Abstract
Background
Acne is a chronic skin disease characterised by inflamed spots and blackheads on the face, neck, back, and chest. Cysts and scarring can also occur, especially in more severe disease. People with acne often turn to complementary and alternative medicine (CAM), such as herbal medicine, acupuncture, and dietary modifications, because of their concerns about the adverse effects of conventional medicines. However, evidence for CAM therapies has not been systematically assessed.
Objectives
To assess the effects and safety of any complementary therapies in people with acne vulgaris.
Search methods
We searched the following databases from inception up to 22 January 2014: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL; 2014,Issue 1), MEDLINE (from 1946), Embase (from 1974), PsycINFO (from 1806), AMED (from 1985), CINAHL (from 1981), Scopus (from 1966), and a number of other databases listed in the Methods section of the review. The Cochrane CAM Field Specialised Register was searched up to May 2014. We also searched five trials registers and checked the reference lists of articles for further references to relevant trials.
Selection criteria
We included parallel‐group randomised controlled trials (or the first phase data of randomised cross‐over trials) of any kind of CAM, compared with no treatment, placebo, or other active therapies, in people with a diagnosis of acne vulgaris.
Data collection and analysis
Three authors collected data from each included trial and evaluated the methodological quality independently. They resolved disagreements by discussion and, as needed, arbitration by another author.
Main results
We included 35 studies, with a total of 3227 participants. We evaluated the majority as having unclear risk of selection, attrition, reporting, detection, and other biases. Because of the clinical heterogeneity between trials and the incomplete data reporting, we could only include four trials in two meta‐analyses, with two trials in each meta‐analysis. The categories of CAM included herbal medicine, acupuncture, cupping therapy, diet, purified bee venom (PBV), and tea tree oil. A pharmaceutical company funded one trial; the other trials did not report their funding sources.
Our main primary outcome was 'Improvement of clinical signs assessed through skin lesion counts', which we have reported as 'Change in inflammatory and non‐inflammatory lesion counts', 'Change of total skin lesion counts', 'Skin lesion scores', and 'Change of acne severity score'. For 'Change in inflammatory and non‐inflammatory lesion counts', we combined 2 studies that compared a low‐ with a high‐glycaemic‐load diet (LGLD, HGLD) at 12 weeks and found no clear evidence of a difference between the groups in change in non‐inflammatory lesion counts (mean difference (MD) ‐3.89, 95% confidence interval (CI) ‐10.07 to 2.29, P = 0.10, 75 participants, 2 trials, low quality of evidence). However, although data from 1 of these 2 trials showed benefit of LGLD for reducing inflammatory lesions (MD ‐7.60, 95% CI ‐13.52 to ‐1.68, 43 participants, 1 trial) and total skin lesion counts (MD ‐8.10, 95% CI ‐14.89 to ‐1.31, 43 participants, 1 trial) for people with acne vulgaris, data regarding inflammatory and total lesion counts from the other study were incomplete and unusable in synthesis.
Data from a single trial showed potential benefit of tea tree oil compared with placebo in improving total skin lesion counts (MD ‐7.53, 95% CI ‐10.40 to ‐4.66, 60 participants, 1 trial, low quality of evidence) and acne severity scores (MD ‐5.75, 95% CI ‐9.51 to ‐1.99, 60 participants, 1 trial). Another trial showed pollen bee venom to be better than control in reducing numbers of skin lesions (MD ‐1.17, 95% CI ‐2.06 to ‐0.28, 12 participants, 1 trial).
Results from the other 31 trials showed inconsistent effects in terms of whether acupuncture, herbal medicine, or wet‐cupping therapy were superior to controls in increasing remission or reducing skin lesions.
Twenty‐six of the 35 included studies reported adverse effects; they did not report any severe adverse events, but specific included trials reported mild adverse effects from herbal medicines, wet‐cupping therapy, and tea tree oil gel.
Thirty trials measured two of our secondary outcomes, which we combined and expressed as 'Number of participants with remission'. We were able to combine 2 studies (low quality of evidence), which compared Ziyin Qinggan Xiaocuo Granule and the antibiotic, minocycline (100 mg daily) (worst case = risk ratio (RR) 0.49, 95% CI 0.09 to 2.53, 2 trials, 206 participants at 4 weeks; best case = RR 2.82, 95% CI 0.82 to 9.06, 2 trials, 206 participants at 4 weeks), but there was no clear evidence of a difference between the groups.
None of the included studies assessed 'Psychosocial function'.
Two studies assessed 'Quality of life', and significant differences in favour of the complementary therapy were found in both of them on 'feelings of self‐worth' (MD 1.51, 95% CI 0.88 to 2.14, P < 0.00001, 1 trial, 70 participants; MD 1.26, 95% CI 0.20 to 2.32, 1 trial, 46 participants) and emotional functionality (MD 2.20, 95% CI 1.75 to 2.65, P < 0.00001, 1 trial, 70 participants; MD 0.93, 95% CI 0.17 to 1.69, 1 trial, 46 participants).
Because of limitations and concerns about the quality of the included studies, we could not draw a robust conclusion for consistency, size, and direction of outcome effects in this review.
Authors' conclusions
There is some low‐quality evidence from single trials that LGLD, tea tree oil, and bee venom may reduce total skin lesions in acne vulgaris, but there is a lack of evidence from the current review to support the use of other CAMs, such as herbal medicine, acupuncture, or wet‐cupping therapy, for the treatment of this condition. There is a potential for adverse effects from herbal medicines; however, future studies need to assess the safety of all of these CAM therapies. Methodological and reporting quality limitations in the included studies weakened any evidence. Future studies should be designed to ensure low risk of bias and meet current reporting standards for clinical trials.
Plain language summary
Complementary therapies for acne vulgaris
Background
Acne is a chronic skin disease, which causes spots to occur simultaneously on several areas of the body, including the face, neck, back, and chest. Besides the current commonly used treatments, complementary and alternative medicines (CAM) are of increasing interest to people who often use them in addition to conventional treatments as additive or single therapies to treat acne.
The review question
Can any complementary therapies improve the clinical symptoms of acne vulgaris?
Study characteristics
We searched relevant databases and trials registers up to 22 January 2014. We identified 35 randomised controlled trials, with 3227 participants, which used 6 kinds of CAM (herbal medicine, acupuncture, wet cupping, diet, purified bee venom, and tea tree oil). A pharmaceutical company funded one trial; the other trials did not report their funding sources.
Key results
For our primary outcome, we combined two studies that compared a low‐ with a high‐glycaemic‐load diet (LGLD, HGLD), but found no clear evidence of a difference between the 2 groups at 12 weeks for a change in non‐inflammatory lesion counts. Only one of these two trials provided usable data to show potential benefit of LGLD for reducing inflammatory and total skin lesion counts. Tea tree oil and pollen bee venom were found to reduce total skin lesion counts in single trials, respectively. The remaining 31 included trials gave mixed results about whether complementary therapies might reduce the total number of skin lesion counts.
Twenty‐six trials reported adverse events. The herbal medicine group found some mild side‐effects, such as nausea, diarrhoea, and stomach upset. The acupuncture group found some itch or redness and pain following needle insertion. Participants who used tea tree oil reported itchiness, dryness, and flaking of the skin. None of the trials reported severe adverse effects.
For our secondary outcome, there was no clear evidence of a difference in the number of participants with remission between Ziyin Qinggan Xiaocuo Granule and minocycline according to a meta‐analysis of two studies.
Quality of the evidence
There is some low‐quality evidence from single trials that a low‐glycaemic‐load diet, tea tree oil, and bee venom may reduce skin lesions in acne vulgaris, but there is a lack of evidence from the current review to support the use of other CAMs. Methodological and reporting quality limitations in the included studies weakened any evidence.
Summary of findings
Summary of findings for the main comparison. Low‐glycaemic‐load diet versus high‐glycaemic‐load diet for acne vulgaris.
| Low‐glycaemic‐load diet versus high‐glycaemic‐load diet for acne vulgaris | ||||||
| Patient or population: people with acne vulgaris Settings: university hospital Intervention: low‐glycaemic‐load diet versus high‐glycaemic‐load diet | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| High‐glycaemic‐load diet | Low‐glycaemic‐load diet | |||||
| Primary outcome: change in non‐inflammatory lesion count (medium‐term data) Follow‐up: 10 to 12 weeks | The mean primary outcome: change in non‐inflammatory lesion count (medium‐term data) in the control groups was ‐1.15 | The mean primary outcome: change in non‐inflammatory lesion count (medium‐term data) in the intervention groups was 3.89 lower (10.07 lower to 2.29 higher) | ‐ | 75 (2 studies) | ⊕⊕⊝⊝ low¹,² | Only results from a meta‐analysis with 2 poor‐quality trials contributed to the assessment of quality of the evidence for this comparison, which showed a low level of evidence. No conclusion could be drawn for safety outcomes of this intervention |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹downgraded by one point due to serious study limitations: There were potential risk of performance and attrition bias. (Blinding of participants and personnel was not applied; missing data in one of the trials were treated inappropriately). ²downgraded by one point due to inconsistency results between trials: Result from each individual trial showed different estimate effect of interventions, and there was potential statistical heterogeneity between trials (I² statistic > 50%).
Summary of findings 2. Herbal medicine versus antibiotics for acne vulgaris.
| Herbal medicine versus antibiotics for acne vulgaris | ||||||
| Patient or population: people with acne vulgaris Settings: outpatient dermatology department of Chinese Hospital Intervention: herbal medicine versus antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antibiotics | Herbal medicine | |||||
| Secondary outcome: number of participants with remission (ITT‐best case, short‐term data) ‐ Ziyin‐Qinggan‐Xiaocuo Granule versus minocycline (at 4 week) Follow‐up: mean 4 weeks | 79 per 1000 | 222 per 1000 (69 to 713) | RR 2.82 (0.88 to 9.06) | 206 (2 studies) | ⊕⊕⊝⊝ low¹ | Only results from a meta‐analysis with 2 poor‐quality trials contributed to the assessment of quality of the evidence for this comparison, which showed a low level of evidence. No conclusion could be drawn for safety outcomes of this intervention |
| Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data) ‐ Ziyin‐Qinggan‐Xiaocuo Granule versus minocycline (at 4 week) Follow‐up: mean 4 weeks | 213 per 1000 | 105 per 1000 (19 to 540) | RR 0.49 (0.09 to 2.53) | 206 (2 studies) | ⊕⊕⊝⊝ low¹ | |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹downgraded by two points due to very serious study limitations: There were high risk of performance, detection, and reporting bias. (Blinding of participants, personnel, and outcome assessors were not applied; there was a difference between the trial protocol and final report).
Background
Please see the glossary in Table 3 for an explanation of the terms we have used.
1. Glossary.
| Term | Description |
| Follicular hyperkeratinisation | A chronic skin disease characterised by small follicular papules; disseminated reddish‐brown scaly patches; and often, palmoplantar hyperkeratosis. The papules are about the size of a pin and topped by a horny plug |
| Gerson diet | A specific diet that was designed by Dr Max B Gerson for cancer and other ailments to detoxify the body while boosting the immune system and improving metabolism |
| Antineoplaston | A mixture of sodium salts of phenylacetic acid and phenylacetylglutamine in the ratio 4:1 |
| Chelation therapy | Therapy of heavy metal poisoning using agents that sequester the metal from organs or tissues and bind it firmly within the ring structure of a new compound, which can be eliminated from the body |
| Immunoaugmentation therapy | A treatment aimed to restore the immune system to enable it to destroy cancer cells, by evaluation‐measuring deficiencies of the immune systems and therapy‐replenishing deficient factors by self‐injection of sera |
| Hyperlipidaemia | Conditions with excess lipids in the blood |
| Teratogenicity | Ability to cause birth defects |
| Decoction | The extraction of the water‐soluble substances of a drug or medicinal plants by boiling |
Description of the condition
Acne is a chronic inflammatory condition. It most commonly affects areas where the sebaceous glands are largest and most abundant ‐ for example, the face, anterior trunk, and upper back (Simon 2005). It is a skin disease that causes spots to occur simultaneously on several areas of the body, including the face, neck, back, and chest. Comedones, also known as blackheads, which are dilated pores with a plug of keratin, characterise mild acne. Whiteheads (small, cream‐coloured, dome‐shaped papules), red papules, pustules, or cysts may be found with moderate or severe acne. Scars, both those on the skin and emotional scars, can last a lifetime (Oberemok 2002a; Webster 2002).
Acne is a common condition affecting 80% of adolescents (most commonly from 12 years of age), but it may also affect 54% of adult women and 40% of adult men (including those in their early‐ or mid‐20s) (Ramos‐e‐Silva 2009). Two surveys suggest that the age of onset of acne has fallen, with a mean age of onset of 11.6 years from a survey period between 2006 to 2008 and a mean age of 11.92 years from a survey period between 1991 to 1993 (Sørensen 2010).
Bacterial colonisation, sebum, hormone production, follicular hyperkeratinisations, and inflammation all contribute to acne causation (Friedlander 2010). According to the lesion type, acne can be classified into four main categories: non‐inflammatory (purely comedone acne), mild papular, scarring papular, and nodular; the latter three are inflammatory acne lesions (Webster 2002). Besides the obvious skin lesions, acne may produce permanent scarring and have significant psychosocial consequences (Fried 2010; Oberemok 2002).
Description of the intervention
Acne treatment aims to lessen the inflammatory or non‐inflammatory acne lesions, improve appearance, prevent or minimise potential adverse effects, and minimise any scarring (Oberemok 2002a). Ignoring prescribed drugs, many people still rely on herbal medications, skin hygiene routines, and dietary modifications (Webster 2002).
Complementary and alternative medicine (CAM) usually refers to therapies that are used in addition to conventional treatment, and many such therapies remain unproven (Verhoef 1999). 'Complementary therapy' is usually used in addition to conventional treatments, while 'alternative therapy' is often used instead of conventional treatment. In 1992, the US Office of Alternative Medicine classified complementary therapies, using the classification established by congressional mandate at the National Institutes of Health (Workshop 1992). According to this classification, complementary therapies are divided into the following categories: diet and nutrition (e.g., macrobiotics, Gerson diet, antioxidants); mind‐body interventions (e.g., meditation, imagery, hypnosis, support groups); bioelectromagnetics (e.g., electromagnetics, electroacupuncture); traditional and folk remedies (e.g., naturopathy, Ayurveda, Traditional Chinese Medicine, homeopathy); pharmacological and biological treatments (e.g., antineoplaston, chelation therapy, immuno‐augmentation therapy, shark cartilage); manual healing methods (e.g., massage, chiropractic, therapeutic touch); and herbal medicine.
Complementary and alternative medicines are a significant subset of healthcare practices not integral to conventional western care but used by people in their healthcare decisions (Lewith 2001). Though lacking biomedical explanation, some of them (physical therapies, diet, acupuncture) have become widely accepted, but others, such as those interventions linked to the theory of humours (an old term to describe liquids within the body ‐ an example of which was the 'letting' of blood as a form of treatment) and radium therapy, quietly fade away (Lewith 2001).
Tradtional Chinese Medicine (TCM), a traditional healthcare system that is based on the beliefs and practices of Chinese culture and ancient philosophy, includes herbal medicine; acupuncture or moxibustion (combustion of the moxa or mugwort herb); massage; cupping therapy; therapeutic exercise, such as tai chi; and dietary therapy. Traditional Chinese Medicine is one of the most important components of complementary and alternative medicines.
How the intervention might work
Acne develops as a result of increased sebum production, hyperkeratinisation, increase in Propionibacterium acnes, and inflammation (Spencer 2009), and treatments aim to address some or all of these elements (Webster 2002).
Chinese herbal medicine, manual healing therapies (such as acupuncture and massage), and other traditional and folk remedies may follow similar mechanisms in the treatment of acne. According to the theory of Traditional Chinese Medicine, several factors may cause acne, including the following: overheating of the lung or stomach, damp and heat toxin with blood stasis, and stagnation of Qi (the life force, or vital energy, of a living thing) and blood. As the condition becomes protracted, heat may rise and lodge in the skin and tissues, thus, producing the lesions (Shen 1995). All of the above therapies may help the body to regulate the Qi and blood, eliminate dampness, relieve heat toxicity, and enhance the immunologic function to improve the remission of acne (Shen 1995). Some studies also mention that acupuncture can stimulate and balance androgen levels to inhibit the over‐secretion of the sebaceous gland (Li 2009).
It is usually recommended that people with acne restrict their consumption of chocolate and oily or fatty foods (El‐Akawi 2006). One review concluded that some components of western diets, particularly dairy products, may be associated with an increased risk of acne (Spencer 2009). Some researchers have concluded that genetic predisposition and hormonal influences play a more important role in acne than diet (Magin 2005; Wolf 2004). However, despite the genetic regulation of sebum excretion and other determinants of acne, diet may act as a modifier of gene expression that may account for the increased acne risk (Walton 1988). The effect of other kinds of complementary therapies and medicines on treating acne are currently unclear due to insufficient evidence.
Commonly used treatments aim to reduce the number of inflammatory lesions, inhibit comedones (Webster 2002), suppress the growth of Propionibacterium acne (Toyoda 1998), or reduce sebaceous gland size and secretory activity (Enders 2003). Topical retinoids (such as tretinoin, adapalene, or tazarotene) can usually inhibit comedonal acne, the non‐inflammatory type of acne. These medications may reduce the number of inflammatory lesions, but local irritation can accompany them (Webster 2002). Benzoyl peroxide (BP) alone or combined with either clindamycin or retinoid, which exert a synergistic and antimicrobial effect, can treat mild papulopustular acne. Oral antibiotics (Simonart 2005) are also used to suppress the growth of acne and reduce the production of inflammatory factors (Toyoda 1998), while oral doxycycline (Garner 2009) or minocycline plus topical retinoid can be used in treating severe papulopustular or nodular acne. Although isotretinoin (optimal dosage 0.5 to 1 mg/kg/day) (Katsambas 2004) may be effective in reducing sebaceous gland size and secretory activity, decreasing comedone formation, and reducing follicular colonisation with acne (Enders 2003), its teratogenicity and adverse effects profile (such as dry skin, hyperlipidaemia, and proposed increased risk of depression) is a concern (Marqueling 2007; Webster 2002).
Because of the inadequate treatment response or potential side‐effects of current topical treatments for acne, there is increasing interest in the use of complementary therapies as adjuvant or single therapies alone. In America, 9% of people reported having skin conditions in the past 12 months, 7% reported that they had used a complementary medicine, and 2% reported seeing a complementary medicine practitioner for their condition (Eisenberg 1998).
Traditional Chinese Medicine has been widely used to treat acne for many years. Herbal medicine, including decoction and patent medicine, is used based on a diagnosis from a TCM perspective according to the different syndromes of acne (Shen 1995).
Although there are no systematic reviews of herbal medicine for the treatment of acne, there has been a systematic summary of the therapeutic effect of herbal medicine for the treatment of bacterial infections (Martin 2003), where the authors found similar results to those for conventional treatments. Another evaluation assessed 17 TCM randomised controlled trials (RCTs) (Li 2009), and the findings from the analysis suggested acupuncture and moxibustion were better than routine western medicine at reducing symptoms of acne. A systematic review was conducted (Magin 2006), which included 23 trials of topical and oral CAM to treat acne.
Why it is important to do this review
Despite the widespread use of complementary and alternative medicines (CAM), there is no systematic review comprehensively assessing the evidence of CAM. In particular, a publication bias exists with the inclusion of English‐language texts only. There is a need to undertake a comprehensive and systematic review of the effectiveness and safety of CAM for the treatment of acne.
Objectives
To assess the effects and safety of any complementary therapies in people with acne vulgaris.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomised controlled trials (RCT) and only the first phase data of randomised cross‐over trials in any data analysis.
Types of participants
We assessed participants of any age and gender with a diagnosis of acne vulgaris or papulopustular, inflammatory, juvenile, or polymorphic acne.
Types of interventions
Eligible interventions included any kind of complementary and alternative medicine, including diet and nutrition; mind‐body interventions; bioelectromagnetics; traditional and folk remedies; biological treatments; manual healing methods; and herbal medicine, compared with no treatment; placebo; or other active therapies. Comparisons included a combination of CAM plus other therapies versus other active therapies alone.
Types of outcome measures
According to the report of the Consensus Conference on Acne Classification 1990 (Pochi 1991), the evaluation of lesions and their complications are important to assess the severity of acne. Psychosocial impact, failure to respond to previous therapies, and occupational disability are three additional factors used in grading acne (Pochi 1991).
The following were timing of outcomes where data were available:
short‐term data ‐ data collected within 30 days after randomisation;
medium‐term data ‐ data collected between 31 and 90 days after randomisation; and
long‐term data ‐ data collected 90 days after randomisation.
Primary outcomes
Improvement of clinical signs assessed through skin lesion counts (total of inflamed and non‐inflamed counted separately) and acne severity scores.
Adverse effects assessed by reporting early study discontinuations, the worsening of acne in participants, and other adverse events during the treatment and follow‐up period.
Secondary outcomes
Physicians' global evaluation (after treatment).
Participants' self assessment of change in specific types of lesion (such as comedones, papules, pustules, or nodules).
Psychosocial function outcomes (such as the Hamilton Depression Rating Scale (HAMD)).
Quality of life (QoL).
We combined the Physicians' global evaluation and Participants' self assessment of change in specific types of lesion and expressed them as the 'Number of participants with remission' as this had a clear definition and was easy to assess. The word "remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine" (Zheng 2002), which means lesions totally faded (or > 95% faded) and only mild pigmentation and scars remaining.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 22 January 2014:
the Cochrane Skin Group Specialised Register using the strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2014,Issue 1), using the search strategy in Appendix 2;
MEDLINE (from 1946) using the strategy in Appendix 3;
Embase (from 1974) using the strategy in Appendix 4;
PsycINFO (from 1806) using the strategy in Appendix 5;
AMED (Allied and Complementary Medicine Database, from 1985) using the strategy in Appendix 6;
CINAHL (Cumulative Index to Nursing and Allied Health Literature, from 1981) using the strategy in Appendix 7; and
PubMed (from 1966) using the CAM subset and the strategy in Appendix 8.
We searched the following databases up to 20 January 2014:
Scopus (from 1966 to the present);
ScienceDirect (from inception to the present);
MD Consult (from inception to the present);
BioMed Central (from 1997 to the present);
Current Contents Connect® (from 1998 to the present); and
ProQuest Health and Medical Complete (from inception to the present).
We also searched the following Chinese databases up to 24 January 2014:
China National Knowledge Infrastructure (CNKI, from 1979 to the present);
VIP Journal Integration Platform (VJIP, from 1989 to the present);
Wanfang Data Chinese databases (from 1985 to the present); and
Chinese Biomedical Literature database (CBM, from 1978 to the present).
We searched the following database up to 21 May 2014:
the Cochrane CAM Field Specialised Register using the search term 'acne'.
Searching other resources
We searched the following trials registers up to 24 January 2014:
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
CenterWatch (www.centerwatch.com/).
We also searched unpublished postgraduate theses in Chinese databases.
We handsearched the reference lists of all relevant articles found electronically for further references to relevant trials.
Adverse effects
We did not perform a separate search for adverse effects of the target interventions. We examined data on adverse effects from the included studies only.
Data collection and analysis
Selection of studies
Three authors (HC, GY, and YW) evaluated the titles and abstracts independently. We retrieved full‐text versions for all potentially relevant studies. The authors (HC, GY, and YW) resolved disagreements by discussion, and another author (JPL) arbitrated when needed.
Data extraction and management
Three authors (HC, GY, and YW) extracted the data from the included trials independently, with disagreements resolved by discussion with a fourth author (JPL). They extracted information about the following:
study methods: type of study design, method of random number generation and allocation concealment, details of blinding methods;
participants: inclusion/exclusion criteria, sample size, characteristics of participants (such as age, gender, duration of disorder, et al);
intervention and control: type of complementary therapy, details of treatment and control;
follow‐up data: duration of follow up, reasons and rates for withdrawal in each group;
outcome data: types of outcome, data for each outcomes per group (with unit if available); and
analysis data: methods of dealing with missing data, comparability of groups at baseline (yes/no), statistical methods.
Assessment of risk of bias in included studies
We used the method for assessment of risk of bias provided by theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to generate a 'Risk of bias' assessment table for each study. We assessed the following categories of bias:
(a) selection bias (random sequence generation and allocation concealment); (b) performance bias (blinding of participants and personnel); (c) detection bias (blinding of outcome assessment); (d) attrition bias (incomplete outcome data); (e) reporting bias (selective reporting); and (f) other bias.
For each included study, we described any important concerns we had about other possible sources of bias ‐ e.g., was there a potential source of bias related to the specific study design? Was the trial stopped early because of some data‐dependent process? Was there extreme baseline imbalance? Had the study been claimed to be fraudulent? (Higgins 2011)
The Cochrane Collaboration's 'Risk of bias' tool consists of six items. There are three potential bias judgements: 'low risk', 'high risk', or 'unclear risk'. If the paper reported insufficient detail regarding what happened in the study, the judgement was usually 'unclear'. We also made an 'unclear' judgement if we knew what happened in the study but the risk of bias was unknown or if an item was not relevant to the study at hand (particularly for assessing blinding and incomplete outcome data or when the study had not measured the outcome assessed).
Measures of treatment effect
We summarised data using risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes or mean difference (MD) with 95% CI for continuous outcomes.
Unit of analysis issues
We planned to include cross‐over trials; however, as there were minimal data on the effectiveness of washout periods for treatment, we included only the first phase of intervention.
Dealing with missing data
We made contact with the authors of the included trials to acquire missing information.
We conducted intention‐to‐treat analysis for dichotomous outcomes. We made 'best‐case' and 'worst‐case' assumptions for those leaving the study early (Gamble 2005). In best‐case assumptions, we analysed participants who dropped out of the intervention group as effective or improved after treatment, and we counted those who dropped out of the control group as having ineffective results. By contrast, for worst‐case analysis, we considered missing data in the intervention group as having an ineffective outcome and missing data in the control group as having an effective outcome. We undertook sensitivity analyses to test how prone the primary outcomes were to change under each assumption.
We reported completer‐only data for continuous outcomes.
Assessment of heterogeneity
We assessed clinical heterogeneity according to the characteristics of the included studies and the participants, details of the intervention or control, and types of outcome measurements. We assessed statistical heterogeneity by using the I² statistic. We assessed heterogeneity as substantial if the I² statistic was greater than 50%.
Assessment of reporting biases
We planned to generate funnel plots (effect size against standard error) if we found a sufficient number of trials. Asymmetry can be due to publication bias, but it can also be due to a relationship between the trial size and effect size (Higgins 2011).
Data synthesis
We performed statistical analyses with Cochrane's Review Manager software. One reviewer (HC) was responsible for entering data into the software, which a second reviewer (YW) checked. We meta‐analysed trials if the I² statistic was less than 85% and characteristics of trials were similar (same intervention or comparison (including drug type)). Forest plots visualised the results of the meta‐analysis. We used the random‐effects model unless the degree of heterogeneity was readily explainable, or if the measure of heterogeneity, the I² statistic, was less than 25%, we used the fixed‐effect model was used (Higgins 2011).
We generated 'Summary of findings' (SOF) tables using GRADEPro software (Version 3.2 for Windows). The SOF tables evaluated the overall quality of the body of evidence for clinical outcomes only from results of meta‐analysis, which used Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias).
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses for different groups split by age; sex; subtypes or severity of acne (inflammatory acne or non‐inflammatory acne); or treatment duration (short‐term, medium‐term, or long‐term), to assess whether the treatment effects were different in different subgroups. Because of insufficient data, we only undertook subgroups for different treatment duration.
Sensitivity analysis
Usually at least one characteristic can be found for a study in a meta‐analysis that makes it different from the others, i.e., an outlier. Heterogeneity may be due to the presence of one or two outlying studies with results that conflict with the rest of the studies (Higgins 2011). We planned to perform analyses both with and without outlying studies as part of a sensitivity analysis; however, we were unable to do this because of insufficient trials in each meta‐analysis.
Results
Description of studies
Results of the search
The searches conducted for the review retrieved a total of 7230 citations, 7222 following deduplication. Title and abstract screening excluded 6635 of these citations. We subsequently obtained full texts of the remaining 587 citations for further inspection. We screened a further 550 citations out with reasons stated in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (please refer to Figure 1). We deemed no citations to be eligible for inclusion, but required further information on outcome measures. Please refer to the 'Characteristics of studies awaiting classification' tables for further details. After full‐text screening, we included 35 studies in the qualitative syntheses and 4 in the quantitative syntheses of this review (please see the 'Characteristics of included studies' tables).
1.
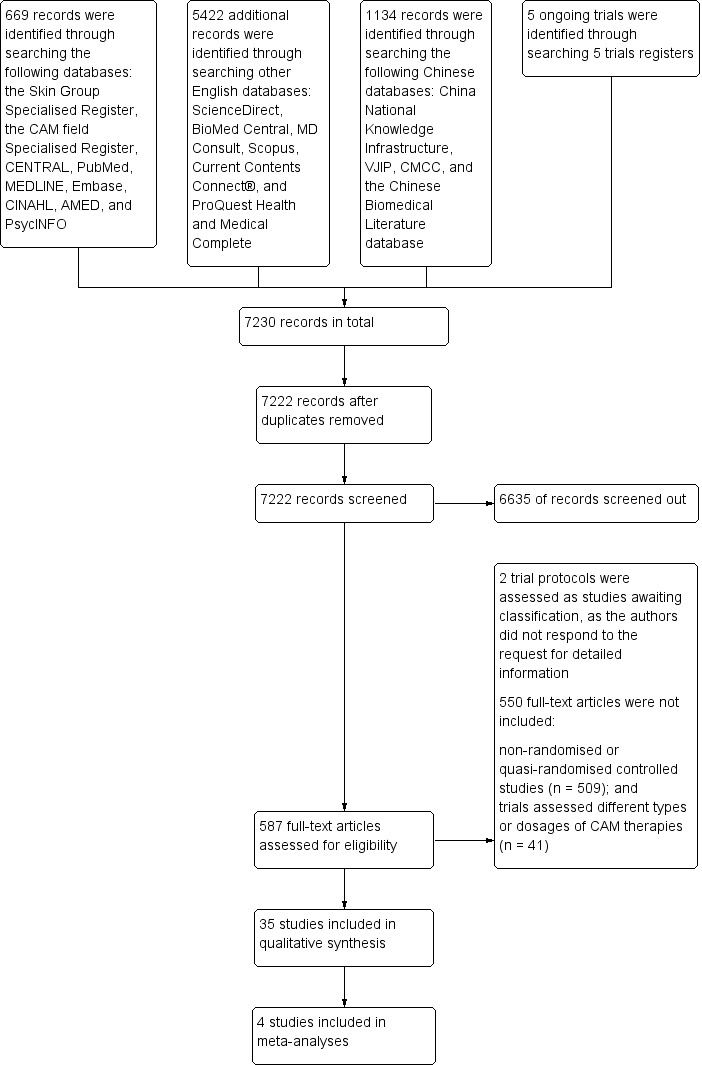
Study flow diagram
Included studies
Thirty‐five trials (from a total of 39 papers), with a total of 3227 participants (average = 46 per group), were eligible for inclusion. Please refer to the 'Characteristics of included studies' tables for detailed information on the included studies. Three papers reported Smith 2007; two papers reported Chen 2012; and two papers reported Li 2007.
Setting
Thirty of the 35 trials were conducted in China, which included 18 publications in Chinese Journals (Chen 2012; Li 2007; Lin 2013; Liu 2004; Liu 2008; Liu 2012; Mo 2011; Peng 2012; Shi 2012; Song 2007; Tang 2011; Wen 2012; Wu 2008; Wu 2008a; Yu 2008; Yu 2011; Zhang 2012; Zhu 2007) and 12 unpublished dissertations (Chen 2009; Feng 2005; Han 2010; Huang 2006; Liu 2006; Liu 2007; Ni 2008; Wang 2012; Wei 2012; You 2012; Zhang 2010; Zhou 2009). For the remaining five trials, which were published in English, three were conducted in Korea (Han 2013; Kim 2012; Kwon 2012), one was conducted in Australia (Smith 2007), and the other was conducted in Iran (Enshaieh 2007).
Design
There were 4 3‐arm trials (Chen 2009; Liu 2007; Liu 2012; Wu 2008) and 1 4‐arm trial (Kim 2012); the remaining 30 trials had a parallel 2‐arm design.
Interventions
We have listed the interventions under the following six headings: Diet, Acupuncture, Herbal medicine, Wet‐cupping therapy, Tea tree oil, and Purified bee venom (PBV).
Diet
Two trials assessed the daily diet for acne participants (Kwon 2012; Smith 2007).
Smith 2007 conducted 1 trial (with 3 publications), which had 12 weeks' duration, to investigate the therapeutic effect of a low‐glycaemic‐load diet (LGLD) where glucose is slowly released into the bloodstream compared with a high‐glycaemic‐load diet for participants with acne. Kwon 2012 also investigated the effect of LGLD for acne (with 10 weeks' duration). These 2 trials used a similar study design, and the LGLD was composed of 25% energy from protein, 45% from low‐glycaemic‐index carbohydrates, and 30% from fat.
Acupuncture
Three trials assessed the therapeutic effect of acupuncture for acne: Two arms of the four‐arm trial, Kim 2012, compared a combination of acupuncture and herbal medicine with herbal medicine alone and also compared acupuncture and a control of four weeks on the waiting list. Han 2010 compared acupuncture with isotretinoin (20 mg daily for the first month and 10 mg daily for the second month) over an 8‐week period. Huang 2006 compared acupuncture with azithromycin (500 mg daily) and clindamycin phosphate gel (externally applied) for 3 weeks.
Herbal medicine
Twenty trials investigated the therapeutic effect of herbal medicine on improving the clinical symptoms of acne. We list components of each herbal product (extract, patent, or decoction) in Table 4.
2. Components of the herbal medicine in relevant trials.
| Study ID | Name | Components | Methods of use |
| Chen 2012 | Xiaocuo decoction | Flos Lonicerae, Fructus forsythiae, Herba taraxaci, Herba violae, Flos chrysanthemi indici, Herba oldenlandiae, Herba lobeliae chinensis, Gypsum fibrosum, Radix scutellariae, Semen coicis, Fructus crataegi, Cortex mori, Radix glycyrrhizae, Radix salviae miltiorrhizae | 40 ml every time, 3 times daily for 4 weeks |
| Chen 2009 | Individualised herbal decoction according to syndrome differentiation | For Wind‐heat Syndrome: Rhizoma seu radix notoperygii 10 g, Radix saposhnikoviae 10 g, Fructus gardeniae 6 g, Rhizoma chuanxiong 10 g, Fructus tribuli 10 g, Radix glycyrrhizae 10 g, Flos chrysanthemi 10 g | Twice daily for 4 weeks |
| For Damp‐heat Syndrome: Fructus forsythiae 10 g, Radix angelicae dahuricae 10 g, Fructus gardeniae 10 g, Radix scutellariae 10 g, Rhizoma coptidis 10 g, Radix sophorae flavescentis 10 g, Herba schizonepetae 10 g, Rhizoma chuanxiong 6 g, Bulbus fritillariae thunbergii 10 g, Cortex mori 10 g, Radix glycyrrhizae 10 g | |||
| For Blood stasis Syndrome: Radix saposhnikoviae 10 g, Radix angelicae dahuricae 10 g, Gypsum fibrosum 10 g, Radix aconiti lateralis preparata 6 g, Pericarpium citri reticulatae 6 g, Rhizoma chuanxiong 6 g, Flos inulae 12 g, Rhizoma pinelliae 10 g, Rhizoma arisaematis 10 g, Bombyx batryticatus 6 g, Sporpio 6 g, Radix glycyrrhizae 10 g | |||
| Feng 2005 | Ziyin Qinggan Xiaocuo Granule | Herba Houttuyniae 15 g, Radix bupleuri 12 g, Radix rehmanniae 20 g, Radix glycyrrhizae 6 g, Radix salviae miltiorrhizae 30 g, Radix curcumae 20 g, Fructus ligustri lucidi 20 g, Herba ecliptae 20 g | Once daily for 4 weeks |
| Kim 2012 | Keigai‐rengyo‐to‐extract (KRTE) | Schizoneptae herba 0.27 g, Forsythiae fructus 0.34 g, Ledebouriellae radix 0.65 g, Angelicae gigantis radix 0.70 g, Cnidii rhizoma 0.65 g, Paeoniae radix 0.32 g, Bupleuri radix 0.59 g, Aurantii fructus 0.86 g, Scutellariae radix 0.67 g, Gardeniae fructus 0.89 g, Angelicae dahuricae radix 0.35 g, Platycodi radix 0.51 g, Glycyrrhizae radix 0.67 g. Total amount is 7.4 g | 3 times daily after meals for 4 weeks |
| Lin 2013 | Herbal medicinal mask | Radix Angelicae Dahuricae, Rhizoma Bletillae, Cortex Dictamni, Herba Taraxaci, Fructus Forsythiae, et al | 30 minutes every time, once every 2 days for 4 weeks |
| Liu 2012 | Herbal medicinal mask | Flos Lonicerae, Herba Violae, Flos chrysanthemi indici, Herba oldenlandiae, Radix angelicae dahuricae, Cortex moutan, Radix aconiti lateralis preparata, Margarita. Total amount is 35 g | 30 minutes every time, twice per week for 4 weeks |
| Liu 2004 | Xiaocuo decoction | Herba Taraxaci 20 g, Radix scutellariae 30 g, Rhizoma coptidis 9 g, Radix et rhizoma rhei 6 g, Radix glycyrrhizae 6 g, Radix salviae miltiorrhizae 30 g, Fructus ligustri lucidi 20 g, Herba ecliptae 20 g | 50 ml every time, twice daily for 4 weeks |
| Liu 2007 | Modified Wuwei Xiaodu Yin and Yinchenhao decoction | Flos Lonicerae 20 g, Fructus forsythiae 10 g, Herba Violae 15 g, Flos chrysanthemi indici 10 g, Folium isatidis 15 g, Herba oldenlandiae 30 g, Herba portulacae 15 g, Rhizoma coptidis 9 g, Radix et rhizoma rhei 6 g, Herba artemisiae scopariae 15 g, Radix glycyrrhizae 6 g, Radix salviae miltiorrhizae 20 g | 100 ml every time, twice daily for 8 weeks |
| Li 2007 | Ziyin Qinggan Xiaocuo Granule | Herba Houttuyniae 15 g, Radix rehmanniae 20 g, Radix et rhizoma rhei 30 g, Radix glycyrrhizae 6 g, Radix salviae miltiorrhizae 30 g, Fructus ligustri lucidi 20 g, Herba ecliptae 20 g | Once daily for 4 weeks |
| Mo 2011 | Yinqiao Liangxue decoction | Flos Lonicerae 20 g, Fructus forsythiae 20 g, Folium isatidis 20 g, Herba oldenlandiae 15 g, Radix trichosanthis 15 g, Cortex moutan 20 g, Radix paeoniae rubra 15 g, Bulbus fritillariae thunbergii 12 g, Cortex mori 12 g, Rhizoma anemarrhenae 15 g | 50 ml every time, twice daily for 4 weeks |
| Peng 2012 | Da Huang Zhe Chong Pills | Radix et Rhizoma Rhei, Eupolyphaga seu Steleophaga, Hirudo, Tabanus, Grub, Resina Toxicodendri, Semen Persicae, Semen Armeniacae Amarum, Radix Scutellariae, Radix Rehmanniae, Radix Paeoniae Alba, Radix Glycyrrhizae | 3 g half hour before meal, taken with Dan Quan rice wine 10 ml (Guangxi Dan Quan wine industry limited by share Ltd.), twice daily for 8 weeks |
| Shi 2012 | Jiawei Xiaodu Yin | Flos Lonicerae 10 g, Herba Taraxaci 10 g, Herba Violae 10 g, Flos chrysanthemi indici 10 g, Radix angelicae dahuricae 10 g, Radix scutellariae 10 g, Medulla tetrapanacis 10 g, Bulbus fritillariae thunbergii 12 g, Cortex mori 10 g, radix astragali 30 g, Spina gleditsiae 10 g | Twice daily for 15 days |
| Tang 2011 | Xiaocuo Granule | Herba Violae 15 g, Flos chrysanthemi indici 12 g, Herba oldenlandiae 15 g, Radix bupleuri 10 g, Radix angelicae dahuricae 10 g, Fructus gardeniae 10 g, Cortex moutan 10 g, Radix glycyrrhizae 6 g, Radix paeoniae alba 12 g, Radix angelicae sinensis 12 g, Radix salviae miltiorrhizae 15 g, Herba menthae 6 g | Twice daily for 5 weeks |
| Herbal decoction external used by steaming | Herba Taraxaci 30 g, Radix angelicae dahuricae 15 g, Herba leonuri 30 g, Radix angelicae sinensis 15 g, Radix salviae miltiorrhizae 15 g | 20 minutes every time, twice daily for 5 weeks | |
| Wang 2012 | Modified Cuochuang decoction | Herba Taraxaci 30 g, Herba Violae 30 g, Herba oldenlandiae 10 g, Herba portulacae 30 g, Radix scutellariae 10 g, Radix rehmanniae 15 g, Radix paeoniae rubra 10 g, Herba artemisiae scopariae 15 g, Bulbus fritillariae thunbergii 30 g, Folium eriobotryae 15 g, Cortex mori 10 g, Radix glycyrrhizae 5 g, Radix angelicae sinensis 5 g, Radix salviae miltiorrhizae 30 g, Flos chrysanthemi 30 g | Twice daily for 6 weeks |
| Wei 2012 | Herbal decoction aim to clear liver heat and dampness | Herba Taraxaci 20 g, Radix bupleuri 6 g, Fructus gardeniae 10 g, Radix scutellariae 10 g, Radix gentianae 10 g, Radix rehmanniae 20 g, Semen coicis 20 g, Semen plantaginis 10 g, Rhizoma alismatis 10 g, Rhizoma atractylodis macrocephalae 10 g, Radix glycyrrhizae 5 g, Radix paeoniae alba 10 g, Radix salviae miltiorrhizae 10 g, Radix angelicae sinensis 10 g | 200 ml every time, twice daily after meals for 8 weeks |
| Wu 2008 | Qingre Twenty‐five Ingredient pills | Lignum santali albi, Fructus toosendan, Semen euphorbiae, Flos carthami, Fructus amomi rotundus, Flos caryophylli, Fructus alpiniae oxyphyllae, Fructus tsaoko, Pericarpium citri reticulatae viride, Borneolum syntheticum, Radix aucklandiae, Caulis clematidis armandii, Gypsum fibrosum, Fructus aurantii immaturus, Fructus chebulae, Fructus cardeniae, Cortex phellodendri, Flos chrysanthemi, Lignum aquilariae resinaltum, Rhizoma belamcandae, et al | 2 g every time, twice daily for 30 days |
| Yu 2011 | Compound Danshen tablet | Guangdong Yikang Pharmaceutical Ltd Co (Z44020873). Main ingredient: Radix salviae miltiorrhizae, Radix notoginseng, Borneolum syntheticum | 0.412 g/tablet, 3 tablets every time, 3 times daily for 4 weeks |
| Yu 2008 | Shugan Ziyin decoction | Fructus toosendan, Rhizoma cyperi, Radix salviae miltiorrhizae, Semen cuscutae, Fructus ligustri lucidi, Herba ecliptae, Radix stellariae | 50 ml once daily for 8 weeks |
| Zhang 2012 | Qingbu decoction | Fructus forsythiae 15 g, Herba Taraxaci 30 g, Herba oldenlandiae 30 g, Spica prunellae 10 g, Fructus gardeniae 9 g, Radix bupleuri 10 g, Semen coicis 30 g, Herba artemisiae scopariae 15 g, Fructus aurantii 10 g, Radix pseudostellariae 30 g, Radix glycyrrhizae 6 g, Radix salviae miltiorrhizae 30 g, Fructus ligustri lucidi 12 g, Herba ecliptae 12 g, Spina gleditsiae 10 g | 150 mg every time, twice daily after meals for 4 weeks |
| Zhu 2007 | Modified Cuochuang decoction | Radix scutellariae 10 g, Cortex phellodendri 10 g, Folium eriobotryae 10 g, Cortex mori 10 g, Radix glycyrrhizae 10 g, Radix salviae miltiorrhizae 30 g, Flos carthami 10 g, Semen persicae 10 g, Rhizoma anemarrhenae 10 g | Twice daily for 6 weeks |
Half of the four‐arm trial, Kim 2012, compared a combination of herbal decoction and acupuncture with acupuncture alone and herbal decoction with a stay on the waiting list, respectively, with four weeks' treatment duration.
Fifteen trials compared herbal medicine with western medications; of these, Wang 2012 employed herbal decoction applied externally to skin lesions. Another trial, Tang 2011, assessed herbal medicine (taken orally and externally applied) compared with roxithromycin (300 mg daily) and viaminate capsules (150 mg daily). The remaining 12 trials all used herbal decoction administered orally and taken twice daily. In the control groups, seven trials (Feng 2005; Li 2007; Liu 2004; Mo 2011; Tang 2011; Wei 2012; Yu 2008) employed antibiotics, including minocycline, doxycycline, azithromycin, and tetracycline. The dosage for the antibiotic drugs used as controls varied (details are in the Effects of interventions section), as well as the treatment duration from four to eight weeks. Eight out of these trials used vitamin A acid (including viaminate capsule, viaminate cream, isotretinoin soft capsules, and tretinoin cream) as a control, in which three (Liu 2007; Shi 2012; Wang 2012) used cream or gel for external application. The oral doses in 5 other trials were 20 to 75 mg per day (Chen 2012; Peng 2012; Tang 2011; Zhang 2012; Zhu 2007). The treatment duration varied from two to eight weeks. One of the 15 trials, Wu 2008, used herbal decoction (used singly or combined with staphylococcus injection) compared with staphylococcus injection; the injection was used 0.2 to 0.5 ml daily for 4 weeks.
Yu 2011 observed a combination of herbal decoction and antibiotics compared with antibiotics (doxycycline, 200 mg daily). Liu 2012 investigated the effect of herbal decoction combined with blue and red light‐emitting diode (LED) phototherapy compared with LED alone.
The remaining trial, Chen 2009, investigated the combination of herbal medicinal mask and wet‐cupping therapy compared with wet‐cupping therapy alone. The main treatment principle of herbal medicine was to clear the liver or lung's heat and dampness, nourishing yin, and removing the blood stasis.
Wet‐cupping therapy
This is a method involving sterilising the selected points with alcohol and making a very small incision with a triangle‐edged needle or a plum‐blossom needle and firmly tapping the point for a short time to cause bleeding (Cao 2010), then creation of a vacuum by applying suction with a hot cup or jar on the desired points or the specific affected body surface, in order to increase the blood supply to the area under suction.
Four trials evaluated the therapeutic effect of wet‐cupping therapy (Chen 2009; Song 2007; Wu 2008a; Zhou 2009): One trial, Zhou 2009, chose GV14 (Dazhui) and BL13 (Feishu) as pricking point, according to the principle of clearing and expelling the lung's heat, compared with viaminate cream. Two trials used tetracycline as control treatment (1000 mg daily in Wu 2008a and 1500 mg daily in Song 2007); however, Wu 2008a chose the tender point on the back as the target point for pricking and cupping, and Song 2007 used blood‐letting on the ear lobe plus wet cupping on the back. One trial, Chen 2009, compared a combination of wet‐cupping therapy and herbal decoction with herbal decoction alone. The treatment duration of these four trials was two and four weeks.
Six trials observed the therapeutic effect of a combination of wet‐cupping therapy and acupuncture for acne (Liu 2006; Liu 2007; Liu 2008; Wen 2012; You 2012; Zhang 2010): Two trials (Liu 2006; Wen 2012) employed minocycline (100 mg daily) as a control treatment, and the other four (Liu 2007; Liu 2008; You 2012; Zhang 2010) used vitamin A acid (viaminate cream, isotretinoin soft capsules 30 mg daily, tretinoin cream, or viaminate capsule 150 mg daily, respectively) as control therapy. Both intervention group and control group used a herbal medicinal mask as an additional treatment in one trial (You 2012). The points GV14 (Dazhui) and BL13 (Feishu) were chosen as the main points for wet‐cupping therapy in all of these six trials. The treatment duration varied from 4 to 10 weeks.
One trial, Lin 2013, used wet‐cupping therapy combined with herbal medicinal mask compared with viaminate capsule (30 mg daily). The treatment duration was four weeks.
One trial, Ni 2008, employed a combination of wet cupping, acupuncture, and massage as intervention therapy and used zinc gluconate oral liquid (40 ml daily) as a control treatment. The treatment duration was three weeks.
Tea tree oil
One trial, Enshaieh 2007, compared tea tree oil gel with placebo (vehicle gel alone) in 60 participants. Tea tree oil gel or placebo gel was applied externally to the skin lesion area for 20 minutes daily for 45 days.
Purified bee venom (PBV)
One trial, Han 2013, used cosmetics with or without purified bee venom (PBV) as the comparison in 12 participants. Cosmetics were applied topically on the whole face with an amount of 4 mL per day for 2 weeks.
Excluded studies
Out of 7230 citations retrieved by the search (7222 after removal of duplicates), our title and abstract screening excluded 6635. We obtained for further inspection the full texts of the remaining 587 citations. We screened out a further 550 citations with reasons stated in the PRISMA flow chart (please refer to Figure 1).
Studies awaiting classification
We identified two studies that are awaiting classification because although they were marked as 'completed', we were unable to get any more information about them from the authors.
Risk of bias in included studies
We present the risk of bias of the included studies in Figure 2 and a summary in Figure 3.
2.
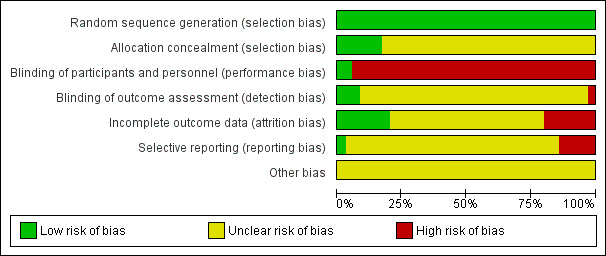
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
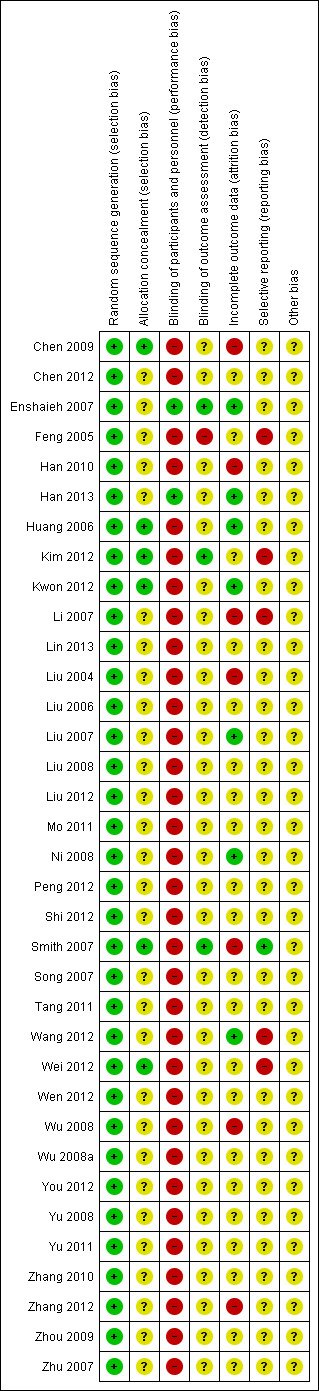
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
We had strict inclusion criteria that all the included studies should clearly state the method of randomisation, so we judged all the included trials as at a low risk of random sequence generation. According to the reports, a random number table or computer software (such as SPSS, SAS) generated the random numbers.
Six trials reported the methods of allocation concealment. Three trials kept the random number in an opaque envelope (Chen 2012; Huang 2006; Wei 2012); in two trials, a third party (research nurse) performed the allocation (Kwon 2012; Smith 2007). The remaining trial, Kim 2012, used two separate databases (participant database and randomisation database) to keep the basic information or allocations, respectively. The participant database was accessible to any researcher; however, the randomisation database was password‐protected. We evaluate the other 29 included trials as at an unclear risk of bias because of the insufficient information about the allocation concealment.
Blinding
Two trials, Enshaieh 2007; Han 2013, reported sufficient information to permit a judgement of a low risk of bias for performance bias, and three trials, Enshaieh 2007; Kim 2012; Smith 2007, reported sufficient information to permit a judgement for detection bias. One trial, Feng 2005, reported the study as a "single blind" study; another trial provided detailed information of blinding to personnel (Kim 2012). However, in those studies, intervention and control (non‐placebo) were not similar in either appearance or taste, so it is hard to believe that blinding of participants or personnel was possible. The remaining trials did not outline the details about blinding methods. Therefore, we evaluated 33 out of 35 of the included trials (except Enshaieh 2007; Han 2013) as at a high risk of performance bias. We evaluated 1 trial as at a high risk of bias for detection bias, and we evaluated 31 out of 35 included trials as at an unclear risk of bias for this domain.
Incomplete outcome data
Six trials had no missing data (Enshaieh 2007; Han 2013; Kwon 2012; Liu 2007; Ni 2008; Wang 2012), and one trial, Huang 2006, stated the reasons for missing data and used intention‐to‐treat analysis to impute the missing data: We evaluated these seven trials as at a low risk of bias for this domain. We assessed seven trials as at a high risk of bias for this domain because of the outcome‐related reason for missing data and the potentially inappropriate application of simple imputation (Chen 2009; Han 2010; Li 2007; Liu 2004; Smith 2007; Wu 2008; Zhang 2012). The other 21 studies reported insufficient information to permit judgement of the risk of bias.
Selective reporting
We evaluated only one, Smith 2007, of the 34 included studies evaluated as at a low risk of bias because it provided the registered protocol of the study, and they had reported their prespecified outcomes in the prespecified way.
Kim 2012 also provided the registered protocol of the study but incompletely reported outcome data as was the case in two other trials (Enshaieh 2007; Kwon 2012), so could not be entered in a meta‐analysis. However, Enshaieh 2007 provided 95% confidence intervals (CIs) for each primary outcome, and Kwon 2012 reported the P value of 2 groups between baseline and the end of the treatment, which made it possible to calculate standard deviations. Besides Kim 2012, four trials did not report at least one of their prespecified primary outcomes, so we assessed them as at a high risk of bias for this domain (Feng 2005; Li 2007; Wang 2012; Wei 2012).
We judged the other 28 studies as having an unclear risk of bias.
Other potential sources of bias
We assessed the following items for other potential bias:
whether or not the study addressed the sample size calculation methods;
whether or not the inclusion or exclusion criteria (of the trial) was appropriate;
whether or not the baseline data between groups were comparable and if not, whether the study applied any statistical methods to adjust for this;
whether or not the study clarified the funding source; and
whether there was any other potential methodological flaw that may have influenced the overall assessment.
According to the trial reports, no funding issue or methods of sample size calculation were apparent in most of the included trials; it was difficult to determine whether a study was fraudulent. We evaluated all of the trials as at an unclear risk of bias.
Effects of interventions
In total, the included studies assessed the therapeutic effects of herbal medicine, acupuncture, wet‐cupping therapy, low‐glycaemic‐load diet (LGLD), tea tree oil gel, and purified bee venom (PBV) for acne. As we pre‐defined in our protocol, we were going to use intention‐to‐treat (ITT) methods if data were available. However, after contacting the study authors, we failed to obtain original data, so we could not use ITT methods for continuous data. For dichotomous data, we conducted ITT analysis based on consideration of 'best‐case' and 'worst‐case' scenarios for trials that had missing data.
None of the trials reported long‐term data (as we defined above); we analysed short‐term data and medium‐term data from relevant included trials separately in different subgroups. We could not conduct other types of subgroup analysis because of insufficient data.
Primary outcomes
1. Improvement of clinical signs assessed through skin lesion counts (change of inflamed and non‐inflamed counted separately and change of total skin lesion counts) and acne severity scores
We have reported this outcome as 'Change in inflammatory and non‐inflammatory lesion counts', 'Change of total skin lesion counts', 'Skin lesion scores', and 'Change of acne severity score'. For trials that did not report the change of skin lesion counts, if the baseline data were available and comparable, we reported the 'Number of inflamed and non‐inflamed counted at the end of the treatment' separately.
Seven trials reported total skin lesion counts (inflammatory or non‐inflammatory skin lesion counts) (Enshaieh 2007; Kim 2012; Kwon 2012; Smith 2007; Wei 2012; Wu 2008; Yu 2008). Han 2013 reported Korean Acne Grading System (KAGS) scores based on numbers of inflammatory and non‐inflammatory lesions.
2. Adverse effects assessed by reporting early study discontinuations, the worsening of acne in participants, and other adverse events during the treatment and follow‐up period
In total, 26 trials reported adverse effects, but none of the trials found any severe adverse effects. Nine trials did not record whether any adverse effects were reported during the treatment and follow‐up period (Han 2013; Kwon 2012; Liu 2007; Ni 2008; Shi 2012; Smith 2007; Song 2007; Tang 2011; Wu 2008a). The types of adverse events differed between groups and among trials, so we could not synthesise the results quantitatively. We present a list of the reported adverse effects from the included trials in Table 5.
3. Adverse events.
| Study ID | Intervention group | Control group | ||
| Description of adverse events | Sample size | Description of adverse events | Sample size | |
| Chen 2012 | 2 participants had mild nausea 2 participants reported dry skin and desquamation |
35 | 1 participant had mild nausea 5 participants reported dry skin and desquamation |
35 |
| Chen 2009 | 15 participants had violaceous mark on application of the cupping, which faded away a few days later | 20 | 2 participants had minimal pruritus and little burning sensation | 20 |
| Enshaieh 2007 | 3 participants had minimal pruritus 1 participant reported a little burning sensation on application of the drug 1 participant had minimal scaling |
30 | 2 participants had minimal pruritus 2 participants reported a little burning sensation on application of the drugs |
30 |
| Feng 2005 | 19 participants reported loose stools once or twice daily | 29 | 12 participants reported mild dizziness | 29 |
| Han 2010 | 4 participants reported topical pruritus 7 participants reported subcutaneous black and blue spot 5 participants felt a sharp prick when the needle went into their skin |
46 | 47 participants reported bearable dry mouth 10 participants had dry and desquamation of skin |
47 |
| Huang 2006 | 10 participants reported slight redness of skin where the acupuncture applied 24 hours after the treatment | 105 | 18 participants reported mild dry mouth, dizziness, nausea, and abdominal pain | 105 |
| Kim 2012 | No adverse events were reported in both groups | |||
| Kwon 2012 | Unknown: Adverse events were not mentioned in the report | |||
| Li 2007 | 3 participants reported mild abdominal pain and diarrhoea 4 participants reported sore throat 3 participants terminated the treatment due to adverse events |
60 | 1 participant reported skin pruritus 8 participants reported dizziness and nausea 6 participants terminated the treatment due to adverse events |
60 |
| Lin 2013 | No adverse events were reported in the intervention group | 36 | 12 participants reported dry mouth and desquamation of skin | 36 |
| Liu 2006 | 7 participants had violaceous mark on application of the cupping, which faded away in 2 weeks | 20 | 1 participant reported stomach upset 1 participant reported nausea 3 participants reported dizziness 1 participant reported loss of appetite |
20 |
| Liu 2008 | 2 participants had a violaceous mark on application of the cupping, which faded away in 2 weeks | 26 | 15 participants reported dry mouth, dry skin and desquamation, pruritus, or cheilitis 1 participant had raised triglycerides, which went back to normal level after they stopped taking drugs |
26 |
| Liu 2012 | 6 out of 94 participants in 2 groups reported redness, dry, and burning sensation after LED therapy; the feelings disappeared after externally using viaminate cream | |||
| Liu 2004 | 20 participants reported redness, pruritus, pain, and burning sensation of skin 4 participants reported stomach upset |
80 | 33 participants reported redness, pruritus, pain, and burning sensation of skin 18 participants reported stomach upset |
80 |
| Liu 2007 | Unknown: Adverse events were not mentioned in the report | |||
| Mo 2011 | No adverse events were reported in both groups | |||
| Ni 2008 | Unknown: Adverse events were not mentioned in the report | |||
| Peng 2012 | 9 participants reported mild abdominal pain and increased stool frequency | 52 | 30 participants reported dry mouth and dry skin 3 participants had mild cheilitis 1 participant reported nosebleed 1 participant reported mild gastrointestinal reaction |
47 |
| Shi 2012 | Unknown: Adverse events were not mentioned in the report | |||
| Smith 2007 | Unknown: Adverse events were not mentioned in the report | |||
| Song 2007 | Unknown: Adverse events were not mentioned in the report | |||
| Tang 2011 | Unknown: Adverse events were not mentioned in the report | |||
| Wang 2012 | No adverse events were reported in the intervention group | 40 | 5 participants reported redness, desquamation, and burning sensation of the skin | 40 |
| Wei 2012 | 1 participant reported mild diarrhoea | 50 | 5 participants reported mild nausea and vomiting 2 participants reported mild dizziness |
50 |
| Wen 2012 | No adverse events were reported in the intervention group | 30 | 7 participants reported stomach upset 3 participants reported nausea 4 participants reported dizziness 3 participants reported loss of appetite |
30 |
| Wu 2008 | Combination group: 21 participants reported chills and fever; 9 participants reported red and swollen skin after injection | 86 | Western drug group: 15 participants reported chills and fever; 8 participants reported red and swollen skin after injection | 87 |
| Herbal medicine group: No adverse events were reported | 84 | |||
| Wu 2008a | Unknown: Adverse events were not mentioned in the report | |||
| You 2012 | 23 participants had a violaceous mark on application of the cupping, which faded away in 2 weeks | 30 | 25 participants reported desquamation and burning sensation of skin | 30 |
| Yu 2011 | 4 participants reported stomach upset 2 participants reported dizziness |
65 | 2 participants reported a little nausea 3 participants reported dizziness |
65 |
| Yu 2008 | No adverse events were reported in the intervention group | 28 | Some participants reported a little nausea and stomach upset | 20 |
| Zhang 2012 | No adverse events were reported in intervention group | 30 | 7 participants reported stomach upset 3 participants reported nausea 4 participants reported dizziness 3 participants reported loss of appetite |
30 |
| Zhang 2010 | 14 participants had a violaceous mark on application of the cupping, which faded away in 2 weeks | 30 | 21 participants reported pruritus, dry skin, and desquamation | 30 |
| Zhou 2009 | No adverse events were reported in both groups | |||
| Zhu 2007 | 2 participants reported dry mouth, cheilitis, dry skin, and desquamation 3 participants reported mild stomach upset |
40 | 35 participants reported dry mouth, cheilitis, dry skin, and desquamation 2 participants reported mild stomach upset 1 participant had raised alanine aminotransferase, which went back to normal level after they stopped taking drugs 3 participants had raised triglycerides, which went back to normal level after they stopped taking drugs |
40 |
Secondary outcomes
1. Physicians' global evaluation (after treatment)
2. Participants' self assessment of change in specific types of lesion (such as comedones, papules, pustules, or nodules)
We combined these two secondary outcomes and expressed them as the 'Number of participants with remission' as this has a clear definition and is easy to assess. We defined the term "remission" according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded) and with only mild pigmentation and scars remaining. All trials that reported the number of participants with remission employed this definition. Thirty trials measured these two secondary outcomes, which we combined and expressed as 'Number of participants with remission'.
3. Psychosocial function outcomes (such as the Hamilton Depression Rating Scale (HAMD))
No trial observed the effect of complementary therapies for improving psychosocial function outcomes of participants with acne vulgaris.
4. Quality of life (QoL)
The QoL‐acne is a questionnaire for assessing facial acne status in clinical trials (Martin 2001). It has three subscales that evaluate participants' social function (five items), self‐reported feeling (five items), and emotional function (four items). Each item is scored from one to six, with a higher score indicating better status of quality of life.
Two studies reported acne quality of life scores: Ni 2008 in a comparison of combination therapy of acupuncture, massage, and wet‐cupping therapy with zinc gluconate, and Liu 2007, which compared herbal medicine, or a combination of acupuncture and wet‐cupping therapy, with viaminate cream. Both Ni 2008 and Liu 2007 reported the scores of QoL subscales.
Comparisons assessed
Diet
Low‐glycaemic‐load diet versus high‐glycaemic‐load diet
Two trials compared low‐glycaemic‐load diet (LGLD) with high‐glycaemic‐load diet (HGLD) (Kwon 2012; Smith 2007). We calculated the standard deviation (SD) of mean changes in non‐inflammatory counts for both groups in Kwon 2012 according to the mean difference of changes, reduction after treatment, and the P value (which the authors provided). No statistically significant difference was shown for our primary outcome 'Change in non‐inflammatory lesion counts' (MD ‐3.89, 95% CI ‐10.07 to 2.29, P = 0.10, I² statistic = 62%, 2 trials, random‐effects model, 75 participants; Analysis 1.1; Figure 4) from baseline to the end of the treatment at 10 weeks (Kwon 2012) or 12 weeks (Smith 2007).
1.1. Analysis.
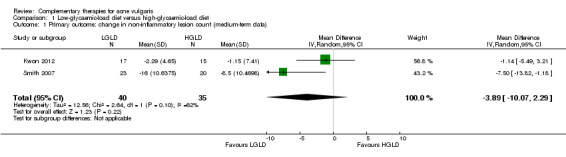
Comparison 1 Low‐glycaemic‐load diet versus high‐glycaemic‐load diet, Outcome 1 Primary outcome: change in non‐inflammatory lesion count (medium‐term data).
4.
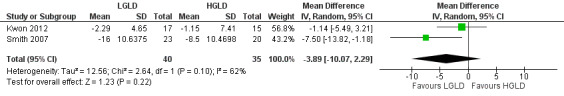
Forest plot of comparison: 1 Low‐glycaemic‐load diet versus high‐glycaemic‐load diet, outcome: 1.1 Primary outcome: change in non‐inflammatory lesion count (medium‐term data)
Smith 2007 found statistically significant differences in favour of LGLD in reducing inflammatory lesion counts (MD ‐7.60, 95% CI ‐13.52 to ‐1.68, P = 0.01, 53 participants; Analysis 1.2) and total lesion counts (MD ‐8.10, 95% CI ‐14.89 to ‐1.31, P = 0.02, 53 participants; Analysis 1.3) of acne. Data regarding inflammatory and total lesion counts from Kwon 2012 were incomplete and unusable in synthesis.
1.2. Analysis.
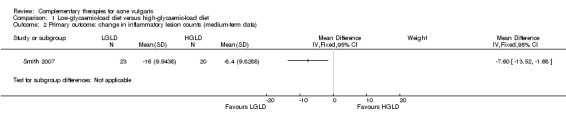
Comparison 1 Low‐glycaemic‐load diet versus high‐glycaemic‐load diet, Outcome 2 Primary outcome: change in inflammatory lesion counts (medium‐term data).
1.3. Analysis.
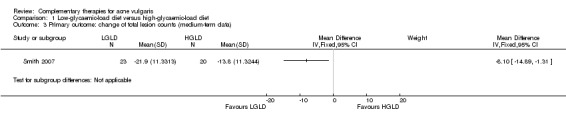
Comparison 1 Low‐glycaemic‐load diet versus high‐glycaemic‐load diet, Outcome 3 Primary outcome: change of total lesion counts (medium‐term data).
Acupuncture
Two studies reported our primary outcome 'Adverse effects' (Han 2010; Huang 2006), which employed acupuncture and found that 23% and 9.7% participants respectively reported pruritus or redness at the application site for acupuncture or sensations of pain following needle insertion.
Acupuncture versus waiting list
Only one four‐arm trial evaluated the effect of acupuncture compared with waiting list (Kim 2012). Our primary outcome 'Change in inflammatory and non‐inflammatory lesion counts' showed no difference between acupuncture and waiting list in reducing the number of inflammatory (MD ‐6.30, 95% CI ‐15.46 to 2.86, 22 participants; Analysis 2.1) or non‐inflammatory lesions (MD ‐0.90, 95% CI ‐28.53 to 26.73, 22 participants; Analysis 2.2) at 4 weeks.
2.1. Analysis.
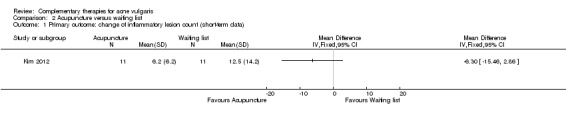
Comparison 2 Acupuncture versus waiting list, Outcome 1 Primary outcome: change of inflammatory lesion count (short‐term data).
2.2. Analysis.
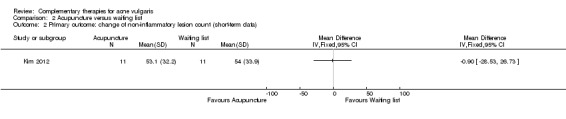
Comparison 2 Acupuncture versus waiting list, Outcome 2 Primary outcome: change of non‐inflammatory lesion count (short‐term data).
Acupuncture versus western drugs
Two trials assessed the effect of acupuncture compared with western drugs, while the method of acupuncture and the type of drugs were different.
With regard to our secondary outcome 'Number of participants with remission', Huang 2006 showed a statistically significant difference in favour of acupuncture when the acupuncture was compared with the western drugs azithromycin (500 mg daily) combined with clindamycin phosphate gel (external application) at 3 weeks. Intention‐to‐treat analysis with 'best‐' or 'worst‐case' assumptions for this trial and outcome demonstrated similar results (RR 3.67, 95% CI 2.22 to 6.06, n = 210; 'worst case' Analysis 3.1) (RR 4.38, 95% CI 2.56 to 7.51, n = 210; 'best case' Analysis 3.2). The other trial showed that there was no statistically significant difference between acupuncture and isotretinoin (20 mg daily for the first month and 10 mg daily for another month) for this outcome (Han 2010). Intention‐to‐treat analysis with 'best' or 'worst case' for this outcome demonstrated similar results (RR 0.69, 95% CI 0.44 to 1.09, n = 100; 'worst case' Analysis 3.3) (RR 0.96, 95% CI 0.62 to 1.48, n = 100; 'best case' Analysis 3.4).
3.1. Analysis.

Comparison 3 Acupuncture versus western drugs, Outcome 1 Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data).
3.2. Analysis.

Comparison 3 Acupuncture versus western drugs, Outcome 2 Secondary outcome: number of participants with remission (ITT‐best case, short‐term data).
3.3. Analysis.

Comparison 3 Acupuncture versus western drugs, Outcome 3 Secondary outcome: number of participants with remission (ITT‐worst case, medium‐term data).
3.4. Analysis.

Comparison 3 Acupuncture versus western drugs, Outcome 4 Secondary outcome: number of participants with remission (ITT‐best case, medium‐term data).
Herbal medicines
Fifteen trials compared herbal medicine with western drugs: antibiotics (n = 7), vitamin A acid (n = 7), and staphylococcus injection (n = 1).
For our primary outcome 'Adverse effects', participants who received herbal medicine as their main treatment reported dizziness (2 out of 65 participants), mild nausea (2 out of 35 participants), abdominal pain (12 out of 112 participants), diarrhoea (20 out of 79 participants), or stomach upset (7 out of 105 participants). However, those who used western drugs reported more cases of adverse events. These adverse events included nausea, stomach upset, dizziness, abdominal pain, dry mouth, cheilitis, nosebleed, or loss of appetite. The external application of western creams may cause pruritus, dryness and desquamation of skin, or a burning sensation on application of the drugs (Chen 2012; Feng 2005; Li 2007; Liu 2004; Liu 2012; Wang 2012; Wei 2012; Wu 2008; Yu 2008; Yu 2011; Zhang 2012; Zhu 2007).
Herbal medicine versus waiting list
One trial compared herbal medicine with a waiting list control (Kim 2012). This trial combined the data of participants who accepted herbal medicine together with those who accepted both acupuncture and herbal medicine and compared this with those who did not use herbal medicine (whether acupuncture was employed or not) and were the waiting list group. The results showed no difference between herbal medicine and waiting list control for our primary outcome 'Change in inflammatory or non‐inflammatory lesions' (inflammatory lesions: MD ‐2.80, 95% CI ‐12.26 to 6.66, 22 participants; Analysis 4.1) (non‐inflammatory lesions: MD ‐12.10, 95% CI ‐39.28 to 15.08, 22 participants; Analysis 4.2) at 4 weeks.
4.1. Analysis.
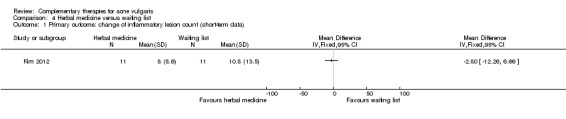
Comparison 4 Herbal medicine versus waiting list, Outcome 1 Primary outcome: change of inflammatory lesion count (short‐term data).
4.2. Analysis.
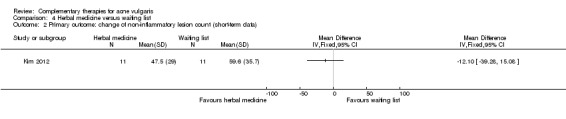
Comparison 4 Herbal medicine versus waiting list, Outcome 2 Primary outcome: change of non‐inflammatory lesion count (short‐term data).
Herbal medicine versus antibiotics
Two studies reported our primary outcome 'Change of total skin lesion counts at eight weeks': 1 trial with 100 participants, Wei 2012, showed a statistically significant difference in favour of herbal medicine when comparing herbal decoction with minocycline (100 mg daily) (MD ‐5.20, 95% CI ‐9.45 to ‐0.95, P = 0.02, 100 participants; Analysis 5.1); the other trial with 48 participants, Yu 2008, showed no difference between Shugan Zishen decoction and tetracycline (1000 mg daily) (MD ‐3.63, 95% CI ‐10.08 to 2.82, P = 0.27, 48 participants; Analysis 5.1).
5.1. Analysis.
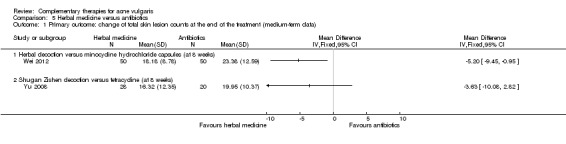
Comparison 5 Herbal medicine versus antibiotics, Outcome 1 Primary outcome: change of total skin lesion counts at the end of the treatment (medium‐term data).
For our secondary outcome 'Number of participants with remission', results from six studies showed inconsistent effects on whether herbal medicine was statistically significantly better than antibiotics in increasing the number of participants with remission. Four trials reported short‐term data for this outcome (Feng 2005; Li 2007; Liu 2004; Mo 2011). According to our meta‐analysis, we did not find any difference in the number of participants with remission between those who received Ziyin Qinggan Xiaocuo Granule and those who received minocycline (100 mg daily), and the direction of the effect estimate changed between results of ITT 'worst‐case' analysis (RR 0.49, 95% CI 0.09 to 2.53, P = 0.08, I² statistic = 68%, 2 trials, random‐effects, 206 participants; Analysis 5.2) and ITT 'best‐case' analysis (RR 2.82, 95% CI 0.88 to 9.06, P = 0.08, I² statistic = 44%, 2 trials, random‐effects, 206 participants; Analysis 5.3) at 4 weeks.
5.2. Analysis.
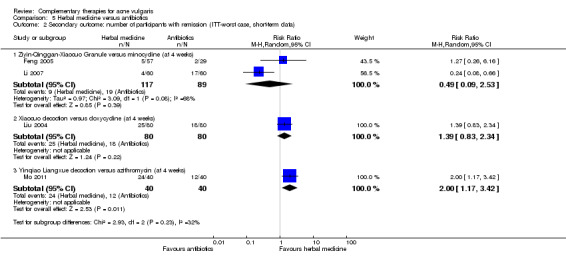
Comparison 5 Herbal medicine versus antibiotics, Outcome 2 Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data).
5.3. Analysis.
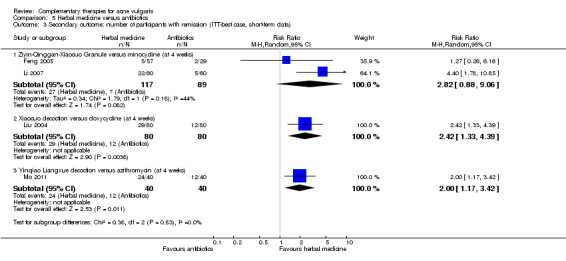
Comparison 5 Herbal medicine versus antibiotics, Outcome 3 Secondary outcome: number of participants with remission (ITT‐best case, short‐term data).
For the other two trials (Liu 2004; Mo 2011), we found that Xiaocuo decoction for the ITT 'worst‐case' analysis was no different to doxycycline (200 mg daily) (RR 1.39, 95% CI 0.83 to 2.34, P = 0.22, 160 participants, Liu 2004), but Yinqiao Liangxue decoction was statistically significantly better than azithromycin (500 mg daily) according to the number of participants with remission at 4 weeks (RR 2.00, 95% CI 1.17 to 3.42, P = 0.01, 80 participants, Mo 2011) (see Analysis 5.2). In the ITT 'best‐case' analysis, both Xiaocuo decoction and Yinqiao Liangxue decoction were statistically significantly better than the respective antibiotics (see Analysis 5.3).
Wei 2012 and Yu 2008 reported medium‐term data for this outcome, which found no difference between groups at 8 weeks (see Analysis 5.4).
5.4. Analysis.
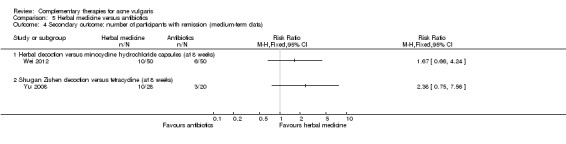
Comparison 5 Herbal medicine versus antibiotics, Outcome 4 Secondary outcome: number of participants with remission (medium‐term data).
Herbal medicine versus vitamin A acid
Seven trials compared herbal decoction with vitamin A acid alone for our secondary outcome 'Number of participants with remission', and four reported short‐term data (see Analysis 6.1).
6.1. Analysis.
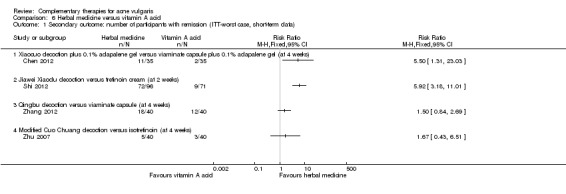
Comparison 6 Herbal medicine versus vitamin A acid, Outcome 1 Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data).
Xiaocuo decoction (plus 0.1% adapalene gel used externally) (RR 5.50, 95% CI 1.31 to 23.03, P = 0.02, 70 participants, Chen 2012) was better than viaminate capsule (75 mg daily, plus 0.1% adapalene gel used externally), with an increased number of participants with remission at 4 weeks. One trial, Shi 2012, showed Jiawei Xiaodu decoction was better than tretinoin cream, with a greater number of participants with remission (RR 5.92, 95% CI 3.18 to 11.01, P < 0.00001, 167 participants) at 2 weeks. Qingbu decoction (RR 1.50, 95% CI 0.84 to 2.69, P = 0.03, 80 participants, Zhang 2012) was no better than viaminate capsule at 4 weeks. However, no difference was shown (RR 1.67, 95% CI 0.43 to 6.51, P = 0.46, 80 participants) between modified Cuochuang decoction and isotretinoin soft capsules (20 mg daily) (Zhu 2007). Intention‐to‐treat with 'best‐' or 'worst‐case' for these studies for this outcome demonstrated similar results for Chen 2012, Shi 2012, and Zhu 2007, but not Zhang 2012 (see Analysis 6.1 and Analysis 6.2).
6.2. Analysis.
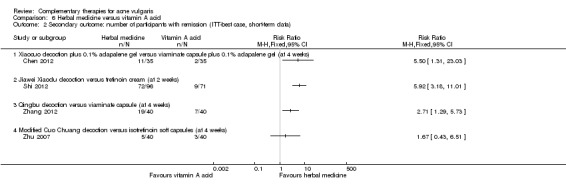
Comparison 6 Herbal medicine versus vitamin A acid, Outcome 2 Secondary outcome: number of participants with remission (ITT‐best case, short‐term data).
The remaining three trials reported medium‐term data for this outcome (Liu 2007; Peng 2012; Wang 2012). No statistically significant difference could be found between participants who used Da Huang Zhe Chong Pillso (RR 0.74, 95% CI 0.34 to 1.63, P = 0.45, 99 participants), modified Wuwei Xiaodu yin plus Yinchenhao decoction (RR 2.00, 95% CI 0.19 to 21.06, P = 0.56, 70 participants), or externally applied herbal decoction (RR 1.13, 95% CI 0.67 to 1.88, P = 0.65, 80 participants) and those who received adapalene externally applied, viaminate cream externally applied, and isotretinoin soft capsule (20 mg daily), respectively (see Analysis 6.3).
6.3. Analysis.
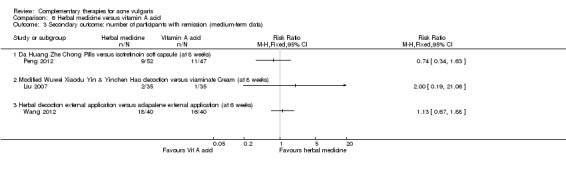
Comparison 6 Herbal medicine versus vitamin A acid, Outcome 3 Secondary outcome: number of participants with remission (medium‐term data).
For our secondary outcome 'Acne quality of life score', 1 trial, Liu 2007, showed no difference between modified Wuwei Xiaodu yin plus Yinchenhao decoction compared with viaminate cream applied externally on a quality of life score measuring functioning in society (MD 0.30, 95% CI ‐0.71 to 1.31, P = 0.56) at 8 weeks. However, a statistically significant difference in favour of herbal medicine was found on 2 other quality of life measures: on feelings of self‐worth (RR 1.51, 95% CI 0.88 to 2.14, P < 0.00001) and emotional functionality (RR 2.20, 95% CI 1.75 to 2.65, P < 0.00001) (see Analysis 6.4).
6.4. Analysis.
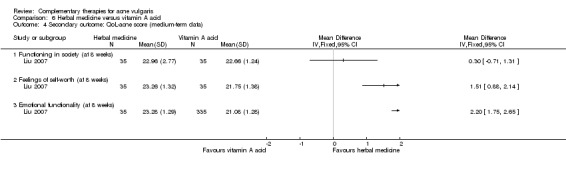
Comparison 6 Herbal medicine versus vitamin A acid, Outcome 4 Secondary outcome: QoL‐acne score (medium‐term data).
Herbal medicine versus antibiotics and vitamin A acid
One trial, Tang 2011, compared a herbal decoction of externally applied Xiaocuo decoction with the antibiotic, roxithromycin (300 mg daily), which they combined with viaminate capsules (150 mg daily). There were a statistically significantly increased number of participants with remission with the herbal decoction (RR 2.00, 95% CI 1.26 to 3.18, P = 0.003, 219 participants; Analysis 7.1) at 5 weeks.
7.1. Analysis.

Comparison 7 Herbal medicine versus antibiotics and vitamin A acid, Outcome 1 Secondary outcome: number of participants with remission (medium‐term data).
Herbal medicine versus staphylococcus injection
One trial, Wu 2008, compared Qingre Twenty‐five Pills with staphylococcus injection (0.2 to 0.5 ml daily). A statistically significant difference was found in favour of herbal medicine in reduction of skin lesion score (MD ‐1.28, 95% CI ‐2.10 to ‐0.46, P = 0.002, 171 participants; Analysis 8.1), but no difference was found in the number of participants with remission between the 2 groups (RR 0.93, 95% CI 0.70 to 1.23, P = 0.60, 171 participants; Analysis 8.2) at 4 weeks.
8.1. Analysis.
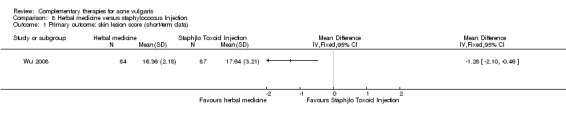
Comparison 8 Herbal medicine versus staphylococcus Injection, Outcome 1 Primary outcome: skin lesion score (short‐term data).
8.2. Analysis.
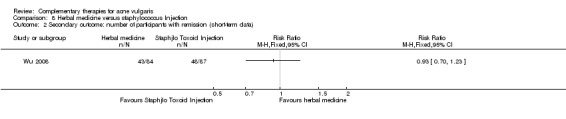
Comparison 8 Herbal medicine versus staphylococcus Injection, Outcome 2 Secondary outcome: number of participants with remission (short‐term data).
Herbal medicine plus western drugs versus western drugs
A combination of Qingre Twenty‐five Ingredients pill and staphylococcus Injection was found to be statistically significantly better than the staphylococcus injection alone for our primary outcome 'Change of total skin lesion counts' (MD ‐9.54, 95% CI ‐10.42 to ‐8.66, P < 0.00001, 173 participants; Analysis 9.1) at 4 weeks (Wu 2008).
9.1. Analysis.
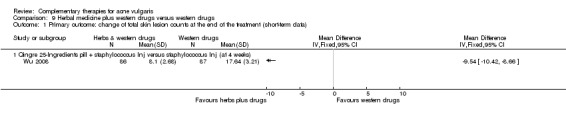
Comparison 9 Herbal medicine plus western drugs versus western drugs, Outcome 1 Primary outcome: change of total skin lesion counts at the end of the treatment (short‐term data).
Two trials compared herbal decoction plus western drugs versus western drugs alone in increasing the number of participants with remission at four weeks (Wu 2008; Yu 2011). Qingre Twenty‐five Ingredients pill plus staphylococcus Injection (0.2 to 0.5 ml daily) was better than staphylococcus Injection alone (RR 1.45, 95% CI 1.17 to 1.81, P = 0.0007, 173 participants) (Wu 2008). Danshen tablet plus doxycycline (200 mg daily) was better than doxycycline (200 mg daily) alone (RR 1.82, 95% CI 1.23 to 2.69, P = 0.003, 130 participants; see Analysis 9.2) (Yu 2011).
9.2. Analysis.
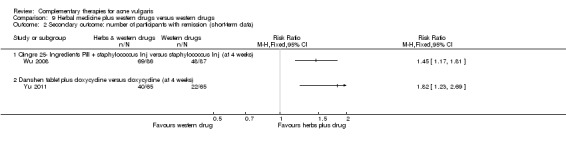
Comparison 9 Herbal medicine plus western drugs versus western drugs, Outcome 2 Secondary outcome: number of participants with remission (short‐term data).
Herbal medicine plus other treatment versus other treatment alone
Another trial compared herbal medicinal mask plus blue and red light‐emitting diode (LED) phototherapy with LED alone (Liu 2012). No statistically significant difference was found between the two groups with regard to the number of participants with remission at four weeks. Intention‐to‐treat with 'best' or 'worst case' for this outcome gave similar results (see Analysis 10.1 and Analysis 10.2).
10.1. Analysis.

Comparison 10 Herbal medicine plus LED versus LED, Outcome 1 Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data).
10.2. Analysis.

Comparison 10 Herbal medicine plus LED versus LED, Outcome 2 Secondary outcome: number of participants with remission (ITT‐best case, short‐term data).
Herbal medicine plus wet cupping versus other treatment alone
One trial compared individualised herbal decoction plus wet‐cupping therapy with wet‐cupping therapy alone (Chen 2009). No difference was found between the 2 groups with regard to the number of participants with remission (RR 2.33, 95% CI 0.67 to 8.18, P = 0.19, 60 participants; Analysis 11.1) at 4 weeks.
11.1. Analysis.
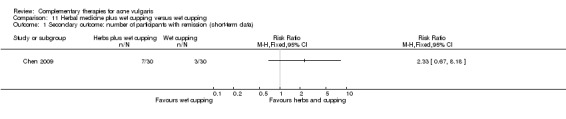
Comparison 11 Herbal medicine plus wet cupping versus wet cupping, Outcome 1 Secondary outcome: number of participants with remission (short‐term data).
Wet cupping
WIth regard to our primary outcome 'Adverse effects', wet‐cupping therapy may leave black and blue spots on the skin after removing the cups. Five trials reported this (Chen 2009; Liu 2006; Liu 2008; You 2012; Zhang 2010).
Wet cupping versus western drugs
In the study by Zhou 2009, a statistically significant difference was found in favour of wet cupping for our primary outcome 'Change in acne severity score'. There was a reduction in the acne severity score, which the Global Acne Grading System (GAGS) measured (MD ‐5.95, 95% CI ‐8.28 to ‐3.62, P < 0.00001, 40 participants; Analysis 12.1) at 4 weeks.
12.1. Analysis.
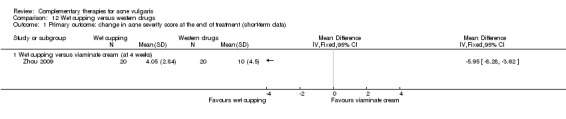
Comparison 12 Wet cupping versus western drugs, Outcome 1 Primary outcome: change in acne severity score at the end of treatment (short‐term data).
Zhou 2009 compared wet‐cupping therapy with externally applied viaminate cream. No difference was found between groups with regard to our secondary outcome 'Number of participants with remission' (RR 5.00, 95% CI 0.26 to 98.00, P = 0.29, 40 participants; Analysis 12.2) at 4 weeks. Two other trials showed a statistically significant increase in the number of participants with remission in the wet‐cupping group: Wu 2008a, which compared wet‐cupping therapy with tetracycline (1000 mg) and ketoconazole cream (external application) (RR 2.50, 95% CI 1.31 to 4.77, P = 0.005, 60 participants), and Song 2007, which compared wet‐cupping (with blood‐letting on ear points) therapy with tetracycline (1500 mg daily) (RR 2.83, 95% CI 1.29 to 6.22, P = 0.009, 76 participants) at 2 weeks and at 4 weeks (See Analysis 12.2).
12.2. Analysis.
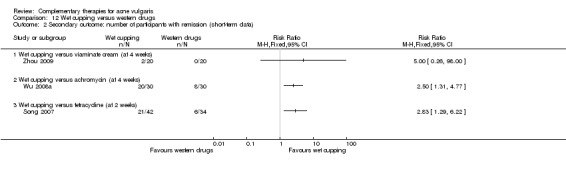
Comparison 12 Wet cupping versus western drugs, Outcome 2 Secondary outcome: number of participants with remission (short‐term data).
Wet cupping plus herbal medicine versus herbal medicine alone
One trial, Chen 2009, showed no difference between wet‐cupping therapy plus individualised herbal decoction compared with individualised herbal decoction alone with regard to the number of participants with remission (RR 2.33, 95% CI 0.67 to 8.18, P = 0.19, 60 participants; Analysis 13.1) at 4 weeks.
13.1. Analysis.
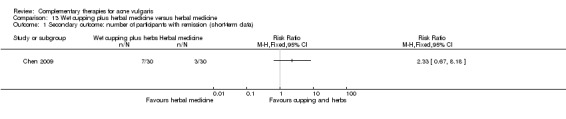
Comparison 13 Wet cupping plus herbal medicine versus herbal medicine, Outcome 1 Secondary outcome: number of participants with remission (short‐term data).
Wet cupping plus acupuncture versus western drugs
Six trials assessed the therapeutic effect of wet‐cupping therapy plus acupuncture compared with western drugs (Liu 2006; Liu 2007; Liu 2008; Wen 2012; You 2012; Zhang 2010). Two trials used antibiotics (including minocycline 100 mg daily and tetracycline 1500 mg daily) as controls, and 4 trials used vitamin A acid (including internal or external application) as a control, respectively.
Our primary outcome 'Skin lesion score' showed no difference between cupping with acupuncture compared with antibiotics at four weeks (see Analysis 14.1). Except for 2 trials (Liu 2007, RR 8.00, 95% CI 1.06 to 60.63, 70 participants; Zhang 2010, RR 2.50, 95% CI 1.12 to 5.56, 60 participants), results from each of the remaining 4 trials consistently showed no difference between the combination therapies and vitamin A acid (including viaminate capsule, viaminate cream, tretinoin cream, and isotretinoin soft capsules) with regard to our secondary outcome 'Number of participants with remission' (see Analysis 14.2).
14.1. Analysis.
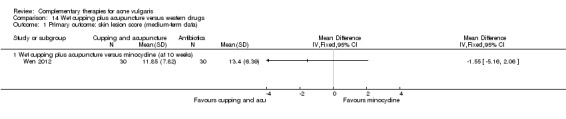
Comparison 14 Wet cupping plus acupuncture versus western drugs, Outcome 1 Primary outcome: skin lesion score (medium‐term data).
14.2. Analysis.
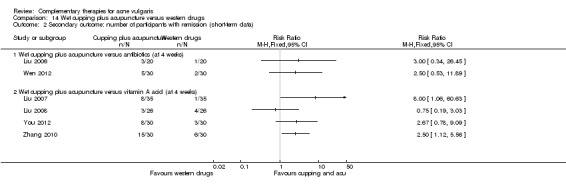
Comparison 14 Wet cupping plus acupuncture versus western drugs, Outcome 2 Secondary outcome: number of participants with remission (short‐term data).
For our secondary outcome 'Acne quality of life score', 1 trial showed wet cupping plus acupuncture was better than vitamin A acid cream for 3 quality of life measures (Liu 2007): functioning in society (MD 4.51, 95% CI 3.95 to 5.07, P < 0.00001, 70 participants), feelings of self‐worth (MD 4.97, 95% CI 4.39 to 5.55, P < 0.00001), and emotional functionality (MD 4.73, 95% CI 4.31 to 5.15, P < 0.00001, 70 participants) at 8 weeks (see Analysis 14.3).
14.3. Analysis.
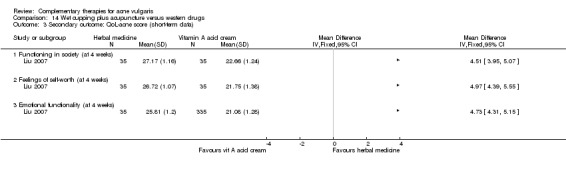
Comparison 14 Wet cupping plus acupuncture versus western drugs, Outcome 3 Secondary outcome: QoL‐acne score (short‐term data).
Wet cupping plus herbal medicine versus western drugs
One trial, Lin 2013, showed no difference between wet‐cupping therapy plus herbal medicinal mask and viaminate capsule (30 mg daily) in the number of participants with remission (RR 1.80, 95% CI 0.67 to 4.85, P = 0.24, 72 participants; Analysis 15.1) at 4 weeks.
15.1. Analysis.
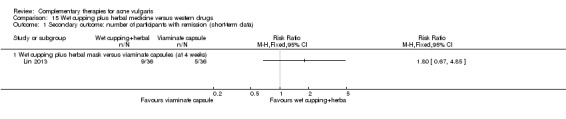
Comparison 15 Wet cupping plus herbal medicine versus western drugs, Outcome 1 Secondary outcome: number of participants with remission (short‐term data).
Combination of wet cupping, acupuncture, and massage versus zinc gluconate
One trial, Ni 2008, compared the combination of wet‐cupping therapy, acupuncture, and massage, with zinc gluconate oral liquid (40 ml daily). A statistically significant difference was found in favour of combination therapies with regard to the primary outcome 'Skin lesion scores' (MD ‐3.87, 95% CI ‐6.97 to ‐0.77, P = 0.01; 46 participants; Analysis 16.1).
16.1. Analysis.
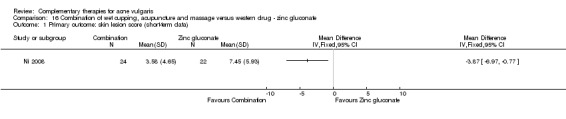
Comparison 16 Combination of wet cupping, acupuncture and massage versus western drug ‐ zinc gluconate, Outcome 1 Primary outcome: skin lesion score (short‐term data).
There was a borderline statistical significant difference between the 2 groups with regard to the secondary outcome 'Number of participants with remission' (RR 4.13, 95% CI 1.00 to 17.04, P = 0.05, 46 participants; Analysis 16.2).
16.2. Analysis.
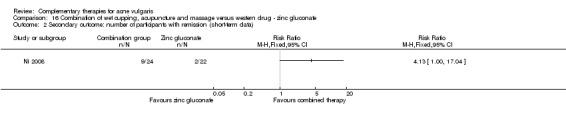
Comparison 16 Combination of wet cupping, acupuncture and massage versus western drug ‐ zinc gluconate, Outcome 2 Secondary outcome: number of participants with remission (short‐term data).
There was a statistically significant difference also in favour of combination therapies for our secondary outcome 'Acne quality of life score'. Three quality of life measures: functioning in society (MD 1.47, 95% CI 0.29 to 2.65, P = 0.01, 46 participants), feelings of self‐worth (MD 1.26, 95% CI 0.20 to 2.32, P = 0.02, 46 participants), and emotional functionality (MD 0.93, 95% CI 0.17 to 1.69, P = 0.02, 46 participants) at 3 weeks (see Analysis 16.3).
16.3. Analysis.
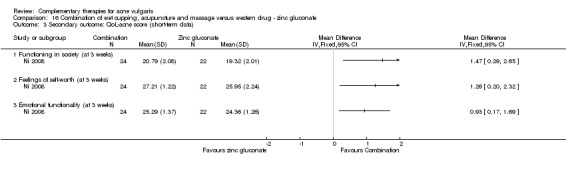
Comparison 16 Combination of wet cupping, acupuncture and massage versus western drug ‐ zinc gluconate, Outcome 3 Secondary outcome: QoL‐acne score (short‐term data).
Tea tree oil
Tea tree oil versus placebo
For our primary outcome 'Change of total skin lesion counts', one trial, Enshaieh 2007, compared tea tree oil gel with placebo (vehicle gel alone) in 60 participants with acne. A statistically significant difference was found in favour of tea tree oil gel (MD ‐7.53, 95% CI ‐10.40 to ‐4.66, P < 0.00001, 60 participants; Analysis 17.1).
17.1. Analysis.
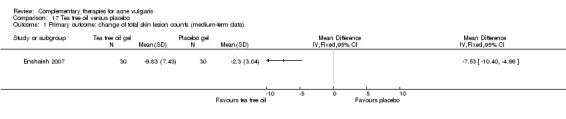
Comparison 17 Tea tree oil versus placebo, Outcome 1 Primary outcome: change of total skin lesion counts (medium‐term data).
For our primary outcome 'Change in acne severity score', there was an improvement in the acne severity index (MD ‐5.75, 95% CI ‐9.51 to ‐1.99, P = 0.003, 60 participants; Analysis 17.2) in the tea tree oil gel group compared with the placebo gel group, from baseline to the end of 45 days of treatment.
17.2. Analysis.
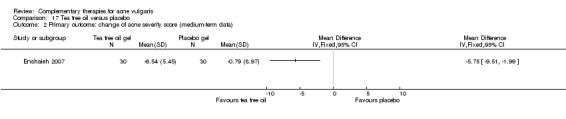
Comparison 17 Tea tree oil versus placebo, Outcome 2 Primary outcome: change of acne severity score (medium‐term data).
For our primary outcome 'Adverse effects', Enshaieh 2007 reported minimal pruritus (3 out of 30 participants in the tea tree oil group and 2 out of 30 participants in the control group), a little burning sensation on the skin area where the oil was applied (1/30 participants in the intervention group and 2/30 participants in the control group), and minimal scaling (1 case in the tea tree oil group).
Purified bee venom (PBV)
Cosmetics containing PBV versus cosmetics without PBV
For our primary outcome 'Change of skin lesion scores', Han 2013 compared cosmetics with or without PBV (0.06 mg/mL) in 12 participants. Purified bee venom was found to be statistically significantly better than the no PBV control when the post‐treatment Korean Acne Grading System (KAGS) scores were measured, which were based on the number of lesions (MD ‐1.17, 95% CI ‐2.06 to ‐0.28, P = 0.01, 12 participants; Analysis 18.1).
18.1. Analysis.

Comparison 18 Cosmetics with PBV versus cosmetics without PBV, Outcome 1 Primary outcome: change in KAGS scores based on number of inflammatory and non‐inflammatory acne lesions.
Discussion
Summary of main results
This review included 35 studies: 23 published studies and 12 unpublished studies. The included studies measured effectiveness of herbal medicine, acupuncture, wet‐cupping therapy, low‐glycaemic‐load diet, tea tree oil, and purified bee venom (PBV); however, because of the clinical heterogeneity between trials and incomplete data reporting, we could only conduct two meta‐analyses, with two trials in each meta analysis.
For our pre‐defined primary outcomes (including improvement of skin lesion counts and adverse events), the results showed no statistically significant difference between low‐glycaemic‐load diet (LGLD) and high‐glycaemic‐load diet (HGLD) on reducing non‐inflammatory lesion counts (mean difference (MD) ‐3.89, 95% CI ‐10.07 to 2.29, 2 trials, 75 participants) at 12 weeks. Results from single trials showed potential benefit of LGLD (MD ‐7.60, 95% CI ‐13.52 to ‐1.68, 43 participants, 1 trial), tea tree oil (MD ‐7.53, 95% CI ‐10.40 to ‐4.66, 60 participants, 1 trial), and PBV (MD ‐1.17, 95% CI ‐2.06 to ‐0.28, 12 participants, 1 trial) in reducing inflammatory skin lesion counts, total skin lesion counts, and skin lesion scores, respectively. Other trials showed inconsistent effects as to whether herbal medicine, acupuncture, or wet cupping were superior to controls in terms of the number of participants with remission or reduction in the total number of skin lesions.
We created 'Summary of findings' tables for comparisons of LGLD and HGLD (Table 1) and comparisons between Ziyin Qinggan Xiaocuo Granule and minocycline (Table 2). We found low‐quality evidence for both LGLD and Ziyin Qinggan Xiaocuo Granule compared with control in improving the clinical symptoms of acne (reducing the number of skin lesions or improving the number of participants with remission) because of the small sample sizes and poor methodological quality of the included studies.
None of the studies reported severe adverse effects. Adverse effects of herbal medicine and orally taken western drugs included dizziness; dry mouth; and mild gastrointestinal effects, such as nausea, diarrhoea, or stomach upset. Wet‐cupping therapy may leave black and blue spots on the skin after removing the cups. Itch or redness and pain following needle insertion were reported as potential adverse effects from acupuncture. For tea tree oil gel or western drugs, adverse effects, which were anticipated, included itchiness, dryness and flaking of the skin, or even a little burning sensation at the application site of the drugs.
Thirty trials measured the secondary outcome 'Number of participants with remission'. Low‐quality evidence from a meta‐analysis comparing Ziyin Qinggan Xiaocuo Granule and the antibiotic minocycline (100 mg daily) showed no difference between the groups (risk ratio (RR) 0.49, 95% CI 0.09 to 2.53, P = 0.08, I² statistic = 68%, 2 trials, random‐effects, 206 participants at 4 weeks). No trial assessed 'Psychosocial function'. Two trials assessed 'Quality of life' according to a QoL‐acne questionnaire, and significant differences in favour of complementary and alternative medicine (CAM) were found in both of them for feelings of self‐worth (MD 1.51, 95% CI 0.88 to 2.14, P < 0.00001, 1 trial, 70 participants) (MD 1.26, 95% CI 0.20 to 2.32, 1 trial, 46 participants) and emotional functionality (MD 2.20, 95% CI 1.75 to 2.65, P < 0.00001, 1 trial, 70 participants) (MD 0.93, 95% CI 0.17 to 1.69, 1 trial, 46 participants).
Overall completeness and applicability of evidence
We searched a comprehensive range of databases, including Chinese‐language databases and ongoing trials registration systems. We placed no language restrictions on the search and screening stages. Subsequently, we included 18 Chinese‐language publications, 12 Chinese‐language unpublished studies, and 5 English publications in this review. We retrieved two trials, which are awaiting assessment. Both the English and Chinese searches were updated before finalisation of the review.
Three trials out of the 35 included studies did not provide complete data (Enshaieh 2007; Kim 2012; Kwon 2012). These authors did not, or could not, respond to requests for additional information. We analysed the data from these three studies as reported; however, these trials are at risk of bias from incomplete reporting. Because of the clinical or statistical heterogeneity of the included studies, we only performed meta‐analyses for the outcomes 'Change in inflammatory lesion counts' and 'Number of participants with remission' for the comparisons between LGLD and HGLD and between herbal medicine and western drugs from four studies. This may also downgrade the level of evidence of the review.
We included 18 types of comparisons between CAM and control groups, including herbal medicine, acupuncture, and cupping. Within these interventions, there was significant variation in the design of the intervention ‐ for example, herbal medicine (e.g., dosage, forms, prescription), wet‐cupping therapy (e.g., method of blood‐letting, time of retaining the cups), and acupuncture (e.g., methods of hand stimulation, depth or strength of insertion, numbers of needling points). The dosage and treatment duration of western drugs are unlikely to be exactly the same in clinics between China and other countries. As such, the results of this review are more clinically relevant for application in traditional Chinese medicine and potentially less clinically relevant for application within western medicine.
Quality of the evidence
Although all of the included trials had a low risk of bias for the "random sequence generation" domain, only six trials reported the methods of allocation concealment. The lack of blinding of participants for 33 studies was a source of potential bias. Only two published studies used placebo as the control (Enshaieh 2007; Han 2013); these studies were assessed as unclear risk of bias for "allocation concealment", "selective reporting", and "other bias" domains.
We employed in this review the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria ratings to assess the quality of the evidence; factors that may downgrade the quality level include imprecision, inconsistency, indirectness, limitations, and bias of the evidence. Factors that may upgrade the quality level could be large effect, plausible confounding, and dose‐response gradient. For two meta‐analyses in this review, we downgraded the level of the evidence because of the potential performance, attrition, and reporting bias and the inconsistency of the results between the included studies. Thus, we could find only low‐quality evidence for LGLD in reducing non‐inflammatory lesion counts and for herbal medicine (Ziyin Qinggan Xiaocuo Granule) in improving the number of participants with remission.
Overall, because of limitations and concerns about the quality of the included studies, we cannot draw a robust conclusion for consistency, size, and direction of outcome effects in this review.
Potential biases in the review process
We did not include over 500 studies (the majority of which were conducted in China) in this review due to insufficient information describing the process of randomisation; our judgement on reading the full text of these articles was that they were not randomised (Figure 1).
Twenty included studies evaluated the effect of herbal medicine, 12 trials assessed the effect of acupuncture or wet‐cupping therapy, and 3 trials assessed diet and the role of tea tree oil. It is only recently that research in traditional Chinese medicine has used a placebo or control group as a standard feature of clinical studies, for which significant criticism has been made in the past. For 32 out of our 35 included trials, lack of blinding was an important issue.
Agreements and disagreements with other studies or reviews
Two other reviews were found on this topic. One review assessed acupuncture and moxibustion compared with western drugs on treating acne vulgaris (Li 2009). All of the 17 included trials in that review were conducted and published in China. The results of their meta‐analysis showed that acupuncture (and moxibustion) or a combination of acupuncture and western drugs were better than western drugs alone at increasing the number of participants with remission. However, there is insufficient high‐quality evidence to determine the effectiveness and efficacy of CAM. In our review, we included only three of the trials included in the Li 2009 review. We did not include the other 14 studies because of insufficient information about random sequence generation. However, in our review, we did not conduct meta‐analysis for these three trials (Huang 2006; Liu 2006; Song 2007), because of the obvious clinical heterogeneity (different kind or usage of western drugs in control groups among studies).
Another review examined the effect of a broad range of CAM treatments (Magin 2006). We included 23 trials of topical and oral CAM to treat acne; evidence from the review suggested that many of these therapies are biologically plausible. However, most included trials in Magin's review were of poor methodological quality, only English databases were searched, and that review included studies from no other languages. Our review reached similar conclusions on the effect of tea tree oil for acne improvement.
There are some studies concerning the safety of herbal medicine that reported that herbal supplements may contain no active ingredients (O'Connor 2013) or unlabelled toxins, such as arsenic, mercury, or lead (Mail Online 2013; Starling 2013). However, those conclusions are mainly based on findings from case reports (Wu 2013) or a cross‐sectional study (Buettner 2009), and similar conclusions could not be drawn directly from our review. We only found dizziness, dry mouth, and mild gastrointestinal effects as mild adverse events of herbal medicine. More research (including experimental studies and clinical studies) with regard to the safety of herbal products should be done to testify the active ingredients and the potential adverse effects of herbal supplements.
Authors' conclusions
Implications for practice.
Some evidence from single studies showed that low‐glycaemic‐load diet, tea tree oil, and pollen bee venom (PBV) may have an effect on reducing total skin lesion counts and acne severity scores for people with acne vulgaris. However, small sample sizes and poor methodological quality limited the strength of the evidence. Evidence from other existing randomised controlled trials does not support the use of herbal medicine, acupuncture, or wet‐cupping therapy for the treatment of acne vulgaris. The evidence for a secondary outcome of this review 'Number of participants with remission' for herbal medicine versus antibiotic was uncertain. There were mixed findings regarding total skin lesion counts and number of participants with remission from each single included study. However, two trials that reported quality of life showed the benefit of herbal medicine compared with western drugs.
Our review highlights potential adverse effects from herbal medicine, e.g., dizziness, dry mouth, nausea, diarrhoea, or stomach upset; acupuncture may cause pain following needle insertion, itchiness, or redness of skin; wet‐cupping therapy may leave black and blue spots for two weeks after removing the cups; and tea tree oil gel may cause pruritus, dryness and flaking of the skin, and even a little burning sensation on application of the drugs.
Methodological and reporting quality limitations weaken the evidence base for treating acne with several kinds of complementary therapies (including herbal medicine, acupuncture, wet‐cupping therapy, low‐glycaemic‐load diet, PBV, and tea tree oil). This review evaluated herbal medicine, wet‐cupping therapy, and acupuncture in a traditional Chinese medicine context; therefore, results might be less generalisable to western medicine.
Implications for research.
Research into the efficacy and safety of complementary therapies for the treatment of acne vulgaris is in its infancy (especially in China). Much of the research conducted thus far is difficult to evaluate because of poor‐quality reporting. Future studies should be designed to ensure low risk of bias and meet current reporting standards for clinical trials. Although blinding of participants and practitioners is difficult for studies of complementary therapies (such as herbal medicine, acupuncture, cupping therapy, etc.), blinding of outcome assessors and statisticians is recommended. Recommendations to improve the evidence base of complementary therapies for acne vulgaris include the following: increasing the sample size for each study and evaluating efficacy in people by measuring the total number of skin lesion counts or total number of inflammatory and non‐inflammatory lesions, respectively. Standard usage of control drugs (such as antibiotics or vitamin A acid) within a reasonable, consistent treatment duration is recommended in future studies. There is a need for collection and full reporting of adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 25 January 2016 | Amended | A search of PubMed, CENTRAL and Embase in January 2016 did identify some new relevant studies, but the authors consider that they have low quality evidence which would not change the conclusion. Thus, an update has not been considered necessary at this time. Our Information Specialist will run a new search in January 2017 to re‐assess whether an update is needed. |
Notes
There was a version of this protocol published by a different team in issue 4 of The Cochrane Library, 2006.
The original authoring team consisted of Tina Leonard, Anne Eady, and Jo Leonardi‐Bee. They relinquished responsibility for the review in July 2010.
A search of PubMed, CENTRAL and Embase in January 2016 did identify some new relevant studies, but the authors consider that they have low quality evidence which would not change the conclusion. Thus, an update has not been considered necessary at this time. Our Information Specialist will run a new search in January 2017 to re‐assess whether an update is needed.
Acknowledgements
The authors wish to thank the Cochrane Skin Group editorial team and Ms Jun Xia from the Cochrane Schizophrenia Group for their assistance in the preparation of this review.
This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM). The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
The Cochrane Skin Group editorial base wishes to thank Robert Dellavalle, who was the Cochrane Dermatology Editor for this review; Matthew Grainge, who was the Statistical Editor; Ching‐Chi Chi, who was Methods Editor; the clinical referees, Alison Devine and Parker Magin; and the consumer referee, Karen Thomas.
Appendices
Appendix 1. Skin Group Specialised Register search strategy
Two searches run as there were too many terms for one search string:
1. acne and (diet* or nutrition* or macrobiotic* or Gerson or antioxidant* or “mind‐body” or meditation or imagery or hypnosis or “support group*” or bioelectromagnetic* or electromagnetic* or electroacupuncture or traditional or folk or naturopathy or ayurved* or “Traditional Chinese Medicine” or TCM or homeopathy or antineoplaston or chelation or immunoaugmentation or shark or “manual healing” or massage or chiropractic or “therapeutic touch” or herb* or “physical therap*” or acupuncture or moxibustion or “cupping therapy” or “therapeutic exercise*” or “Tai Chi” or complementary or alternative or aromatherapy or reflexology or reiki or cam or yoga or music or “tea tree” or “aloe vera”)
2. acne and (anthroposophy or "auricular therapy" or "holistic health" or "horticultural therapy" or Arabic or Asian or African or Kampo or unani or shamanism or mesotherapy or psychology or “breathing exercise*” or "laughter therapy" or "mental healing" or psychodrama or psychophysiology or "relaxation therapy" or Taiji or acupressure or kinesiology or manipulation or "tissue therapy" or eclecticism or reflexotherapy or rejuvenation or “acoustic stimulation” or "art therapy" or "colour therapy" or "dance therapy" or Speleotherapy or "faith healing" or magic or occultism or Radiesthesia or witchcraft or biofeedback or “immuno augmentation therapy” or “natural remed*” or “drama therapy” or visualisation)
Appendix 2. CENTRAL (The Cochrane Library) search strategy
#1 (diet* or nutrition* or macrobiotic* or Gerson or antioxidant* or "mind‐body" or meditation or imagery or hypnosis or "support group*" or bioelectromagnetic* or electromagnetic* or electroacupuncture or traditional or folk or naturopathy or ayurved* or "Traditional Chinese Medicine" or TCM or homeopathy or antineoplaston or chelation or immunoaugmentation or shark or "manual healing" or massage or chiropractic or "therapeutic touch" or herb* or "physical therap*" or acupuncture or moxibustion or "cupping therapy" or "therapeutic exercise*" or "Tai Chi" or complementary or alternative or aromatherapy or reflexology or reiki or cam or yoga or music or "tea tree" or "aloe vera") #2 (anthroposophy or "auricular therapy" or "holistic health" or "horticultural therapy" or Arabic or Asian or African or Kampo or unani or shamanism or mesotherapy or psychology or "breathing exercise*" or "laughter therapy" or "mental healing" or psychodrama or psychophysiology or "relaxation therapy" or Taiji or acupressure or kinesiology or manipulation or "tissue therapy" or eclecticism or reflexotherapy or rejuvenation or "acoustic stimulation" or "art therapy" or "colour therapy" or "dance therapy" or Speleotherapy or "faith healing" or magic or occultism or Radiesthesia or witchcraft or biofeedback or "immuno augmentation therapy" or "natural remed*" or "drama therapy" or visualisation) #3 (#1 OR #2) #4 (acne):ti,ab,kw #5 MeSH descriptor Acne Vulgaris explode all trees #6 (#4 OR #5) #7 (#3 AND #6)
Appendix 3. MEDLINE (OVID) search strategy
1. acoustic stimulation.mp. or exp Acoustic Stimulation/ 2. acupressure.mp. or exp Acupressure/ 3. exp Acupuncture/ or acupuncture.mp. 4. exp Medicine, African Traditional/ 5. anthroposophy.mp. or exp Anthroposophy/ 6. antioxidants.mp. or exp Antioxidants/ 7. arabic medicine.mp. or exp Medicine, Arabic/ 8. aromatherapy.mp. or exp Aromatherapy/ 9. art therapy.mp. or exp Art Therapy/ 10. auricular therapy.mp. 11. exp Medicine, Ayurvedic/ 12. ayurved$.mp. 13. biofeedback.mp. or exp Biofeedback, Psychology/ 14. breathing exercise$.mp. or exp Breathing Exercises/ 15. exp Color Therapy/ 16. (color therap$ or colour therap$).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 17. exp complementary therapies/ or exp acupuncture therapy/ or exp anthroposophy/ or exp auriculotherapy/ or exp holistic health/ or exp homeopathy/ or exp horticultural therapy/ or exp medicine, traditional/ or exp mesotherapy/ or exp mind‐body therapies/ or exp musculoskeletal manipulations/ or exp naturopathy/ or exp organotherapy/ or exp phytotherapy/ or exp reflexotherapy/ or exp rejuvenation/ or exp sensory art therapies/ or exp speleotherapy/ or exp spiritual therapies/ 18. dance therapy.mp. or exp Dance Therapy/ 19. exp Medicine, East Asian Traditional/ 20. eclecticism.mp. or exp Eclecticism, Historical/ 21. electroacupuncture.mp. or exp Electroacupuncture/ 22. faith healing.mp. or exp Faith Healing/ 23. holistic health.mp. 24. homeopathy.mp. 25. horticultural therapy.mp. 26. hypnosis.mp. or exp Hypnosis/ 27. exp "Imagery (Psychotherapy)"/ 28. kampo.mp. or exp Medicine, Kampo/ 29. exp Kinesiology, Applied/ or kinesiology.mp. 30. laughter therapy.mp. or exp Laughter Therapy/ 31. magic.mp. or exp Magic/ 32. manipulation.mp. or exp Manipulation, Chiropractic/ 33. exp Massage/ or massage.mp. 34. meditation.mp. or exp Meditation/ 35. mental healing.mp. or exp Mental Healing/ 36. mesotherapy.mp. or exp Mesotherapy/ 37. moxibustion.mp. or exp Moxibustion/ 38. music therapy.mp. or exp Music Therapy/ 39. exp Naturopathy/ or naturopath$.mp. 40. natural remed$.mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 41. occultism.mp. or exp Occultism/ 42. exp Psychodrama/ or psychodrama.mp. 43. drama therapy.mp. 44. Psychology/ or psychology.mp. 45. psychophysiology.mp. or exp Psychophysiology/ 46. mind body relation$.mp. 47. radiesthesia.mp. or exp Radiesthesia/ 48. reflexotherapy.mp. or exp Reflexotherapy/ 49. rejuvenation.mp. or exp Rejuvenation/ 50. relaxation therapy.mp. or exp Relaxation Therapy/ 51. shamanism.mp. or exp Shamanism/ 52. speleotherapy.mp. or exp Speleotherapy/ 53. Tai Ji/ 54. tai chi.mp. 55. therapeutic touch.mp. or exp Therapeutic Touch/ 56. exp Medicine, Unani/ or unani.mp. 57. witchcraft.mp. or exp Witchcraft/ 58. yoga.mp. or exp Yoga/ 59. visualisation.mp. 60. immunoaugmentation.mp. 61. Tissue Therapy/ 62. Medicine, Chinese Traditional/ 63. tcm.mp. 64. antineoplaston.mp. 65. chelation.mp. 66. shark.mp. 67. electromagnetic$.mp. 68. "Tea Tree Oil"/ 69. Aloe/ 70. reflexology.mp. 71. reiki.mp. 72. herb$.mp. 73. physical therapy.mp. 74. cupping therapy.mp. 75. therapeutic exercise.mp. 76. folk.mp. 77. bioelectromagnetic$.mp. 78. Diet/ 79. nutrition.mp. 80. Diet, Macrobiotic/ 81. gerson.mp. 82. support group.mp. or Self‐Help Groups/ 83. or/1‐82 84. acne.mp. or exp Acne Vulgaris/ 85. randomized controlled trial.pt. 86. controlled clinical trial.pt. 87. randomized.ab. 88. placebo.ab. 89. clinical trials as topic.sh. 90. randomly.ab. 91. trial.ti. 92. 85 or 86 or 87 or 88 or 89 or 90 or 91 93. (animals not (humans and animals)).sh. 94. 92 not 93 95. 83 and 84 and 94
Appendix 4. Embase (OVID) search strategy
1. exp *acne vulgaris/ 2. acne.ti,ab. 3. 1 or 2 4. random$.mp. 5. factorial$.mp. 6. (crossover$ or cross‐over$).mp. 7. placebo$.mp. or PLACEBO/ 8. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 10. (assign$ or allocat$).mp. 11. volunteer$.mp. or VOLUNTEER/ 12. Crossover Procedure/ 13. Double Blind Procedure/ 14. Randomized Controlled Trial/ 15. Single Blind Procedure/ 16. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17. acoustic stimulation.mp. or auditory stimulation/ 18. diet/ 19. nutrition/ 20. exp alternative medicine/ or macrobiotic diet/ 21. antioxidant/ 22. psychophysiology/ 23. meditation/ 24. imagery/ 25. hypnosis/ 26. support group/ 27. acupuncture/ or electroacupuncture/ 28. exp traditional medicine/ 29. Ayurveda/ 30. Chinese medicine/ 31. homeopathy/ 32. chelation/ 33. massage/ 34. chiropractic/ 35. herbal medicine/ 36. physiotherapy/ 37. moxibustion/ 38. Tai Chi/ or kinesiotherapy/ 39. aromatherapy/ 40. reflexology/ 41. Reiki/ 42. yoga/ 43. music therapy/ 44. "tea tree oil"/ 45. Aloe vera/ 46. horticultural therapy/ 47. African medicine/ 48. kampo/ 49. psychology/ 50. breathing exercise/ 51. psychodrama/ 52. relaxation training/ 53. acupressure/ 54. eclecticism/ 55. rejuvenation/ 56. art therapy/ 57. speleotherapy/ 58. spiritual healing/ 59. magic/ 60. mysticism/ 61. witchcraft/ 62. feedback system/ 63. (diet$ or nutrition$ or macrobiotic$ or Gerson or antioxidant$ or mind‐body or meditation or imagery or hypnosis or support group$ or bioelectromagnetic$ or electromagnetic$ or electroacupuncture or traditional or folk or naturopathy or ayurved$ or Traditional Chinese Medicine or TCM or homeopathy or antineoplaston or chelation or immunoaugmentation or shark or manual healing or massage or chiropractic or therapeutic touch or herb$ or physical therap$ or acupuncture or moxibustion or cupping therapy or therapeutic exercise$ or Tai Chi or complementary or alternative or aromatherapy or reflexology or reiki or cam or yoga or music or tea tree or aloe vera or anthroposophy or auricular therapy or holistic health or horticultural therapy or Arabic or Asian or African or Kampo or unani or shamanism or mesotherapy or psychology or breathing exercise$ or laughter therapy or mental healing or psychodrama or psychophysiology or relaxation therapy or Taiji or acupressure or kinesiology or manipulation or tissue therapy or eclecticism or reflexotherapy or rejuvenation or acoustic stimulation or art therapy or colour therapy or dance therapy or Speleotherapy or faith healing or magic or occultism or Radiesthesia or witchcraft or biofeedback or immuno augmentation therapy or macrobiotic$ or natural remed$ or drama therapy or visualisation).ti,ab. 64. or/17‐63 65. 3 and 16 and 64
Appendix 5. PsycINFO (OVID) search strategy
1. nutrition/ 2. antioxidant/ 3. psychophysiology/ 4. meditation/ 5. imagery/ 6. hypnosis/ 7. massage/ 8. physiotherapy/ 9. yoga/ 10. music therapy/ 11. psychology/ 12. art therapy/ 13. (diet$ or nutrition$ or macrobiotic$ or Gerson or antioxidant$ or mind‐body or meditation or imagery or hypnosis or support group$ or bioelectromagnetic$ or electromagnetic$ or electroacupuncture or traditional or folk or naturopathy or ayurved$ or Traditional Chinese Medicine or TCM or homeopathy or antineoplaston or chelation or immunoaugmentation or shark or manual healing or massage or chiropractic or therapeutic touch or herb$ or physical therap$ or acupuncture or moxibustion or cupping therapy or therapeutic exercise$ or Tai Chi or complementary or alternative or aromatherapy or reflexology or reiki or cam or yoga or music or tea tree or aloe vera or anthroposophy or auricular therapy or holistic health or horticultural therapy or Arabic or Asian or African or Kampo or unani or shamanism or mesotherapy or psychology or breathing exercise$ or laughter therapy or mental healing or psychodrama or psychophysiology or relaxation therapy or Taiji or acupressure or kinesiology or manipulation or tissue therapy or eclecticism or reflexotherapy or rejuvenation or acoustic stimulation or art therapy or colour therapy or dance therapy or Speleotherapy or faith healing or magic or occultism or Radiesthesia or witchcraft or biofeedback or immuno augmentation therapy or macrobiotic$ or natural remed$ or drama therapy or visualisation).ti,ab. 14. Faith healing/ 15. Acupuncture/ 16. Shamanism/ 17. Psychology/ 18. Dance therapy/ 19. Visualization/ 20. Diets/ 21. Support Groups/ 22. exp Alternative Medicine/ or exp Folk Medicine/ 23. Holistic Health/ 24. Aromatherapy/ 25. exp Horticulture Therapy/ 26. Shamanism/ 27. Relaxation Therapy/ 28. Biofeedback/ 29. Psychodrama/ 30. Occultism/ 31. Witchcraft/ 32. or/1‐31 33. acne.mp. 34. double‐blind.tw. 35. random$ assigned.tw. 36. control.tw. 37. 34 or 35 or 36 38. 32 and 33 and 37
Appendix 6. AMED (OVID) search strategy
1. diet/ 2. nutrition/ 3. antioxidant/ 4. psychophysiology/ 5. meditation/ 6. imagery/ 7. hypnosis/ 8. exp traditional medicine/ 9. homeopathy/ 10. chelation/ 11. massage/ 12. chiropractic/ 13. physiotherapy/ 14. moxibustion/ 15. reflexology/ 16. Reiki/ 17. yoga/ 18. music therapy/ 19. "tea tree oil"/ 20. Aloe vera/ 21. psychology/ 22. breathing exercise/ 23. acupressure/ 24. art therapy/ 25. (diet$ or nutrition$ or macrobiotic$ or Gerson or antioxidant$ or mind‐body or meditation or imagery or hypnosis or support group$ or bioelectromagnetic$ or electromagnetic$ or electroacupuncture or traditional or folk or naturopathy or ayurved$ or Traditional Chinese Medicine or TCM or homeopathy or antineoplaston or chelation or immunoaugmentation or shark or manual healing or massage or chiropractic or therapeutic touch or herb$ or physical therap$ or acupuncture or moxibustion or cupping therapy or therapeutic exercise$ or Tai Chi or complementary or alternative or aromatherapy or reflexology or reiki or cam or yoga or music or tea tree or aloe vera or anthroposophy or auricular therapy or holistic health or horticultural therapy or Arabic or Asian or African or Kampo or unani or shamanism or mesotherapy or psychology or breathing exercise$ or laughter therapy or mental healing or psychodrama or psychophysiology or relaxation therapy or Taiji or acupressure or kinesiology or manipulation or tissue therapy or eclecticism or reflexotherapy or rejuvenation or acoustic stimulation or art therapy or colour therapy or dance therapy or Speleotherapy or faith healing or magic or occultism or Radiesthesia or witchcraft or biofeedback or immuno augmentation therapy or macrobiotic$ or natural remed$ or drama therapy or visualisation).ti,ab. 26. acoustic stimulation.mp. 27. exp Complementary medicine/ or exp Complementary therapies/ 28. Social support/ 29. Ayurvedic medicine/ 30. Traditional medicine chinese/ 31. Aroma therapy/ 32. Horticulture/ 33. Herbal drugs/ or Plants medicinal/ 34. Medicine kampo/ 35. Drama/ 36. Acupuncture therapy/ 37. Relaxation/ or Biofeedback/ 38. Faith healing/ 39. Folk remedies/ 40. Naturopathy/ 41. Therapeutic touch/ or Holistic health/ 42. Applied kinesiology/ 43. Tai chi/ 44. Acupuncture/ 45. Anthroposophical medicine/ 46. Shamanism/ 47. Psychology/ 48. Laughter therapy/ 49. Mental healing/ 50. Manipulation/ 51. Color therapy/ 52. Dance therapy/ 53. Visualization/ 54. or/1‐53 55. exp Acne vulgaris/ or acne.mp. 56. randomized controlled trial$/ 57. random allocation/ 58. double blind method/ 59. single blind method.mp. 60. exp Clinical trials/ 61. (clin$ adj25 trial$).mp. [mp=abstract, heading words, title] 62. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$ or dummy)).mp. [mp=abstract, heading words, title] 63. (placebo$ or random$).mp. [mp=abstract, heading words, title] 64. research design/ or clinical trials/ or comparative study/ or double blind method/ or random allocation/ 65. prospective studies.mp. 66. cross over studies.mp. 67. Follow up studies/ 68. control$.mp. 69. (multicent$ or multi‐cent$).mp. [mp=abstract, heading words, title] 70. ((stud or design$) adj25 (factorial or prospective or intervention or crossver or cross‐over or quasi‐experiment$)).mp. [mp=abstract, heading words, title] 71. 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 72. 54 and 55 and 71
Appendix 7. CINAHL (EBSCO) search strategy
S1 (MM "Acne Vulgaris") S2 TI acne S3 AB acne S4 S1 or S2 or S3 S5 (MH "Clinical Trials+") S6 PT clinical trial S7 TX (clinic* n1 trial*) S8 (MH "Random Assignment") S9 TX random* allocat* S10 TX placebo* S11 (MH "Placebos") S12 (MH "Quantitative Studies") S13 TX allocat* random* S14 "randomi#ed control* trial*" S15 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) S16 S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 S17 (MH "Alternative Therapies") OR (MH "Color Therapy") OR (MH "Manual Therapy") S18 (MH "Diet") OR (MH "Macrobiotic Diet") S19 (MH "Nutrition") S20 (MH "Antioxidants") S21 (MH "Meditation") OR (MH "Yoga") S22 (MH "Mental Healing") S23 (MH "Hypnosis") S24 (MH "Support Groups") S25 (MH "Medicine, Chinese Traditional") OR (MH "Medicine, African Traditional") OR (MH "Medicine, Arabic") OR (MH "Medicine, Ayurvedic") OR (MH "Medicine, Oriental Traditional") OR (MH "Medicine, Herbal") S26 (MH "Naturopathy") S27 (MH "Homeopathy") S28 (MH "Chelation Therapy") S29 (MH "Massage") S30 (MH "Chiropractic") S31 (MH "Therapeutic Touch") S32 (MH "Physical Therapy") S33 (MH "Acupuncture") S34 (MH "Moxibustion") S35 (MH "Therapeutic Exercise") S36 (MH "Tai Chi") S37 (MH "Aromatherapy") S38 (MH "Reflexology") S39 (MH "Reiki") S40 (MH "Music Therapy") S41 (MH "Tea Tree Oil") S42 (MH "Aloe") S43 (MH "Anthroposophy") S44 (MH "Holistic Health") S45 (MH "Shamanism") S46 (MH "Psychology") S47 (MH "Breathing Exercises") S48 (MH "Psychodrama") S49 (MH "Psychophysiology") OR (MH "Biofeedback") S50 (MH "Acupressure") OR (MH "Auriculotherapy") S51 (MH "Kinesiology") S52 (MH "Rejuvenation") S53 (MH "Acoustic Stimulation") S54 (MH "Art Therapy") S55 (MH "Dance Therapy") S56 (MH "Spiritual Healing") S57 (MH "Magic") S58 (MH "Occultism") S59 (MH "Witchcraft") S60 TX diet* or nutrition* or macrobiotic* or Gerson or antioxidant* or “mind‐body” or meditation or imagery or hypnosis or “support group*” or bioelectromagnetic* or electromagnetic* or electroacupuncture or traditional or folk or naturopathy or ayurved* or “Traditional Chinese Medicine” or TCM or homeopathy or antineoplaston or chelation or immunoaugmentation or shark or “manual healing” or massage or chiropractic or “therapeutic touch” or herb* or “physical therap*” or acupuncture or moxibustion or “cupping therapy” or “therapeutic exercise*” or “Tai Chi” or complementary or alternative or aromatherapy or reflexology or reiki or cam or yoga or music or “tea tree” or “aloe vera” or anthroposophy or “auricular therapy” or “holistic health” or “horticultural therapy” or Arabic or Asian or African or Kampo or unani or shamanism or mesotherapy or psychology or “breathing exercise*” or “laughter therapy” or “mental healing” or psychodrama or psychophysiology or “relaxation therapy” or Taiji or acupressure or kinesiology or manipulation or “tissue therapy” or eclecticism or reflexotherapy or rejuvenation or “acoustic stimulation” or “art therapy” or “colour therapy” or “dance therapy” or Speleotherapy or “faith healing” or magic or occultism or Radiesthesia or witchcraft or biofeedback or “immuno augmentation therapy” or macrobiotic* or “natural remed*” or “drama therapy” or visualisation S61 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 S62 S4 and S16 and S61
Appendix 8. PubMed search strategy (Using CAM subset)
#4 Search #3 Limits: Complementary Medicine #3 Search #1 and #2 #2 Search (acne vulgaris[MeSH Terms]) OR acne[Text Word] #1 Search ((randomized controlled trial[Publication Type] OR controlled clinical trial[Publication Type]) OR randomized[Title/Abstract]) OR placebo[Title/Abstract] OR "clinical trials as topic"[MeSH Terms:noexp] OR randomly[Title/Abstract] OR trial[Title] NOT (animals[MeSH Terms] NOT (humans[MeSH Terms]) AND animals[MeSH Terms])
Data and analyses
Comparison 1. Low‐glycaemic‐load diet versus high‐glycaemic‐load diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: change in non‐inflammatory lesion count (medium‐term data) | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐3.89 [‐10.07, 2.29] |
| 2 Primary outcome: change in inflammatory lesion counts (medium‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Primary outcome: change of total lesion counts (medium‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 2. Acupuncture versus waiting list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: change of inflammatory lesion count (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Primary outcome: change of non‐inflammatory lesion count (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Acupuncture versus western drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fire acupuncture versus Clindamycin phosphate gel and azithromycin capsule | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: number of participants with remission (ITT‐best case, short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fire acupuncture versus Clindamycin phosphate gel and azithromycin capsule | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Secondary outcome: number of participants with remission (ITT‐worst case, medium‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Acupuncture versus isotretinoin soft capsules | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Secondary outcome: number of participants with remission (ITT‐best case, medium‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Acupuncture versus isotretinoin soft capsules | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Herbal medicine versus waiting list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: change of inflammatory lesion count (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Primary outcome: change of non‐inflammatory lesion count (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Herbal medicine versus antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: change of total skin lesion counts at the end of the treatment (medium‐term data) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Herbal decoction versus minocycline hydrochloride capsules (at 8 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Shugan Zishen decoction versus tetracycline (at 8 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Ziyin‐Qinggan‐Xiaocuo Granule versus minocycline (at 4 weeks) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.09, 2.53] |
| 2.2 Xiaocuo decoction versus doxycycline (at 4 weeks) | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.83, 2.34] |
| 2.3 Yinqiao Liangxue decoction versus azithromycin (at 4 weeks) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [1.17, 3.42] |
| 3 Secondary outcome: number of participants with remission (ITT‐best case, short‐term data) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Ziyin‐Qinggan‐Xiaocuo Granule versus minocycline (at 4 weeks) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.88, 9.06] |
| 3.2 Xiaocuo decoction versus doxycycline (at 4 weeks) | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.33, 4.39] |
| 3.3 Yinqiao Liangxue decoction versus azithromycin (at 4 weeks) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [1.17, 3.42] |
| 4 Secondary outcome: number of participants with remission (medium‐term data) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Herbal decoction versus minocycline hydrochloride capsules (at 8 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Shugan Zishen decoction versus tetracycline (at 8 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Herbal medicine versus vitamin A acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Xiaocuo decoction plus 0.1% adapalene gel versus viaminate capsule plus 0.1% adapalene gel (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Jiawei Xiaodu decoction versus tretinoin cream (at 2 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Qingbu decoction versus viaminate capsule (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Modified Cuo Chuang decoction versus isotretinoin (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: number of participants with remission (ITT‐best case, short‐term data) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Xiaocuo decoction plus 0.1% adapalene gel versus viaminate capsule plus 0.1% adapalene gel (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Jiawei Xiaodu decoction versus tretinoin cream (at 2 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Qingbu decoction versus viaminate capsule (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Modified Cuo Chuang decoction versus isotretinoin soft capsules (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Secondary outcome: number of participants with remission (medium‐term data) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Da Huang Zhe Chong Pills versus isotretinoin soft capsule (at 8 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Modified Wuwei Xiaodu Yin & Yinchen Hao decoction versus viaminate Cream (at 8 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Herbal decoction external application versus adapalene external application (at 6 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Secondary outcome: QoL‐acne score (medium‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Functioning in society (at 8 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Feelings of self‐worth (at 8 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Emotional functionality (at 8 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Herbal medicine versus antibiotics and vitamin A acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: number of participants with remission (medium‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Xiaocuo Granule + herbal decoction externally used versus roxithromycin & viaminate capsules (at 5 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Herbal medicine versus staphylococcus Injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: skin lesion score (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Secondary outcome: number of participants with remission (short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Herbal medicine plus western drugs versus western drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: change of total skin lesion counts at the end of the treatment (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Qingre 25‐Ingredients pill + staphylococcus Inj versus staphylococcus Inj (at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: number of participants with remission (short‐term data) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Qingre 25‐ Ingredients Pill + staphylococcus Inj versus staphylococcus Inj (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Danshen tablet plus doxycycline versus doxycycline (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Herbal medicine plus LED versus LED.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: number of participants with remission (ITT‐worst case, short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Secondary outcome: number of participants with remission (ITT‐best case, short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Herbal medicine plus wet cupping versus wet cupping.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: number of participants with remission (short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Wet cupping versus western drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: change in acne severity score at the end of treatment (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Wet cupping versus viaminate cream (at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: number of participants with remission (short‐term data) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Wet cupping versus viaminate cream (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Wet cupping versus achromycin (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Wet cupping versus tetracycline (at 2 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Wet cupping plus herbal medicine versus herbal medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: number of participants with remission (short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 14. Wet cupping plus acupuncture versus western drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: skin lesion score (medium‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Wet cupping plus acupuncture versus minocycline (at 10 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: number of participants with remission (short‐term data) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Wet cupping plus acupuncture versus antibiotics (at 4 weeks) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Wet cupping plus acupuncture versus vitamin A acid (at 4 weeks) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Secondary outcome: QoL‐acne score (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Functioning in society (at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Feelings of self‐worth (at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Emotional functionality (at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Wet cupping plus herbal medicine versus western drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: number of participants with remission (short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Wet cupping plus herbal mask versus viaminate capsules (at 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. Combination of wet cupping, acupuncture and massage versus western drug ‐ zinc gluconate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: skin lesion score (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Secondary outcome: number of participants with remission (short‐term data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Secondary outcome: QoL‐acne score (short‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Functioning in society (at 3 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Feelings of self‐worth (at 3 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Emotional functionality (at 3 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. Tea tree oil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: change of total skin lesion counts (medium‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Primary outcome: change of acne severity score (medium‐term data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 18. Cosmetics with PBV versus cosmetics without PBV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: change in KAGS scores based on number of inflammatory and non‐inflammatory acne lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2009.
| Methods | Randomised controlled trial | |
| Participants | There were 90 participants in the study, with 30 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 22.93 (6.03); C‐A = 21.37 (5.12); C‐B = 21.83 (6.07) Duration of disease (years): I = 4.31 (3.24); C‐A = 2.98 (2.40); C‐B = 2.60 (1.92) |
|
| Interventions |
Intervention: 1. Chinese herbal medicine according to syndromes, orally taken; 2. blood‐letting plus cupping by a 0.65 mm * 3.5 mm blood collection needle for bleeding of 3 cc, then cupping for 10 min; 3. acupuncture was done during retaining cupping according to syndromes by a 0.35 mm * 40 mm filiform needle, manipulated with neutral supplementation and drainage method, twice per week for 4 weeks Control A: blood‐letting ‐ dose and method were the same as for the treatment group Control B: Chinese herbal medicine according to syndromes ‐ 1 decoction daily for 4 weeks |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using random number table to gain the random number" Comment: The study reported enough detailed information to indicate that random sequence generation was appropriate |
| Allocation concealment (selection bias) | Low risk | Quote: "...using sequentially numbered, opaque, sealed envelope..." Comment: The study reported enough detailed information to indicate that allocation concealment was appropriate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details. However, because participants in the intervention group were given a TCM decoction plus cupping and blood‐letting, while participants in control A group were given only cupping and blood‐letting, and the control B group were only given TCM decoction, it seems likely that there was no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study randomised 90 participants, which were all included in the analysis: "During the follow‐up of 3 months after treatment, there was no recurrence reported among the seven remission cases in treatment group, while for remission cases in control A and B, two out of the three remission cases recurred in both groups." It seems likely that there were no incomplete outcome data in this study. However, as the exclusion criteria noted "...participants with incomplete data will be excluded" and follow‐up only focused on the remissions, it is probable that the study had incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was difficult to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Chen 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 70 participants in the study, with 35 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.54 (4.64); C = 23.34 (4.37) Sex ratio, male/female: I = 14/21; C = 13/22 Duration of disease (years): I = 4.48 (2.58), C = 4.31 (2.47) |
|
| Interventions |
Intervention: Xiao Cuo decoction (hospital preparation by the First Affiliated Hospital of Hunan University of Chinese Medicine) taken orally ‐ 40 ml per time, 3 times daily for 4 weeks ‐ plus 0.1% adapalene gel externally used on lesion area per night Control: viaminate capsules (Wei An Zhi capsules, Chong Qing Hua Bong pharmaceutical limited by share Ltd.) ‐ 25 mg, 3 times daily ‐ plus 0.1% adapalene gel externally used on lesion area once per night |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to intervention and control groups, with 35 patients [in] each group…the random numbers were generated by random number table…" Comment: Even though the study provided no more detailed information to describe how randomisation was assigned, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, as the intervention and control medicines were not similar in appearance and taste, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 70 participants were randomised, and all were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, due to the exclusion criteria, participants who dropped out during the treatment were excluded, so there was insufficient information about this item |
| Selective reporting (reporting bias) | Unclear risk | In the methods section, the study authors mentioned that the blood test, liver/kidney function test would be measured, but these were not reported in the results section. However, this information may not have influenced the final analysis since they were not primary outcomes. As we could not find the protocol for this trial, it was hard to judge whether they reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Enshaieh 2007.
| Methods | Randomised controlled trial | |
| Participants | There were 60 participants in the study, with 30 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria: unclear Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I =19.3 (3.1); C = unclear Sex ratio, male/female: I = 7/23; C = 6/24 Duration of disease (years): unclear |
|
| Interventions |
Intervention: applied 5% tea tree oil gel (provided by Cinere Company, Iran) over the affected area for 20 mins and then washed it off with tap water twice daily for 45 days Control: vehicle gel (Cinere Company, Iran) composed of the carbomer that had no anti‐acne activities per se, which was the same colour, texture, package size, but different label than the intervention drug, applied twice daily over the affected area for 20 min, then washed off with tap water |
|
| Outcomes | Measured every 15‐day period Primary outcome:
Secondary outcome:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to intervention and control groups… the random numbers were generated by random allocation software…" Comment: Even though no more detailed information was provided to describe how randomisation was assigned, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both investigators and the patients were blinded to the type of treatment" Quote: "Tea tree oil and placebo gels were composed the same colour, texture, package size but with different label" Comment: It seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A second investigator blinded to the type of treatment was responsible for counting the lesions before and after the treatment" Quote: "At the end of the study, the data were analysed by the third investigator using SPSS program and then the labels were revealed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All of the participants completed the study according to the authors' report |
| Selective reporting (reporting bias) | Unclear risk | No standard deviation was reported for all the outcome data in the results section |
| Other bias | Unclear risk | Cinere company financial supported the study, which also provided the intervention and control drugs for the study No sample size calculation was provided |
Feng 2005.
| Methods | Randomised controlled trial (2:1 randomised) | |
| Participants | There were 86 participants in the study, with 57 participants in the intervention group and 29 participants in the control group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 24.67 (4.53); C = 24.52 (4.54) Sex ratio, male/female: I = 0/57; C = 0/29 Duration of disease (years): I = 4.25 (3.63), C = 4.95 (3.12) |
|
| Interventions |
Intervention: herbal decoction (Zi Yin Qing Gan Xiao Cuo decoction: particle‐free granules from Jiangyin Tianjiang Pharmaceutical Company) ‐ 250 ml administered orally once daily for 4 weeks ‐ plus Sanhuang lotion externally applied on lesion area twice daily Control: minocin capsule ‐ 50 mg twice daily (Jiangsu Suzhou Lida Pharmaceutical Company) ‐ plus Sanhuang lotion externally applied on lesion area twice daily |
|
| Outcomes | Measured at weeks 0, 1, 2, and 4
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to intervention and control groups by random number table…" Comment: No more detailed information was provided to describe how randomisation was assigned; it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors reported the study as "single blind" without details; however, as the intervention and control medicines were not similar in appearance and taste, it seems likely that blinding of participants and personnel was not maintained |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias; however, authors reported the study as "single blind" with regard to participants. Therefore, the outcome assessors may not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 86 participants were recruited and analysed according to the report; however, there was insufficient information about whether all participants completed the study to permit judgement of 'low' or 'high' risk of bias |
| Selective reporting (reporting bias) | High risk | Skin lesion integral was reported as a primary outcome, but was not reported after the treatment finished |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Han 2010.
| Methods | Randomised controlled trial | |
| Participants | There were 100 participants in the study, with 50 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 25.83 (5.25); C = 24.68 (4.36) Sex ratio, male/female: I = 18/28; C = 14/33 Duration of disease (years): I = 2.35 (0.86), C = 2.15 (0.82) |
|
| Interventions |
Intervention: acupuncture on abdomen point (CV13, CV12, CV4, CV6, K13, R6, E24) for 30 minutes, once every 2 days for 8 weeks Control: isotretinoin soft capsules (Shanghai Donghai Pharmaceutical Company) ‐ 10 mg twice daily for a month, then once daily for the second month |
|
| Outcomes | Outcomes measured at weeks 0, 4, and 8, and 1 month after treatment
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to intervention and control groups by random number table…" Comment: No more detailed information was provided to describe how randomisation was assigned; it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 100 participants were randomised into 2 groups: 4 in the intervention group and 3 in the control group dropped out of the study due to personal reasons. These 7 participants' data were not included in the statistical analysis |
| Selective reporting (reporting bias) | Unclear risk | We could not find any protocol information to assist us with judgement |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Han 2013.
| Methods | Randomised controlled trial | |
| Participants | There were 12 participants in the study, with 6 participants in each group Diagnostic criteria: not reported Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): not reported Sex ratio, male/female: not reported Duration of disease (years): not reported |
|
| Interventions | Intervention: cosmetics containing PBV twice daily in the morning and evening for 2 weeks. Cosmetics were applied topically on the whole face with an amount of 4 mL per day. The concentration of PBV was 0.06 mg/mL in PBV‐containing cosmetics Control: cosmetics without PBV were applied twice daily in the morning and evening for 2 weeks. The usage method was the same as in the intervention group | |
| Outcomes | Outcomes measured at weeks 0 and 2
|
|
| Notes | The severity of acne was assessed using KAGS, proposed by the consensus conference in 2004. The severity score (grades 1 to 6) was as follows: grade 1, papules ≤ 10; grade 2, papules 11 to 30; grade 3, papules ≥ 31, nodules ≤ 10; grade 4, nodules 11 to 20, ± mild ongoing scars; grade 5, nodules 21 to 30, ± moderate ongoing scars; grade 6, nodules ≥ 30, ± severe ongoing scars and ± sinus tract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoted "Korean female and male subjects...participated in a doubled‐blind, placebo‐controlled, split face study with left‐right randomisation that was carried out June 2012" Comment: No more detailed information was provided to describe how randomisation was assigned; it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quoted "Korean female and male subjects...participated in a doubled‐blind, placebo‐controlled, split face study with left‐right randomisation that was carried out June 2012" Comment: Cosmetics with or without PBV were applied in the intervention or control group; usage method of cosmetics and treatment frequency was exactly the same in both groups. Though details of blinding were not mentioned, it seems that blinding of participants and personnel could be conducted successfully |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants were included in the trial; no‐one dropped out of the study during treatment. The number of participants in the per‐protocol set and intention‐to‐treatment analysis was 6 per group |
| Selective reporting (reporting bias) | Unclear risk | We could not find any protocol information to assist us with judgement |
| Other bias | Unclear risk | No funding issue was apparent No baseline information was provided to determine comparability No sample size calculation was provided |
Huang 2006.
| Methods | Multicentre randomised controlled trial | |
| Participants | There were 210 participants in the study, with 105 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.52 (5.38); C = 24.94 (6.26) Sex ratio, male/female: I = 73/32; C = 62/43 Duration of disease (months): I = 49.93 (43.58), C = 51.27 (54.12) |
|
| Interventions |
Intervention: acupuncture on lesion points BL 13 and BL 20; the needle was inserted and kept for 5 seconds before withdrawal ‐ done 5 times for each point, once every 4 days for 3 weeks Control: clindamycin phosphate gel (Suzhou Pharmaceutical Company) ‐ 10 g externally applied on lesion area twice daily ‐ plus 0.5 g azithromycin capsule (Shandong Lukang Medical Company) once daily for 3 weeks |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...random number was generated by SPSS software…" Comment: It was probably generated appropriately |
| Allocation concealment (selection bias) | Low risk | Quote: "...random number was kept in envelope, then sent to each clinical centre..." Comment: It was probably concealed appropriately |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants dropped out (2 cases in each group); reasons were outlined; intention‐to‐treat analysis was also used for a primary outcome |
| Selective reporting (reporting bias) | Unclear risk | We could not find any protocol information to assist us with judgement |
| Other bias | Unclear risk | No funding issue was apparent |
Kim 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 44 participants in the study, with 11 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 21.5 (3.6); C = 23.3 (4.1) Sex ratio, male/female: I = 22/0; C = 22/0 Duration of disease (years): I = 5.8 (3.4), C = 6.6 (4.7) |
|
| Interventions |
Intervention 1: Keigai‐rengyo‐to extract ‐ 3 times daily after meals for 4 weeks Intervention 2: acupuncture on ST2, ST6, LI20, LI11, PC6, HT8, SP3, SP6, SP10, and LR3 for 15 minutes each time; disposable stainless steel needles were inserted at a vertical or oblique angle to a depth of 1 to 5 minutes at each point, twice per week for 4 weeks Intervention 3: Keigai‐rengyo‐to extract ‐ 3 times daily after meals, plus acupuncture (same points' selection as intervention 2) twice per week for 4 weeks Control: waiting list for 4 weeks |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of four groups. Random number were generated by a computerized random number generator through the block‐randomisation method of a software program…" Comment: It was probably generated appropriately |
| Allocation concealment (selection bias) | Low risk | Quote: "...two separate databases were created: a 'patients' database, which lists basic information, including patient name, contact details, and so on, and a 'randomisation' database, which holds data on which patients have been registered on trial along with their allocations. The 'patients' database was accessible to any researcher, whereas the 'randomisation' database was password protected, so that it was accessible only by the principal investigator and a nominated statistician..." Comment: The study probably concealed allocation appropriately |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The assessor, who was the acupuncture practitioner and KRTE supplier, and who was blinded to the allocation results until the end of study, performed the outcome assessment" Comment: It seems likely that personnel were blinded, but participants were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The assessor, who was the acupuncture practitioner and KRTE supplier, and who was blinded to the allocation results until the end of study, performed the outcome assessment" Comment: The study outcome assessor was blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 participants dropped out during the study, with a total dropout rate of 22.7%. The number of participants that dropped out was different among groups (3:1:2:4), but the reason for dropout was not clarified with detailed information Quote: "The two most common reasons for discontinuation were 'lost to follow‐up' or 'personal reason'" Comment: Though an intention‐to‐treat analysis was used with LOCF methods, they may have had unclear risk of attrition bias that influenced the overall outcome assessment |
| Selective reporting (reporting bias) | High risk | The study provided a protocol, but primary and secondary outcomes were incompletely reported for all groups |
| Other bias | Unclear risk | No sample size calculation was provided |
Kwon 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 17 participants in the intervention group and 15 participants in the control group Diagnostic criteria: unclear Inclusion criteria:
Exclusion criteria: unclear Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.5 (3.2); C = 23.7 (2.6) Sex ratio, male/female: I = 13/4; C = 11/4 Duration of disease (years): unclear |
|
| Interventions |
Intervention: low‐glycaemic‐load diet (LGLD) with 25% energy from protein, 45% low‐glycaemic‐index carbohydrates, and 30% energy from fat ‐ once daily for 10 weeks Control: carbohydrate‐rich foods ‐ once daily for 10 weeks |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A blocked random allocation sequence was created by computer‐generated random number…" Comment: Random sequence generation was generated appropriately |
| Allocation concealment (selection bias) | Low risk | Quote: "...allocation to specific groups was performed by a research nurse..." Comment: Allocation was concealed appropriately |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, intervention and control were not similar in colour and taste. It seems likely that participants and personnel were not blind to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All of the participants completed the study according to the authors' report |
| Selective reporting (reporting bias) | Unclear risk | No standard deviation was reported for all of the outcome data. Also, the mean difference of primary outcome for control group was not provided |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Li 2007.
| Methods | Randomised controlled trial | |
| Participants | There were 120 participants, with 60 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 25.45 (4.45); C = 26.67 (7.06) Sex ratio, male/female: I = 0/60; C = 0/60 Duration of disease (months): I = 26.40 (18.59), C = 25.60 (18.01) |
|
| Interventions |
Intervention: herbal medicine Ziyin‐Qinggan‐Xiaocuo granule (Jiangyin Tianjiang Pharmaceutical Company) ‐ 200 ml given orally once daily for 4 weeks ‐ plus Cuoling Tincture externally used twice daily on the lesion area Control: 50 mg minocycline capsules taken twice daily (Jiangsu Suzhou Lida Pharmaceutical Company), plus Cuoling Tincture externally used twice daily |
|
| Outcomes | Outcomes were measured at weeks 0, 1, 2, 3, and 4
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to intervention and control groups by random number table…" Comment: Even though no more detail information was provided to describe how randomisation was assigned, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 20 participants were excluded from the final analysis; 9 of them were excluded because of the potential adverse effects of the drug, and 11 of them were lost to follow up. No appropriate statistical methods was used to handle the incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Primary outcomes including the number, location, and type of the lesion were not reported in the results section |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Lin 2013.
| Methods | Randomised controlled trial | |
| Participants | There were 72 participants in the study, with 36 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 18 to 35; C = 18 to 28 Sex ratio, male/female: I = 10/26; C = 8/28 Duration of disease (years): I = 0.25 to 3, C = 0.17 to 3 |
|
| Interventions |
Intervention: wet cupping plus herbal mask; pricking with triangle‐edged needle on 2 points selected from GV14, BL13, BL15, BL18, BL20, BL21, and BL25, then cups were applied on the points, retained for 5 min ‐ once every 2 days, 4 times as a treatment session for a month; herbal mask for 30 minutes once every 2 days ‐ 15 times as a session for a month Control group: 10 mg viaminate capsule (brand name: Taiersi, Shanghai Yan'an Phamarceutical company) taken 3 times daily for a month |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to intervention and control groups by random number table…" Comment: Even though no more detailed information was provided to describe how randomisation was assigned, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Because there was no information about the dropout and follow‐up rate, it was difficult to judge whether we should apply a 'low' or 'high' risk of bias to this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was difficult to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Liu 2004.
| Methods | Randomised controlled trial | |
| Participants | There were 160 participants in the study, with 80 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 22.70 (2.54); C = 21.20 (2.37) Sex ratio, male/female: I = 32/44; C = 31/41 Duration of disease (years): I = 2.78 (1.05), C = 2.67 (0.82) |
|
| Interventions | 2 groups both used compound tretinoin cream and compound vitamin nitrate whistle cream, made by their hospital. Compound vitamin nitrate whistle cream was used 1˜2 times during the daytime, and compound tretinoin cream was used once at night ‐ 4 weeks for a course Intervention: modified Xiao Cuo decoction according to syndrome differentiation, 100 ml per time, twice per day Control: doxycycline, orally administered 0.1 g per time, twice per day |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the sequence generation process to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the sequence generation process to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total, 160 participants were included, but 4 in the intervention group and 6 in the control group were excluded from the final analysis. 7 of them were excluded due to the potential adverse effects of the drug, and 3 of them were lost follow up. No appropriate statistical methods were used to handle the incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Liu 2006.
| Methods | Randomised controlled trial | |
| Participants | There were 40 participants in the study, with 20 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.70 (3.11); C = 23.70 (3.83) Sex ratio, male/female: I = 6/14; C = 5/15 Duration of disease (years): I = 4.66 (3.78), C = 3.61 (3.11) |
|
| Interventions |
Intervention group: acupuncture treatment once every other day, 15 times for 1 course, 2 courses in total; pricking and cupping treatment, once every 3 days, 8 times for 1 course, 2 courses in total control group: "Mei man du su" capsule (minocycline), oral administration, 1 capsule once, twice per day, 1 month for 1 course, 2 courses in total |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the sequence generation process to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Because there was no information about the dropout and follow‐up rate, it was hard to judge whether we should apply a 'low' or 'high' risk of bias to this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Liu 2007.
| Methods | Randomised controlled trial | |
| Participants | There were 105 participants in the study, with 35 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): 19.16 Sex ratio, male/female: I = 20/15; C1 = 19/16; C2 = 19/16 Duration of disease (months): 20.23 |
|
| Interventions |
Western medicine group: drug for exterior use: viaminate cream (vitamin A acid cream, Chongqing Huabang Pharmaceutical Co. Ltd), used once per day before sleeping for 8 weeks in total Chinese medicine group: Qingre Chushi Jiedu decoction, 100 ml per time, taken once every morning and night for 8 weeks in total Acupuncture group: acupuncture, once per day, 5 times from Monday to Friday; pricking and cupping, twice per week for 8 weeks in total |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table generated the random sequence |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the sequence generation process to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicine were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the sequence generation process to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 105 participants were randomly divided into 3 groups, and all were included in the analysis Quote: "All participants completed the study" |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Liu 2008.
| Methods | Randomised controlled trial | |
| Participants | There were 52 participants in the study, with 26 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.25 (1.25); C = 22.75 (1.96) Sex ratio, male/female: I = 8/18; C = 10/16 Duration of disease (years): I = 2.79 (2.01), C = 2.57 (2.36) |
|
| Interventions |
Intervention: acupuncture plus pricking and cupping, twice per week, 4 weeks in total Control: isotretinoin soft capsules, orally administered, 10 mg per time, 3 times per day, 4 weeks in total |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the sequence generation process to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicine were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Because there was no information about the dropout and follow‐up rate, it was hard to judge whether we should apply a 'low' or 'high' risk of bias to this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Liu 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 94 participants in the study, with 31, 31, or 32 participants in the intervention, control 1, and control 2 group, respectively Diagnostic criteria: unclear Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 22.3; C1 = 9.8; C2 = 21.3 Sex ratio, male/female: I = 15/16; C1 = 17/14, C2 = 14/18 |
|
| Interventions | 3 groups were all treated with "Fu Fang Zhen Zhu An Chuang" tablets and lotion for acne vulgaris Intervention: traditional Chinese medical mask combined with blue and red LED phototherapy ‐ 15 minutes for blue LED, 15 minutes for red LED, after that, using traditional Chinese medical mask twice per week, 8 times for 1 course Control group 1: blue and red LED phototherapy Control group 2: traditional Chinese medical mask twice per week 3 groups were all treated for 4 weeks |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "94 patients were randomly divided into 3 groups by number" |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the sequence generation process to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant in each group failed to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Mo 2011.
| Methods | Randomised controlled trial | |
| Participants | There were 80 participants in the study, with 40 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 22.35 (5.55); C = 21.20 (5.48) Sex ratio, male/female: I = 12/28; C = 14/26 Duration of disease (years): I = 2.50 (3.52); C = 2.60 (3.60) |
|
| Interventions |
Intervention group: (Chinese medicine) modified Yin Qiao Liang Xue Tang, oral administration, once daily externally applied drug: Sheng Ji Zhen Hua San 2 g, Qing Ha San 5 g, Xiao Yan Xuan Shi Gao 7.5 g. 2 h per time, once daily Control group: (western medicine) azithromycin, 0.5 g per time, oral administration |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "80 patients were randomised into two groups" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicine were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 80 participants were randomised, and all were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Ni 2008.
| Methods | Randomised controlled trial | |
| Participants | There were 46 participants in the study, with 24 participants in the intervention group and 22 participants in the control group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 22.35 (5.55); C = 21.20 (5.48) Sex ratio, male/female: I = 12/28; C = 14/26 Duration of disease (years): I = 2.50 (3.52); C = 2.60 (3.60) |
|
| Interventions |
Intervention: acupuncture, 3 times per week, 3 weeks for a course; pricking and cupping, twice per week, 3 weeks for a course; massage, 3 times per week, 3 weeks for a course Control: (western medicine) zinc gluconate, 20 ml per time, twice per day, 3 weeks for a course Giving participants some life guides, such as keeping optimistic and avoiding fat, spicy, and sugary foods |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using the method of block randomizations according to patients' visiting sequence" |
| Allocation concealment (selection bias) | Unclear risk | There was no information to permit judgement of allocation concealment as 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicine were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information to permit judgement of blinding method of outcome assessors as 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46 participants were randomised, and all were included in the analysis. No participants dropped out or were lost to follow up in either group |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Peng 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 99 participants in the study, with 52 participants in the intervention group and 47 participants in the control group Diagnostic criteria:
Inclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 22.70 (4.30); C = 21.70 (3.60) Sex ratio, male/female: I = 32/20; C = 28/19 Duration of disease (years): I = 2.62 (1.42), C = 2.33 (1.25) |
|
| Interventions |
Intervention: Da Huang Zhe Chong Pills (Beijing Tongrentang Limited by share Ltd.) 3 g 0.5 h before meal, taken with Dan Quan rice wine 10 ml (Guangxi Dan Quan wine industry limited by share Ltd.) twice daily for 8 weeks Control: isotretinoin soft capsule (trade name: Taiersi, Shanghai Xin Yi Yan'an Phamarceutical Limited by share Ltd.) 10 mg twice daily for 4 weeks, then 10 mg once daily for another 4 weeks |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided into two groups by random number table" |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicine were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 99 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Shi 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 167 participants in the study, with 96 participants in the intervention group and 71 participants in the control group Diagnostic criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.3 (3.12); C = 23 (2.43) Sex ratio, male/female: I = 54/42; C = 40/31 |
|
| Interventions |
Intervention: (Chinese medicine) modified Jia Wei Xiao Du Yin taken twice per day, in the morning and evening ‐ 15 days for a course Control: (western medicine) tretinoin cream (Di Wei Cream, Chongqing Huabang Pharmaceutical Co. Ltd) twice per day ‐ 15 days for a course |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided into two groups by random number table" |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 167 participants were randomised, and all were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Smith 2007.
| Methods | Randomised controlled trial | |
| Participants | There were 54 participants in the study, with 27 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 18.2 (0.5); C = 18.5 (0.5) |
|
| Interventions |
Intervention: The recommended LGLD consisted of 25% energy from protein, 45% from low GI carbohydrates, and 30% energy from fats Control: The control group received carbohydrate‐dense staples and were instructed to eat these or similar foods daily. The foods provided had moderate to high GI values; participants were urged to include carbohydrates as a regular part of their diet All participants were provided with a topical cleanser and were advised to use it in place of their normal wash, soap, or cleanser |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by computer‐generated random numbers..." |
| Allocation concealment (selection bias) | Low risk | Quote: "...allocation to groups was performed by a third party" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants were informed that the study was comparing the carbohydrate to protein ratio in the diet and were not informed of the study's hypothesis" Comment: It seems likely that the trial blinded participants. However, blinding of personnel may have been hard to do due to the difference between the intervention and control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This study was designed as a parallel dietary intervention study with investigator‐masked dermatology assessments" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Seven participants did not complete the study (5 in control and 2 in LGL groups), and 4 were removed from data set (two were noncompliant; two began medications known to affect acne, an exclusion criterion)" Comment: The dropout rate was 15% and 25% in the intervention and control groups, respectively. Intention‐to‐treat analysis was not applied |
| Selective reporting (reporting bias) | Low risk | We could retrieve the protocol, which reported all primary and secondary outcomes |
| Other bias | Unclear risk | No funding issues were apparent The study appeared to be free of other sources of bias |
Song 2007.
| Methods | Randomised controlled trial | |
| Participants | There were 76 participants in the study, with 42 participants in the intervention group and 34 participants in the control group Diagnostic criteria:
Inclusion criteria: unclear Exclusion criteria: unclear Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Sex ratio, male/female: I = 24/18; C = 19/15 |
|
| Interventions |
Intervention group: pricking ear point, then pricking the Back‐Shu point and cupping, once daily ‐ 15 times for a course control group: (western medicine) tetracycline, 500 mg per time, 3 times per day cleaning the face, using Cuo Chuang Ping cream, once every morning and night ‐ 15 days for a course |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 76 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Tang 2011.
| Methods | Randomised controlled trial | |
| Participants | There were 219 participants in the study, with 120 participants in the intervention group and 99 participants in the control group Diagnostic criteria:
Inclusion criteria: unclear Exclusion criteria: unclear Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 24.81 (5.41); C = 23.54 (5.57) Duration of disease (years): I = 4.35; C = 4.21 |
|
| Interventions |
Intervention: (Chinese medicine) Qin Gan Jie Yu Xiao Cuo particles orally taken, twice per day, 1 bag per time. Exterior use Chinese medicine, once every morning and night Chloramphenicol Tincture, external application, twice every morning and night Control: roxithromycin, orally taken, 150 mg, twice daily viaminate capsules, 50 mg, 3 times daily Chloramphenicol tincture, external application, twice every morning and night |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table…" |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Wang 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 80 participants in the study, with 40 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 24.1 (5.49); C = 24.35 (6.33) Sex ratio, male/female: I = 14/26; C = 16/24 Duration of disease (years): I = 1.99; C = 2.13 |
|
| Interventions |
Intervention: modified Cuo Chuang Fang, orally taken, 100 ml, 2 times per day; herbs steaming, used Automatic Chinese Medicine Steaming Instrument (provided by Changzhou Zhengrong Medical Equipment Co. Lid, type: XZQ‐V) to prepare herbs for steaming, 15 minutes per time, 2 times per week for 6 weeks Control: modified Cuo Chuang Fang; 0.1% adapalene Gel (Vitamin A acid) (trade name: Differin, provided by Laboratoires Galderma S.A.) externally used once per night |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table…" Comment: Random sequence generation was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, as participants in the treatment group were given steamed TCM, which were not given in the control group, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80 participants were randomised, and all of these were included in the analysis Quote: "All participants completed the study" |
| Selective reporting (reporting bias) | High risk | In the methods section, authors did not mention that the relapse rate would be measured, but they reported it in the results section. As we could not find the protocol for this trial, it might not have reported all of the primary outcomes for this trial |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Wei 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 100 participants in the study, with 50 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 28 (2.02); C = 27 (3.8) Sex ratio, male/female: I = 25/25; C = 26/24 Duration of disease (months): I = 1.53 (1.39); C = 1.60 (1.41) |
|
| Interventions |
Intervention: decoction with function of clearing liver and eliminating dampness, 200 ml, orally taken 30 min after meal, twice daily for 8 weeks Control: minocycline hydrochloride capsules (Wyeth Pharmaceutical Company, Ltd., H10960010), 50 mg per time, 2 times daily for 8 weeks |
|
| Outcomes | Measured at 2, 4, and 8 weeks:
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using SPSS 17.0 software to generate random number, patients were assigned to two groups…" |
| Allocation concealment (selection bias) | Low risk | Quote: "...took the random number into opaque envelopes…" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicines were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 100 participants were randomised, and all of these were included in the analysis. The study reported that during the follow‐up of 1 month, there was no recurrence in the treatment group and 2 cases in the control group. It seems likely that there were no incomplete outcome data in this study. However, because there was no detailed information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | High risk | In the methods section, authors mentioned that skin lesion score would be measured and did not mention that number of rashes. However, in the results section, only skin lesion score before treatment was reported, and the number of rashes before and after treatment of each group were reported. The skin lesion score was the primary outcome. Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Wen 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 60 participants in the study, with 30 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.70 (3.11); C = 23.70 (3.83) Sex ratio, male/female: I = 16/14; C = 12/18 Duration of disease (months): I = 4.66 (3.78); C = 3.61 (3.11) |
|
| Interventions |
Intervention: 1. facial blood‐letting and quick cupping on the local area of acne by disposable blood collection needle (No. 4) inserting in the centre of the acne, then used quick cupping 3 times for bleeding of 0.5 to 1 ml. Twice per week for 10 weeks. 2. body acupuncture on LI 11, SJ 6, ST 36, ST 40, and ST 44 by filiform needle (0.25 * 40 mm) inserting upright for 0.5 to 1.2 inch, manipulated needles with neutral supplementation and drainage method, and retained the needles for 30 min. Twice per week for 10 weeks Control: minocycline hydrochloride capsules (Wyeth Pharmaceutical Company, Ltd.), 50 mg per time, twice daily for 10 weeks |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to treatment group and control group by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, because participants in the treatment group were treated by acupuncture plus moving cupping and blood‐letting, while participants in the control group were given a capsule, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | In the methods section, the study authors did not report the outcome measures. In addition, since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the outcomes in the article |
| Other bias | Unclear risk | No sample size calculation was provided |
Wu 2008.
| Methods | Randomised controlled trial | |
| Participants | There were 270 participants in the study, with 86, 84, or 87 participants in the combination, herbal medicine, or control group, respectively Diagnostic criteria:
Inclusion criteria:
Exclusion criteria: not reported Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (range), years: 21.78 (16 to 56) Sex ratio, male/female: 224/46 Duration of disease (weeks): 23.7 |
|
| Interventions |
Intervention: Qingre Twenty‐five Ingredients Pill, 2 g per time, twice daily for 30 days Control A: staphylococcus Injection, once daily. The first injection was 0.2 ml, then increased by 0.1 ml till 0.5 ml, for 30 days Control B: staphylococcus Injection plus Qingre Twenty‐five Ingredients Pill. Dose and course were the same as for the other 2 groups |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "270 patients were randomly assigned to three groups by drawing lots…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding details were outlined; however, the intervention and control medicine were totally different therapies, so it seems unlikely that blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 270 participants were randomised, and 3 participants in control A group, 6 participants in intervention group, and 4 participants in control B group dropped out during the treatment. The outcome data of these dropout participants were not included in the data analysis. No ITT analysis was used |
| Selective reporting (reporting bias) | Unclear risk | Since in the methods section, the study authors did not report the outcome measures and the protocol for this trial was not available, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Wu 2008a.
| Methods | Randomised controlled trial | |
| Participants | There were 60 participants in the study, with 30 participants in each group Diagnostic criteria:
Inclusion criteria: not reported Exclusion criteria: not reported Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 26.13 (2.54); C = 24.83 (3.07) Sex ratio, male/female: I = 9/21; C = 7/23 Duration of disease (weeks): I = 15.20 (1.36); C = 14.53 (1.87) |
|
| Interventions |
Intervention: blood‐letting and cupping Selection of points: Ashi points in the first lateral line of bladder channel on the back, or Feishu (BL 13) if no obvious Ashi points Method: pricked the points quickly 10 times with a 3‐edged needle, then used cupping on the points for bleeding of 7 to 8 ml ‐ once daily, 10 times as 1 treatment course Course: 1 month Control: achromycin, orally taken, 0.25 g per time, 4 times daily; 2% Cremorketoconazoli, externally used, once daily |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned into intervention group and control group by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, because participants in the treatment group were treated by cupping and blood‐letting, while participants in the control group were given western medicine, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since in the methods section, the study authors did not mention outcome measure and we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
You 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 60 participants in the study, with 30 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 24.83 (4.54); C = 24.67 (5.33) Sex ratio, male/female: I = 17/13; C = 16/14 Duration of disease (months): I = 23.36 (9.74); C = 22.81 (8.43) |
|
| Interventions |
Intervention: 1. acupuncture on EX‐HN3, EX‐HN5, SI 18, RN 24, BL 13, BL19, BL 20, BL 23, DU 14, LI 11, LI 4, ST 36, and KI 3, with supplement deficiency and unblocking the collaterals method, using "three‐three manipulation", and retained the needles for 30 minutes, once daily; 2. blood‐letting by 3‐edged needle or No.7 needle for injection to prick the points for bleeding of 3 to 5 ml, once every 5 days, 6 times as 1 treatment course; 3. self‐made Chinese herbal facial mask, externally used, once every 3 days for 10 times Control: 1. self‐made Chinese herbal facial mask, external use, once every 3 days for 10 times; 2. tretinoin Cream (provided by Chongqing Huapont Pharm. Co., Ltd, H50021817), externally used every night, 4 weeks as 1 treatment course, 2 courses in total. There was a 5‐day break before the next course |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, because participants in the treatment group were treated by acupuncture plus cupping and blood‐letting, while participants in the control group were given western medicine and facial mask, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since in the methods section authors did not mention outcome measures, and we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Yu 2008.
| Methods | Randomised controlled trial | |
| Participants | There were 48 participants in the study, with 28 participants in the intervention group and 20 participants in the control group Diagnostic criteria: not reported Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 26.4 (5.8); C = 25.8 (6.7) Duration of disease (months): I = 24.8 (12.6); C = 25.2 (14.9) Sex: female |
|
| Interventions |
Intervention: Shugan Zishen Fang (prepared in hospital preparation room), orally taken, 100 ml, twice per day for 8 weeks Control: tetracycline, orally taken, 0.5 g, twice per day for 8 weeks |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "48 patients were randomly assigned to two groups by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, because participants in the treatment group were given TCM decoction, while participants in the control group were given western medicine, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 48 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | In the methods section, authors mentioned the condition of skin lesion including acne, papilla, pustule before and after treatment would be measured, and the result was reported as composite outcome like total effective rate. In addition, since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Yu 2011.
| Methods | Randomised controlled trial | |
| Participants | There were 130 participants in the study, with 65 participants in each group Diagnostic criteria:
Inclusion criteria: not reported Exclusion criteria: not reported Participants' baseline data presented in total, not for each group: Average age (years): 21 (4) Sex ratio, male/female: 73/57 |
|
| Interventions |
Intervention: compound Danshen tablets (provided by Guangdong Yikang Pharm. Co. Lid.), 3 tablets per time, 3 times daily; Doxycycline Hyclate Tablets (provided by Guangdong Yikang Pharm. Co. Lid.) 0.1 g per time, twice daily for 4 weeks Control: Doxycycline Hyclate tablets, 0.1 g per time, twice daily for 4 weeks Participants in both groups externally used 2% Chloramghenicol for cleaning skin |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table, and 65 cases in each group…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, because participants in the treatment group were TCM patent on the basis of western medicine, while participants in the control group were given only western medicine, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 130 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Zhang 2010.
| Methods | Randomised controlled trial | |
| Participants | There were 60 participants in the study, with 30 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age in total (years): 22.83 Sex ratio, male/female: I = 17/13; C = 16/14 Duration of disease (months): I = 30.65 (9.53); C = 27.63 (8.83) |
|
| Interventions |
Intervention: 1. acupuncture on EX‐HN5, SI 18, ST 7, LI 11, LI 4, and SJ 5 by Huatuo needle inserted the points, and manipulated needles with lifting‐thrusting for supplementation/drainage method. Retained the needles for 20 minutes. Once every other day, 6 times as 1 treatment course, 2 courses in total. There was a 3‐day break between 2 courses; 2. blood‐letting and cupping by 3‐edged needle to prick DU 14, BL 13, BL 21, BL 25, BL 20, BL 17 for bleeding of 2 to 3 ml, retained the cupping for 5 min. Once every other day, 6 times as 1 treatment course. 2 courses in total. There was a 3‐day break between 2 courses; 3. self‐made Chinese herbal facial mask, externally used, once every 3 days, 10 times as 1 treatment course, for 6 weeks Control: viaminate capsules (provided by Chongqing Huapton Pharm. Co., Ltd., 25 mg*20s/pack) 50 mg per time, 3 times daily for 6 weeks |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, because participants in the treatment group were treated by acupuncture plus cupping and blood‐letting, while participants in the control group were given western medicine, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since in the methods section, the authors had not mentioned outcome measures, and we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Zhang 2012.
| Methods | Randomised controlled trial | |
| Participants | There were 80 participants in the study, with 40 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.60 (3.66); C = 23.5 (3.70) Sex ratio, male/female: I = 13/27; C = 15/25 Duration of disease (months): I = 6.78 (4.34); C = 7.64 (4.30) |
|
| Interventions |
Intervention: Qing Bu Tang (herbal medicine), orally taken, 150 ml, twice daily for 4 weeks Control: viaminate capsules (Wei An Zhi Capsules, provided by Chongqing Huapton Pharm. Co., Ltd.) 25 mg per time, 3 times daily for 4 weeks |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by random number table, and 40 case in each group…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, because participants in the treatment group were given TCM decoction, while participants in the control group were given western medicine, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 80 participants were randomised, and "during the treatment 1 case in treatment group dropped out due to no returning visit and 5 cases in control group due to adverse events or no returning visit." The data of these participants were not included in data analysis. No ITT analysis was used |
| Selective reporting (reporting bias) | Unclear risk | Since in the methods section, the study authors had not reported outcome measures, and we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes for this trial |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Zhou 2009.
| Methods | Randomised controlled trial | |
| Participants | There were 40 participants in the study, with 20 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 23.95 (4.58); C = 24.50 (4.35) Sex ratio, male/female: I = 11/9; C = 11/9 Duration of disease (months): I = 18.75 (7.43), C = 18.80 (6.88) |
|
| Interventions |
Intervention: wet‐cupping therapy. Prick on Dazhui (DU14) and Feishu (BL13) (plus other points selected according to syndrome differentiation), insert the needle 0.2 to 0.5 cm under skin, then apply the cups on the selected area until bleeding 3 to 5 ml ‐ once every 2 days, 6 times a course, 3 days between the courses for 2 courses (27 days) Control: viaminate cream (Chongqing Hua Bong pharmaceutical limited by share Ltd) externally applied on lesion skin, once every night |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to intervention and control groups...sequence was generated by SAS software 6.12…" Comment: Even though no more detailed information was provided to describe how randomisation was assigned, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, as the intervention and control medicines were not similar in appearance and taste, it seems likely that there was no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 40 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, there was insufficient information about this item to permit judgement of 'low' or 'high' risk of bias |
| Selective reporting (reporting bias) | Unclear risk | We could not find any protocol information to assist us with judgement |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
Zhu 2007.
| Methods | Randomised controlled trial | |
| Participants | There were 80 participants in the study, with 40 participants in each group Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Participants' baseline data presented as mean (SD) for intervention (I) and control (C) groups: Average age (years): I = 22.68; C = 23.30 Sex ratio, male/female: I = 15/25; C = 12/28 Duration of disease (years): I = 16.8; C = 19.4 |
|
| Interventions |
Intervention: modified Cuo Chuang Fang, orally taken, twice daily for 6 weeks Control: isotretinoin soft capsules (trade name: Taiersi, provided by Shanghai Xinyi Yanan Pharm. Co., Ltd.) 10 mg, twice per day, taken after meal for 6 weeks |
|
| Outcomes |
|
|
| Notes | *"remission" was defined according to the "Guiding principle of clinical research on new drugs of traditional Chinese medicine", which means lesions totally faded (or > 95% faded), and only mild pigmentation and scars remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "80 patients all outpatients,and they were randomly assigned by random number table…" Comment: Even though no more detailed information was provided, it was probably generated appropriately |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information about the allocation concealment to permit judgement of 'low' or 'high' risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not outline any blinding details; however, because participants in the intervention group were given TCM decoction, while participants in the control group were given western medicine, it seems unlikely that blinding of participants and personnel was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information about the blinding method of outcome assessors to permit judgement of 'low' or 'high' risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 80 participants were randomised, and all of these were included in the analysis. It seems likely that there were no incomplete outcome data in this study. However, because there was no information about the dropout and follow‐up rate, it was hard to judge whether there was 'low' or 'high' risk of bias for this domain |
| Selective reporting (reporting bias) | Unclear risk | Since we could not find the protocol for this trial, it was hard to judge whether the study reported all of the primary outcomes in the article |
| Other bias | Unclear risk | No funding issue was apparent No sample size calculation was provided |
ASI = Acne Severity Index. ATP = adenosine triphosphate. I = intervention. IL = interleukin. ITT = intention‐to‐treat. BMI = body mass index. BUN = blood urea nitrogen. C = control group. h = hour. intervention = intervention group. IL = interleukin. KAGS = Korean Acne Grading System. GAGS = Global Acne Grading System. GI = glycaemic index. KRTE = Keigai‐rengyo‐to‐extract. mean (SD) = mean (standard deviation). min = minutes. No. = number. LED = light‐emitting diode. LGLD = low‐glycaemic‐load diet. LOCF = last observation carried forward. PBV = purified bee venom. TCM = Tradtional Chinese Medicine. TLC = total lesion count. QoL = quality of life.
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12605000233628.
| Methods | Randomised controlled trial |
| Participants |
Sample size: 18 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: tea tree oil formulation Control group: placebo |
| Outcomes |
|
| Notes | We found this study in the clinical trials registry system; the status was "complete". However, we sent an email and got no response from the contact author |
ACTRN12607000140459.
| Methods | Pilot study for a randomised controlled trial |
| Participants |
Sample size: 20 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: 3 products will be used twice daily in the trial. A topical gel containing nicotinamide (4% w/w) and sandalwood oil (2% w/w) as the actives, a cosmetic facial cleanser and an oral supplement containing a combination of vitamins and minerals (zinc 12.5 mg, nicotinamide 25 mg, vitamin B6 25 mg vitamin E 25 IU, vitamin C 125 mg, linseed oil 250 mg, and vitamin A 1250 IU). Products will be used twice daily, i.e., face will be cleansed with facial cleanser, topical gel will be applied to affected area, and 1x capsule will be taken. This process is repeated twice daily, i.e., morning and night. These products will be compared against matching placebos, i.e., identical formulations as the treatment minus the actives. There will be 2 treatment groups ‐ active and placebo. Active group will take active topical gel, active facial cleanser, and active oral supplement Control group: Placebo group will take placebo topical gel, placebo facial cleanser, and placebo oral supplement. Trial duration is 12 weeks |
| Outcomes |
|
| Notes | We found this study in the clinical trials registry system; the status was "complete". However, we sent an email and got no response from the contact author |
Differences between protocol and review
We changed the Data extraction and management section from the protocol, but it is now more concise. We created 'Summary of findings' tables and used GRADE criteria to evaluate the overall quality of the body of evidence.
Our primary outcome is 'Improvement of clinical signs assessed through skin lesion counts (total of inflamed and non‐inflamed counted separately) and acne severity scores'. We decided to report this as 'Change in inflammatory and non‐inflammatory lesion counts', 'Change of total skin lesion counts', 'Skin lesion scores', and 'Change of acne severity score'.
When we assessed the secondary outcomes, we combined the 'Physicians' global evaluation' and 'Participants' self assessment of change in specific types of lesion' and expressed them as the 'Number of participants with remission' as this had a clear definition and was easy to assess. We added description in the Methods section to clarify this type of outcome.
For missing data, we mentioned in the protocol that 'we planned to use the method of last observation carried forward for continuous data if available'; however, we could not obtain original individual participant data to carry out last observation carried forward (LOCF). Thus, we only applied intention‐to‐treat analyses to dichotomous outcomes.
Contributions of authors
JPL was the contact person with the editorial base. HC co‐ordinated contributions from the co‐authors and wrote the final draft of the review. CS and JPL commented on drafts of protocol and the review. HC, GY, and YW screened papers against eligibility criteria. HC obtained data on ongoing and unpublished studies. HC, GY, and YW appraised the quality of papers. HC, GY, and YW extracted data for the review and sought additional information about papers. HC, GY, and YW entered data into RevMan. HC analysed and interpreted data. HC, HL, CS, and JPL worked on the methods sections. HC, HL, and JPL drafted the clinical sections of the background and responded to the clinical comments of the referees. HC, CS, and JPL responded to the methodology and statistics comments of the referees. YL was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. HC and JPL is the guarantor of the update.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health, UK.
Sources of support
Internal sources
Centre for Evidence‐Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
The research capacity establishment grant of Beijing University of Chinese Medicine (grant number 201207007 and 2011‐CXTD‐09), China.
External sources
-
Grant No R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM) of the US National Institutes of Health, USA.
International Cooperation Project (Grant No 2009DFA31460) from the Ministry of Science and Technology, China.
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group
Declarations of interest
Huijuan Cao: nothing to declare. Guoyan Yang: nothing to declare. Yuyi Wang: nothing to declare. Jian Ping Liu: nothing to declare. Caroline A Smith: nothing to declare. Hui Luo: nothing to declare. Yueming Liu: nothing to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Chen 2009 {published data only}
- Chen YQ, Lai XS. A clinical study on the effect of blood‐letting therapy on acne. Dissertation for a masters degree from Guangzhou University of Chinese Medicine 2009.
Chen 2012 {published data only}
- Chen LF, Xi JY. Clinical observation on effective of XiaoZuo Mixture for 35 acne patients with Lung‐stomach heat syndrome. Dissertation for a masters degree of Guangzhou University of Chinese Medicine 2009.
- Chen LF, Xi JY. Clinical observation on effective of XiaoZuo Mixture for 35 acne patients with Lung‐stomach heat syndrome. Guiding Journal of Traditional Chinese Medicine & Pharmacy 2012;18(9):56‐8. [Google Scholar]
Enshaieh 2007 {published data only}
- Enshaieh S, Jooya A, Siadat AH, Iraji F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: A randomised, double‐blind placebo‐controlled study. Indian Journal of Dermatology, Venereology & Leprology 2007;73(1):22‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feng 2005 {published data only}
- Feng JY, Fan RQ. Zi Yin Qing Gan Xiao Guo Fang in the treatment of female acne and epidemiological study of high school students in Guangzhou. Dissertation for a masters degree from Guangzhou University of Chinese Medicine 2005.
Han 2010 {published data only}
- Han B, Mi JP. A clinical study on abdominal acupuncture for acne by conditioning liver and kidney. Dissertation for a masters degree from Guangzhou University of Chinese Medicine 2010.
Han 2013 {published data only}
- Han SM, Lee KG, Pak SC. Effects of cosmetics containing purified honeybee (Apis mellifera L.) venom on acne vulgaris. Journal of Integrative Medicine 2013;11(5):320‐326. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang S, Zhang Y. Clinical assessment on therapeutic effect of fire needling for acne with nodular and cystic. Dissertation for a masters degree from Chengdu University of Chinese Medicine 2006.
Kim 2012 {published data only}
- Kim KS, Kim YB. Anti‐inflammatory effect of Keigai‐rengyo‐to extract and acupuncture in male patients with acne vulgaris: a randomised controlled pilot trial. Journal of Alternative & Complementary Medicine 2012;18(5):501‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kwon 2012 {published data only}
- Kwon HH, Yoon JY, Hong JS, Jung JY, Park MS, Suh DH. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomised controlled trial. Acta Dermato‐Venereologica 2012;92(3):241‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2007 {published data only}
- Li ZZ, Chi FH, Fan RQ. Clinical observation on Ziyin Qinggan Xiaocuo Prescription combined with Cuo Ling Tincture for adult female acne vulgaris with yin deficiency and liver heat. Dissertation for a masters degree of Guangzhou University of Chinese Medicine 2007.
- Li ZZ, Chi FH, Fan RQ. Clinical observation on Ziyin Qinggan Xiaocuo Prescription combined with Cuo Ling Tincture for adult female acne vulgaris with yin deficiency and liver heat. Journal of Guangzhou University of Traditional Chinese Medicine 2007;24(1):30‐2. [Google Scholar]
Lin 2013 {published data only}
- Lin J. Clinical observation of wet cupping therapy on Back Shu points combined with herbal medicinal mask for acne vulgaris. China Health Care & Nutrition 2013;2:964‐5. [Google Scholar]
Liu 2004 {published data only}
- Liu W, Sun Y, Jiang Y, Li XP. Clinical observation on Xiaocuoyin in treatment of acne. Conference proceedings of dermatology branch of Chinese medicine association 2004.
Liu 2006 {unpublished data only}
- Liu CZ, Lin H. Clinical Study on Treatment of Acne Conglobata with Body Acupuncture Combined with Ventouse and Venesection;. Dissertation for a masters degree from Guangzhou College of Chinese Medicine 2006.
Liu 2007 {published data only}
- Liu YF. Theoretical and clinical study on treating acne of moist and heat in lung and stomach with combined therapy of acupuncture. Dissertation for a masters degree from Shandong University of Chinese Medicine 2007.
Liu 2008 {published data only}
- Liu CZ, Lei B, Zhen JF. Randomized control study on the treatment of 26 cases of acne conglobata with encircling acupuncture combined with venesection and cupping. Chen Tzu Yen Chiu Acupuncture research 2008;33(6):406‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu L, Li BL, Chen WH, Li BY. Therapeutic effect of blue and red LED photography combined with traditional Chinese medical mask on acne vulgaris. Chinese Journal of Aesthetic Medicine 2012;21(9):1626‐8. [Google Scholar]
Mo 2011 {published data only}
- Mo LL. Clinical study of treating acne with Yinqiao Liangxue Decotion. China Journal of Chinese medicine 2011;26(153):229‐30. [Google Scholar]
Ni 2008 {unpublished data only}
- Ni Q. Clinical Observation on Treatment of Acne about Accumulation of Heat in Lung and Stomach with Acupuncture and Massage. Dissertation for a masters degree from Guangzhou University of Chinese Medicine 2008.
Peng 2012 {published data only}
- Peng HH. Clinical study of Da Huang Zhe Chong Pills for 52 cases of acne tuberata. Forum on Traditional Chinese Medicine 2012;27(6):6‐8. [Google Scholar]
Shi 2012 {published data only}
- Shi QL. Clinical observation on Xiaoduyin modified in treatment of acne. Seek Medical and Ask The Medicine 2012;10(5):77. [Google Scholar]
Smith 2007 {published data only}
- Smith RN, Baue A, Varigos GA, Mann NJ. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. Journal of Dermatological Science 2008;50(1):41‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smith RN, Mann NJ, Braue A, Makelainen H, Varigos GA. A low‐glycemic‐load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. American Journal of Clinical Nutrition 2007;86(1):107‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smith RN, Mann NJ, Braue A, Makelainen H, Varigos GA. The effect of a high‐protein, low glycemic‐load diet versus a conventional, high glycemic‐load diet on biochemical parameters associated with acne vulgaris:A randomized, investigator‐masked, controlled trial. Journal of the American Academy of Dermatology 2007;57(2):247‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2007 {published data only}
- Song SJ. Observation on therapeutic effect of ear point blood‐letting combined with cupping on Back‐shu points for treatment of acne vulgaris. Zhongguo Zhenjiu: Chinese Acupuncture & Moxibustion 2007;27(8):626‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Tang 2011 {published data only}
- Tang ZD. Xiaocuo Qinggan Jieyu particles combined with external use traditional Chinese medicine in treatment of female's acne vulgaris of 120 cases. Journal of Guangxi traditional Chinese medicine 2011;34(5):260‐1. [Google Scholar]
Wang 2012 {unpublished data only}
- Wang N, Song W. Clinical observation on treating acne vulgaris by Chinese medicine steaming. Dissertation for a Masters Degree of Guangzhou University of Chinese Medicine 2012.
Wei 2012 {unpublished data only}
- Wei X, Zhu S. A clinical observation on treatment of liver meridian of damp‐heat acne through clearing liver and eliminating dampness. Dissertation for a Masters Degree of Guangzhou University of Chinese Medicine 2012.
Wen 2012 {published data only}
- Wen N, Hao J, Jin Z. Clinical study on facial pricking blood and quick cupping plus body acupuncture for the treatment of acne conglobata. Beijing Journal of Traditional Chinese Medicine 2012;31(3):363‐6. [Google Scholar]
Wu 2008 {published data only}
- Wu Q, Chen L. Observation on the effect of Staphylococcus aureus toxins plus Qingre Twenty‐five Ingredients Pill in treating acne vulgaris. Journal of Clinical Dermatology 2008;37(5):327‐8. [Google Scholar]
Wu 2008a {published data only}
- Wu Y. The therapeutics effect on acne by pricking blood adding cupping therapy on back. Journal of Practical Traditional Chinese Internal Medicine 2008;22(10):61‐2. [Google Scholar]
You 2012 {unpublished data only}
- You M, Liu G. The clinical study of acupuncture (Bu Xu Tong Luo Method) treatment of face acne. Dissertation for a Masters Degree of Guangzhou University of Chinese Medicine 2012.
Yu 2008 {published data only}
- Yu X, Chen J, Lin C, Chen M. From perspective of liver constraint and kidney deficiency to treat 48 cases of acne vulgaris. Journal of Liaoning University of TCM 2008;10(6):93‐4. [Google Scholar]
Yu 2011 {published data only}
- Yu J, Chen R, Zhang G, Liang G, Wang J. Clinical observation on Compound Danshen Tablets plus Doxycycline Hyclate Tablets for the treatment of acne. Chinese Journal of Postgraduates of Medicine 2011;34(9):40‐1. [Google Scholar]
Zhang 2010 {unpublished data only}
- Zhang X, Chai T. A clinical study on acne treated with Jin's three‐needle technique binding syndrome differentiation. Dissertation for a Masters Degree of Guangzhou University of Chinese Medicine 2010.
Zhang 2012 {published data only}
- Zhang H, Wang Q, Zhou C. Observation of the effect of Qing Bu Tang for severe acne. Shandong Journal of Traditional Chinese Medicine 2012;31(6):404‐5. [Google Scholar]
Zhou 2009 {unpublished data only}
- Zhou D, Feng S. A clinical study on the effect of blood‐letting therapy on acne. The clinical study of using blood‐vessel pricking to bleed at the Back‐shu points for treat acne. Dissertation for a Masters Degree of Guangzhou University of Chinese Medicine 2009.
Zhu 2007 {published data only}
- Zhu Z. Observation of therapeutic effect of acne vulgaris with the herb of Cuo Chuang Prescription. Chinese Journal of Dermatovenereology 2007;21(5):31‐311. [Google Scholar]
References to studies awaiting assessment
ACTRN12605000233628 {published data only}
- ACTRN12605000233628. The effects of Tea Tree oil on acne. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12605000233628 (accessed 15 August 2013).
ACTRN12607000140459 {published data only}
- ACTRN12607000140459. Pilot study in patients with mild to moderate acne utilising a facial cleanser, topical gel and oral supplement compared to placebo for treatment of acne. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12607000140459 (accessed 15 August 2013).
Additional references
Buettner 2009
- Buettner C, Mukamal KJ, Gardiner P, Davis RB, Phillips RS, Mittleman MA. Herbal supplement use and blood lead levels of United States adults. Journal of General Internal Medicine 2009;24(11):1175‐1182. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2010
- Cao H, Han M, Li X, Dong S, Shang Y, Wang Q, et al. Clinical research evidence of cupping therapy in China: a systematic literature review. BMC Complementary & Alternative Medicine 2010;10:70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenberg 1998
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Rompay M, et al. Trends in alternative medicine use in the United States, 1990‐1997: results of a follow‐up national survey. Journal of the American Medical Association 1998;280(18):1569‐75. [EMBASE: 1998386275] [DOI] [PubMed] [Google Scholar]
El‐Akawi 2006
- El‐Akawi Z, Nemr NA, Abdul‐Razzak K, Al‐Aboosi M. Factors believed by Jordanian acne patients to affect their acne condition. Eastern Mediterranean Health Journal 2006;12(6):840‐6. [EMBASE: 2007118895] [PubMed] [Google Scholar]
Enders 2003
- Enders SJ, Enders JM. Isotretinoin and psychiatric illness in adolescents and young adults. Annals of Pharmacotherapy 2003;37(7‐8):1124‐7. [EMBASE: 2003295056] [DOI] [PubMed] [Google Scholar]
Fried 2010
- Fried R, Friedman A. Psychosocial sequelae related to acne: looking beyond the physical. Journal of Drugs in Dermatology 2010;9(5 Suppl):s50‐2. [EMBASE: 20518360] [PubMed] [Google Scholar]
Friedlander 2010
- Friedlander SF, Eichenfield LF, Fowler JF, Fried RG, Levy ML, Webster GF. Acne epidemiology and pathophysiology. Seminars in Cutaneous Medicine & Surgery 2010;29(2 Suppl 1):2‐4. [EMBASE: 20610306] [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [EMBASE: 2005224467] [DOI] [PubMed] [Google Scholar]
Garner 2009
- Garner SE, Eady A, Popescu CM, Newton J, Li Wan Po A. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD002086] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011 ]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katsambas 2004
- Katsambas AD, Stefanaki C, Cunliffe WJ. Guidelines for treating acne. Clinics in Dermatology 2004;22(5):439‐44. [EMBASE: 2004499754] [DOI] [PubMed] [Google Scholar]
Lewith 2001
- Lewith G, Jonas WB, Walach H. Clinical Research in Complementary Therapies: Principals, Problems and Solutions. London: Churchill Livingstone, 2001. [Google Scholar]
Li 2009
- Li B, Chai H, Du YH, Xiao L, Xiong J. Evaluation of therapeutic effect and safety for clinical randomized and controlled trials of treatment of acne with acupuncture and moxibustion. Chinese Acupuncture & Moxibustion 2009;29(3):247‐51. [EMBASE: 19358513] [PubMed] [Google Scholar]
Magin 2005
- Magin P, Pond D, Smith W, Watson A. A systematic review of the evidence for "myths and misconceptions" in acne management: diet, face‐washing and sunlight. Family Practice 2005;22(1):62‐70. [EMBASE: 2005101150] [DOI] [PubMed] [Google Scholar]
Magin 2006
- Magin PJ, Adams J, Pond CD, Smith W. Topical and oral CAM in acne: A review of the empirical evidence and a consideration of its context. Complementary Therapies in Medicine 2006;14(1):62‐76. [EMBASE: 2006073257] [DOI] [PubMed] [Google Scholar]
Mail Online 2013
- Reporter. Chinese medicines contain 'dangerously high' levels of lead, mercury and arsenic, warns health watchdog. www.dailymail.co.uk/health/article‐2398192/Health‐watchdog‐warns‐dangerously‐high‐levels‐lead‐mercury‐arsenic‐unlicensed‐Chinese‐medicines.html (accessed 28 February 2013).
Marqueling 2007
- Marqueling AL, Zane LT. Depression and suicidal behavior in acne patients treated with isotretinoin: a systematic review. Seminars in Cutaneous Medicine & Surgery 2007;26(4):210‐20. [EMBASE: 2008157859] [DOI] [PubMed] [Google Scholar]
Martin 2001
- Martin AR, Lookingbill DP, Botek A, Light J, Thiboutot D, Girman CJ. Health‐related quality of life among patients with facial acne ‐‐ assessment of a new acne‐specific questionnaire. Clinical & Experimental Dermatology 2001;26(5):380‐385. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martin 2003
- Martin KW, Ernst E. Herbal medicines for treatment of bacterial infections: a review of controlled clinical trials. Journal of Antimicrobial Chemotherapy 2003;51(2):241‐6. [EMBASE: 2003083760] [DOI] [PubMed] [Google Scholar]
O'Connor 2013
- O'Connor Ahahad. Herbal supplements are not often what they seem. www.nytimes.com/2013/11/05/science/herbal‐supplements‐are‐often‐not‐what‐they‐seem.html (accessed 4 November 2014).
Oberemok 2002
- Oberemok SS, Shalita AR. Acne vulgaris, I: pathogenesis and diagnosis. Cutis 2002;70(2):101‐5. [EMBASE: 12234155] [PubMed] [Google Scholar]
Oberemok 2002a
- Oberemok SS, Shalita AR. Acne vulgaris, II: treatment. Cutis 2002;70(2):111‐4. [EMBASE: 12234157] [PubMed] [Google Scholar]
Pochi 1991
- Pochi PE, Shalita AR, Strauss JS, Webster SB. Report of the Consensus Conference on Acne Classification, Washington, DC, March 24 and 25, 1990. Journal of the American Academy of Dermatology 1991;24(3):495–500. [EMBASE: 1991105563] [DOI] [PubMed] [Google Scholar]
Ramos‐e‐Silva 2009
- Ramos‐e‐Silva M, Carneiro SC. Acne vulgaris: review and guidelines. Dermatology Nursing 2009;21(2):63‐8. [EMBASE: 19507372] [PubMed] [Google Scholar]
Shen 1995
- Shen DH, Wang N, Wu XF. Manual of Dermatology in Chinese Medicine. WA: Eastland Press, 1996. [Google Scholar]
Simon 2005
- Simon C, Everitt H, Kendrick T. Oxford Handbook of General Practice. 2nd Edition. New York: Oxford University Press, 2005. [Google Scholar]
Simonart 2005
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. British Journal of Dermatology 2005;153(2):395‐403. [EMBASE: 2005396090] [DOI] [PubMed] [Google Scholar]
Spencer 2009
- Spencer EH, Ferdowsian HR, Barnard ND. Diet and acne: a review of the evidence. International Journal of Dermatology 2009;48(4):339‐47. [EMBASE: 2009151447] [DOI] [PubMed] [Google Scholar]
Starling 2013
- Starling S. 'Very serious health hazard': Swedes bust Chinese herbs containing arsenic. www.nutraingredients.com/Regulation/Very‐serious‐health‐hazard‐Swedes‐bust‐Chinese‐herbs‐containing‐arsenic (accessed on 28 February 2013).
Sørensen 2010
- Sørensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: Associations with body mass index. Journal of Clinical Endocrinology & Metabolism 2010;95(1):263‐70. [EMBASE: 2010069119] [DOI] [PubMed] [Google Scholar]
Toyoda 1998
- Toyoda M, Morohashi M. An overview of topical antibiotics for acne treatment. Dermatology 1998;196(1):130‐4. [EMBASE: 1998082842] [DOI] [PubMed] [Google Scholar]
Verhoef 1999
- Verhoef MJ, Hilsden RJ, O'Beirne M. Complementary therapies and cancer care: an overview. Patient Education & Counseling 1999;38(2):93‐100. [EMBASE: 14528701] [DOI] [PubMed] [Google Scholar]
Walton 1988
- Walton S, Wyatt EH, Cunliffe WJ. Genetic control of sebum excretion and acne ‐ a twin study. British Journal of Dermatology 1988;118(3):393‐6. [EMBASE: 1988157488] [DOI] [PubMed] [Google Scholar]
Webster 2002
- Webster GF. Acne vulgaris. British Medical Journal 2002;325(7362):475‐9. [EMBASE: 2002318657] [PMC free article] [PubMed] [Google Scholar]
Wolf 2004
- Wolf R, Matz H, Orion E. Acne and diet. Clinics in Dermatology 2004;22(5):387‐93. [EMBASE: 2004499746] [DOI] [PubMed] [Google Scholar]
Workshop 1992
- Alternative medicine: expanding medical horizons. [Workshop on Alternative Medicine]. A report to the National Institutes of Health on alternative medical systems and practices in the United States. Washington DC: US Government Printing Office, 1992.
Wu 2013
- Wu ML, Deng JF, Lin KP, Tsai WJ. Lead, mercury, and arsenic poisoning due to topical use of traditional Chinese medicines. American Journal of Medicine 2013;126(5):451‐454. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zheng 2002
- Zheng XY. Guiding principle of clinical research on new drugs of traditional Chinese medicine. Beijing: The Medicine Science & Technology Press of China, 2002:292‐5. [Google Scholar]


