Abstract
Few studies have clearly linked long-term monitoring with in situ experiments to clarify potential drivers of observed change at a given site. This is especially necessary when findings from a site are applied to a much broader geographic area. Here, we document vegetation change at Barrow and Atqasuk, Alaska, occurring naturally and due to experimental warming over nearly two decades. An examination of plant cover, canopy height, and community indices showed more significant differences between years than due to experimental warming. However, changes with warming were more consistent than changes between years and were cumulative in many cases. Most cases of directional change observed in the control plots over time corresponded with a directional change in response to experimental warming. These included increases in canopy height and decreases in lichen cover. Experimental warming resulted in additional increases in evergreen shrub cover and decreases in diversity and bryophyte cover. This study suggests that the directional changes occurring at the sites are primarily due to warming and indicates that further changes are likely in the next two decades if the regional warming trend continues. These findings provide an example of the utility of coupling in situ experiments with long-term monitoring to accurately document vegetation change in response to global change and to identify the underlying mechanisms driving observed changes.
Keywords: Arctic, biodiversity, Cassiope tetragona, climate change, community change, ITEX, Poa arctica
Introduction
Identifying the drivers of documented change in natural ecosystems is a challenge due to the many changing abiotic and biotic factors occurring at a given location and over time (Jeltsch et al. 2008; Thuiller et al. 2008). Yet if reasonable forecasts are to be made, it is critical that the primary driver(s) be identified. Determining clearly whether climate change is the primary driver is especially challenging due to the large variability in weather between years. Arctic ecosystems have been studied intensively to determine the impacts of climate change because warming in the Arctic has been documented since the 1800s and has been occurring at faster rates in recent decades (Kaufman et al. 2009; IPCC 2013). The response of arctic plant communities to climate change is of particular interest for the following reasons. Small changes in environmental conditions can have large effects on the plant community (Billings 1952; Chapin et al. 1995). These changes in plant community dynamics have been associated with alterations in ecosystem function and nutrient cycling (Shaver and Chapin 1991; Hobbie and Chapin 1998). Alterations to plant community structure can have far-reaching consequences as they provide shelter for animals and are the base of the food web (Sørensen et al. 2008; Joly et al. 2010; Tape et al. 2010). Changes in canopy structure and physiology of plants could greatly influence the energy balance, which can impact regional climate and permafrost dynamics (Chapin et al. 2005). Finally, shifts in community dynamics and changes in ecosystem function have the potential to shift Arctic tundra ecosystems from a carbon sink to a source (Oechel et al. 1993) that could provide a significant feedback to climate change.
Many studies have been conducted to examine how tundra plant communities respond to environmental changes, such as increased temperatures and nutrient availability (Arft et al. 1999; Dormann and Woodin 2002; Callaghan et al. 2004). Yet most studies span 5 years or less and are unable to address whether or not plant community responses are maintained in the long term. Few studies address the tundra vegetation changes that occur after prolonged periods of environmental change (Hudson et al. 2011; Elmendorf et al. 2012a; Michelsen et al. 2012). Earlier studies have given insights into how plant communities may shift beyond the initial responses to changes in their environment (Chapin et al. 1995; Hollister et al. 2005). Temperature gradient studies, paleoecological investigations, and modeling efforts clearly show that with warming, tundra vegetation moves from an open canopy (with limited vascular plant cover) toward a closed canopy that gets taller due to the increased abundance of graminoids, then shrubs, and, ultimately with enough warming, trees (Oechel et al. 1997). Given that preexisting plant communities can resist change (Hudson and Henry 2010; Svenning and Sandel 2013), the ultimate question is as follows: Will modest warming cause clear directional change in tundra communities, and if so, how quickly will the change occur?
To answer this question, researchers have conducted warming experiments throughout many regions of the world (Rustad et al. 2001). The tundra has received special attention for the reasons listed above and most of the warming studies in tundra collaborate as part of the International Tundra Experiment (ITEX) network (http://ibis.geog.ubc.ca/itex/). ITEX researchers have agreed on standard protocols that allow for detailed comparisons across sites. The primary focus has been on documenting the response of tundra vegetation to passive experimental warming imposed by open-top chambers. Now that many of the sites have monitored vegetation for over a decade, changes in the control plots have become increasingly important for documenting the impact of climate change (Elmendorf et al. 2012b). The goal of this study is to document the effects of experimental warming on plant community dynamics over nearly two decades and to evaluate whether the changes in plant communities associated with this experimental warming are consistent with the natural trends observed in the control plots. Specifically, we examined changes at the species level in plant cover, canopy height, and species diversity at four sites which span a moisture and climate gradient. We focus on the consistency of the response over time to look for clear directional trends that the community may be moving toward.
Materials and Methods
Site descriptions
This study consisted of four study sites; two sites were located near Barrow, AK (71°19′N, 156°36′W), and two were approximately 100 km south near Atqasuk, AK (70°27′N, 157°24′W) (Fig.1). At each location, a wet and a dry site were established. These locations are representative of two bioclimate zones; Barrow is classified in the circumpolar vegetation map (CAVM 2003) and by Raynolds et al. (2006) as Biozone C and Atqasuk as Biozone D. Both locations have a deep heritage of research; Barrow was an International Biological Tundra Biome site in the early 1970s (Brown et al. 1980), and Atqasuk was the focus of the Research on Arctic Tundra Environments (Batzli 1980). The sites near Barrow include a dry (BD) and wet (BW) site; both have a mean July temperature of ∼4°C (Brown et al. 1980). In Barrow, snowmelt occurs in early to mid-June and maximum thaw depth is typically between 50 and 100 cm. The BD site is situated on a well-drained beach ridge above a drained thaw lake dominated by Cassiope tetragona, Salix rotundifolia, and Luzula confusa. The BW site is in a frequently inundated transitional zone between the beach ridge of the dry site and a drained lake basin, and is dominated by Carex aquatilis, Dupontia fisheri, and Eriophorum spp. The sites near Atqasuk also include a dry (AD) and wet (AW) site; both have a mean July temperature of ∼9°C (Batzli 1980). Snowmelt in Atqasuk occurs in late May, and maximum thaw depth is typically between 90 and 120 cm. The AD site is on a well-drained ridge above a thaw lake and is dominated by Cassiope tetragona, Ledum palustre, and Luzula confusa. The AW site is located at the edge of a thaw lake in a frequently inundated meadow and is dominated by Carex aquatilis, Eriophorum spp., and Salix pulchra. Topographic changes are small (<0.5 m) at the sites; however, even small differences may be associated with significant shifts in plant community composition and soil moisture (Webber 1978; Komárková and Webber 1980).
Figure 1.

Images of the four sites: Atqasuk dry (AD), Atqasuk wet (AW), Barrow dry (BD), and Barrow wet (BW).
The four sites were established between 1994 and 1996 and have been monitored since using standard ITEX protocols. Each site consists of 48 ∼1 m2 plots (24 control and 24 warmed). Warming was achieved using hexagonal open-top chambers (OTCs) constructed of Sun-Lite HPTM fiberglass according to the guidelines in the ITEX manual (Molau and Mølgaard 1996). OTCs were installed every year shortly after snowmelt and removed at the end of the growing season. OTCs have been shown to warm the surface air temperature an average of 0.6 to 2.2°C (Hollister et al. 2006), and, despite experimental artifacts (Bokhorst et al. 2013), they have been shown to realistically simulate climate change in the tundra (Hollister and Webber 2000).
Climate monitoring
Weather stations were established in 1998 at the dry sites at both Barrow and Atqasuk. Readings of temperature at screen height (2 m, 107 temperature probe) and precipitation (35 cm, TE525 tipping bucket rain gage) were taken every 15 min, averaged (temperature) or summed (precipitation), and recorded every hour (CR10X datalogger; the above instruments were produced by Campbell Scientific Inc., Logan, UT). At each of the four sites, two plots were also monitored for soil moisture at 7.5 cm depth (HYD-10-A hydra probe, Stevens Vitel Hydrological and Meteorological Systems, Chantilly, VA). Voltages from the soil moisture probe were recorded every hour and were converted to water fraction by volume (WFV). The focus of the measurements was relative change between years; thus, readings were not calibrated with gravimetric methods. During times prior to the weather station establishment or instrument malfunction, readings from a nearby station were substituted (for details see Hollister et al. 2006).
Vegetation sampling
All four sites were sampled four separate times (1995–97, 2000, 2007–08, and 2012; Table1) according to the nondestructive point frame method outlined in the ITEX Manual (Molau and Mølgaard 1996). A 75 cm2 100-point grid with measurement points every 7 cm was leveled above the plant canopy using permanent markers that allow for reasonably accurate resampling of the same point over multiple years. At each point on the grid, a graduated ruler was lowered to the first contact (uppermost) within the plant canopy and then to the lowermost contact at that point. This shortcut, omitting intermediate contacts, has been shown to be effective at detecting vegetation change in tundra communities, especially at sites with a leaf area index less than two; however, it does artificially limit cover to 200% (May and Hollister 2012). At each contact, the species, live/dead status, and height were recorded. Some species were difficult to identify in situ and were grouped into the closest secure taxon; this included only recording cryptograms to growth form (i.e., acrocarpous moss). Taxa were also grouped into broad growth forms (i.e., bryophytes) for analysis of growth form trends (see Table2 for grouping schemes).
Table 1.
The years when vegetation was sampled and the associated number of summers of warming between samplings (cumulative number of years of warming in parentheses). Sites are Atqasuk dry (AD), Atqasuk wet (AW), Barrow dry (BD), and Barrow wet (BW)
| Site | Year | Summers of warming | ||||||
|---|---|---|---|---|---|---|---|---|
| Sampling 1 | Sampling 2 | Sampling 3 | Sampling 4 | Sampling 1 | Sampling 2 | Sampling 3 | Sampling 4 | |
| AD | 1997 | 2000 | 2007 | 2012 | 2 (2) | 3 (5) | 7 (12) | 5 (17) |
| AW | 1997 | 2000 | 2007 | 2012 | 2 (2) | 3 (5) | 7 (12) | 5 (17) |
| BD | 1995 | 2000 | 2008 | 2012 | 2 (2) | 5 (7) | 8 (15) | 4 (19) |
| BW | 1996 | 2000 | 2008 | 2012 | 2 (2) | 4 (6) | 8 (14) | 4 (18) |
Table 2.
Change in plant cover over time in control plots and in response to warming at the four sites. The average cover and standard error are presented at sampling 1, sampling 2, sampling 3, and sampling 4 for control (C1, C2, C3, and C4) and experimentally warmed (E1, E2, E3, and E4) plots. For convenience, the warming response is also presented as the differences between control and experimental plots at the four samplings (W1, W2, W3, and W4). The change over time in response to the ambient environment and to experimental warming is categorized as no change (.), inconsistent change (I), nondirectional change (N), and cumulative directional change (D+ – increase, D− – decrease); because the response to warming could also be considered relative to the change in the control plots, the warming response could be categorized as consistent change (C+ – increase, C− – decrease) and a cumulative directional change observed only in relation to the control plots is noted with an italicized D (see methods for further details). Taxa include all growth forms (in bold) present at a site and vascular plant species or narrower growth from (for nonvascular plants) that occurred in at least half the plots
| Taxa | C1 | C2 | C3 | C4 | E1 | E2 | E3 | E4 | W1 | W2 | W3 | W4 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atqasuk dry (AD) site | ||||||||||||||
| Deciduous Shrub | 0.5 (0.3) | 0.4 (0.3) | 0.6 (0.4) | 0.4 (0.2) | . | 0.3 (0.2) | 0.3 (0.2) | 0.5 (0.2) | 0.6 (0.4) | . | −0.3 | −0.1 | −0.2 | 0.2 |
| Evergreen Shrub | 29.2 (1.5) | 22.6 (1.5) | 35.5 (1.7) | 23.2 (2.1) | N | 29.8 (2.3) | 26.1 (2.4) | 33.0 (1.7) | 26.9 (1.9) | . | 0.6 | 3.5 | −2.5 | 3.8 |
| Cassiope tetragona | 6.3 (0.5) | 4.7 (0.5) | 6.0 (0.6) | 3.3 (0.7) | N | 7.2 (0.9) | 5.5 (0.8) | 7.7 (1.2) | 6.2 (0.9) | D+ | 0.9 | 0.8 | 1.7 | 2.8 |
| Diapensia lapponica | 3.8 (0.7) | 2.2 (0.7) | 3.8 (0.7) | 2.6 (0.6) | . | 3.5 (0.6) | 3.3 (0.5) | 3.9 (0.6) | 2.5 (0.5) | . | −0.2 | 1.0 | 0.1 | −0.1 |
| Ledum palustre | 11.5 (1.6) | 9.8 (1.6) | 14.5 (1.6) | 9.8 (1.3) | . | 11.9 (1.6) | 10.6 (1.8) | 13.8 (1.6) | 11.5 (1.3) | . | 0.3 | 0.8 | −0.7 | 1.7 |
| Vaccinium vitis-idaea | 7.6 (0.8) | 6.0 (0.8) | 11.3 (1.4) | 7.4 (1.1) | N | 7.2 (0.8) | 6.8 (0.7) | 7.6 (0.7) | 6.7 (0.9) | . | −0.4 | 0.8 | −3.6 | −0.7 |
| Forb | 0.7 (0.3) | 0.4 (0.3) | 0.8 (0.3) | 0.6 (0.2) | . | 0.7 (0.2) | 0.8 (0.3) | 1.5 (0.4) | 1.4 (0.5) | . | 0.0 | 0.5 | 0.7 | 0.8 |
| Graminoid | 12.3 (1.7) | 9.0 (1.7) | 18.3 (1.9) | 10.7 (1.4) | N | 12.6 (1.6) | 6.4 (1.0) | 15.9 (1.4) | 9.2 (1.4) | . | 0.4 | −2.6 | −2.5 | −1.5 |
| Hierochloe alpina | 3.0 (0.8) | 1.4 (0.8) | 3.3 (0.8) | 2.3 (0.5) | . | 2.8 (0.7) | 1.3 (0.3) | 4.9 (0.9) | 2.2 (0.5) | . | −0.1 | −0.1 | 1.6 | −0.1 |
| Luzula confusa | 5.3 (0.8) | 4.4 (0.8) | 9.4 (1.1) | 3.6 (0.6) | N | 6.3 (1.1) | 3.1 (0.6) | 7.1 (1.1) | 3.4 (0.7) | . | 0.9 | −1.3 | −2.3 | −0.3 |
| Bryophyte | 9.8 (0.8) | 12.0 (0.8) | 7.9 (0.7) | 6.4 (0.6) | N | 10.5 (1.3) | 11.3 (1.1) | 7.9 (1.0) | 6.5 (1.0) | . | 0.7 | −0.7 | 0.0 | 0.1 |
| Acrocarpous Moss | 8.0 (0.7) | 10.2 (0.7) | 4.5 (0.6) | 5.3 (0.6) | N | 7.2 (0.9) | 8.3 (0.9) | 4.6 (0.8) | 5.3 (0.9) | . | −0.8 | −2.0 | 0.2 | 0.0 |
| Lichen | 60.1 (1.8) | 56.3 (1.8) | 39.3 (2.3) | 23.8 (1.7) | D− | 67.2 (2.2) | 55.8 (3.5) | 37.4 (2.3) | 18.9 (1.6) | D− | 7.0 | −0.5 | −2.0 | −4.9 |
| Crustose Lichen | 1.8 (0.3) | 8.1 (0.3) | 2.1 (0.4) | 4.8 (0.8) | N | 1.5 (0.3) | 7.8 (1.0) | 2.3 (0.5) | 4.3 (0.8) | . | −0.3 | −0.3 | 0.1 | −0.6 |
| Foliose Lichen | 16.6 (1.2) | 12.3 (1.2) | 10.7 (0.8) | 5.8 (0.7) | D− | 15.9 (1.1) | 8.5 (0.8) | 9.2 (0.9) | 4.8 (0.6) | C− | −0.8 | −3.8 | −1.5 | −1.1 |
| Fruticose Lichen | 41.8 (1.7) | 36.0 (1.7) | 26.5 (2.1) | 13.2 (1.1) | D− | 49.8 (1.9) | 39.5 (2.6) | 25.9 (1.8) | 9.9 (1.0) | D− | 8.0 | 3.5 | −0.6 | −3.3 |
| Atqasuk wet (AW) site | ||||||||||||||
| Deciduous Shrub | 8.0 (1.5) | 8.6 (1.5) | 8.0 (1.5) | 7.8 (1.2) | . | 6.2 (0.9) | 8.0 (1.2) | 7.0 (0.9) | 7.5 (0.9) | . | −1.8 | −0.6 | −1.0 | −0.3 |
| Salix pulchra | 6.5 (1.5) | 7.3 (1.5) | 5.5 (1.1) | 5.0 (1.1) | . | 5.3 (0.9) | 6.6 (1.2) | 6.0 (0.8) | 4.7 (1.0) | . | −1.3 | −0.7 | 0.5 | −0.3 |
| Forb | 0.5 (0.2) | 0.5 (0.2) | 0.3 (0.1) | 0.1 (0.1) | . | 0.5 (0.2) | 0.3 (0.2) | 0.2 (0.1) | 0.2 (0.1) | . | 0.0 | −0.1 | −0.2 | 0.1 |
| Graminoid | 27.8 (1.5) | 19.7 (1.5) | 32.8 (1.4) | 26.8 (1.1) | N | 26.5 (1.5) | 23.6 (1.8) | 40.0 (1.7) | 32.3 (1.8) | I | −1.3 | 3.9 | 7.1 | 5.5 |
| Carex aquatilis | 19.8 (1.2) | 12.6 (1.2) | 24.5 (1.2) | 18.1 (1.1) | N | 18.5 (1.2) | 15.2 (1.0) | 30.1 (1.1) | 23.0 (1.4) | I | −1.3 | 2.6 | 5.6 | 4.9 |
| Eriophorum angustifolium | 3.3 (0.8) | 4.5 (0.8) | 4.6 (0.8) | 4.9 (0.6) | . | 3.1 (0.6) | 3.6 (0.7) | 4.9 (0.7) | 5.7 (0.7) | . | −0.2 | −0.9 | 0.3 | 0.8 |
| Eriophorum russeolum | 4.5 (0.8) | 2.4 (0.8) | 2.8 (0.5) | 3.2 (0.4) | . | 4.5 (0.6) | 4.5 (0.8) | 3.8 (0.6) | 3.1 (0.6) | . | 0.1 | 2.1 | 1.0 | −0.1 |
| Bryophyte | 87.8 (0.9) | 91.8 (0.9) | 86.5 (2.6) | 55.0 (3.1) | N | 86.7 (1.2) | 90.8 (1.4) | 94.1 (0.8) | 47.9 (3.0) | I | −1.1 | −1.1 | 7.6 | −7.1 |
| Acrocarpous Moss | 31.5 (4.1) | 32.0 (4.1) | 29.5 (3.9) | 22.5 (3.4) | . | 31.8 (3.4) | 31.0 (4.6) | 29.0 (3.4) | 17.0 (2.5) | . | 0.2 | −1.0 | −0.5 | −5.4 |
| Pleurocarpous Moss | 49.7 (3.9) | 54.9 (5.2) | 48.8 (5.5) | 27.1 (2.7) | N | 48.6 (3.1) | 55.9 (4.2) | 56.9 (3.6) | 24.0 (2.6) | . | −1.1 | 1.0 | 8.1 | −3.1 |
| Sphagnum Moss | 3.5 (0.9) | 4.5 (0.9) | 8.1 (1.8) | 5.4 (1.5) | . | 3.8 (1.4) | 3.5 (1.6) | 8.2 (2.7) | 6.7 (2.7) | . | 0.3 | −1.0 | 0.0 | 1.3 |
| Lichen | 1.0 (0.3) | 0.6 (0.3) | 0.3 (0.1) | 0.5 (0.2) | . | 1.0 (0.3) | 0.3 (0.2) | 0.2 (0.1) | 0.3 (0.1) | . | 0.0 | −0.3 | −0.1 | −0.3 |
| Barrow Dry (BD) site | ||||||||||||||
| Deciduous Shrub | 15.0 (1.1) | 28.5 (1.1) | 24.5 (1.5) | 17.1 (1.6) | N | 14.9 (1.3) | 24.3 (2.0) | 20.0 (1.8) | 14.7 (1.2) | C− | −0.1 | −4.2 | −4.5 | −2.5 |
| Salix rotundifolia | 15.0 (1.1) | 28.5 (1.1) | 24.5 (1.5) | 17.1 (1.6) | N | 14.9 (1.3) | 24.3 (2.0) | 20.0 (1.8) | 14.7 (1.2) | C− | −0.1 | −4.2 | −4.5 | −2.5 |
| Evergreen Shrub | 11.4 (1.0) | 20.4 (1.0) | 16.7 (1.2) | 26.8 (1.7) | N | 15.2 (1.1) | 24.8 (1.8) | 23.1 (2.0) | 33.0 (2.5) | D+ | 3.8 | 4.4 | 6.4 | 6.2 |
| Cassiope tetragona | 11.3 (1.0) | 19.8 (1.0) | 16.6 (1.3) | 26.5 (1.8) | N | 15.2 (1.1) | 24.8 (1.8) | 23.1 (2.0) | 33.0 (2.5) | D+ | 3.9 | 5.0 | 6.5 | 6.5 |
| Forb | 4.5 (0.6) | 7.7 (0.6) | 7.2 (0.9) | 5.5 (0.9) | N | 4.3 (0.4) | 6.6 (0.8) | 10.9 (1.8) | 7.0 (1.3) | . | −0.3 | −1.0 | 3.8 | 1.5 |
| Potentilla hyparctica | 2.0 (0.5) | 2.4 (0.5) | 3.0 (0.7) | 2.5 (0.7) | . | 1.8 (0.3) | 1.2 (0.4) | 4.0 (0.8) | 3.0 (0.6) | . | −0.2 | −1.3 | 1.1 | 0.4 |
| Graminoid | 3.0 (0.6) | 7.3 (0.6) | 7.2 (0.7) | 6.2 (0.8) | N | 4.5 (0.8) | 12.3 (1.9) | 16.3 (1.8) | 12.0 (1.4) | I | 1.4 | 5.0 | 9.1 | 5.8 |
| Luzula confusa | 1.3 (0.4) | 3.0 (0.4) | 3.6 (0.5) | 2.3 (0.3) | N | 1.3 (0.3) | 2.8 (0.5) | 4.4 (0.6) | 2.2 (0.4) | . | 0.0 | −0.2 | 0.8 | −0.1 |
| Poa arctica | 0.5 (0.1) | 1.6 (0.1) | 1.8 (0.3) | 2.1 (0.4) | D+ | 0.6 (0.2) | 3.8 (0.4) | 6.2 (1.0) | 5.8 (0.9) | D+ | 0.2 | 2.1 | 4.4 | 3.6 |
| Bryophyte | 11.0 (1.0) | 19.8 (1.0) | 11.7 (1.0) | 17.3 (1.9) | N | 8.4 (0.9) | 13.8 (1.4) | 6.3 (0.9) | 9.4 (1.4) | C− | −2.6 | −6.0 | −5.4 | −7.9 |
| Acrocarpous Moss | 7.5 (0.9) | 11.8 (0.9) | 7.7 (0.9) | 7.9 (1.0) | N | 5.4 (0.6) | 9.6 (1.1) | 3.6 (0.5) | 5.6 (0.9) | C− | −2.1 | −2.2 | −4.1 | −2.3 |
| Pleurocarpous Moss | 2.0 (0.6) | 5.7 (0.6) | 3.0 (0.7) | 8.9 (1.8) | N | 1.3 (0.5) | 3.2 (0.8) | 2.1 (0.5) | 3.5 (1.0) | I | −0.7 | −2.5 | −0.9 | −5.4 |
| Lichen | 27.0 (1.2) | 37.9 (1.2) | 31.9 (2.0) | 41.0 (1.9) | N | 25.8 (2.0) | 24.7 (2.7) | 15.9 (1.9) | 17.0 (2.3) | D− | −1.3 | −13.2 | −16.0 | −24.1 |
| Foliose Lichen | 6.3 (0.9) | 8.5 (0.9) | 8.5 (0.7) | 9.0 (0.9) | . | 6.0 (0.7) | 6.0 (0.6) | 4.3 (0.6) | 5.2 (0.7) | D− | −0.3 | −2.5 | −4.3 | −3.8 |
| Fruticose Lichen | 16.7 (1.0) | 26.3 (1.0) | 22.8 (1.6) | 31.0 (1.7) | N | 15.7 (1.6) | 15.8 (2.2) | 11.1 (1.6) | 11.1 (1.9) | D− | −1.0 | −10.5 | −11.8 | −19.9 |
| Barrow Wet (BW) site | ||||||||||||||
| Deciduous Shrub | 0.2 (0.1) | 0.0 (0.1) | 0.0 (0.0) | 0.2 (0.1) | . | 0.3 (0.2) | 0.7 (0.4) | 1.8 (1.0) | 1.2 (0.6) | C+ | 0.2 | 0.6 | 1.8 | 1.0 |
| Forb | 17.8 (1.8) | 14.6 (1.8) | 13.2 (1.8) | 8.5 (1.6) | D− | 15.6 (1.7) | 13.1 (1.9) | 15.7 (2.0) | 6.8 (0.9) | . | −2.2 | −1.5 | 2.5 | −1.8 |
| Saxifraga cernua | 2.0 (0.4) | 2.1 (0.4) | 1.9 (0.5) | 0.9 (0.2) | . | 2.5 (0.4) | 1.9 (0.4) | 3.8 (0.7) | 1.4 (0.3) | C+ | 0.4 | −0.2 | 1.9 | 0.5 |
| Stellaria laeta | 4.0 (0.8) | 2.1 (0.8) | 1.8 (0.4) | 1.3 (0.3) | N | 4.0 (0.9) | 3.1 (0.9) | 1.6 (0.3) | 1.1 (0.2) | . | 0.0 | 1.0 | −0.2 | −0.2 |
| Graminoid | 43.3 (1.8) | 63.0 (1.8) | 41.3 (2.4) | 49.0 (2.2) | N | 44.4 (1.2) | 60.4 (6.8) | 43.0 (2.2) | 50.3 (2.8) | . | 1.1 | −2.5 | 1.7 | 1.3 |
| Carex aquatilis | 18.5 (2.0) | 14.6 (2.0) | 19.0 (1.4) | 20.3 (1.6) | N | 23.1 (2.0) | 16.6 (1.5) | 26.6 (1.3) | 25.5 (1.8) | C+ | 4.5 | 2.0 | 7.7 | 5.3 |
| Dupontia fisheri | 7.8 (0.9) | 12.9 (0.9) | 7.8 (1.0) | 13.6 (2.0) | N | 6.1 (0.8) | 9.0 (1.4) | 4.0 (0.6) | 9.2 (1.5) | C− | −1.6 | −3.8 | −3.8 | −4.4 |
| Eriophorum angustifolium | 9.9 (1.5) | 19.0 (1.5) | 4.9 (1.0) | 7.8 (1.5) | N | 8.4 (1.4) | 18.6 (4.1) | 4.0 (0.7) | 7.0 (1.9) | . | −1.5 | −0.4 | −0.9 | −0.8 |
| Eriophorum russeolum | 2.3 (0.4) | 4.9 (0.4) | 5.0 (0.6) | 2.6 (0.6) | N | 2.8 (0.6) | 4.0 (0.9) | 4.7 (0.7) | 3.1 (0.7) | . | 0.6 | −0.9 | −0.3 | 0.5 |
| Poaceae spp.2 | 3.8 (0.7) | 11.3 (0.7) | 4.1 (0.8) | 4.4 (0.7) | N | 2.7 (0.4) | 11.8 (1.5) | 2.8 (0.7) | 5.3 (0.9) | . | −1.1 | 0.5 | −1.3 | 0.9 |
| Bryophyte | 42.0 (3.1) | 56.4 (3.1) | 24.9 (3.0) | 31.6 (4.2) | N | 42.0 (4.1) | 45.6 (3.3) | 16.1 (2.2) | 18.4 (3.0) | D− | 0.0 | −10.8 | −8.8 | −13.2 |
| Acrocarpous Moss | 17.2 (1.9) | 25.1 (1.9) | 9.1 (1.3) | 14.3 (2.4) | N | 16.5 (2.7) | 20.6 (2.7) | 7.4 (1.0) | 8.7 (1.8) | C− | −0.7 | −4.5 | −1.7 | −5.6 |
| Pleurocarpous Moss1 | 23.8 (2.2) | 30.9 (2.9) | 15.7 (2.7) | 16.9 (4.0) | N | 24.9 (3.0) | 24.0 (2.1) | 8.7 (1.7) | 9.4 (2.0) | D− | 1.1 | −6.9 | −7.0 | −7.5 |
| Lichen | 2.5 (0.8) | 3.3 (0.8) | 5.5 (1.8) | 5.8 (2.1) | . | 1.8 (0.7) | 1.8 (0.9) | 1.7 (0.7) | 1.9 (0.8) | D− | −0.8 | −1.5 | −3.8 | −3.9 |
| Foliose Lichen | 2.5 (0.8) | 3.3 (0.8) | 5.5 (1.8) | 5.8 (2.1) | . | 1.6 (0.7) | 1.7 (0.9) | 1.6 (0.7) | 1.8 (0.8) | D− | −0.9 | −1.6 | −3.8 | −4.0 |
Pleurocarpous moss included leafy liverworts at the wet sites due to difficulties with identification underwater.
Calamagrostis holmii, Hierochloe pauciflora, and Poa arctica were lumped due to difficulties identifying sterile tillers.
Data analysis
Cover, height, and diversity indices were calculated for each plot. All encounters of equipment (i.e., individual tags) were removed from the dataset before analysis (<1% total cover). Cover estimates were calculated by summing all contacts from each grouping examined (e.g., taxon, live contacts, dead contacts). The cover and canopy height of all taxa were based on live encounters only (except for litter, standing dead, and open canopy cover). Open canopy was calculated by summing the cover of all mosses, lichens, litter, and bare ground encountered in the top contacts only. Dead plant matter was considered standing dead if it was attached or litter if it was unattached. Height for each contact was calculated by taking the difference between the encountered plant contact and the ground contact. Canopy height was calculated using only the tallest encounter of each grouping (species, growth form or other) in each plot. Species richness and Shannon index were calculated per plot based on cover estimates of all live taxa using the computer program PC-ORD 4.0 (McCune and Mefford 1999).
The cover, height, and diversity indices at each sampling time were used to calculate estimates of vegetation change occurring in the control plots and due to warming. To determine whether the control plots were changing, a one factor repeated-measures ANOVA was performed (using year) on the control plots only (Fig.2). If the difference between years was significant, the taxon was considered “responsive” and a correlation was performed between year and the yearly average value to determine whether the change was directional. If the response was not directional, it was considered “nondirectional.” To determine whether the plants were responding to experimental warming, a 2 factor repeated-measures ANOVA was performed (using year, treatment, and the interaction between them; Fig.2). If treatment was significant or there was a significant interaction between year and treatment, then the taxon was considered “responsive.” To determine whether the response was directional, a correlation was performed between year and the yearly average value for experimental plots or between year and the yearly average difference between experimental and control plots. If the response was not considered directional, then it was considered “inconsistent” if there was a significant interaction between year and treatment or “consistent” if the interaction was not significant. All results were considered statistically significant with a Type 1 error probability of 5% or less using R version 2.13.1 statistical platform (R Development Core Team 2011). Repeated-measures ANOVAs were conducted using linear mixed effect models (the “lme” function in the R package “nlme”). Regressions between the average value of a year versus year (for significant responses) were considered “directional” if the R2 was greater than 0.8. Cases that varied significantly from normality were either log- or square-root-transformed or tested with an equivalent nonparametric test (Kruskal–Wallis).
Figure 2.

Decision tree used to determine the response categorization (see Methods for details).
Results
Temperature, precipitation, and soil moisture
Mean July temperature varied at both Atqasuk and Barrow throughout the duration of this study (Fig.3). Temperatures during the summers when the vegetation was sampled varied greatly. Both regions showed increasing temperature trends over the duration of the study, although neither trend was statistically significant. Precipitation and soil moisture also varied greatly between years (Fig.3), and the AD site has had consistent low soil moisture from 2007 through 2012.
Figure 3.
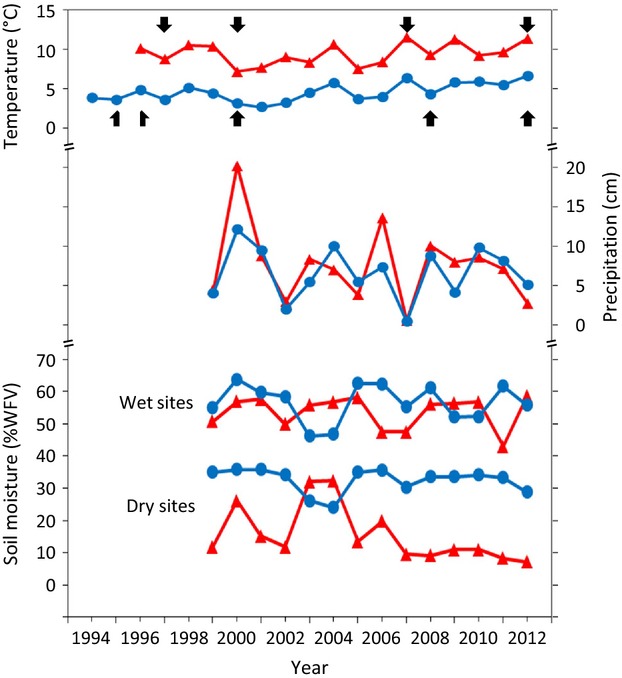
Mean temperature (top), total precipitation (middle), and mean soil moisture (bottom) at the sites in Atqasuk (red triangles) and Barrow (blue circles) in July during the years of the study. Years when vegetation was sampled are noted with arrows. No precipitation or soil moisture information was available before 1999.
Change within sites
At the AD site, lichens decreased over time in the control plots and with warming (Tables2 and 3, Fig.4). Vascular plant diversity decreased, whereas changes in the cover of evergreen shrubs, graminoids, bryophytes, total live plants, standing dead, litter, and open canopy and species richness were nondirectional over time (Tables2 and 3, Fig.4). Cover of Cassiope tetragona and standing dead increased with warming. Although not quantitatively measured, it was clear that the site was heavily impacted by caribou grazing the winter before the second sampling; the effect of this can be seen by the decrease in canopy heights and the decrease in the cover of total live plants and standing dead (Tables3 and 4).
Table 3.
Change in community indices over time in control plots and in response to warming at the four sites (AD – Atqasuk dry, AW – Atqasuk wet, BD – Barrow dry, and BW – Barrow wet). The community indices used were cover of live, standing dead, and dead unattached (litter) plant material; the percent of the canopy that was not occupied by vascular plants (Open Canopy); and the diversity metrics species richness and Shannon index. See Table2 for an explanation of the table layout
| Site | C1 | C2 | C3 | C4 | E1 | E2 | E3 | E4 | W1 | W2 | W3 | W4 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Live Plants | ||||||||||||||
| AD | 112.6 (2.3) | 100.7 (2.3) | 102.6 (3.1) | 65.3 (2.7) | N | 121.0 (3.2) | 100.8 (4.6) | 96.1 (2.3) | 63.8 (2.6) | . | 8.4 | 0.1 | −6.5 | −1.5 |
| AW | 125.0 (1.3) | 121.2 (1.3) | 128.0 (2.2) | 90.5 (3.5) | N | 121.0 (1.7) | 122.9 (1.7) | 141.4 (1.4) | 88.4 (2.5) | I | −4.0 | 1.8 | 13.4 | −2.1 |
| BD | 72.0 (1.0) | 121.4 (1.0) | 99.3 (1.8) | 114.1 (2.4) | N | 73.0 (1.5) | 106.4 (3.9) | 92.6 (1.8) | 93.1 (2.7) | I | 1.0 | −15.0 | −6.7 | −21.0 |
| BW | 106.1 (3.2) | 137.3 (3.2) | 85.3 (2.2) | 97.8 (5.0) | N | 104.5 (4.7) | 121.7 (6.8) | 78.5 (2.9) | 81.0 (3.7) | I | −1.6 | −15.7 | −6.8 | −16.8 |
| Standing Dead | ||||||||||||||
| AD | 14.6 (1.2) | 7.1 (1.2) | 17.1 (1.9) | 26.3 (2.5) | N | 18.0 (1.4) | 11.0 (1.4) | 18.2 (1.6) | 28.3 (2.4) | C+ | 3.4 | 3.8 | 1.1 | 2.0 |
| AW | 36.2 (1.4) | 43.8 (1.4) | 18.0 (2.1) | 60.8 (2.8) | N | 41.5 (1.6) | 48.5 (2.2) | 24.0 (2.0) | 62.0 (2.7) | C+ | 5.3 | 4.8 | 6.0 | 1.3 |
| BD | 15.1 (0.8) | 11.9 (0.8) | 20.9 (1.9) | 41.2 (3.3) | N | 19.2 (1.3) | 17.7 (1.4) | 35.8 (1.9) | 53.2 (3.6) | D+ | 4.1 | 5.8 | 14.9 | 12.0 |
| BW | 37.1 (2.6) | 36.5 (2.6) | 45.7 (2.7) | 40.0 (2.1) | . | 40.8 (2.3) | 45.0 (4.4) | 61.3 (4.3) | 43.1 (3.4) | C+ | 3.8 | 8.5 | 15.6 | 3.2 |
| Litter | ||||||||||||||
| AD | 22.0 (1.0) | 13.0 (1.0) | 9.9 (1.2) | 49.1 (2.2) | N | 20.8 (1.2) | 16.3 (2.2) | 13.7 (1.5) | 51.0 (2.7) | . | −1.3 | 3.2 | 3.8 | 1.9 |
| AW | 8.4 (0.9) | 4.2 (0.9) | 9.7 (1.9) | 27.8 (2.4) | N | 9.4 (1.1) | 4.3 (0.8) | 3.7 (0.7) | 31.9 (2.5) | I | 1.0 | 0.0 | −6.0 | 4.1 |
| BD | 10.1 (0.7) | 8.1 (0.7) | 12.3 (0.9) | 15.3 (1.6) | N | 12.5 (1.2) | 9.5 (1.5) | 12.4 (1.0) | 32.0 (2.6) | I | 2.4 | 1.3 | 0.0 | 16.7 |
| BW | 27.6 (1.5) | 9.4 (1.5) | 26.9 (1.5) | 43.2 (4.3) | N | 32.8 (1.4) | 13.5 (1.7) | 24.7 (2) | 60.2 (3.8) | I | 5.2 | 4.1 | −2.2 | 17.0 |
| Open Canopy | ||||||||||||||
| AD | 53.0 (2.4) | 61.2 (2.4) | 42.8 (2.2) | 45.4 (3.5) | N | 54.5 (2.4) | 56.3 (3.1) | 46.2 (1.8) | 40.5 (3.5) | . | 1.5 | −4.9 | 3.4 | −5.0 |
| AW | 24.6 (1.9) | 23.6 (1.9) | 38.5 (1.9) | 13.1 (1.7) | N | 22.3 (1.9) | 15.5 (1.6) | 26.5 (2.3) | 9.8 (1.2) | I | −2.3 | −8.2 | −12.0 | −3.3 |
| BD | 48.4 (1.6) | 35.9 (1.6) | 40.0 (2.4) | 30.4 (1.7) | N | 41.8 (1.9) | 27.9 (2.3) | 24.6 (1.9) | 13.6 (1.8) | D− | −6.6 | −8.0 | −15.5 | −16.8 |
| BW | 15.2 (2.2) | 9.6 (2.2) | 28.3 (2.4) | 9.8 (1.6) | N | 11.3 (1.2) | 8.0 (0.9) | 21.3 (2.3) | 6.7 (1.0) | C− | −3.9 | −1.7 | −7.0 | −3.2 |
| Species Richness | ||||||||||||||
| AD | 6.4 (0.2) | 5.7 (0.2) | 6.8 (0.2) | 6.0 (0.2) | N | 6.3 (0.2) | 5.8 (0.2) | 6.6 (0.2) | 6.2 (0.2) | . | −0.1 | 0.1 | −0.2 | 0.2 |
| AW | 5.0 (0.2) | 4.9 (0.2) | 5.3 (0.3) | 5.2 (0.1) | . | 4.9 (0.2) | 4.8 (0.1) | 5.0 (0.2) | 5.1 (0.2) | . | −0.1 | −0.1 | −0.3 | 0.0 |
| BD | 5.3 (0.2) | 6.5 (0.2) | 6.5 (0.2) | 6.0 (0.2) | N | 5.5 (0.2) | 6.7 (0.2) | 7.5 (0.2) | 6.6 (0.2) | C+ | 0.2 | 0.2 | 1.0 | 0.6 |
| BW | 7.3 (0.2) | 7.6 (0.2) | 7.3 (0.3) | 6.9 (0.2) | . | 7.3 (0.3) | 7.4 (0.3) | 7.1 (0.2) | 6.3 (0.2) | . | 0.0 | −0.2 | −0.2 | −0.5 |
| Shannon Index | ||||||||||||||
| AD | 0.97 (0.01) | 1.02 (0.02) | 0.99 (0.02) | 0.92 (0.02) | D− | 0.95 (0.01) | 1.00 (0.02) | 1.00 (0.01) | 0.93 (0.02) | . | −0.02 | −0.02 | 0.00 | 0.01 |
| AW | 0.32 (0.01) | 0.37 (0.02) | 0.34 (0.02) | 0.35 (0.02) | N | 0.33 (0.01) | 0.35 (0.01) | 0.31 (0.01) | 0.32 (0.01) | . | 0.01 | −0.02 | −0.02 | −0.03 |
| BD | 0.76 (0.01) | 0.77 (0.02) | 0.80 (0.01) | 0.69 (0.01) | N | 0.75 (0.02) | 0.71 (0.02) | 0.70 (0.02) | 0.63 (0.02) | D− | −0.01 | −0.05 | −0.10 | −0.07 |
| BW | 0.44 (0.02) | 0.44 (0.02) | 0.41 (0.01) | 0.37 (0.01) | N | 0.41 (0.02) | 0.40 (0.01) | 0.40 (0.01) | 0.33 (0.01) | C− | −0.03 | −0.04 | 0.00 | −0.04 |
Figure 4.
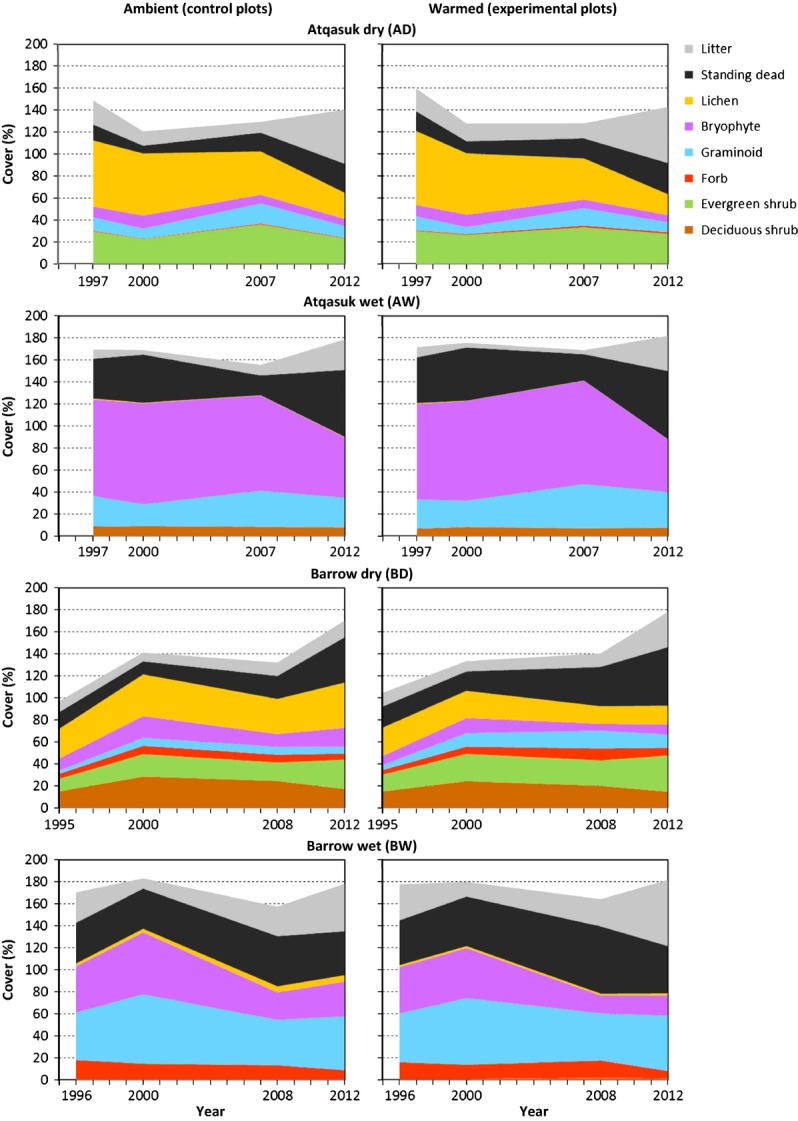
Changes in cover over time in the ambient environment and with warming at the four sites. Years sampled are shown on the axis.
Table 4.
Change in canopy height over time in control plots and in response to warming at the four sites. Height was calculated as the maximum height recorded in a plot for each taxon. See Table2 for an explanation of the table layout
| C1 | C2 | C3 | C4 | E1 | E2 | E3 | E4 | W1 | W2 | W3 | W4 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atqasuk dry (AD) site | ||||||||||||||
| Plot Maximum | 9.6 (1.2) | 7.0 (1.2) | 12.7 (0.9) | 11.0 (0.9) | N | 11.7 (1.2) | 6.4 (0.5) | 14.9 (1.2) | 12.5 (1.2) | . | 2.1 | −0.6 | 2.2 | 1.6 |
| Evergreen Shrub | 3.7 (0.4) | 3.5 (0.4) | 4.2 (0.4) | 4.6 (0.5) | . | 3.1 (0.2) | 2.6 (0.2) | 3.9 (0.3) | 5.1 (0.3) | . | −0.6 | −0.9 | −0.3 | 0.5 |
| Cassiope tetragona | 3.5 (0.4) | 2.2 (0.4) | 4.1 (0.4) | 3.6 (0.4) | N | 2.7 (0.2) | 2.4 (0.2) | 2.7 (0.4) | 4.5 (0.3) | I | −0.8 | 0.1 | −1.4 | 0.8 |
| Diapensia lapponica | 0.3 (0.2) | 0.3 (0.2) | 0.7 (0.3) | 0.6 (0.3) | . | 0.1 (0.0) | 0.2 (0.1) | 0.5 (0.2) | 0.6 (0.3) | . | −0.3 | −0.1 | −0.2 | 0.0 |
| Ledum palustre | 2.1 (0.2) | 2.3 (0.2) | 1.8 (0.2) | 3.2 (0.5) | N | 1.9 (0.2) | 1.9 (0.2) | 2.9 (0.5) | 4.1 (0.4) | . | −0.3 | −0.4 | 1.1 | 0.9 |
| Vaccinium vitis-idaea | 1.1 (0.1) | 1.1 (0.1) | 0.5 (0.1) | 1.3 (0.3) | . | 1.4 (0.2) | 1.0 (0.2) | 0.2 (0.1) | 1.2 (0.2) | . | 0.2 | −0.1 | −0.2 | 0.0 |
| Graminoid | 9.4 (1.3) | 6.6 (1.3) | 12.2 (1.0) | 10.9 (1.0) | N | 11.6 (1.3) | 6.3 (0.5) | 14.9 (1.2) | 12.0 (1.2) | . | 2.2 | −0.3 | 2.7 | 1.1 |
| Hierochloe alpina | 7.7 (1.6) | 4.9 (1.6) | 11.9 (1.3) | 9.6 (1.3) | N | 10.0 (1.7) | 5.3 (0.6) | 12.1 (1.6) | 10.1 (1.4) | . | 2.3 | 0.4 | 0.2 | 0.6 |
| Luzula confusa | 5.6 (0.8) | 4.7 (0.8) | 7.5 (0.8) | 7.5 (1.0) | N | 6.5 (0.9) | 3.8 (0.5) | 9.6 (1.2) | 6.2 (0.8) | . | 0.8 | −0.9 | 2.0 | −1.4 |
| Atqasuk wet (AW) site | ||||||||||||||
| Plot Maximum | 21.9 (1.1) | 19.2 (1.1) | 24.1 (1.2) | 20.7 (1.0) | N | 24.4 (0.9) | 22.7 (0.8) | 27.8 (1.1) | 24.2 (1.1) | C+ | 2.5 | 3.5 | 3.7 | 3.5 |
| Deciduous Shrub | 9.5 (0.9) | 10.2 (0.9) | 10.9 (0.4) | 11.4 (0.6) | . | 10.2 (0.9) | 12.1 (0.9) | 13.6 (1.2) | 12.8 (0.9) | C+ | 0.6 | 1.8 | 2.7 | 1.3 |
| Salix pulchra | 10.0 (0.9) | 9.9 (0.9) | 10.6 (0.6) | 11.2 (0.7) | . | 10.7 (0.9) | 12.2 (1.0) | 13.4 (1.2) | 11.9 (1.2) | C+ | 0.7 | 2.2 | 2.8 | 0.7 |
| Graminoid | 21.9 (1.1) | 19.2 (1.1) | 24.1 (1.2) | 20.5 (1.0) | N | 24.4 (0.9) | 22.7 (0.8) | 27.7 (1.1) | 24.2 (1.1) | C+ | 2.5 | 3.5 | 3.5 | 3.7 |
| Carex aquatilis | 21.8 (1.2) | 18.8 (1.2) | 23.5 (1.3) | 19.9 (1.2) | N | 24.0 (0.9) | 22.3 (0.8) | 27.5 (1.1) | 24.0 (1.1) | C+ | 2.2 | 3.5 | 4.1 | 4.2 |
| Eriophorum angustifolium | 12.0 (1.1) | 12.6 (1.1) | 15.7 (1.3) | 12.5 (0.9) | . | 14.3 (1.4) | 14.6 (0.8) | 16.6 (1.1) | 15.3 (1.1) | C+ | 2.3 | 2.0 | 1.0 | 2.8 |
| Eriophorum russeolum | 12.6 (1.0) | 9.8 (1.0) | 12.3 (1.1) | 10.7 (1.1) | . | 13.2 (1.0) | 14.0 (1.1) | 14.4 (1.1) | 13.0 (1.0) | C+ | 0.6 | 4.2 | 2.2 | 2.3 |
| Barrow Dry (BD) site | ||||||||||||||
| Plot Maximum | 3.6 (0.4) | 6.0 (0.4) | 8.1 (0.7) | 7.9 (0.6) | D+ | 6.6 (0.6) | 9.3 (1.4) | 12.8 (0.7) | 12.7 (0.8) | D+ | 2.9 | 3.3 | 4.6 | 4.8 |
| Deciduous Shrub | 0.1 (0.1) | 1.3 (0.1) | 0.6 (0.2) | 1.8 (0.1) | N | 0.3 (0.1) | 1.6 (0.2) | 0.2 (0.1) | 2.2 (0.2) | . | 0.2 | 0.3 | −0.4 | 0.4 |
| Salix rotundifolia | 0.1 (0.1) | 1.3 (0.1) | 0.6 (0.2) | 1.8 (0.1) | N | 0.3 (0.1) | 1.6 (0.2) | 0.2 (0.1) | 2.2 (0.2) | . | 0.2 | 0.3 | −0.4 | 0.4 |
| Evergreen Shrub | 2.1 (0.5) | 3.1 (0.5) | 4.2 (0.2) | 5.4 (0.3) | D+ | 3.3 (0.5) | 4.1 (0.3) | 5.2 (0.3) | 7.9 (0.5) | D+ | 1.2 | 1.0 | 0.9 | 2.5 |
| Cassiope tetragona | 2.1 (0.5) | 3.1 (0.5) | 4.2 (0.2) | 5.4 (0.3) | D+ | 3.3 (0.5) | 4.1 (0.3) | 5.2 (0.3) | 7.9 (0.5) | D+ | 1.2 | 1.0 | 0.9 | 2.5 |
| Forb | 1.1 (0.4) | 3.0 (0.4) | 4.9 (0.8) | 5.1 (0.8) | D+ | 3.5 (0.7) | 4.4 (1.2) | 7.0 (1.2) | 7.8 (1.2) | D+ | 2.4 | 1.4 | 2.1 | 2.8 |
| Potentilla hyparctica | 1.0 (0.6) | 2.5 (0.6) | 3.3 (0.8) | 3.2 (0.5) | D+ | 2.2 (0.5) | 1.0 (0.5) | 6.9 (1.2) | 8.7 (1.2) | D+ | 1.2 | −1.5 | 3.6 | 5.5 |
| Graminoid | 2.1 (0.4) | 4.9 (0.4) | 6.6 (0.6) | 5.9 (0.5) | N | 4.0 (0.7) | 7.0 (1.3) | 11.7 (0.7) | 10.5 (0.8) | D+ | 1.9 | 2.1 | 5.1 | 4.7 |
| Luzula confusa | 1.3 (0.4) | 3.2 (0.4) | 4.9 (0.4) | 4.2 (0.4) | D+ | 2.2 (0.4) | 2.8 (0.4) | 7.2 (0.8) | 6.6 (0.5) | D+ | 0.8 | −0.4 | 2.3 | 2.4 |
| Poa arctica | 0.2 (0.2) | 2.4 (0.2) | 4.2 (0.6) | 3.9 (0.5) | D+ | 0.6 (0.2) | 3.2 (0.5) | 8.9 (0.9) | 8.5 (0.9) | D+ | 0.4 | 0.8 | 4.7 | 4.6 |
| Barrow Wet (BW) site | ||||||||||||||
| Plot Maximum | 8.9 (0.6) | 11.4 (0.6) | 13.1 (0.6) | 14.2 (0.4) | D+ | 11.4 (0.5) | 12.9 (0.6) | 15.0 (0.5) | 16.8 (0.8) | D+ | 2.5 | 1.6 | 1.9 | 2.7 |
| Forb | 3.5 (0.5) | 3.4 (0.5) | 5.4 (0.9) | 7.0 (0.9) | D+ | 5.6 (0.9) | 3.2 (0.6) | 7.4 (1.2) | 7.8 (1.1) | . | 2.1 | −0.1 | 2.0 | 0.8 |
| Saxifraga cernua | 0.4 (0.2) | 0.9 (0.2) | 2.7 (1.0) | 3.8 (1.3) | D+ | 1.5 (0.6) | 0.8 (0.4) | 2.5 (1.2) | 4.4 (1.0) | . | 1.1 | −0.1 | −0.2 | 0.6 |
| Stellaria laeta | 2.1 (0.6) | 2.3 (0.6) | 3.0 (0.4) | 2.8 (0.5) | . | 3.7 (0.6) | 2.1 (0.7) | 3.0 (0.6) | 3.8 (0.6) | . | 1.6 | −0.2 | 0.0 | 1.0 |
| Graminoid | 8.9 (0.6) | 11.4 (0.6) | 12.8 (0.5) | 14.1 (0.4) | D+ | 10.5 (0.6) | 12.9 (0.6) | 14.7 (0.5) | 16.4 (0.8) | D+ | 1.6 | 1.6 | 1.9 | 2.3 |
| Carex aquatilis | 8.0 (0.5) | 9.9 (0.5) | 11.0 (0.6) | 12.7 (0.4) | D+ | 10.3 (0.7) | 12.7 (0.6) | 14.0 (0.4) | 14.8 (0.6) | D+ | 2.3 | 2.8 | 3.0 | 2.1 |
| Dupontia fisheri | 6.6 (0.7) | 9.0 (0.7) | 10.6 (0.7) | 11.3 (0.6) | D+ | 6.1 (0.6) | 8.1 (0.7) | 10.5 (0.8) | 12.5 (1.0) | . | −0.5 | −0.9 | −0.1 | 1.1 |
| Eriophorum angustifolium | 3.9 (0.4) | 7.6 (0.4) | 6.6 (0.7) | 9.4 (0.4) | N | 4.9 (0.5) | 8.6 (0.8) | 9.0 (0.9) | 9.9 (0.7) | C+ | 1.1 | 1.0 | 2.4 | 0.5 |
| Eriophorum russeolum | 2.6 (0.3) | 4.4 (0.3) | 6.7 (0.4) | 6.0 (0.5) | D+ | 2.4 (0.5) | 5.3 (0.8) | 8.5 (0.7) | 7.6 (0.8) | D+ | −0.2 | 0.9 | 1.8 | 1.6 |
| Poaceae spp.1 | 4.2 (0.5) | 4.7 (0.5) | 6.8 (0.7) | 8.4 (1.3) | D+ | 3.6 (0.5) | 4.3 (0.5) | 8.4 (0.9) | 10.0 (1.3) | . | −0.5 | −0.4 | 1.6 | 1.5 |
Calamagrostis holmii, Hierochloe pauciflora, Poa arctica.
At the AW site, the canopy height of all the shrubs and graminoids consistently increased with warming, but these differences were realized at the first sampling and remained relatively constant (Table4). Changes in the cover of graminoids, bryophytes, total live plants, litter, and open canopy were nondirectional over time and inconsistent with warming (Tables2 and 3, Fig.4). Changes in the cover of standing dead and the canopy height of graminoids were nondirectional over time. Cover of standing dead increased with warming.
At the BD site, canopy height increased over time and with warming (Tables3 and 4, Fig.4). Canopy height of evergreen shrubs, forbs, and the dominant graminoid species increased over time and with warming, resulting in more than a doubling of maximum canopy height over the 18 years of sampling (Table4). Poa arctica was particularly responsive and increased both canopy height and cover over time and with warming (Tables2 and 4). Changes in the cover of graminoids, total live plants, and litter were nondirectional over time and inconsistent with warming. Changes in the cover of shrubs, forbs, bryophytes, lichens, and open canopy and species richness were nondirectional over time, while the cover of deciduous shrubs, bryophytes, lichens, and open canopy and diversity decreased and the cover of evergreen shrubs and standing dead and species richness increased with warming.
At the BW site, the canopy height of graminoids increased over time and with warming (Tables3 and 4, Fig.4). Cover of total live plants and litter was nondirectional over time and inconsistent with warming (Table3, Fig.4). Forbs decreased over time, and changes in the cover of graminoids, bryophytes, and open canopy were nondirectional over time (Tables2 and 3, Fig.4). Cover of deciduous shrubs and standing dead increased, while cover of bryophytes, lichens, and open canopy and diversity decreased with warming. Cover of graminoids and forbs did not change significantly with warming despite significant changes in species within each group. Height of forbs increased over time but did not change with warming.
Comparisons across sites
The number of taxa that showed significant changes in cover over time was greater in the control plots than in response to warming (39 taxa vs. 28 of 58, Tables2 and 5). However, in the control plots, only five taxa showed a directional change; the rest (34) changed in ways that were inconsistent across years and therefore nondirectional. Of these, the four that decreased were in either the AD site or BW site, and they included one forb and three lichens; these taxa also decreased with warming except the forb at the BW site. The only taxon that increased in the control plots was a grass, Poa arctica, at the BD site, which also increased with warming. With warming, fewer taxa changed, but of the ones that did, all but five showed either a cumulative directional change (13 taxa) or a consistent change (10 taxa). The AW site was the only site where no taxa showed a consistent warming response. At the AD site, one taxon increased and three decreased; at the BD site, three taxa increased and seven decreased; and at the BW site, three taxa increased and six decreased. Taxa that increased with warming included one deciduous shrub, three evergreen shrubs, one forb, and two graminoids. Taxa that decreased with warming included two deciduous shrubs, one graminoid, five bryophytes, and eight lichens. Of the taxa that showed a cumulative directional change with warming, shrubs and graminoids increased and bryophytes and lichens decreased.
Table 5.
Summary of the consistency of changes over time in cover of taxa from Table2. The changes in control plots (ambient) between years and in response to experimental warming (warmed) are shown. The table tabulates the number of taxa categorized as no change (.), changed nondirectionally (N), changed inconsistently (I), changed consistently (decrease C− or increase C+), and changed directionally over time (decrease D− or increase D+) grouped by site and growth form
| Ambient | Warmed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | N | D− | D+ | . | I | C− | C+ | D− | D+ | |
| Site | ||||||||||
| Atqasuk Dry | 5 | 8 | 3 | 0 | 12 | 0 | 1 | 0 | 2 | 1 |
| Atqasuk Wet | 8 | 4 | 0 | 0 | 9 | 3 | 0 | 0 | 0 | 0 |
| Barrow Dry | 2 | 12 | 0 | 1 | 3 | 2 | 4 | 0 | 3 | 3 |
| Barrow Wet | 4 | 10 | 1 | 0 | 6 | 0 | 2 | 3 | 4 | 0 |
| Growth Form | ||||||||||
| Deciduous Shrub | 4 | 2 | 0 | 0 | 3 | 0 | 2 | 1 | 0 | 0 |
| Evergreen Shrub | 2 | 5 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 3 |
| Forb | 4 | 2 | 1 | 0 | 6 | 0 | 0 | 1 | 0 | 0 |
| Graminoid | 3 | 12 | 0 | 1 | 10 | 3 | 1 | 1 | 0 | 1 |
| Bryophyte | 2 | 10 | 0 | 0 | 5 | 2 | 3 | 0 | 2 | 0 |
| Lichen | 4 | 3 | 3 | 0 | 2 | 0 | 1 | 0 | 7 | 0 |
| Total | 19 | 34 | 4 | 1 | 30 | 5 | 7 | 3 | 9 | 4 |
The community indices generally showed nondirectional changes over time except species diversity which decreased at the AD site (Table3). With warming, there was an increase in the cover of standing dead at all sites and a decrease in the cover of open canopy and diversity at the two sites in Barrow and an increase in the number of vascular taxa at the BD site.
Changes in canopy height were greater at Barrow than Atqasuk (Table4). In Atqasuk, there was either no change or an inconsistent change in height over time and with warming, except at the wet site which showed an increase in canopy height for all taxa with warming only, however, this change was observed in the first sampling and was not cumulative. At Barrow, most, but not all, taxa showed a cumulative increase in canopy height over time and with warming.
In summary, from an examination of the tables, it is clear that the magnitude of change was almost always greater between years than in response to experimental warming. In addition, the number of significant responses was greater over time than with warming. However, the change over time was mostly nondirectional. Of the 21 instances where change over time was directional (this included changes in community indices and cover of taxa), all but six showed a corresponding directional change with warming.
Discussion
Temperature trends in Barrow and Atqasuk regions followed similar trends to those found elsewhere in high latitude regions (Serreze et al. 2000; ACIA 2005; IPCC 2013). Both regions had variability in mean July temperatures between years with a small increasing trend across the duration of the study. While this trend was not statistically significant, it was consistent with documented trends (earlier snowmelt and warmer summers) in the region (Stone et al. 2002; Hinzman et al. 2005; Lynch and Brunner 2007; Wendler et al. 2014).
Overall, the vegetative changes in control plots between samplings were larger than the responses to warming. This should be expected given that temperature varied more between years than in response to experimental warming, and precipitation and soil moisture varied greatly between years as did herbivore intensity (the impact of herbivory was documented in adjacent areas by Villarreal et al. (2012) and shown to be a strong determinant of plant community composition). In most cases, changes in control plots were inconsistent through time, whereas responses to warming, while fewer, were mostly consistent. A directional change is likely due to a clear competitive advantage (or disadvantage) resulting in a cumulative increase (or decrease) over time. A consistent response may be due to a release from temperature restraints that causes a physiological response which does not accumulate, such as an increase in growth/biomass of a preexisting individual.
The instances where directional changes in the control plots were matched by a directional change in response to warming provide strong evidence that the observed change in the control plots is due to warming. These included a decrease in cover of lichens at the AD site, increased canopy height at both sites in Barrow, and an increase in canopy height and cover of Poa arctica at the BD site. A decrease in the abundance of lichens is consistent with the majority of warming studies (Cornelissen et al. 2001; Elmendorf et al. 2012a; Lang et al. 2012). Canopy height has been shown by a number of studies to increase with warming and across natural temperature gradients (Walker et al. 2006; Elmendorf et al. 2012a), and in fact is one of the most consistent responses seen when examining warming studies (Elmendorf et al. 2012a). Poa arctica is commonly associated with disturbance (Potter 1972; Bliss and Peterson 1992) and may be responding directly to warming or indirectly to disturbances caused by warming which may include increased nutrient availability given that the species is generally more common in fertilized areas (Gartner et al. 1983).
The warming experiment provides a possible look into future vegetation at the sites. From this, we would expect over the next two decades to see further changes in the control plots in addition to those already observed assuming the region continues to warm. These include decreases in the cover of lichens at all sites except the AW site (which is already nearly devoid of lichens), decreases in bryophytes at the BW site, and an increase in the cover of evergreen shrub, Cassiope tetragona at the dry sites, an increase in standing dead at the BD site, a decrease in the cover of open canopy at the BD site, and a decrease in diversity at the BD site. Decreases in bryophytes with warming, while originally proposed, have been recently questioned; mechanistically an increase in stature of vascular plants may benefit mosses by providing shade (Zona et al. 2011; Jägerbrand et al. 2012). Thus, the mixed result shown here of some sites showing a decrease in the cover of moss and others showing no response is consistent with recent studies (Lang et al. 2012). The increased cover of Cassiope tetragona with warming is consistent with a large volume of literature that has examined the species (Havström et al. 1993; Weijers et al. 2012); in fact, the species is often used as a climate proxy because of its tight coupling between growth and seasonal temperature (Callaghan et al. 1989; Weijers et al. 2012). The increased cover of standing dead with warming is consistent with previously accepted ideas of arctic plants holding their dead leaves in the canopy (Bliss 1962; Savile 1972) and is consistent with warming experiments that have found similar increases (Elmendorf et al. 2012a). Increased standing dead may be due to increased growth in the early years of the experiment and the resulting growth senescing then being retained as standing dead. The decrease in the openness of the canopy has been less well documented, but given that the consensus findings are increases in graminoids and shrubs and decreases in lichens with warming (Elmendorf et al. 2012a), this is consistent with a general loss of open space in the canopy. It is important to note that the Barrow sites, where the canopy became less open with warming, have very short canopy heights, and the open canopy is a more or less colonizable area for vascular plants, whereas at Atqasuk, the canopy height is much taller and the open area is heavily shaded (Fig.1). Diversity of vascular plants is expected to ultimately increase in tundra with warming (Walker 1995; Francis and Currie 2003); however, this study and a synthesis of experimental warming studies in tundra have shown a decrease in diversity (Walker et al. 2006) or no change (Elmendorf et al. 2012a).
Predicting community change based on growth form may be problematic. At several sites, the taxa within growth forms increased while others decreased resulting in a muted warming response; this was especially true at the BW site. This disparity in how taxa within growth forms respond may be explained by grouping taxa by other attributes, such as home range, maximum plant height, or leaf density (Cornelissen et al. 2003; Kattge et al. 2011). Such grouping schemes, or a suite of them, may better identify traits that respond similarly and make predicting community changes in response to changing environmental conditions more accurate (Suding et al. 2008; Dorji et al. 2013; Soudzilovskaia et al. 2013).
Variability in weather, especially temperature, between years may explain much of the nondirectional change observed over time (Chapin and Shaver 1996; Arft et al. 1999). Microclimate differences within sites could also allow for conditions between plots to vary enough that a species may be successful in some plots and not others (Hudson and Henry 2009). Confounding effects may have led to variations in warming responses between years (Walker et al. 1994; Cooper et al. 2011). For example, it appears that experimental warming is in general limiting growth at the AD site and it is likely this is because the site is water stressed (especially in later years) and temperature is not as limiting a factor, whereas at the other three sites, canopy height is clearly responsive to warming. Nontemperature factors may prove helpful in the future when incorporated into investigations about arctic plant community changes (Phoenix and Lee 2004). However, it is difficult to separate the drivers of directional change from the many factors that fluctuate between years without long-term repeated annual sampling. Furthermore, the cumulative nature of directional change makes it difficult to correlate change in community composition to factors other than year.
This study shows the power of coupling an in situ experiment with long-term monitoring. Clearly in most cases, species fluctuate between years in ways that are difficult to decipher. However, in cases where there are clear directional changes in natural communities, it is not possible to identify the driver without additional information. Therefore, when it is important to identify the driving factors at a given site, we advocate for coupling long-term monitoring with in situ experiments. This is especially true in cases where results from an intensely studied site are generalized to a much larger region. Assuming that manipulation is low cost and logistically simple, the addition of manipulations can add greatly to the utility of new and existing monitoring programs. Monitoring programs such as these are needed to inform policy decisions as ecologists grapple with global change.
Acknowledgments
This project has been run over many years and has relied on the efforts of many individuals especially Pat Webber, Christian Bay, and Brian Noyle. We have received many insights from our colleagues especially those of the ITEX network and several anonymous reviewers. This material is based upon work supported by the National Science Foundation.
Data Accessibility
All data are available at ACADIS managed by the National Snow and Iced Data Center (http://nsidc.org/acadis/).
Conflict of Interest
None declared.
References
- ACIA. Impacts of a warming arctic: Arctic climate impact assessment. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Arft AM, Walker MD, Gurevitch J, Alatalo JM, Bret-Harte MS, Dale MRT, et al. Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecol. Monogr. 1999;64:491–511. [Google Scholar]
- Batzli GO. 1980. Research on Arctic Tundra Environments (RATE) program. Special issue of Arctic and Alpine Research. 12(4)
- Billings WD. The environmental complex in relation to plant growth and distribution. Q. Rev. Biol. 1952;27:251–265. doi: 10.1086/399022. [DOI] [PubMed] [Google Scholar]
- Bliss LC. Adaptations of arctic and alpine plants to environmental conditions. Arctic. 1962;15:117–144. [Google Scholar]
- Bliss LC. Peterson KM. Plant succession, competition, and the physiological constraints of species in the Arctic. In: Chapin FS III, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J, editors; Arctic ecosystems in a changing climate. An ecophysiological perspective. San Diego, CA: Academic Press; 1992. pp. 111–138. [Google Scholar]
- Bokhorst S, Huiskes A, Aerts R, Convey P, Cooper EJ, Dalen L, et al. Variable temperature effects of Open Top Chambers at polar and alpine sites explained by irradiance and snow depth. Glob. Change Biol. 2013;19:64–74. doi: 10.1111/gcb.12028. [DOI] [PubMed] [Google Scholar]
- Brown J, Miller PC, Tieszen LL. Bunnell FL. An arctic ecosystem: the coastal tundra at Barrow, Alaska. Stroudsburg, PA: Dowden, Hutchinson, & Ross, Inc; 1980. p. 571. [Google Scholar]
- Callaghan TV, Carlsson BÅ. Tyler NJC. Historical records of climate-related growth in Cassiope tetragona from the Arctic. J. Ecol. 1989;77:823–837. [Google Scholar]
- Callaghan TV, Björn LO, Chernov Y, Chapin T, Christensen TR, Huntley B, et al. Climate Change and UV-B impacts on arctic tundra and polar desert ecosystems: effects on the structure of arctic ecosystems in the short- and long-term perspectives. Ambio. 2004;33:436–447. doi: 10.1579/0044-7447-33.7.436. [DOI] [PubMed] [Google Scholar]
- CAVM. 2003. Anchorage, Alaska U.S. Fish and Wildlife Service Team (Circumpolar Arctic Vegetation Map. Scale 1: 7,500000. Conservation of Arctic Flora and Fauna (CAFF) Map No. 1.
- Chapin FS., III Shaver GR. Physiological and growth responses of arctic plants to a field experiment simulating climatic change. Ecology. 1996;77:822–840. [Google Scholar]
- Chapin FS, III, Shaver GR, Giblin AE, Nadelhoffer KJ. Laundre JA. Response of arctic tundra to experimental and observed changes in climate. Ecology. 1995;76:696–711. [Google Scholar]
- Chapin FS, III, Sturm M, Serreze MC, McFadden JP, Key JR, Lloyd AH, et al. Role of land-surface changes in arctic summer warming. Science. 2005;310:657–660. doi: 10.1126/science.1117368. [DOI] [PubMed] [Google Scholar]
- Cooper EJ, Dullinger S. Semenchuk P. Late snowmelt delays plant development and results in lower reproductive success in the High Arctic. Plant Sci. 2011;180:157–167. doi: 10.1016/j.plantsci.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Callaghan TV, Alatalo JM, Michaelson A, Graglia E, Hartley AE, et al. Global change and arctic ecosystems: is lichen decline a function of increases in vascular plant biomass? J. Ecol. 2001;89:984–994. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003;51:335–380. [Google Scholar]
- Dorji T, Totland Ø, Moe SR, Hopping KA, Pan JB. Klein JA. Plant functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Glob. Change Biol. 2013;19:459–472. doi: 10.1111/gcb.12059. [DOI] [PubMed] [Google Scholar]
- Dormann CF. Woodin SJ. Climate change in the Arctic: using plant functional types in a meta-analysis of field experiments. Funct. Ecol. 2002;16:4–17. [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, Bjork RG, Bjorkman AD, Callaghan TV, et al. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol. Lett. 2012a;15:164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, Björk RG, Boulanger-Lapointe N, Cooper EJ, et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Chang. 2012b;2:453–457. [Google Scholar]
- Francis AP. Currie DJ. A globally consistent richness-climate relationship for angiosperms. Am. Nat. 2003;161:523–536. doi: 10.1086/368223. [DOI] [PubMed] [Google Scholar]
- Gartner BL. Shaver FS., III Chapin GR. Demographic patterns of seedling establishment and growth of native graminoids in an Alaskan tundra disturbance. J. Appl. Ecol. 1983;20:965–980. [Google Scholar]
- Havström M, Callaghan TV. Jonasson S. Differential growth responses of Cassiope tetragona, an arctic dwarf-shrub, to environmental perturbations among three contrasting high- and sub- arctic sites. Oikos. 1993;66:389–402. [Google Scholar]
- Hinzman LD, Bettez N, Chapin FS, III, Dyurgerov M, Fastie CL, Griffith B, et al. Evidence and implications of recent climate change in terrestrial regions of the Arctic. Clim. Change. 2005;72:251–298. [Google Scholar]
- Hobbie SE. Chapin FS., III The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology. 1998;79:1526–1544. [Google Scholar]
- Hollister RD. Webber PJ. Biotic validation of small open-top chambers in a tundra ecosystem. Glob. Change Biol. 2000;6:835–842. [Google Scholar]
- Hollister RD, Webber PJ. Tweedie CE. The response of Alaskan arctic tundra to experimental warming: differences between short- and long-term responses. Glob. Change Biol. 2005;11:525–536. [Google Scholar]
- Hollister RD, Webber PJ, Nelson FE. Tweedie CE. Soil thaw and temperature response to air warming varies by plant community: results from an open-top chamber experiment in northern Alaska. Arct. Antarct. Alp. Res. 2006;38:206–215. [Google Scholar]
- Hudson JMG. Henry GHR. Increased plant biomass in a High Arctic heath community from 1981 to 2008. Ecology. 2009;90:2657–2663. doi: 10.1890/09-0102.1. [DOI] [PubMed] [Google Scholar]
- Hudson JMG. Henry GHR. High Arctic plant community resists 15 years of experimental warming. J. Ecol. 2010;98:1035–1041. [Google Scholar]
- Hudson JMG, Henry GHR. Cornwell WK. Taller and larger: shifts in arctic tundra leaf traits after 16 years of experimental warming. Glob. Change Biol. 2011;17:1013–1021. [Google Scholar]
- IPCC. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2013. p. 1535. [Google Scholar]
- Jägerbrand AK, Kudo G, Alatalo JM. Molau U. Effects of neighboring vascular plants on the abundance of bryophytes in different vegetation types. Polar Sci. 2012;6:200–208. [Google Scholar]
- Jeltsch F, Moloney KA, Schurr FM, Köchy M. Schwager M. The state of plant population modelling in light of environmental change. Perspect. Plant Ecol. Evol. Syst. 2008;9:171–189. [Google Scholar]
- Joly K, Klein FS., III Chapin DR. Winter habitat selection by caribou in relation to lichen abundance, wildfires, grazing, and landscape characteristics in northwest Alaska. Ecoscience. 2010;17:321–333. [Google Scholar]
- Kattge J, Díaz S, Lavorel S, Prentice C, Leadley P, Bönisch G, et al. TRY - a global database of plant traits. Glob. Change Biol. 2011;17:2905–2935. [Google Scholar]
- Kaufman DS, Schneider DP, Mckay NP, Ammann CM, Bradley RS, Briffa KR, et al. Recent warming reverses long-term arctic cooling. Science. 2009;325:1236–1239. doi: 10.1126/science.1173983. [DOI] [PubMed] [Google Scholar]
- Komárková V. Webber PJ. Two low Arctic vegetation maps near Atkasook, Alaska. Arct. Alp. Res. 1980;4:447–472. [Google Scholar]
- Lang SI, Cornelissen JHC, Shaver GR, Ahrens M, Callaghan TV, Molau U, et al. Arctic warming on two continents has consistent negative effects on lichen diversity and mixed effects on bryophyte diversity. Glob. Change Biol. 2012;18:1096–1107. [Google Scholar]
- Lynch AH. Brunner RD. Context and climate change: an integrated assessment for Barrow, Alaska. Clim. Change. 2007;82:93–111. [Google Scholar]
- May JL. Hollister RD. Validation of a simplified point frame method to detect change in tundra vegetation. Polar Biol. 2012;35:1815–1823. [Google Scholar]
- McCune B. Mefford MJ. Multivariate analysis of ecological data 4.0. Gleneden Beach, OR: MjM Software; 1999. [Google Scholar]
- Michelsen A, Rinnan R. Jonasson S. Two decades of experimental manipulations of heaths and forest understory in the subarctic. Ambio. 2012;41:218–230. doi: 10.1007/s13280-012-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molau U. Mølgaard P. International tundra experiment (ITEX) manual. Second edition. Copenhagen, Denmark: Danish Polar Center; 1996. (53 + XXI pp. [Google Scholar]
- Oechel WC, Hastings SJ, Vourlitis GL, Jenkins MA, Riechers G. Grulke N. Recent change of arctic tundra ecosystems from a net carbon dioxide sink to a source. Nature. 1993;361:520–523. [Google Scholar]
- Oechel WC, Callaghan TV, Gilmanov TG, Holten JI, Maxwell B, Molau U, et al., editors. Global change and Arctic terrestrial ecosystems. New York, NY: Springer-Verlag; 1997. p. 493. [Google Scholar]
- Phoenix GK. Lee JA. Predicting impacts of Arctic climate change: past lessons and future challenges. Ecol. Res. 2004;19:65–74. [Google Scholar]
- Potter LD. Plant ecology of the Walakpa Bay Area, Alaska. Arctic. 1972;25:115–130. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Raynolds MK, Walker DA. Maier HA. Alaska Arctic tundra vegetation map. 1:4,000,000. Anchorage, Alaska: U.S. Fish and Wildlife Service; 2006. [Google Scholar]
- Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia. 2001;126:543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- Savile DBO. Arctic adaptations in plants. Canada Dept. Agric. Mon. 1972;6:1–81. [Google Scholar]
- Serreze MC, Walsh JE, Osterkamp FS., III Chapin TE. Dyurgerov M, Romanovsky VE, et al. Observational evidence of recent change in the northern high-latitude environment. Clim. Change. 2000;46:159–207. [Google Scholar]
- Shaver GR. Chapin FS., III Production: biomass relationships and element cycling in contrasting arctic vegetation types. Ecol. Monogr. 1991;61:1–31. [Google Scholar]
- Sørensen LI, Mikola J. Kytöviita MM. Defoliation effects on plant and soil properties in an experimental low arctic grassland community - the role of plant community structure. Soil Biol. Biochem. 2008;40:2596–2604. [Google Scholar]
- Soudzilovskaia NA, Elumeeva TG, Onipchenko VG, Shidakov II, Salpagarova FS, Khubiev AB, et al. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proc. Natl Acad. Sci. USA. 2013;110:18180–18184. doi: 10.1073/pnas.1310700110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RS, Dutton EG, Harris JM. Longenecker D. Earlier spring snowmelt in northern Alaska as an indicator of climate change. J. Geophys. Res. Atmos. 2002;107(D10):1–15. [Google Scholar]
- Suding KN, Lavorel S, Cornelissen FS., III Chapin JHC, Díaz S, Garnier E, et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob. Change Biol. 2008;14:1125–1140. [Google Scholar]
- Svenning JC. Sandel B. Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 2013;100:1266–1286. doi: 10.3732/ajb.1200469. [DOI] [PubMed] [Google Scholar]
- Tape KD, Lord R, Marshall HP. Ruess RW. Snow-mediated ptarmigan browsing and shrub expansion in arctic Alaska. Ecoscience. 2010;17:186–193. [Google Scholar]
- Thuiller W, Albert C, Araújo MB, Berry PM, Cabeza M, Guisan A, et al. Predicting global change impacts on plant species’ distributions: future challenges. Perspect. Plant Ecol. Evol. Syst. 2008;9:137–152. [Google Scholar]
- Villarreal S, Hollister RD, Johnson DR, Lara MJ, Webber PJ. Tweedie CE. Tundra vegetation change near Barrow, Alaska (1972–2010) Environ. Res. Lett. 2012;7:1–10. 015508. [Google Scholar]
- Walker MD. Patterns and causes of arctic plant community diversity. In: Chapin FS III, Körner C, editors. Arctic and alpine biodiversity patterns, causes and ecosystem consequences. New York, NY: Springer-Verlag; 1995. pp. 3–20. [Google Scholar]
- Walker MD, Webber PJ, Arnold EH. Ebert-May D. Effects of interannual climate variation on aboveground phytomass in alpine vegetation. Ecology. 1994;75:393–408. [Google Scholar]
- Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, et al. Plant community responses to experimental warming across the tundra biome. Proc. Natl Acad. Sci. USA. 2006;103:1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber PJ. Spatial and temporal variation of the vegetation and its production, Barrow, Alaska. In: Tieszen LL, editor. Vegetation and production ecology of an Alaskan Arctic tundra. New York, NY: Springer-Verlag; 1978. pp. 37–112. [Google Scholar]
- Weijers S, Alsos IG, Eidesen PB, Broekman R, Loonen MJJE. Rozema J. No divergence in Cassiope tetragona: persistence of growth response along a latitudinal temperature gradient and under multi-year experimental warming. Ann. Bot. 2012;110:653–665. doi: 10.1093/aob/mcs123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler G, Moore B. Galloway K. Strong temperature increase and shrinking sea ice in Arctic Alaska. Open Atmos. Sci. J. 2014;8:7–15. [Google Scholar]
- Zona D, Oechel WC, Richards JH, Hastings S, Kopetz I, Ikawa H, et al. Light-stress avoidance mechanisms in a Sphagnum-dominated wet coastal Arctic tundra ecosystem in Alaska. Ecology. 2011;92:633–644. doi: 10.1890/10-0822.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available at ACADIS managed by the National Snow and Iced Data Center (http://nsidc.org/acadis/).


