Abstract
Hippocampal CA1 and CA3 neurons sampled randomly in large numbers in primate brain show conclusive examples of hierarchical encoding of task specific information. Hierarchical encoding allows multi-task utilization of the same hippocampal neural networks via distributed firing between neurons that respond to subsets, attributes or “categories” of stimulus features which can be applied in events in different contexts. In addition, such networks are uniquely adaptable to neural systems unrestricted by rigid synaptic architecture (i.e. columns, layers or “patches”) which physically limits the number of possible task-specific interactions between neurons. Also hierarchical encoding is not random; it requires multiple exposures to the same types of relevant events to elevate synaptic connectivity between neurons for different stimulus features that occur in different task-dependent contexts. The large number of cells within associated hierarchical circuits in structures such as hippocampus provides efficient processing of information relevant to common memory-dependent behavioral decisions within different contextual circumstances.
Keywords: Hippocampal neurons, memory, hierarchical encoding, retention and retrieval, nonlinear hierarchical model, nonhuman primates
1. Neurons and Networks
1.1 Requirements for Neural Encoding
Individual neurons change their firing activity based on the nature of the synaptic inputs they receive which could be related to the nature of the transmitter involved, or frequency of membrane depolarization from repetitive single, or temporally convergent multiple, synaptic inputs. The resulting long-term potentiation (LTP) from such repetitive synaptic activation provides the primary basis for sustained increases in firing tendencies, under the same circumstances, at the single neuron level (Abraham, 2003; Bliss and Collingridge, 1993; Lynch, 2004; Lynch et al., 2014). An established feature of neuron encoding (Cox et al., 2014) is modulation of neuronal efficacy in response to single or convergent synaptic inputs, dependent upon whether such inputs exhibit higher firing rates during critical events. However, from the perspective of circuit operation and large scale information integration, if firing of the involved neural cells is not synchronized in a spatiotemporal manner, adequate recruitment and altered responsiveness is not likely to happen (Hampson et al., 1999; Brasselet et al., 2012; Lynch et al., 2013).
1.2 Relation to Organized Circuit Operation
Synchronized synaptic inputs and firing frequencies are primary factors in coordinating connections between neurons in the same functional circuits. Factors such as LTP promote the chance that groups of neurons with multiple synaptic connections will tend to fire in coordinated spatiotemporal patterns that underlie information processing for specific sensory events when frequently repeated. In previous investigations a nonlinear multi-input multi-output (MIMO) math model was employed to analyze cell firing across large neuron populations in the same structures (Berger et al 2011; Hampson et al., 2012b; Hampson et al., 2012c). In those studies it was demonstrated that the derived spatiotemporal firing patterns across all cells in the population were hierarchical, and provided important information-specific ‘firing codes’ during performance of complex memory tasks. This convergence of synaptic inputs from two or more Simple neurons that encode item-specific information, onto other single neurons at the next stage of input-output circuit transmission through the structure, provides “Conjunctive neurons” for functional hierarchical circuits. Firing of only a few Conjunctive cells can therefore reflect several different task features dependent of the temporal synchrony of inputs from different convergent Simple cells activated by specific task-related events. Hence, it is likely that functional hierarchical circuits are the bases for the spatiotemporal firing patterns that represent effective task-related performance codes developed in brain areas that process information required for complex decision-making situations.
1.3 Categorization-Constraints and Plasticity
As mentioned with respect to the role of neuron population encoding in task-dependent circumstances, it is assumed that hierarchical formats of multi-neuron connectivity are what logically encodes features related to memory demands and cognitive processing. New hierarchical neural networks therefore are required to be constructed for encoding additional task-dependent features that were previously not relevant to successful performance. However, the evolution of successful hierarchical encoding depends to a large extent on frequent re-exposure to task events that can be categorized via specific firing of directly responsive “Simple” cells for eventual selective increased synaptic connectivity with “Conjunctive” and “Trial Type” (TT) cells (Marmarelis et al., 2013; Hampson et al., 2012c; Deadwyler and Hampson, 2004; Hampson and Deadwyler, 2003; Hampson et al., 2001). The resulting outcome of such hierarchically controlled plasticity is faster processing of previously unfamiliar information due to the sharing of some of the same Simple and Conjunctive cells in a previously hierarchical circuit established for other circumstances.
2. Neural Dynamics of Memory Formation and Retrieval
2.1 Pattern Identity and Extraction
There are several studies which have established the features of pattern identification by brain processes (Kaliukhovich and Vogels, 2013; Beyeler et al., 2013; Safaai et al., 2013; Cerda and Girau, 2013; Brasselet et al., 2012). In order to extract spatiotemporal patterns of multi-neuron firing that have specificity for cognition and memory in primate brain requires that the patterns be obtained within subareas representative of input-output flow of information through the structure which has been previously shown for hippocampus and prefrontal cortex (Hampson et al 2012b. 2013). Inherent anatomic distributions of cells and intrinsic connectivity within such cell groupings are defining factors as to where and how such hierarchical information processing circuits are formatted. In classic cortical structures where pyramidal cells communicate via columnar connectivity, this micro-anatomic substrate serves as the basis for information segregation, derivation and transmission. However, in structures that do not have such detailed anatomic microcircuit capacity the only way of processing information within or across cell layers in a proficient input-output manner, is via hierarchical encoding of item specific information. Such encoding is produced via spatiotemporal convergence of synaptic inputs across Simple, Conjunctive, and one or two TT neurons within a time frame required to satisfy the event-specific constraints of the memory demanding circumstances.
2.2 Temporal Dynamics
Maintained temporal relations between the firing of individual neurons is critical for preserving appropriate information processing in hierarchical circuits. Spatiotemporal firing patterns have been extracted by the experimental diagnosis of nonlinear ‘input-output’ characteristics of multi-cell firing recorded from synaptically connected neurons to reveal hierarchical encoding of information during cognitive processing (Safaai et al., 2013; Mathis et al., 2012; Brasselet et al., 2012; Hampson et al., 2012c; Hampson et al., 2004; Hampson and Deadwyler, 2003; Hampson et al., 2001). Temporal specificity is a key element in terms of the critical (postsynaptic) outputs that result from specific (presynaptic) input patterns, which is required for effective hierarchical coding. Fortunately, as shown below, employment of nonlinear multi input-multi output (MIMO) models can reveal the spatiotemporal dynamics critical for effective operation of hierarchically organized neural systems (Berger et al., 2011; Berger et al., 2012a; Hampson et al., 2012b; Hampson et al., 2013).
2.3 Specificity of Neural Representation
The nature of neural representation described above involves continuous dimensional and categorically defined formatting of information that provides the means to relate, extract, infer, or even reconstruct events via temporally congruent firing in hierarchical arrays. Prior investigations showed that cellular encoding of conjunctive logical relations of recurring events within a stable behavioral context was the basis for the emergence of “trial-type” (TT) cells whose firing signaled particular combinations of events within the task (Hampson et al., 2012c). Such hierarchically determined categorized firing served as the “code” for specific types of events and/or contexts and provided information required for successful performance. The functional basis of such codes is related to firing of “Simple” cells that are primarily responsive to task-related sensory elements and have convergent synaptic connections to higher level cells that receive input from more than one Simple cell, to form ‘Conjunctive’ cells. Conjunctive cells fire maximally to the combined occurrence of events encoded simultaneously by both Simple cells rather than one or the other event alone. In addition, such hierarchically constructed circuits can overlap at lower levels and employ some of the same Simple cells to project to other Conjunctive cells to provide input to different hierarchical circuits that encode different task events or contexts using some of the same task elements. Thus, event occurrence initiates temporally synchronized firing across large numbers of hierarchically connected cells and provides the means for extraction of spatiotemporal task-dependent firing codes that support performance related to memory encoding, retrieval and cognitive assessment (Berger et al., 2011; Hampson et al., 2013;).
3. Generalization and Extraction of Task-Specific Information
The basis for generalized encoding within a group of neurons is dependent upon hierarchical interconnections built from similarities in events and contexts that recur enough in Simple cell input to provide rapid activation of Conjunctive cells that encode the entire context of temporally synchronous task-relevant events. One feature which is not appreciated in trying to understand human memory is the continuous high frequency of execution of memory processes related to similar circumstances which occur within common daily routines. These “practice states” employ much of the same neural circuitry required for new or unfamiliar events, in the form of layer common Simple cells from different hierarchies that fire synchronously to new or unfamiliar events. Thus, even though familiar circumstances within the formation of new memory processes provide only partial features to construct a new encoding hierarchical circuit, much of the needed event representation and circuit related conjunctive format can already be present. Therefore, the extent to which established complex hierarchies are employed daily to rapidly recognize sensory elements and perform involved tasks, provides an effective basis for “tuning” new encoding processes that can utilize common neural components from those established hierarchical circuits.
3.1 Generalization: Extent of Hierarchical Circuit Overlap
The various stages of hierarchical neural circuit construction described above indicate that further information encoding and retrieval can occur when such interconnected Simple and Conjunctive neurons fire in the same timeframes to allow simultaneous transmission between Conjunctive cells to establish more detailed logical relations between events. Transformation from one micro-anatomic hierarchical circuit with few synaptic connections to a larger more encompassing hierarchy retaining the same firing dependencies, can occur via convergent connections from separate Conjunctive cells onto Trial Type (TT) cells. Such TT cells fire only when the specific events extracted by the Conjunctive cells in both micro-hierarchies occur in the same timeframe. Thus TT cells are activated only when both hierarchically encoded circumstances occur simultaneously, not when either of the same Conjunctive cell-encoded stimulus elements occurs in isolation on different trials. Once this occurs, the entire context of tobe-remembered elements can be integrated into a spatiotemporal code signaled by activation of only one higher level TT cell dependent on conjunctive firing across all neurons in the combined hierarchies. This type of condition would promote “generalization” since the firing of TT cells reflects spatiotemporal overlap in the activation of multiple, but different, hierarchical circuits. While it is clear that degree of exposure and similarity of contexts provide a basis for generalization, hierarchical circuitry also provides selective access to “shared” information which would not be available if circuits were more specific such as for sensory detection or response selection (Rotman and Klyachko, 2013; Plakke et al., 2013; Cerda and Girau, 2013; Brasselet et al., 2012). Thus ‘generalization’ is a natural outcome of hierarchical representation because of shared elements encoded and combined in different ways due to different Conjunctive cells activated at the same time. However, such tendencies can only be exploited when common contextual features are the major objective. If more detailed selection and retrieval is necessary it can only result from the activation of specific Simple cells or encoded Conjunctive cells with no connections to TT cells to limit the involvement of other hierarchies (Hampson et al., 2012c).
3.2 Memory Extraction or “How We Know”
Culmination of the efficacy of the above described hierarchical memory processing requires a means of information extraction that is relatively instantaneous and modifiable on a large scale, similar to other functional circuits such as those used in visual detection and motor control. Categorization requires simultaneous detection of similar elements with the ability to shift and compare different hierarchical representations that share the same Simple and Conjunction cells. Much of this is related to online spatiotemporal firing episodes since patterns related to processing by different hierarchies produces unique patterns or ‘output codes’ for particular types of events. Also, it is likely that other brain regions responsible for the implementation of hierarchically extracted (i.e. encoded) information are modified via prior exposures to recognize certain spatiotemporal output codes. Once constructed, the spatiotemporal output code becomes the basis for online detection by other neural systems involved in response execution which connect only to the TT cells since they represent the essence of the information extracted via hierarchical connections. This has been confirmed in a number of studies in rodents and primates utilizing multi-neuron recording techniques to extract “strong code” (i.e. correct) spatiotemporal firing patterns with nonlinear models during the task and (Deadwyler et al., 2013; Hampson et al., 2013; Hampson et al., 2012b; Berger et al., 2011). As shown below, task related short-term memory is dependent on presence of such “strong codes” and it is possible to detect on error trials, the absence of such codes. A major requirement for detecting such codes is the ability to record from multiple neurons (> 15) in known synaptically connected regions related to the input-output circuitry of the structure, i.e. CA3 and CA1 fields in hippocampus.
4. Hierarchical Encoding Utilized in a Primate Memory Task
4.1 Rule-Based Delayed Match to Sample (DMS) Task
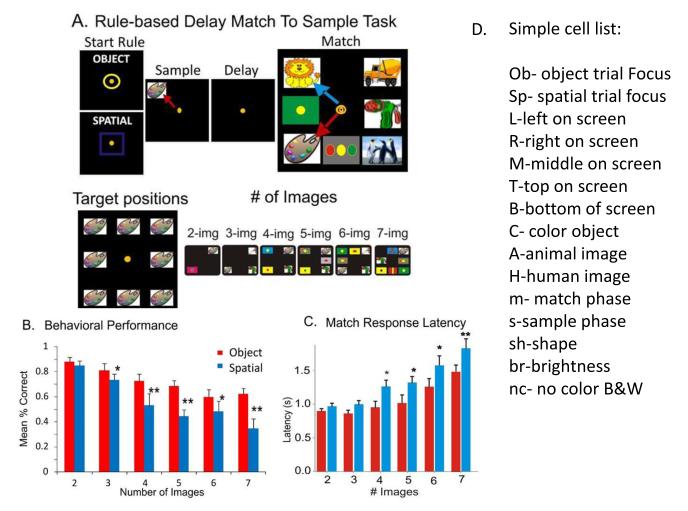
The goal of understanding how memories are established, represented and extracted has been facilitated by employing a complex Delayed Match to Sample (DMS) task in which each trial requires retention of one of two possible reward dependent rules or criteria. The reward contingent rules are trial specific and can change with each trial presentation (Hampson et al 2013). Figure 1 illustrates the task and the two different aspects of Sample information required to be retained across the variable delay interval of 10-90s. A major difference from standard DMS tasks is the requirement conveyed by the trial “Start rule” signal, as to which feature of the next presented Sample image, either 1) the image itself (Object trial) or 2) the spatial location of the image on the screen (Spatial trial), must be retained across the intervening delay interval and selected in the Match phase to obtain a reward. All sample images utilized for trials within and across sessions were unique, selected daily from a website buffer of 5,000 different images. At the onset of the Match phase of the task, following termination of the delay interval, the Sample image is presented in conjunction with a variable number (2-7) of other different ‘distracter’ images. Distracter images can be in the same spatial location on the screen as occupied by the Sample image in the prior Sample phase of the same trial. Thus the task requirement in the Match phase is to 1) remember the Start-rule for the type of trial signaled by the activated Start-Rule signal image and 2) remember that related feature to select in the Match phase as either a) the same screen location irrespective of the image that occupies it (Spatial trial) or b) the same Sample image presented randomly in a one of the 8 different screen locations (Object trial). These task features along with associated performance for each type of trial are illustrated in Figure 1,B&CA.
Figure 1. Dual Trial-Delayed Match to Sample Memory Task.
A. The Rule-based DMS task: NHPs are seated in a primate chair with a shelf-counter in front of them facing a large display screen. During task performance the right hand position on the counter top is tracked by a small LCD camera positioned 30 cm above the hand and displayed as a bright yellow cursor on the projection screen. The screen displayed 2-8 clipart images (# of images) per trial which were placed randomly across each of the 8 possible screen positions (Target positions). Representative screen displays and screen images are shown for successive Phases of the DMS task: Start-Rule, Sample, Delay and Match, in which two types of trials are possible. The display of the Match phase on the right (Match) shows correct selections for both types of trial indicated by the Start-Rule image (left) as either: 1)a Spatial trial in which the correct response (blue arrow) was placement of the cursor into the same spatial location on the screen in which the Sample image was displayed and responded to in the Sample phase, irrespective of image features, or; 2) an Object trial in which the same image shown in the Sample phase was selected (red arrow) irrespective of which location on the screen it occupied. The Delay interval between the occurrence of the Sample response and presentation of the Match phase was of variable duration (5.0-90.0s) on each trial. B&C. Average behavioral performance (% correct) and Match response latencies of trained animals (n=6) on each type of trial, shown as a function of the number of distracter images presented in the Match phase. D. List of abbreviated labels for task-related attributes of images presented in Sample phase recorded consistently from hippocampal cells during either Spatial or Object trials for at least 60 daily sessions consisting of 100 trials. Abbreviated labels denote Simple cell features in the hierarchical encoding arrays shown in Figures 2-4.
4.2 Specificity of Hierarchical Encoding
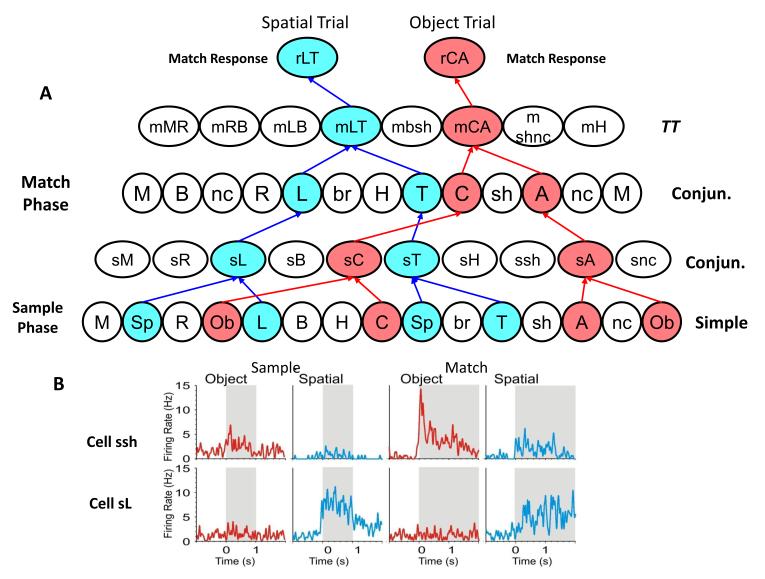
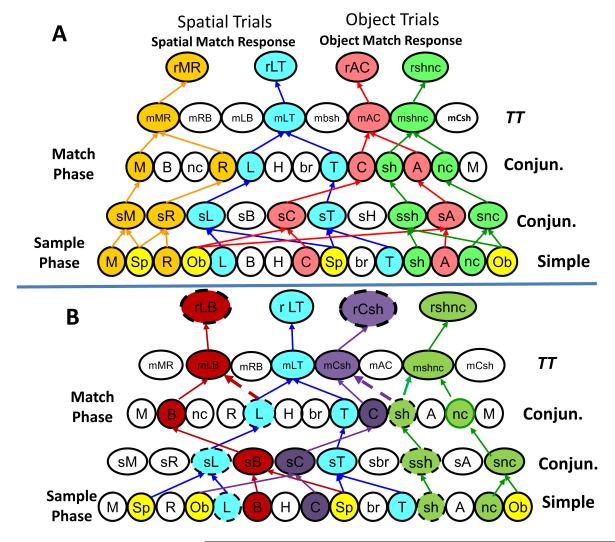
Hierarchical encoding can only work appropriately if all connected cells fire in a manner that corresponds to task demand and context exposure. This requires adequate spatial proximity of cells within the neural structure to provide rapid synaptic activation as well as temporal synchrony with respect to exposure to the task events to be encoded or detected. Simple cell firing features shown in the list in Fig. 1D, extracted from recordings in hippocampus over several different sessions in an animal performing the DMS task (Hampson et al., 2004), are plotted in Figs 2-4 to demonstrate different hierarchical pairings with other Conjunctive and TT cells recorded at the same time. In Figure 2 formation of Conjunctive cell firing is shown by the simultaneous inputs from specific Simple cells in the lower layer of the hierarchical circuit that are temporally synchronized. This can be extracted with multi-neuron spatiotemporal firing displays but only if recording locations encompass cell layers or patches that correspond to the input/output regions of synaptic connections within the structure. If large numbers of cells with known synaptic connections are recorded simultaneously during task performance, the extracted spatiotemporal firing patterns will reveal logical connections in the form of Conjunctive cell firing as shown in Figure 2 (Conjun.). Such Conjunctive cells fire to more than one image feature (i.e. sL = spatial trial + left screen position of Sample image) encoded by Simple cells (lower layer) in the Sample and/or Match phases of the task (Fig 2B). Figure 3A shows how this hierarchical encoding within the same cell population can be exploited to detect different image features on 4 different trials by activation of different convergent connections to separate Conjunctive cells (rMR. rRL, rAC rshnc) in the Match phase of the task. However it is also possible for the same Simple cells to participate in the encoding of different task events on different trials, as shown for Simple cells R and sh in Figure 3B, where images with other stimulus features (i.e. bottom vs. top spatial position; color vs. no color for same shape image) are presented in the Sample phase. The fact that the group of cells can logically encode up to 4 different trial events (Fig 3A) shows that hierarchical representation is effective even though some of the same Simple cells are utilized for encoding on other trials (Fig 3B). Thus a wide range of different task attributes can be encoded hierarchically over the same population of hippocampal neurons irrespective of topographic relations in terms of cellular location, the only criteria is for potential synaptic connections for all hierarchically relatable cells.
Figure 2. Hierarchical Encoding of Spatial and Object Trials.
A: Diagram depicts hierarchical encoding of hippocampal cells recorded on Spatial (blue) and Object (red) trials as time transitions from the Sample (lower 2 levels) to Match (upper two levels) phase of the DMS task. Labels of Simple cells in the lower two rows show designation of trial type from the prior Start Rule response (spatial-Sp and object-Ob), and for the different attributes of images presented in the Sample phase of the task as listed in Figure 1D. Arrows (blue and red) indicate associated synaptic connections with Conjunctive cells in the Sample phase (sL, sT; sC, sH) that transfer firing to the Match phase (L,T,C, H) and project to higher category TT cells (mLT and mHC). TT cells then project to appropriate Match Response cells (rLT, rHC) for selection of either the appropriate image (Object trial hierarchy), or location on the screen (Spatial trial hierarchy), corresponding to the Sample phase response. B: Average firing rate profiles are shown for two Conjunctive cells (sH and sL) in the above hierarchy for both phases of the task (Sample and Match) during each type of DMS trial (Spatial and Object).
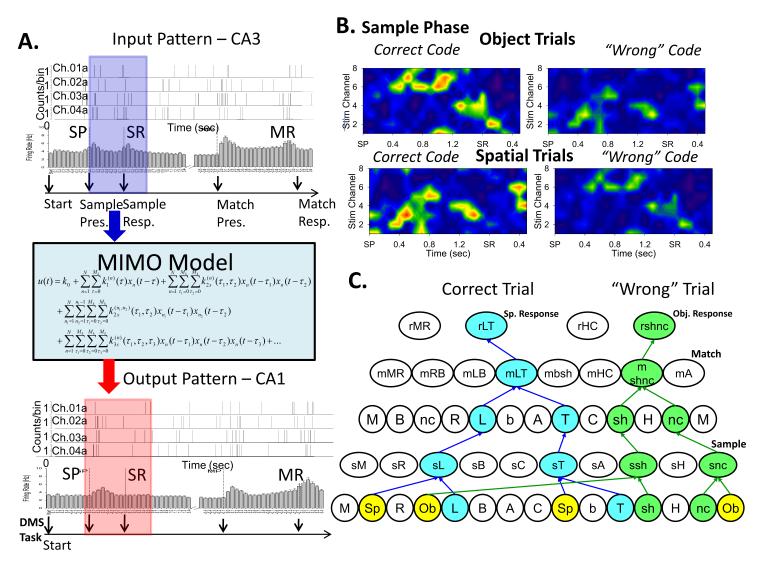
Figure 4. Extraction of Hierarchical Spatiotemporal Codes via Nonlinear MIMO Model.
A. Depiction of spatiotemporal firing patterns extracted by a multi-input multi-output (MIMO) nonlinear model (Berger et al., 2011) applied to simultaneously recorded cells (Chs. 01-04) in CA3 (top) and CA1 (bottom) with two separate multi-array probes. B. MIMO model extracted firing patterns of multiple cells (n=8) displayed in a “heat map” style to emphasize spatiotemporal patterns recorded at the time of Sample phase image presentation (SP) and response (SR) on both correct and error, Spatial vs. Object trials. Intensity of firing is scaled across cells from low (4 Hz, green) to high (10 Hz, red) rates during Sample presentation (SP) and response (SR) depicted by the blue and red arrows in A. Spatiotemporal heat map firing patterns differ as a function of the type of trial as well as whether the trial was responded to correctly (Correct code) or incorrectly (“Wrong” code). C. Extraction of hierarchical spatiotemporal firing in this manner provides insight into why some trials may not be responded to correctly. “Wrong” code trial firing patterns are less intense but on some occasions also reflect suppressed hierarchical processing of the opposite trial type (i.e. Object trial) as shown in the heat maps in B and the hierarchical diagrams in C (“Wrong” code). Since retention of the Start-Rule code indicating the ‘type of trial’ is also critical for effective performance in the Match phase (C: Correct Trial Sp response), errors may also indicate encoding by the wrong Start-Rule Simple cells in the Sample phase of the task (C: ‘Wrong Trial’ object response).
Figure 3. Multiple Hierarchical Array Encoding.
Hierarchical representation within the same hippocampal cell population of trial-specific images on 4 different trials. A. This illustrates the high degree of overlap and cross-connectivity that is possible within the same homogeneous cell population in which connectivity with Conjunctive cells is controlled by categorized Simple cell image encoding in the Match phase. B. Shared hierarchical of encoding by the same Simple hippocampal cells that encode events on more than one type of trial due to the occurrence of similar features in images presented on other trials. The green and blue labeled hierarchies involve the same Simple and Conjunctive hippocampal cells shown in Fig 3A, however the red and purple hierarchies employ some of those same Simple and Conjunctive cells (L and sh) to encode different spatial (B) or image (C) features on a different trials as shown by the dotted connections to separate TT cells in the Match phase (mLB & mCsh).
4.3 Nonlinear Multi-input, Multi-output (MIMO) Model to Detect Hierarchical Spatiotemporal Firing Patterns
Multi-neuron recording over the same temporal intervals allows assessment of firing between synaptically connected neurons with respect to prediction of “output” (correlated spikes within a fixed timeframe) on the basis of “input” (spikes correlated with the onset of external events) from different neurons known to be separated by at least one synaptic connection (i.e. CA3 to CA1). The MIMO model shown in Figure 4A has been employed effectively in prior studies to extract task-related spatiotemporal firing in multi-neuron hippocampal cell recordings in rodents and NHPs and in both circumstances encoding of task information by neuron populations was found to be hierarchical with respect to retrieval of Sample information in memory tasks (Berger et al., 2011; Hampson et al., 2013). When examining strong (correct) vs. weak (error) MIMO derived spatiotemporal ‘codes’ on both types of trials, it was found that strong codes appeared only when the same cell populations exhibited hierarchical encoding associated with correct selection in Match phases on prior trials. In addition the DMS task verified the selectivity of the strong codes by consistently showing different spatiotemporal patterns for the two types of trials which required Spatial vs. Object encoding of Sample information (Fig 3A&B). This is shown in Figure 4B as “heat map” displays of multiple cell firing profiles for information encoded by neurons recorded in CA3 and CA1 of hippocampus during performance of each type of randomly presented trial during the session. A key element related to performance was the differential Start-rule signal (square or circle, Fig 1A) which had to be registered by Simple cells to properly encode the Sample stimulus for later selection in the Match phase on the same trial. Figures 2&3 show how this type of information was hierarchically represented differentially with respect to the key image features encoded by Simple cells. These included: 1) the spatial position of the Sample image on the screen (Spatial trial), a firing bias described originally and analyzed completely for hippocampal cells by J. O’keefe 1971, Okeefe &Nadel 1978, Moser, Kropff, Moser (2008) who recently received the Nobel Prize for this discovery (Nobelprize.prg Nobel Media AB 2014), or, 2) Sample image shape and/or color within any spatial position on the screen (Object trial). It is clear that different hierarchical codes can coexist in the same populations of synaptically connected cells via activation of functionally different conjunctive interconnections. Selectivity for differential encoding of specific types of trial information at the time of the Sample stimulus response was detected by two different but compatible neural population measures: 1) hierarchical conjunctive encoding (Figure 2B), and 2) differences in spatiotemporal firing patterns on correct vs. error trials (Figure 4B).
4.4 Specificity of Model Extracted Codes for Task Performance
The presence of strong, weak or ‘wrong’ spatiotemporal codes during performance of a cognitive task provides insight into how neural populations encode information differentially in hierarchical fashion. Figure 3A shows that a wide range of different task attributes can be encoded hierarchically over the same population of neurons that have mutual synaptic connections. Different Conjunction neuron firing contingent on simultaneous inputs from different sets of Simple cells was established from prior presentations (i.e. trials) during task training. Therefore it is possible for different types of information to be extracted via firing of some of the same Simple cells due to connections with other Conjunctive cells to form a different hierarchy (as shown in Fig 3B). Given this arrangement of hierarchical overlap, specificity of information encoding is only possible if the firing of Conjunctive cells in different hierarchies is temporally isolated via presentation of only one Sample image on each trial, however, since such representations are usually task-related, differently encoded events do not occur at the same time. Therefore, the occurrence of stimulus elements unique to a given trial produces temporally selective firing of tuned Simple cells in the hierarchy with established and unique convergent synaptic connections to Conjunctive cells that fire maximally only when facilitated by multiple occurring synaptic inputs (i.e. LTP) provided by convergent Simple cells (Figs 2&3). As shown in Figure 4A this can be assessed with a nonlinear multi-input multi-output (MIMO) model that re-constructs the hierarchical spatiotemporal firing pattern related to the specificity of encoding for the event presented. Patterns related to correct performance are considered “strong codes” and when dissected provide the means to reconstruct the underlying functional hierarchy in real-time as shown by the “heat map” type neuron firing displays in Figure 4B. This distinction with respect to firing specificity and encoding can be determined when other events occur also (Figure 4B, spatial vs. object trials), as well as by comparison with “wrong codes” for a given event produced during errors in performance (Figure 4C). What is critical is the fact that the different spatiotemporal codes shown in Figure 4B are distinct for specific events (spatial and object trials) and they reflect in real-time the firing of different sets of Conjunctive and TT cells related to the operation of separate functional hierarchies within the same anatomic structure (i.e. hippocampus).
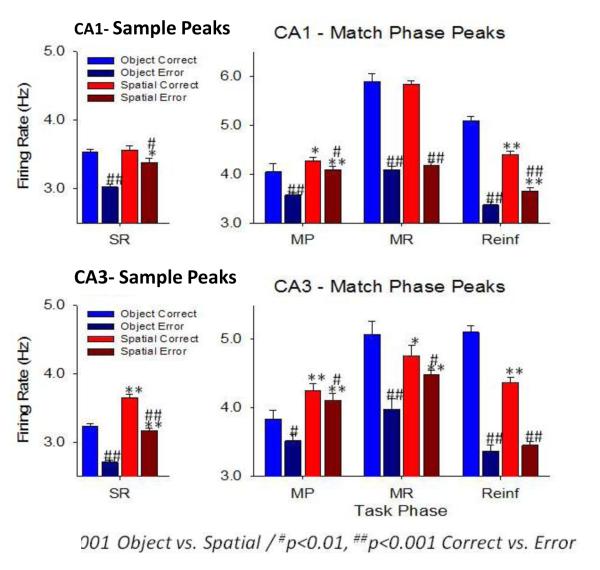
4.5 Relation to Successful Performance Requiring Accurate Memory
The accuracy of the spatiotemporal code can be examined in terms of firing intensity of cells within the population sampled to extract correct vs. error trial patterns of cell activity in critical phases of the task. Figure 5 shows the average firing rates of recorded cells comprising different hierarchical codes calculated across all the different spatial and object trials presented in the same session (Figures 1&2). It is clear that on correct vs. error trials encoding in the Sample and Match phases of the task was higher on both spatial and object trials. This supports the notion that hierarchy firing in Match phases utilized the same Simple cells triggered in the Sample phase in order to activate encoded Conjunctive and TT cells required to select the correct “Match” behavioral response in that phase of the task. Figure 5 also shows that there were similar firing profiles for CA3 and CA1 cells in both task phases which supports the relevance of hierarchical encoding since synaptic connections between cells in these two areas constitute the main input-output circuitry of hippocampus (Hampson et al., 2013; Berger et al., 2011; Zanos et al., 2008; Brasselet et al., 2012; Li et al., 2014; Kimura et al., 2011; Knierim et al., 2006; Vinogradova, 2001).
Figure 5. Memory Encoding in Hippocampus.
An important issue with respect to the functional significance of hierarchical encoding is whether processing eventually conforms to the overall input-output processing features of the structure involved. This is required otherwise the processed information does not get transmitted to the appropriate effector (i.e. behavioral response) system. The average peak firing rates of cells involved in hierarchical processing in CA3 and CA1 were compared and showed similar firing tendencies in the critical phases of both types of trial, as well as on error trials in which the output structure CA1 showed the same decrease as in CA3 across all task phases. Although there are other reasons why such correspondence in average firing may not occur, such as initiation of the “wrong” code as shown in Figure 4B, the substrates required for successful performance are not possible without ‘consistent utilization’ of the appropriate Conjunctive and TT cells in the CA1output region of hippocampus.
5. The Neural Basis of Memory
The above description and analyses of how hippocampal neurons, sampled randomly in large numbers in primate brain, exhibited hierarchical encoding of task specific information, can be related directly to how human memory operates daily in terms of multiple hierarchical processes. As stated previously, one factor not stressed in memory studies in humans, is the influence of prior exposure and training in the “life-long” construction of frequently employed hierarchical retrieval schemes. Hierarchical processing provides for inherent multi-task application of the same hippocampal systems which can access conjunctive (via Simple cell) firing that may have established sensitivity to features of items that are necessary to encode in different contexts (Brasselet et al., 2012). Therefore, detection via multi-electrode arrays of task-encoded information in NHPs as described here reflects hierarchical encoding designed to improve performance in new contexts, as would be expected from hippocampal neural systems. In fact, because of the necessity for frequent use, such systems are likely well engrained and routinely initiated. Hence, operation of such established systems is one factor controlling task-specific encoding of new vs. familiar events, which under some circumstances can also produce “wrong” encoding followed by erroneous performance (Figures 4B&C).
These features of hierarchical processing are uniquely adaptable to neural elements that have high levels of synaptic connections with multiple cells. Hierarchical array encoding is the only way to segregate information in a manner that can be labeled and identified on subsequent occurrences of the same events. The extent to which such systems are utilized also provides a more rapid means of processing and less necessity to establish every new functional synaptic connection for different events. In addition, hierarchical systems can become more efficient via specific synaptic enhancement as a function of repetitive employment in similar types of encoding and recognition conditions. Hierarchical encoding is not random and is a logical means of representing differential information in smaller numbers of cells with multiple interconnections. Because establishment of hierarchical encoding depends on elevated synaptic connectivity under specific environmental conditions, it is likely that use-dependent plasticity via synaptic enhancement such as LTP is required for the establishment of Conjunctive and TT cells. This is evidenced by hierarchical processing’s reliance on temporal synchrony, revealed by spatiotemporal firing in which input-output patterns occur within the timeframe necessary for correct behavioral responding (Figs 2&4). Hence, detecting hierarchical encoding in neural systems such as hippocampus is expected, given the degree of processing required for the type of information retention and retrieval associated with hippocampal-dependent cognitive tasks (O’Keefe and Nadel, 1978; Zola-Morgan et al., 1986; Lynch et al., 2008; Lynch et al., 2013; Hampson et al., 2013).
Without such hierarchical encoding and associated use-dependent plasticity, memory processes are inflexible and incapable of adapting to changes in the environment as reflected in many clinical circumstances. The large number of Simple cells within associated hierarchical circuits in structures like hippocampus (Fig 3A&B) provides the necessary basis for efficient encoding and recognition within unrestricted cognitive and behavioral contexts. This also provides the means to rapidly alter feature extraction after errors (Fig 4B&C), because hierarchical circuits contain at least some of the synaptic connections required to re-encode elements appropriately on subsequent trials. By showing how large groups of neurons parcel and differentiate stimulus features critical for behavioral decisions, important insight into how brain structures like hippocampus can self-organize to provide adaptation to environmental circumstances, have now been obtained and applied (Goonawardena et al., 2010; Chan et al., 2011; Marmarelis et al., 2011; Song et al., 2013). Knowing that these structures have this capability provides the additional capacity for extending the neural basis of memory to neuroprosthetics that can restore or enhance memory in disease situations where some of the same neural circuitry has been impaired (Berger et al., 2011; Hampson et al., 2012a; Hampson et al., 2013).
Highlights.
Hippocampal neurons encode information via hierarchical networks of task features
Neuron ensembles form spatio-temporal “codes” for information to be remembered
Single hierarchically activated hippocampal cells can encode entire memory contexts
Hierarchical networks allow for different memories in the same neural population
Acknowledgements
The authors thank the following individuals for their technical and analytic assistance: Andrew J. Sweatt, Ph.D., Vasilis Z. Marmarelis, Ph.D., Lucas M. Santos, Ph.D., Jeff Atwell, Joshua Long, Joseph Noto, Brian Parrish, Christina Dyson, Chad Collins, and Frances Miller. Research contributing to this article was supported in part by National Institutes of Health Grants DA06634, DA023573, DA026487 (to S.A.D.); by Defense Advanced Research Projects Agency (DARPA) contracts N66001-09-C-2080 & N66001-14-C-4016 (to S.A.D.) and N66001-09-C-2081 (to T.W.B.); and by National Science Foundation grant EEC-0310723 (to T.W.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham WC. How long will long-term potentiation last? Philos Trans R Soc Lond B Biol Sci. 2003;358:735–744. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger TW, Hampson RE, Song D, Goonawardena A, Marmarelis VZ, Deadwyler SA. A cortical neural prosthesis for restoring and enhancing memory. Journal of Neural Engineering. 2011;8:046017. doi: 10.1088/1741-2560/8/4/046017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger TW, Song D, Chan RH, Marmarelis VZ, LaCoss J, Wills J, Hampson RE, Deadwyler SA, Granacki JJ. A hippocampal cognitive prosthesis: multi-input, multi-output nonlinear modeling and VLSI implementation. IEEE Trans Neural Syst Rehabil Eng. 2012;20:198–211. doi: 10.1109/TNSRE.2012.2189133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyeler M, Dutt ND, Krichmar JL. Categorization and decision-making in a neurobiologically plausible spiking network using a STDP-like learning rule. Neural Netw. 2013;48:109–24. doi: 10.1016/j.neunet.2013.07.012. doi: 10.1016/j.neunet.2013.07.012. Epub@2013 Aug 14.:109-124. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 6.Brasselet R, Panzeri S, Logothetis NK, Kayser C. Neurons with stereotyped and rapid responses provide a reference frame for relative temporal coding in primate auditory cortex. J Neurosci. 2012;32:2998–3008. doi: 10.1523/JNEUROSCI.5435-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerda M, Girau B. Asymmetry in neural fields: a spatiotemporal encoding mechanism. Biol Cybern. 2013;107:161–178. doi: 10.1007/s00422-012-0544-0. [DOI] [PubMed] [Google Scholar]
- 8.Chan RH, Song D, Goonawardena AV, Bough S, Sesay J, Hampson RE, Deadwyler SA, Berger TW. Tracking the changes of hippocampal population nonlinear dynamics in rats learning a memory-dependent task. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3326–3329. doi: 10.1109/IEMBS.2011.6090902. [DOI] [PubMed] [Google Scholar]
- 9.Cox CD, Rex CS, Palmer LC, Babayan AH, Pham DT, Corwin SD, Trieu BH, Gall CM, Lynch G. A map of LTP-related synaptic changes in dorsal hippocampus following unsupervised learning. J Neurosci. 2014;34:3033–3041. doi: 10.1523/JNEUROSCI.4159-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deadwyler SA, Berger TW, Sweatt AJ, Song D, Chan RH, Opris I, Gerhardt GA, Marmarelis VZ, Hampson RE. Donor/recipient enhancement of memory in rat hippocampus. Front Syst Neurosci. 2013;7:120. doi: 10.3389/fnsys.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42:465–476. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- 12.Goonawardena AV, Robinson L, Riedel G, Hampson RE. Recruitment of hippocampal neurons to encode behavioral events in the rat: Alterations in cognitive demand and cannabinoid exposure. Hippocampus. 2010;20:1083–1094. doi: 10.1002/hipo.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampson RE, Deadwyler SA. Temporal firing characteristics and the strategic role of subicular neurons in short-term memory. Hippocampus. 2003;13:529–541. doi: 10.1002/hipo.10119. [DOI] [PubMed] [Google Scholar]
- 14.Hampson RE, Gerhardt GA, Marmarelis VZ, Song D, Opris I, Santos L, Berger TW, Deadwyler SA. Facilitation and restoration of cognitive function in primate prefrontal cortex by a neuroprosthesis that utilizes minicolumn-specific neural firing. J Neural Eng. 2012a;9:056012. doi: 10.1088/1741-2560/9/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hampson RE, Pons TP, Stanford TR, Deadwyler SA. Categorization in the monkey hippocampus: A possible mechanism for encoding information into memory. Proc Natl Acad Sci U S A. 2004;101:3184–3189. doi: 10.1073/pnas.0400162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampson RE, Simeral JD, Deadwyler SA. Distribution of Spatial andNonspatial Information in Dorsal Hippocampus. Nature. 1999;402:610–614. doi: 10.1038/45154. [DOI] [PubMed] [Google Scholar]
- 17.Hampson RE, Simeral JD, Deadwyler SA. What ensemble recordings reveal about functional hippocampal cell encoding. Prog Brain Res. 2001;130:345–57. doi: 10.1016/s0079-6123(01)30023-7. 345-357. [DOI] [PubMed] [Google Scholar]
- 18.Hampson RE, Song D, Chan RH, Sweatt AJ, Riley MR, Gerhardt GA, Shin DC, Marmarelis VZ, Berger TW, Deadwyler SA. A nonlinear model for hippocampal cognitive prosthesis: memory facilitation by hippocampal ensemble stimulation. IEEE Trans Neural Syst Rehabil Eng. 2012b;20:184–197. doi: 10.1109/TNSRE.2012.2189163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampson RE, Song D, Chan RH, Sweatt AJ, Riley MR, Goonawardena AV, Marmarelis VZ, Gerhardt GA, Berger TW, Deadwyler SA. Closing the loop for memory prosthesis: detecting the role of hippocampal neural ensembles using nonlinear models. IEEE Trans Neural Syst Rehabil Eng. 2012c;20:510–525. doi: 10.1109/TNSRE.2012.2190942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hampson RE, Song D, Opris I, Santos LM, Shin DC, Gerhardt GA, Marmarelis VZ, Berger TW, Deadwyler SA. Facilitation of memory encoding in primate hippocampus by a neuroprosthesis that promotes task-specific neural firing. J Neural Eng. 2013;10:066013. doi: 10.1088/1741-2560/10/6/066013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaliukhovich DA, Vogels R. Decoding of repeated objects from local field potentials in macaque inferior temporal cortex. PLoS One. 2013;8:e74665. doi: 10.1371/journal.pone.0074665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura R, Kang S, Takahashi N, Usami A, Matsuki N, Fukai T, Ikegaya Y. Hippocampal polysynaptic computation. J Neurosci. 2011;31:13168–13179. doi: 10.1523/JNEUROSCI.1920-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- 24.Li CJ, Lu Y, Zhou M, Guo LJ. Electrophysiological properties of hippocampal-cortical neural networks, role in the processes of learning and memory in rats. J Neural Transm. 2014;121:583–592. doi: 10.1007/s00702-014-1164-8. [DOI] [PubMed] [Google Scholar]
- 25.Lynch G, Cox CD, Gall CM. Pharmacological enhancement of memory or cognition in normal subjects. Front Syst Neurosci. 2014;8:90. doi: 10.3389/fnsys.2014.00090. doi: 10.3389/fnsys.2014.00090. eCollection@2014.:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch G, Kramar EA, Babayan AH, Rumbaugh G, Gall CM. Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology. 2013;64:27–36. doi: 10.1016/j.neuropharm.2012.07.006. doi: 10.1016/j.neuropharm.2012.07.006. Epub@2012 Jul@20.:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 29.Marmarelis VZ, Shin DC, Song D, Hampson RE, Deadwyler SA, Berger TW. Dynamic nonlinear modeling of interactions between neuronal ensembles using principal dynamic modes. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3334–3337. doi: 10.1109/IEMBS.2011.6090904. [DOI] [PubMed] [Google Scholar]
- 30.Marmarelis VZ, Shin DC, Song D, Hampson RE, Deadwyler SA, Berger TW. Nonlinear modeling of dynamic interactions within neuronal ensembles using Principal Dynamic Modes. J Comput Neurosci. 2013;34:73–87. doi: 10.1007/s10827-012-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathis A, Herz AV, Stemmler M. Optimal population codes for space: grid cells outperform place cells. Neural Comput. 2012;24:2280–2317. doi: 10.1162/NECO_a_00319. [DOI] [PubMed] [Google Scholar]
- 32.Moser E, Kropff E, Moser M. Place cells, grid cells, and the brain’s spatial representation system. Annual review of neuroscience. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 33.O’Keefe JA. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 34.O’Keefe JA, Nadel L. The Hippocampus as a Cognitive Map. Clarendon Press; Oxford: 1978. [Google Scholar]
- 35.Plakke B, Diltz MD, Romanski LM. Coding of vocalizations by single neurons in ventrolateral prefrontal cortex. Hear Res. 2013;305:135–43. doi: 10.1016/j.heares.2013.07.011. doi: 10.1016/j.heares.2013.07.011. Epub@2013 Jul 26.:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotman Z, Klyachko VA. Role of synaptic dynamics and heterogeneity in neuronal learning of temporal code. J Neurophysiol. 2013;110:2275–2286. doi: 10.1152/jn.00454.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safaai H, von HM, Sorando JM, Diamond ME, Maravall M. Coordinated population activity underlying texture discrimination in rat barrel cortex. J Neurosci. 2013;33:5843–5855. doi: 10.1523/JNEUROSCI.3486-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song D, Wang H, Tu CY, Marmarelis VZ, Hampson RE, Deadwyler SA, Berger TW. Identification of sparse neural functional connectivity using penalized likelihood estimation and basis functions. J Comput Neurosci. 2013;35:335–357. doi: 10.1007/s10827-013-0455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- 40.Zanos TP, Hampson RE, Deadwyler SA, Berger TW, Marmarelis VZ. Functional connectivity through nonlinear modeling: An application to the rat hippocampus. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5522–5525. doi: 10.1109/IEMBS.2008.4650465. [DOI] [PubMed] [Google Scholar]
- 41.Zola-Morgan SM, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. Journal of Neuroscience. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]