Abstract
Widespread prostate-specific antigen (PSA) screening detects many cancers that would have otherwise gone undiagnosed. To estimate the prevalence of unsuspected prostate cancer, we reviewed 19 studies of prostate cancer discovered at autopsy among 6024 men. Among men aged 70-79, tumor was found in 36% of Caucasians and 51% of African-Americans. This enormous prevalence, coupled with the high sensitivity of PSA screening, has led to the marked increase in the apparent incidence of prostate cancer. The impact of PSA screening on clinical practice is well-recognized, but its effect on epidemiologic research is less appreciated. Before screening, a larger proportion of incident prostate cancers had lethal potential and were diagnosed at advanced stage. However, in the PSA era, overall incident prostate cancer mainly is indolent disease, and often reflects the propensity to be screened and biopsied. Studies must therefore focus on cancers with lethal potential, and include long follow-up to accommodate the lead time induced by screening. Moreover, risk factor patterns differ markedly for potentially lethal and indolent disease, suggesting separate etiologies and distinct disease entities. Studies of total incident or indolent prostate cancer are of limited clinical utility, and the main focus of research should be on prostate cancers of lethal potential.
Keywords: Prostate cancer, autopsy, PSA-screening, epidemiology, lethal prostate cancer
Introduction
Prostate-specific antigen (PSA) screening has led to a dramatic increase in the apparent incidence of prostate cancer and drastically changed its epidemiologic characterization. By 2010, over half of U.S. men over age 40 were screened in the previous two years1. The high sensitivity of PSA screening, and the long lead time of approximately eleven years2-5 has led to widespread overdiagnosis and overtreatment6 of prostate cancer and complicated large-scale screening trials, leading some to advocate that routine screening be abandoned7-9.
These far-reaching effects of PSA screening are a direct consequence of the large prevalence of undiagnosed prostate cancer. In Part I of this review, we present data estimating the prevalence of asymptomatic prostate cancer. The high prevalence of prostate cancer at autopsy is often cited to indicate widespread presence of clinically insignificant disease7. However, no previous study has provided a systematic analysis. By combining data from 19 studies, encompassing over 6,000 men, we could define the age-related prevalence with much greater precision than previous studies, the largest of which only included 1,185 men10. In Part II, we discuss the implications of the high prevalence of indolent prostate cancer for observational studies and clinical trials. We show that risk factor patterns for potentially lethal prostate cancer diverge sharply from those for indolent disease, suggesting different etiologies and distinct entities. Studies of overall incident prostate cancer mainly reflect indolent disease; future research should instead focus on clinically relevant tumors of lethal potential.
Part I: Prevalence of asymptomatic prostate cancer at autopsy and random biopsy
Search strategy and selection criteria
To identify studies of prostate cancer found at autopsy, we searched Medline (PubMed) using the following search terms: ‘latent prostate cancer’, ‘prostate cancer autopsy’ in addition to manual searches of the bibliographies. We found 40 studies published in English between 1935 and January 2014; all but two before widespread PSA screening became common (1990). In the 19 included studies, prostates were fully removed and fixed, and entirely step-sectioned in 3-5mm slices11. We excluded studies that included men with a clinical diagnosis of prostate cancer or did not stratify by age. When age categories in an individual study did not line up exactly, we assigned those data to the upper age range. For example, the data from age category 45-54 was grouped in our 50-59 age category. A total of 6024 prostates from 19 studies were included11-30. We calculated the age-specific prevalence by dividing the total number of cases within an age category by the total number of prostates examined within each age category across all studies.
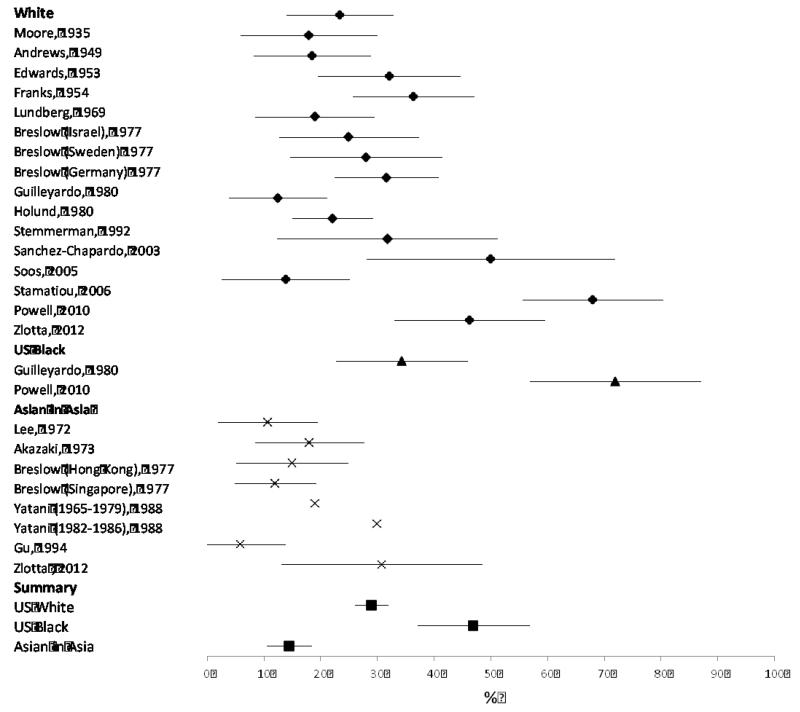
Table 1 provides the details of each study included in this analysis. Ten were from the US, and 17 countries were represented. We observed a marked age-related increase in the prevalence of incidental prostate cancer discovered at autopsy (Table 2), with a prevalence of 47.3% among US White and European men aged 80+. These findings are provided in graphical form in Figures 1-3. These data underestimate the true cumulative incidence of total prostate cancer because they do not include clinically diagnosed cases. The number of diagnosed cancers with low-risk features, though substantial, is therefore small compared to the millions of undiagnosed cases. Projected to the current age and racial distribution, these data suggest roughly 45 million cases of potentially detectable prostate cancer in the US. By contrast, an estimated 2.9 million US men are living with a prostate cancer diagnosis31.
Table 1. Included autopsy publications.
| Author, publication year | Collection dates | Number of prostates examined |
Population location |
|---|---|---|---|
| US White and European | |||
| Moore, 1935 (8) | 1931-1932 | 304 | Austria |
| Andrews, 1949 (9) | 142 | United Kingdom | |
| Edwards, 1953 (10) | 1942-1945 | 173 | Canada |
| Franks, 1954 (11) | 220 | U.S. | |
| Halpert*, 1965 (12) | 70 | U.S. veterans | |
| Lundberg, 1969 (13) | 1967 | 292 | Sweden |
| Breslow*, 1977 (14) | 594 | Israel, Sweden, Germany |
|
| Guileyardo*, 1980 (15) | 293 | U.S. | |
| Holund, 1980 (16) | 1971-1977 | 223 | Denmark |
| Stemmermann, 1992 (17) | 1970-1990 | 293 | U.S. men of Japanese descent |
| Sakr*, 1993 (18) | 54 | U.S. | |
| Sanchez-Chapado, 2003 (19) | 146 | Spain | |
| Soos, 2005 (20) | 139 | Hungary | |
| Stamatiou, 2006 (21) | 2002-2004 | 212 | Greece |
| Powell*, 2010 (22) | 1993-2004 | 426 | U.S. |
| Zlotta*, 2013 (23) | 2008-2011 | 220 | Russia |
| US Black | |||
| Halpert*, 1965 (12) | 30 | U.S. veterans | |
| Guileyardo*, 1980 (15) | 207 | U.S. | |
| Sakr*, 1993 (18) | 98 | U.S. | |
| Powell*, 2010 (22) | 1993-2004 | 630 | U.S. |
| Asian | |||
| Lee, 1972 (24) | 156 | Singapore | |
| Akazaki, 1973 (25) | 1969-1972 | 239 | Native Japanese in Japan |
| Breslow*, 1977 (14) | 415 | Hong Kong, Singapore |
|
| Yatani, 1988 | 1965-1979 1982-1986 |
576 660 |
Japan |
| Gu, 1994 (26) | 1989-1992 | 350 | China |
| Zlotta*, 2013 (23) | 2008-2011 | 100 | Japan |
Study includes more than one race. Collection dates were not reported in all studies.
Table 2.
Prostate cancer diagnosed at autopsy by race: Comprehensive summary of all 19 published studies, including 6024 men. Values rounded from available data
| 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | >80 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| N | PC (%) |
N | PC (%) |
N | PC (%) |
N | PC (%) |
N | PC (%) |
N | PC (%) |
N | PC (%) |
N | PC (%) |
|
| US White and European (17 groups) |
109 | 4 (3.7) |
245 | 38 (15.5) |
505 | 117 (23.2) |
625 | 138 (22.1) |
914 | 265 (29.0) |
962 | 343 (35.7) |
439 | 208 (47.4) |
379 9 |
1113 (29.3) |
| US Black (3 groups) |
194 | 13 (6.7) |
168 | 51 (30.4) |
291 | 103 (35.4) |
111 | 51 (45.9) |
98 | 46 (46.9) |
103 | 52 (50.5) |
965 | 316 (32.7) |
||
| Asian (6 groups) |
112 | 2 (1.8) | 111 | 1 (0.9) | 72 | 2 (2.8) | 240 | 19 (7.9) |
296 | 43 (14.5) |
277 | 59 (21.3) |
152 | 44 (28.9) |
126 0 |
170 (13.5) |
Figure 1.
Prostate cancer diagnosed at autopsy by race: comprehensive summary of all 19 published studies, including 6024 men.
Figure 3. Forest plot of autopsy studies of undiagnosed prostate cancer among men age 70-79.
We observed pronounced ethnic differences: in men 70-79, the prevalence was 50·5% in U.S. Blacks, 35·7% of whites, and 21·2% in Asian autopsies. These trends parallel observed incidence rates by ethnicity32, suggesting that ethnic discrepancies in prostate cancer incidence are not solely due to different PSA screening patterns.
These studies of prostate cancer at autopsy were published over the span of 71 years, but we found no strong trends for changes in prevalence over calendar time. The findings are limited by sparse data, particularly among US Black men, but the estimates are generally consistent within racial groups. These findings do not support the adage33 that the prevalence of prostate cancer is equal to the decade of age, e.g. 70% among those aged 70-79. Nonetheless, based on autopsy findings and diagnosed low-risk tumors we estimate that among 70-79 year olds, more than one-third of Caucasian men and half of African American men have indolent prostate cancer that would not cause harm if undiagnosed and untreated.
The prevalence of undiagnosed prostate cancer at autopsy is consistent with the high prevalence found upon routine (i.e., not screen-directed) prostate biopsy, as illustrated by findings from the 7-year randomized trial of Finasteride among men (median age 63.2) with a normal digital rectal exam and PSA ≤ 3·0 ng/ml. At the end of the trial, all men who had not been diagnosed with prostate cancer were offered an end-of-trial biopsy. This was performed in 3820 men in the placebo arm, which revealed 576 cases (15·1%) of unsuspected prostate cancer34. Overall, 24% of the men in the placebo arm were diagnosed with prostate cancer by per-protocol end of trial biopsy or biopsy as indicated by PSA-screening. In comparison, we observed that 22% of men 50-59 years old and 29% of men 60-69 years old had undetected prostate cancer on autopsy. The prevalence in that trial likely underestimates the true prevalence of indolent prostate cancer in that age group due to the low PSA requirement for study selection, the exclusion of men diagnosed with prostate cancer during the trial and the imperfect nature of non-targeted sextant biopsy sampling.
The high prevalence of asymptomatic and unsuspected prostate cancer, as demonstrated by these autopsy and biopsy studies, underlies the potential for widespread diagnosis of cases of prostate cancer that would have caused no clinical harm had they remained undetected. The overdetection of prostate cancer has obvious adverse clinical consequences, since most treated men experience no direct clinical benefit from treatment35. It has been estimated that 42-66% of diagnosed prostate cancers would have caused no clinical harm had they remained undetected2.
Part II: Implications of PSA screening for population research
Distinct Etiologies of Lethal and Indolent Prostate Cancer
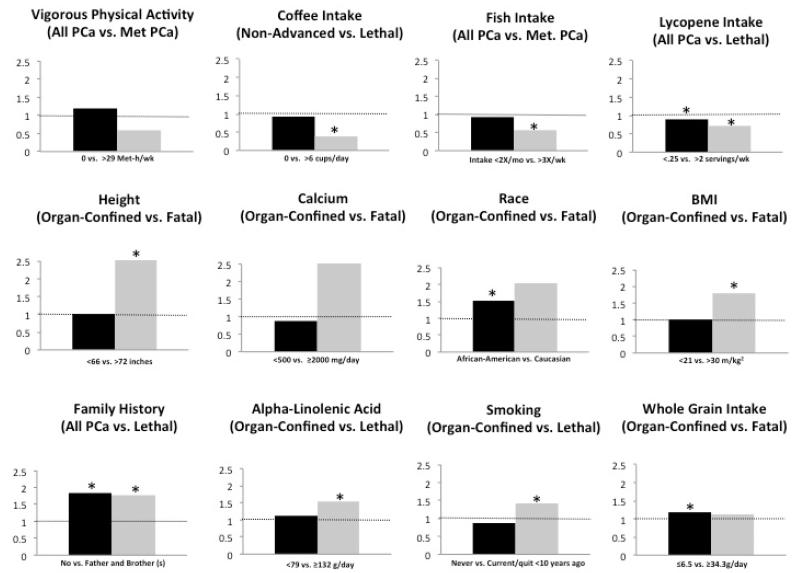
A major question is whether the apparently indolent disease merely represents an earlier stage that would eventually become lethal, or if the two represent etiologically distinct entities. The high prevalence of undetected prostate cancer at young ages – 23% for white men in their 40’s – might argue for different entities. Indeed, if even half those cases became lethal within a thirty-year time frame, the death rate from prostate cancer would be much higher than observed. Moreover, Giovannucci et al. have pointed out that prostate cancers of lethal potential, defined here as prostate cancer that has the potential to cause death or clinical metastases, have a different pattern of risk factors as compared with indolent disease36. Figure 4 summarizes the marked differences in relative risk estimates for lethal and indolent disease for a variety of risk factors in the Health Professionals Follow-Up Study. To reduce variability in outcome definitions and avoid publication bias, we only included results from this cohort in the figure, but a similar pattern of differences is observed in other cohorts. Smoking is related to higher risk of lethal prostate cancer (Hazard Ratio (HR): 1.41; 95% Confidence Interval (CI): 1.04-1.91), but not to risk of indolent disease (HR: 0.88; 95% CI: 0.77-1.01)36. Similarly, height has a robust association with lethal (HR: 2.06; 95% CI: 1.24-5.44) but not indolent disease (HR: 1.05; 95% CI:0.88-1.27)36; this is unlikely due to detection bias and instead supports the biologic distinction between indolent cancers and those with lethal potential. Other protective factors including coffee37, lycopene38, fish consumption39, vigorous physical activity40, and statin use41, have all been linked to lower risk of lethal disease, but the findings are weaker and much less consistent for overall or indolent prostate cancer.
Figure 4. Differing patterns of risk factors for indolent and lethal prostate cancers.
Multivariable relative risks for the highest category versus reference category for selected variables from published results separately for total, incident, or organ-confined prostate cancer (blue) and lethal/advanced/fatal prostate cancer (red) in the most recent Health Professionals Follow-Up Study (HPFS). Data were selected with a preference for indolent or organ-confined and lethal or fatal outcomes as indicated. See original publications for covariates that were adjusted for in Cox models36, 37, 39, 40, 59.*indicates statistical significance.
In addition to the Health Professionals Follow-Up Study, other studies have observed differences in risk factors for lethal and total incident prostate cancer outcomes. For example, among men in the screened group of the PLCO, cruciferous vegetable intake was inversely associated with extraprostatic cancer (HR: 0.60, 95%CI: 0.36-0.98, p-trend: 0.02), but was not significantly related to total prostate cancer (HR: 0.85, 95%CI: 0.64-1.14). That study population was PSA-screened annually, thus reducing possible detection bias. Note that in these and other studies that compare advanced or lethal disease to overall prostate cancer, the advanced cases are included in the total; thus, the contrast between potentially lethal disease with indolent is underestimated. Our findings for differences in risk factors for lethal versus indolent prostate cancer were replicated in meta-analyses of body mass index, smoking, and fish intake42-44.
Differences in findings between studies of prostate cancer epidemiology in the pre-PSA versus the PSA era are instructive because a much larger fraction of cases diagnosed before PSA screening had lethal potential. Before screening was introduced, potentially lethal cases could be identified as those with advanced stage (T3b or higher) at diagnosis, but in the PSA era, over 90% of cases present with early stage disease45. Of course, even before PSA screening was initiated, some cases were indolent and diagnosed in the course of assessing urinary symptoms unrelated to the cancer. The same is true for cases diagnosed in countries without systematic PSA screening. Nonetheless, pre-PSA cases were clearly enriched with those of lethal potential, as compared with the present distribution in screened populations. Hence, epidemiologic studies of overall prostate cancer in the pre-PSA era tended to observe relative risk estimates closer to those found for lethal disease in contemporary studies. The differing pattern of risk factors for lethal versus indolent disease implies that prostate cancers of lethal potential should not be considered simply as a subgroup of total prostate cancer, but rather as a distinct entity that should be the main outcome for research. We recognize the possibility of a spectrum of aggressiveness, and of course cannot exclude the potential for some apparently indolent disease to eventually become lethal. We also recognize that in practice, there is inevitable misclassification between these two categories. Any man with metastatic disease or prostate-specific death would be correctly classified as “lethal”, but some men with potentially lethal disease die of another cause before clinical metastases appear, and some are cured through early treatment, and would be misclassified as indolent. However, from both a clinical and research perspective, a dichotomy of indolent versus potentially lethal disease serves as a useful approximation to guide study design and investigation.
The limited success in identifying genetic variants that preferentially predict lethal prostate cancer – in contrast with the many confirmed risk SNPs for overall prostate cancer – combined with the more consistent data supporting a role of diet and lifestyle factors for development of lethal, but not total, prostate cancer - suggests the possibility that genetic factors mainly provide more susceptibility, and environmental factors may play a greater role in determining risk of progression to lethal outcomes. This hypothesis is supported by the finding that family history is a risk factor of similar magnitude for lethal and indolent prostate cancer, in contrast to many of the risk factors showing marked differences between those two outcomes.
Surrogate Endpoints for Lethal Prostate Cancer
A common approach to dealing with the difficulty of the diminishing number of advanced cases at diagnosis in the PSA-era is to use surrogate markers of lethal disease, particularly high grade Gleason disease, or biochemical recurrence (i.e., PSA rise) after primary treatment. Certainly both of these are predictors of lethal prostate cancer, but they are poor surrogates for lethal disease. Most men diagnosed with Gleason 7 die of a cause other than prostate cancer46, 47, and the same is true for men with PSA rise after treatment48. Gleason score was not widely reported in the studies of prostate cancer at autopsy, and changes in the Gleason scoring criteria over time further complicate this review. However, Zlotta and colleagues reported 37% of 320 prostate cancers autopsied in Caucasians between 2008 and 2011 were Gleason score 7-10 and 11% had extraprostatic extension26. Thus, the presence of Gleason pattern 4 is insufficient to identify potentially lethal disease.
The challenges of prostate cancer epidemiology in the PSA era are well illustrated by recent studies of marine fatty acids (largely reflecting fish intake). Brasky and colleagues reported positive associations of high plasma levels of long-chain ω-3 polyunsaturated fatty acids (marine fatty acids) and risk of total and high-grade prostate cancer49. This finding stands in stark contrast to the results of several studies focusing on risk of prostate cancer mortality, which show significant inverse relations with fish intake (meta-analysis of 4 cohort studies HR: 0.37; 95% CI: 0.18-0.74) and a null relationship with total incident prostate cancer (meta-analysis of 12 cohort studies HR: 1.01; 95% CI: 0.90-1.14)50. The association reported by Brasky et al. with higher grade Gleason score was that of similar magnitude as the overall association, and cannot be considered to reflect risk of lethal disease.
These conflicting results might be explained by detection bias (i.e., the probability of being diagnosed with prostate cancer was associated with the exposure) in Brasky’s study. That study of 834 cases included only one above stage T3. In US populations, fish consumption is a characteristic of health-conscious behavior and is directly correlated with intensity of PSA screening51. Thus, the modest apparent increase in risk of overall prostate cancer was likely due to more screening in men with higher fish consumption and hence more diagnoses of prostate cancer. Indeed, in the Physicians’ Health Study we found an apparent increase in overall prostate cancer associated with higher fish consumption. After adjustment for PSA screening, this apparent increase disappeared51.
Likewise, because screening and early treatment can reduce prostate cancer specific mortality52 researchers must be alert to the possibility that an apparent protective factor for lethal disease might simply be a marker of screening propensity. In the studies of fish intake, an inverse association with lethal prostate cancer remained strong even after excluding all PSA-detected cases. Thus, to avoid simply identifying markers of screening behavior, epidemiologic analyses must carefully control for PSA-screening.
Implications for Clinical Trials
Another consequence of PSA screening is that the follow-up for studies focused on prostate cancer prevention must be very long to be informative for lethal prostate cancer, due to the long lead-time of PSA-detected cases. Lead times estimates vary depending upon the methods and definitions, but estimates of 8-12 years are well founded3. Hence, the follow-up period of trials in the PSA era must be at least 8-12 years just to get to the median point at which cases would have been diagnosed in the pre-PSA era. Even trials of this length will have only modest power to detect any effect of the intervention on clinically significant, prostate cancers of lethal potential since men can live many years beyond the time of clinical detection. Indeed, in the most recent report from the Swedish trial of surgical intervention versus watchful waiting, findings for a marked benefit of surgery for prostate-specific and total prostate cancer emerged clearly only after 15 years of follow-up53. In contrast, shorter-term studies are likely to be uninformative. For example, the SELECT trial was conducted among 35,533 men with prior PSA screening to test selenium and vitamin E as preventive agents. The trial was terminated, with null results reported for both interventions, after a median of 5.5 years. By that time, 1,758 cases of prostate cancer were diagnosed, but only 10 (0·6%) were stage T3 or higher and one prostate cancer death54. Thus, this trial could not provide an adequate test regarding the effect of selenium or vitamin E supplements on risk of lethal prostate cancer.
Trials of PSA Screening on Mortality
Similarly, because of the long lead-time created by PSA screening, the full impact of PSA screening on mortality due to prostate cancer cannot emerge until 15-20 years have elapsed. The challenge of maintaining an adequate contrast between screened and non-screened groups in randomized controlled trials over such a long interval is formidable. This is illustrated by the PLCO screening trial, in which the “unscreened” group had considerable screening before the trial began, and had 2.7 PSA tests on average in the 6 years of intervention versus an average of 5 PSA tests in the screened group55. Indeed, the PLCO control group had more intensive screening on average (only 21% had no PSA test) than the screening arms of the European trials, which showed a reduction in fatal prostate cancer56. Thus, the null findings in PLCO are not surprising, though the U.S. Preventive Services Task Force missed this critical distinction in their summary of the evidence9, as reviewed by Carlsson57. Indeed, the Göteborg PSA screening trial has the longest follow-up, and with adequate distinction of screened versus unscreened men, demonstrated a significant reduction in prostate cancer mortality only after a median of 14 years of follow-up58.
Conclusion
Autopsy studies confirm a high prevalence of asymptomatic and undiagnosed prostate cancers in men as young as 30 years of age, and the prevalence increases with age such that about half of Caucasian men over 80 years likely have indolent prostate cancer. By combining data from 19 studies, encompassing over 6,000 men, we could define the age-related prevalence with much greater precision than previous studies10. In conjunction with the downward stage shift and long lead-time for PSA screening, this has had a major impact on the epidemiology of prostate cancer. In the PSA era, studies of total incident prostate cancer largely describe men diagnosed through PSA screening who may not otherwise experience clinical symptoms. In many instances, the risk factors for total prostate cancer merely reflect propensity for screening, and when compared with risk factors for lethal disease, suggest distinct etiologies between indolent and lethal prostate cancers. Until better surrogates are identified and validated, population and prevention research should focus on risk of potentially lethal prostate cancer as the primary outcome.
Figure 2. Forest plot of autopsy studies of undiagnosed prostate cancer among men age 60-69.
Acknowledgments
Financial support: This work was supported by the Dana-Farber/Harvard Cancer Center Prostate SPORE (5P50CA090381-08); and the National Cancer Institute (5R01CA141298, R01CA133891, UM1 CA167552).
Footnotes
Potential conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Mariotto AB, Etzioni R, Krapcho M, Feuer EJ. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109:1877–86. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 2.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, Feuer E, de Koning H. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. Journal of the National Cancer Institute. 2009;101:374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RAM, Schröder FH, de Koning HJ. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute. 2003;95:868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 4.Auvinen A, Määttänen L, Stenman U-H, Tammela T, Rannikko S, Aro J, Juusela H, Hakama M. Lead-time in prostate cancer screening (Finland) Cancer Causes and Control. 2002;13:279–85. doi: 10.1023/a:1015040231402. [DOI] [PubMed] [Google Scholar]
- 5.Finne P, Fallah M, Hakama M, Ciatto S, Hugosson J, de Koning H, Moss S, Nelen V, Auvinen A. Lead-time in the European Randomised Study of Screening for Prostate Cancer. European Journal of Cancer. 2010;46:3102–8. doi: 10.1016/j.ejca.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Loeb S, Bjurlin M, Nicholson J, Tammela TL, Penson D, Carter HB, Carroll P, Etzioni R. Overdiagnosis and Overtreatment of Prostate Cancer. European Urology. 2014 doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter HB. American Urological Association (AUA) guideline on prostate cancer detection: process and rationale. BJU international. 2013;112:543–7. doi: 10.1111/bju.12318. [DOI] [PubMed] [Google Scholar]
- 8.Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158:761–9. doi: 10.7326/0003-4819-158-10-201305210-00633. [DOI] [PubMed] [Google Scholar]
- 9.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 10.Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15:3866–71. [PMC free article] [PubMed] [Google Scholar]
- 11.Moore RA. The morphology of small prostatic carcinoma. J Urol. 1935;33:224–34. [Google Scholar]
- 12.Andrews GS. Latent Carcinoma of the Prostate. [Google Scholar]
- 13.Edwards CN, Steinthorsson E, Nicholson D. An autopsy study of latent prostatic cancer. Cancer. 1953;6:531–54. doi: 10.1002/1097-0142(195305)6:3<531::aid-cncr2820060311>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Franks LM. Latent carcinoma of the prostate. The Journal of pathology and bacteriology. 1954;68:603–16. doi: 10.1002/path.1700680233. [DOI] [PubMed] [Google Scholar]
- 15.Halpert B, Schmalhorst WR. Carcinoma of the prostate in patients 70 to 79 years old. Cancer. 1966;19:695–8. doi: 10.1002/1097-0142(196605)19:5<695::aid-cncr2820190515>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg S, Berge T. Prostatic carcinoma: an autopsy study. Scandinavian journal of urology and nephrology. 1970;4:93–7. doi: 10.3109/00365597009137581. [DOI] [PubMed] [Google Scholar]
- 17.Breslow N, Sternby NH, Tulinius H, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. International journal of cancer Journal international du cancer. 1977;20:680. doi: 10.1002/ijc.2910200506. [DOI] [PubMed] [Google Scholar]
- 18.Guileyardo JM, Johnson WD, Welsh RA, Akazaki K, Correa P. Prevalence of latent prostate carcinoma in two U.S. populations. Journal of the National Cancer Institute. 1980;65:311–6. [PubMed] [Google Scholar]
- 19.Holund B. Latent prostatic cancer in a consecutive autopsy series. Scandinavian journal of urology and nephrology. 1980;14:29–35. doi: 10.3109/00365598009181186. [DOI] [PubMed] [Google Scholar]
- 20.Stemmermann GN, Nomura AM, Chyou PH, Yatani R. A prospective comparison of prostate cancer at autopsy and as a clinical event: the Hawaii Japanese experience. Cancer Epidemiology Biomarkers & Prevention. 1992;1:189. [PubMed] [Google Scholar]
- 21.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150:379–85. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. The Prostate. 2003;54:238–47. doi: 10.1002/pros.10177. [DOI] [PubMed] [Google Scholar]
- 23.Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B. The Prevalence of Prostate Carcinoma and Its Precursor in Hungary: An Autopsy Study. European Urology. 2005;48:739–44. doi: 10.1016/j.eururo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Stamatiou K, Alevizos A, Perimeni D, Sofras F, Agapitos E. Frequency of impalpable prostate adenocarcinoma and precancerous conditions in Greek male population: an autopsy study. Prostate Cancer and Prostatic Diseases. 2006;9:45–9. doi: 10.1038/sj.pcan.4500847. [DOI] [PubMed] [Google Scholar]
- 25.Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792–6. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlotta AR, Aldaoud N, Fleshner N, Finelli A, Klotz L, Sykes J, Lockwood G, van der Kwast TH, Egawa S, Pushkar D, Govorov A, Kimura T, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. Journal of the National Cancer Institute. 2013;105:1050. doi: 10.1093/jnci/djt151. [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Shanmugaratnam K. Latent prostate carcinoma in Singapore Chinese. Singapore Med. 1972;13:1–6. [PubMed] [Google Scholar]
- 28.Akazaki K, Stemmerman GN. Comparative study of latent carcinoma of the prostate among Japanese in Japan and Hawaii. Journal of the National Cancer Institute. 1973;50:1137–44. doi: 10.1093/jnci/50.5.1137. [DOI] [PubMed] [Google Scholar]
- 29.Gu FL, Xia TL, Kong XT. Preliminary study of the frequency of benign prostatic hyperplasia and prostatic cancer in China. Urology. 1994;44:688–91. doi: 10.1016/s0090-4295(94)80207-6. [DOI] [PubMed] [Google Scholar]
- 30.Yatani R, Shiraishi T, Nakakuki K, Kusano I, Takanari H, Hayashi T, Stemmermann GN. Trends in frequency of latent prostate carcinoma in Japan from 1965-1979 to 1982-1986. J Natl Cancer Inst. 1988;80:683–7. doi: 10.1093/jnci/80.9.683. [DOI] [PubMed] [Google Scholar]
- 31.Society AC. Cancer Treatment and Survivorship Facts & Figures 2014-2015. American Cancer Society; 2014. [Google Scholar]
- 32.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 33.Fundamentals of treatment: Undiagnosed: Prostate Oncology Specialists. 2014. [Google Scholar]
- 34.Thompson IM, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman JCA, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. The New England journal of medicine. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman KE, Niu J, Shen Y, Jiang J, Davis JW, Kim J, Kuban DA, Perkins GH, Shah JB, Smith GL, Volk RJ, Buchholz TA, et al. Physician Variation in Management of Low-Risk Prostate Cancer: A Population-Based Cohort Study. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2014.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. International journal of cancer Journal international du cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson KM, Kasperzyk JL, Rider JR, Kenfield S, van Dam RM, Stampfer MJ, Giovannucci E, Mucci LA. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. Journal of the National Cancer Institute. 2011;103:876–84. doi: 10.1093/jnci/djr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, Giovannucci E. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. Journal of the National Cancer Institute. 2014;106:djt430. doi: 10.1093/jnci/djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augustsson K, Michaud D, Rimm E, Leitzmann M, Stampfer M, Willett W, Giovannucci E. A Prospective Study of Intake of Fish and Marine Fatty Acids and Prostate Cancer. Cancer Epidemiology Biomarkers & Prevention. 2003;12:64. [PubMed] [Google Scholar]
- 40.Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165:1005–10. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- 41.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. Journal of the National Cancer Institute. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 42.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 43.Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. The American journal of clinical nutrition. 2010;92:1223–33. doi: 10.3945/ajcn.2010.29530. [DOI] [PubMed] [Google Scholar]
- 44.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Djenaba JA, Soman A, Rim SH, Master VA. Recent trends in prostate cancer incidence by age, cancer stage, and grade, the United States, 2001-2007. Prostate cancer. 2012;2012:691380–8. doi: 10.1155/2012/691380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andren O, Fall K, Franzen L, Andersson SO, Johansson JE, Rubin MA. How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol. 2006;175:1337–40. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 47.Stark JR, Loda M, Giovannucci EL, Rubin MA, Mucci LA, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, Ma J, Fiorentino M, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:3459–64. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bianco JFJ, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66:83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 49.Brasky TM, Parnes HL, Klein EA, Kristal AR, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, Meyskens JFL, Goodman GE, Minasian LM. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. Journal of the National Cancer Institute. 2013;105:1132. doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. The American journal of clinical nutrition. 2010;92:1223–33. doi: 10.3945/ajcn.2010.29530. [DOI] [PubMed] [Google Scholar]
- 51.Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. The American journal of clinical nutrition. 2008;88:1297. doi: 10.3945/ajcn.2008.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Maattanen L, Lilja H, Denis LJ, Recker F, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014 doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bill-Axelson A, Spångberg A, Andrén O, Palmgren J, Steineck G, Adami H-O, Johansson J-E, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. The New England journal of medicine. 2014;370:932. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lippman SM, Hartline JA, Parsons JK, Bearden rJD, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Klein EA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA: the journal of the American Medical Association. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinsky PF, Black A, Parnes HL, Grubb R, David Crawford E, Miller A, Reding D, Andriole G. Prostate cancer specific survival in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Cancer epidemiology. 2012;36:e401–e6. doi: 10.1016/j.canep.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stampfer MJ, Jahn JL, P HG. Further Evidence that Prostate-Specific Antigen Screening Reduces Prostate Cancer Mortality. Journal of the National Cancer Institute. 2014 doi: 10.1093/jnci/dju026. [DOI] [PubMed] [Google Scholar]
- 57.Carlsson S, Vickers AJ, Roobol M, Eastham J, Scardino P, Lilja H, Hugosson J. Prostate cancer screening: facts, statistics, and interpretation in response to the US Preventive Services Task Force Review. J Clin Oncol. 2012;30:2581–4. doi: 10.1200/JCO.2011.40.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, Pihl CG, Stranne J, Holmberg E, Lilja H. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nimptsch K, Kenfield S, Jensen MK, Stampfer MJ, Franz M, Sampson L, Brand-Miller JC, Willett WC, Giovannucci E. Dietary glycemic index, glycemic load, insulin index, fiber and whole-grain intake in relation to risk of prostate cancer. Cancer causes & control: CCC. 2011;22:51–61. doi: 10.1007/s10552-010-9671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]