Abstract
This article is aimed at providing a review of the progress made in the field over the period 2011 to present in order to expand in parts on two previous reviews (S. Karenga and Z. El Rassi, Electrophoresis, 2011, 32, 90-104; D. Gunasena and Z. El Rassi, Electrophoresis, 2012, 33, 251-261). In brief, this review article describes progress made in nonpolar and polar monoliths used in reversed phase HPLC and CEC (RPC/RP-CEC) and in hydrophilic interaction liquid chromatography/CEC (HILIC/HI-CEC), respectively. This article is by no means an exhaustive review of the literature; it is rather a survey of the recent progress made in the field with 69 references published on nonpolar and polar polymeric monoliths.
Keywords: Organic monoliths, Capillary electrochromatography, High performance liquid chromatography, Reversed phase chromatography, Hydrophilic interaction liquid chromatography, Hydrophilic interaction capillary electrochromatography
1 Introduction
Organic polymer monolithic stationary phases have been in the past two decades a subject of interest to the separation science community since they are known to possess, among other things, the ability to withstand a wide range of operational conditions and to provide solutions for a variety of separation problems. Thus, it is the aim of this current review article to describe the latest developments (2011- present) regarding organic monolithic columns including their fabrication strategies, solute retention principles and applications. Briefly, there are two main approaches for the fabrication of monolithic columns: (i) functionalization by direct copolymerization and (ii) post-polymerization modification [1]. The second strategy is adopted when significant portion of the functional ligands remain buried in the polymer network [2]. Both routes involve the judicious choice of the components of the polymerization mixture, namely, the functional monomer, crosslinker, porogenic mixture and initiator and the mode of the external stimulus used to initiate the polymerization process, such as heat or UV irradiation etc. [3]. Therefore, this review article is attempting to provide an adequate description of the various parameters involved in the preparation of polar and nonpolar monoliths that have been published over the past 4 years.
This review article is composed of two distinct parts. The first part is about nonpolar organic monoliths while the second part is concerned with polar organic monoliths and their variants. The nonpolar organic monolith part is subdivided into (i) methacrylate/acrylate based monoliths, (ii) styrene divinylbenzene based monoliths and (iii) hypercrosslinked monoliths. On the other hand, the part on polar organic monoliths involving mostly methacrylate-derived monoliths classifies the monoliths according to their surface charged groups (e.g., neutral, anionic, cationic, and zwitterionic monoliths) as well as their post-polymerization functionalization.
2 Nonpolar organic monoliths for RPC separations
There are three commonly used organic monolithic stationary phases, namely, acrylamide- [4], acrylate/methacrylate- [5] and styrene-based [6] monoliths. The latter two are the most suited monoliths for RPC and RP-CEC applications.
2.1 Methacrylate/acrylate copolymers based monoliths
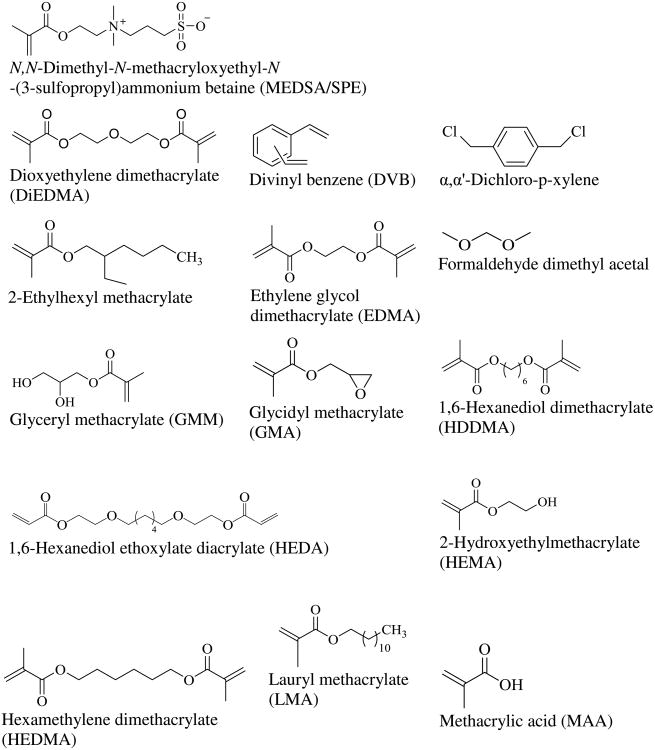
Generally, the acrylate/methacrylate-based monoliths are more polar than their styrene-divinylbenzene (SDVB) monolith counterparts. Various approaches were introduced for the formation of different kinds of monoliths mainly by the choice of the functional monomers and crosslinking monomers. These two main variables will be discussed in the sections below. For the sake of clarity and convenience the structures of the functional and crosslinking monomers used for nonpolar and polar monoliths are shown in Fig. 1.
Figure 1.
Structures of functional monomers and crosslinkers used in the preparation of nonpolar and polar monoliths described in this article. For convenience, the monomers and crosslinkers are arranged alphabetically.
2.1.1 Nature of functional monomers
In order to obtain a generalization about the effect of alkyl chain length and shape of the functional monomer on the structural features of the monolith, Urban et al. used different alkyl methacrylate monomers with butyl, cyclohexyl, 2-ethyl hexyl, lauryl, and stearyl functional groups in the polymerization mixture with ethylene glycol dimethacrylate (EDMA) as the crosslinker (for structures of monomers, see Fig. 1). 1-Propanol and 1,4-butanediol (BDO) were chosen as the porogens in all cases [7]. Alkylbenzenes and standard proteins were used as test solutes, and the standard parameters such column permeability, methylene and phenyl selectivity were assessed. The highest permeability was observed for butyl methacrylate (BMA) monolith and the least for the lauryl methacrylate (LMA) while the other three monoliths showed similar permeability. When it comes to methylene and phenyl selectivity there was no direct correlation with the size of the monomer molecule. The lowest selectivity was observed on the LMA-based monolith, while BMA-based monolith showed the highest phenyl selectivity and the stearyl methacrylate(SMA)-based monolith possessed the highest methylene selectivity. Lauryl and cyclohexyl methacrylate provided marginally better separations for the tested standard proteins by gradient elution in HPLC.
The effect of the alkyl chain length of the functional monomers on solute retention was also investigated by Puangpila et al [8] in CEC. The authors reported neutral monoliths (void of fixed charges) to completely eliminate electrostatic interactions nuisance created between charged solutes and the otherwise surface attached charged moieties, an approach that was originally described by Okanda and El Rassi [9] and recently by Karenga and El Rassi [10]. Two different series of monolithic columns were prepared with C8, C12 and C16 surface bound chains using the same crosslinking monomer pentaerythritol triacrylate (PETA). One series of monoliths (called series A) was produced by adjusting the composition of functional monomers and crosslinker to obtain comparable solute retention regardless of the alkyl chain length and another series of monoliths (referred to as series B) was prepared by maintaining the same composition of functional monomers and crosslinker yielding chromatographic retention, which increased as expected in the order of increasing the n-alkyl chain length. A ternary porogenic solvent made of cyclohexanol, ethylene glycol and water was used. The C16-monolith of the A series yielded the highest separation efficiency towards small solutes, but the A columns series were inadequate for protein separation. The C8-monolith of the B series provided the best separation efficiency for proteins. For tryptic peptide mapping, the C16-monolith of the A series seems to provide the best separation. For large protein molecules, the energetically “softer” C8 surface allowed faster sorption kinetics and in turn improved efficiency, while an energetically “harder” C16 surface favored better separation of the smaller size peptide solutes. In brief, neutral, polar and charged solutes (e.g., proteins and peptides) were separated efficiently and the results were in agreement with previously reported work on neutral monoliths by El Rassi and coworkers [9, 11].
In a novel approach to achieving unique selectivity, mixed ligand monolithic (MLM) columns were prepared for CEC experiments [12]. These columns were made by the copolymerization of different compositions of octadecyl acrylate (ODA) and 2-naphthyl methacrylate (NAPM) functional monomers in the presence of trimethylolpropane trimethacrylate (TRIM) crosslinker and a ternary porogenic solvent made up of cyclohexanol, ethylene glycol and water. The combined retentive property of the ODA ligand, which is solely hydrophobic, with that of the NAPM ligand, which is both hydrophobic and π-interaction provider was exploited in the CEC of neutral, polar and charged solutes. As expected the magnitude of the electroosmotic flow (EOF) changed with the composition of the MLM. As the percent of the monomer ODA in the polymerization mixture was increased, the EOF increased to a maximum at 50-mol% ODA and then leveled off at 75-mol% and 100-mol% ODA an indication that the ODA ligand in general exhibited a higher binding for the mobile phase ions than the NAPM monomer. This is due to the fact that the ODA is an acrylate-based monomer whereas the NAPM is a methacrylate based monomer. It was found out that columns with a given composition of both ligands yielded a unique selectivity for a given set of solutes that was not matched by columns made by either ODA or NAPM alone (see Fig. 2 for illustration). Several test mixtures were used in the evaluation of the MLM columns including polycyclic aromatic hydrocarbons, alkyl phenyl ketones, nitroalkanes, alkylbenzenes, toluene derivatives, peptides, and proteins. Peptide mapping of the tryptic digest of the standard lysozyme protein was also studied.
Figure 2.

Electrochromatograms showing the separation of 5 alkylbenzenes and 5 PAHs on monolithic columns with different mol fraction ODA/NAPM. Capillary column, 20 cm effective length, 27 cm total length × 100 μm ID; mobile phase, 70 % ACN, 1mM sodium phosphate monobasic, pH 7.0, running voltage 20 kV; electrokinetic injection for 3 s at 10 kV. Solutes: 1, benzene; 2, toluene, 3, ethylbenzene; 4, 1-nitronaphthalene; 5, butylbenzene; 6, fluorene;7, 9-anthracenecarbonitrile; 8, heptylbenzene; 9, 9-nitroanthracene; and 10, fluoranthene. EOF tracer: thiourea. Reproduced with permission from Ref. [12].
Furthermore, Karenga and El Rassi also developed a series of segmented monolithic columns (SMC) [13] and demonstrated their applications in CEC. These SMCs were made up of two adjoining segments each filled with a different monolith in the aim of controlling and manipulating the EOF, retention and selectivity in RP-CEC. In these SMCs one capillary segment was filled with a naphthyl methacrylate monolith (NMM) to provide hydrophobic and π-interactions while the other capillary segment was filled with an octadecyl monolith (ODM) to provide solely hydrophobic interaction. The ODM segment not only provided hydrophobic interactions but also functioned as the EOF accelerator segment (see Fig. 3). The average EOF of the SMC increased linearly with increasing the fractional length of the ODM segment. The neutral SMC provided a convenient way for tuning EOF, selectivity and retention in the absence of annoying electrostatic interactions and irreversible solute adsorption. The SMCs allowed the separation of a wide range of neutral solutes including polycyclic aromatic hydrocarbons (PAHs) that are difficult to separate using conventional alkyl bonded stationary phases. In all cases, the k´ of a given solute was a linear function of the fractional length of the ODM or NMM segment in the SMCs, thus facilitating the tailoring of a given SMC to solve a given separation problem. At some ODM fractional length, the fabricated SMC allowed the separation of charged solutes such as peptides and proteins that could not otherwise be achieved on a monolithic column made from NMM as an isotropic stationary phase due to the lower EOF exhibited by this monolith.
Figure 3.

Variation of EOF velocity (mm/s) with fractional length of capillary filled with ODM/NMM. Capillary column, 20 cm effective length, 27 cm total length × 100 μm i.d.; mobile phase, 70% ACN, 5mM sodium phosphate monobasic, pH 7.0; running voltage, 20 kV. EOF tracer, thiourea. Reproduced with permission from Ref. [13].
In a recent work, Niu et al [14] reported a monolith made of bisphenol A epoxy vinyl ester resin (VER) as the functional monomer along with EDMA as the crosslinker using a ternary porogenic system composed of 1-propanol, BDO and water. The monolith produced was found to have a high surface area of about 500 m2g-1 as determined by nitrogen adsorption. Polymerization was carried out in fused-capillaries (150 μm i.d.) and the ratio of VER to EDMA was carefully adjusted along with the porogens to obtain very high permeability. The mechanical stability of the column was also evaluated by studying the backpressures at the column inlet vs. the flow rate. Furthermore, the separation of benzene derivatives was demonstrated by HPLC, with a column efficiency of around 200,000 plates/m. The separations seemed to be superior on this column when compared to that obtained on a poly (BMA-co-EDMA) monolith.
Triallyl isocyanurate (TAIC) [15] (see Fig. 1 for structure of TAIC) was used as a functional monomer along with trimethylolpropane triacrylate (TMPTA) as the crosslinker to prepare a monolith using atom transfer radical polymerization (ATRP) with CCl4 as an initiator and FeCl2 as a catalyst. The monolith was prepared in stainless steel columns (5 cm × 4.6 mm i.d.) and in fused-silica capillaries (100 mm × 150 μm i.d.) for their applications in HPLC and CLC, respectively. The composition of the porogens and the polymerization temperature by ATRP process were further optimized to get the desired mechanical characters and the monoliths were characterized by using FT-IR and SEM imaging. The backpressures obtained at the column inlet showed a fairly linear relationship with the linear velocity of the mobile phase. Separation of some aromatic compounds was demonstrated in both columns of analytical and capillary format.
In order to evaluate the repeatability in column preparation of a reversed-phase C18 monolith, Kositarat et al. [16] prepared the monolith poly(SMA-co-EDMA) by varying the polymerization temperature and performing the polymerization in three different polymerizing apparatus, namely water bath, hot air and GC oven. The obtained monolithic columns were evaluated by studying the retention times of neutral aromatic compounds in CEC experiments using fused-silica capillaries of 100-μm i.d. × 23.5 cm effective length and 32 cm total length. Firstly, increasing the polymerization time gave increased retention times; this is common for all heating apparatus, which might be due to formation of smaller pores at high temperatures as a result of fast polymerization. Also, the formation of free radical initiators at elevated temperatures might be another reason for the small pores. The temperatures 55°C and 60°C were selected for water bath and ovens, respectively, for conducting the polymerization and the % relative standard deviation for the results obtained for the three columns prepared were 9.0, 6.5 and 12.5% for GC oven, hot air oven and water bath, respectively. This indicates that hot air oven produced more reproducible results than the rest. Furthermore, the interday and intraday repeatability studies showed good precision for data obtained for retention times, peak area and efficiency for the hot air oven. Apart from these studies, selectivity of the monolithic columns with respect to the separation of tocopherols (TOH) or vitamin E homologues were performed and compared with other C-8 and C-18 monolithic columns and silica counterparts which were previously reported. The difficult to separate components, mainly the β-TOH, γ-TOH isomers were baseline resolved with Rs of 1.1 (see Fig. 4A). The authors claim that this monolith offer a better selectivity due to presence of the residual polar ester groups on the surface which would provide the HILIC advantage in interacting with the hydroxyl groups of the tocopherols. The previously reported C-8 microparticulate columns and the C-18 silica based columns could not separate the β and the γ isomers (Rs = 0.0).
Figure 4.

(A) CEC separation of tocopherols homologues on the SMA-EDMA monolithic column. Conditions: capillary column dimensions 23.5 cm × 100 μm i.d.; detection, 200 nm; applied voltage +30 kV, 7:93(v/v); 20mM Tris Buffer pH 9: acetonitrile; column temperature, 30°C; electrokinetic injection at +10 kV for 30s. Reproduced with permission from Ref. [16] (B) A Nano LC separation of tocopherol homologues on the poly (AOD-co-EDMA) monolithic column. Conditions: column dimensions 180mm × 100 μm i.d.; mobile phase: MeOH/H2O (93.5/6.5, v/v); detection, 292 nm; flow rate: 500nl/min; injection volume: 20nl. Reproduced with permission from [17].
Recently, a novel monolithic column with double C-18 chains was introduced by Duan et al. [17] for RPC whereby the functional monomer 3-methylacrylol-3-oxapropyl-3-(N,N-dioctadecylcarbomyl)-propionate (AOD) was co-polymerized with the crosslinker EDMA. Fused-silica capillary columns (100 μm i.d.) were used in the preparation of the monolithic column for μHPLC. For comparison studies, a C-18 monolith namely poly (SMA-co-EDMA) was also prepared according to the authors previous work [18]. A binary porogenic mixture of 2-methyl-1-propanol and BDO was used. When the monomer percentage was varied from 50-70 % w/w an increase in the backpressure from 0.6-13.0 MPa at a flow rate of 500 nL/min was observed. Furthermore, it was also observed that increasing the percentage of 2-methyl-1-propanol from 75% to 95% w/w increased the backpressure from 0.6-13.0 MPa. The SEM pictures were in agreement with these results obtained. Poly (AOD-co-EDMA) monolith showed better theoretical plate height of 19.2μm than poly (SMA-co-EDMA) monolith, which was 32.1μm at linear velocity of 0.85 mm/s at similar conditions. This column showed good permeabilities with ACN, MeOH and water. Chemical stability was investigated by running highly acidic and basic buffer for prolonged 30 h period and no prominent degradation was observed. The run-to-run, day-to-day repeatability studies and batch-to-batch reproducibility studies were investigated, which also gave satisfactory results. Van Deemter plots and the methylene selectivity studies were also carried out to evaluate the column efficiency and the reversed phase retention behavior, respectively. Alkylphenones were used as test solutes and the methylene selectivity was observed to be as 1.68, which was similar to 1.70 obtained with the poly (SMA-co-EDMA) monolithic column. 16 polyaromatic hydrocarbons (PAH) were separated on the poly (AOD-co-EDMA) monolithic column by using gradient elution. Again TOH was used to test this column, and a complete separation of α-, β-, γ-, and δ- TOH isomers was obtained in in less than 30 min (see Fig. 4B). Baseline separation was achieved for three different standard proteins in less than 8 min in reversed phase gradient elution.
2.1.2 Nature of crosslinking monomers
A set of eight different crosslinkers were evaluated in the preparation of a series of reversed-phase monolithic capillary columns for use in nano LC and their effect on the separation efficiency was systematically investigated [19]. The eight different dimethacrylate crosslinking monomers were copolymerized with LMA as the functional monomer in the presence of the porogens BDO and 1-propanol using a thermally initiated free radical polymerization. The crosslinkers used have repeated methylene and oxymethylene units, including EDMA, tetramethylene dimethacrylate, hexamethylene dimethacrylate, dioxyethylene dimethacrylate, trioxyethylene dimethacrylate, tetraoxyethylene dimethacrylate, pentaerythritol dimethacrylate, and bisphenol A dimethacrylate (see Fig. 1 for structures of monomers). The effects of crosslinker on the monolith morphological features such as pore size distribution and permeability were studied along with the separation efficiencies of the columns using alkylbenzenes and standard proteins as the test solutes. The separation efficiency of the monolithic columns of small molecules significantly improved with the increasing number of repeat nonpolar methylene groups in the crosslinking monomers, which correlated with increasing number of small pores with size less than 50 nm. The thinnest plate height for alkylbenzenes of 25 μm was obtained on the columns prepared with hexamethylene dimethacrylate. The authors reported that the columns prepared with polar (poly)oxyethylene dimethacrylate crosslinkers showed also enhanced separation efficiency with increasing chain length and in particular better performance than that of the (poly)methylene dimethacrylate crosslinkers of comparable size with less apparent effect of the chain lengths on pore size distribution. In fact, a thinner plate height of 15 μm was obtained on the columns made with tetraoxyethylene dimethacrylate crosslinker with approx. 70,000 plates/m. Also, good permeability and column-to-column reproducibility were achieved with the monolith prepared with the tetraoxyethylene dimethacrylate crosslinker. On the other hand, for the separation of proteins, baseline resolution was achieved for column prepared with hexamethylene dimethacrylate as the crosslinker. Four standard proteins, namely insulin from bovine serum pancreas, cytochrome C, bovine serum albumin and β-lactoglobulin were separated on the three monolithic columns by gradient elution in nano LC method. The separation improved from the EDMA based column to the tetramethylene dimethacrylate based column and baseline separation was achieved in less than 4 min for the hexamethylene dimethacrylate based monolith. On the other hand, the monoliths made with the crosslinkers of repetitive oxymethylene groups (see Fig. 1 for structures) showed very poor efficiency for the separation of proteins.
In a very recent work, Liu et al. [20] introduced a monolithic column for HPLC by the free-radical co-polymerization of a mixture of two functional monomers, TMPTA along with N-isopropylacrylamide (NIPAAm) and the crosslinker EDMA. The polymerization process yielded a highly crosslinked poly (TMPTA-co-NIPAAm-co-EDMA) monolith in a 50 mm × 4.6 mm i.d. stainless steel column. Flow rates of up to 7 mL/min were achieved indicating good mechanical stability and permeability of the column. Furthermore, separation of benzene, biphenyl and phenanthrene was demonstrated on the column by isocratic elution. Reproducibility and stability of the column were evaluated along with the effect of column temperature on solute retention. As expected, the analysis time was shortened from ∼20 min to ∼10 min by increasing the column temperature from 25°C to 70°C, which is typical for temperature effect in LC. In another work from the same laboratory, Bai et al. [21] used the TMPTA along with EDMA to produce a dense network of nonpolar monolith in a 50 mm × 4.6 mm i.d. stainless steel column and used it for the RPC separations of small aromatic molecules.
1,6-Hexanediol ethoxylate diacrylate, a new crosslinker (see Fig. 1 for structure), was reported by Lin et al. [22] for making nonpolar monoliths for RPC separations in capillaries. The suitability of this new crosslinker was studied with three alkyl methacrylate functional monomers, namely BMA, LMA and SMA. The co-polymerization was achieved in the presence of the porogenic mixture of 1-propanol and BDO. For comparing the retentive abilities of these monoliths, the authors also made alkyl methacrylate monolithic columns with EDMA and 1,6-hexanediol dimethacrylate as the crosslinker in 250-μm i.d. fused silica capillaries. In situ thermally initiated free radical polymerization was performed in all cases. Owing to the presence of the two ether linkages, the 1,6-hexanediol ethoxylate diacrylate monolithic columns has an added advantage of dipole-dipole interactions in the separation of polar analytes. A 30% (w/w) alkyl methacrylate concentration was maintained in all the cases, and the conversion of the monomer was maximal with 1,6-hexanediol ethoxylate diacrylate than for EDMA and 1,6-hexanediol dimethacrylate. The permeabilities, porosities and the separation efficiencies of the 18 different monoliths were compared of which the 1,6-hexanediol ethoxylate diacrylate based alkyl methacrylate monoliths was found to have the highest column efficiencies of up to 14,800 plates/m for uracil. The poor performance for the EDMA and the 1,6-hexanediol dimethacrylate column was attributed to their poor conversion rate in the formation of the polymer. The 1,6-hexanediol ethoxylate diacrylate based columns were investigated for their reproducibility and separation of mixture of eight phenyl urea herbicides samples.
In a strategy to increase the surface area of the monolith, Li et al. [23] used the approach of solely using crosslinking monomers to produce nonpolar monoliths a concept that was originally introduced by El Rassi and coworkers [9, 24, 25]. Bisphenol A dimethacrylate, bisphenol A ethoxylate diacrylate and pentaerythritol diacrylate monostearate were used to produce nonpolar monolithic capillary columns for RPC of small molecules. Tetrahydrofuran (THF) and decanol proved useful porogens for the formation of bisphenol A ethoxylate diacrylate (where EO/phenol = 4) monoliths, while dimethylformamide (DMF) along with decanol were adequate for the formation of bisphenol A dimethacrylate monoliths. The selection of the porogens was on the basis of the dipole moment and the solubility parameter. Selecting porogenic mixture for the poly(pentaerythritol diacrylate monostearate) was found to be challenging because of the rigid structure of the pentaerythritol diacrylate monostearate, and most of the generally used porogenic solvents gave either a hard monolith or a soft gel like substance with this monomer. Since the structure of the pentaerythritol diacrylate monostearate is similar to a surfactant, the authors predicted that a nonionic surfactant would be a potential candidate for the preparation of the monolith. Tri block poly(ethylene oxide)-poly(propylene oxide)-poly(ethyleneoxide) (PEO-PPO-PEO) or PPO-PEO-PPO, was used as the porogenic solvent. Different compositions of the porogenic mixtures were tried and for all the copolymers photo polymerization was carried out for 4 min and alkylbenzenes and alkylparabens were used as test solutes in both gradient and isocratic nano LC. Baseline separations were achieved for alkylbenzenes and alkylparabens when used with poly(bisphenol A ethoxylate diacrylate), poly(bisphenol A dimethacrylate) and poly(pentaerythritol diacrylate monostearate) monolithic columns in gradient elution. The poly (bisphenol A ethoxylate diacrylate) yielded an average plate number of 30,000 plates/m, whereas the poly (bisphenol A dimethacrylate) and the poly (pentaerythritol diacrylate monostearate) exhibited a maximum of around 61,000 plates/m and 21,000plates/m, respectively. These columns showed good permeabilities and highly repeatable results. This may be due to the single monomer approach in the synthesis of the monoliths, which involves lesser variables in the synthesis.
In a more recent work from the same laboratory, Lui et al. [26] further exploited the above concept of using single crosslinking monomers to produce highly crosslinked polymeric monoliths for RPC in 75 μm i.d. fused-silica capillary columns. Alkanediol methacrylates monomers, namely, 1,4-butanediol dimethacrylate, 1,5-pentanediol dimethacrylate, 1,6-hexanediol dimethacrylate, 1,10-decanediol dimethacrylate, 1,12-dodecanediol dimethacrylate, 1,3-butanediol dimethacrylate, and neopentyl glycol dimethacrylate were used in this study (see Fig. 1 for structures). Dodecanol and methanol were selected as the porogens for the synthesis of the monoliths. The columns showed good mechanical stability, permeability and reproducibility. Alkylbenzenes and parabens were separated using gradient elution and baseline separations were achieved in both cases. The plate numbers for all the monolithic columns were observed between 14,000 and 35,000 plates/m.
In a further report, the same group demonstrated the use of highly crosslinking polymers made from various dimethacrylate of C6 functional groups namely, 1,6-hexanediol dimethacrylate, cyclohexanediol dimethacrylate and 1,4-phenylene diacrylate in the separation of small molecules (e.g., alkylbenzenes) by RPC using the nano LC format. The 1,6-hexanediol dimethacrylate column exhibited the highest efficiency (86,000 plates/m) with the least permeability. Van Deemter plots were also used to illustrate the column separation efficiency vs. the linear flow velocity of the mobile phase. Also, the run-to-run and column-to-column reproducibilities were evaluated with the test analytes alkylbenzenes, which were separated with baseline resolution in less than 10 min on all the columns. Single monomer approach might be the reason for the repeatability of the monolith fabrication [27]. Isocratic elution of alkylbenzenes was performed at an optimum flow rate of 300 nL/min on the capillary columns made with monolith from these monomers. Whereas the 1,6-hexanediol dimethacrylate and the 1,4-cyclohexanediol dimethacrylate-based monoliths offered baseline resolution for the alkylbenzenes investigated, the 1,4-phenylene diacrylate based monolith showed very poor resolution.
2.1.3 Modified methacrylate monoliths
Recently, Chambers et al. [28] reported porous monoliths functionalized through copolymerization of a C60 fullerene-containing methacrylate monomer for the separations of small molecules. The monoliths consisted of poly(glycidyl methacrylate (GMA)-co-EDMA) and poly(BMA-co-EDMA) capillary columns, which incorporated the new monomer [6,6]-phenyl-C61-butyric acid 2-hydroxyethyl methacrylate (PCB-HEM; see Fig. 1 for structure). Both reported monoliths showed added advantages in the separation of alkylbenzenes. A very poor separation of alkylbenzenes was observed for the parent poly(GMA-co-EDMA) monolith with a number of plates of around 4400 plates/m for benzene, which is the first retained compound exhibiting the highest efficiency. Upon the addition of PCB-HEM, the plate number for benzene increased to around 72,000 plates/m. On the other hand, the parent poly(BMA-co-EDMA) monolith, which is a more hydrophobic monolith than the poly(GMA-co-EDMA) monolith yielded enhanced retention toward alkylbenzene solutes than the less hydrophobic poly(GMA-co-EDMA) monolith, and afforded monolithic columns with an efficiency for benzene exceeding 110,000 plates/m. Column-to-column and run-to-run repeatability were shown.
Apart from C60 fullerenes, Chambers et al. also reported the use of carbon nanotubes in poly(GMA-co-EDMA) monoliths in order to improve the retention abilities of the monolith towards small molecules [29]. Entrapment of the multiwalled carbon nanotubes (MWCNT) in the methacrylate monoliths significantly improved the column efficiency to around 35,000 plates/m, when compared to the parent GMA-EDMA monolith, which was on the order of 1,800 plates/m. In addition, the authors tried to attach the MWCNT onto the surface of the monolith by passing the oxidized nanotubes onto amino modified GMA-EDMA monolith as a second approach. Six alkylbenzenes were separated on this new MWCNT modified monolithic columns exhibiting an efficiency of 44,000 plates/m.
In a different recent investigation, Aqel et al. reported the incorporation of MWCNT in benzyl methacrylate-co-EDMA monolithic columns in capillary LC [30]. The amount of nanotubes was varied between 0 and 0.4 mg/mL in the polymerization solution with the porogens cyclohexanol, BDO and butanol. Capillaries of 0.32 mm i.d. were used for making the monolithic columns. Alkylphenones and phenol, which were chosen as the test solutes showed significant improvement in separation efficiencies between the parent monolith and the one, which was incorporated with MWCNT.
Very recently, Mayadunne and El Rassi [31] reported the incorporation of MWCNT into monolithic columns for the separations of small and large molecules in HPLC. Two approaches for incorporating carbon nanotubes into monolithic columns were described in this report. They pertain to the investigation of carbon nanotubes either (i) as entities to modulate solute retention on monolithic columns bearing well defined retentive ligands or (ii) as entities that constitute the stationary phase responsible for solute retention and separation. Approach (i) involved the incorporation of carbon nanotubes into ODM columns while approach (ii) concerns the preparation and evaluation of an ideal monolithic support and coating it with carbon nanotubes to yield a real “carbon nanotube stationary phase” for the HPLC separation of a wide range of solutes. First, an ODM column based on the in situ polymerization of ODA and TRIM was optimized for use in HPLC separations of small and large solutes (e.g., proteins). To further modulate the retention and separation of proteins, small amounts of carbon nanotubes were incorporated into the octadecyl monolith column. In approach (ii), an inert, relatively polar monolith based on the in situ polymerization of glyceryl monomethacrylate (GMM) and EDMA proved to be the most suitable support for the preparation of “carbon nanotube stationary phase”. This carbon nanotube “coated” monolith proved useful in the HPLC separation of a wide range of small solutes including enantiomers (see Fig. 5). In approach (ii), a more homogeneous incorporation of carbon nanotubes into the diol monolithic columns (i.e., GMM/EDMA) was achieved when hydroxyl functionalized carbon nanotubes were incorporated into the GMM/EDMA monolithic support. In addition, high power sonication for a short time enhanced further the homogeneity of the monolith incorporated with nanotubes. In all cases, nonpolar and π interactions were responsible for solute retention on the monolith incorporated carbon nanotubes.
Figure 5.

Separation of the enantiomers of 2,4-dichlorophenoxypropionic acid, Dns-phenylalanine, Dns-methionine and bupivacaine in (A), (B), (C) and (D), respectively, obtained on MN3-15 monolithic column. Mobile phase in (A), ACN:H2O at 40:60 (v/v) containing 50 mM sodium acetate, pH 4.1; mobile phase in (B) and (C): ACN:H2O at 35:65 (v/v) containing 25 mM sodium acetate, pH 4.1; mobile phase in (D): ACN:H2O at 55:45 (v/v) containing 0.1% TFA. Reproduced with permission from Ref. [31]
Apart from MWCNT and the C60 fullerenes, graphene oxide (GO) is another carbonaceous nanoparticles that have attracted some attention in recent years due to its unique structure and excellent physiochemical and mechanical characteristic features, including the high surface area and the π-π stacking ability of GO. Thus, the incorporation of GO into a monolithic column for CEC separations was introduced [32] and this incorporation not only imparted the monolith a high surface area, π-π interactions and hydrophobicity but also introduced into the monolith oxide functional groups for efficient EOF generation for CEC separations. GO incorporated into poly (methacrylic acid (MAA)-co-EDMA) was prepared in fused-capillaries of 75-μm i.d. This monolith showed enhanced separation of some neutral and polar aromatic compounds than its parent monolith. Furthermore, the separation of some aniline compounds was also demonstrated on the column. Along the same lines, another column with GO incorporated into the poly(GMA-co-EDMA) monolith was made which facilitated the separation of small molecules via RPC than the parent monolith [33]. The GO was modified with 3-(trimethoxysilyl)propyl methacrylate and polymerized along with GMA and EDMA in a 50.0 mm × 4.6 mm i.d. stainless steel column. Separation of some steroids and aniline compounds was demonstrated on the column.
2.2 Styrene divinyl benzene-based monoliths
The effects of MAA on the morphological and chromatographic properties of the traditional poly(styrene-co-divinylbenzene (DVB)) monoliths were studied by Svobodova et al [34]. Poly(styrene-co-DVB-co-MAA) was prepared and applied for the separation of some aromatic compounds by RPC in the CLC format. The results were compared to those obtained on the monolith prepared without adding MAA. Baseline separation was achieved with a column efficiency of around 28,000 plates/m. These results indicate that the contribution of the MAA is not only merely imparting charge to the monolith surface for the generation of EOF in CEC but also affect the morphological features of the separation medium. Figure 6 shows the influence of the presence of MAA on the separation of a typical set of small molecules on the poly(styrene-co-DVB-co-MAA) monolith. While no separation was achieved using the column polymerized without MAA and all the components co-eluted in a single large peak, the monolith with added MAA allowed the baseline separation of all the eight analytes, with a separation efficiency of 28,000 plates/m.
Figure 6.

A CLC separation of small organic molecules using poly (styrene-co-divinyl benzene) columns (A) without MAA and (B) with MAA. Conditions: mobile phase, acetonitrile/water (65/35, v/v); UV detection, 214 nm; flow rate, 4 μl/min; inj, 100 nL; effective column length, 170 mm. Peak identification (1) thiourea, (2) phenol, (3) aniline, (4) benzene, (5) toluene, (6) ethyl benzene, (7) propyl benzene and (8) butyl benzene. The concentrations of the analytes in the mixture were in the range 0.2-0.5 mg/ml. Reproduced with permission from Ref. [34].
The porosity of the monoliths can be efficiently controlled by organotellurium mediated living radical copolymerization [35]. This way of finely tuning the monolith porosity has been found to be effective in optimizing the micro/mesopores ratio, which has facilitated the separation of small molecules. Different textures of monolith can be prepared compared to that of traditional poly(styrene-co-DVB) monolith. Alkylbenzenes were taken as probe solutes to demonstrate the separation ability of the column under isocratic elution. The controlled living polymerization opted by the researchers gave highly regular macropores and intrinsically less heterogeneous networks, which facilitated the separation of small molecules in RPC by isocratic elution.
A new monolith for RPC and ion-pair RPC was prepared by using N-vinylcarbazole (NVC) and DVB by thermally initiated free radical copolymerization [36] in both 200 μm and 100 μm i.d. capillary columns and the retentive ability of different peptides, proteins and oligonucleotides were investigated by nano LC. The surface area and the morphological characters of the monolith thus obtained were characterized by N2 adsorption, mercury intrusion porosimetery and SEM. The multipoint BET gave a very high surface area of about 160 m2g-1. These monoliths exhibited high permeability for the common reversed-phase solvents, namely, acetonitrile, methanol and tetrahydrofuran. A peptide mixture sample containing 9 different standard peptides were separated in less than 7 min in the 200 μm i.d. column, however when using a 100 μm i.d. column a better resolution could be achieved, see Fig. 7. Furthermore, baseline separation of six standard proteins was obtained in less than 7 and 5 min on 100-μm i.d. and 200-μm i.d. columns, respectively. According to the authors, this might be due to the fact that the inner diameter determines the optimal flow rate for the monolith, which in turn affects the separation efficiency. The peptides vasopressin [Arg8] and methionine encephalin were not baseline separated on the 200 μm i.d. column, but the 100 μm i.d. column gave better separation and the resolution increased from 1.04 to1.79 and the average plate height decreased nearly 37%. Ion-pair RPC was performed by adding 0.1 M triethylammonium acetate to the mobile phase, thus allowing the separation of oligonucleotides by isocratic elution. The measured separation efficiency was around 41,000 plates per column.
Figure 7.

Separation of 9 peptides, namely, 1) bradykinin fragment 1-5, 2) vasopressin [Arg8], 3) methionine enkephalin, 4) leucine enkephalin, 5) oxytocin, 6) bradykinin, 7) LHRH, 8) bombesin and 9) substance P, on columns with different dimensions, A) 70 mm × 0.2 mm i.d. separation conditions: 1-30% B in 7.5 min, 60°C, UV 214 nm, inj.: 1μL, 1 ng each peptide, flow rate: 4 μL min-1. B) 70 mm × 0.1 mm i.d. column; separation conditions: 1-30% B in 5 min, 70°C, UV 214 nm, inj.: 100nl, 0.2 ng each peptide. Reproduced with permission from Ref. [36].
2.3 Hypercrosslinked monoliths
Another post polymerization modification aiming at producing highly crosslinked networks involves an old technique introduced by Davankov [37] nearly four decades ago. This technique uses Friedel-Crafts alkylation to further connect the free ends of styrene and chloromethyl styrene monomers. By adopting this approach, new class of columns called hypercrosslinked monolithic columns have evolved with substantially increased specific surface areas when compared to that of the precursor or parent monoliths [38, 39].
Recently, two monolithic columns, namely poly(styrene-co-vinylbenzyl chloride (VBC)-co-DVB) poly(4-methylstyrene(MST)-co-VBC-co-DVB) were hypercrosslinked and evaluated in CEC [40]. The hypercrosslinking was achieved by flushing the two monolithic columns with 1,2-dichloroethane in the presence of Fe3+ as the catalyst. These monoliths showed enhanced specific surface areas over the parent monolith (i.e., without hypercrosslinking). Owing to the increase in the specific surface area a 34-fold increase in EOF was observed for hypercrosslinked poly(styrene-co-VBC-co-DVB) monoliths and a 21-fold increase in EOF was achieved for hypercrosslinked poly(MST-co-VBC-co-DVB). These results confirm the positive effect of the enlarged surface area upon hypercrosslinking on the adsorption of ions from the mobile phase and the generation of EOF. The EOF was stable over a long period of time as was demonstrated with numerous separations using the same column. Six alkylbenzenes were separated in less than 8 min and 6 min on poly(styrene-co-VBC-co-DVB) and poly (MST-co-VBC-co-DVB), respectively.
In another work, new type of hypercrosslinked poly(styrene-co-DVB) monoliths were formed by using a Fe3+ catalyzed Friedel–Crafts alkylation involving three external crosslinkers [41], namely 4,4´-bis(chloromethyl)-1,1´-biphenyl, α,α ´-dichloro-p-xylene and formaldehyde dimethyl acetal (see Fig. 1 for structures of the crosslinkers). Of the three external crosslinkers, 4,4´-bis(chloromethyl)-1,1´-biphenyl was found to produce monoliths with the best chromatographic performance. The effects of a number of variables affecting the hypercrosslinking reaction were studied in detail. Monoliths with extremely large surface areas reaching up to 900 m2/g were obtained using a precursor monolith polymerized for only 2.5 h, and hypercrosslinked with 4,4´-bis(chloromethyl)-1,10-biphenyl. The hypercrosslinked monoliths in capillary columns were evaluated in RPC using a mixture of solute probes composed of acetone and six alkylbenzenes. Under isocratic elution conditions, separation efficiencies exceeding 70,000 plates/m were readily achieved for retained analytes.
3 Polar organic monoliths for HILIC separations
Although normal phase chromatography (NPC), which uses polar stationary phases with nonpolar organic mobile phases, provides a variety of chromatographic solutions for the separation, purification and fractionation of a wide range of solutes, it has its own limitations in separating highly polar analytes. NPC of hydrophilic samples is difficult because of the problems associated with dissolving hydrophilic materials in non-aqueous mobile phases [42]. Furthermore, NPC suffers from poor reproducibility for hydrophilic compounds and poor ionization efficiency for mass spectrometry [43]. Also, most of the hydrophilic samples are poorly retained on reversed phase columns eluting near the dead time of the column or exhibiting little or no resolution. All these facts together triggered Alpert about 25 years ago to introduce a novel chromatographic technique called “hydrophilic interaction liquid chromatography” (HILIC) for the separation of peptides, nucleic acids and other polar compounds [44]. In HILIC, a polar (hydrophilic) stationary phase is used with an organic rich hydro-organic mobile phase in order to separate polar analytes with better separation efficiencies. The use of organic-rich mobile phase in HILIC provides some additional advantages making this technique more popular. These advantages include low column backpressure, which allows fast separation of analytes with shorter analysis time and suitability of HILIC to direct coupling with MS detection (e.g., ESI-MS).
Similarly to other modes of interactive chromatography, the mobile phase composition has strong influence on the retention of polar solutes in HILIC and hydrophilic interaction capillary electrochromatography (HI-CEC). Usually, HILIC/HI-CEC is run using a high organic, low aqueous mobile phase. Under these HILIC conditions, a water-rich layer is formed on the monolithic surface. The separation is achieved by the partitioning of polar solutes in between this adsorbed water layer on the stationary phase surface and the organic rich hydro-organic mobile phase. However, a simple retention mechanism is not possible for most compounds since in addition to a partition mechanism, hydrogen bonding, dipole-dipole, ion-dipole, and ion-ion interactions are also involved [43].
As a complementary technique to RPC (see above section), HILIC often provides an excellent separation for polar analytes [43]. Porous organic polymer monolithic columns have been used extensively as HILIC stationary phases during the last few years due to their distinct advantages including (i) high permeability which leads to lower backpressure, (ii) low resistance to mass transfer, (iii) simple preparation procedure and ease of surface functionalization, (iv) stability under extreme pH conditions, and (v) a wide selection of functional monomers [43, 45]. A variety of organic polymer monoliths with amino [46, 47], amide [48], hydroxyl [48], sulfoalkylbetaine [43, 49], boronic acid [50], and some other functionalities have been reported for use in HILIC/HI-CEC separations.
There have been relatively large numbers of nonpolar organic polymer monoliths reported (see the previous section), in contrast to a relatively lesser number of HILIC monoliths that have been reported in the literature. This is mainly due to the lack of commercially available polar monomers and limited solubility of highly polar monomers in commonly used porogenic solvents [43, 45, 51].
Several excellent articles can be found in the literature, which reviews the preparation, applications and recent advances in designing monolithic columns for HILIC [52-54]. Recently, Gunasena and El Rassi have reviewed organic monoliths for HI-CEC/HILIC and immunoaffinity chromatography as well [55].
3.1 Monomer and crosslinker composition in HILIC/HI-CEC
As mentioned in the introduction of section 2, organic polymer monoliths can be classified into three major categories based on their polymer backbone chemistry, which directly depends on the nature of the crosslinker. These are (i) styrene-, (ii) acrylamide- and (iii) acrylate/methacrylate-based monoliths. Among them, the majority of the reported HILIC organic polymer monoliths are based on methacrylate derived monoliths because of their unique properties including high chemical stability over a wide pH range, excellent mechanical properties, flexibility for modification and good reproducibility. The chemistry of the crosslinking monomer is an important parameter affecting the properties of HILIC monolithic columns prepared by using single-step polymerization procedures [45, 56].
Staňková et al. [57] have studied the effect of crosslinkers on the performance of polar monolithic columns intended for use in HILIC applications. They have synthesized seven different polymethacrylate monolithic capillary columns using N,N-dimethyl-N-methacryloxyethyl-N-(3-sulfopropyl) ammonium betaine (MEDSA) functional monomer and various crosslinking monomers differing in polarity and size including ethylene glycol dimethacrylate (EDMA), tetramethylene dimethacrylate (BUDMA), hexamethylene dimethacylate (HEDMA), dioxyethylene dimethacrylate (DiEDMA), PETA, bisphenol A dimethacrylate (BIDMA) and bisphenol A glycerolate dimethacrylate (BIGDMA). The authors have come to several important conclusions based on their experimental results: (i) the type of the crosslinker affects the pore size and distribution, (ii) the efficiency of monolithic columns for polar, low molecular weight compounds in the HILIC mode depends rather on the polarity than on the size of the crosslinker molecules and no clear correlation is apparent between the crosslinker methylene chain length and the column separation efficiency, (iii) the proportion of mesopores in the polar zwitterionic polymethacrylate monolithic columns depends on the length of the crosslinking dimethacrylate in the polymerization mixture, however, the mesopore size and distribution depend more significantly on the polarity of the crosslinkers and (iv) the zwitterionic monolithic columns prepared with polar crosslinkers show dual RP-HILIC separation mechanisms, depending on the sample properties, and on the mobile phase composition [57].
Jandera et al. have prepared 0.53 and 0.32 mm i.d. microcolumns by the in situ copolymerization of zwitterionic sulfobetaine functional monomer MEDSA with bisphenol A glycerolate dimethacrylate (BIGDMA) and dioxyethylene dimethacrylate (DiEDMA) crosslinker in the presence of 1-propanol, BDO, and water as the porogens [58]. The separation efficiency and selectivity of the 0.53 mm i.d.poly(MEDSA-co-BIGDMA) column did not change significantly even after more than three weeks of continuous use, which is much better than the 0.53 mm i.d. poly(MEDSA-co-DiEDMA) column, which lost approximately half of its separation efficiency after 30 h of use, probably because the pore morphology in the monolith prepared with the DiEDMA crosslinker is less stable over the longer operation time across a larger cross-section in comparison to BIGDMA crosslinker. Some significant differences between the separation selectivity on the poly(MEDSA-co-DiEDMA) and poly(MEDSA-co-BIGDMA) monolithic columns were observed including the reverse order of elution for some solutes. The poly(MEDSA-co-BIGDMA) column provided a remarkable baseline separation of diastereomers (+) catechin and (-) epicatechin, containing two chiral carbon atoms, whereas those two compounds completely co-eluted on the poly(MEDSA-co-DiEDMA) column. Both poly(MEDSA-co-BIGDMA) and poly(MEDSA-co-DiEDMA) columns were used for the separation of phenolic acids and flavonoid compounds in one-dimensional chromatography (1D) in the HILIC mode. The HILIC analysis of a mixture of antioxidants containing 32 phenolic acids and flavonoids on the 0.53 mm i.d. monolithic columns under gradient conditions showed an incomplete separation. Therefore, Jandera and coworkers have coupled these zwitterionic HILIC columns with a short nonpolar core shell column in the second dimension, for comprehensive 2D LC separations of phenolic and flavonoid compounds [58].
Some of the commonly used crosslinkers and functional monomers in the preparation of HILIC monoliths are illustrated in Fig. 1. The polarity and charge of the functional monomer is also very important in designing a HILIC/HI-CEC monolithic column. In another classification, the polarity and the charge of the stationary phases can be used to classify HILIC monoliths into (i) neutral monoliths, (ii) cationic monoliths, (iii) anionic monoliths and (iv) zwitterionic monoliths.
3.1.1 Neutral monoliths
Neutral monoliths are widely used as HILIC/HI-CEC separation media. Gunasena and El Rassi have reported a hydroxyl monolith for HI-CEC containing polar PETA as the crosslinker and glyceryl methacrylate (GMM) as the functional monomer [48]. Cyclohexanol, dodecanol and water were used as porogens in the polymerization mixture. The functional monomer GMM and the crosslinker PETA have hydroxyl groups in their structures, which impart the GMM/PETA monolith with hydrophilic interaction sites. Compared to silica-based stationary phases, the polymer backbone of methacrylate-based monolith is hydrophobic [51]. Hence, in order to prepare more hydrophilic polymer backbone, a polar crosslinker PETA has been used instead of the traditional EDMA crosslinker. The hydroxyl sublayer of PETA can give increased hydrophilicity to the monolithic backbone. Although without fixed charges on the surface, the hydroxyl monolith showed cathodal EOF. It was believed that the EOF was due to the adsorption of mobile phase ions (e.g., acetate ions) onto the monolithic surface, which imparts the monolith with the zeta potential necessary to generate an adequate EOF. Most interestingly, the magnitude of the EOF was increased with increasing the %ACN in the mobile phase, thus confirming the mobile phase ion adsorption onto the neutral polar monolithic surface. This hydroxyl monolith was successfully used to separate polar solutes including phenols and phenolic derivative, nucleic acid bases and nucleosides (see Fig. 8 for illustration) [48].
Figure 8.

Separation of three phenols (A), four phenol derivatives (B) and three other compounds (C) obtained on the neutral hydroxy monolith. Condition: hydro-organic mobile phase, 5 mM NH4Ac (pH 8.0) at 95% ACN (v/v), running voltage 20 kV, column temperature 20 °C, sample injection, pressure at 5 bar for 10 s. Solutes in C are DMF, formamide and thiourea (left to right). Reproduced with permission from Ref. [48]
A novel poly(N-acryloyltris(hydroxymethyl)aminomethane-co-pentaerythritol triacrylate), poly(NAHAM-co-PETA) monolith has been prepared by Chen et al. and its usefulness investigated in CLC [51]. The polymer monolith was synthesized by the in situ copolymerization of the functional monomer NAHAM and the crosslinker PETA in the presence of polyethylene glycol (PEG) in dimethyl sulfoxide as the porogens. The poly(NAHAM-co-PETA) monolith thus obtained can be viewed as a neutral but polar monolith with hydroxyl and amide functionalities. Separations of polar analytes on this column are mainly due to hydrophilic interactions between the separated analytes and the surface hydroxyl groups of the monolith. The ratios of monomer (NAHAM) to crosslinker PETA were optimized by increasing the NAHAM content. Using the homogeneity and backpressure as assessment criteria, the authors have found that the monolith was detached when the NAHAM content was increased, which can be explained by the fact that the decreased content of the crosslinker led to an insufficient formation of firm bonds of the monolith to the inner wall of the capillary. In order to find the appropriate permeability and the highest possible specific surface area, five types of PEG with different molecular weight were investigated as the porogenic solvents. It has been found that the backpressure of the monoliths decreased when the molecular weight of PEG increased. In addition, the specific surface areas of the monoliths decreased monotonously with increasing the molecular weight of PEG, which indicated that increasing the chain length of PEG can result in larger through-pore and smaller surface area. The performance of poly(NAHAM-co-PETA) monolith column for the separation of neutral polar and charged compounds has been evaluated in details. Efficient separation of five nucleosides, seven benzoic acids and five anilines were achieved on this monolithic column. In addition, by combining the poly(NAHAM-co-PETA) monolith with another extraction monolith, an online solid phase micro-extraction was developed, which was successfully applied for the rapid and sensitive determination of four nucleosides in urine samples.
3.1.2 Cationic monoliths
Monoliths with positively charged surfaces have been used in HILIC/HI-CEC as well. These cationic monolithic columns have been viewed as hydrophilic interaction/strong anion-exchange (HI-SAX) monoliths in many studies.
Recently, Gunasena and El Rassi [48] have reported a cationic HI-CEC monolith column designated as AP monolith that resulted from the in situ copolymerization of the crosslinker EDMA and the functional monomer N-(3-aminopropyl) methacrylamide hydrochloride (NAPMH) in the presence of methanol, cyclohexanol and dodecanol as porogens. This cationic AP monolith possesses amine/amide functionalities on its surface. Significant changes in the chromatographic properties including retention factor (k′) and selectivity factor (α) were observed with subtle changes in the monomer composition in the polymerization mixture. The k′ values for polar solutes decreased as the percentage of the crosslinker EDMA in the polymerization solution was decreased. This could be due to fact that when the percentage of the crosslinker EDMA was decreased it might have affected the structure and the porosity of the AP-monolith, hence decreasing the monolith performance. The solubility of the polar monomer NAPMH, which is a solid compound at room temperature, was limited in the binary porogenic mixture consisting of cyclohexanol and dodecanol and a clear solution could not be obtained. Therefore, a sufficient amount of methanol has been added to the polymerization mixture in order to solubilize NAPMH and obtain a clear solution. Over the pH range studied, the AP monolith showed a strong anodal EOF. The EOF velocity was increased with increasing the pH of the mobile phase up to pH 6 and thereafter the EOF velocity remained unchanged. This behavior was explained by the ionization of surface amino groups and the interplay of the ionization and the shielding effects of the adsorbed mobile phase ions to the surface of the monolithic stationary phase. With increasing the mobile phase pH, the ionization of the amino groups of the AP monolith decreased, which would lower the electrostatic attractions and in turn the neutralization/shielding effect exercised by the negatively charged acetate ions of the mobile phase. This led to an apparent increase in the EOF velocity with increasing pH in the range 4-6. The AP monolith was characterized using various polar solutes including, phenols, substituted phenols and amides. The elution order for these analytes was in agreement with the typical hydrophilic interaction behavior where the most polar analytes were eluted last.
Lin et al. have developed a HILIC monolith using vinylbenzyl trimethylammonium chloride (VBTA) as the functional monomer, bisphenol A glycerolate dimethacrylate (BisGMA) as the crosslinker and methanol plus dodecanol as the porogenic solvents [47]. BisGMA, acting as a polar crosslinking dimethacrylate for polymeric monoliths, is superior to other dimethacrylate crosslinkers due to its relatively high molecular weight and stiff partial aromatic molecular structure, providing low polymerization shrinkage, low volatility and outstanding mechanical properties for the polymeric monolith. BisGMA contains multiple polar hydroxyl and ester groups, which can give hydrophilic backbone to the polymeric monolith [47]. Ammonium groups in VBTA functional monomer afforded strong electrostatic interaction, and vinylbenzyl group with rigid phenyl framework could be employed to fabricate a stable polymeric structure. With increasing the VBTA concentration in the polymerization solution, the EOF velocities and the k′ values for polar analytes were increased slightly while the permeability of the monolith was decreased. Also, with a high monomer to porogen ratio, the permeability of the obtained poly(VBTA-co-bisGMA) monolith was poor. Furthermore, Lin et al. have also investigated the effect of porogenic solvent on monolithic column performance. These studies showed that the permeability of the column decreased but the column separation efficiencies increased with increasing the methanol content of the polymerization mixture. These observations imply that increasing the methanol content might leads to a smaller pore size in the polymeric aggregation. This reported poly(VBTA-co-bisGMA) monolith afforded hydrophilic and electrostatic interactions for HI-CEC. Also, this monolith showed RP-HILIC mixed mode retention behavior depending on the organic content in the mobile phase. Finally, this poly(VBTA-co-bisGMA) monolith was successfully used to separate benzoic acid derivatives, phenols, nucleosides and nucleobases.
The same research group has reported another HI-SAX monolith for CEC [46]. This HI-SAX monolith was prepared by the copolymerization of 2-(methacryloyloxy) ethyltrimethylammonium methyl sulfate (META) as the functional monomer, PETA as the crosslinker in the presence of binary porogenic solvent mixture consisting of cyclohexanol and ethylene glycol. As the percentage of META increased while keeping the monomer to porogen ratio constant, a noticeable increase in the permeability of the monolith was observed. This observation was the result of reduced polymeric aggregation caused by the decrease in the amount of crosslinker. Also, increasing the percentage of META leads to increasing the hydrophilicity of the monolith as well as the monolith charge density with further increase in the monolith zeta potential. The ammonium groups on the surface can generate a stable anodic EOF. The retention patterns of polar analytes including phenolic compounds and various amides clearly showed hydrophilic interaction/anion exchange mixed mode behavior. Polar phenols and charged basic nucleic acid bases and nucleosides were also used for the evaluation of the separation mechanism of the column (see Fig. 9 for illustration). This HI-SAX monolith was successfully used to separate carboxylic phytohormones with high resolution.
Figure 9.
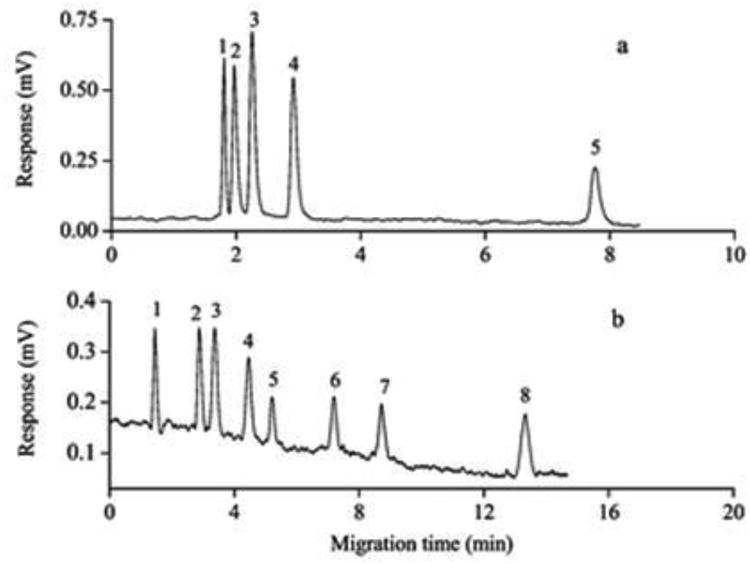
Electrochromatographic profiling of phenols (a) and nucleic acid bases and nucleosides (b) on poly(META-co-PETA) monolith. The profile (a) was recorded in the mobile phase 5 mM ammonium formate, pH 3.0, at 95% (v/v) ACN; pump flow: 0.02 mL min−1; backpressure 250 psi; applied voltage: +10 kV. Solutes: (1)phenol; (2) catechol; (3) hydroquinone; (4) resorcinol; (5) pyrogallol. The profile (b) was recorded in the mobile phase 5 mM ammonium formate, pH 5.0, at 90% (v/v) ACN; the other conditions were the same as for (a). Solutes: (1) uracil; (2) adenine; (3) adenosine; (4) cytosine; (5) uridine; (6)guanine; (7) cytidine; (8) guanosine. Reproduced with permission from Ref. [46].
3.1.3 Anionic monoliths
Monoliths containing surface bound negative charges have been used in HILIC/HI-CEC. For instance, Chen et al. have prepared an anionic poly(methacrylic acid-co-ethylene glycol dimethacrylate), poly(MAA-co-EDMA) monolith for capillary HILIC (cHILIC). The carboxylic acid group on the monolithic surface provides an anionic surface upon deprotonating at high pH. This monolithic column showed homogeneous and continuous column bed, good permeability and narrow pore size distribution. To evaluate the effect of the monomer amount on the porous structure of the polymer monolith, the concentrations of MAA in the polymerization mixture were varied. The results showed that the permeability increased at elevated ratio of MAA to EDMA. Normally, large ion-exchange capacity can be achieved at increased percentage of MAA, but the high content of MAA resulted in heterogeneous column bed [59].
Porosity, permeability and separation efficiencies of polymer based monolithic column depend greatly on the porogenic solution composition in the polymerization mixture. Therefore, the selection of the correct porogenic solvent and the systematic optimization of the composition of the porogenic solvent are very important to generate a better HILIC monolithic column with high separation efficiencies. Chen et al. have studied the effect of hydrophilic and hydrophobic porogenic systems on the porous properties of the poly(MAA-co-EDMA) monolith and its separation efficiencies in HILIC. Two different sets of poly(MAA-co-EDMA) monoliths were prepared using hydrophilic porogen system consisting of PEG in dimethyl sulfoxide and hydrophobic porogen system containing dodecanol and toluene. Scanning electron microscopic (SEM) examinations showed that the homogeneity of hydrophobic porogen monolith was not as good as that in the hydrophilic porogen one. Chromatographic analysis revealed that the separation efficiency of the hydrophilic porogen monolith column was much higher than the hydrophobic porogen column. Compared with the hydrophobic porogen column, the larger ion exchange capacity of the hydrophilic porogen column provides stronger hydrophilic interaction for polar compounds as well as more prominent electrostatic interactions for charged compounds. These results indicated that the hydrophilic porogen might promote the exposure of carboxylic acid groups of MAA on the monolith surface and then lead to a large ion exchange capacity. In addition, the effects of the PEG content on the polymer morphology have been studied. Results showed that with increasing the molecular weight of PEG, the permeability increased but the specific surface area decreased. Increasing the molecular weight of PEG resulted in a solvated system with higher steric hindrance, and therefore larger through pore. And also the permeability of the prepared monoliths increased with increasing the amount of PEG in the polymerization mixture. Under HILIC conditions, five nucleosides were baseline separated using poly(MAA-co-EDMA) monolith. Moreover, this monolithic column was used to separate aniline and benzoic acid derivatives as shown in Fig. 10. Retention patterns of aniline and benzoic acid solutes exhibited a typical hydrophilic interaction with the anionic monolith. Moreover, electrostatic repulsion experienced by the acidic solutes revealed the presence of ion exchange type of interactions in the column. The poly(MAA-co-EDMA) monolith column was used to separate the tryptic digest of bovine serum albumin (BSA) [59].
Figure 10.
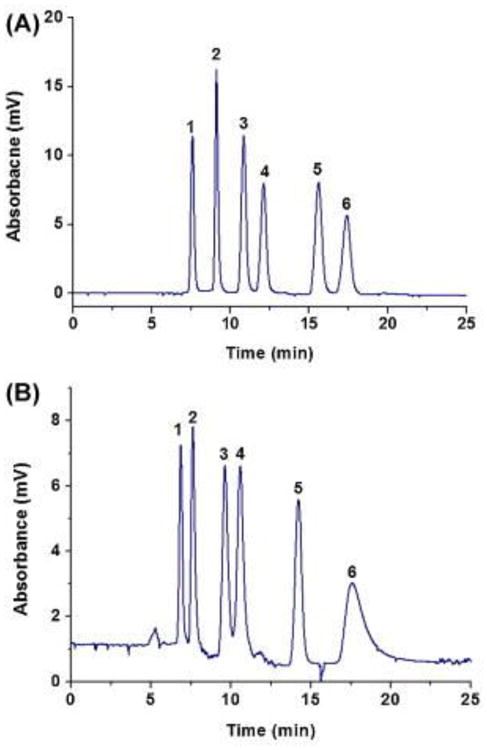
Separation of anilines and benzoic acids on anionic poly (MAA-co-EDMA) monolith (A) Chromatogram of anilines under optimized conditions. (B) Chromatogram of benzoic acids under optimized conditions. Experimental conditions: monolithic capillary column, 100 μm i.d. × 30 cm; UV detection at 254 nm for anilines, 214 nm for benzoic acids; flow rate: 400 nL/min; mobile phase for the separation of anilines: ACN/10 mM ammonium hydroxide (pH 5.0) (90/10, v/v); mobile phase for the separation of benzoic acids: ACN/50 mM ammonium formate (pH 4.0) (90/10, v/v). Order of peaks for (A): (1) toluene; (2) p-nitroaniline; (3) aniline; (4) N-methylaniline; (5) p-phenylenediamine; (6) N,N-dimethylaniline. Order of peaks for (B): (1) Toluene; (2) p-nitrobenzoic acid; (3) benzoic acid; (4) 3,5-dinitrobenzoic acid; (5) p-aminobenzoic acid; (6) o-aminobenzoic acid. Reproduced with permission from Ref. [59].
A novel hydrophilic polymethacrylate based monolith for HI-CEC applications has been prepared by Lin et al. [56]. This monolith was generated by the copolymerization of 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS) functional monomer and PETA crosslinker in the presence of a ternary porogenic solvent consisting of methanol, ethyl ether and water. A decrease in the column permeability and pore size was observed with increasing wt% of AMPS. In addition, an increase in the average pore size and column permeability was observed with increasing the methanol wt% in the polymerization solution. Water was selected as one of the porogen to help dissolve AMPS, because the solubility of AMPS in organic solvents is low. Anionic stationary phase surface was observed at pH 4.5 and above due to the ionization of the sulfonate groups of the AMPS monomer (pKa 1.67). The AMPS molecule contains the hydrophilic sulfonate groups and the hydrophilic acrylamido group, which are connected to a short alkyl chain. This polar AMPS monomer together with the polar PETA crosslinker give a hydrophilic character to the poly(AMPS-co-PETA) monolith. Additionally, a mixed mode of hydrophilic interaction (HI) and strong cation-exchange (SCX) could be also obtained in the analysis of charged peptides, and high column efficiency was achieved without peak tailing. The favorable features of the poly(AMPS-co-PETA) monolith are high hydrophilicity and satisfying stability. Due to the relatively high hydrophilicity of this monolith, good retention and efficient separation of polar analytes such as neutral polar amides, phenols and charged peptides could be obtained with an ACN content of only about 50% v/v in the mobile phase.
Lin et al. have reported a novel polymer monolith with three modes of interactions including reversed phase, hydrophilic and cation-exchange interactions. This monolith was prepared by the in situ copolymerization of GMA and 4-vinylphenylboronic acid (VPBA) functional monomers with the EDMA crosslinker [50]. Similarly to the poly(AMPS-co-PETA) anionic monolith, the boronic acid groups on the monolithic surface become ionized at high pH values and form an anionic monolithic surface. VPBA was chosen as one of the monomers to prepare the mixed mode monolith because it was believed that monoliths containing hydrophilic/ionizable B(OH)2 groups would afford more flexible adjustment of selectivity in terms of hydrophobic, hydrophilic, as well as cation-exchange interactions. Furthermore, VPBA has proven to offer excellent biocompatibility to proteins. Higher content of VPBA in the polymerization mixture showed the formation and aggregation of smaller microglobules inside the capillary during the copolymerization process, which led to a higher backpressure. The polar functionalities of boronic acid-bonded monolith could contribute to the separation of polar analytes in the HILIC mode. Since boronic acid functionalities are weakly acidic(pKa = 8.86), the monolithic surface become more negatively charged as the pH of the mobile phase is increased from pH 4.0 to 9.0. The authors have considered several binary porogenic solvents preferentially including, 1-propanol/BDO, dodecanol/DMF, dodecanol/cyclohexanol, ethyleneglycol/diethylene glycol (DEG), 1-propanol/DEG, BDO/ethylene glycol, and BDO/DEG when preparing the poly (GMA-co-VPBA-co-EDMA) monolith. It was determined that the binary porogen of BDO/DEG was best suited for the solubilization of hydrophobic GMA and EDMA, and hydrophilic VPBA in order to prepare the polymerization solution. Because the polymerization is temperature sensitive, the effect of temperature on the morphology and permeability of the resultant monolithic column was also studied. These results suggested that a high reaction temperature leads to a fast polymerization and tends to form denser and smaller flow-through pores. The column performance was assessed by the separation of series of amides and anilines. Poly(GMA-co-VPBA-co-EDMA) monolith was used to separate alkaloids and proteins successfully due to its RPC and HILIC mixed mode retention behavior.
The preparation of a novel hydrophilic methacrylate based monolith has been reported by Cheng et al. [45] via the in situ copolymerization of the functional monomer 2-hydroxyethylmethacrylate (HEMA) and the polar crosslinker N,N′-methylenebisacrylamide (MBAA). The authors have successfully used this column to separate amines, nucleosides and narcotics with good reproducibility in the HI-CEC mode [45]. During the polymerization process, AMPS was incorporated into the poly(HEMA-co-MBAA) monolithic surface in order to generate an EOF. The zeta potential was mainly provided by the sulfonic acid groups of AMPS, which are totally dissociated when the pH value of the mobile phase exceeds 3.0, which would result in a stable charge density of the monolith. Although the authors have claimed this monolith as a neutral monolith some anionic fixed charges are present on the monolithic surface at pH values greater than 3.0, due to the incorporation of AMPS. The polar sites on the surface of the monolith responsible for hydrophilic interactions are due to the presence of hydroxyl, amide and sulfonic acid groups. A typical HI-CEC behavior was observed on this polar stationary phase. An increase in the content of AMPS increased the charge density of the monolith, and in turn increased the EOF velocity and reduced the analysis time. Dimethyl sulfoxide (DMSO), DMF and water saturated with Na2HPO4 have been used when preparing the poly(HEMA-co-MBAA) monolith. Since MBAA is soluble only in DMSO, DMF, or H2O, a mixed solution containing these three solvents was used. The mixed solution has been saturated with Na2HPO4 because the presence of Na2HPO4 in the porogenic solution can facilitate the aggregation of polymeric chains into the monolithic stationary phase.
3.1.4 Zwitterionic monoliths
Zwitterionic molecules combine both anion and cation groups in a single particle, thus expanding ion-exchange selectivity [43]. Zwitterionic phases exhibit great potential for the separation of polar analytes since they offer the advantage of weak electrostatic interactions between charged analytes and zwitterionic functional groups combined with the high efficiency and selectivity of hydrophilic interactions [60]. Polar sulfoalkylbetaine monomers, possessing both positively charged quaternary ammonium and negatively charged sulfonic groups, have been widely used in the preparation of zwitterionic monolithic columns to be used in HILIC/HI-CEC applications [43, 49, 60, 61].
Recently, Liu et al. described a novel monolithic HILIC column through the in situ copolymerization of the zwitterionic monomer N,N-dimethyl-N-(3-methacryl-amidopropyl)-N-(3 sulfopropyl)ammonium betaine (SPP) and the crosslinker EDMA. In this work, the influence of the EDMA content of the monolith was investigated. Increasing the EDMA weight fraction in the polymerization mixture resulted in a decrease of theoretical plate height. It was also observed that the porosity of monolithic column increased significantly with increasing the weight proportion of EDMA. SEM measurements showed that increasing the weight fraction of EDMA yielded a polymer with bigger microglobules. This observation was not consistent with the previously reported effect of EDMA, and Liu et al. further investigated the reason for this behavior [60]. Methanol has been used as the porogen since it provides sufficient solubility to both the functional monomer and the crosslinker when preparing poly(SPP-co-EDMA) monolith [60]. It was also observed that the porosity of the monolithic column slightly increased with increasing the proportion of methanol in the polymerization mixture. The zwitterionic surface of the poly(SPP-co-EDMA) monolithic column offers the possibility of weak electrostatic interactions with charged analytes, making it possible to develop HILIC separations by manipulating the pH value, ionic strength, and the organic solvent content of the mobile phase. Typical HILIC retention mechanism was exhibited by the poly(SPP-co-EDMA) monolithic column at high ACN content (>60% v/v). This poly(SPP-co-EDMA) monolith was successfully used to separate benzoic acids, phenols and a series of basic and neutral compounds. Also, highly polar ascorbic acid and dehydroascorbic acid were simultaneously separated using the poly(SPP-co-EDMA) monolithic column.
Yuan et al. have used the highly polar monomer N,N-dimethyl-N-acryloyloxyethyl-N-(3-sulfopropyl)ammonium betaine (SPDA) and the hydrophilic crosslinker N,N′-methylenebisacrylamide (MBA) for the preparation of a HILIC monolith to be used in micro-HPLC [49]. Simultaneously, they have prepared another novel monolithic column containing SPDA as the functional monomer and EDMA as the crosslinker. The polarity and separation efficiency of poly(SPDA-co-MBA) monolith column was compared with that of the poly(SPDA-co-EDMA) monolithic column. The functional monomer SPDA showed poor solubility in commonly used porogenic solvents including methanol. To overcome this problem water was used as the co-solvent. But, the crosslinker EDMA showed a low solubility in water. The contrasting solubility between SPDA and EDMA provided a very narrow space for the optimization of the polymerization mixture composition of poly(SPDA-co-EDMA) monolith. Therefore, a relatively high content of MeOH in the porogen was required to dissolve both SPDA and EDMA. Compared to EDMA, the hydrophilic crosslinker MBA showed good solubility in water and it allowed the preparation of monoliths with higher proportion of the functional monomer SPDA in poly(SPDA-co-MBA) monolith. The authors have observed a decrease of column permeability when the weight content of the crosslinker MBA was increased in the polymerization mixture while all other conditions remained the same. Porosity of the poly(SPDA-co-MBA) monolith column clearly increased as the weight content of the porogen increased. The authors have reported that the poly(SPDA-co-MBA) monolith yields better overall separation efficiencies, and greater polarity than the poly (SPDA-co-EDMA) monolith. Also, a significantly enhanced hydrophilicity was observed for the poly(SPDA-co-MBA) monolith compared to the previously reported zwitterionic sulfobetaine monolithic columns, which could be evidenced by the lowered critical composition of the mobile phase corresponding to the transition from the HILIC to the RPC retention mode. It was suggested that the increased hydrophilicity could be the result of the incorporation of the more hydrophilic functional monomer SPDA over that of the highly polar crosslinker MBA. The retention mechanism studies showed that electrostatic interactions could also contribute to the overall retention of charged analytes. The final optimized poly(SPDA-co-MBA) monolith was successfully applied to the separation of a series of polar compounds, such as phenols, bases, benzoic acid derivatives and peptides. It was also suitable for the separation of highly polar compounds, such as allantoin and urea and their determination in one cosmetic product.
In another report, Chen et al. have described a porous monolith for HILIC applications using N,N-dimethyl-N-methacryloxyethyl-N-(3-sulfopropyl)ammonium betaine (SPE) as the functional monomer and poly(ethylene glycol) diacrylate (PEGDA) as the crosslinker in a binary porogenic system comprising isopropanol and decanol. PEGDA, which has an acrylate group at each end of the molecule and a three unit ethylene glycol connecting chain, is a good crosslinker that has been shown to be more biocompatible and more hydrophilic than EDMA [43]. SPE has positively and negatively charged functionalities that can participate in both hydrophilic and ion-exchange interaction mechanism. Methanol was used as the porogenic solvent for preparing poly(SPE-co-PEGDA) monolith and no monolith was observed during the first attempt. This indicates that the methanol was a poor solvent, leading to large pore formation. Therefore, isopropanol was substituted for methanol as the porogen, and a monolith was obtained. Finally, a long chain alcohol, decanol was selected to complement the short chain alcohol isopropanol, as a secondary porogenic solvent. Combination of SPE with the relatively hydrophilic crosslinker PEGDA, yielded poly(SPE-co-PEGDA) monolith, and this monolith showed typical HILIC retention mechanism when the content of ACN in the mobile phase was higher than 60%. Since SPE contains both positive and negative charges, the poly(SPE-co-PEGDA) monolith may exhibit ionic interactions with charged compounds in addition to hydrophilic interactions. As expected, varying the organic solvent concentration, pH and salt concentration of the mobile phase largely affected the selectivity, resolution and peak shapes. The poly(SPE-co-PEGDA) monolith was successfully used to separate amides, phenols and benzoic acid derivatives.
Another zwitterionic monolith has been reported by Foo et al. [61]. It was prepared by the co-polymerization of the zwitterionic functional monomer SPE and the crosslinker 1,2-bis(p-vinylphenyl) ethane (BVPE) in the presence of porogens toluene and methanol. The polymerization reaction was carried out for 1,2,4, 8 and 12h inside a capillary at 75 °C in order to produce the poly (SPE-co-BVPE) monolithic column. SEM images showed that the polymer clusters that are formed become bigger as the polymerization time was increased. Since the mesoporosity can be regarded as a function of the size of microglobules, then it follows that the smaller the microglobules, the higher the fraction of mesopores. SEM images showed that the fraction of mesopores and thus the specific surface area were optimal when the polymerization time was reduced. Furthermore, the column backpressure was increased with increasing the polymerization time. Thus, 2h polymerization time showed the best column efficiencies and this polymerization time has been used for further studies. The chromatographic properties of the optimized poly(SPE-co-BVPE) monolithic column were evaluated with test mixtures containing both basic and neutral compounds in the HILIC mode using a gradient elution. This poly(SPE-co-BVPE) monolith was successfully applied for the rapid and high resolution separation of low molecular weight compounds such as pyrimidines and purines producing sharp and symmetrical peaks using HILIC gradient separation mode.
3.2 Hypercrosslinking and surface modification of HILIC monoliths
The chemical composition and the morphology of polymer based monolithic surface play an important role in determining the selectivity and separation efficiency of a given stationary phase. In this regard, many studies have been devoted to design well defined surface chemistries on monolithic surfaces [62]. Post-polymerization functionalization is a practiced strategy to introduce functional groups on the internal pore surface of monoliths while avoiding tedious re-optimization of the polymerization conditions from monolith to monolith. One of the major advantages of this strategy is that starting from one generic template monolith matrix with optimized mechanical and flow through porous properties, one can prepare other monoliths with the bonded surface ligands of choice [48, 62-66]. To date, glycidyl methacrylate was mainly used as the reactive functional monomer enabling original and smart post-polymerization modification strategies to fine tune the surface chemistry of monolithic columns. During recent years, there was a strong interest among chromatographers to use “click reactions” for the surface modification of template monolithic columns in order to obtain HILIC stationary phases.
In 2001, Sharpless et al. described a new concept for conducting organic reactions, called “click reactions” [67]. The main characteristics of modular click reactions include a) high yields with by-products (if any) that are removable by non-chromatographic process, b) regiospecificity and stereospecificity, c) insensitivity to oxygen or water, d) mild, solventless (or aqueous) reaction conditions, e) orthogonality with other common organic synthesis reactions and f) amenability to a wide variety of readily available starting compounds. Molecular processes considered to fit all or most of these criteria include certain cycloaddition reactions and nucleophilic ring opening reactions. The reaction of thiols with enes, whether proceeding it by a radical (termed thiol-ene reaction) or by an anionic chain (termed thiol Michael addition), carry many of the attributes of click reactions including achieving quantitative yields, having rapid reaction rates, and requiring essentially no clean up. Accordingly, both thiol-ene radical and thiol Michael addition reactions are now routinely referred to in the literature as thiol click reactions (see Fig. 11A) [68].
Figure 11.
(A), Thiol-ene click reactions by (a.) free radical and (b.) Michael addition reactions. (B), Schematic illustration of the synthetic pathway for the preparation of poly(NAS-co-EDMA) with surface-grafted oligo(ethylene glycol) chains. Poly(NAS-co-EDMA) is prepared by photoinitiated free radical copolymerization of NAS and EDMA in the presence of toluene as porogen and AIBN as initiator. Alkene-containing monolith is prepared by surface functionalization of poly(NAS-co-EDMA) through nucleophilic substitution reaction with allylamine, and is used for sequential grafting of thiol-containing oligo-ethylene glycol and mercaptoethanol via thiol-ene photoaddition. Adapted from Ref. [62].
A HILIC monolithic column containing hydroxysuccinimide reactive sites for post polymerization modifications has been designed by Tijunelyte et al. [62]. The functional monomer N-acryloxysuccinimide (NAS) and the crosslinker EDMA were copolymerized in the presence of toluene as the porogenic solvent to yield poly(NAS-co-EDMA) monolith with surface succinimide groups. This macroporous monolith was used as a starting material for the double step surface thiol-ene click post polymerization functionalization. During the first step, allylamine was used for the grafting of alkene units on the surface of poly(NAS-co-EDMA) monolith, via hydroxysuccinimide/alkene nucleophilic substitution. The obtained polymeric network with the surface-grafted allyl units serves as a “clickable” monolith that allows modification of its interfacial properties via the thiol-ene click reaction. During the second step, the pore surface with pendant allyl moieties was further functionalized via two step thiol-ene click reaction with thiol containing oligo(ethylene glycol) and mercaptoethanol as shown in Fig. 11B. This surface grafting yielded a neutral monolith for HI-CEC. Oligo(ethylene glycol) (OEG) was selected as a good candidate because it is well-established that ethylene oxide based molecular fragments tethered on solid surface are prone to hydration. After the OEG grafting, the remaining and accessible double bonds were grafted with mercaptoethanol in a second step thiol-ene click coupling giving a hydroxyl terminus.
A poly(NAS-co-EDMA) monolith has been used by Guerrouache et al. for developing amine based monolith via thiol-yne click surface grafting [65]. During the two-step grafting procedure, the monolith was first reacted with propargylamine, which leads to an amide bond attachment of alkyne groups. In the second step, click grafting of cysteamine was performed under UV irradiation in the presence of 2-hydroxy-2-methyl-1-phenyl-propane as a photoinitiator. These surface grafting yielded NH2 groups on the monolithic pore surface. Stable anodic EOF was observed up to pH 5, above which its magnitude decreased. Reversal of the EOF direction was observed above a pH value of 7 and further increase in the buffer pH values led to a stable cathodal EOF. Though thiol-yne reaction is mechanistically comparable to the thiol-ene one, it may allow higher grafting density because the surface-grafted alkyne can react with two thiols. This monolith was successfully used to separate hydroxyl-substituted aromatic solutes under HILIC conditions.
Yongqin and coworkers have prepared a porous polymer monolith with zwitterionic functionalities via the “thiol-ene” click chemistry [66]. The popular poly(GMA-co-EDMA) monolith was used for this purpose. The epoxy groups of the generic GMA-EDMA monolith were reacted first with cystamine followed by snipping the disulfide bond using tris(2-carboxylethyl)phosphine (TCEP) that liberates the desired thiol groups. Most of the cystamine reacts through both amine functionalities to deliver a more substantial degree of functionalization. Then, the thiol-containing monolith was clicked with the zwitterionic SPE functional monomer. This reaction yielded a monolith with a hydrophilic surface chemistry suitable for separation in HILIC. This monolith was successfully used to separate six peptides by performing gradient elution with decreasing percentage of acetonitrile in the mobile phase. Four nucleosides were also selectively separated on this HILIC monolith with high separation efficiencies.
The surface of the monolith columns can be further modified to yield double layered or stratified monolithic stationary phases. Gunasena and El Rassi have further exploited previously discussed AP-monolith in HI-CEC by modifying its surface with neutral, mono- and oligosaccharides to produce a series of the so-called sugar modified AP-monoliths (SMAP-monolith), which are considered as stratified hydrophilic monoliths possessing a sub-layer of polar amine/amide groups and a top layer of sugar (i.e., a polyhydroxy top layer) [48]. In this regards, N-acetylglucosamine (GlcNAc), chitotriose, and chitopentose were covalently attached to the amine bearing monolithic surface via reductive amination, which converted the primary amine to the much less reactive secondary amines. The SMAP-monolith yielded an anodal EOF, due to the fact that the reductive amination does not remove the amine groups; it rather transforms the primary amines to secondary amines, and therefore the positive charges are still present on the surface of the monoliths to give the positive zeta potential required to maintain the anodal EOF. By modifying the AP-monolith with sugars, different HILIC retention properties were observed. Furthermore, the polarity of the stationary phases and the k′ values of polar solutes increased with increasing number of repeating sugar units in the immobilized saccharides.
Lv and coworkers have developed a novel approach to porous polymer hypercrosslinked monoliths to obtain large surface area with zwitterionic functionalities through the attachment of gold nanoparticles (GNPs) in a layered architecture [64]. The generic poly(4-methylstyrene-co-vinylbenzyl chloride-co-divinylbenzene) monolith was prepared in a capillary and hypercrosslinked via a Friedel-Crafts alkylation forming a plethora of mesopores within the monolith. Then, the free radical bromination was used to manipulate the surface chemistry of the monolith. Brominated monolith was reacted with cystamine in a simpler, more efficient and straightforward approach in a microwave oven. Then, GNPs were attached to this thiol containing monolithic surface. In order to prepare HILIC monolithic column, these GNPs were further modified with cysteine and polyethyleneimine (PEI). Cysteine is a naturally zwitterionic compound that can be utilized for the preparation of monoliths suitable for HILIC separations. Similarly, PEI is known to be an excellent anion-exchange ligand, which also exhibit good hydrophilicity. When comparing the two monoliths, the attachment of PEI produced a column exhibiting better performance than those modified with cysteine alone mainly because of the formation of a dense hydrophilic polymer layer on the surface of the GNPs. Finally, the authors have prepared monolithic columns with layered architecture by embedding a second layer of nanoparticles after modification with PEI, which served as a spacer enabling construction of a dual layer of GNPs that were then functionalized with cysteine. This approach formed a hydrophilic layer consisting of both PEI and cysteine functionalities held together via interactions with GNPs. This dual layered monolith was successfully used for the separation of nucleosides and peptides with higher separation efficiency and resolution.
Škeříková and Urban have used a two-step surface modification of poly(styrene-co-vinylbenzyl chloride-co-divinylbenzene) monolithic stationary phase, including hypercrosslinking and thermally initiated surface grafting of [2-(methacryloyloxy)ethyl]dimethyl(3-sulfopropyl)ammonium hydroxide [63]. The prepared monolith column provided a dual retention mechanism, combining RPC and HILIC. Surface grafting reaction was optimized using response surface methodology. During the model evaluation, three experimental factors including activation time, time of grafting and temperature of grafting were investigated while all other variables were kept constant. According to response surface methodology, the main factor that affects surface modification of hypercrosslinked monoliths was the synergistic effect of grafting time.
4 Concluding remarks
This review article has outlined the various strategies developed over the past four years for making nonpolar and polar monolithic columns for RPC/RP-CEC and HILIC/HI-CEC, respectively. Although polymer-based monolithic columns can be readily prepared and confined in columns in all sizes, these media suffer from low surface area and in turn limited retention toward small solutes. However, their low surface areas make monolithic columns ideal for the efficient separations of biopolymers (e.g., proteins) using gradient elution in HPLC or simply isocratic elution in capillary electrochromatography (CEC). A few nano entities, including carbon nanotubes, carbon and gold nanoparticles and fullerenes have been incorporated in monolithic columns to increase the retention of small solutes in both HPLC and CEC. Also, hypercrosslinked monolithic materials were developed for the same purpose. It is expected that more work will be seen in the near future on post functionalization of monolithic columns for achieving enhanced selectivity and at the same time improving the chromatography of a wide range small molecules.
Table 1. Summary of nonpolar monolithic columns.
| Name of the monolith[poly(functional monomer-co-crosslinker)] | Porogens used | Column i.d./material | Polymerization conditions | Format/Separated Analytes | Ref. |
|---|---|---|---|---|---|
| poly(BMA-co-EDMA) poly(cyclohexyl methacrylate-co-EDMA) poly(2-ethylhexyl methacrylate-co-EDMA) poly(LMA-co- EDMA) poly(SMA-co- EDMA) |
1-propanol BDO | 0.32 mm i.d./fused-silica | thermal initiated polymerization | LC/alkyl benzenes, gradient separation of standard proteins | [7] |
| poly (octyl methacrylate-co-PETA) poly(dodecyl acrylate-co-PETA) poly(cetyl methacrylate-co-PETA) |
cyclohexanol, ethylene glycol, water | 100 μm i.d./fused-silica | thermal initiated polymerization | CEC/alkyl benzenes, proteins, peptides and tryptic digest | [8] |
| Poly(ODA-co-TRIM) poly(NAPM-co-TRIM) |
cyclohexanol, ethylene glycol, water | 100μm i.d./fused-silica | thermal initiated polymerization | CEC/alkyl benzenes, benzene derivatives, poly aromatic hydrocarbons, proteins and peptides | [12, 13] |
| poly(bisphenol A epoxy vinyl ester resin(VER)-co-EDMA) | 1-propanol, BDO, water | 150 μm i.d./fused-silica | thermal initiated polymerization | LC/benzene derivatives halogenated benzenes | [14] |
| poly(TAIC-co-TMPTA) | PEG 200, 1,2-propanediol, dodecanol, 1-hexanediol | 4.6 mm i.d. × 50 mm/stainless steel | atom transfer radical polymerization (ATRP) | HPLC/aromatic compounds | [15] |
| poly(SMA-co-EDMA) | 100μm i.d./fused silica | thermal initiation polymerization | CEC/aromatic compounds/tocopherols | [16] | |
| poly(LMA-co-EDMA) poly(BMA-co-EDMA) poly(GMA-co-EDMA) |
4.6mm i.d. × 50 mm/stainless steel | thermal initiation polymerization | GPEC/polystyrene standards | [69] | |
| poly(AOD-co-EDMA) poly(SMA-co-EDMA) |
2-methyl-1-propanol, BDO | 100μm i.d. fused silica | thermal initiation polymerization | LC/alkylphenones, benzene derivatives, standard proteins, tocopherols | [17] |
| poly(LMA-co-EDMA) poly(LMA-co-tetramethylene dimethacrylate), poly(LMA-co- hexamethylene dimethacrylate), poly(LMA-co-dioxyethylene dimethacrylate), poly(LMA-co- trioxyethylene dimethacrylate), poly(LMA-co-tetraoxyethylene dimethacrylate), poly(LMA-co- pentaerythritol triacrylate) poly(LMA-co- bisphenol A dimethacrylate) |
BDO 1-propanol | 320μm i.d. fused capillaries | thermal initiation polymerization | nano LC/alkyl benzenes, proteins | [19] |
| poly(TMPTA-co-N-isopropylacrylamide-co-EDMA) | PEG, methanol | 4.6 mm i.d. × 50 mm/stainless steel | thermal initiation polymerization (70°C/24h/AIBN) | HPLC/benzene and its substituents | [20] |
| poly(TMPTA-co-EDMA) | PEG, methanol | 4.6 mm i.d. × 50 mm/stainless steel | thermal initiation polymerization (60°C/24h/AIBN) | HPLC/benzene and its substituents | [21] |
| poly(BMA-co-1,6-hexanediol ethoxylate diacrylate), poly(LMA-co-1,6-hexanediol ethoxylate diacrylate), poly(SMA-co-1,6-hexanediol ethoxylate diacrylate) poly(BMA-co-1,6-hexanediol dimethacrylate), poly(LMA-co-1,6-hexanediol dimethacrylate), poly(SMA-co-1,6-hexanediol dimethacrylate) |
1-propanol, BDO | 250 μm i.d./fused silica | thermal initiation polymerization (60°C/2h/AIBN) | LC/phenolic compounds, phenyl urea herbicides | [22] |
| poly(bisphenol A dimethacrylate), poly(bisphenol A ethoxylate diacrylate), poly(pentaerythritol diacrylate monostearate) | PEP-2700, EPE-2800, THF, IPA | 75μm i.d./fused silica | UV irradiation polymerization (390 nm/4 min/DMPA) | LC/alkyl benzenes, alkyl parabens, | [23] |
| poly(1,4-butanediol dimethacrylate), poly(1,5-pentanediol dimethacrylate), poly(1,6-hexanediol dimethacrylate), poly(1,10-decanediol dimethacrylate), poly(1,12-dodecanediol dimethacrylate), poly(1,3-butanediol dimethacrylate), poly(neopentyl glycol dimethacrylate) | methanol, dodecanol | 75μm i.d./fused silica | UV irradiation polymerization (390 nm/3.5 min/DMPA) | LC/alkyl benzenes, alkyl parabens, | [26] |
| poly(1,6-hexanediol dimethacrylate), poly(1,4-cyclohexanediol dimethacrylate), poly(1,4-phenylene diacrylate) | methanol, dodecanol, THF | 75μm i.d./fused silica | UV irradiation polymerization (390 nm/3-4 min/DMPA) | LC/alkyl benzenes | [27] |
Table 2. Summary of polar monolithic columns.
| Category | Name of the monolith [poly(functional monomer-co-crosslinker)] |
Porogens used | Column i.d./ material |
Polymerization conditions |
Format/Separated Analytes |
Ref. |
|---|---|---|---|---|---|---|
| Neutral | Poly(GMM-co-PETA) (hydroxyl monolith) | cyclohexanol, dodecanol and water | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for 15 h with AIBN | HI-CEC/phenols, phenolic derivatives, nucleobases and nucleosides | [48] |
| Neutral | Poly(NAHAM-co-PETA) | PEG in DMSO | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for 12 h with AIBN | CLC/nucleosides, benzoic acids, anilines, nucleosides in urine | [51] |
| Cationic | Poly(NAPMH-co-EDMA) (AP monolith) | cyclohexanol, dodecanol and methanol | 100 μm id/fused silica | Thermal initiation polymerization 50 °C for 24 h with AIBN | HI-CEC/phenols, substituted phenols and amides | [48] |
| Cationic | Poly(VBTA-co-BisGMA) | Methanol and dodecanol | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for 10 h with AIBN | HI-pCEC/benzoic acid derivatives, phenols, nucleosides and nucleobases | [47] |
| Cationic | Poly(META-co-PETA) | Cyclohexanol and ethyleneglycol | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for 20 h with AIBN | pCEC/carboxylic phytohormones | [46] |
| Anionic | Poly(MAA-co-EDMA) | PEG in DMSO or dodecanol and toluene | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for 12 h with AIBN | cHILIC/anilines, benzoic acid derivatives, nucleosides and tryptic digest of BSA, | [59] |
| Anionic | Poly(AMPS-co-PETA) | Methanol, ethylether and water | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for 20 h with AIBN | HI-CEC/neutral polar amides, phenols and charged peptides | [56] |
| Anionic | Poly(GMA-co-VPBA-co-EDMA) | DEG and BDO | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for 24 h with AIBN | CLC/alkaloids and proteins | [50] |
| Anionic | Poly(HEMA-co-MBAA) | DMSO, DMF and H2O with Na2HPO4 | 100 μm id/fused silica | Thermal initiation polymerization 70 °C for 24 h with AIBN | HI-CEC/nucleosides and some narcotics | [45] |
| Zwitterionic | Poly(SPP-co-EDMA) | Methanol | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for various time with AIBN | HILIC/benzoic acid derivatives, phenols, Separation of ascorbic acid from dehydroascorbic acid | [60] |
| Zwitterionic | Poly(SPDA-co-MBA)/Poly(SPDA-co-EDMA) | Methanol and water | 100 μm id/fused silica | Thermal initiation polymerization 60 °C for 12 h with AIBN | HILIC/phenols, benzoic acid derivatives, peptides. Separation of aliantoin and urea in cosmetic products | [49] |
| Zwitterionic | Poly(SPE-co-PEGDA) | Isopropanol and decanol | 75 μm id/fused silica | 3 min under UV radiation with 2,2-dimethoxy, 2-phenyl acetophenone | HILIC/amides, benzoic acid derivatives and phenols | [43] |
| Zwitterionic | Poly(SPE-co-BVPE) | Methanol and toluene | 200 μm id/fused silica | Thermal initiation polymerization 75 °C for 24 h with AIBN | HILIC gradient separation/nucleobases | [61] |
| Zwitterionic | poly(MEDSA-co-BIGDMA) poly(MEDSA-co-DiEDMA) | 1-propanol, BDO and water | 0.53 mm and 0.32 mm id/fused silica | Thermal initiation polymerization 60 °C for 20 h with AIBN | 1D and 2D LC/phenolic and flavonoid compounds | [58] |
Acknowledgments
The financial support by a Grant No. 1R15GM096286-01 from the National Institutes of Health is greatly appreciated.
Nonstandard abbreviations
- ACN
acetonitrile
- AMPS
2-acrylamido-2-methyl-1-propanesulfonic acid
- AOD
3-methylacrylol-3-oxapropyl-3-(N,N-dioctadecylcarbomyl)-propionate
- ATRP
atom transfer radical polymerization
- BDO
1,4-butanediol
- BIDMA
bisphenol A dimethacrylate
- BisGMA/BIGDMA
bisphenol A glycerolate dimethacrylate
- BMA
butyl methacrylate
- BSA
bovine serum albumin
- BUDMA
tetramethylene dimethacrylate
- BVPE
1,2-bis(p-vinyl phenyl)ethane
- CEC
capillary electrochromatography
- cHILIC
capillary hydrophilic interaction liquid chromatography
- CLC
capillary liquid chromatography
- DEG
diethylene glycol
- DiEDMA
dioxyethylene dimethacrylate
- DMF
dimethylformamide
- DMSO
dimethylsulfoxide
- DVB
divinylbenzene
- EDMA
ethylene glycol dimethacrylate
- EOF
electroosmotic flow
- ESI-MS
electrospray ionization mass spectrometry
- GlcNAc
N-acetylglucosamine
- GMA
glycidyl methacrylate
- GMM
glyceryl methacrylate
- GNP
gold nanoparticles
- GO
graphene oxide
- HDDMA
1,6-hexanediol dimethacrylate
- HEDA
1,6-hexanediol ethoxylate diacrylate
- HEDMA
hexamethylene dimethacrylate
- HEMA
2- hydroxyethyl methacrylate
- HIC
hydrophobic interaction chromatography
- HI-CEC
hydrophilic interaction capillary electrochromatography
- HILIC
hydrophilic interaction liquid chromatography
- HI-SAX
hydrophilic interaction/strong anion exchange
- i.d.
inner diameter
- ILs.
ionic liquids
- LCST
lower critical solution temperature
- LMA
lauryl methacrylate
- MAA
methacrylic acid
- MBAA/MBA
N,N′-methylenebisacrylamide
- MEDSA/SPE
N,N-dimethyl-N-methacryloxyethyl-N-(3-sulfopropyl) ammonium betaine
- META
2-(methacryloyloxy) ethyltrimethylammonium methyl sulfate
- MST
4-methylstyrene
- MWCNTs
multi-walled carbon nanotubes
- NAHAM
N-acryloyl tris(hydroxymethyl) aminomethane
- NAPMH
N-(3-aminopropyl) methacrylamide hydrochloride
- NAPM
2-napthyl methacrylate
- NMM
naphthyl methacrylate monolith
- NAS
N-acryloxysuccinimide
- NIPAAm
N-isopropylacrylamide
- NPC
normal phase chromatography
- NVC
N-vinylcarbazole
- ODA
octadecyl acrylate
- ODM
octadecyl monolith
- PAH
polyaromatic hydrocarbons
- PCB-HEM
[6,6]-phenyl-C61-butyric acid 2-hydroxyethyl methacrylate
- PEG
polyethylene glycol
- PEGDA
poly(ethylene glycol) diacrylate
- PEI
polyethyleneimine
- PETA
pentaerythritol triacrylate
- RPC
reverse phase chromatography
- SEM
scanning electron microscope
- SMA
stearyl methacrylate
- SPDA
N,N-dimethyl-N-acryloyloxyethyl-N-(3-sulfopropyl)ammonium betaine
- SPP
N,N-dimethyl-N-(3-methacryl-amidopropyl)-N-(3-sulfopropyl)ammonium betaine
- TAIC
triallyl isocyanurate
- TEAA
triethyl ammonium acetate
- TECP
tris(2-carboxylethyl)phosphine
- TMPTA
trimethylolpropanetriacrylate
- TOH
tocopherol
- TRIM
trimethylolpropane trimethacrylate
- VBC
vinylbenzyl chloride
- VBTA
vinylbenzyl trimethylammonium chloride
- VPBA
4-vinylphenylboronic acid
References
- 1.Tong S, Liu S, Wang H, Jia Q. Chromatographia. 2014;77:5–14. [Google Scholar]
- 2.Svec F. J Chromatogr A. 2010;1217:902–924. doi: 10.1016/j.chroma.2009.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nischang I, Teasdale I, Brüggemann O. Anal Bioanal Chem. 2011;400:2289–2304. doi: 10.1007/s00216-010-4579-6. [DOI] [PubMed] [Google Scholar]
- 4.Hjerten S, Liao JL, Zhang R. J Chromatogr A. 1989;473:273–275. [Google Scholar]
- 5.Tennikova T, Svec F, Belenkii B. J Liq Chromatogr. 1990;13:63–70. [Google Scholar]
- 6.Wang QC, Svec F, Frechet JM. Anal Chem. 1993;65:2243–2248. doi: 10.1021/ac00065a013. [DOI] [PubMed] [Google Scholar]
- 7.Urban J, Jandera P, Langmaier P. J Sep Sci. 2011;34:2054–2062. doi: 10.1002/jssc.201100174. [DOI] [PubMed] [Google Scholar]
- 8.Puangpila C, Nhujak T, Rassi ZE. Electrophoresis. 2012;33:1431–1442. doi: 10.1002/elps.201200018. [DOI] [PubMed] [Google Scholar]
- 9.Okanda FM, El Rassi Z. Electrophoresis. 2005;26:1988–1995. doi: 10.1002/elps.200500073. [DOI] [PubMed] [Google Scholar]
- 10.Karenga S, El Rassi Z. Electrophoresis. 2011;32:90–104. doi: 10.1002/elps.201000490. [DOI] [PubMed] [Google Scholar]
- 11.Karenga S, El Rassi Z. J Sep Sci. 2008;31:2677–2685. doi: 10.1002/jssc.200800310. [DOI] [PubMed] [Google Scholar]
- 12.Karenga S, Rassi ZE. Electrophoresis. 2011;32:1044–1053. doi: 10.1002/elps.201000632. [DOI] [PubMed] [Google Scholar]
- 13.Karenga S, El Rassi Z. Electrophoresis. 2011;32:1033–1043. doi: 10.1002/elps.201000563. [DOI] [PubMed] [Google Scholar]
- 14.Niu W, Wang L, Bai L, Yang G. J Chromatogr A. 2013;1297:131–137. doi: 10.1016/j.chroma.2013.04.076. [DOI] [PubMed] [Google Scholar]
- 15.Zhong J, Hao M, Li R, Bai L, Yang G. J Chromatogr A. 2014;1333:79–86. doi: 10.1016/j.chroma.2014.01.072. [DOI] [PubMed] [Google Scholar]
- 16.Kositarat S, Smith NW, Nacapricha D, Wilairat P, Chaisuwan P. Talanta. 2011;84:1374–1378. doi: 10.1016/j.talanta.2011.03.083. [DOI] [PubMed] [Google Scholar]
- 17.Duan Q, Liu C, Liu Z, Zhou Z, Chen W, Wang Q, Crommen J, Jiang Z. J Chromatogr A. 2014;1345:174–181. doi: 10.1016/j.chroma.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Smith NW, David Ferguson P, Robert Taylor M. J Biochem Biophys Methods. 2007;70:39–45. doi: 10.1016/j.jbbm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Jandera P, Staňková M, Škeříková V, Urban J. J Chromatogr A. 2013;1274:97–106. doi: 10.1016/j.chroma.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Bai X, Wei D, Yang G. J Chromatogr A. 2014;1324:128–134. doi: 10.1016/j.chroma.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Bai X, Liu H, Wei D, Yang G. Talanta. 2014;119:479–484. doi: 10.1016/j.talanta.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 22.Lin SL, Wu YR, Lin TY, Fuh MR. J Chromatogr A. 2013;1298:35–43. doi: 10.1016/j.chroma.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Tolley HD, Lee ML. J Chromatogr A. 2011;1218:1399–1408. doi: 10.1016/j.chroma.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Bedair M, El Rassi Z. Electrophopresis. 2002;23:2938–2948. doi: 10.1002/1522-2683(200209)23:17<2938::AID-ELPS2938>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Bedair M, El Rassi Z. J Chromatogr A. 2003;1013:35–45. doi: 10.1016/s0021-9673(03)01031-8. [DOI] [PubMed] [Google Scholar]
- 26.Liu K, Tolley HD, Lee ML. J Chromatogr A. 2012;1227:96–104. doi: 10.1016/j.chroma.2011.12.081. [DOI] [PubMed] [Google Scholar]
- 27.Liu K, Tolley HD, Lawson JS, Lee ML. J Chromatogr A. 2013;1321:80–87. doi: 10.1016/j.chroma.2013.10.071. [DOI] [PubMed] [Google Scholar]
- 28.Chambers SD, Holcombe TW, Svec F, Frechet JMJ. Anal Chem. 2011;83:9478–9484. doi: 10.1021/ac202183g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambers SD, Svec F, Frechet JMJ. J Chromatogr A. 2011;1218:2546–2552. doi: 10.1016/j.chroma.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aqel A, Yusuf K, Al-Othman ZA, Badjah-Hadj-Ahmed AY, Alwarthan AA. Analyst. 2012;137:4309–4317. doi: 10.1039/c2an35518c. [DOI] [PubMed] [Google Scholar]
- 31.Mayadunne E, El Rassi Z. Talanta. 2014;129:565–574. doi: 10.1016/j.talanta.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang MM, Yan XP. Anal Chem. 2011;84:39–44. doi: 10.1021/ac202860a. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Qi L, Ma H. Analyst. 2013;138:5470–5478. doi: 10.1039/c3an01122d. [DOI] [PubMed] [Google Scholar]
- 34.Svobodova A, Krizek T, Sirc J, Salek P, Tesarova E, Coufal P, Stulik K. J Chromatogr A. 2011;1218:1544–1547. doi: 10.1016/j.chroma.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa G, Kanamori K, Ishizuka N, Nakanishi K. ACS Appl Mater Interfaces. 2012;4:2343–2347. doi: 10.1021/am300552q. [DOI] [PubMed] [Google Scholar]
- 36.Koeck R, Bakry R, Tessadri R, Bonn GK. Analyst. 2013;138:5089–5098. doi: 10.1039/c3an00377a. [DOI] [PubMed] [Google Scholar]
- 37.Davankov V, Rogozhin S, Tsyurupa M. US Patent Appl. 1971;3:729. [Google Scholar]
- 38.Urban J, Svec F, Fréchet JM. J Chromatogr A. 2010;1217:8212–8221. doi: 10.1016/j.chroma.2010.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban J, Svec F, Fréchet JM. Anal Chem. 2010;82:1621–1623. doi: 10.1021/ac100008n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen XJ, Dinh NP, Zhao J, Wang YT, Li SP, Svec F. J Sep Sci. 2012;35:1502–1506. doi: 10.1002/jssc.201200138. [DOI] [PubMed] [Google Scholar]
- 41.Maya F, Svec F. Polymer. 2014;55:340–346. [Google Scholar]
- 42.Strege MA. Anal Chem. 1998;70:2439–2445. doi: 10.1021/ac9802271. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Tolley HD, Lee ML. J Sep Sci. 2011;34:2088–2096. doi: 10.1002/jssc.201100155. [DOI] [PubMed] [Google Scholar]
- 44.Alpert AJ. J Chromatogr A. 1990;499:177–196. doi: 10.1016/s0021-9673(00)96972-3. [DOI] [PubMed] [Google Scholar]
- 45.Cheng J, Chen X, Cai Y, He Y, Chen Z, Lin Z, Zhang L. Electrophoresis. 2013;34:1189–1196. doi: 10.1002/elps.201200523. [DOI] [PubMed] [Google Scholar]
- 46.Lin X, Li Y, Xu D, Yang C, Xie Z. Analyst. 2013;138:635–641. doi: 10.1039/c2an36354b. [DOI] [PubMed] [Google Scholar]
- 47.Lin X, Feng S, Jia W, Ding K, Xie Z. J Chromatogr A. 2013;1316:104–111. doi: 10.1016/j.chroma.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Gunasena DN, El Rassi Z. J Chromatogr A. 2013;1317:77–84. doi: 10.1016/j.chroma.2013.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan G, Peng Y, Liu Z, Hong J, Xiao Y, Guo J, Smith NW, Crommen J, Jiang Z. J Chromatogr A. 2013;1301:88–97. doi: 10.1016/j.chroma.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 50.Lin Z, Huang H, Sun X, Lin Y, Zhang L, Chen G. J Chromatogr A. 2012;1246:90–97. doi: 10.1016/j.chroma.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 51.Chen ML, Wei SS, Yuan BF, Feng YQ. J Chromatogr A. 2012;1228:183–192. doi: 10.1016/j.chroma.2011.07.061. [DOI] [PubMed] [Google Scholar]
- 52.Jandera P. Anal Chim Acta. 2011;692:1–25. doi: 10.1016/j.aca.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Z, Smith NW, Liu Z. J Chromatogr A. 2011;1218:2350–2361. doi: 10.1016/j.chroma.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 54.Gama MR, da Costa Silva RG, Collins CH, Bottoli CB. TrAC. 2012;37:48–60. [Google Scholar]
- 55.Gunasena DN, El Rassi Z. Electrophoresis. 2012;33:251–261. doi: 10.1002/elps.201100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin J, Liu S, Lin J, Lin X, Xie Z. J Chromatogr A. 2011;1218:4671–4677. doi: 10.1016/j.chroma.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 57.Staňková M, Jandera P, Škeříková V, Urban J. J Chromatogr A. 2013;1289:47–57. doi: 10.1016/j.chroma.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 58.Jandera P, Staňková M, Hájek T. J Sep Sci. 2013;36:2430–2440. doi: 10.1002/jssc.201300337. [DOI] [PubMed] [Google Scholar]
- 59.Chen ML, Li LM, Yuan BF, Ma Q, Feng YQ. J Chromatogr A. 2012;1230:54–60. doi: 10.1016/j.chroma.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 60.Liu Z, Peng Y, Wang T, Yuan G, Zhang Q, Guo J, Jiang Z. J Sep Sci. 2013;36:262–269. doi: 10.1002/jssc.201200682. [DOI] [PubMed] [Google Scholar]
- 61.Foo HC, Heaton J, Smith NW, Stanley S. Talanta. 2012;100:344–348. doi: 10.1016/j.talanta.2012.07.083. [DOI] [PubMed] [Google Scholar]
- 62.Tijunelyte I, Babinot J, Guerrouache M, Valincius G, Carbonnier B. Polymer. 2012;53:29–36. [Google Scholar]
- 63.Škeříková V, Urban J. J Sep Sci. 2013;36:2806–2812. doi: 10.1002/jssc.201300395. [DOI] [PubMed] [Google Scholar]
- 64.Lv Y, Lin Z, Svec F. Anal Chem. 2012;84:8457–8460. doi: 10.1021/ac302438m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerrouache M, Mahouche-Chergui S, Chehimi MM, Carbonnier B. Chem Comm. 2012;48:7486–7488. doi: 10.1039/c2cc33134a. [DOI] [PubMed] [Google Scholar]
- 66.Lv Y, Lin Z, Svec F. Analyst. 2012;137:4114–4118. doi: 10.1039/c2an35706b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 68.Hoyle CE, Bowman CN. Angew Chem Int Ed. 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 69.Maksimova E, Vlakh E, Sinitsyna E, Tennikova T. J Sep Sci. 2013;36:3741–3749. doi: 10.1002/jssc.201300852. [DOI] [PubMed] [Google Scholar]