Abstract
Voltage-gated sodium channels (Nav channels) are critical for electrical signaling in the nervous system and are the primary targets of the insecticides DDT and pyrethroids. In Drosophila melanogaster, besides the canonical Nav channel, Para (also called DmNav), there is a sodium channel-like cation channel called DSC1 (Drosophila sodium channel 1). Temperature-sensitive paralytic mutations in DmNav (parats) confer resistance to DDT and pyrethroids, whereas DSC1 knockout flies exhibit enhanced sensitivity to pyrethroids. To further define the roles and interaction of DmNav and DSC1 channels in DDT and pyrethroid neurotoxicology, we generated a DmNav/DSC1 double mutant line by introducing a parats1 allele (carrying the I265N mutation) into a DSC1 knockout line. We confirmed that the I265N mutation reduced the sensitivity to two pyrethroids, permethrin and deltamethrin of a DmNav variant expressed in Xenopus oocytes. Computer modeling predicts that the I265N mutation confers pyrethroid resistance by allosterically altering the second pyrethroid receptor site on the DmNav channel. Furthermore, we found that I265N-mediated pyrethroid resistance in parats1 mutant flies was almost completely abolished in parats1;DSC1−/− double mutant flies. Unexpectedly, however, the DSC1 knockout flies were less sensitive to DDT, compared to the control flies (w1118A), and the parats1;DSC1−/− double mutant flies were even more resistant to DDT compared to the DSC1 knockout or parats1 mutant. Our findings revealed distinct roles of the DmNav and DSC1 channels in the neurotoxicology of DDT vs. pyrethroids and implicate the exciting possibility of using DSC1 channel blockers or modifiers in the management of pyrethroid resistance.
Keywords: DSC1, voltage-gated sodium channel, DDT, pyrethroids insecticide resistance
Introduction
Insect pests and disease vectors pose tremendous medical, agricultural and economic threats to humans. As such, various control methods, including the use of insecticides, have been developed to mitigate their impact (Perry et al., 1998). DDT and pyrethroids (particularly type I pyrethroids) have a long history of wide usage due to their rapid knockdown and high potency (Narahashi, 2002). Pyrethroids continue to be used extensively in controlling arthropod pests and disease vectors due to their low mammalian toxicity and favorable environmental properties (Narahashi et al., 2007). Although the use of DDT is largely banned in most parts of the world due to its detrimental impact on the ecosystem, it is still one of 12 pesticides recommended by the WHO for indoor residual spray programs in malaria control in African countries (http://www.who.int/mediacentre/news/releases/2006/pr50/en/).
DDT and pyrethroids affect the function of voltage-gated sodium channels (Nav), which are critical for electrical signaling in excitable cells (Narahashi, 1971). Early studies revealed characteristic repetitive discharge in cockroach nerves treated with DDT or pyrethrins (Eaton and Sternburg, 1964; Lalonde and Brown, 1954). Subsequent studies demonstrated that the insecticide-induced repetitive discharge was the result of prolonged sodium currents (Hille, 1968; Narahashi, 1962; Narahasi and Haas, 1967). Since then, numerous studies have shown that DDT and pyrethroids prolong the opening of Nav channels by inhibiting channel deactivation and inactivation and stabilizing the open state of sodium channels (Bloomquist, 1996; Bloomquist and Soderlund, 1988; Narahashi, 1992, 2002, 2000; Vijverberg et al., 1982). To date, more than 50 Nav mutations have been identified that are associated with resistance to pyrethroids in various arthropod pests and disease vectors, and some of them are also responsible for DDT resistance (Rinkevich et al., 2013). Elucidation of mechanisms of pyrethroid resistance led to the identification of two pyrethroid receptor sites, PyR1 and PyR2 (formally Site 1 and Site 2, respectively) on the Nav channel (Du et al., 2013; O'Reilly et al., 2006), and provided the molecular basis of the action of DDT and pyrethroids on Nav channels (Dong et al., 2014; Soderlund, 2005).
In D. melanogaster, besides the canonical voltage-gated Nav channel, DmNav, (also known as Para) (Loughney et al., 1989), there is a Nav channel-like gene called DSC1 (Drosophila Sodium Channel 1, also known as NaCP60E in Flybase). DSC1 was considered as a putative Nav channel gene based on its high sequence similarity to Nav channels in the transmembrane regions (Salkoff et al., 1987). The overall topology of the DSC1 channel is similar to that of the DmNav channel, consisting of four homologous domains, each of which has six transmembrane segments (Kulkarni et al., 2002; Zhang et al., 2011). However, functional analysis of the DSC1 channel and a DSC1 ortholog from the German cockroach, BSC1, in Xenopus oocytes revealed that BSC1/DSC1 channels represent a novel family of voltage-gated cation channels with high permeability to Ca2+ (Zhang et al., 2011; Zhou et al., 2004). A recent study showed that the DSC1 knockout line of D. melanogaster exhibits enhanced sensitivity to pyrethroids (Zhang et al., 2013), suggesting potential functional interactions between DSC1 channels and Nav channels in modulating insecticide neurotoxicity. DSC1 orthologs were isolated from B. germanica and Heliothis virescens (Liu et al., 2001; Park et al., 1999) and have now also been found in all insect species with a complete genome sequence (Cui et al., 2012)
To further discern the role of the DSC1 channel in the toxicology of DDT and pyrethroids, we introduced a temperature-sensitive paralytic allele (parats1), carrying an I265N mutation in DmNav (Loughney et al., 1989; Pittendrigh et al., 1997) into a DSC1 knockout line. We then conducted bioassays to compare the susceptibilities of the mutant lines to DDT, permethrin and deltamethrin. In addition, we also evaluated the effects of the I265N mutation on the response of DmNav to DDT and pyrethroids. Our analyses revealed surprisingly distinct roles of DmNav and DSC1 channels in mediating the toxicological effects of DDT and pyrethroids.
MATERIALS AND METHODS
Site-directed mutagenesis
We introduced the parats1 (I265N) mutation into DmNav22, a pyrethroid-sensitive Nav channel variant from D. melanogaster (Olson et al., 2008). Site-directed mutagenesis was performed by polymerase chain reaction (PCR) using primers (Forward Primer GGCCTGAAGACGATCGTCGGCGCCGTCAACGAATCGGTGAAGAATCTGCGCGATGTG; Reverse Primer CACATCGCGCAGATTCTTCACCGATTCGTTGACGGCGCCGACGATCGTCTTCAGGCC) and Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA). Mutagenesis was verified by DNA sequencing.
Expression of DmNav22 and mutant channels in Xenopus oocytes
The procedures for oocyte preparation and cRNA injection were identical to those described previously (Olson et al., 2008). For robust expression of sodium currents, cRNA was co-injected into oocytes with Drosophila melanogaster tipE cRNA (1:1 ratio), which enhances sodium channel expression (Feng, 1995; Warmke, 1997).
Measurement of effects of DDT and pyrethroids on of DmNav22 and mutant channels expressed in Xenopus oocytes
The method for application of DDT and pyrethroids in the recording system was identical to that described previously (Tan et al., 2002b). The effects of pyrethroids were measured 10 min after their application. The pyrethroid-induced tail current was recorded during a 100-pulse train of 5-ms step depolarizations from – 120 to 0 mV with 5-ms interpulse intervals (Vais et al., 2000). The percentage of channels modified by pyrethroids was calculated using the equation M = {[Itail/(Eh – ENa)]/[INa/(Et – ENa)]} × 100 (Tatebayashi and Narahashi, 1994), where Itail is the maximal tail current amplitude, Eh is the potential to which the membrane is repolarized, ENa is the reversal potential for sodium current determined from the current-voltage curve, INa is the amplitude of the peak current during depolarization before pyrethroid exposure, and Et is the potential of step depolarization.
We incubated oocytes expressing DmNav22 and I265N channels in DDT solutions overnight in order to detect the inhibitory effect of DDT on fast inactivation. The degree of DDT inhibition was assayed by measuring the remaining current at the end of 20 ms depolarization to -10 mV from the holding potential of -120 mV and normalized to peak current. Because of an intrinsic non-inactivating current from DmNav22 channels (Olson et al., 2008) as well as I265N channels, we subtracted it from the normalized non-inactivating current in the presence of DDT to obtain the percentage of DDT inhibition of fast inactivation.
Fly Strains
Four D. melanogaster strains were used in this study: w1118A, DSC1−/−, parats1 and parats1; DSC1−/−. The w1118A line was obtained from Steve Crews’ lab in the University of North Carolina, Chapel Hill, and was used in generating the DSC1 knockout strain, DSC1−/−. DSC1−/− is one of two DSC1 knockout founder lines (Zhang et al., 2013). parats1 is a temperature-sensitive paralytic mutant, which has an I265N mutation in the linker connecting transmembrane segments S4 and S5 of domain I of DmNav (Pittendrigh et al., 1997).
To generate the parats1;DSC1−/− double mutants, the following crosses were carried out. Virgin females of parats1 were crossed to males from a stock carrying dominant second chromosome marker Bc (Black cell) to generate F1 male parats1 with second chromosome marked by Bc because the DSC1 gene resides on the second chromosome. They were then back-crossed to parats1 females to obtain F2 parats1 virgin females with second chromosome carrying marker Bc, which were further crossed to homozygous DSC1−/− males to generate F3 parats1 males carrying heterozygous DSC1 over Bc marker on the second chromosome. These flies were crossed with virgin females from a double balancer stock, with attached-X (X^X) and the second chromosome balancer CyO (Curly Oster). The progeny from this final cross yields a balanced stock with parats1 or XX chromosomes over Y, and DSC1 over CyO. Since the double mutant parats1;DSC1−/− stock was kept over X^X, the females were X^X/Y; DSC1−/− and only males were hemizygous for parats1 and homozygous for DSC1−/−, which were used in bioassays.
Bioassays
Contact bioassays with deltamethrin, permethrin and DDT (Chem Service, West Chester PA) were performed as described previously with 1-3 day old male flies (Rinkevich and Scott, 2012). Probit analyses were performed using Minitab (State College, PA). The LC50 values were considered to be significantly different if the 95% confidence intervals (CI) did not overlap.
Deltamethrin and DDT Knockdown
Scintillation vials were coated with 0.5 ml of 0.02 mg/ml deltamethrin in acetone (40 µM, approximately 475x the LC50 for w1118A) or 2 mg/ml DDT (5 mM, approximately 333x the LC50 for w1118A) and allowed acetone to evaporate completely under a fume hood for 1 hr. Ten 1- to 3-day old male flies of each strain were placed in separate vials. The number of flies that were ataxic or unable to stand without exaggerated wobbling was recorded as knocked down at 5 minute intervals for 30 minutes for deltamethrin and 60 minutes for DDT.
Computer modeling
Previous experimental and molecular-modeling studies proposed models of PyR1 and PyR2 in insect sodium channels. Both are homology models based on the X-ray structure of the open voltage-gated potassium channel Kv1.2. The model for PyR1 contains residues from transmembrane helices IIS5 and IIIS6, as well as from a linker-helix, IIL45, that connects IIS4 and IIS5 (O'Reilly et al., 2006; Usherwood et al., 2007). The model for PyR2 is formed by residues from helices IL45, IS5, IS6, and IIS6 (Du et al., 2013). Within all these helices, the amino acid sequences of the DmNav channel are identical to those in the mosquito AaNav1-1 channel. Therefore, we used this structural similarity to generate a Kv1.2-based homology model of the open DmNav channel with deltamethrin (Du et al., 2013) {Du, 2013 #10077; Du, 2013 #10077} and explore possible effects of the I265N mutation on binding of pyrethroids. The energy of the deltamethrin-bound complexes of DmNav channel and its mutant was Monte Carlo minimized as described before (Du et al., 2013).
RESULTS
The parats1 (I265N) mutation reduced the sensitivity of DmNav22 channels expressed in Xenopus oocytes to pyrethroids and DDT
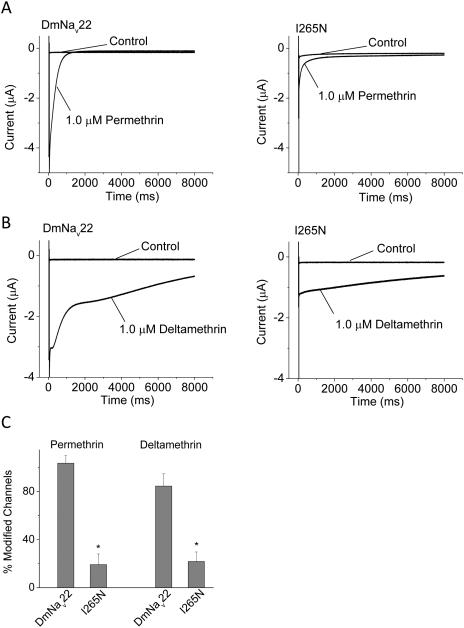
DmNav22 and I265N channels were expressed in Xenopus oocytes and examined for channel gating and sensitivity to DDT, permethrin and deltamethrin. The I265N mutation did not alter the voltage-dependence of channel activation or inactivation (Table 1). A 100-pulse train of 5-ms depolarizations from -120 to 0 mV with a 5-ms interpulse interval was used to elicit pyrethroid-induced tail currents from oocytes expressing DmNav22 and I265N mutant channels (Vais et al., 2000). The resulting pyrethroid-induced tail currents serve as a measure of channel sensitivity to pyrethroids (Tatebayashi and Narahashi, 1994). As shown in Fig. 1, large tail currents were induced by 1 µM of permethrin (Fig. 1A) and 1 µM of deltamethrin (Fig. 1B) in oocytes expressing DmNav22 channels, indicating that DmNav22 is highly sensitive to pyrethroids. There was no difference between DmNav22 and I265N mutant channels in the kinetics of decay of permethrin or deltamethrin-induced tail currents (Fig. 1). However, the amplitude of pyrethroid-induced tail currents was reduced for I265N channels (Fig. 1), indicating that the I265N mutation reduced DmNav22 channel sensitivity to both permethrin and deltamethrin.
Table 1.
Effects of the parats1 mutation I265N (I1k12N) on the voltage-dependence of sodium channel activation and inactivation
| Activation | Inactivation | ||||
|---|---|---|---|---|---|
|
|
|||||
| Na+ Channel Type | V1/2 (mV) | k (mV) | V1/2 (mV) | k (mV) | n |
|
|
|
|
|||
| DmNav22 | −25.98 ± 0.47 | 5.46 ± 0.23 | −46.06 ± 0.28 | 5.40 ± 0.13 | 8 |
| I265N | −23.90 ± 1.40 | 7.46 ± 0.60 | −45.86 ± 0.90 | 5.85 ± 0.19 | 10 |
Figure 1.
The parats1 (I265N) channels are less sensitive to the pyrethroids permethrin and deltamethrin than DmNav22. A and B. Tail-current induced by permethrin (A) or deltamethrin (B) from DmNav22 or I265N channels. The I265N mutation is located in the linker connecting S4 and S5 of domain I. C. Percent channel modification by pyrethroids. The number of oocytes for each channel was >5. The protocols and quantitative analysis of pyrethroid-induced tails are described in the Materials and Methods. Data are the average ± SEM. * indicates significantly different from DmNav22 channels (Student’s t-test, P < 0.05).
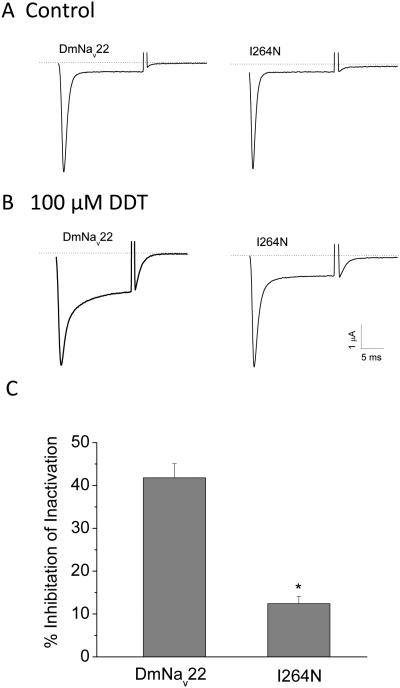
Like pyrethroids, DDT induced a tail current of one DmNav channel variant expressed in oocytes (Burton et al., 2011; Usherwood et al., 2005). Apparently this effect was observed only after overnight incubation of oocytes expressing DmNav channels in a DDT solution (Ian Mellor, personal communication). However, we found that DDT-induced tail currents from DmNav22 or I265N channels were still too small for quantitative analysis even after overnight incubation with DDT at high concentrations (~100 µM). Instead, the more drastic effect of DDT on sodium channel gating was the inhibition of fast inactivation. DDT at 100 µM induced a non-inactivating current that is 41% of peak current at the end of 20 ms depolarization of DmNav22 channels (Fig. 2). However, DDT at the same concentration induced a non-inactivating current of I265N channels that is only 12% of peak current (Fig. 2) indicating that the I265N mutation reduced DmNav22 channel sensitivity to DDT.
Figure 2.
The parats1 (I265N) channels are less sensitive to DDT than DmNav22 channels. A. Representative sodium current traces from oocytes expressing DmNav22 and I265N channels. B. Representative sodium current traces from DmNav22 and I265N channels after overnight incubation in 100 µM DDT. C. Inhibition of fast inactivation by DDT. The number of oocytes for each channel was >15. The protocol and quantitative analysis of DDT inhibition are described in the Materials and Methods. Data are the average ± SEM. * indicates significantly different from DmNav22 channels (Student’s t-test, P < 0.05).
Knockout of DSC1 antagonizes I265N-mediated pyrethroid resistance
A recent study showed that knockout of DSC1 caused enhanced sensitivity to pyrethroids in D. melanogaster (Zhang et al., 2013). To investigate any potential functional interactions between DSC1 and DmNav channels in modulating pyrethroid toxicology, we conducted bioassays to examine the sensitivities of w1118A, parats1, DSC1−/, and parats1;DSC1−/− flies to permethrin and deltamethrin.
Compared to w1118A, the parats1 or DSC1−/− strains were 4.6-fold less sensitive or 5-fold more sensitive to permethrin, respectively. However, the parats1;DSC1−/− double mutant was 1.8-fold more sensitive to permethrin compared with w1118A (Table 2). This value is lower than what would be expected if the interactions between the parental parats1 and DSC1−/− were merely additive, as simply adding the Resistance Ratios (RRs) would yield an expected RR of -0.4. Furthermore, averaging the mortality at each dose from DSC1−/− and parats1 yields a RR of 2.4-fold. Recalculating the LC50 by Probit analysis on the combined bioassay data produced a RR of 1-fold. The 1.8-fold increase in sensitivity to permethrin observed for parats1;DSC1−/− is significantly lower than all of these expected values. Therefore, these results suggest that the absence of DSC1 has an epistatic effect on permethrin resistance conferred by the parats1 mutation.
Table 2.
Toxicity of permethrin and deltamethrin against w1118A, parats1, DSC1−/− and parats1/DSC1−/−
| Strain | Permethrin | |||
|---|---|---|---|---|
|
| ||||
| n | LC50 | Slope | RR | |
| w1118A | 1140 | 2.0 (1.9-2.1) | 3.8 (±0.2) | 1.0a |
| DSC1−/− | 720 | 0.4 (0.3-0.5) | 1.8(±0.1) | −5.0b |
| parats1 | 760 | 9.2 (8.0-10.5) | 2.1 (±0.1) | 4.6c |
| parats1/DSC1−/− | 903 | 1.1 (1.0-1.2) | 2.7 (±0.2) | −1.8d |
|
| ||||
| Deltamethrin | ||||
|
|
||||
| n | LC50 | Slope | RR | |
|
| ||||
| w1118A | 780 | 0.011(0.009-0.012) | 2.6 (±0.2) | 1.0a |
| DSC1−/− | 400 | 0.004 (0.004-0.005) | 3.4 (±0.3) | −2.4b |
| parats1 | 899 | 0.067 (0.058-0.078) | 1.4 (±0.1) | 6.3c |
| parats1/DSC1−/− | 948 | 0.015 (0.014-0.016) | 3.8 (±0.3) | 1.4d |
|
| ||||
| DDT | ||||
|
|
||||
| n | LC50 | Slope | RR | |
|
| ||||
| w1118A | 760 | 0.6 (0.6-0.7) | 3.4 (±0.3) | 1.0a |
| DSC1−/− | 980 | 2.5 (2.1-2.9) | 1.2 (±0.1) | 4.0b |
| parats1 | 820 | 7.7 (6.2-9.3) | 1.1 (±0.1) | 12.1c |
| parats1/DSC1−/− | 960 | 11.6 (7.9-15.5) | 0.7 (±0.1) | 18.4c |
The DSC1 knockout significantly antagonizes permethrin and deltamethrin resistance conferred by the parats1 mutation, but enhances DDT resistance in an additive manner. The LC50 values are in units of μg/vial with the 95% CI in parenthesis. The values in parenthesis next to the slope value are the ± SE of the slope. The Resistance Ratios (RR) are calculated relative to the LC50 of w1118A. Letters in the RR column represent significant differences between strains within each insecticide treatment.
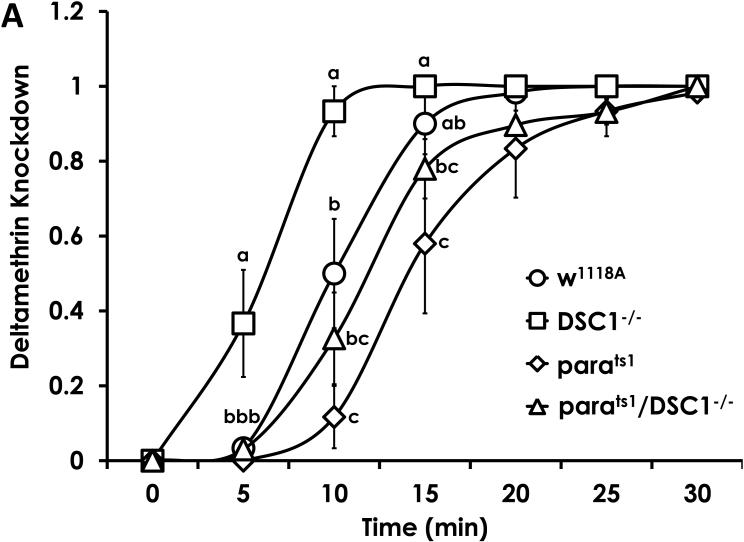
A similar pattern of epistasis was observed with sensitivity of these fly strains to deltamethrin. Compared to w1118A, the parats1 strain was more than 6-fold less sensitive to deltamethrin, but the DSC1−/− strain was more than 2-fold more sensitive. However, the parats1;DSC1−/− double mutant was 1.4-fold less sensitive to deltamethrin compared with w1118A (Table 2). Adding RRs, averaging mortality, and recalculating the LC50 using Probit analysis of the combined data from DSC1−/− and parats1 gives RRs of 3.9, 3.4 and 2.4, respectively, for parats1;DSC1−/−. The 1.4 RR observed for parats1;DSC1−/− was significantly lower than all of these expected values.
The onset of knockdown due to deltamethrin exposure was more rapid for DSC1−/− mutant flies. The proportion of flies knocked down by deltamethrin was higher for DSC1−/− than all the other strains during the 5th and 10th min (Fig. 3A). The differences in the proportion of DSC1−/− flies knocked down compared to parats1 and parats1;DSC1−/− continued through the 15th minute. There were differences in the speed of knockdown between w1118A and parats1 at 10, and 15 min, and no differences between w1118A and parats1;DSC1−/− or between parats1 and parats1;DSC1−/− at any time. There were no differences in knockdown proportion between any of the strains over the last 10 minutes of the knockdown bioassay. In addition, the time to knock down 50% of population was significantly faster for DSC1−/− than for all other strains. It took significantly longer to knockdown parats1 flies than to knockdown w1118A flies, but no such difference was observed between parats1 and parats1;DSC1−/− or between w1118A and parats1;DSC1−/− (Table 3).
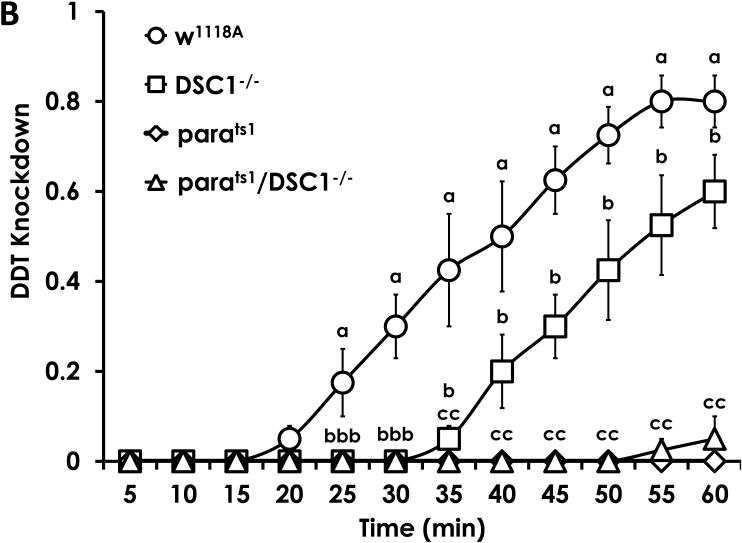
Figure 3.
The time course of knockdown of D. melanogaster exposed to deltamethrin (A) and DDT (B). DSC1−/− exhibited increased knockdown by deltamethrin, but demonstrated delayed knockdown by DDT. There was no significant knockdown of parats1 or parats1;DSC1−/− by DDT. Data are the average ± SEM. Letters above each data point at every 5 minute intervals indicate significant differences at each time point (One-Way ANOVA, Fishers LSD, P<0.05).
Table 3.
Time (min) to knockdown (KD50) for 50% of flies exposed to 0.02 mg/ml deltamethrin or 2 mg/ml DDT
| Strain |
KD50 | |
|---|---|---|
|
| ||
| Deltamethrin |
DDT |
|
| w1118A | 8.8 (±0.6)a | 41.4 (±3.4)a |
| DSC1−/− | 5.1 (±0.7)b | 59.1 (±3.5)b |
| parats1 | 15.0 (±2.2)c | NA |
| parats1/DSC1−/− | 11.0 (±1.2)ac | NA |
KD50 values are shown ± SE. We were unable to calculate KD50 values for DDT against parats1 and parats1/DSC1−/− since there was no significant knockdown. Different letters within each column indicate significance of differences (See Fig. 3)
Knockout of DSC1 confers resistance to DDT
Unexpectedly, DSC1−/− flies were 4-fold less sensitive to DDT compared to w1118A (Table 2). The slope of the mortality response curve is significantly lower for DSC1−/− flies compared to w1118A flies demonstrating a significant increase in the resistance ratios at higher concentrations (i.e. RR @ LC95 = 27, Table 2). The lower slope of the mortality response curve also demonstrates a more heterogeneous response for DSC1−/− flies, suggesting DSC1 knockout produces a number of changes in the nervous system to shape the mortality response curve. Consistent with previous results (Pittendrigh et al., 1997), the parats1 mutation conferred a 12-fold decrease in DDT insensitivity. The 18-fold increase in the RR of parats1;DSC1−/− flies was within the range to be considered additive with respect to both individual mutant lines.
Consistent with the mortality results, it took longer for DSC1−/− flies to be knocked down by exposure to DDT than w1118A starting at minute 25 and continuing through the rest of the duration of the assay (Fig. 3B). The time to knock down 50% of the population was significantly faster for w1118A compared to DSC1−/− (Table 3). Both parats1 and parats1;DSC1−/− flies were knocked down significantly less than w1118A and DSC1−/− at 25 and 35 minutes, respectively, and these differences continued throughout the rest of the experiment. Both parats1 and parats1;DSC1−/− flies were not affected by DDT during the time course of this experiment, and these results are consistent with bioassay results demonstrating high levels of DDT insensitivity in those strains.
Computer modeling of the DmNav 22 channel carrying the I265N mutation
We designate the I265 residue as I1k12 using the nomenclature that is universal for sodium channels and other P-loop ion channels (Du et al., 2013; Zhorov and Tikhonov, 2004). This designation reflects the location of the I1k12N mutation: domain D1, linker-helix IL45 (k), and relative position 12 within the linker. The linker helix contains two recently identified pyrethroid-sensing residues I1k7 and V1k11 (Du et al., 2013). In our model of PyR2 in the mosquito sodium channel, the side chains of both I1k7 and V1k11 extend towards helix IIS6 and interact directly with the halogen-containing end of the pyrethroid molecule, which binds in the domain I/II interface, between helices IL45 and IIS6. In contrast the side chain of I1k12 faces lipids and does not form contacts with the bound pyrethroid molecule (Du et al., 2013).
In our model of the DmNav I1k12N channel with deltamethrin bound to PyR2 (Fig. 4), the N1k12 side chain also faces lipids and occurs too far from the pyrethroid molecule to noticeably interact with it. In high-resolution X-ray structures, 26.4% of asparagine residues participate in side chain-backbone hydrogen bonds. In the three most populated motifs the asparagine side chain accepts an H-bond from the backbone NH group two or three residues downstream or donates an H-bond to the backbone carbonyl four residues upstream (Vasudev et al., 2012). We used distance constraints to impose such H-bonds for N1k12 and Monte Carlo-minimized respective channel-deltamethrin complexes. The N1k12 H-bonds with the NH groups of two other pyrethroid sensing residues in IS5 S1o2 (Wang et al., 2014) and V1o3 (Du et al., 2013) caused noticeable backbone deformation in IL45 (Fig. 4). However, in all the structures the N1k12 side chain remained far from deltamethrin. Thus, the effect of I1k12N mutation on pyrethroid sensitivity is likely allosteric.
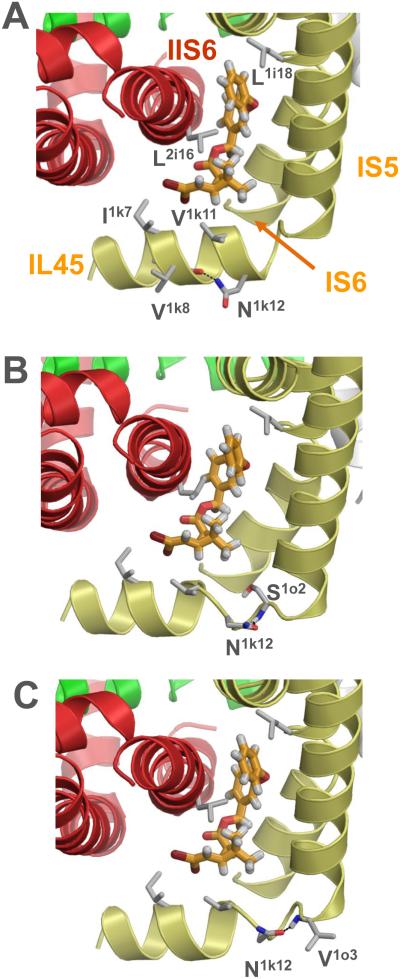
Figure 4.
Kv1.2-based model of the open DmNav channel phenotype N1k12 (N265) with a deltamethrin molecule docked to PyR2 as described before (Du et al., 2013). Helices in domains I, II, III and IV are shown, respectively, by yellow, red, green, and white ribbons. Pyrethroid-sensing residues in the I/II domain interface, as well as N1k12 and its possible H-bonding partners V1k8 (A), S1o2 (B) and V1o3 (C) are shown as sticks. Asparagine N1k12 is far from deltamethrin and does not contribute to the pyrethroid binding site. However, an H-bond between the N1k12 side chain and a backbone atom can deform the backbone and thus affect the mutual disposition of deltamethrin and pyrethroid-sensing residues in PyR2.
DISCUSSION
It is well established that both DDT and pyrethroids act on Nav channels and modify the gating of Nav channels (Bloomquist and Soderlund, 1988; Narahashi, 2002; Soderlund, 2010). Identification of naturally occurring mutations in insect Nav channels that confer cross-resistance to DDT and pyrethroids further confirms that DDT and pyrethroids have a rather common mode of action. Although the DSC1 protein shares a high sequence homology with the DmNav channel, the role of the DSC1 channel in the molecular action of DDT and pyrethroids remain unclear. In this study, we first examined the effects of DDT and pyrethroids on DmNav channels carrying the parats1 mutation I265N (I1k12N) in Xenopus oocytes and then evaluated the toxicity of DDT and pyrethroids against DSC1 knockout D. melanogaster flies as well as DSC1 knockout flies carrying the parats1 mutation (I265N). Our findings revealed striking differences in the activities of DDT and pyrethroids on flies lacking expression of DSC1 channels, providing the first molecular evidence that DSC1 channels play distinct roles in the neurotoxicity of DDT and pyrethroids in vivo.
Our results from both bioassays and functional analysis corroborate the fact that the parats1 mutation (I265N) causes reduced sensitivity to DDT and pyrethroids (permethrin and deltamethrin). Our finding is therefore different from the finding from an early study, which argued for a hypersensitivity of parats1 flies to deltamethrin (Pedra et al., 2004). It is likely that Pedra and colleagues compared the sensitivity of the parats1 mutant to that of another susceptible strain Canton-S, which had an LC50 of 0.343 µg deltamethrin/vial, whereas the w1118A strain used in this study had a much lower LC50 value of 0.011 (95% CI 0.009-.012) µg deltamethrin/vial. The LC50 of deltamethrin for parats1 flies in our experiments was 0.067 (95% CI 0.058-0.078) µg/vial (Table 2), which is in agreement with the LC50 of deltamethrin (0.049 µg /vial; 95% CI 0.019-0.091) previously reported (Pedra et al., 2004). Furthermore, the parats2 strain which carries the same I265N mutation is also more resistant to deltamethrin (Pittendrigh et al., 1997). Taken together, these results demonstrate that the I265N mutation is responsible for resistance of parats1 flies to DDT and pyrethroids.
The mechanism through which the I265N mutation confers DDT and pyrethroid resistance appears to be a unique mechanism that is not currently known because the mutation does not affect channel gating properties and, based on computer modeling, the I265N mutation does not appear to be located within either of the known pyrethroid-binding sites. In contrast, many other Nav channel mutations that reduce pyrethroid sensitivity occur at residues that either are located within the two pyrethroid-binding sites or that are involved in regulating channel kinetics and voltage-dependent gating (Du et al., 2013; Soderlund and Knipple, 2003; Usherwood et al., 2007). Nevertheless, the I265N (I1k12N) mutation appeared to allosterically deform pyrethroid receptor PyR2 resulting in reduced pyrethroid binding. Isoleucine has long been known to stabilize α-helices, whereas asparagine is a prominent α-helix breaker (Chou and Fasman, 1974). Therefore, the I265N mutation could destabilize the α-helical structure of the IL45 linker. Notably, there is another helix-breaking residue, G1k9, three positions upstream of I265N (I1k12N). The combined effects of two helix-destabilizing residues, G1k9 and N265 (N1k12), may be likely sufficient to deform the linker helix. This deformation then may change the mutual disposition of the pyrethroid-sensing residues, I1k7 and V1k11, on the one hand, and helix IIS6, on the other hand, thus distorting the geometry of PyR2.
Unlike the parats1 mutation, DSC1 knockout increased sensitivity of flies to pyrethroids (this study; Zhang et al., 2013), suggesting that the DSC1 channel is not a direct target of pyrethroid insecticides, but has epistatic effects on pyrethroid action on the Nav channels. In addition, DSC1 knockout did not alter the expression of Nav channel transcripts in these flies (Zhang et al., 2013). Our previous study showed that loss of the DSC1 channel results in membrane hyperexcitability and the DSC1 channel stabilizes the nervous system likely through regulating synaptic transmission (Zhang et al., 2013). The membrane hyperexcitability in the DSC1 knockout is expected to potentiate the activating effects of pyrethroids on sodium channels/the nervous system, which could explain why DSC1 knockout flies are more sensitive to pyrethroids. Consistent with this expectation, we found that the permethrin and deltamethrin resistance caused by parats1 is significantly antagonized by the DSC1 knockout mutation. The antagonism of permethrin and deltamethrin resistance between the parats1 and DSC1 mutations is a unique example in which two homologous genes have a negative epistatic interaction to modulate insecticide toxicity by exerting opposing effects (inhibitory by DSC1 channels and excitatory by Nav channels) on membrane excitability.
The epistatic effects that DSC1 knockout has on pyrethroid resistance may have significant practical implications. For example, identifying compounds that affect the activity of DSC1 channels could theoretically be used to enhance the sensitivity of pyrethroid-resistant insects to pyrethroids. Importantly, the DSC1 family of genes appears to be restricted to only insects and several invertebrate phyla; they are not found in mammals and other vertebrates (Cui et al., 2012). In this regard, the DSC1/BSC1 family of ion channels represents an attractive target for future development of new and safe chemicals against insect pests.
Because DDT and pyrethroids exert rather similar effects on Nav channels, we had expected that knockout of the DSC1 channel would also potentiate the action of DDT. Surprisingly, however, DSC1 knockout flies were less sensitive to DDT compared to w1118A flies in both toxicity and knockdown bioassays. Furthermore, the parats1;DSC1−/− double mutant showed an additive effect of these two gene mutations, which is in contrast to the pyrethroid antagonism described above. These results raise the intriguing possibility that the DSC1 channel could be a major target of DDT activity. This is an important finding because although the Nav channel has been the focus for understanding the mechanism of DDT resistance, DDT-resistance has been linked to a mutation in the DSC1 channel (Amichot et al., 1992). As such, a significant implication of this study is that future investigations of the DDT mode of action and resistance mechanisms to DDT in mosquitoes should include characterization of the DSC1 gene, in addition to the Nav gene.
The involvement of the DSC1 channel in the action of DDT, but not of pyrethroids, could also explain some puzzling findings in earlier studies. For example, injection of a sublethal dose of the sodium channel blocker tetrodotoxin (TTX) protects cockroach peripheral nervous system (PNS) from the effects of allethrin, a pyrethroid insecticide (Gammon, 1978). However, TTX does not protect cockroach PNS from the effect of DDT, demonstrating that DDT acts on a target aside from a Nav channel (Gammon, 1977). We predict that the DSC1 channel might be the non-sodium channel target because TTX does not block the DSC1 channel (Zhang et al., 2011).
ACKNOWLEDGEMENTS
We thank Dr. Kris Silver for critical review of the manuscript. The research was supported by grants from the National Institutes of Health (GM 080255 to K.D. and C-F Wu and GM057445 to K.D. and B.S.Z).
Literature Cited
- Amichot M, Castella C, Cuany A, Berge JB, Pauron D. Target modification as a molecular mechanism of pyrethroid resistance in Drosophila melanogaster. Pestic. Biochem. Physiol. 1992;44:183–190. [Google Scholar]
- Beeman RW. Recent advances in mode of action of insecticides. Annu. Rev. Entomol. 1982;27:253–281. doi: 10.1146/annurev.en.27.010182.001345. [DOI] [PubMed] [Google Scholar]
- Bloomquist JR, Mittler TE, Radovsky FJ, Resh VH. Ann. Rev. Entomol. Annual Reviews, Inc.; Palo Alto: 1996. Ion Channels as Targets for Insecticides; pp. 163–190. [DOI] [PubMed] [Google Scholar]
- Bloomquist JR, Soderlund DM. Pyrethroid insecticides and DDT modify alkaloid-dependent sodium channel activation and its enhancement by sea anemone toxin. Molec. Pharmacol. 1988;33:543–550. [PubMed] [Google Scholar]
- Burton MJ, Mellor IR, Duce IR, Davies TGE, Field LM, Williamson MS. Differential resistance of insect sodium channels with kdr mutations to deltamethrin, permethrin and DDT. Insect Biochem. Mol. Biol. 2011;41:723–732. doi: 10.1016/j.ibmb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Conformational parameters for amino acids in helical, β-sheet, and random coil regions calculated from proteins. Biochemistry. 1974;13:211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Cui Y-J, Yu L-L, Xu H-J, Dong K, Zhang C-X. Molecular characterization of DSC1 orthologs in invertebrate species. Insect Biochem. Molec. Biol. 2012;42:353–359. doi: 10.1016/j.ibmb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, Silver K, Zhorov BS. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Molec. Biol. 2014;50:1–17. doi: 10.1016/j.ibmb.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, Zhorov B, Dong K. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. PNAS. 2013;110:11785–11790. doi: 10.1073/pnas.1305118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JL, Sternburg J. Temperature and the mode of action of DDT on the nervous system of Periplaneta americana. J. Ins. Physiol. 1964;10:471–485. [Google Scholar]
- Farley JM, Narahashi T, Holan G. The mechanism of action of a DDT analog on the crayfishneuromuscular junction. Neurotoxic. 1979;1:191–207. [Google Scholar]
- Feng G, Deák P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Gammon DW. Neural correlates of insecticide poisoning in the cockroach. University of Cambridge; 1977. [Google Scholar]
- Gammon DW. Neural effects of allethrin on the free walking cockroach, Periplaneta americana: an investigation using defined doses at 15 and 32°C. Pestic. Sci. 1978;9:79–91. [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J. Gen. Physiol. 1968;51:199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni NH, Yamamoto AH, Robinson KO, Mackay TFC, Anholt RRH. The DSC1 channel, encoded by the smi60E locus, contributes to odor-guided behavior in Drosophila melanogaster. Genetics. 2002;161:1507–1516. doi: 10.1093/genetics/161.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde DIV, Brown AWA. The effects of insecticides on teh action potentials of insect nerve. Canadian J. Zool. 1954;32:74–81. [Google Scholar]
- Narahashi T. Effect of the insecticide allethrin on membrane potentials of cockroach giant axons. J. Cellular Comp. Physiol. 1962;59:61–65. doi: 10.1002/jcp.1030590108. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Mode of Action of Pyrethroids. Bulletin of the World Health Organization; International Conference on Alternative Insecticides for Vector Control; Atlanta. World Health Organization; 1971. [Google Scholar]
- Narahashi T. Nerve Membrane Na+ Channels as Targets of Insecticides. TiPS Rev. 1992;13:236–241. doi: 10.1016/0165-6147(92)90075-h. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Nerve membrane ion channels as the target site of insecticides. Mini Rev. Medic. Chem. 2002;2:419–432. doi: 10.2174/1389557023405927. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Neuroreceptors and ion channels as the basis for drug action: past, present, and future. J Pharmacol Exp Ther. 2000;294:1–26. [PubMed] [Google Scholar]
- Narahashi T, Zhao X, Ikeda T, Nagata K, Yeh J. Differential actions of insecticides on target sites: basis for selective toxicity. Human & Experimental Toxicology. 2007;26:361–366. doi: 10.1177/0960327106078408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahasi T, Haas HG. DDT: Interaction with nerve membrane conductance changes. Science. 1967;157:1438–1440. doi: 10.1126/science.157.3795.1438. [DOI] [PubMed] [Google Scholar]
- Olson R, Liu Z, Nomura Y, Song W, Dong K. Molecular and functional characterization of voltage-gated sodium channel variants from Drosophila melanogaster. Insect Biochem. Molec. Biol. 2008;38:604–610. doi: 10.1016/j.ibmb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly AO, Khambay BPS, Williamson MS, Field LA, Wallace BA, Davies TGE. Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem. J. 2006;396:255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedra JHF, Hostetler A, Gaffney PJ, Reenan RA, Pittendrigh B. Hyper-susceptibility to deltamethrin in parats-1 DDT resistance Drosophila melanogaster. Pestic. Biochem. Physiol. 2004;78:58–66. [Google Scholar]
- Perry AS, Yamamoto I, Ishaaya I, Perry RY. Insecticides in agriculture and environment; Retrospects and prospects. Springer Publishing Co.; New York, NY: 1998. [Google Scholar]
- Pittendrigh B, Reenan R, ffrench-Constant RH, Ganetzky B. Point mutations in the Drosophila sodium channel gene para associated with resistance to DDT and pyrethroid insecticides. Mol. Gen. Genet. 1997;256:602–610. doi: 10.1007/s004380050608. [DOI] [PubMed] [Google Scholar]
- Rinkevich FD, Du Y, Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids Pestic. Biochem. Physiol. 2013;106:93–100. doi: 10.1016/j.pestbp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich FD, Scott JG. Reduction of dADAR activity affects the sensitivity of Drosophila melanogaster to spinosad and imidacloprid. Pesticide Biochemistry and Physiology. 2012;104:163–169. [Google Scholar]
- Salkoff L, Butler A, Wei A, Scavarda N, Giffen K, Ifune C, Goodman R, Mandel G. Genomic organization and deduced amino acid sequence of a putative sodium channel gene in Drosophila. Science. 1987;237:744–749. doi: 10.1126/science.2441469. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA. Effects of pyrethroids on voltage-sensitive calcium channels: a critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Tox. Appl. Pharmacol. 2004;196:303–318. doi: 10.1016/j.taap.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Gilbert LI, Iatrou K, Gill SS. Comprehensive molecular insect science. Elsevier; Amsterdam: 2005. Sodium channels; pp. 1–24. [Google Scholar]
- Soderlund DM. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pesticide Biochemistry and Physiology. 2010;97:78–86. doi: 10.1016/j.pestbp.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM, Knipple DC. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem. Molec. Biol. 2003;33:563–577. doi: 10.1016/s0965-1748(03)00023-7. [DOI] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetradotoxin-sensitive and tetradotoxin-resistant sodium channels. J. Pharmacol. Exper. Therap. 1994;270:595–603. [PubMed] [Google Scholar]
- Usherwood PNR, Davies TGE, Mellor IR, O'Reilly AO, Peng F, Vais H, Khambay BPS, Field LM, Williamson MS. Mutations in DIIS5 and the DIIS4-S5 linker of Drosophila melanoaster sodium channel define binding domains for pyrethroids and DDT. FEBS Letters. 2007;581:5485–5492. doi: 10.1016/j.febslet.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Usherwood PNR, Vais H, Khambay BPS, Davies TGE, Williamson MS. Sensitivity of the Drosophila para sodium channel to DDT is not lowered by the super-kdr mutation M918T on the IIS4-S5 linker that profoundly reduces sensitivity to permethrin and deltamethrin. FEBS Lett. 2005;579:6317–6325. doi: 10.1016/j.febslet.2005.09.096. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Goodson SJ, Devonshire AL, Warmke JW, Usherwood PN, Cohen CJ. Activation of Drosophila sodium channels promotes modification by deltamethrin. Reductions in affinity caused by knock-down resistance mutations. J Gen Physiol. 2000;115:305–318. doi: 10.1085/jgp.115.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudev PG, Banerjee M, Ramakrishnan C, Balaram P. Asparagine and glutamine differ in their propensities to form specific side chain-backbone hydrogen bonded motifs in proteins. Proteins: Structure, Function, and Bioinformatics. 2012;80:991–1002. doi: 10.1002/prot.24001. [DOI] [PubMed] [Google Scholar]
- Vijverberg HPM, vanden Zalm JM, vanden Bercken J. Similar mode of action of pyrethroids and DDT on sodiumchannel gating in myelinated nerves. Nature. 1982;295:601–603. doi: 10.1038/295601a0. [DOI] [PubMed] [Google Scholar]
- Warmke J, Reenan RA, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Van der Ploeg LH, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology. J Gen Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Liu Z, Song W, Du Y, Dong K. Molecular characterization and functional expression of the DSC1 channel. Insect Biochem. Molecular Bio. 2011;41:451–458. doi: 10.1016/j.ibmb.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wang Z, Wang L, Luo N, Jiang L, Liu Z, Wu CF, Dong K. Role of the DSC1 channel in regulating neuronal excitability in Drosophila melanogaster: Extending nervous system stability under stress. PLoS Genetics. 2013;9:e1003327. doi: 10.1371/journal.pgen.1003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhorov BS, Tikhonov DB. Potassium, sodium, calcium, and glutamate-gated channels: Pore architechture and ligand action. J. Neurochem. 2004;88:782–799. doi: 10.1111/j.1471-4159.2004.02261.x. [DOI] [PubMed] [Google Scholar]
- Zhou W, Chung I, Goldin A, Dong K. A voltage-gated calcium-selective channel encoded by a sodium channel-like gene. Neuron. 2004;42:101–112. doi: 10.1016/s0896-6273(04)00148-5. Z., L. [DOI] [PMC free article] [PubMed] [Google Scholar]