Abstract
Bladder cancer is the 4th most common cancer among men in the U.S. and more than half of patients experience recurrences within 5 years after initial diagnosis. Additional clinically informative and actionable biomarkers of the recurrent bladder cancer phenotypes are needed to improve screening and molecular therapeutic approaches for recurrence prevention. MicroRNA-34a (miR-34a) is a short non-coding regulatory RNA with tumor suppressive attributes. We leveraged our unique, large, population-based prognostic study of bladder cancer in New Hampshire, U.S. to evaluate miR-34a expression levels in individual tumor cells to assess prognostic value. We collected detailed exposure and medical history data, as well as tumor tissue specimens from bladder patients and followed them long-term for recurrence, progression and survival. Fluorescence-based in situ hybridization assays were performed on urothelial carcinoma tissue specimens (n=229). A larger proportion of the non-muscle invasive tumors had high levels of miR-34a within the carcinoma cells compared to those tumors that were muscle invasive. Patients with high miR-34a levels in their baseline non-muscle invasive tumors experienced lower risks of recurrence (adjusted hazard ratio (HR) 0.57 95%CI 0.34–0.93). Consistent with these observations, we demonstrated a functional tumor suppressive role for miR-34a in cultured urothelial cells, including reduced matrigel invasion and growth in soft agar. Our results highlight the need for further clinical studies of miR-34a as a guide for recurrence screening and as a possible candidate therapeutic target in the bladder.
Keywords: miR, miRNA, bladder cancer, urothelial carcinoma, recurrence
Introduction
Bladder cancer is the 4th most common cancer among men in the U.S. and the 8th most common in women.1 Diagnosis is commonly made based on symptoms of gross painless hematuria using cystoscopy and urine cytology, followed by resection and histopathologic confirmation.2 The median age of diagnosis is 653 and the majority (>90%) of cases are urothelial (transitional cell) carcinoma.4 Bladder cancer risk is up to four-fold higher among cigarette smokers compared with non-smokers.5 Approximately 14,680 deaths occur annually in the U.S. from bladder cancer, which constitutes the leading cause of cancer death in men ≥ age 80.6 Northern New England, the region of focus for our study, has among the highest bladder cancer incidence and mortality rates in the U.S.7, 8
Non-muscle invasive tumors are prevalent in the population, with an estimated 500,000 patients with a history of urothelial carcinoma currently residing in the U.S.9 Bladder cancer recurrences are common10 and tumor behavior within a single histopathologic group is highly heterogeneous.11 Of patients diagnosed with non-muscle invasive bladder cancer, 50% to 75% experience recurrences within 6 to 12 years of diagnosis and 10% to 30% of tumors progress to muscle-invasive disease.12 This high rate of disease recurrence is a major challenge in patient management.13 The need to screen for these recurrences (typically every 6 months by the invasive cystoscopy procedure) makes bladder cancer one of the most expensive malignancies, costing the U.S. an estimated $3.7 billion in 2001.14, 15 Known predictors of recurrent bladder cancer include a history of recurrences and primary tumor clinicopathologic characteristics, including multiplicity, tumor size, T category (depth of invasion), presence of carcinoma in situ, tumor grade; and patient gender.16 Additional clinically informative and actionable biomarkers of the recurrent bladder cancer phenotypes are needed to improve screening and target molecular therapeutic approaches for recurrence prevention.
Non-coding RNAs are a class of RNA molecules that perform their regulatory biological functions through RNA:RNA interaction, without ever being translated into a protein.17, 18 MicroRNAs (miRNAs) are one class of non-coding RNAs that has received great attention in cancer biology in recent years.19–22 MiRNAs regulate their target genes by binding to specific sites, usually in the 3′ untranslated region of the target gene. The miRNA can modulate protein output of target genes via translational repression, cleavage, degradation, and/or sequestration.18, 21 The miRNA can concurrently regulate expression of many target genes in a cell type- and context-dependent manner, providing an extensive and flexible gene control mechanism.17,22 Expression profiling studies using whole tissue specimens have indicated that RNA levels of miR-34a are altered in bladder cancer. In these studies, miR-34a levels were lower in tumors compared to normal tissue, consistent with its tumor suppressive role in other cancer types.23–26 A positive-feedback loop links miR-34a to expression and activity of the genome gate-keeper p53 in some contexts,27 but there is also evidence of p53-independent functions of miR-34 and dispensability of miR-34a for p53-mediated processes.28–30 The fact that TP53 is frequently altered or inactivated in bladder tumors as they become invasive made miR-34a of particular interest as our candidate miRNA for investigation as a marker of bladder cancer prognosis. Few studies have characterized altered miRNA expression in specific cellular compartments of urothelial cancer lesions and/or investigated associations between miRNA expression and bladder cancer outcomes.31, 32
We have leveraged our population-based prognostic study of urothelial carcinoma to evaluate cancer cell-specific expression of miR-34a levels in relation to recurrence and also demonstrate a functional tumor suppression role for miR-34a in cultured urothelial cells.
Materials and Methods
Population
Bladder cancer patients diagnosed in the state of New Hampshire between January 1, 2002 – July 31, 2004 were obtained from a population-based case-control study. Eligible cases were identified using the State Cancer Registry, hospital pathology departments, and hospital cancer registries (30–79 years of age and residents of the state of New Hampshire at the time of diagnosis), as described.33 A standardized histopathology review and case verification and was performed by the study pathologist (ARS).34 Staging was performed using the TNM criteria of the American Joint Committee on Cancer (AJCC). For all cases with registry T values of T2b (tumor invading greater than one-half of the muscle wall) or higher, the registry T values were used; otherwise histologic grade and tumor stage was assigned by the study pathologist. Tumors were classified using the World Health Organization (WHO)/International Society of Urologic Pathology (ISUP) consensus system into either carcinoma in situ (CIS), papillary urothelial neoplasm of low malignant potential (PUN-LMP), low-grade papillary urothelial carcinoma (Pap.Ca-LG), high-grade papillary urothelial carcinoma (Pap.Ca-HG), non-papillary urothelial carcinoma (noPap.Ca-HG). Of the 389 patients with histologically confirmed urothelial carcinoma of the bladder, n=229 had tumor tissue samples available for the in situ hybridization assay. Informed consent was obtained from each participant and all procedures and study materials were approved by the Committee for the Protection of Human Subjects at Dartmouth College.
Information on bladder cancer recurrences was obtained from medical records provided by the treating hospital(s) (both in and outpatient records, including any pathology reports) covering the follow-up period. Records were reviewed by an experienced, certified tumor registrar to abstract the data on bladder tumors occurring subsequent to the incident tumor. Hospital registry data were used if the medical record could not be obtained. Data on the size and multiplicity of the primary tumor, as well as the initial course of treatment were obtained from the State Cancer Registry and were verified by medical record review (immunotherapy, chemotherapy, radiotherapy, transurethral resection, cystectomy).
The first recurrent tumor was defined as any tumor identified following a disease-free remission period, more than 90 days after the date of initial primary bladder tumor diagnosis. These recurrent tumors include those of the same level of invasiveness, as well as those which have progressed to higher stage/grade. Persistent primary tumors that did not have a remission period were excluded from the analysis of recurrence (n=19). Time to recurrence was calculated as the time between the initial diagnosis date and the date of the first recurrence event. For progression the event was the diagnosis of a tumor with a greater stage or grade than the initial primary bladder tumor. If no events were reported, the date the patient was last seen documented in the medical record was used for censoring. Status (alive or dead) was determined as of January 13, 2011 using the Social Security and the National Death Indices (NDI). Survival time was calculated from the date of initial diagnosis to date of death.
In Situ Hybridization
We performed in situ hybridization (ISH) for miR-34a on a tissue microarray slide representing n=229 urothelial carcinoma cases, as previously described.35 Briefly, 5′-,3′-fluorescein-tagged locked nucleic acid (LNA)-modified DNA probe complementary to entire miR-34a sequence (probe:5′FAM/A+CAA+CCA+GCT+AAG+ACA+CTG+CCA/3′FAM; +N denotes LNA modification) was added at 50 nM to 50% deionized formamide, 5× SSC, 500 μg/ml yeast tRNA, 1× Denhardt’s solution, 0.01% tween*20 solution and hybridized against tissue at 45°C for 75 min in Leica Bond-MAX automated staining station. miR-34a probe labeling was revealed by horseradish peroxidase (HRP)-mediated deposition of fluorescein-conjugated substrate. Then, expression of cytokeratin 19 protein levels was revealed with another round of HRP-mediated deposition of Dylight 594-conjugated substrate, after sequential incubations with primary anti-CK19 (50 μg/mL; MU246-UC, Biogenex) and secondary anti-mouse HRP conjugated antibody (1 μg/mL; 170–6516, Biorad). Tissue was counterstained with DAPI. RNA expression was assessed by fluorescence microscopy after manual selection of the cellular area of interest (i.e. urothelial carcinoma cells) using the ImagePro system. MiR-34a expression is presented as the miRNA fluorescence score (0,1+,2+,or 3+) within CK19-positive cancer cells (Figure 1).
Figure 1.
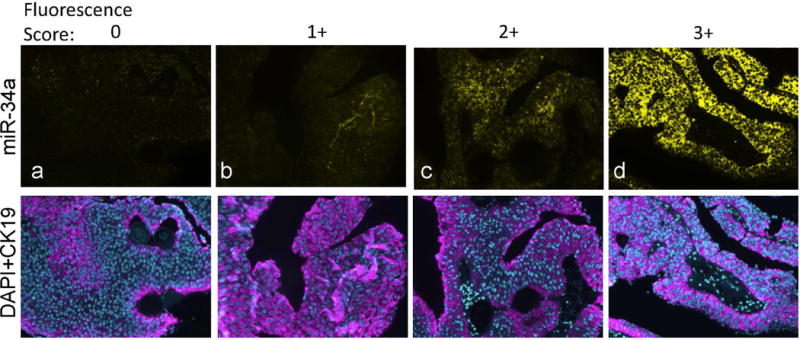
Detection of miR-34a by in situ hybridization on bladder tumor tissue specimens (Ta low grade). Expression of miR-34a (yellow) and the carcinoma cell marker cytokeratin 19 (CK19; pink) was detected by consecutive rounds of HRP-mediated deposition of fluorescent substrates. MiR-34a levels were scored as 0,1+,2+,3+ based on fluorescence intensity (representative slides are shown in panels a,b,c,d respectively). Tissue sections were counterstained with nuclear marker DAPI (blue).
Immunohistochemistry
Immunohistochemical staining of paraffin-embedded slides was performed using the avidin-biotin complex technique. Briefly, slides were deparaffinized and hydrated into water. Slides underwent antigen retrieval in Citra solution using the Biocare Decloaking Chamber (Biocare Medical). Staining of p53 was performed using a monoclonal antibody (Clone D0-7), at a 1:100 dilution on the Optimax I-6000 Immunostainer with a mouse secondary antibody (BioGenex, San Ramon, CA). The intensity of nuclear staining and the percentage of positively staining tumor cells was scored by the study pathologist (ARS). High staining intensity was classified as ≥3+ intensity staining.
Cell culture models
UROtsa normal urothelial cells were grown in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with an additional 1 mg/ml glucose, 5% fetal bovine serum and 1% penicillin/streptomycin.36, 37 HTB-2 cells (human urinary bladder epithelial cells with transitional cell papilloma) were cultured in McCoy′s 5A medium with L-glutamine and sodium bicarbonate supplemented with 10% fetal bovine serum.
Functional studies were carried out by transient transfection of synthetic miR-34a activity modulator compounds (anti-miR-34a or pre-miR-34a; AM17000, 17100, Applied Biosystems). Scramble RNA sequences were used as negative controls. We transfected these nucleotide-based compounds (20nM) using siPORT NeoFX reagent in Opti-Mem medium (Ambion).38, 39 Taqman miRNA specific probes were used to verify the miRNA expression levels post-transfection using the MicroRNA Reverse Transcription Kit and Taqman Universal PCR Master Mix (Applied Biosystems). Transfection with miR-34 activity modulators typically resulted in 3.5 – 4.5 fold differences in miR-34a expression levels compared to the negative control.
Tumorigenesis assays
Functional assays of miRNA dysregulation were performed to assess the tumorigenic potential of cells following miRNA dysregulation in cell culture.40 Capacity for anchorage-independent growth was evaluated by assays for colony formation in soft agar. HTB-2 cells with normal vs. dysregulated miR-34a levels were removed from culture flasks with trypsin-EDTA and suspended in DMEM containing 5% v/v FBS and 1% antibiotic–antimycotic supplemented with 0.5% agar. The agar enriched with cells were overlaid onto 0.7% agar medium in a 24-well plate with a density of 1×104 cells per well. After 7 days of incubation, colonies were manually counted with a microscope. Data represent colonies counted from 5 fields chosen at random within each well.
Matrigel invasion assays were performed to assess whether dysregulated miRNA levels modify the invasive potential of bladder cells. Using the BD BioCoat Tumor Invasion system, cells were suspended in serum-free DMEM in transwell membrane filter inserts (8 um pore size) coated with Matrigel matrix mimicking a reconstituted basement membrane placed inside tissue culture plates containing fetal bovine serum. Cells that invaded into the underside of the chamber after 16 hours at 37°C were stained with Calcein AM and counted with a fluorescent microscope.
Gene expression microarray
Differential mRNA target gene expression was assessed in miR-34a low (anti-miR-34a) vs. high UROtsa cells (negative control) using Illumina Human HT-12 gene expression microarrays containing ~25,000 annotated genes. Data were processed and cubic spline normalized using Illumina Genome Studio (GSGX Version 1.8.0). We focused on a subset of 133 genes with the most dramatic expression differences, selected by filtering using expression ratios >1.9 or <0.4, which had false discovery rate (FDR) adjusted P-values below 1×10−7. A heat map was constructed by clustering genes by the expression ratio and mean centering the log2 intensities of each array to allow visualization. We then compared the lists of differentially expressed mRNAs observed with miR-34a dysregulation against the in silico sequence-predicted miR-34a ‘target genes’ from a combination of sources (MicroT, Microcosm, Pictar, Segal, TargetScan).
RT-PCR
Additional urothelial carcinoma patients were recruited from Dartmouth-Hitchcock Medical Center for RNA isolation of sections of their primary (n=5) and recurrent tumor tissue samples (n=12). Total RNA was isolated from formalin-fixed paraffin embedded (FFPE) bladder tissue samples using the Qiagen Deparaffinization Reagent followed by miRNeasy FFPE RNA isolation kits, according to the manufacturer’s instructions (Qiagen). Taqman miR-34a- or U6-snRNA specific probes were used to verify the miRNA expression levels using the MicroRNA Reverse Transcription Kit and Taqman Universal PCR Master Mix (Applied Biosystems). S100P mRNA levels were assessed by Qiagen Reverse Transcriptase followed by Taqman S100P or GAPDH primer-probe sets and Taqman Universal PCR Master Mix (Applied Biosystems). A standard curve was constructed to convert expression level Ct values into ng cDNA41. The miR-34a levels were normalized to U6, while S100P levels were normalized to GAPDH. Expression levels were assessed in tumors relative to histologically normal urothelial tissue.
Statistical analysis
Median times to first recurrence, progression or survival were calculated using the Kaplan-Meier method. Multivariate analysis of time to the first bladder tumor recurrence, progression and survival analyses were performed using Cox-proportional hazards regression analysis in SAS version 9.3. Analyses were performed separately for non-muscle invasive tumors (stage 0,I) and muscle invasive tumors (stage II, III, IV). MiR-34a was analyzed by assigning low levels to tissue samples with fluorescence scores 0–1+, and high levels for scores 2–3+. The standard prognostic model included adjustment for age at diagnosis of first bladder tumor, gender, smoking (never – <100 lifetime cigarettes, former – quit >1 year prior to diagnosis, or current – continuing within a year of diagnosis), as well as size (<3, 3+cm), multiplicity (single, multiple), stage/grade (non-muscle invasive low grade, high grade, presence of Cis, or stages II–IV), and treatment in the model. Treatment was coded as transurethral resection, +/− immunotherapy, chemotherapy, radiotherapy, or cystectomy for non-muscle invasive patients; or as cystectomy, chemotherapy, or other for muscle-invasive patients). P values represent two-sided statistical tests.
Results
This analysis addressed the hypothesis that lower levels of miRNA-34a expression within neoplastic cells increased risk of bladder cancer recurrence. MiR-34a levels did not differ significantly by characteristics including gender, age, smoking status, tumor size, or multiplicity (Table 1). As this is a population-based cohort, the majority of our cases are non-muscle invasive (stage 0,I) at diagnosis. Most (95%) of the specimens with high miR-34a levels were non-muscle invasive tumors (P=0.003). Medical data subsequent to diagnosis were reviewed to obtain longitudinal follow-up information on 86% of the cases, with an average duration of follow-up of 3.8 years and 75% of the cases (292 out of 389) were followed >7.0 years. Half of the cases experienced recurrences, with 4% (n=13) representing progression to a higher stage lesion. Characteristics of the n=229 urothelial carcinoma patients with tissue available for in situ hybridization were very similar to the overall study population (Supplemental Table 2), although there were more large-sized lesions (≥ 3cm 54% vs. 40%).
Table 1.
Characteristics of urothelial carcinoma patients by miR-34a.
| miR-34a level
|
||||||
|---|---|---|---|---|---|---|
| Low (0–1+) | High (2–3+) | |||||
| N | % | N | % | P-value | ||
| Gender | male | 127 | 77% | 44 | 70% | 0.30 |
| female | 39 | 23% | 19 | 30% | ||
| total | 166 | 63 | ||||
| Age | <40 | 1 | 1% | 1 | 2% | 0.39 |
| 40–49 | 13 | 8% | 5 | 8% | ||
| 50–59 | 38 | 23% | 9 | 14% | ||
| 60–69 | 45 | 27% | 24 | 38% | ||
| 70+ | 69 | 42% | 24 | 38% | ||
| Smoking | never | 28 | 17% | 7 | 11% | 0.56 |
| former | 85 | 52% | 34 | 55% | ||
| current | 51 | 31% | 21 | 34% | ||
| Tumor size | <3 cm | 74 | 45% | 30 | 48% | 0.52 |
| 3 cm+ | 90 | 54% | 30 | 48% | ||
| Multiplicity | single | 121 | 73% | 46 | 73% | 0.99 |
| multiple | 42 | 25% | 16 | 25% | ||
| Stage | 0,I | 131 | 79% | 60 | 95% | 0.003 |
| II–IV | 35 | 21% | 3 | 5% | ||
Missing data: smoking n=3, size n=5, multiplicity n=4.
We assessed the level and cell type distribution of miR-34a in bladder tumor tissue sections by multi-color in situ hybridization (ISH) assay. Representative cases in Figure 1 show miR-34a expression within the urothelial carcinoma cells of these Ta low grade tumors. MiR-34a levels were scored from low to high (0, 1+,2+, 3+, as represented by Figure 1 panels a – d). Analysis of scored miR-34a levels exclusively within urothelial carcinoma cells across all 229 tumors indicates that a smaller proportion of the muscle invasive tumors have high levels of miR-34a, compared to non-muscle invasive (P=0.006) (Figure 2a). We observed a trend towards higher miR-34a levels among the papillary lesions, particularly the PUN-LMP and low grade papillary lesions (Pap.Ca-LG), but could not rule out the possibility of chance (Figure 2b). MiR-34a levels did not differ by P53 protein staining level in the tumor tissue, although among the muscle invasive tumors fewer negative scores were observed in p53 low vs. high tumors (Figure 2c).
Figure 2.
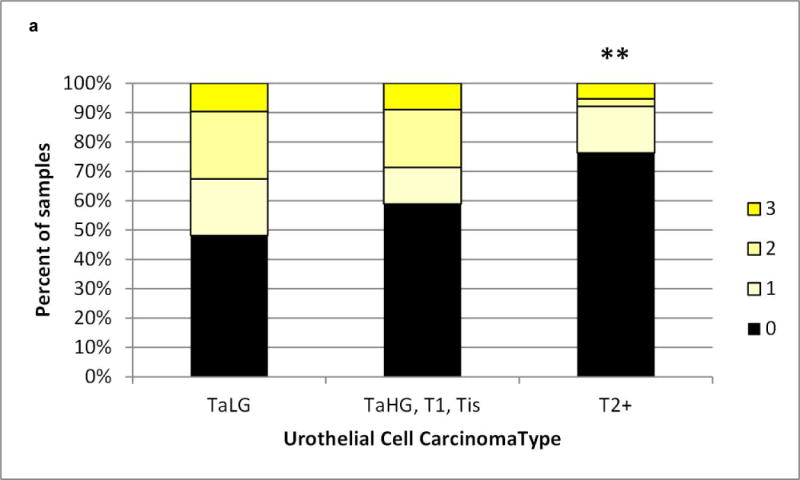
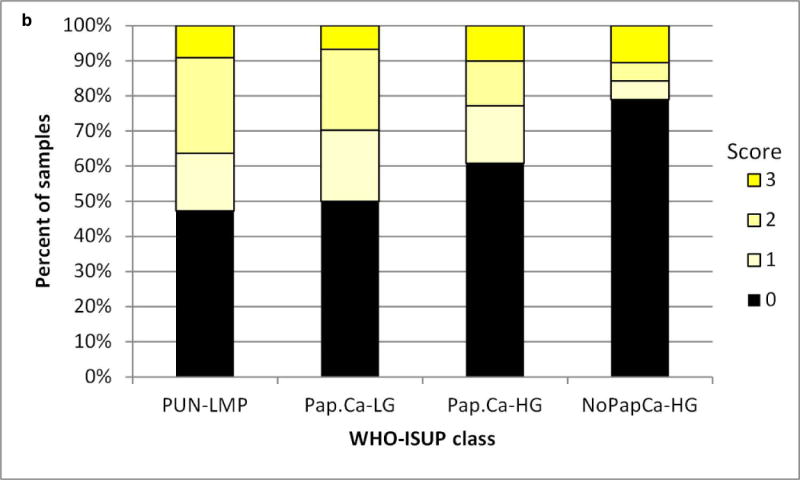
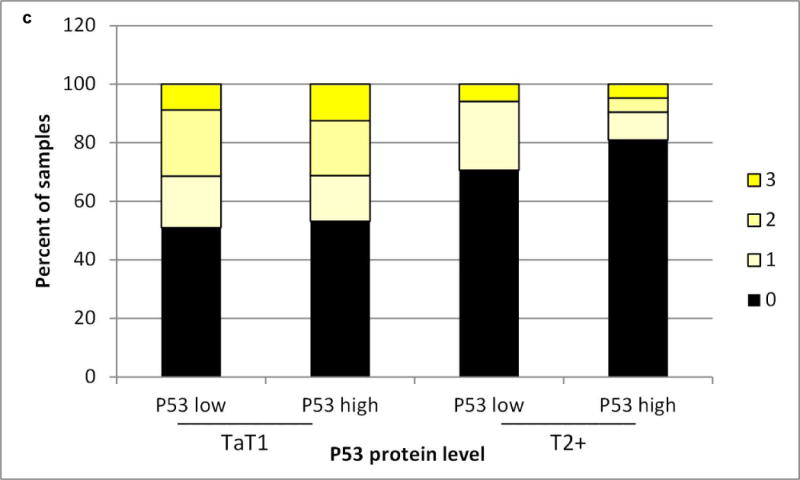
a) Urothelial carcinomas that are non-muscle invasive (Ta low grade (TaLG) or Ta high grade (TaHG)/T1/Tis tumors have a greater frequency of high miR-34a levels than muscle invasive tumors (T2+), chi-square P=0.008. b) Papillary urothelial carcinomas trend towards a greater frequency of high miR-34a levels than non-papillary tumors. c) MiR-34a levels do not differ by p53 protein level, assessed by immunohistochemical staining, either in non-muscle invasive (TaT1) or muscle invasive (T2+) tumors.
We assessed the prognostic significance of miR-34a using Kaplan-Meier plots. Among non-muscle invasive tumors, median time to first recurrence was longer (2.84 years) for those with high miR-34a (scores 2–3+), compared to 1.15 years for those with low miR-34a levels (scores 0–1+) in the cancer-cell compartment (log-rank P-value 0.04) (Figure 3). Similar results were obtained when analyses were restricted to the Ta low grade (log-rank P-value 0.03), or the TaHG/T1/Tis subsets. Score-specific analysis showed that patients with an miR-34a intensity score of 3+ had the longest time to recurrence (positive predictive value for no recurrence 0.75), while those negative for miR-34a (score=0) had the shortest time (negative predictive value for recurrence 0.68) (Supplementary Figure 1). In the multivariate Cox regression models, lower risk of recurrence was associated with having high miR-34a levels in the baseline non-muscle invasive tumor (n=63) compared to low miR-34a levels (n=166), with a hazard ratio (HR) 0.57 95%CI 0.34–0.93, adjusted for age, gender, smoking, size, multiplicity, stage, grade, and treatment. We did not observe differences in time to progression (log-rank P-value 0.34) or survival (log-rank P-values 0.49) of patients with non-muscle invasive disease by miR-34a status, based on 9 and 35 events respectively.
Figure 3.
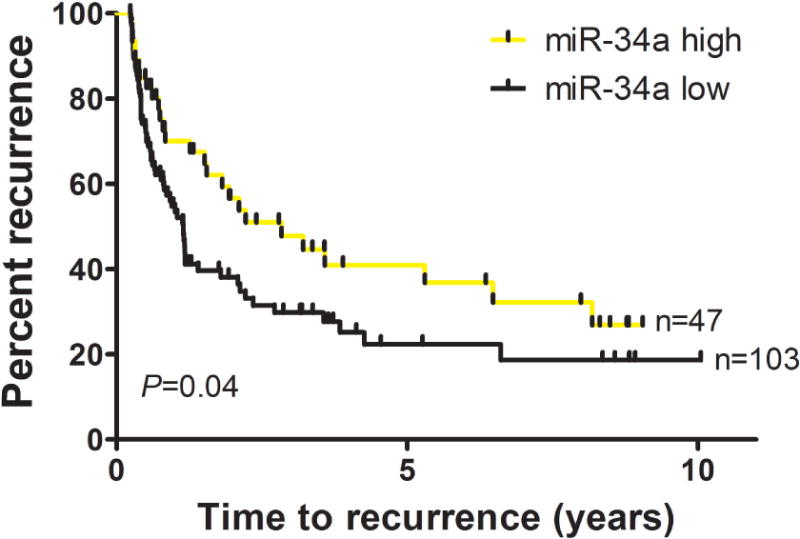
Individuals with high miR-34a levels in their non-muscle invasive baseline tumors experience longer time to recurrence. Median time to first recurrence was 2.84 years for those with high miR-34a, scores 2–3, compared to 1.15 years for those with low miR-34a, scores 0–1, (Log-rank P=0.04).
We went on to perform functional assays in cells derived from the bladder urothelium. Specifically, we assessed whether modulation of miR-34a expression in a controlled experimental setting could modify the behavior of cells in tumorigenic assays using cultured bladder epithelial cell lines. Cultured urothelial carcinoma cells with more miR-34a had a reduced propensity invade through a matrigel plug, compared to those with lower levels (Figure 4a). Likewise, the number of urothelial carcinoma colonies able to grow in soft-agar was significantly reduced in cells with more miR-34a (Figure 4b).
Figure 4.
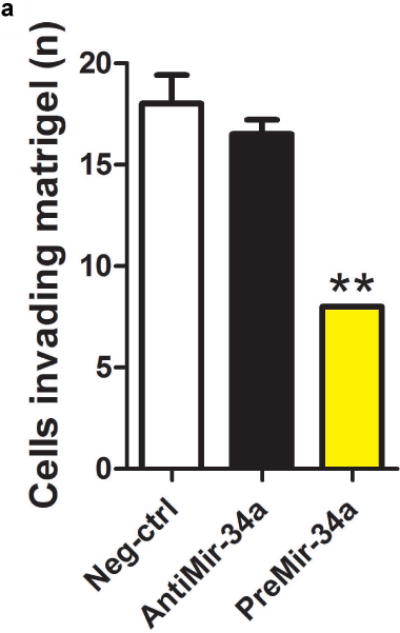
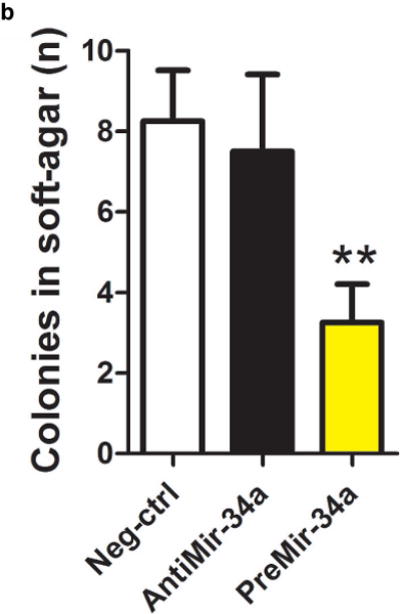
Functional assays of cultured urothelial cells support a tumor suppressive role for miR-34a. Cultured urothelial cells were transfected with negative control, antimiR-34a or preMir-34a constructs. Raising miR-34a levels using preMir-34a significantly reduced both the number of a) cells invading matrigel and b) colonies able to grow in soft agar.
The downstream mRNA level changes occurring with miR-34a loss (anti-miR-34a, low level) were assessed experimentally by comparing to the expression of genes in cultured urothelial cells with high miR-34a levels (control, high level). A heat map illustrates mRNA levels for the 133 genes with the most dramatic expression differences (Supplementary Figure 2). 45 mRNAs were expressed at higher levels in cells with more miR-34a, while 88 mRNAs were down-regulated in cells with more miR-34a (listed in Supplementary Table 2). Of the subset of differentially expressed genes that met our expression ratio and P-value thresholds, analysis overlaying our gene expression data and in silico, sequence predicted target genes revealed S100P as a novel sequence-predicted miR-34a target gene. Translating this observation from cell culture into human tissue, we observed that S100P levels were also inversely related to miR-34a levels (Figure 5). GAPDH-normalized S100P levels were 4.6-fold higher in the bladder tumor specimens vs. histologically normal tissue, while U6-normalized miR-34a levels in primary or recurrent tumor specimens were approximately one-third those of the normal tissue.
Figure 5.
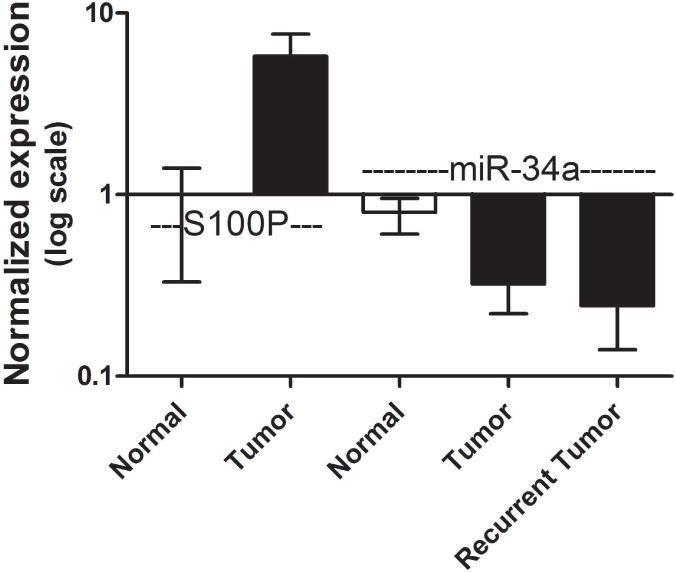
Expression level of S100P shows opposing expression to miR-34a in tumor tissue. Tissue S100P mRNA or miR-34a levels were assessed by RT-PCR with normalization to GAPDH and U6-snRNA, respectively. Relative to histologically normal adjacent urothelial tissue, primary tumor tissue samples (n=5), had low miR-34a levels, but 4-fold higher levels of S100 calcium binding protein P (S100P). Recurrent tumor tissue samples (n=12) also had low miR-34a levels.
Discussion
We studied the prognostic value of altered miR-34a expression in newly diagnosed urothelial carcinomas. We observed a lower risk of recurrence for non-muscle invasive bladder cancer patients with high miR-34a levels within the cancer cell compartment of their baseline primary tumors. To our knowledge, miR-34a in baseline primary tumor tissue has not been previously evaluated in relation to metachronous bladder cancer recurrence or its expression characterized at single-cell resolution in complex bladder cancer lesions. Tumor vs. normal comparisons consistently show altered levels of miR-34a in bladder cancer samples. For example, miR-34a levels were 3-fold higher in whole normal bladder tissues compared to that of either non-muscle invasive (P=0.0023), or muscle invasive bladder tumor tissues (P=0.0070) (n=7 per group).32 Array analysis of urothelial tissue revealed 25- fold higher miR-34a levels in n=10 non-diseased controls compared to the histologically normal urothelium distant from the site of the tumor in n=10 UCC patients (P=0.013).23 This observation in normal urothelial tissue, along with our determination of altered miR-34 expression within cancer cells of urothelial origin, suggests a potential for miR-34a level monitoring as part of tumor surveillance procedures.
Our functional assays in non-muscle invasive urothelial carcinoma cell lines demonstrated that high miR-34a levels decreased the cell’s ability to grow in soft agar and lowered the invasive capacity. Similarly, transfection of muscle invasive cell lines with pre-miR-34a significantly decreased their clonogenic potential and increased the percentage of senescent and apoptotic cells in the cultures.43 We did not observe a significant phenotype change with anti-miR-34a treatment in these urothelial carcinoma cells, as the levels of miR-34a are already low.
The mechanisms underlying miR-34a dysregulation in cancer are being elucidated. In other cell types, the downregulation of miR-34a was not correlated with deletion of the chromosomal region encoding miR-34a (1p36), nor with P53 mutation.25 Although P53 is capable of modulating miR-34a levels in other contexts,27 we did not observe a statistically significant correlation between miR-34a and P53 protein levels in urothelial cell tumors. It has become apparent that there are multiple other P53-independent pathways and mechanisms controlling miR-34a, which acts as a universal tumor suppressor.28 MiR-34a is upregulated by the transcription factor ELK1.28 MiR-34a is silenced by methylation of CpGs in the promoter in several types of cancer and methylation was observed in two of six bladder cell lines (33%).25 Likewise, five out of the seven (71%) muscle invasive urothelial cell tumor tissue samples tested in a previous study had methylated miR-34a CpGs.44
MiR-34a’s tumor suppressive activity likely involves regulation of mRNAs. Recent work in invasive bladder tumor cell lines suggests that mir-34a expression can inhibit cell migration and invasion by antagonizing Notch1 signaling.26 Our study focused on miR-34a in non-muscle invasive bladder tumors. Anti-miR-34a treatment of normal urothelial cells to simulate loss of miR-34 revealed a variety of downstream differences in mRNA expression (Supplementary Figure 1). Our gene expression data demonstrated an elevation in levels of the in silico sequence predicted miR-34a target gene S100P when cultured urothelial cells loose miR-34a due to anti-miR-34a treatment. We reaffirmed this opposing relationship in bladder tissue with the observation that tumors showing loss of miR-34a also show 4-fold higher expression of S100P compared to normal tissue. Of the n=300 urothelial carcinomas assessed previously by immunohistochemistry, 22% stained negatively for S100P protein, 7% were equivalent, 16% weak, and 62% strong, demonstrating frequent overexpression with a range of levels.45 S100P positivity was also previously associated with 7-fold shorter breast cancer survival, poor clinical outcomes for gastric cancer patients, and is a marker of aggressive, hormone-resistant and metastatic prostate cancer.46 Mechanistic studies suggest that S100P promotes tumor cell motility and transendothelial migration, in part by activating the cell-matrix attachment mediator ezrin (reviewed in46), and by binding to the cell surface receptor for advanced glycation end products (RAGE).47 Recent work has demonstrated that reducing S100P overexpression by siRNA treatment of endothelial cancer cells blocked cell proliferation, nuclear beta-catenin protein level, cyclin D1 and c-myc mRNA.48 Our data suggest down-regulation of miR-34a could be related to the strong S100P levels observed in some urothelial tumors and support potential benefits of further clinical testing for miR-34a restoration therapies.
MiR-34a is a front-runner of miRNA-based restoration therapy.19, 49 Forced expression of miR-34 interferes with growth a several cancer cell lines and can prevent or reduce progression in mouse models of lung cancer and hepatocarcinoma.50 Phase I clinical trial using a miR-34a mimic drug called MRX34 has been initiated for patients with hepatocarcinoma or liver metastasis to test safety of miR-34 restoration therapy.49, 51 The bladder is a very accessible organ and thus would be well suited for implementation of miR-34 restoration therapy in near future, if supported by on-going clinical trials.
Limitations of our study include few events available for the progression and survival analyses. The proportion of larger lesions was slightly higher among the in situ hybridized samples compared to the overall population, however the results remained significant in the multivariate models adjusted for tumor size.
Our findings have potential utility in refining the clinical management of non-muscle invasive bladder cancers. Our results encourage validation of miR-34a as a biomarker for guiding optimal bladder tumor recurrence screening intervals. Our data also suggest miR-34a as a candidate for future testing of miRNA restoration therapies that could improve patient outcomes.
Supplementary Material
Supplementary Figure 1. Time to recurrence plotted by in situ hybridization miR-34a intensity score (0, 1+, 2+, 3+). The yellow line shows the most intense miR-34a level (score 3+) associated with a longer time to first recurrence (log rank P=0.03).
Supplementary Figure 2. Genes differentially expressed by miR-34 level in cultured urothelial cells. The mean centered log2 intensity is plotted with higher expression levels shown in gold and lower expression are shown in blue tones.
Novelty and Impact.
An estimated 500,000 patients in the US population have a history of urothelial carcinoma. The high rate of disease recurrence is a major challenge in patient management. High levels of tumor suppressive miR-34a were associated with a lower risk of tumor recurrence. The value of miR-34a expression as a prognostic marker to guide surveillance intervals and the promise for miR-34a-based restoration therapy should be evaluated in clinical studies.
Acknowledgments
This publication was funded in part by grant numbers K07CA102327, CA182659, LM009012, GM103506 and GM103534, RR024475, RR028309, CA102327, CA121382, CA141017, CA099500, CA82354, CA078609, ES00002, 5P42ES05947, RR018787, and ES07373 from the National Cancer Institute, NIH, from the National Institute of Environmental Health Sciences, NIH, the National Center for Research Resources, NIH, and the National Institute of General Medical Sciences, NIH; by the Jay and Betty Van Andel Foundation (LFS); and by the Hitchcock Foundation (LFS). The New Hampshire State Cancer Registry is supported by the Centers for Disease Control and Prevention’s National Program of Cancer Registries through cooperative agreement U58/DP000798 awarded to the New Hampshire Department of Health and Human Services, Division of Public Health Services, Bureau of Public Health Statistics & Informatics, Health Statistics and Data Management Section.
Abbreviations
- HR
adjusted hazard ratio
- miR-34a
microRNA-34a
- ISH
in situ hybridization
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalgo ML, Jones PA. Rapid quantitation of methylation differences at specific sites using methylation-sensitive single nucleotide primer extension (Ms-SNuPE) 1997;25:2529–31. doi: 10.1093/nar/25.12.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montie J, Shipley W. Bladder Cancer Including Upper Tract Tumors and Transitional Cell Carcinoma of the Prostate. In: National Comprehensive Cancer Network, editor. National Comprehensive Cancer Network Practice Guidelines in Oncology. 2004. [Google Scholar]
- 4.Silverman DT, Morrison AS, Devesa SS. Bladder Cancer. In: Schottenfeld DFJ, editor. Cancer Epidemiology and Prevention. 2. New York: Oxford University Press; 1996. pp. 1156–79. [Google Scholar]
- 5.Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, Sesterhenn IA, Tachibana M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 7.Brown LM, Zahm SH, Hoover RN, Fraumeni JF., Jr High bladder cancer mortality in rural New England (United States): an etiologic study. 1995;6:361–8. doi: 10.1007/BF00051412. [DOI] [PubMed] [Google Scholar]
- 8.Michaud DS, Clinton SK, Rimm EB, Willett WC, Giovannucci E. Risk of bladder cancer by geographic region in a U.S. cohort of male health professionals. Epidemiology. 2001;12:719–26. doi: 10.1097/00001648-200111000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Carroll P, Raghavan D, Stein J, Zietman A. The Treatment of Bladder Cancer- Stage by Stage. 2001 AUA Annual Meeting. 2001 [Google Scholar]
- 11.Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, Malats N. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urol. 2007;25:285–95. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 12.Petrovich Z, Baert L, Boyd SD, Brady LW, D’Hallewin M, Heilmann HP, Jakse G, Jones PA, Van Der Meijden AP, Oyen RH, Van Poppel H, Rotman M, et al. Management of carcinoma of the bladder. Am J Clin Oncol. 1998;21:217–22. doi: 10.1097/00000421-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Honma I, Masumori N, Sato E, Takayanagi A, Takahashi A, Itoh N, Tamagawa M, Sato MA, Tsukamoto T. Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology. 2004;64:744–8. doi: 10.1016/j.urology.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 15.Strope SA, Montie JE. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J Urol. 2008;180:31–7. doi: 10.1016/j.juro.2008.03.045. ; discussion 7. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz-Drager BJ. Identifying risk factors in patients with non-muscle-invasive bladder cancer: clinical implications. Eur Urol. 2011;60:721–3. doi: 10.1016/j.eururo.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 17.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–87. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trang P, Weidhaas JB, Slack FJ. MicroRNAs as potential cancer therapeutics. Oncogene. 2008;27(Suppl 2):S52–7. doi: 10.1038/onc.2009.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009;136:586–91. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sempere LF, Kauppinen S. Translational Implications of MicroRNAs in Clinical Diagnostics and Therapeutics. In: Bradshaw RA, Dennis EA, editors. Handbook of Cell Signaling. Oxford: Academic Press; 2009. pp. 2965–81. [Google Scholar]
- 22.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clinical pharmacology and therapeutics. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 23.Catto JW, Miah S, Owen HC, Bryant H, Myers K, Dudziec E, Larre S, Milo M, Rehman I, Rosario DJ, Di Martino E, Knowles MA, et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res. 2009;69:8472–81. doi: 10.1158/0008-5472.CAN-09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veerla S, Lindgren D, Kvist A, Frigyesi A, Staaf J, Persson H, Liedberg F, Chebil G, Gudjonsson S, Borg A, Mansson W, Rovira C, et al. MiRNA expression in urothelial carcinomas: important roles of miR-10a, miR-222, miR-125b, miR-7 and miR-452 for tumor stage and metastasis, and frequent homozygous losses of miR-31. Int J Cancer. 2009;124:2236–42. doi: 10.1002/ijc.24183. [DOI] [PubMed] [Google Scholar]
- 25.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Yao Z, Zhu M, Ma X, Shi T, Li H, Wang B, Ouyang J, Zhang X. Inhibitory effects of microRNA-34a on cell migration and invasion of invasive urothelial bladder carcinoma by targeting notch1. J Huazhong Univ Sci Technolog Med Sci. 2012;32:375–82. doi: 10.1007/s11596-012-0065-z. [DOI] [PubMed] [Google Scholar]
- 27.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–4. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, Klausen M, Pilpel Y, Nielsen FC, Oren M, Lund AH. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ. 2010;17:236–45. doi: 10.1038/cdd.2009.109. [DOI] [PubMed] [Google Scholar]
- 29.Lal A, Thomas MP, Altschuler G, Navarro F, O’Day E, Li XL, Concepcion C, Han YC, Thiery J, Rajani DK, Deutsch A, Hofmann O, et al. Capture of microRNA-bound mRNAs identifies the tumor suppressor miR-34a as a regulator of growth factor signaling. PLoS Genet. 2011;7:e1002363. doi: 10.1371/journal.pgen.1002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Lammers P, Torrance CJ, Bader AG. TP53-independent function of miR-34a via HDAC1 and p21(CIP1/WAF1) Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:1678–86. doi: 10.1038/mt.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyrskjot L, Ostenfeld MS, Bramsen JB, Silahtaroglu AN, Lamy P, Ramanathan R, Fristrup N, Jensen JL, Andersen CL, Zieger K, Kauppinen S, Ulhoi BP, et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–60. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- 32.Pignot G, Cizeron-Clairac G, Vacher S, Susini A, Tozlu S, Vieillefond A, Zerbib M, Lidereau R, Debre B, Amsellem-Ouazana D, Bieche I. Microrna expression profile in a large series of bladder tumors: Identification of a 3-mirna signature associated with aggressiveness of muscle-invasive bladder cancer. Int J Cancer. 2012 doi: 10.1002/ijc.27949. [DOI] [PubMed] [Google Scholar]
- 33.Marsit CJ, Houseman EA, Christensen BC, Gagne L, Wrensch MR, Nelson HH, Wiemels J, Zheng S, Wiencke JK, Andrew AS, Schned AR, Karagas MR, et al. Identification of methylated genes associated with aggressive bladder cancer. PLoS One. 2010;5:e12334. doi: 10.1371/journal.pone.0012334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schned AR, Andrew AS, Marsit CJ, Kelsey KT, Zens MS, Karagas MR. Histological classification and stage of newly diagnosed bladder cancer in a population-based study from the Northeastern United States. Scand J Urol Nephrol. 2008;42:237–42. doi: 10.1080/00365590801948166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sempere LF, Preis M, Yezefski T, Ouyang H, Suriawinata AA, Silahtaroglu A, Conejo-Garcia JR, Kauppinen S, Wells W, Korc M. Fluorescence-based codetection with protein markers reveals distinct cellular compartments for altered MicroRNA expression in solid tumors. Clin Cancer Res. 2010;16:4246–55. doi: 10.1158/1078-0432.CCR-10-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sens DA, Park S, Gurel V, Sens MA, Garrett SH, Somji S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol Sci. 2004;79:56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
- 37.Sempere LF. Fully automated fluorescence-based 4-color multiplex assay for co-detection of microRNA and protein biomarkers in clinical tissue specimens. In: Nielsen BS, editor. In situ Hybridization, Methods in Molecular Medicine. Springer; Science & Business Media, LLC; in press. [DOI] [PubMed] [Google Scholar]
- 38.Neely LA, Rieger-Christ KM, Neto BS, Eroshkin A, Garver J, Patel S, Phung NA, McLaughlin S, Libertino JA, Whitney D, Summerhayes IC. A microRNA expression ratio defining the invasive phenotype in bladder tumors. Urol Oncol. 2010;28:39–48. doi: 10.1016/j.urolonc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Sempere LF, Ouyang H, Memoli VA, Andrew AS, Luo Y, Demidenko E, Korc M, Shi W, Preis M, Dragnev KH, Li H, et al. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest. 2010;120:1298–309. doi: 10.1172/JCI39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiyomaru T, Enokida H, Tatarano S, Kawahara K, Uchida Y, Nishiyama K, Fujimura L, Kikkawa N, Seki N, Nakagawa M. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102:883–91. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS One. 2011;6:e21210. doi: 10.1371/journal.pone.0021210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamura S, Saini S, Majid S, Hirata H, Ueno K, Deng G, Dahiya R. MicroRNA-34a Modulates c-Myc Transcriptional Complexes to Suppress Malignancy in Human Prostate Cancer Cells. PLoS One. 2012;7:e29722. doi: 10.1371/journal.pone.0029722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinall RL, Ripoll AZ, Wang S, Pan CX, Devere White RW. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of p53-Rb pathway status. Int J Cancer. 2011 doi: 10.1002/ijc.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogt M, Munding J, Gruner M, Liffers ST, Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A, Hermeking H. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–22. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JP, Kaygusuz G, Wang L, Montgomery K, Mason V, Zhu SX, Marinelli RJ, Presti JC, Jr, van de Rijn M, Brooks JD. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31:673–80. doi: 10.1097/01.pas.0000213438.01278.5f. [DOI] [PubMed] [Google Scholar]
- 46.Tothova V, Gibadulinova A. S100P, a peculiar member of S100 family of calcium-binding proteins implicated in cancer. Acta Virol. 2013;57:238–46. doi: 10.4149/av_2013_02_238. [DOI] [PubMed] [Google Scholar]
- 47.Penumutchu SR, Chou RH, Yu C. Structural insights into calcium-bound S100P and the V domain of the RAGE complex. PLoS One. 2014;9:e103947. doi: 10.1371/journal.pone.0103947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo L, Chen S, Jiang H, Huang J, Jin W, Yao S. The expression of S100P increases and promotes cellular proliferation by increasing nuclear translocation of beta-catenin in endometrial cancer. Int J Clin Exp Pathol. 2014;7:2102–12. [PMC free article] [PubMed] [Google Scholar]
- 49.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–65. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bader AG. miR-34 – a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirna Therapeutics I. A PhaseI Study of MRX34 Given Intravenously in Patients With Unresectable Primary Liver Cancer or Metastatic Cancer With Liver Involvement. 2013;2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Time to recurrence plotted by in situ hybridization miR-34a intensity score (0, 1+, 2+, 3+). The yellow line shows the most intense miR-34a level (score 3+) associated with a longer time to first recurrence (log rank P=0.03).
Supplementary Figure 2. Genes differentially expressed by miR-34 level in cultured urothelial cells. The mean centered log2 intensity is plotted with higher expression levels shown in gold and lower expression are shown in blue tones.


