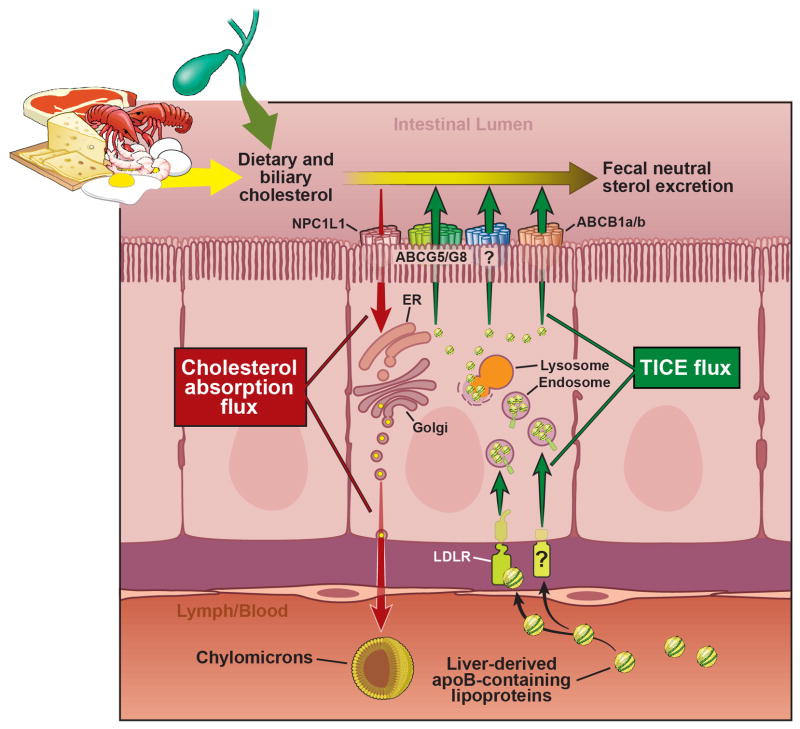
Figure 2. Model for Cholesterol Flux Across the Intestinal Enterocyte.
The intestinal enterocyte is a gatekeeper of cholesterol balance with two opposing pathways delivering cholesterol from opposite sides of this polarized cell. First, the cholesterol absorption pathway is initiated by NPC1L1-dependent delivery of dietary and biliary cholesterol to the ER, where it is packaged into nascent chylomicron particles for delivery into the lymphatics. Directly opposing the absorptive pathway, TICE flux is driven by the delivery of liver-derived apoB-containing lipoproteins that are taken up by the LDL receptor and likely other receptors. Once internalized these lipoproteins are trafficked through endosomal and lysosomal compartments, and ultimately effluxed from the apical membrane through ABCG5/ABCG8, ABCB1a/b, and likely other transporter. A portion of this newly effluxed cholesterol can be excreted into the feces. Collectively, these two opposing influx pathways (absorption and TICE) along with endogenous cholesterol synthesis comprise the total enterocyte cholesterol pool. Abbreviations used: ABCB1a/b = ATP-binding cassette transporter 1a/b; ABCG5/G8 = ATP-binding cassette transporters G5 and G8; apoB = apolipoprotein B; ER = endoplasmic reticulum; LDLR = low density lipoprotein receptor; NPC1L1 = Niemann-Pick C1-like 1; TICE = transintestinal cholesterol excretion; ? = unknown receptor mediating the uptake of TICE lipoproteins.
Points of therapeutic intervention
Common HDL-driven centripetal flux of cholesterol back to the liver
The common component of RCT encompasses the multistep process of HDL-driven centripetal flux of cholesterol from peripheral tissues back to the liver (Figure 1) [7,8]. This initial portion of centripetal flux is what most people define as RCT [7–9], but HDL-driven movement of cholesterol back to the liver is only one part of the RCT pathway. It is important to note that once HDL-derived cholesterol is delivered to the liver, a poorly understood sorting process occurs to dictate whether this new cholesterol pool is used locally or shunted into biliary or non-biliary RCT pathways for ultimate excretion into the feces to complete removal from the body (Figure 1). In the context of atherosclerosis, the common centripetal RCT pathway starts by removal of cholesterol from the macrophage foam cell in the artery wall, and ends by HDL-driven delivery of macrophage-derived cholesterol to the liver (Figure 1). Unequivocally, elevation of apoA-I or HDL can prevent or even regress established atherosclerosis in animal models and humans, and it is assumed that removal of excess cholesterol from lesional macrophages is a primary mechanism supporting this atheroprotection [84–95]. However, apoA-I and HDL have many biological properties including their ability to suppress inflammation [96], regulate hematopoetic stem cell proliferation [97], and serve as circulating carriers of diverse cargo such as bioactive lipids, proteins/enzymes, hormones, vitamins and small non-coding RNAs [98–100]. Any of these diverse functions of HDL could potentially regulate the pathogenesis of atherosclerosis. Although apoA-I and HDL can stimulate cholesterol efflux from macrophage foam cells in culture, recent evidence suggests that genetic modulation of the early steps in HDL biogenesis has very minor or non-existent effects on RCT flux into the bile or feces [53–57,100,101]. First, mice with extremely low levels of HDL due to defects in synthesis or maturation (ABCA1−/−, apoA-I−/−, LCAT−/−) have normal levels of RCT into bile and feces [53–57]. In a recent elegant study, John Parks and colleagues demonstrated that genetic deletion of ABCA1 in hepatocytes (the primary site of HDL biogenesis) had no effect on macrophage RCT into bile or feces, and also did not alter atherosclerosis progression in LDLr−/− mice [100]. Likewise, maturation of HDL driven by LCAT-mediated cholesterol esterification is not a major determinant of macrophage RCT into bile or feces in mice [101]. Collectively, these recent insights show that although centripetal flux of cholesterol to the liver is generally considered to determine the quantity of cholesterol removed from the body through bile and feces [7,9], it is the subsequent steps within the liver that dictate the amount of cholesterol ultimately secreted into biliary and non-biliary excretory routes (i.e. the end result of RCT). Thus, future drug discovery efforts may benefit from targeting hepatic pathways that traffic cholesterol into either the biliary or non-biliary branch points of RCT (Figure 1).
Increasing flux through the biliary pathway in the liver
In the classic view of RCT [7–9], it is assumed that centripetal movement of cholesterol to the liver will result in the secretion of this HDL-derived cargo into bile, which will ultimately lead to fecal excretion. However, the assumption that HDL-derived cholesterol moves exclusively into bile is not strongly supported by recent findings in mice with altered HDL biogenesis [53–57,100,101]. As mentioned previously, mice lacking the ability to synthesize apoA-I-containing HDL have normal biliary and fecal cholesterol levels [53–57,100,101]. Instead of being primarily governed by HDL-driven RCT flux, biliary cholesterol secretion is under dynamic regulation by both protein-mediated and biophysical mechanisms [19–23,102,103]. The first stage of control for biliary cholesterol secretion is at the level of the HDL receptor SR-BI. SR-BI has the unique ability to facilitate directional (basolateral to apical) trafficking of cholesterol in polarized cells [74–77], and SR-BI-dependent selective uptake promotes biliary cholesterol secretion in vivo [78]. This ability of SR-BI to directionally traffic cholesterol across the hepatocyte likely plays a major role in linking centripetal RCT flux to biliary cholesterol secretion. Once cholesterol arrives at the cannalicular membrane (the site of bile formation), the major mechanism of biliary secretion involves protein-mediated translocation of sterols across the cannalicular membrane by the heterodimeric half-transporters ABCG5 and ABCG8 [19,20]. However, a small amount of cholesterol can still be secreted into bile in ABCG5/ABCG8−/− mice through alternative mechanisms involving ATB8B1 and diffusion [21,23]. Importantly, protein-mediated and diffusion-driven movement of cholesterol into bile requires the presence of a biliary acceptor “micelle” which is assembled during the simultaneous transport of bile acids and phospholipids into bile by dedicated protein transporters such as bile salt export pump (BSEP) and multi-drug resistance protein 2 (Mdr2) [11,23,44,102]. Given the requirement of the “micellar” acceptor, the rate of biliary cholesterol secretion is highly dependent on the rate of bile acid and phospholipid secretion [11,23,44,102,103]. Once cholesterol is secreted into bile, another control mechanism exists in humans to prevent excessive cholesterol loss through the bile [22]. Much like its role in the small intestine, the sterol transporter NPC1L1 can transport newly secreted biliary cholesterol back into hepatocytes [22], and this re-uptake mechanism can instead initiate flux through the non-biliary TICE pathway (Figure 1). There have been a number of molecular targets identified controlling the flux of cholesterol into the bile, yet therapeutic interventions selectively targeting enhanced biliary secretion lack efficacy in atheroprotection and can cause unwanted side effects.
Like other concepts in the RCT field, the assumption that specifically increasing biliary cholesterol loss will protect against atherosclerosis is not strongly supported by experimental evidence. For instance, hepatocyte-specific overexpression of ABCG5 and ABCG8 results in ~2-fold increase in biliary cholesterol secretion, but this chronic increase in biliary cholesterol levels does not equate to protection against atherosclerosis in either apoE or LDLr knockout mice [104]. Subsequent studies have demonstrated that the elevated biliary cholesterol in these ABCG5/ABCG8 transgenic mice can be efficiently recovered by NPC1L1 in the small intestine, and this intestinal re-absorption pathway opposes the atheroprotection expected by removing cholesterol from the body [105]. These studies suggest that in order for drugs targeting enhanced biliary cholesterol secretion to efficaciously protect against atherosclerosis, they need to be given in combination with an intestinal cholesterol absorption inhibitor such as ezetimibe [104,105]. Another extremely important consideration when designing drugs to stimulate biliary cholesterol flux is potential deleterious side effects, given the critical role that biliary cholesterol secretion plays in gallstone formation [102,103]. It has long been known excessive biliary cholesterol secretion can result in supersaturation of bile, promoting cholesterol nucleation and gall stone formation [102,103]. Although most assume that RCT flux into bile is a fundamental pathway mediating atheroprotection [7–9], it is not guaranteed that targeted stimulation of biliary cholesterol loss will indeed result in a negative cholesterol balance due to intestinal absorption [104,105]. Further, there may be unwanted side effects of stimulating biliary cholesterol secretion such as cholesterol gallstones [102,103]. With these caveats in mind, all current data suggests that targeting the non-biliary branch of RCT holds untapped therapeutic potential with no predicted side effects. Although not discussed here due to space limitations, there is a large capacity of hepatic cholesterol to be converted into bile acids, which can ultimately contribute substantially to biliary RCT [6–8].
Increasing flux through the non-biliary TICE pathway
Although much is known regarding mechanisms regulating common centripetal RCT flux back to the liver and hepatobiliary secretion, we are still in our infancy in understanding the non-biliary TICE pathway. However, based on research performed within the last decade, there are several steps in the non-biliary TICE pathway that could be potentially therapeutically exploited.
Hepatic lipoprotein production
Through the function of ABCA1, the liver produces nascent HDL that upon maturation carries the majority of circulating HDL cholesterol [62]. Until recently it was widely believed that hepatic HDL production was an important contributor to RCT. However, Bi and colleagues found that Ldlr−/− mice with hepatocyte-specific deletion of ABCA1 had an ~50% reduction in HDL cholesterol but no change in either macrophage-to-feces RCT or aortic atherosclerosis development [100]. Moreover, it had been previously reported that mice with whole-body ABCA1 deficiency (Abca1−/−), which have virtually no circulating HDL cholesterol, have normal biliary and fecal cholesterol levels [55,56]. Most recently, Vrins and colleagues also reported that the rate of TICE in Abca1−/− mice was not significantly different than that of wild-type littermates [106]. Thus, current data suggests that therapeutic strategies that raise hepatic HDL production will not likely stimulate TICE.
Unlike HDL, liver-derived apoB-containing lipoproteins appear to promote non-biliary RCT (Figure 1). We have previously reported that mice with hepatic knockdown of acyl-CoA:cholesterol acyltransferase (ACAT2), also known as sterol o-acyltransferase (Soat2HKD) have normal biliary cholesterol secretion, but increased fecal neutral sterol excretion, suggesting increased TICE [13]. In this study, we found that the small intestine accumulated a greater amount of cholesterol originating from nascent VLDL from Soat2HKD compared to control mice [13]. In a follow up study, we have shown that acute Soat2HKD in cholesterol-fed mice promotes rapid increases in fecal neutral sterol loss without changes in biliary cholesterol levels, and this increased TICE flux was accompanied by acute elevation of circulating apoB-containing lipoproteins [79]. In addition, we recently tested the hypothesis that reduced hepatic VLDL secretion would limit TICE flux. To address this, NPC1L1LiverTg (a mouse model of chronically activated TICE) and littermate controls were treated with antisense oligonucleotides (ASOs) to knockdown the hepatic expression of microsomal triglyceride transfer protein (MTP), which is needed for proper lipidation and secretion of apoB-containing lipoproteins. Hepatic knockdown of MTP (MTPHKD) significantly reduced hepatic triglyceride secretion but did not impact biliary cholesterol concentration [107]. Fecal neutral sterol excretion was significantly reduced with MTPHKD in NPC1L1LiverTg but not littermate controls leading to the conclusion that hepatic VLDL secretion is necessary for TICE [107]. Our conclusion was supported by the findings of Dikkers and colleagues who showed that macrophage-to-feces RCT was decreased in mice with hepatic MTP deficiency [108]. Thus, stimulating the secretion of hepatic apoB-containing lipoproteins may promote TICE. However, therapeutic targeting of this step in the non-biliary RCT pathway would likely be problematic since it may elevate circulating LDL levels and consequently increase the risk of atherosclerotic CVD.
Intestinal lipoprotein uptake
The uptake of cholesterol from high density lipoproteins (HDL) by the liver is an important step in the hepatobiliary RCT pathway. Hepatic overexpression of SR-BI, which mediates the selective uptake of HDL cholesterol, increases macrophage-to-feces RCT in mice [109]. Since SR-BI is also expressed in enterocytes, we determined whether increasing intestinal SR-BI would promote TICE [80]. SR-BIhApoCIII-ApoAIV-Tg mice that overexpress SR-BI in the small intestine, and to a lesser extent in the liver, have reduced HDL cholesterol but no change in cholesterol absorption and fecal neutral sterol excretion [80]. SR-BIhApoCIII-ApoAIV-Tg mice were also treated with ezetimibe to block cholesterol absorption and potentially unmask an increase in TICE. However, SR-BIhApoCIII-ApoAIV-Tg mice and wild-type littermate controls had similar fecal neutral sterol excretion when treated with ezetimibe [80]. We also crossed NPC1L1LiverTg mice, which have increased TICE, with SR-BIhApoCIII-ApoAIV-Tg mice. The NPC1L1LiverTg and double transgenic mice had similar biliary cholesterol concentration, cholesterol absorption, and fecal neutral sterol excretion [80]. From these results, we concluded that intestinal SR-BI overexpression does not increase TICE. Collectively, we do not believe that TICE will be stimulated by therapies that increase SR-BI in the gut.
Since the current data indicates that liver-derived apoB-containing lipoproteins are responsible for supplying cholesterol for TICE, increasing intestinal LDL receptor (LDLr) expression should drive non-biliary RCT (Figure 1). In a recent study, Le May and colleagues showed that conditions stimulating LDLr expression, such as Pcsk9 deficiency and statin treatment, increased TICE in mice undergoing intestinal perfusion [18]. Unexpectedly, they also found that TICE was increased in Ldlr−/− mice [18]. We have also reported that Ldlr−/− with hepatic Soat2 knockdown have increased TICE flux [13]. Moreover, LXR activation has been shown to significantly increase TICE in mice [10,11,14], but at the same time nearly eliminates intestinal LDLr protein due to the action of the E3 ubiquitin ligase Idol [110]. These results indicate that stimulating LDLr expression should promote TICE, but also that other receptor systems feed cholesterol from apoB-containing lipoproteins into the non-biliary RCT pathway. Other members in the LDL receptor family such as LRP, VLDL receptor (VLDLR), and apoER2 are expressed in the small intestine [111,112], and could conceivably act as primary or secondary receptors for apoB-containing lipoproteins. It is also conceivable that like in the liver [113], heparin sulfate proteoglycans mediate the uptake of VLDL remnants and consequently support TICE. Currently, there are many unanswered questions concerning the intestinal receptors involved in TICE flux. Hence, defining the lipoprotein receptor systems that provide TICE-derived cholesterol to the small intestine has the potential to uncover new therapeutic targets.
Intestinal specific LXR activation
Systemic activation of liver X receptor (LXRs) with synthetic agonists has been reported to increase RCT by stimulating both the hepatobiliary and TICE pathways [9,11,14,113]. However, activation of LXRs in the liver increases de novo lipogenesis resulting in the unwanted side effect of hepatic steatosis [114]. Moreover, LXR agonists increase Idol in primate but not rodent liver consequently decreasing LDLr protein and elevating LDL concentration [115]. Because of these adverse effects, systematic LXR agonists have only been used to increase RCT in preclinical trials. Intestinal specific activation of LXRs may be one way to increase RCT and eliminate the side effects associated with systemic LXR agonists. Yasuda and colleagues reported that an intestinal specific LXR agonist was able to significantly increase macrophage-to-feces RCT [116]. In addition, Lo Sasso and colleagues [117] created a mouse model with constitutively active LXRα in enterocytes, and found that these animals had decreased cholesterol absorption and increased fecal excretion of macrophage-derived neutral sterol. In both studies, the heterodimeric sterol transporters ABCG5 and ABCG8 were increased exclusively in intestine therefore leading to the conclusion that the elevation in macrophage RCT was the result of reduced cholesterol absorption [116,117]. However, it is also possible that increased ABCG5 and ABCG8 in enterocytes promote TICE. The recent study by Wang and colleagues highlights the possible role that ABCG5 and ABCG8 play in non-biliary RCT [45]. In this study, mice with liver (L-G5G8−/−), intestinal (I-G5G8−/−) or whole body (G5G8−/−) knockout of ABCG5 and ABCG8 were created. In order to gauge the importance of hepatic and intestinal ABCG5/ABCG8 in RCT, the mice were injected with 3H-cholesterol, and the levels of 3H-cholesterol in bile and feces were measured. The percentage of 3H-cholesterol in the bile of the mice was qualitatively similar to that observed for biliary cholesterol mass (WT = I-G5G8−/− > L-G5G8−/− > G5G8−/−). Reduced biliary cholesterol secretion undoubtedly contributed to the decrease in fecal 3H-cholesterol excretion for the L-G5G8−/− and G5G8−/− versus WT mice. I-G5G8−/− compared to WT mice also had decreased fecal 3H-cholesterol excretion, which could have resulted from increased cholesterol absorption. However, fractional cholesterol absorption was similar for I-G5G8−/− and WT mice. Therefore, the reduction in fecal 3H-cholesterol excretion for the I-G5G8−/− mice could have been due to decreased TICE. Collectively, these studies suggest that intestinal specific LXR agonists could promote RCT by decreasing cholesterol absorption and increasing TICE.
Inhibition of cholesterol absorption with ezetimibe
Ezetimibe is a clinically-approved drug used to treat hypercholesterolemia. It is well established that ezetimibe lowers LDL cholesterol by inhibiting NPC1L1 in the small intestine consequently reducing cholesterol absorption [82]. In species such as humans that express NPC1L1 in liver, ezetimibe may also decrease LDL cholesterol by blocking the recycling of biliary cholesterol by hepatocytes [22]. In theory, cholesterol coming from the hepatobiliary or transintestinal pathway could be absorbed by the small intestine prior to being excreted into the feces. Therefore, by blocking cholesterol absorption, ezetimibe should stimulate both biliary and non-biliary RCT. Several groups have shown in rodent models in which the biliary pathway should predominate that ezetimibe treatment significantly increases macrophage-to-feces RCT [83,118–120]. Moreover, ezetimibe appears to increase RCT in humans since ezetimibe stimulated the excretion of stable isotope labeled cholesterol that had been IV infused into hyperlipidemic patients [121]. There are two recent studies that have addressed whether ezetimibe increases RCT through the TICE pathway. To eliminate any contribution to RCT from the hepatobiliary pathway, Uto-Kondo et al performed bile duct ligations on ezetimibe-treated hamster and then measured macrophage-to-feces RCT [120]. Ezetimibe significantly increased fecal excretion of macrophage-derived 3H-neutral sterol in bile duct ligated hamsters. However, macrophage-to-feces RCT was stimulated to a much greater extent in sham operated compared to bile duct ligated animals. Therefore, it can be concluded that that ezetimibe can stimulate TICE but requires the hepatobiliary pathway to maximally stimulate RCT.
We previously reported that despite very low biliary cholesterol secretion, NPC1L1LiverTg mice have normal macrophage-to-feces RCT and therefore concluded that NPC1L1LiverTg mice have increased TICE [9]. We also found that ezetimibe treatment could normalize biliary cholesterol concentration in NPC1L1LiverTg mice [22]. To determine whether inhibiting hepatic NPC1L1 with ezetimibe would facilitate macrophage RCT, Xie and colleagues created NPC1L1LiverTg mice lacking intestinal Npc1l1 (NPC1L1LiverOnly) and fed the mice a low cholesterol diet, which caused the vast majority of excreted cholesterol to come from the either the hepatobiliary or TICE pathway [122]. Compared to Npc1l1 whole body knockouts (Npc1l1−/−), the NPC1L1LiverOnly mice had extremely reduced biliary cholesterol as expected [122]. Unlike what we reported for NPC1L1LiverTg mice with intact intestinal NPC1L1 function, mass and macrophage-derived fecal neutral sterol excretion was significantly reduced in the NPC1L1LiverOnly compared to Npc1l1−/− mice [122]. Treatment of the NPC1L1LiverOnly mice with ezetimibe normalized biliary cholesterol levels and macrophage-to-feces RCT [122]. From these results, the authors concluded that biliary cholesterol secretion is needed for maximal macrophage-to-feces RCT, and that ezetimibe can promote RCT by inhibiting hepatic NPC1L1. We do not dispute the authors’ conclusions, but do believe their data does not exclude the possibility that TICE is stimulated in NPC1L1LiverTg mice. Because of the lack of intestinal Npc1l1, all of the mice used in their study had significantly reduced cholesterol absorption and consequently much higher than normal fecal neutral sterol excretion. The NPC1L1LiverOnly mice displayed much greater mass and macrophage-derived fecal neutral sterol excretion compared to what we previously reported for NPC1L1LiverTg mice [122]. Therefore, in the face of a >80% decrease in biliary cholesterol concentration, it is likely that TICE contributed to the residual macrophage RCT observed in the NPC1L1LiverOnly mice. Given that NPC1L1LiverOnly have to rely solely on endogenous cholesterol synthesis for their total body cholesterol pool (i.e. cannot absorb dietary or bilary cholesterol), this model represents a pathophysiologic state where multiple pathways of cholesterol homeostasis are altered. Overall, it can be concluded that ezetimibe can promote RCT by blocking cholesterol absorption and inhibiting the reclamation of cholesterol from the bile by liver. In addition, ezetimibe can stimulate TICE but likely requires the hepatobiliary pathway to maximally stimulate RCT.
THE UNEXPECTED ROLE OF HEPATIC FLAVIN MONOOXYGENASE 3 IN BALANCING BILIARY AND NON-BILARY RCT
Several lines of evidence suggest there is some mechanism of cross-talk between biliary and non-biliary pathways that synergize to maintain the set point of cholesterol excretion. For example, animals with genetic or surgical diversion of biliary cholesterol have normal or increased fecal cholesterol loss [9,11,33–44], indicating the liver was able to sense a defect in the biliary pathway and in a compensatory fashion upregulate TICE to maintain cholesterol balance. Recently, we set out to identify novel factors that regulate TICE and serendipitously uncovered a new pathway balancing both biliary and non-biliary RCT flux [123]. In an attempt to identify novel regulators of TICE we performed transcriptional profiling in NPC1L1-LiverTg mice (which exhibit chronic TICE stimulation [9]) and mice with hepatic knockdown of sterol o-acyltransferase (Soat2HKD, which exhibit acute TICE stimulation [79]). From this screening, we found that the hepatic expression of flavin-containing monooxygenase 3 (FMO3) was coordinately downregulated in mouse models of stimulated TICE [123]. Several independent studies show that plasma levels of FMO3’s enzymatic product trimethylamine-N-oxide (TMAO) are highly predictive of atherosclerosis in humans, and TMAO is proatherogenic in mice [124–130]. Given that we identified alterations in the TMAO-producing enzyme FMO3 in TICE mouse models, and independent groups identified TMAO as a proatherogenic metabolite in humans, we initially took a loss-of-function approach to study the role of FMO3 in cholesterol metabolism and RCT [123]. In a TICE-like manner, ASO-mediated knockdown reduced biliary cholesterol secretion, while stimulating fecal neutral sterol loss [123]. Likewise, FMO3 knockdown promoted both basal and LXR-stimulated macrophage RCT, while reducing biliary cholesterol secretion [123]. Intriguingly, FMO3 knockdown closely phenocopies our preferred chronic TICE mouse model (NPC1L1-LiverTg) [123], which displays severely reduced biliary cholesterol levels with normal fecal cholesterol loss [9,22]. It is also important to note that LXR activation alone significantly reduces hepatic FMO3 mRNA [123]. Collectively, our recent findings demonstrate that FMO3 gene expression is coordinately repressed in several mouse models of augmented TICE and that FMO3 knockdown promotes basal and LXR agonist-stimulated nonbiliary RCT [123]. However, it is important to point out that FMO3 knockdown alters multiple steps of cholesterol balance, making it difficult to know whether FMO3 is primarily affecting TICE or another component of cholesterol metabolism. We also saw deleterious effects of FMO3 including hepatic inflammation, further dampening enthusiasm for this potential RCT modulating target [123]. Overall, these recent observations identify the gut microbiota-driven FMO3/TMAO pathway as a key integrator of lipid metabolism, and specifically identify FMO3 as a novel regulator of sterol balance and RCT. Although these studies have identified FMO3 as a critical regulator of both biliary and non-biliary RCT pathways, targeting FMO3 is not likely a safe therapeutic strategy and would not specifically stimulate non-biliary RCT [123,124].
Concluding remarks
The process of RCT has long been thought to play an atheroprotective role, yet recent failed clinical trials and Mendelian randomization studies have caused many to question the therapeutic benefit of HDL elevation. In the face of this growing doubt, there is little question that improving HDL function, facilitating removal of cholesterol from macrophage foam cells in the artery wall, and the excretion of cholesterol out of the body (all different steps in a common pathway) would provide therapeutic benefit to those suffering from CVD. Research focused on the RCT pathway during the last decade has been full of unexpected surprises, prompting substantial revision of the theoretical model of RCT flux. The new model includes classic centripetal flux back to the liver, but also includes additional steps where the liver determines whether to secrete cholesterol into bile or shunt a portion into the non-biliary TICE pathway. Undoubtedly, there are a number of outstanding questions that need to be addressed (Box 1). Although nearly all current RCT elevating strategies being tested in clinical trials are focused on HDL cholesterol elevation, there is no guarantee that raising HDL cholesterol will have a therapeutic benefit, given recent lessons learned. Moving forward it will be important to pay attention to the evolving RCT model, and design new therapies targeting specific branches of the RCT pathway that hold the most therapeutic promise for the patient. In particular, therapeutic strategies that specifically enhance the non-biliary TICE pathway hold immense untapped potential with no predicted side effects.
OUTSTANDING QUESTIONS BOX.
In order to more fully understand therapeutic opportunities targeting the non-biliary TICE pathway (Figure 1), the following outstanding questions will need to be addressed:
Can we establish standardized quantitative methods to measure biliary and non-biliary fecal sterol loss in humans?
Are the apoB-containing lipoproteins that initiate TICE bona fide VLDL or a novel lipoprotein replete with its own proteome and cargo that allow for unique intravascular metabolism and recognition at the proximal small intestine?
What are the intestinal lipoprotein receptors/transporters that clear the hepatic apoB-containing lipoproteins that drive TICE?
What is the trafficking itinerary of TICE lipoproteins once they are taken up by intestinal enterocytes?
Can we identify drug targets to specifically modulate the intestinal component of RCT, while avoiding excessive biliary cholesterol secretion?
HIGHLIGHTS.
Reverse cholesterol transport (RCT) involves both biliary and non-biliary pathways.
The non-biliary pathway normally constitutes ~30% of RCT, but it can be stimulated.
Flux through biliary and non-biliary RCT pathways is coordinated by the liver.
Targeting the intestine as a cholesterol excretory organ holds therapeutic promise.
Acknowledgments
This work was supported by grants provided by the National Institutes of Health: R00 HL096166 (J.M.B.), R01 HL122283 (J.M.B.), and R01 HL111932 (R.E.T).
Footnotes
Illustrations were created by David Schumick, B.S, C.M.I, and reprints are available with the permission of the Cleveland Clinic Center for Medical Art & Photography © 2015.
The authors report that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, et al. Executive summary: heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Rader DJ, Tall AR. The not-so-simply HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–1346. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 3.Boden WE, et al. AIM-HIGH investigators. (2011) Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, et al. Effect of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 5.Voight BF, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 7.Rader DJ, et al. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenson RS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temel RE, et al. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 2010;12:96–102. doi: 10.1016/j.cmet.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Temel RE, Brown JM. Biliary and nonbiliary contributions to reverse cholesterol transport. Curr Opin Lipidol. 2012;23:85–90. doi: 10.1097/MOL.0b013e3283508c21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruit JK, et al. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–156. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Van der Velde AE, et al. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Brown JM, et al. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem. 2008;283:10522–10534. doi: 10.1074/jbc.M707659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Veen JN, et al. Activation of liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrins CL, et al. PPARδ activation leads to increased trans intestinal cholesterol efflux. J Lipid Res. 2009;50:2046–2054. doi: 10.1194/jlr.M800579-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Velde AE, et al. Regulation of direct transintestinal cholesterol excretion in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G203–G208. doi: 10.1152/ajpgi.90231.2008. [DOI] [PubMed] [Google Scholar]
- 17.Jakulj L, et al. Ezetimibe stimulates faecal neutral sterol excretion depending on abcg8 function in mice. FEBS Lett. 2010;584:3625–3628. doi: 10.1016/j.febslet.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Le May C, et al. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler Thromb Vasc Biol. 2013;33:1484–1493. doi: 10.1161/ATVBAHA.112.300263. [DOI] [PubMed] [Google Scholar]
- 19.Yu L, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graf GA, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 21.Wiersma H, et al. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology. 2009;50:1263–1272. doi: 10.1002/hep.23112. [DOI] [PubMed] [Google Scholar]
- 22.Temel RE, et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentrations and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groen A, et al. Abcg5/8 independent biliary cholesterol excretion in Atp8b1-deficient mice. Gastroenterology. 2008;134:2091–2100. doi: 10.1053/j.gastro.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 24.Sieber MH, Thummel CS. Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab. 2012;15:122–127. doi: 10.1016/j.cmet.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babin PJ, et al. Both apolipoprotein E and A-I genes are present in a nonmammalian vertebrate and are highly expressed during embryonic development. Proc Natl Acad Sci USA. 1997;94:8622–8627. doi: 10.1073/pnas.94.16.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, et al. Efficient delivery of miR-122 to regulate cholesterol metabolism using a non-covalent peptide-based strategy. Mol Med Rep. 2013;8:1472–1478. doi: 10.3892/mmr.2013.1691. [DOI] [PubMed] [Google Scholar]
- 27.Temel RE, Brown JM. A new framework for reverse cholesterol transport: non-biliary contributions to reverse cholesterol transport. World J Gastroenterol. 2010;16:5946–5952. doi: 10.3748/wjg.v16.i47.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tietge UJ, Groen AK. Role of TICE?: advancing the concept of transintestinal cholesterol excretion. Arterioscler Thromb Vasc Biol. 2013;33:1452–1453. doi: 10.1161/ATVBAHA.113.301562. [DOI] [PubMed] [Google Scholar]
- 29.Van der Velde AE, et al. Transintestinal cholesterol efflux. Curr Opin Lipidol. 2010;21:167–171. doi: 10.1097/MOL.0b013e3283395e45. [DOI] [PubMed] [Google Scholar]
- 30.Kruit JK, et al. Emerging roles of the intestine in control of cholesterol metabolism. World J Gastroenterol. 2006;12:6429–6439. doi: 10.3748/wjg.v12.i40.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brufau G, et al. Reverse cholesterol transport revisited: contribution of biliary versus intestinal cholesterol excretion. Arterioscler Thromb Vasc Biol. 2011;31:1726–1733. doi: 10.1161/ATVBAHA.108.181206. [DOI] [PubMed] [Google Scholar]
- 32.Jakulj L, et al. Intestinal cholesterol secretion: future clinical implications. Neth J Med. 2013;71:459–465. [PubMed] [Google Scholar]
- 33.Cheng SH, Stanley MM. Secretion of cholesterol by intestinal mucosa in patients with complete common bile duct obstruction. Proc Soc Exp Biol Med. 1959;101:223–225. doi: 10.3181/00379727-101-24890. [DOI] [PubMed] [Google Scholar]
- 34.Stanley M, et al. Serum cholesterol esters and intestinal cholesterol secretion and absorption in obstructive jaundice due to cancer. N Engl J Med. 1959;261:368–373. doi: 10.1056/NEJM195908202610802. [DOI] [PubMed] [Google Scholar]
- 35.Simmonds WJ, et al. Absorption of cholesterol from a micellular solution: intestinal perfusion studies in man. J Clin Invest. 1967;46:874–890. doi: 10.1172/JCI105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deckelbaum RJ, et al. Failure of complete bile diversion and oral bile acid therapy in the treatment of homozygous familial hypercholesterolemia. N Engl J Med. 1977;296:465–470. doi: 10.1056/NEJM197703032960901. [DOI] [PubMed] [Google Scholar]
- 37.Hellman L, et al. Isotopic studies of plasma cholesterol of endogenous and exogenous origins. J Clin Invest. 1955;34:48–60. doi: 10.1172/JCI103062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenfeld R, Hellman L. The relation of plasma and biliary cholesterol to bile acid synthesis in man. J Clin Invest. 1959;38:1334–1338. doi: 10.1172/JCI103908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperry WM. Lipid Excretion IV. A study of the relationship of the bile to the fecal lipids with special reference to certain problems of sterol metabolism. J Biol Chem. 1927;71:351–378. [Google Scholar]
- 40.Pertsemlidis D, et al. Regulation of cholesterol metabolism in the dog I. Effects of complete bile diversion and of cholesterol feeding on absorption, synthesis, accumulation, and excretion rates measured during life. J Clin Invest. 1973;52:2353–2367. doi: 10.1172/JCI107424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietschy JM. The role of bile salts in controlling the rate of intestinal cholesterogenesis. J Clin Invest. 1968;47:286–300. doi: 10.1172/JCI105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietschy JM, Siperstein MD. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965;44:1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bandsma RH, et al. Contribution of newly synthesized cholesterol to rat plasma and bile determined by mass isotopomer distribution analysis: bile-salt flux promotes secretion of newly synthesized cholesterol into bile. Biochem J. 1998;329:699–703. doi: 10.1042/bj3290699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voshol PJ, et al. Reduced plasma cholesterol and increased fecal sterol loss in multidrug resistance gene 2 P-glycoprotien-deficient mice. Gastroenterology. 1998;114:1024–1034. doi: 10.1016/s0016-5085(98)70323-3. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, et al. Relative roles of ABCG5/ABCG8 in liver and intestine. J Lipid Res. 2015;56:319–330. doi: 10.1194/jlr.M054544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glomset JA. The plasma lecithin:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 47.Glomset JA, Norum KR. The metabolic role of lecithin: cholesterol acyltransferase: perspectives from pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- 48.Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothblatt GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietschy JM, et al. Role of the liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 51.Acton S, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 52.Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 53.Osono Y, et al. Centripetal cholesterol flux from extrahepatic organs to the liver is independent of the concentration of high density lipoprotein-cholesterol in plasma. Proc Natl Acad Sci USA. 1996;93:4114–4119. doi: 10.1073/pnas.93.9.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jolley CD, et al. Centripetal cholesterol flux to the liver is dictated by events in the peripheral organs and not by the plasma high density lipoprotein or apolipoprotein A-I concentration. J Lipid Res. 1998;39:2142–2149. [PubMed] [Google Scholar]
- 55.Groen AK, et al. Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J Clin Invest. 2001;108:843–850. doi: 10.1172/JCI12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie C, et al. ABCA1 plays no role in centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse. J Lipid Res. 2009;50:1316–1329. doi: 10.1194/jlr.M900024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plosch T, et al. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J Biol Chem. 2002;277:33870–33877. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- 58.Hellerstein M, Turner S. Reverse cholesterol transport fluxes. Curr Opin Lipidol. 2014;25:40–47. doi: 10.1097/MOL.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 59.Briand F. The use of dyslipidemic hamsters to evaluate drug-induced alterations in reverse cholesterol transport. Curr Opin Investig Drugs. 2010;11:289–297. [PubMed] [Google Scholar]
- 60.Castro-Perez J, et al. Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in Syrian golden hamsters. J Lipid Res. 2011;52:1965–1973. doi: 10.1194/jlr.M016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barter PJ, et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160–167. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 62.Timmins JM, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunham L, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kunnen S, Van Eck M. Lecithin:cholesterol acyltransferase: old friend or foe in atherosclerosis. J Lipid Res. 2012;53:1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yazdanyar A, et al. Role of phospholipid transfer protein in high-density lipoprotein-mediated reverse cholesterol transport. Curr Atheroscler Rep. 2011;13:242–248. doi: 10.1007/s11883-011-0172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zannis VI, et al. HDL biogenesis, remodeling, and catabolism. Handb Exp Pharmacol. 2015;224:53–111. doi: 10.1007/978-3-319-09665-0_2. [DOI] [PubMed] [Google Scholar]
- 67.Mulya A, et al. Initial interaction of apoA-I with ABCA1 impacts in vivo metabolic fate of nascent HDL. J Lipid Res. 2008;49:2390–2401. doi: 10.1194/jlr.M800241-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ji A, et al. Impact of phospholipid transfer protein on nascent high-density lipoprotein formation and remodeling. Arterioscler Thromb Vasc Biol. 2014;34:1910–1916. doi: 10.1161/ATVBAHA.114.303533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JY, et al. Prebeta high density lipoprotein has two metabolic fates in human apolipoprotein A-I transgenic mice. J Lipid Res. 2004;45:716–728. doi: 10.1194/jlr.M300422-JLR200. [DOI] [PubMed] [Google Scholar]
- 70.Martinez LO, et al. Ectopic β-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- 71.Fabre AC, et al. P2Y13 receptor is critical for reverse cholesterol transport. Hepatology. 2010;52:1477–1483. doi: 10.1002/hep.23897. [DOI] [PubMed] [Google Scholar]
- 72.Serhan N, et al. Chronic pharmacologic activation of P2Y13 receptor in mice decreases HDL-cholesterol by increasing hepatic HDL uptake and bile acid secretion. Biochim Biophys Acta. 2013;1831:719–725. doi: 10.1016/j.bbalip.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Jacquet S, et al. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell Mol Life Sci. 2005;62:2508–2515. doi: 10.1007/s00018-005-5194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silver DL, et al. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type I results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J Biol Chem. 2001;276:25287–25293. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]
- 75.Burgos PV, et al. Cholesterol depletion induces PKA-mediated basolateral-to-apical transcytosis of the scavenger receptor class B type I in MDCK cells. Proc Natl Acad Sci USA. 2004;101:3845–3850. doi: 10.1073/pnas.0400295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harder CJ, et al. SR-BI undergoes cholesterol-stimulated transcytosis to the bile canaliculus in polarized WIF-B cells. J Biol Chem. 2007;282:1445–1455. doi: 10.1074/jbc.M604627200. [DOI] [PubMed] [Google Scholar]
- 77.Wustner D, et al. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J Lipid Res. 2004;45:427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- 78.Dikkers A, et al. Scavenger receptor BI and ABCG5/G8 differentially impact biliary sterol secretion and reverse cholesterol transport in mice. Hepatology. 2013;58:293–303. doi: 10.1002/hep.26316. [DOI] [PubMed] [Google Scholar]
- 79.Marshall S, et al. Acute sterol o-acyltransferase 2 (SOAT2) knockdown rapidly mobilizes hepatic cholesterol for fecal excretion. PLoS One. 2014;9:e98953. doi: 10.1371/journal.pone.0098953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bura K, et al. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J Lipid Res. 2013;54:1567–1577. doi: 10.1194/jlr.M034454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brufau G, et al. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS One. 2011;6:e21576. doi: 10.1371/journal.pone.0021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Altmann SW, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 83.Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1296–1297. doi: 10.1161/ATVBAHA.108.165803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plump AS, et al. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paszty C, et al. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choudhury RP, et al. High-density lipoproteins retard the progression of atherosclerosis and favorably remodel lesions without suppressing indices of inflammation or oxidation. Arterioscler Thromb Vasc Biol. 2004;24:1904–1909. doi: 10.1161/01.ATV.0000142808.34602.25. [DOI] [PubMed] [Google Scholar]
- 87.Benoit P, et al. Somatic gene transfer of human ApoA-I inhibits atherosclerosis progression in mouse models. Circulation. 1999;99:105–110. doi: 10.1161/01.cir.99.1.105. [DOI] [PubMed] [Google Scholar]
- 88.Belalcazar, et al. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- 89.Van Craeyveld E, et al. Regression and stabilization of advanced murine atherosclerosis lesions: a comparison of LDL lowering and HDL raising gene transfer strategies. J Mol Med. 2011;89:555–567. doi: 10.1007/s00109-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyazaki A, Sakuma S, Morikawa W, Takiue T, Miake F, Terano T, Sakai M, Hakamata H, Sakamoto Y, Natio M. Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1882–1888. doi: 10.1161/01.atv.15.11.1882. [DOI] [PubMed] [Google Scholar]
- 91.Tangirala RK, et al. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 92.Shah PK, et al. High-dose recombinant apolipoprotein A-I (Milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 93.Badimon JJ, et al. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rong JX, et al. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 95.Feig JE, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci USA. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navab M, et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 97.Tall AR, et al. Cholesterol efflux: a novel regulator of myelopoiesis and atherogenesis. Arterioscler Thromb Vasc Biol. 2012;32:2547–2552. doi: 10.1161/ATVBAHA.112.300134. [DOI] [PubMed] [Google Scholar]
- 98.Shah AS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013;54:2575–2585. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vickers KC, Remaley AT. HDL and cholesterol: life after the divorce? J Lipid Res. 2014;55:4–12. doi: 10.1194/jlr.R035964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bi X, et al. Liver ABCA1 deletion in LDLrKO mice does not impair macrophage reverse cholesterol transport or exacerbate atherogenesis. Arterioscler Thromb Vasc Biol. 2014;33:2288–2296. doi: 10.1161/ATVBAHA.112.301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tanigawa H, et al. Lecithin:cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation. 2009;120:160–169. doi: 10.1161/CIRCULATIONAHA.108.825109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Small DM. Role of ABC transporters in secretion of cholesterol from liver into bile. Proc Natl Acad Sci USA. 2003;100:4–6. doi: 10.1073/pnas.0237205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carey MC, Small DM. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest. 1978;61:998–1026. doi: 10.1172/JCI109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu JE, et al. Hepatic ABCG5 and ABCG8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J Biol Chem. 2004;279:22913–22925. doi: 10.1074/jbc.M402838200. [DOI] [PubMed] [Google Scholar]
- 105.Basso F, et al. Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J Lipid Res. 2007;48:114–126. doi: 10.1194/jlr.M600353-JLR200. [DOI] [PubMed] [Google Scholar]
- 106.Vrins CL, et al. Trans-intestinal cholesterol efflux is not mediated through high density lipoprotein. J Lipid Res. 2012;53:2017–2023. doi: 10.1194/jlr.M022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marshall S, et al. Reduction of VLDL secretion decreases cholesterol excretion in niemann -pick C1-like 1 hepatic transgenic mice. PLoS One. 2014;9:e84418. doi: 10.1371/journal.pone.0084418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dikkers A, et al. Differential impact of hepatic deficiency and total body inhibition of MTP on cholesterol metabolism and RCT in mice. J Lipid Res. 2014;55:816–825. doi: 10.1194/jlr.M042986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, et al. Hepatic overexpression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zelcer N, et al. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Herz J, et al. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garcia-Miranda P, et al. Rat small intestine expresses the reelin-Disabled-1 signaling pathway. Exp Physiol. 2010;95:498–507. doi: 10.1113/expphysiol.2009.050682. [DOI] [PubMed] [Google Scholar]
- 113.Nijstad N, et al. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology. 2011;140:1043–1051. doi: 10.1053/j.gastro.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 114.Schultz JR, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2832–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hong C, et al. The LXR-Idol axis differentially regulates plasma LDL levels in primates and mice. Cell Metab. 2014;20:910–908. doi: 10.1016/j.cmet.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yasuda T, et al. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2010;30:781–786. doi: 10.1161/ATVBAHA.109.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lo Sasso G, et al. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 118.Briand F, et al. Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal cholesterol reabsorption of HDL-derived cholesterol. Clin Transl Sci. 2009;2:127–133. doi: 10.1111/j.1752-8062.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maugeais C, et al. rHDL administration increases reverse cholesterol transport in mice, but is not additive on top of ezetimibe or cholestyramine treatment. Atherosclerosis. 2013;229:94–101. doi: 10.1016/j.atherosclerosis.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 120.Uto-Kondo H, et al. Ezetimibe enhance macrophage reverse cholesterol transport in hamsters: contribution of hepatobiliary pathway. Biochim Biophys Acta. 2014;1841:1247–1255. doi: 10.1016/j.bbalip.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 121.Davidson MH, et al. Inhibition of intestinal cholesterol absorption with ezetimibe increases components of reverse cholesterol transport in humans. Atherosclerosis. 2013;230:322–329. doi: 10.1016/j.atherosclerosis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 122.Xie P, et al. Ezetimibe inhibits hepatic Niemann-Pick C1-Like 1 to facilitate macrophage reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2013;33:920–925. doi: 10.1161/ATVBAHA.112.301187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Warrier M, et al. The TMAO-generating enzyme flavin monooxgenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015 doi: 10.1016/j.celrep.2014.12.036. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brown JM, Hazen SL. Metaorganismal nutrient metabolism as a basis of cardiovascular disease. Curr Opin Lipidol. 2014;25:48–53. doi: 10.1097/MOL.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koeth RA, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang WH, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bennett BJ, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koeth RA, et al. g-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shih DM, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]